Abstract
Introduction
Greater body mass index (BMI) has been associated with less radiographic progression in rheumatoid arthritis (RA). We evaluated the association between BMI and joint damage progression as measured by X-ray and MRI.
Methods
1068 subjects with RA from two clinical trials of golimumab (GO-BEFORE and GO-FORWARD) had radiographs performed at weeks 0, 52 and 104 and evaluated using the van der Heijde–Sharp (vdHS) scoring system. Contrast-enhanced MRIs of the dominant wrist and hand were obtained at weeks 0, 12, 24, 52 and 104. Multivariable logistic regression evaluated the risk of radiographic progression for each BMI category (<25, 25–30, >30 kg/m2). Within GO-BEFORE, piecewise, robust generalised estimating equations marginal models assessed the probability of MRI erosion progression for each BMI category. Multivariable linear regression models assessed baseline associations between BMI and bone oedema (a precursor of bone erosion).
Results
Higher BMI category was associated with a lower probability of progression in vdHS score at weeks 52 and 104 independent of potential confounders. Higher BMI was also independently associated with a lower probability of progression in MRI erosion score over 2 years. Subjects with greater BMI demonstrated less bone oedema independent of differences in other disease severity measures, including MRI synovitis in the same joints.
Conclusions
Greater BMI is associated with a lower risk of progression on X-ray and MRI over 2 years. Subjects with greater BMI also demonstrate less bone oedema at baseline. Greater BMI may indicate a less aggressive RA phenotype and aid in risk stratification.
Several recent observational studies reported reduced radiographic progression in subjects with greater body mass index (BMI) compared with those with lower BMI.1–4 While thin subjects with rheumatoid arthritis (RA) might be hypothesised to have a more severe form of the disease, the explanation for the lower risk among overweight and obese subjects has not been fully elucidated. Adequate power and subject characterisation has not been available to fully evaluate potential confounding factors, including disease activity and treatment effects.5,6
MRI has come to the forefront as a useful tool to visualise and quantify RA intra-articular inflammation and structural joint damage with high sensitivity.7–9 We have shown that early changes synovitis and bone oedema on MRI predict subsequent progression on X-ray.10 MRI measures of synovitis may more accurately reflect ongoing disease burden and, therefore, allow for a more accurate assessment when exploring the confounding effects of RA disease activity on progression.
We aimed to determine whether lower BMI at baseline was associated with a greater risk of progression on X-ray and MRI over 2 years in a cohort of 1068 subjects with RA from two clinical trials of golimumab. We also aimed to determine whether associations were explained by clinically available measures of disease severity and activity (eg, seropositivity, disease duration, disease activity, response to therapy), and MRI measures of disease activity such as the RA MRI (RAMRIS) synovitis score. Finally, we aimed to determine whether subjects with lower BMI had greater MRI bone marrow oedema (a precursor of bone erosion) at baseline independent of potential confounders.
METHODS
This observation cohort study was a secondary analysis of the GO-BEFORE (Clintrials.gov identifier NCT00361335) and GO-FORWARD (NCT00264550) randomised clinical trials. Both were multicentre, double-blind, placebo-controlled trials that evaluated the efficacy of golimumab, a fully human monoclonal antibody to tumour necrosis factor- α, for the treatment of RA.11–13 Both studies evaluated the effect of golimumab in combination with methotrexate compared with methotrexate and golimumab monotherapy. The GO-BEFORE study was performed in methotrexate and biologic-naive subjects, while GO-FORWARD was performed in subjects who previously failed methotrexate. The trials were conducted according to the principles of the Declaration of Helsinki. As such, all patients provided written informed consent before participating in the study.
Data collection
Detailed characteristics of the original study designs and results have been previously published.11–14 Briefly, patient visits occurred at 4-week intervals and included independent, blinded assessments of disease activity scores of 28 joints (DAS28) incorporating C-reactive protein (CRP). Radiographs of the hands and feet were performed at baseline, week 52 and week 104. Radiographs were assessed using the average scores of two, blinded, centralised readers using the van der Heijde–Sharp (vdHS) system.15
We also evaluated MRI erosion outcomes from the GO-BEFORE study. All patients at eligible (based on technical capabilities) and willing study sites participated in the MRI substudy. MRIs of the patient's dominant wrist and metacarpophalangeal joints were obtained at baseline, and at weeks 12, 24, 52 and 104 using 1.5 T MRI with contrast enhancement and scored using the RAMRIS scoring system.9 Methods and results regarding the MRI substudy have been previously published.16 In total, 317 subjects had MRIs scored for bone erosion/bone oedema; 20 of these subjects did not receive gadolinium and, therefore, did not have synovitis scores at baseline (N=297).
Statistical analysis
Data were analysed with STATA V.11 software (StataCorp, LP, College Station, Texas, USA). Weight categories were defined as low-normal weight (<25 kg/m2), overweight (>25 to <30 kg/m2) and obese (≥30 kg/m2), consistent with previous studies.4 Thirty-one subjects had a BMI <18.5 kg/m2 and were included in the low-normal weight group. Associations between BMI category and continuous variables were assessed using analysis of variance to test the hypothesis of equality of means across the BMI categories. Kruskal–Wallis tests were applied for highly skewed (non-normally distributed) variables.
A change of vdHS score or MRI erosion score of >0.5 was considered progression consistent with previous studies.10,17,18,19 This cut-off was chosen to reduce misclassification error. The magnitude of the coefficients of risk was similar when using a change cut-off of >3 in vdHS (not shown). Baseline characteristics were used to inform the development of multivariable logistic regression models for assessing likelihood of radiographic progression. Regression models evaluated the odds of radiographic progression for each category of BMI adjusting for age, race, sex, cyclic citrullinated peptide (CCP) status, corticosteroid use, disease duration, treatment group, DAS28(CRP), change in DAS28(CRP) over the study period (either 52-week or 104-week change), baseline vdHS scores, smoking status and study (GO-BEFORE or GO-FORWARD).
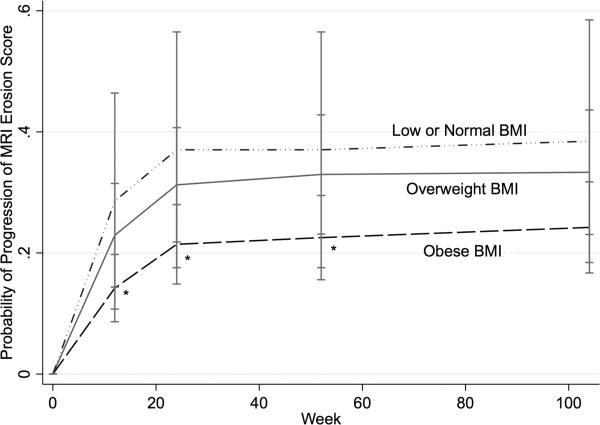
Robust generalised estimating equations marginal models with exchangeable correlation structures were used to assess the likelihood of MRI erosion progression by BMI category over all progression outcomes throughout the study period adjusting for potential confounders. The robust ‘sandwich’ estimator of covariance was applied because it typically gives consistent estimates of the SEs even when the correlation matrix is specified incorrectly. The autoregressive (AR1) and unstructured matrices were implemented (when they would converge) to confirm that the results were consistent according to the choice of correlation structure. Piecewise regression was implemented by placing a knot at 24 weeks where the rate of progression decreased significantly (see figure 1). Initial models included age, sex, race (Caucasian vs non-Caucasian), CCP seropositivity, disease duration, baseline corticosteroid use, baseline DAS28(CRP), baseline RAMRIS synovitis scores and treatment group.
Figure 1.
Probability of MRI erosion score progression >0.5 over the study follow-up by body mass index (BMI) category. *Significantly different from low or normal BMI group.
Bone oedema scores were skewed. Therefore, multivariable ordinal logistic regression was used to evaluate associations between BMI category and greater bone oedema on MRI at baseline. Bone oedema was categorised into quintiles based on baseline RAMRIS bone oedema score. Ordinal logistic regression models evaluated the likelihood of an individual having a bone oedema score in a higher category after adjusting for potential confounders. Brant tests were performed to test for a significant violation of the parallel regression assumption and were non-significant (p=0.1).
RESULTS
Participant characteristics from GO-BEFORE and GO-FORWARD have been previously published.12,13 18 Briefly, compared with GO-BEFORE, the GO-FORWARD study subjects had greater disease duration (p<0.001), greater baseline radiographic damage (p<0.001) and were more likely to be CCP positive (p=0.02) and to take corticosteroids at baseline (p<0.001). There were no study differences in mean BMI (27.1 (6.2) vs 27.1 (5.8); p=0.9).
Demographic and disease characteristics varied according to BMI category (table 1). Specifically, there were differences in age, sex, race, proportion of CCP-positive subjects, vdHS scores at baseline and steroid use at baseline. Within the GO-BEFORE MRI substudy, there were significant differences in age, race and bone oedema scores between BMI category. There was a trend towards differences in CCP positivity and synovitis scores.
Table 1.
Baseline subject characteristics by BMI category
| BMI category |
||||
|---|---|---|---|---|
| BMI <25 kg/m2 | BMI 25-30 kg/m2 | BMI ≥30 kg/m2 | p Value | |
| X-ray cohort (GO-BEFORE+GO-FORWARD) (N=1068) | ||||
| N | 452 | 357 | 272 | |
| Age (years) | 47.4 (13.2) | 51.9 (10.5) | 51.4 (10.7) | <0.001 |
| Female, N (%) | 378 (82%) | 276 (78%) | 220 (82%) | 0.04 |
| Race | ||||
| Caucasian, N (%) | 286 (64%) | 279 (79%) | 228 (85%) | <0.001 |
| Asian, N (%) | 135 (30%) | 37 (10%) | 11 (4%) | <0.001 |
| BMI (kg/m2) | 22.0 (2.2) | 27.4 (1.4) | 35.2 (5.0) | N/A |
| CRP (mg/dL) | 1.2 (0.4, 3.3) | 1.1 (0.4, 2.3) | 1.1 (0.5, 2.2) | 0.3 |
| DAS28 (CRP) | 5.53 (1.09) | 5.66 (1.02) | 5.67 (1.00) | 0.1 |
| CCP-positive, N (%) | 356 (80%) | 276 (78%) | 177 (66%) | <0.001 |
| vdHS score | 12 (2.5, 46.5) | 7 (2.5, 25) | 5.5 (1.5, 18) | <0.001 |
| Steroid use, N (%) | 281 (63%) | 209 (59%) | 143 (54%) | 0.05 |
| Active smoking, N (%) | 72 (18%) | 69 (23%) | 42 (17%) | 0.1 |
| GO-BEFORE MRI substudy (N=317) | ||||
| N | 137 | 111 | 68 | |
| Age | 46.5 | 52.0 | 51.6 | 0.0006 |
| Female, N (%) | 113 (82%) | 85 (77%) | 57 (84%) | 0.4 |
| Race | ||||
| Caucasian, N (%) | 77 (56%) | 73 (66%) | 54 (79%) | <0.001 |
| Asian, N (%) | 55 (40%) | 23 (21%) | 6 (9%) | <0.001 |
| BMI (kg/m2) | 21.9 (2.2) | 27.4 (1.4) | 35.3 (5.3) | N/A |
| CRP (mg/dL) | 1.4 (0.4, 3.1) | 1.2 (0.5, 2.6) | 0.9 (0.5, 2) | 0.4 |
| DAS28(CRP) | 5.61 (1.12) | 5.63 (1.09) | 5.55 (0.99) | 0.9 |
| CCP-positive, N (%) | 110 (80%) | 87 (78%) | 45 (66%) | 0.07 |
| vdHS score | 6 (2, 29.3) | 5.8 (2.9, 16.5) | 4.8 (1.8, 11.3) | 0.2 |
| Steroid use, N (%) | 91 (66%) | 70 (63%) | 37 (54%) | 0.2 |
| Active smoking, N (%) | 20 (17%) | 22 (24%) | 11 (17%) | 0.3 |
| RAMRIS | ||||
| Synovitis | 9 (5.5, 13.5) | 11 (6, 13.8) | 7.5 (4.5, 12.8) | 0.09 |
| Bone oedema | 9 (2.5, 19) | 6.3 (2.5, 13) | 4.8 (1.5, 9.8) | 0.01 |
| Bone erosion | 16 (10, 25) | 14 (11, 21.5) | 13.5 (9.5, 18.3) | 0.1 |
BMI, body mass index; CCP, cyclic citrullinated peptide; CRP, C-reactive protein; DAS28, disease activity score 28; RAMRIS, RA MRI score; vdHS, van der Heijde–Sharp.
BMI category and X-ray progression over 1 and 2 years
The proportion of subjects who had radiographic progression on X-ray at 52 weeks was significantly lower among subjects who were obese (21.2%) and overweight (22.0%) compared with those who were low-normal weight (31.7%) (p=0.002). Greater BMI category at baseline was significantly associated with lower odds of progression in vdHS score over 1 and 2 years of follow-up after adjustment for potential confounders (table 2). Testing for a modified effect by study was performed and was non-significant (p>0.6). Exclusion of the 31 underweight subjects did not alter these results.
Table 2.
Multivariable adjusted association between BMI category and progression of vdHS score at 52 and 104 weeks
| vdHS progression* (52 weeks, N=930) |
vdHS progression* (104 weeks, N=838) |
|||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| BMI category | ||||
| Overweight (25–30 kg/kg2) | 0.63 (0.44 to 0.92) | 0.02 | 0.73 (0.50 to 1.09) | 0.1 |
| Obese (>30 mg/kg2) | 0.63 (0.41 to 0.96) | 0.03 | 0.55 (0.34 to 0.87) | 0.01 |
| DAS28(CRP) (per 1 unit) | 1.58 (1.34 to 1.86) | <0.001 | 1.63 (1.33 to 1.94) | <0.001 |
| Δ DAS28(CRP) (per 1 unit) | 1.17 (1.04 to 1.32) | 0.01 | 1.20 (1.04 to 1.38) | 0.01 |
| CCP positive at baseline | 1.73 (1.16 to 2.58) | 0.007 | 1.36 (0.89 to 2.08) | 0.2 |
| vdHS score at baseline (per 1 unit) | 1.01 (1.00 to 1.01) | <0.001 | 1.01 (1.00 to 1.01) | <0.001 |
Also adjusted for age, sex, race, study and treatment group. Covariables tested but not included in final models: disease duration, active smoking and steroid use at baseline.
Δ=change over the follow-up period (negative change indicating improvement).
BMI, body mass index; CCP, cyclic citrullinated peptide; CRP, C-reactive protein; DAS28, disease activity score 28; vdHS, van der Heijde-Sharp.
BMI category and risk of MRI progression over 2 years
Greater BMI category was associated with lower odds of MRI erosion score progression over the 2-year period (figure 1). In longitudinal models, obese BMI was associated with a significantly lower likelihood of progression in MRI erosion score over 2 years of follow-up compared with low or normal weight (OR 0.29 (0.10 to 0.81); p=0.02) (table 3) after adjustment for hypothesised confounders. Corticosteroid use at baseline was associated with lower odds of progression over the 2 years. Also, the combination (methotrexate plus golimumab) treatment arms were associated with a significantly lower likelihood of progression in MRI erosion score in this model (not shown). However, the baseline DAS28(CRP), CCP seropositivity, disease duration, baseline MRI erosion score and smoking status were not independently associated with a greater risk of progression over time. Exclusion of the 11 underweight subjects from the analysis slightly increased the measured effect of overweight category (OR 0.40 (0.17 to 0.91); p=0.03) and obese category (OR 0.26 (0.092 to 0.74); p=0.01).
Table 3.
Robust generalised estimating equations (GEE) piecewise model evaluating independent effect of BMI category on the change in MRI bone erosion score over 2 years
| Full model* |
||
|---|---|---|
| OR* (95% CI) | p Value | |
| BMI category | ||
| Overweight (25–30 kg/kg2) | 0.45 (0.20 to 1.02) | 0.06 |
| Obese (>30 mg/kg2) | 0.29 (0.10 to 0.81) | 0.02 |
| Baseline synovitis (per 1 unit) | 1.10 (1.01 to 1.19) | 0.03 |
| Baseline DAS28 (CRP) (per 1 unit) | 1.01 (0.66 to 1.54) | 1 |
| CCP positive | 1.27 (0.54 to 3.03) | 0.6 |
| Corticosteroid use | 0.42 (0.20 to 0.90) | 0.03 |
| Disease duration (per 1 year) | 0.97 (0.88 to 1.06) | 0.5 |
| Baseline MRI erosion (per 1 unit) | 1.00 (0.98 to 1.02) | 1 |
| Active smoking | 1.41 (0.56 to 3.52) | 0.5 |
Also adjusted for age, sex, race, treatment group and study week.
BMI, body mass index; CCP, cyclic citrullinated peptide; CRP, C-reactive protein; DAS28, disease activity score 28; RAMRIS, RA MRI scoring system.
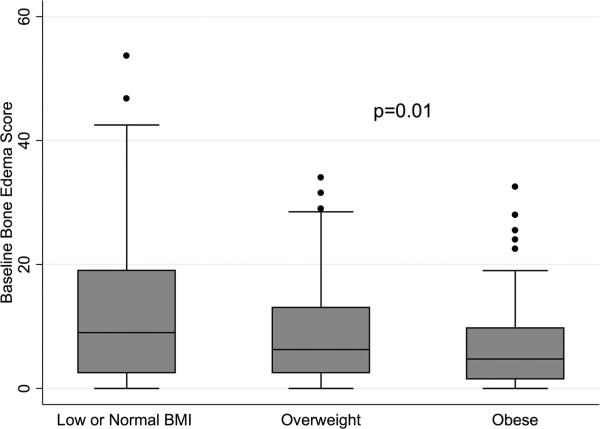
BMI category and baseline bone oedema
There was a negative correlation between bone oedema score and BMI at baseline (Spearman's r: –0.18 (p=0.001)). Significantly higher bone oedema scores were seen among RA subjects with low-normal BMI (figure 2). In multivariable ordinal logistic regression analyses, greater BMI category was associated with lower likelihood of being in a higher oedema score quintile at baseline after controlling adjusting for disease factors (OR 0.47 (0.27 to 0.82); p=0.008) (table 4). This association remained significant with inclusion of RAMRIS synovitis scores from the same joints in the model (OR 0.53 (0.29 to 0.96); p=0.04) (table 4). Factors independently associated with greater bone oedema on MRI in the final model included non-white race, greater disease duration, positive CCP status and greater MRI synovitis.
Figure 2.
Baseline bone oedema by baseline body mass index (BMI) category.
Table 4.
Ordinal multivariable logistic regression demonstrating adjusted associations between baseline BMI category and risk of higher quintile of bone oedema score in GO-BEFORE at baseline
| Model 1* Risk of higher bone oedema (N=313) |
Model 2 (synovitis in model)* Risk of higher bone oedema (N=295) |
|||
|---|---|---|---|---|
| Variable | OR* (95% CI) | p Value | OR* (95% CI) | p Value |
| BMI category | ||||
| Overweight (25-29.9 kg/m2) | 0.68 (0.42 to 1.08) | 0.1 | 0.60 (0.35 to 0.94) | 0.04 |
| Obese (≥30 kg/m2) | 0.47 (0.27 to 0.82) | 0.008 | 0.53 (0.29 to 0.96) | 0.04 |
| Caucasian | 0.48 (0.31 to 0.73) | 0.001 | 0.52 (0.33 to 0.82) | 0.005 |
| CCP positive | 1.51 (0.95 to 2.41) | 0.08 | 2.05(1.24 to 3.39) | 0.005 |
| Disease duration (per 1 year) | 1.12 (1.07 to 1.18) | <0.001 | 1.13 (1.07 to 1.18) | <0.001 |
| DAS28(CRP) (per 1 unit) | 1.32 (1.10 to 1.59) | 0.003 | 0.96 (0.77 to 1.19) | 0.7 |
| Synovitis score (per 1 unit) | – | – | 1.24 (1.18 to 1.31) | <0.001 |
Also adjusted for age and sex (non-significant, p>0.2). Tested but not included in final model (p>0.2): corticosteroid use at baseline, active smoking.
BMI, body mass index; CCP, cyclic citrullinated peptide; CRP, C-reactive protein; DAS28, disease activity score 28.
DISCUSSION
In a large cohort of subjects with RA from two clinical trials, these data demonstrated that greater BMI at baseline was associated with less significant radiographic progression on X-ray at 1 and 2 years independent of traditional measures of disease burden, including disease activity, seropositivity, disease duration, use of steroids, treatment and improvement in disease activity over the follow-up period. These data suggest that treating physicians should consider a patient's BMI when considering long-term risk in RA.
Using MRI to improve detection of disease, we are the first to show that greater baseline BMI is also associated with a lower risk of progression in RAMRIS erosion score, and this association was not explained by differences in RAMRIS synovitis in the same joints at baseline. This novel observation suggests that the association is not easily explained by differences in the inflammatory burden in the affected joints among those with low BMI. Furthermore, the difference in risk of progression by BMI category was not explained by differences in a number of measured potential confounders, including CCP seropositivity, corticosteroid use, DAS28(CRP) and treatment group. The estimates of risk were consistent and robust before and after multivariable adjustment.
Interestingly, there was a strong and significant association between BMI and bone oedema at baseline that was not explained by differences in the amount synovitis quantified in the same joints. These observations could suggest that low BMI (and perhaps weight loss or lack of weight gain) identifies a phenotype of disease that is associated with greater osteitis and subsequent erosion for the same intensity of synovial inflammation. Alternatively, it could suggest that subjects with greater BMI are protected against bone involvement, perhaps through effects of adipokines or through beneficial effects of greater weight on bone remodelling.3,20
The mechanisms underlying these study observations are not yet clear. Low BMI may be the result of early weight loss (or relative lack of weight gain) among patients with more severe disease. Weight loss early in the disease presentation may therefore represent a window of missed opportunity due to under-recognition and/or undertreatment of active disease. Adjustment for clinical disease activity and known measures associated with disease severity did not alter these associations. Furthermore, the current data demonstrated that differences in the burden of MRI synovitis in the individual joints being assessed did not explain the findings.
Adipokines, particularly adiponectin, have also been implicated in some studies.20,21 How adipokines might interact with inflammatory cytokines to affect disease outcomes is an ongoing area of investigation. Finally, the positive effects of greater weight and greater muscle mass on cortical bone remodelling and homeostasis may also act to retard the invasion of inflammatory pannus and support the health of overlying cartilage. New emerging molecules important in bone homeostasis and related to mechanical loading, such as sclerostin, could potentially play a role in this pathway.22,23
There are several limitations worth noting. This study was performed in a clinical trial setting and may not be entirely generalisable to other populations. Our study did not evaluate adipokines, cytokines, growth hormones or other pathways, which may be dysregulated in RA. Furthermore, associations are correlative only and do not address causality. It remains unknown whether low BMI simply is a marker signifying a distinct subgroup of disease or whether there are biological implications to having a low BMI that promote greater joint destruction over time. Finally, BMI is an inadequate measure of total adiposity in diseases associated with altered body composition such as RA. We were unable to evaluate the independent effects of lean and fat mass on radiographic progression.
The identification of distinct phenotypes of RA is a critical goal for rheumatologists in the next decade. This study has demonstrated that low or normal BMI at enrolment was a marker of greater risk of radiographic progression on both X-ray and MRI over 1–2 years independent of other available clinical predictors including synovitis burden as measured by MRI. This work may indicate a distinct RA phenotype and provides another measure for stratification of therapy.
Acknowledgments
Funding JFB is supported by a Veterans Affairs Clinical Science Research and Development Career Development Award. PGC is funded in part by a grant from Arthritis Research UK. This ancillary study did not receive additional funding.
Footnotes
Contributors JFB was primarily responsible for study design, analysis plan, data analysis and manuscript preparation. MØ, PE and PGC contributed to study design, analysis plan and manuscript preparation. MG contributed to the analysis plan and manuscript preparation. DGB contributed to data collection, original study design, analysis plan and manuscript preparation.
Competing interests DGB is an employee of Janssen Biotech. PGC has done speakers bureaus or consultancies for BMS, Janssen, Merck, Pfizer and Roche. MØ has received fees for consultancy or speaker fees and/or research support from Abbott, BMS, Centocor, GSK, Janssen, Merck, Mundipharma, Novo, Pfizer, Schering-Plough, Roche UCB and Wyeth. PE has received consulting fees, speaking fees and/or honoraria from Pfizer, Merck, Abbvie, UCB, Roche, BMS, Lilly and Novartis (less than $10 000 each).
Patient consent Obtained.
Ethics approval University of Pennsylvania.
Provenance and peer review Not commissioned; externally peer reviewed.
To cite: Baker JF, Østergaard M, George M, et al. Ann Rheum Dis Published Online First: [please include Day Month Year] doi:10.1136/annrheumdis-2014-205544
REFERENCES
- 1.Westhoff G, Rau R, Zink A. Radiographic joint damage in early rheumatoid arthritis is highly dependent on body mass index. Arthritis Rheum. 2007;56:3575–82. doi: 10.1002/art.23033. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann J, Kielstein V, Kilian S, et al. Relation between body mass index and radiological progression in patients with rheumatoid arthritis. J Rheumatol. 2003;30:2350–5. [PubMed] [Google Scholar]
- 3.Baker JF, George M, Baker DG, et al. Associations between body mass, radiographic joint damage, adipokines, and risk factors for bone loss in rheumatoid arthritis. Rheumatology (Oxford) 2011;50:2100–7. doi: 10.1093/rheumatology/ker294. [DOI] [PubMed] [Google Scholar]
- 4.van der Helm-van Mil AH, van der Kooij SM, Allaart CF, et al. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis. 2008;67:769–74. doi: 10.1136/ard.2007.078832. [DOI] [PubMed] [Google Scholar]
- 5.Ajeganova S, Andersson ML, Hafstrom I. Obesity is associated with worse disease severity in rheumatoid arthritis as well as with co-morbidities—a long-term follow-up from disease onset. Arthritis Care Res (Hoboken) 2013;65:78–87. doi: 10.1002/acr.21710. [DOI] [PubMed] [Google Scholar]
- 6.Engvall IL, Tengstrand B, Brismar K, et al. Infliximab therapy increases body fat mass in early rheumatoid arthritis independently of changes in disease activity and levels of leptin and adiponectin: a randomized study over 21 months. Arthritis Res Ther. 2010;12:R197. doi: 10.1186/ar3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conaghan PG, Ejbjerg B, Lassere M, et al. A multicenter reliability study of extremity-magnetic resonance imaging in the longitudinal evaluation of rheumatoid arthritis. J Rheumatol. 2007;34:857–8. [PubMed] [Google Scholar]
- 8.Hetland ML, Ejbjerg B, Horslev-Petersen K, et al. MRI bone oedema is the strongest predictor of subsequent radiographic progression in early rheumatoid arthritis. Results from a 2-year randomised controlled trial (CIMESTRA). Ann Rheum Dis. 2009;68:384–90. doi: 10.1136/ard.2008.088245. [DOI] [PubMed] [Google Scholar]
- 9.Ostergaard M, Peterfy C, Conaghan P, et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30:1385–6. [PubMed] [Google Scholar]
- 10.Baker JF, Ostergaard M, Emery P, et al. Early MRI measures independently predict 1- and 2-year X-ray progression: results from a large clinical trial. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-203444. Published Online First 31 July 2013. doi:10.1136/annrheumdis-2013-203444. [DOI] [PubMed] [Google Scholar]
- 11.Keystone EC, Genovese MC, Klareskog L, et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD Study. Ann Rheum Dis. 2009;68:789–96. doi: 10.1136/ard.2008.099010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keystone E, Genovese MC, Klareskog L, et al. Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: 52-week results of the GO-FORWARD study. Ann Rheum Dis. 2010;69:1129–35. doi: 10.1136/ard.2009.116319. [DOI] [PubMed] [Google Scholar]
- 13.Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60:2272–83. doi: 10.1002/art.24638. [DOI] [PubMed] [Google Scholar]
- 14.Kremer J, Ritchlin C, Mendelsohn A, et al. Golimumab, a new human anti-tumor necrosis factor alpha antibody, administered intravenously in patients with active rheumatoid arthritis: Forty-eight-week efficacy and safety results of a phase III randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2010;62:917–28. doi: 10.1002/art.27348. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 1999;26:743–5. [PubMed] [Google Scholar]
- 16.Conaghan PG, Emery P, Ostergaard M, et al. Assessment by MRI of inflammation and damage in rheumatoid arthritis patients with methotrexate inadequate response receiving golimumab: results of the GO-FORWARD trial. Ann Rheum Dis. 2011;70:1968–74. doi: 10.1136/ard.2010.146068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker JF, Conaghan PG, Smolen JS, et al. Development and validation of modified disease activity scores in rheumatoid arthritis: superior correlation with MRI synovitis and X-ray progression. Arthritis Rheum. 2014;66:794–802. doi: 10.1002/art.38304. [DOI] [PubMed] [Google Scholar]
- 18.Emery P, Breedveld F, van der Heijde D, et al. Two-year clinical and radiographic results with combination etanercept-methotrexate therapy versus monotherapy in early rheumatoid arthritis: a two-year, double-blind, randomized study. Arthritis Rheum. 2010;62:674–82. doi: 10.1002/art.27268. [DOI] [PubMed] [Google Scholar]
- 19.Aletaha D, Smolen JS. Joint damage in rheumatoid arthritis progresses in remission according to the Disease Activity Score in 28 joints and is driven by residual swollen joints. Arthritis Rheum. 2011;63:3702–11. doi: 10.1002/art.30634. [DOI] [PubMed] [Google Scholar]
- 20.Giles JT, Allison M, Bingham CO, III, et al. Adiponectin is a mediator of the inverse association of adiposity with radiographic damage in rheumatoid arthritis. Arthritis Rheum. 2009;61:1248–56. doi: 10.1002/art.24789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giles JT, van der Heijde DM, Bathon JM. Association of circulating adiponectin levels with progression of radiographic joint destruction in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1562–8. doi: 10.1136/ard.2011.150813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agholme F, Isaksson H, Li X, et al. Anti-sclerostin antibody and mechanical loading appear to influence metaphyseal bone independently in rats. Acta Orthop. 2011;82:628–32. doi: 10.3109/17453674.2011.625539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moustafa A, Sugiyama T, Prasad J, et al. Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int. 2012;23:1225–34. doi: 10.1007/s00198-011-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]