Abstract
Full body repetitive behaviors, known as motor stereotypic behaviors (MSBs), are one of the most commonly seen abnormal behaviors in captive non-human primates, and are frequently used as a behavioral measure of well-being. The main goal of this paper was to examine the role of environmental factors (i.e., foraging enrichment and socialization) and intrinsic factors (i.e., temperament and origin) in the development of MSB in rhesus macaques living in cages. MSB was assessed during short annual observations in which a trained observer recorded a monkey’s behavior for 5 min, followed by a 3-min novel object test. Data were collected over 11 years, totaling 9805 observations. We compared MSB for animals with and without foraging enrichment, and across three socialization conditions: full contact pairing, protected contact socialization (partners physically separated by widely spaced bars), and single housing. In addition, we evaluated whether individual differences in response to a novel object and ancestral origin (i.e., China vs. India), predicted MSB expression during the annual observations. Data were analyzed using generalized mixed effects modeling, with the best fitting models chosen using Akaike Information Criterion. Subjects were at lowest risk for MSB when a foraging device was present (p < 0.05), and when in full contact social housing (p < 0.001). There was no statistically significant difference in MSB between subjects that were single housed and subjects housed in protected contact pairs. In addition, subjects that never touched the novel object were significantly less likely to exhibit MSB than those that touched the object immediately (p < 0.001) or within 3 min (p < 0.001). Finally, monkeys with some degree of Chinese ancestry were significantly more likely to display MSB than Indian-origin monkeys (p < 0.05). These results add to the growing body of literature on factors that can contribute to the development of MSB.
Keywords: stereotypy, enrichment, abnormal behavior, welfare, foraging, temperament, protected-contact
1. Introduction
Stereotypic behaviors, typically defined as repetitive behaviors caused by central nervous system dysfunction, frustration, or repeated attempts to cope (Mason, 2006), are commonly observed in captive rhesus macaques (Macaca mulatta) (Lutz et al., 2003; Lutz et al., 2011). Full body repetitive behaviors, known as motor stereotypic behaviors (MSBs), are the most commonly seen abnormal behavior in rhesus macaques (Lutz et al., 2003); in a survey of three National Primate Research Centers, 18-49% of singly housed rhesus monkeys were reported as displaying some form of MSB, such as repetitive pacing, bouncing, swaying, rocking, or somersaulting (Lutz et al., 2011). Because they are often linked to frustration, lack of stimulation, lack of environmental control, and/or unavoidable stress (Mason, 1991; Mason and Latham, 2004; Mench and Mason, 1997), MSBs are frequently used as a behavioral metric to help evaluate captive primate well-being (e.g., Bayne et al., 1991; Gottlieb et al., 2013b; Novak et al., 1998). Common remediation strategies include foraging enrichment and socialization.
Previous research has identified multiple environmental risk factors that positively predict the development and expression of MSB in rhesus macaques, including time spent indoors (Gottlieb et al., 2013a), relocations (Gottlieb et al., 2013a), and blood draws (Lutz et al., 2003). Further, being single housed at a young age (Lutz et al., 2003) and reared indoors (as opposed to outdoors) (Gottlieb et al., 2013a; Novak et al., 2006) are both risk factors for the development of MSB. Despite the many known environmental factors, the odds of an individual developing or expressing the behavior differs depending on various individual intrinsic factors. For example, it is well established that males are more likely to express MSB than females (Gottlieb et al., 2013a; Lutz et al., 2003) and that rate of MSB expression decreases with age (Gottlieb et al., 2013a; Lutz et al., 2003). Additionally, there is growing evidence that individual factors such as temperament or personality, and ancestral origin (i.e., China vs. India), may play a role in the development of MSB in rhesus macaques (Champoux et al., 1997; Gottlieb et al., 2013a; Vandeleest et al., 2011). In a recent study, infant rhesus macaques with an active personality were more likely than others to develop MSB later in life (Gottlieb et al., 2013a). While these studies have added to our knowledge of MSB, more work is needed to determine if findings such as these are robust and consistent across multiple environments.
In order to reduce MSB, most, if not all, facilities housing NHPs regularly utilize some form of foraging enrichment, such as devices and substrate covered or filled with food (Baker et al., 2007). Foraging enrichment is designed to increase species typical behaviors and thereby help reduce boredom, a factor often thought to underlie the development of the MSB. This benefit, however, may be short lived, as foraging devices may be ineffective once depleted of food. Furthermore, it is currently unclear whether repeated exposure to foraging enrichment is effective in decreasing stereotypic behaviors (Lutz and Novak, 2005). While some studies evaluating foraging enrichment have shown significant decreases in abnormal behaviors (e.g., Bayne et al., 1991; Novak et al., 1998), others found no such effects (e.g., Byrne and Suomi, 1991; Schapiro and Bloomsmith, 1995; for a review see Lutz and Novak, 2005).
Another commonly utilized strategy for reducing MSB for caged animals is providing a social partner. Compared to single housing, full contact pairing, in which two animals live in two adjoined cages, has reliably been shown to correlate with decreased expression of MSB in rhesus monkeys (Baker et al., 2012a; Baker et al., 2013; Gottlieb et al., 2013a; Lutz et al., 2003). In contrast, protected contact, a kind of housing in which two NHPs are separated by a partially open divider that allows some tactile contact, has not always shown the same reduction. Several studies on rhesus macaques have found higher abnormal behaviors in protected contact than full contact housing (Baker et al., 2013; Baker et al., 2012b; Gottlieb et al., 2013a). However, the separation barriers in these studies were made of mesh or were solid slides with relatively small holes for grooming, and thus provided limited physical contact between partners. In contrast, multiple studies on longtailed macaques (Macaca fascicularis) have utilized widely spaced bars for protected contact housing, allowing for more tactile contact and grooming opportunities. In these studies, there were no statistically significant differences in abnormal behaviors, including MSBs, between protected and full contact housing (Baker et al., 2012b; Lee et al., 2012). While it is possible that the disparate results could be due to species differences, it is also possible that the increased opportunities for tactile contact may have helped reduce MSB. Thus, we examined whether protected contact barriers with widely spaced bars reduced MSB in rhesus macaques.
The first goal of the present study was to evaluate whether long term foraging enrichment and socialization strategies significantly affected MSB expression in indoor-housed rhesus macaques. The second goal of this study was to examined the role of intrinsic factors “ancestry” (all domestically bred rhesus macaques can be traced back to either Indian or Chinese ancestry, or a hybrid between the two (Kanthaswamy et al., 2009)) and “temperament” on the expression of MSB. To assess temperament, we utilized a novel object test (e.g., Coleman et al., 2005) to assess the trait “behavioral inhibition,” defined as withdrawal from or timidity towards the unfamiliar (Kagan et al., 1987). We performed a retrospective analysis, utilizing 11 years of MSB data on over 3000 subjects. Unlike prospective studies, which typically evaluate a short-term change in behavior on a limited number of subjects, our analysis allowed us to evaluate the effect of enrichment and housing condition on a large sample of animals over an extended period of time. This comprehensive approach, which utilizes environmental as well as intrinsic factors from a large sample of individuals, will increase our understanding of stereotypy expression in captive primates.
2. Materials and methods
2.1. Subjects
Subjects in this study were 3823 rhesus macaques (Macaca mulatta) at the Oregon National Primate Research Center (ONPRC) (1419 males, 2404 females), with an average age of 10.14 years (standard deviation = 5.32 years). At the time of the behavioral assessment (see below), subjects were housed indoors in cages 76.2-91.4 cm high with 1.4-2.7 m2 of flooring, depending on the size of the animal, in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council 2011). Animals were housed in one of the following conditions: full contact pair housing, in which partners shared two adjacent cages and had full access to one another, protected contact pair housing, in which partners were physically separated by a partition (half of the partition was solid and the other consisted of widely spaced bars 4.5 cm apart), or single housing, in which animal had visual and auditory but no physical access with conspecifics. All animals had visual access to at least one other conspecific at all times. Subjects were fed standard monkey chow (High Protein Monkey Chow, Ralston-Purina, St. Louis, MO) twice a day (in the morning and afternoon), as well as produce or food enrichment daily, and water was provided ad libitum through automatic lixit systems. All animals were cared for in compliance with protocols approved by the ONPRC Institutional Animal Care and Use Committee, and participated in the ONPRC Behavioral Management plan. The ONPRC animal care program is compliant with the United States Animal Welfare Act regulations (Animal Welfare Act, 1985) and is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC), International.
Over the course of this study, foraging devices such as puzzle balls, peanut feeders, and foraging trays were available to all indoor-housed animals at the ONPRC on a rotating basis. Foraging devices were rotated regularly to avoid habituation. For the majority of this study, most animals were provided with a single device for two weeks, followed by two weeks with no device, and then two weeks with a different device. In 2012, the ONPRC began to phase out the two-week period without the device in some animal rooms (i.e., in these rooms devices were always present but still rotated every two weeks). All devices were affixed to the outside of the animal’s cage and were filled in the afternoon with forage materials such as grains, trail mix, and peanuts at least three days a week (Fig. 1). During periods in which foraging devices were not present, monkeys were given fresh produce and/or other food enrichment directly into or on top of their cages. Additional standard enrichment such as chew toys, television, and radio were provided on a regular basis.
Fig. 1.
Two rhesus macaques utilizing a type of foraging enrichment.
Rearing environment has consistently been a major factor influencing risk of MSB development and expression (Gottlieb et al., 2013a; Novak et al., 2006). To determine the role of the various rearing environments at the ONPRC on the development of MSB, we categorized subjects into one of seven conditions, based on where they spent the first six months of life (Table 1). The majority of subjects were born in groups at the ONPRC, including corrals (1 acre outdoor enclosure), sheltered housing (130 square m outdoor, environmentally controlled enclosures), and indoor groups (indoor housing 6 to 48 sq m with or without an outdoor area). Other animals were reared in a cage with a dam (i.e., indoor mother reared) or in the ONPRC nursery without a dam (i.e., nursery reared). For each of these categories, animals spent at least 75% of their first 6 months in that condition. Animals that spent less than 75% of time in a given condition (e.g., an animal that spent the first 3 months in a corral and then moved to the nursery) were categorized as “mixed rearing.” The majority of “mixed rearing” animals spent time in both cage and group rearing conditions. Finally, animals that were not born at the ONPRC were categorized as “acquired”.
Table 1.
Rearing condition of subjects. Type of housing in which animals spent at least 75% of their first six months of life.
| Rearing Condition | Description | n |
|---|---|---|
| Corral | Monkeys raised in 1-acre outdoor enclosure consisting of 150-250 individuals. |
1230 |
| Sheltered Housing | Monkeys raised in 130 square m outdoor environmentally controlled enclosures with 30-50 individuals. |
485 |
| Indoor-Group Housing | Monkeys raised in small group housing consisting of 6- 15 individuals located indoors with or without an outdoor area. |
152 |
| Indoor Mother Reared | Monkeys raised in indoor caging raised with biological or foster mother. |
379 |
| Nursery Reared | Monkeys raised in indoor nursery without monkey’s mother. Nursery reared monkeys were full paired and given playtime with conspecifics until 6-12 months of age. |
157 |
| Mixed | Monkeys born at the ONPRC that spent more than 25% of time in at least two types of housing. |
256 |
| Acquired | Monkeys not born at ONPRC. Monkeys typically acquired as adults. |
1164 |
While most of the subjects were of Indian descent (n=2722), 937 had some Chinese descent (either pure Chinese or Chinese-Indian hybrid). Given the relatively low number of subjects with Chinese descent, individuals with any Chinese ancestry were categorized as “Chinese” in our analyses. We were not able to track ancestry in 164 animals.
2.2. Behavior assessments
Since 2002, the ONPRC has performed short annual behavior assessments on all indoor-housed macaques, totaling 9805 observations on rhesus macaques from January 2002 until December 2012. A single observer performed the majority of observations (70%), and functioned as the point person responsible for training any additional observers. Animals that were indoor-housed for multiple years were observed once per year. Thus, subjects could have had up to 11 observations. Of the 3823 subjects, 2170 were observed more than once. On average subjects participated in 2.56 (SD= 2.05) behavior assessments. During these assessments, a trained observer monitored 1-4 animals at a time for 5 min, and recorded their current housing condition (e.g., full paired, protected contact paired or singly housed) the presence or absence of an enrichment device, and whether they engaged in various behaviors, including MSBs. MSBs were defined as three or more repeated full body locomotion behaviors, including repetitive pacing, bouncing, rocking, swinging, and somersaulting.
At the end of the 5-min period the observer performed a short temperament test, utilizing a novel object. The observer placed a novel food item inside the animal’s cage, and remained by the cage for 3-min recording the time it took for the subject to inspect the novel food. Inspection was defined as any intentional visual, olfactory, or tactile examination of the object. Responses were classified as bold if the subject inspected the object within 5 seconds, moderate if the subject inspected the object, but not within 5 seconds, and inhibited if the subject did not inspect the object within the 3-min testing period. Multiple novel food items were utilized over the 11-year period to avoid familiarity with the items. Food items were selected that had a shape, color, and/or texture that were unfamiliar to the monkeys, including black licorice, cheese puffs, and fruit rollups. All behavior assessments were performed after the morning feeding and before noon to avoid time of day effects and to control for hunger. Foraging devices had been filled at least 12 hours prior to these observations.
2.3. Data analysis
Data were analyzed using generalized mixed effects modeling with hierarchical model selection in R computational software (R Development Core Team, 2013). Analyses were performed using a binomial distribution, with presence/absence of MSB during the 5-min behavior assessment as the outcome. Because multiple observations were performed on the same individuals, individual was included in the model as a random effect. There were minor changes husbandry practices at the ONPRC from year to year, and to account for this, year of observation was included in the model as a random effect. Covariates rearing condition, age and sex were retained in the model regardless of corresponding p-value due to their established relationship with MSB (Gottlieb et al., 2013a; Lutz et al., 2003; Novak et al., 2006). All reported beta coefficients are conditional values (i.e., they are determined with all other coefficients held constant).
Hierarchical model selection was performed through comparison of multiple models, starting with the simplest possible model that included the intercept, covariates, and random effects. Additional models that included a single new variable were then compared in a predetermined stepwise fashion based on biological significance and increasing complexity of the variables. Variables tested include presence of an enrichment device, pairing status, response to novel object, and animal origin. At each step, Akaike Information Criterion (AIC) was used to evaluate whether the addition of variables improved the model. AIC is a popular information criterion used to compare candidate models, in which models are rewarded for goodness of fit and penalized for over-complexity (Akaike, 1987). If the addition of variables to the model lowered AIC more than 2 points this was considered an improved model of the data (Burnham and Anderson, 2002). The model with the lowest AIC was chosen as the best fit of the data. Contrasts were performed between all levels of categorical variables with more than two levels, and 95% confidence intervals were used to evaluate which levels of the variable were functionally distinct. Model diagnoses were performed at each step to ensure that collinearity did not occur.
3. Results
3.1. Behavior assessment summary statistics
MSB was observed during 14% of all behavior assessments. Almost a quarter (24%) of subjects performed MSB during at least one assessment. The vast majority (84%) of the novel object tests resulted in bold responses (i.e., subject inspected the object within 5 sec), while 8% resulted in moderate responses (i.e., subject inspected the object but not within 5 sec) and 8% resulted in inhibited responses (i.e., subject did not inspect the object). Foraging devices were present during 44% of observations. Subjects were single housed in 62% of observations, full contact pair housed in 26% of observations, and protected contact housed in 12% of observations.
3.2. MSB
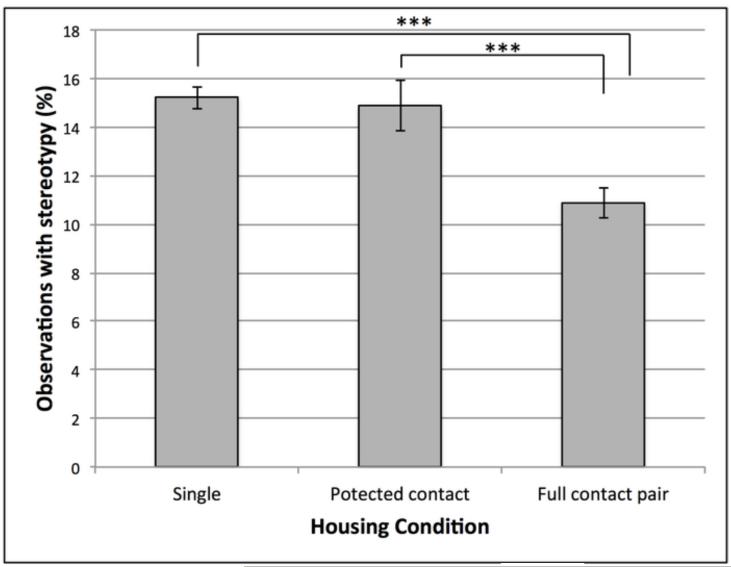
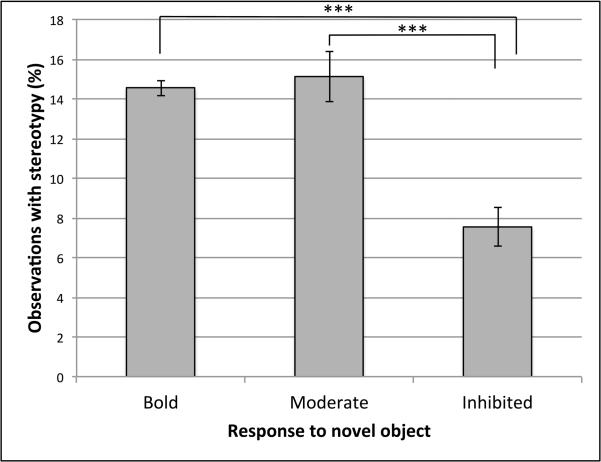
The best fitting model found MSB expression to be predicted by the presence of an enrichment device, the subject’s pairing status, response to novel object, and origin, in addition to the subject’s rearing history, sex, and age. See Table 2 for a comparison of AIC values from competing models, and tables 3 and 4 for a list of beta coefficients, standard error, p-values, 95% confidence intervals, and contrasts for each variable in the model. In the best fitting model, subjects were 15% less likely to display MSB when they had a foraging device hanging on their cage than when no device was present (p < 0.05). Animals were 49% and 41% less likely to display MSB when full contact pair housed than when single housed or protected contact housed, respectively (p < 0.001, p < 0.001). There was no statistical difference in MSB between single housed and protected contact housed subjects (Fig. 2). Animals with bold or moderate responses to the novel object test were 2.73 and 2.27 times as likely to display MSB as subjects with inhibited responses, respectively (p < 0.001, p < 0.001) (Fig. 3). Finally, subjects were 49% more likely to display MSB if they had any degree of Chinese ancestry than if they did not (p < 0.05).
Table 2.
Comparison of AIC of various models. Models were compared in a predetermined stepwise fashion based on biological significance and increasing complexity of the variables. Models with lower AIC are considered better fitting. Best fitting model in italics.
| Model | Variables included in the model | AIC |
|---|---|---|
| 1 | Intercept and random effects for animal and year (null model) | 7415 |
| 2 | Rearing, sex, age, and random effects for animal and year (covariate only model) | 7064 |
| 3 | Rearing, sex, age, origin and random effects for animal and year | 7061 |
| 4 | Rearing, sex, age, origin, response to novel object, and random effects for animal and year |
7027 |
| 5 | Rearing, sex, age, origin, response to novel object, enrichment device, and random effects for animal and year |
7024 |
| 6 |
Rearing, sex, age, origin, response to novel object, enrichment device, pairing,
and random effects for animal and year |
6985 |
Table 3.
Variables included in final model. Variables with positive beta (ß) estimates positively predict MSB, variables with negative beta estimates negatively predict MSB. Categorical variables all have a “referent” variable, the variable to which other levels of the variable are compared.
| Variable | ß Estimate (SE) | Lower 95% CI | Upper 95% CI | p-value |
|---|---|---|---|---|
|
| ||||
|
Model: Motor stereotypic behavior (MSB) ~ rearing, sex, age, origin, response to novel
object, current pair, enrichment device | ||||
| Rearing | ||||
| Corral | – | – | – | – |
| Shelter | 0.55 (0.23) | 0.10 | 1.00 | < 0.05 |
| Indoor-group | 1.60 (0.24) | 1.12 | 2.07 | < 0.001 |
| Indoor-mother | 2.68 (0.18) | 2.32 | 3.04 | < 0.001 |
| Nursery | 2.62 (0.23) | 2.16 | 3.08 | < 0.001 |
| Mix | 1.22 (0.22) | 0.80 | 1.65 | < 0.001 |
| Acquired | 0.45 (0.19) | 0.08 | 0.81 | < 0.05 |
| Sex | ||||
| Female | – | – | – | – |
| Male | 0.23 (0.13) | −0.03 | 0.48 | < 0.10 |
| Age in years (linear predictor) | 0.05 (0.04) | −0.03 | 0.12 | 0.23 |
| Age in years (quadratic predictor) | −0.004 (.002) | −0.01 | 0.00 | < 0.05 |
| Origin | ||||
| Indian | – | – | – | – |
| Chinese | 0.40 (0.17) | 0.07 | 0.73 | < 0.05 |
| Response to novel object | – | |||
| Inhibited | – | – | – | – |
| Moderate | 0.82 (0.25) | 0.34 | 1.30 | < 0.001 |
| Bold | 1.00 (0.21) | 0.60 | 1.41 | < 0.001 |
| Enrichment device | ||||
| Absent | – | – | – | – |
| Present | −0.17 (0.08) | −0.33 | 0.01 | < 0.05 |
| Pairing status | ||||
| Full contact pair housed | – | – | – | – |
| Protected contact housed | 0.53 (0.15) | 0.23 | 0.83 | < 0.001 |
| Single housed | 0.67 (0.11) | 0.46 | 0.89 | < 0.001 |
Table 4.
Contrasts between categorical variables in the final model.
| Variable | ß Estimate (SE) | Lower 95% CI | Upper 95% CI |
|---|---|---|---|
| Rearing | |||
| Shelter – Indoor-group | −1.05 | −1.61 | −0.49 |
| Shelter – Indoor-mother | −2.13 | −2.59 | −1.68 |
| Shelter – Nursery | −2.07 | −2.62 | −1.52 |
| Shelter – Mix | −0.67 | −1.21 | −0.14 |
| Shelter – Acquired | 0.10 | −0.40 | 0.61 |
| Indoor-group – Indoor-mother | −1.08 | −1.57 | −0.59 |
| Indoor-group – Nursery | −1.02 | −1.59 | −0.44 |
| Indoor-group – Mix | 0.38 | −0.18 | 0.93 |
| Indoor-group – Acquired | 1.15 | 0.65 | 1.66 |
| Indoor-mother – Nursery | 0.06 | −0.41 | 0.53 |
| Indoor-mother – Mix | 1.46 | 1.01 | 1.90 |
| Indoor-mother – Acquired | 2.23 | 1.84 | 2.63 |
| Nursery – Mix | 1.39 | 0.86 | 1.93 |
| Nursery – Acquired | 2.17 | 1.67 | 2.67 |
| Mix – Acquired | 0.78 | 0.32 | 1.23 |
| Response to novel object | |||
| Moderate – Bold | −0.19 | −0.49 | 0.12 |
| Pairing Status | |||
| Single housed – Protected contact housed |
0.14 | −0.14 | 0.39 |
Fig. 2.
Percent of observations in which subjects displayed motor stereotypic behavior in housing conditions (single housed, protected contact housed, and full contact pair housed). *** indicates significant differences at P < 0.001. Note: Plot represents raw data, however statistical analyses and effect sizes were calculated while controlling for all other variables.
Fig. 3.
Percent of observations in which subjects displayed motor stereotypic behavior categorized by subject’s response to novel object: inspected the object within 5 sec (Bold), inspected the object in more than 5 sec (Moderate), or did not inspect object (Inhibited). *** indicates significant differences at P < 0.001. Note: Plot represents raw data, however statistical analyses and effect sizes were calculated while controlling for all other variables.
In the final model, males were 26% more likely to display MSB than females, although the effect of sex only approached significance (p = 0.08). Age predicted MSB using a positive linear predictor (p < 0.001) and a negative quadratic predictor (p < 0.05). Specifically, risk of MSB increased until an animal was 6 years old, after which there was an exponential decrease in MSB risk with increasing age.
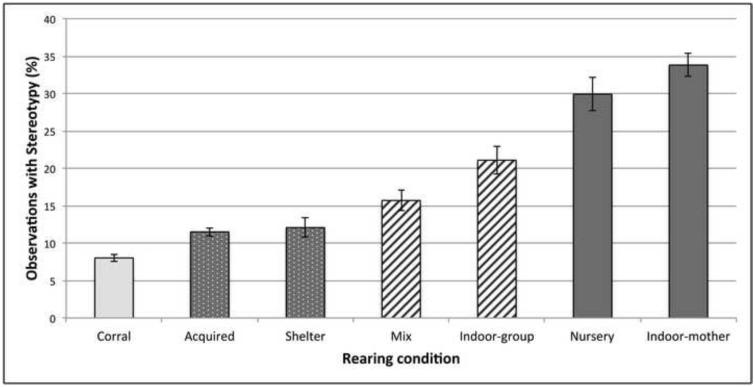
Compared to corral reared animals, risk of MSB increased by 56% for acquired (p < 0.05), 73% for sheltered housed reared (p < 0.05), 240% for mixed rearing (p < 0.001), 395% for indoor group housing reared (p < 0.001), 1270% for nursery reared (p < 0.001), and 1359% for indoor mother reared monkeys (p < 0.001). Contrasts between the 7 rearing conditions with 95% confidence intervals were used to evaluate which rearing environments led to significantly different risks of MSB. Using the 95% confidence intervals, compared to acquired animals, monkeys’ risk of MSB increased by 118% for mixed rearing, 217% for indoor group housing reared, 777% for nursery reared, and 834% for indoor mother reared. When comparing to sheltered housed reared animals, monkeys’ risk of MSB increased by 96% for mixed rearing, 186% for indoor group housing reared, 691% for nursery reared, and 743% for indoor mother reared. Compared to mixed reared animals, monkeys’ risk of MSB increased by 303% for nursery reared, and 329% for indoor mother reared. Finally, compared to indoor-group reared animals, monkeys’ risk of MSB increased by 177% for nursery reared, and 195% for indoor mother reared. Overall, subjects were most likely to express MSB if either nursery reared or indoor mother reared (Fig. 4).
Fig. 4.
Percent of observations in which subjects from various rearing conditions displayed motor stereotypic behavior. Rearing conditions that have the same shading have overlapping 95% confidence intervals and are not considered significantly different. Note: Plot represents raw data, however statistical analyses, and effect sizes, and 95% confidence intervals were calculated while controlling for all other variables.
4. Discussion
4.1. Foraging enrichment
Once developed, stereotypic behaviors across species are most frequently elicited by unavoidable stress, chronic frustration, or low levels of stimulation (Mason, 1991). Enrichment programs that effectively decrease established rates of stereotypic behavior expression are assumed to benefit the animals by either decreasing underlying animal stress or frustration, or providing needed stimulation. In our study, subjects were 15% less likely to exhibit MSB when a foraging device was present on their cage compared to when there was no device. Even though individuals still exhibited MSB when foraging devices were present, these results demonstrate a beneficial effect of foraging enrichment.
Food enrichment was provided daily for each monkey, regardless of whether or not a foraging device was present. Thus, the significant decrease in MSB associated with foraging devices was likely due to the opportunity to forage and work for enrichment afforded by the device, as opposed to the actual food itself. There are several potential reasons for this decrease in MSB. First, MSBs are incompatible with behaviors such as foraging from an enrichment device; subjects are physically unable to simultaneously forage and pace. Thus decreases in MSB seen during observations taken shortly after the provision of foraging enrichment may be explained by the presence of foraging or other incompatible behaviors. In the present study, observations were taken at least 12 hours after foraging devices were filled. Anecdotally, most devices were empty at the time of the observation, making it highly unlikely the observed decrease in MSB was purely caused by incompatible behaviors. Rather, given the known triggers of stereotypic behavior expression, it is reasonable to assume the foraging enrichment decreased MSB by increasing stimulation and/or decreasing stress or frustration. In other words, the simple act of extracting food from the foraging devices, however short lived it may have been, may have provided some basic sort of stimulation. More importantly, providing an opportunity to forage may have decreased overall animal frustration. In the wild, rhesus macaques can spend as much up to half of their daily activity foraging and searching for food (Chancellor and Isbell, 2008; Neville, 1968; Seth and Seth, 1986). In captivity, animals are typically fed once or twice a day, greatly reducing this foraging time. The inability to perform species-normal behavior such as foraging can cause stress and frustration for captive animals (Petherick and Rushen, 1997). Thus, the increase in foraging opportunities may have reduced frustration and, in turn, decreased MSB expression.
4.2. Social enrichment
Monkeys were significantly less likely to demonstrate MSB when full contact pair housed than when single housed. These results were not surprising; several studies have found that single housed macaques are more likely to display anxiety and abnormal behaviors, including MSB, compared pair housed animals (Baker et al., 2012a; Baker et al., 2013; Gottlieb et al., 2013a; Schapiro et al., 1996). What was somewhat surprising was the finding that animals housed in protected contact showed the same amount of MSB as single housed monkeys. While previous studies on rhesus macaques found no significant difference in abnormal behaviors between protected contact housing and single housing (Baker et al., 2013; Gottlieb et al., 2013a), the animals in these studies were separated with relatively restrictive metal dividers (i.e., mesh or a barrier with small holes) that only provided limited opportunities for actual contact. In contrast, our protected contact dividers allowed animals to reach entire limbs into their partner’s cage, promoting greater physical contact and even allowing additional behaviors such as mounting (personal observation). We hypothesized that this increased opportunity for social interaction would result in a decrease in MSB compared to single housing, similar to that seen in studies using equivalent protected contact dividers in longtailed macaques (Baker et al., 2012b; Lee et al., 2012). While more work is needed to determine whether other abnormal behaviors in rhesus monkeys may be decreased with the use of the wide bar protected contact dividers, our results suggest that protected contact housing, even with widely spaced metal bars, produces rates of MSB equivalent to single housing for rhesus macaques, and may not offer the same benefits as full contact housing.
4.3. Response to novel object
In addition to environmental or experiential factors, intrinsic factors such as temperament may also be involved in the development of MSB. We investigated the relationship between MSB and behavioral inhibition using a simple novel object test. The vast majority of animals in the current study immediately investigated the novel food item (i.e., bold response), while inhibited responses, in which the subject never investigated the food, were relatively rare (8%). Interestingly, subjects that never investigated the novel stimulus were significantly less likely to display MSB than those that did inspect the object. These findings support those from a recent study (Gottlieb et al., 2013a). In that study, rhesus macaques that did not touch a novel object when tested at 3 months of age were less likely than others to show MSB later in life.
It is unclear why these inhibited animals show a reduced amount of MSB compared to less inhibited animals. Recent studies have proposed that when faced with relevant triggers (e.g., unavoidable stress, behavioral restriction), individuals with a “proactive” personality develop stereotypic behaviors as a coping strategy, while those with a “reactive” personality exhibit a passive response to stressors, and instead develop depressive-like symptoms and learned helplessness (Ijichi et al., 2013). It is possible that compared to “proactive” monkeys, individuals with a “reactive” personality are both less likely to respond to stress with MSB, and more likely to avoid a novel, and potentially threatening stimulus.
Alternatively, monkeys that display an inhibited response to the novel object test may simply be less likely to display MSB in the presence of a human observer. Inhibited animals are more likely than others to freeze or decrease activity in response to an unfamiliar human standing next to their cage (e.g., Kalin and Shelton, 1989) which is incompatible with MSB. Further, there are several environmental triggers of MSB; some individuals are more likely to engage in MSB when a human is present, while others are more likely to do so in the absence of an observer (Coleman, unpublished data). Future research is needed to determine whether response to the novel object test similarly predicts MSB in the absence of a human stimulus.
4.4. Origin
We found that rhesus macaques with Chinese ancestry were 49% more likely to display MSB than those of pure Indian origin. Previous research has shown that Chinese-Indian hybrids are more likely than pure Indian rhesus macaques to display MSB when placed in single housing during social separations at 6-months of age (Champoux et al., 1997). Our results further demonstrate rhesus macaques with Chinese ancestry display higher MSB than those with Indian ancestry when adults, even when socially housed.
Many known differences between Chinese and Indian origin rhesus exist, including genetic differences (Hernandez et al., 2007), experiential differences (e.g., Chinese ancestry individuals at the ONPRC are more likely to be wild caught or imported from another center than Indian macaques), and temperamental differences (when measured in infancy, Chinese-Indian hybrids show increased arousal, irritability, and anxiety, and decreased fearfulness, compared to pure Indian rhesus (Champoux et al., 1997; Champoux et al., 1994; Jiang et al., 2013)). Although we generally controlled for differences in rearing condition in our analysis, any of the above listed factors may have contributed to the observed difference in MSB expression. Further research is needed to determine the underlying cause of the discrepancy in MSB.
4.5. Rearing condition, age, and sex
Previous research has demonstrated significant effects of rearing condition, age, and sex on MSB in rhesus macaques (Gottlieb et al., 2013a; Lutz et al., 2003; Novak et al., 2006), therefore we included these variables as covariates in our model. Consistent with previous research, we found MSB to be higher in males (although this relationship only approached statistical significance). While previous research has found MSB to decrease with age, we specifically found MSB to decrease only after an animal has reached 6 years old.
We found MSB to be lowest in monkeys raised outdoors (corral and sheltered housed reared) and highest in monkeys raised indoors (indoor mother and nursery reared), with corral raised monkeys having the overall lowest expected rate of MSB. These results are consistent with previous research, in which MSB was found to be lowest in monkeys raised in large outdoor enclosures and highest in indoor reared monkeys (Gottlieb et al., 2013a). Also consistent with previous research (Gottlieb et al., 2013a; Lutz et al., 2003), we did not find a significant difference in MSB between indoor-reared monkeys and nursery reared monkeys. Interestingly, we found that animals raised in indoor social groups, which were significantly smaller than corrals or sheltered housing, expressed intermediate levels of MSB (i.e., less MSB than indoor reared monkeys but more MSB than outdoor reared monkeys). Similarly, mixed reared animals, the majority of which were reared in both group and caged environments, also showed intermediate levels of MSB. These results demonstrate what appears to be a hierarchy of rearing environments, with the development of MSB decreasing as animals are reared in larger, more complex social environments.
5. Conclusions
The relative frequency of motor stereotypies is often used as method of comparing welfare across various environmental conditions. Environments that have the lowest overall development and expression of stereotypic behavior are often considered the most effective in promoting animal well-being (Mason, 1991; Mason and Latham, 2004). For example, animals in enriched environments often show reduced stereotypic behavior and concomitant measures indicative of positive welfare, including reduced heart rate and cortisol levels, compared to those in a barren environment (Mason and Latham, 2004). In the current study, we found that animals with foraging devices and full contact socialization (but not protected contact socialization) expressed significantly less MSB than expected, after controlling for known predictors of MSB such as age and sex, suggesting that these enrichment strategies promote animal well-being.
We also found that intrinsic factors, such as temperament or origin, contribute to the likelihood of developing stereotypic behavior. In our study, bold monkeys and those of Chinese ancestry were more likely to express MSB than inhibited and Indian origin monkeys, respectively. While these findings may appear to indicate that bold and Chinese origin individuals experience relative compromised welfare in captivity, it is important to recognize that individual differences in stereotypic behavior expression cannot necessarily be used to assess individual variation in welfare among subjects (Mason and Latham, 2004). Within a particular environment, animals with high levels of individual MSB do not necessarily have behavioral or physiological signs of poor welfare (Mason and Latham, 2004). Indeed, in some circumstances, MSB may function as an effective coping strategy (Ijichi et al., 2013; Mason and Latham, 2004).
As demonstrated by this study, the expression of MSB by an individual at any given time is a function of multiple factors including past experiences (e.g., rearing history), intrinsic factors (e.g., temperament, origin), and current environment (e.g., socialization status, presence of enrichment). With this in mind, care should be taken to control and/or account for these elements when utilizing MSB to evaluate welfare on either a group or individual level. Knowing factors, both environmental and intrinsic, that contribute to the development and expression of MSB is important to understanding and improving its role as a welfare indicator.
Highlights.
We evaluated stereotypic behavior in 3823 captive rhesus macaques over 11 years
Monkeys displayed less stereotypic behavior when a foraging device was present
Full but not partial contact socialization was associated with decreased stereotypy
Inhibition towards inspecting a novel object was associated with decreased stereotypy
Chinese origin monkeys displayed more stereotypy than those of Indian origin
Acknowledgements
We thank the Oregon National Primate Research Center Behavioral Services Unit staff, interns, and volunteers for their assistance in data collection, Gary Jones for his help in retrospective data retrieval, Andrea Gottlieb for her help in data analysis, and the ONPRC Division of Comparative Medicine for help and support of this project. This project was supported by P51OD011092.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors are not aware of any actual or potential conflicts of interest that could inappropriately influence this research.
6. References
- Animal Welfare Act Animal Welfare act, title 17, subtitle F, food security act. 1985 [Google Scholar]
- Akaike H. Factor-analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, Neu K, Griffis C, Schoof V, Maloney M. Benefits of pair housing are consistent across a diverse population of rhesus macaques. Appl. Anim. Behav. Sci. 2012a;137:148–156. doi: 10.1016/j.applanim.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, Neu K, Griffis C, Schoof VA. Comparing options for pair housing rhesus macaques using behavioral welfare measures. Am J Primatol. 2013 doi: 10.1002/ajp.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Crockett CM, Lee GH, Oettinger BC, Schoof V, Thom JP. Pair housing for female longtailed and rhesus macaques in the laboratory: Behavior in protected contact versus full contact. J Appl Anim Welf Sci. 2012b;15:126–143. doi: 10.1080/10888705.2012.658330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Weed JL, Crockett CM, Bloomsmith MA. Survey of environmental enhancement programs for laboratory primates. Am J Primatol. 2007;69:377–394. doi: 10.1002/ajp.20347. [DOI] [PubMed] [Google Scholar]
- Bayne K, Mainzer H, Dexter S, Campbell G, Yamada F, Suomi S. The reduction of abnormal behaviors in individually housed rhesus-monkeys (Macaca-mulatta) with a foraging grooming board. Am J Primatol. 1991;23:23–35. doi: 10.1002/ajp.1350230104. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: A practical information-theoretic approach. Springer-Verlag; New York: 2002. [Google Scholar]
- Byrne GD, Suomi SJ. Effects of woodchips and buried food on behavior patterns and psychological well-being of captive rhesus-monkeys. Am J Primatol. 1991;23:141–151. doi: 10.1002/ajp.1350230302. [DOI] [PubMed] [Google Scholar]
- Champoux M, Higley JD, Suomi SJ. Behavioral and physiological characteristics of Indian and Chinese-Indian hybrid rhesus macaque infants. Dev psychobiol. 1997;31:49–63. [PubMed] [Google Scholar]
- Champoux M, Suomi SJ, Schneider ML. Temperament differences between captive Indian and Chinese-Indian hybrid rhesus macaque neonates. Lab. Anim. Sci. 1994;44:351–357. [PubMed] [Google Scholar]
- Chancellor RL, Isbell LA. Punishment and competition over food in captive rhesus macaques, Macaca mulatta. Animal Behaviour. 2008;75:1939–1947. [Google Scholar]
- Coleman K, Tully LA, McMillan JL. Temperament correlates with training success in adult rhesus macaques. Am J Primatol. 2005;65:63–71. doi: 10.1002/ajp.20097. [DOI] [PubMed] [Google Scholar]
- Gottlieb DH, Capitanio JP, McCowan B. Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta); animal's history, current environment, and personality. Am J Primatol. 2013a;75:995–1008. doi: 10.1002/ajp.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Coleman K, McCowan B. the effects of predictability in daily husbandry routines on captive rhesus macaques (Macaca mulatta) Appl. Anim. Behav. Sci. 2013b;143:117–127. doi: 10.1016/j.applanim.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RD, Hubisz MJ, Wheeler DA, Smith DG, Ferguson B, Rogers J, Nazareth L, Indap A, Bourquin T, McPherson J. Demographic histories and patterns of linkage disequilibrium in Chinese and Indian rhesus macaques. Science. 2007;316:240–243. doi: 10.1126/science.1140462. [DOI] [PubMed] [Google Scholar]
- Ijichi CL, Collins LM, Elwood RW. Evidence for the role of personality in stereotypy predisposition. Anim Behav. 2013;85:1145–1151. [Google Scholar]
- Jiang J, Kanthaswamy S, Capitanio JP. Degree of Chinese ancestry affects behavioral characteristics of infant rhesus macaques (Macaca mulatta) J Med Primatol. 2013;42:20–27. doi: 10.1111/jmp.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick J, Snidman N. Temperamental variation in response to the unfamiliar. In: Krasnegor N, Blass E, Hofer M, Smotherman W, editors. Perinatal development: a psychobiological perspective. Academic Press; Orlando, FL: 1987. pp. 421–440. [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus-monkeys - environemtal cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kanthaswamy S, Gill L, Satkoski J, Goyal V, Malladi V, Kou A, Basuta K, Sarkisyan L, George D, Smith DG. Development of a Chinese–Indian hybrid (Chindian) rhesus macaque colony at the California National Primate Research Center by introgression. J Med Primatol. 2009;38:86–96. doi: 10.1111/j.1600-0684.2008.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GH, Thom JP, Chu KL, Crockett CM. Comparing the relative benefits of grooming-contact and full-contact pairing for laboratory-housed adult female Macaca fascicularis. Appl. Anim. Behav. Sci. 2012;137:157–165. doi: 10.1016/j.applanim.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz CK, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience. Am J Primatol. 2003;60:1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Lutz CK, Coleman K, Maier A, McCowan B. Abnormal behavior in rhesus monkeys: risk factors within and between animals and facilities. Am J Primatol. 2011;73:41–41. [Google Scholar]
- Lutz CK, Novak MA. Environmental enrichment for nonhuman primates: Theory and application. Ilar Journal. 2005;46:178–191. doi: 10.1093/ilar.46.2.178. [DOI] [PubMed] [Google Scholar]
- Mason G. In: Stereotypic behaviour in captive animals: fundamentals and implications for welfare and beyond. stereotypic animal behaviour: Fundamentals and applications to welfare. Mason G, Rushen J, editors. CABI; Wallingford: 2006. pp. 325–356. [Google Scholar]
- Mason GJ. Stereotypies - a critical-review. Anim Behav. 1991;41:1015–1037. [Google Scholar]
- Mason GJ, Latham NR. Can't stop, won't stop: is stereotypy a reliable animal welfare indicator? Anim. Welf. 2004;13:S57–S69. [Google Scholar]
- Mench JA, Mason GJ. Behaviour. In: Appbleby MC, Hughes BO, editors. Animal welfare. CABI Publishing; Cambridge: 1997. pp. 127–141. [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. Eighth The National Academies Press; 2011. [Google Scholar]
- Neville MK. Ecology and activity of himalayan foothill rhesus monkeys (Macaca Mulatta) Ecology. 1968;49:110–123. [Google Scholar]
- Novak MA, Kinsey JH, Jorgensen MJ, Hazen TJ. Effects of puzzle feeders on pathological behavior in individually housed rhesus monkeys. Am J Primatol. 1998;46:213–227. doi: 10.1002/(SICI)1098-2345(1998)46:3<213::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Novak MA, Meyer JS, Lutz C, Tiefenbacher S. Deprived environments: developmental insights from primatology. In: Mason G, Rushen J, editors. Stereotypic animal behaviour: Fundamentals and applications to welfare. CABI; Wallingford: 2006. pp. 153–189. [Google Scholar]
- Petherick JC, Rushen J. Behavioural restriction. In: Appbleby MC, Hughes BO, editors. Animal welfare. CABI Publishing; Cambridge: 1997. pp. 89–105. [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. r foundation for statistical computing; Vienna, Austria: 2013. [Google Scholar]
- Schapiro SJ, Bloomsmith MA. Behavioral effects of enrichment on singly-housed, yearling rhesus monkeys: An analysis including three enrichment conditions and a control group. Am J Primatol. 1995;35:89–101. doi: 10.1002/ajp.1350350202. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Porter LM, Suarez SA. Enrichment effects on rhesus monkeys successively housed singly, in pairs, and in groups. Appl. Anim. Behav. Sci. 1996;48:159–171. [Google Scholar]
- Seth PK, Seth S. Ecology and behavior of rhesus monkeys in India. In: Else JG, Lee PC, editors. Primate ecology and conversation. Cambridge University Press; Cambridge: 1986. [Google Scholar]
- Vandeleest JJ, McCowan B, Capitanio JP. Early rearing interacts with temperament and housing to influence the risk for motor stereotypy in rhesus monkeys (Macaca mulatta) Appl. Anim. Behav. Sci. 2011;132:81–89. doi: 10.1016/j.applanim.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]