Abstract
Regulation of the Src-related tyrosine kinase Lck is crucial to the outcome of T-cell receptor (TCR) stimulation. It was previously shown that the stability of the constitutively active mutant LckY505F is controlled by Hsp90 (M. J. Bijlmakers and M. Marsh, Mol. Biol. Cell. 11:1585-1595, 2000). Here we establish that following TCR stimulation, endogenous activated Lck in T cells is also degraded in the presence of the Hsp90 inhibitor geldanamycin. Using Lck constructs expressed in COS-7 cells, we show that the presence of activating Lck mutations results not only in the enhanced dependence on Hsp90 but also in enhanced ubiquitination of Lck. Although both processes were induced by mutations Y505F and W97A that release the SH2 and SH3 inhibitory intramolecular interactions, respectively, neither process required Lck kinase activity or activation-dependent phosphorylation at serines 42 and 59 or tyrosine 394. By binding to the ATP-binding site, the Src family inhibitor PP2 reduced ubiquitination and overcame the need for Hsp90 monitoring of active Lck. We conclude that the levels of active Lck are influenced by two opposing processes, targeting for degradation by ubiquitination and rescue from degradation by Hsp90 monitoring. Based on the PP2 result, we propose that activation-induced conformational changes of the Lck kinase domain instigate both regulatory processes.
The tyrosine kinase Lck is a Src family member that is primarily expressed in T lymphocytes, where it plays a key role in signaling from the T-cell antigen receptor (TCR). Engagement of the TCR by major histocompatibility complex and peptide leads to activation of Lck, which then phosphorylates the TCR-associated CD3 and ζ chains, resulting in the recruitment and activation of the tyrosine kinase Zap-70 (26). These events initiate various signaling pathways leading to the production of interleukin-2 and eventually to the proliferation and differentiation of T cells. T cells lacking functional Lck are impaired in early tyrosine phosphorylation events and, as a consequence, in activation (45). Studies on mice with a targeted disruption of the Lck gene have demonstrated that Lck is also essential for thymocyte development. Lck−/− mice show a profound block at the early CD4−/CD8− thymocyte stage, when the pre-TCR is expressed (31). Lck also plays a crucial role at later stages of development, when positive and negative selection and CD4+/CD8+ lineage decisions occur.
Like other members of the Src family, Lck contains a unique domain at its NH2 terminus, followed by a Src homology 3 (SH3), an SH2, a kinase (SH1) domain, and a short regulatory tail. Lck is a membrane-associated protein owing to NH2-terminal myristylation and palmitylation (38). At the plasma membrane, Lck associates with the cytoplasmic domain of TCR coreceptor CD4 or CD8, an interaction mediated by two cysteines in the unique domain that ensures the proximity of Lck to the TCR (40, 47).
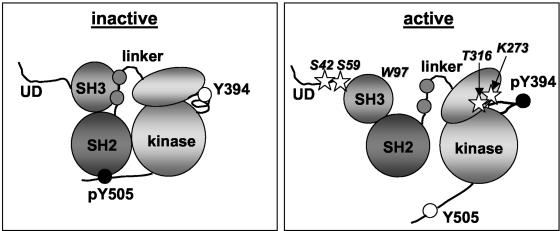
The kinase activity of Src kinases is modulated by intramolecular interactions and the phosphorylation of crucial tyrosines (22, 43, 51, 53, 56). In the inactive state, the tyrosine in the regulatory tail (Y505 in Lck) is phosphorylated and binds to the SH2 domain. Dephosphorylation of this residue or the release of this interaction by competition with SH2 substrates activates the kinase activity (30, 34). A second inhibitory interaction involves the linker between the SH2 and the kinase domain, a stretch of 13 to 14 amino acids that binds to both the SH3 and the kinase domain. Displacement of this SH2-SH1 linker by SH3 substrates can also activate the kinase (12, 30). The kinase domain itself is made up of small NH2-terminal and large COOH-terminal lobes, with the active cleft located between the two lobes. Autophosphorylation of a tyrosine (Y394 in Lck) in the activation loop of the kinase domain induces conformational changes that are essential for kinase activity (43). In the resulting conformation, a lysine (K273 in Lck) in the small lobe is optimally positioned to mediate phosphate transfer to substrates. Thus, the activation of Src kinases is accompanied by changes in phosphorylation status, intramolecular interactions, and conformation (summarized in Fig. 1).
FIG. 1.
Schematic representation of inactive and active Lck. The diagram of inactive Lck is based on crystal structures of inactive Src and Hck (43, 53), and the diagram of active Lck is based on the crystal structure of Lck in the autophosphorylated form (56). No structural information is available on the unique domain (UD). Lck undergoes the following major changes upon activation: (i) the inhibitory interactions between the SH2 domain and the regulatory tail and that between the SH3 domain and the linker are released, (ii) tyrosine 505 in the tail is dephosphorylated and tyrosine 394 in the activation loop is phosphorylated, and (iii) the small lobe of the kinase domain rotates relative to the large lobe. The Lck residues that have been mutated in this study are indicated in the active protein by stars.
A strict control of Lck activity is important for reliable T-cell activation and T-cell development. Moreover, uncontrolled activity of Lck, like that of other Src kinases, can lead to tumorigenesis (1, 28). Hence, several regulatory mechanisms are in place. The best studied is the reversible phosphorylation of the key tyrosines Y394 and Y505 (20). The regulatory tail tyrosine 505 is phosphorylated by the kinase Csk and can be dephosphorylated by the phosphatase CD45, which activates the protein. In contrast, dephosphorylation of Y394 in the kinase domain inactivates Lck. Lck can also be regulated by its recruitment into specialized plasma membrane domains, such as lipid rafts and the immunological synapse, where signaling molecules are concentrated (6, 23). In addition, it has recently been shown that Src proteins in their active states are ubiquitinated and degraded by the proteasome, providing a mechanism of irreversible Src protein downmodulation. For instance, ubiquitination and degradation is observed for Src in embryonic fibroblasts from Csk−/− mice, in which Src is constitutively active owing to a lack of phosphorylation of the regulatory tail tyrosine (13, 15). Furthermore, the active form of Blk, a Src kinase expressed in B cells, is ubiquitinated by the ubiquitin ligase E6AP (33). Constitutively active forms of Lck, Src, Hck, and Fyn were all shown to be ubiquitinated in the presence of Cbl (3, 29, 36, 57), an adaptor protein recently shown to have ubiquitin ligase activity (24). Importantly, ubiquitination of Lck and Blk is induced by T- and B-cell activation, respectively, suggesting that ubiquitination plays a role in downregulating Src kinases activated by receptor stimulation (33, 36).
Previous work on the role of Hsp90 in Lck biosynthesis also suggested that active Lck is targeted for degradation. It was found that a constitutively active Lck mutant, but not wild-type Lck, is unstable when Hsp90 is inhibited (5). Hsp90 is an abundant cytosolic chaperone that facilitates the folding, stabilization, and functional modulation of a select group of client proteins mostly involved in signal transduction (59). The activity of Hsp90 can be selectively inhibited by geldanamycin (GA) (49), a compound that is being tested as an antitumor agent due to its destabilizing effect on Hsp90 substrates, such as ErbB2 (32). Lck requires Hsp90 activity during folding, but once mature only the active form needs chaperoning.
Here we determined that Hsp90 is not only important for the stability of constitutively active Lck but also for Lck activated by TCR stimulation. Having established this role for Hsp90 monitoring in Lck function, we then investigated the relationship between Hsp90 monitoring and ubiquitination of active Lck. The data reveal that these processes are induced by similar Lck determinants and are primarily contingent on the conformation of the active kinase domain. We propose that Lck is subjected to two opposing regulatory influences after activation, targeting for degradation by ubiquitination and rescue from degradation by Hsp90. The balance between these processes is expected to determine the levels of active Lck and thereby to influence TCR signaling.
MATERIALS AND METHODS
Antibodies.
Lck was detected by immunoblotting with monoclonal antibody (MAb) 3A5 (Santa Cruz Biotechnology, Inc.) and by immunoprecipitation with a rabbit polyclonal antibody against residues 39 to 58 (provided by J. Borst, The Netherlands Cancer Institute, Amsterdam, The Netherlands). To detect Lck phosphorylated on Y394, rabbit polyclonal phospho-Src(Tyr416) (Cell Signaling Technology) was used. Tyrosine-phosphorylated proteins were visualized with MAb 4G10 (Upstate). Antiubiquitin MAb F4G7 was purchased from Covance; MAb HA11, against the influenza hemagglutinin (HA) epitope, was from Babco; and MAb 9E10, against the myc epitope, was from Sigma. MAb against Fyn was from Santa Cruz (sc-434), and MAb 327 was used against Src (Oncogene Science). Peroxidase-conjugated goat anti-rabbit and rabbit anti-mouse antibodies were from Perbio. MAb against CD3 (UCHT1) and CD28 (ANC28.1) were from Ancell. Polyclonal antibody H-114 (Santa Cruz Biotechnology, Inc.) was used to detect Hsp90.
Reagents.
Chemicals were from Sigma, unless indicated otherwise. Tissue culture reagents were from Cambrex.
T-cell stimulation.
Peripheral blood mononuclear cells (PBMC) of healthy volunteers were isolated from 50-ml blood samples by using Histopaque. Following isolation, cells were resuspended at a concentration of 106/ml in RPMI supplemented with 10% fetal calf serum (FCS) and 100 U of penicillin/ml and 0.1 mg of streptomycin/ml (pen/strep) at 37°C, 5% CO2. PBMC were stimulated with 6-μm polystyrene beads coated with anti-CD3 and anti-CD28 antibodies. For antibody coating, 2 × 107 Polybead polystyrene beads (Polysciences, Inc.) were washed once with cold phosphate-buffered saline (PBS) and were incubated overnight in 50 μl of PBS at 4°C with the MAb (30 μg/ml each). Beads were then washed twice with PBS and blocked for 1 h in 100 μl of denatured bovine serum albumin (200 μg/ml in PBS) at room temperature. The beads were washed three times and were resuspended in PBS at 1 × 108 to 2 × 108/ml. To stimulate PBMC, 1 × 106 to 2 × 106 cells were taken up in 150 μl of RPMI-10% FCS-pen/strep in a 15-ml conical tube. An equal amount of beads (in 10 μl) was added, and the mixture was centrifuged for 10 s at 250 × g, gently resuspended, and incubated for the indicated length of time at 37°C, 5% CO2. The formation of conjugates between beads and cells was verified by microscopy.
Drug treatment of cells.
GA was used at 5 μM, added from a 20 mM stock in dimethyl sulfoxide (DMSO). T cells stimulated in the presence of GA were pretreated with GA for 30 min. Epoxomycin (Calbiochem) was used at 10 μM and was added from a 1 mM stock in DMSO. PP2 (Calbiochem) was used at 12.5 μM, added from a 25 mM stock in DMSO.
cDNAs and mutagenesis.
Murine Lck, chicken c-Src, and Lck mutants LckY505F and LckK273A, in the vector PSM containing a simian virus 40 promoter, were obtained from D. Littman (Skirball Institute, New York, N.Y). LckY505F was subcloned into pCI (Promega) for high-level expression to allow detection of Hsp90 association. The introduction of mutations K273A, S59A, S42A, W97A, and T316M in Lck or LckY505F and Y527F in c-Src was done by the Quickchange site-directed mutagenesis method (Stratagene). Constructs generated by mutagenesis were completely sequenced to ensure that no further mutations were introduced. The human brain Fyn mutant, FynY531F, was obtained from R. Kypta (Imperial College, London, United Kingdom), HA-ubiquitin cDNA was from M. Treier (EMBL, Heidelberg, Germany), and Tip cDNA was from B. Sefton (Salk Institute, La Jolla, Calif.).
Cell culture and transfections.
COS-7 and 293T human embryonic kidney epithelial cells were maintained at 37°C and 5% CO2 in Dulbecco's modified Eagle medium supplemented with 10% FCS and pen/strep. COS-7 cells were transiently transfected with either Effectene (QIAGEN) or Nucleofection (Amaxa) according to the manufacturers' protocol. When using Effectene, cells were plated the day before transfection in 6-well plates at 2 × 105 cells per well. For GA time course experiments, DNA was mixed with transfection reagents, equal amounts of which were added to each of 4 wells. When using Nucleofection, typically 5 μg of DNA in 100 μl of buffer V was added to 2.5 × 106 cells, and cells were electroporated using Amaxa's cuvettes and Nucleofector. Cells were then taken up in a solution of RPMI, 10% FCS, and pen/strep and were equally divided over 4 wells of a 6-well plate. Cells were used 18 to 24 h after transfection. 293T cells were transfected by using the calcium phosphate method.
SDS-polyacrylamide gel electrophoresis and immunoblotting.
Cells were lysed in NP-40 buffer (1% Nonidet P-40 [Perbio], 20 mM Tris [pH 7.8], 150 mM NaCl, 2 mM MgCl2, 1 mM EDTA, 100 mM NA3VO4) containing the protease inhibitors phenylmethylsulfonyl fluoride (at 1 mM) and CLAP (5-μg/ml concentration each of chymostatin, pepstatin A, and antipain hydrochloride and 10 μg of leupeptin hemisulphate/ml) and were left on ice for 30 min. Nuclei and cell debris were removed by centrifugation at 13,000 × g for 5 min at 4°C. Lysates were denatured for 5 min at 95°C in nonreducing sodium dodecyl sulfate (SDS) sample buffer and were loaded onto SDS-10% polyacrylamide gels. After gel electrophoresis, proteins were transferred to nitrocellulose membranes (Schleicher & Schuell) and were immunoblotted. Incubations with antibodies were in a solution of PBS, 0.1% Tween 20, 10% skim milk or in a solution of PBS, 0.1% Tween 20, 5% bovine serum albumin for anti-phospho-Src(Tyr316), p-Erk, and 4G10. Blots were developed by using chemiluminescence (SuperSignal West Femto; Perbio) and were visualized with autoradiography film (Hyperfilm ECL; Amersham Pharmacia).
Immunoprecipitation.
Cells were lysed in NP-40 buffer or, to detect ubiquitinated Lck, in NP-40 buffer supplemented with 0.1% SDS and 5 mM N-ethylmaleimide. Cell lysates were cleared of nonspecifically binding proteins by incubation with 15 μl of packed protein A-Sepharose beads (Pharmacia Biotech AB) for 30 min at 4°C. To detect ubiquitination of Lck, 1 μl of polyclonal rabbit anti-Lck serum was used for immunoprecipitations. To detect association of Hsp90 with Lck, 10 μl of anti-Lck MAb 3A5 was used. Samples were incubated with antibodies for 45 min on ice. Immune complexes were recovered by incubation with protein A-Sepharose (15 μl of packed beads) for 45 min at 4°C and were washed five times in NP-40 lysis buffer. Immune complexes were eluted by addition of reducing SDS-sample buffer, incubated for 5 min at 95°C, and loaded on SDS-8% polyacrylamide gels. After electrophoresis, proteins were transferred to nitrocellulose membranes and were analyzed by immunoblotting.
Assay for kinase activity.
Kinase activity of Lck constructs was measured using a nonradioactive tyrosine kinase kit (Upstate) according to the manufacturer's guidelines. Briefly, biotinylated Src substrate peptide was coated on a streptavidin-coated 96-well plate. Lysates of cells expressing Lck constructs, diluted 1:5 with kinase buffer, were added to the wells in duplicate. By using active LckY505F, the linear part of the reaction was first determined by varying incubation times. To compare the activity between Lck constructs, reactions were left to develop for a fixed length of time. Following termination of the reaction, plates were incubated with horseradish peroxidase-conjugated antiphosphotyrosine MAb 4G10 to detect phosphorylated peptide and then were developed with horseradish peroxidase substrate 3,3′,5,5′-tetramethylbenzidine. After addition of sulfuric acid, plates were read at 450 nm (Anthos 2010). Cell lysates used in the kinase assays were also analyzed by immunoblotting for Lck, and the blots were quantitated with a Chemidoc system (Bio-Rad). The values of the kinase assay were then equalized for Lck protein levels.
RESULTS
Lck is sensitive to Hsp90 inhibition following TCR stimulation.
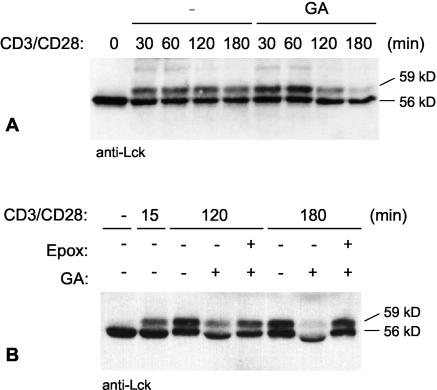
We previously showed that levels of a constitutively active Lck mutant, LckY505F, but not wild-type Lck are greatly reduced within 3 h of treatment with the Hsp90 inhibitor GA (5). Because treatment with cycloheximide, a protein synthesis inhibitor, for the same length of time did not reduce levels of LckY505F, we concluded that the effect of GA is not due to loss of the newly synthesized protein but of the mature protein. LckY505F is constitutively active because of a mutation of the regulatory tail tyrosine (28), which abolishes the inhibitory interaction between the tail and the SH2 domain (Fig. 1). This mutant therefore mimics the active form of Lck dephosphorylated on tyrosine 505. Given the effect of GA on this active mutant, we predicted that Lck activated by TCR stimulation would also be sensitive to Hsp90 inhibition. To investigate this possibility, T cells were activated with anti-CD3 and anti-CD28 antibodies in the absence or presence of GA. In stimulated cells, a molecular size shift from 56 to 59 kDa occurs for Lck as a result of the phosphorylation of serine 59 in the unique domain by Erk1 and Erk2 (Fig. 2A) (42, 48, 52). Because the activation of Erk1 and Erk2 is dependent on Lck activity (42 and data not shown) and still occurred in GA-treated cells, we concluded that the activation of Lck and that of downstream signaling pathways were not affected by Hsp90 inhibition. However, a decrease in Lck levels was observed with time in the presence of GA (Fig. 2A). This loss of Lck was not seen in T cells stimulated in the absence of GA (Fig. 2A) or in T cells treated with GA in the absence of stimulation (data not shown).
FIG. 2.
Lck is degraded after T-cell activation in the absence of active Hsp90. (A) Human PBMC were activated for various lengths of time with anti-CD3- and anti-CD28-coated polystyrene beads either in the absence or presence of the Hsp90 inhibitor GA. PBMC were pretreated with GA for 30 min before activation. Cells were lysed and immunoblotted for Lck. (B) PBMC were activated as described in panel A in the absence or presence of GA and the proteasome inhibitor epoxomycin (Epox) as indicated. Cells were pretreated with epoxomycin for 90 min and with GA for 30 min before activation. Cell lysates were immunoblotted for Lck. The 56-kDa as well as the phosphorylated 59-kDa form of Lck are indicated.
Whereas the levels of both the 56- and the 59-kDa form of Lck were reduced in the presence of GA, the effect was most striking for the 59-kDa form, which was virtually absent after 3 h of activation in the presence of the drug (Fig. 2A). At this time, levels of active Erk1 and Erk2, detected with an antibody specific for the phosphorylated Erk proteins, were only marginally affected (data not shown). Thus, the loss of the 59-kDa form of Lck did not result from a lack of phosphorylation by Erk1 and Erk2. To investigate whether the loss of Lck in the presence of GA is a result of proteasomal degradation, T cells were activated in the presence of either GA alone or together with the proteasome inhibitor epoxomycin. Indeed, more Lck was recovered in the presence of epoxomycin, indicating that Lck was rescued from degradation (Fig. 2B). Given that inhibition of Hsp90 only affects active Lck (5), the greater sensitivity of the 59-kDa form is most likely explained by a relatively larger proportion of it being present in the active state than is the 56-kDa form. Taken together, these results show that Lck activated by TCR stimulation is dependent on Hsp90 monitoring for its stability.
Active Lck is ubiquitinated.
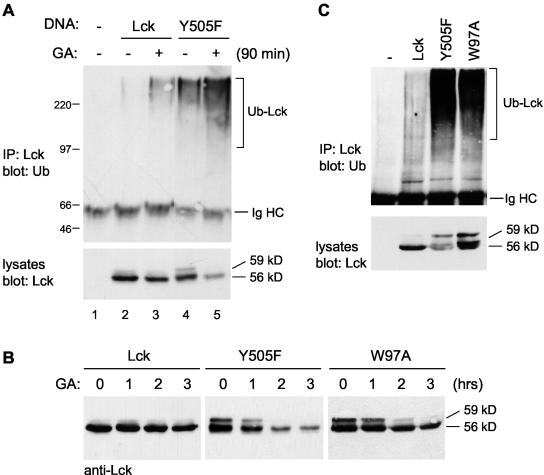
Proteins are normally targeted for proteasomal degradation by polyubiquitination. To examine whether active Lck is subjected to ubiquitination, Lck and LckY505F constructs were coexpressed with HA-tagged ubiquitin in COS-7 cells. Immunoblotting of Lck immunoprecipitates with anti-HA antibodies showed extensive ubiquitination of active LckY505F, whereas that of wild-type Lck was negligible (Fig. 3A, upper gel, lanes 2 and 4). Incubation of cells with GA for 90 min led to a reduction in LckY505F levels (Fig. 3A, lower gel, lanes 4 and 5) as shown before (5). We show below that an interaction between LckY505F and Hsp90 can be detected and that this association is completely disrupted by GA treatment (see Fig. 7B). Thus, the effects of GA can be attributed to this direct action on Lck rather than on other Hsp90 substrates. The constitutively active LckY505F was detected as a 59-kDa as well as a 56-kDa band. As in T cells, the 59-kDa form results from the activation of and phosphorylation by Erk1 and Erk2 (see Fig. 5). However, both the 56-kDa and the 59-kDa forms were active based on their phosphorylation at the autophosphorylation site (Y394) (see Fig. 6B), and consistent with this, both forms were degraded in the presence of GA. Treatment with GA further resulted in an increase in LckY505F ubiquitination (Fig. 3A, upper gel, lanes 4 and 5). This suggests that Hsp90 protects LckY505F from ubiquitination to some extent. For wild-type Lck, a small amount of ubiquitinated protein was observed in the presence of GA, possibly representing a fraction in the active conformation (Fig. 3A, upper gel, lane 3).
FIG. 3.
Active Lck mutants are dependent on Hsp90 for stability and ubiquitinated. (A) COS-7 cells were transfected with HA-tagged ubiquitin together with wild-type Lck (Lck) or the active mutant LckY505F (Y505F). Where indicated, cells were treated with GA for 90 min. Cell lysates were either subjected to immunoprecipitation (IP) with anti-Lck antibodies followed by blotting with anti-HA antibodies to detect ubiquitinated Lck (top) or were blotted for Lck directly (bottom). (B) COS-7 cells transfected with Lck, LckY505F (Y505F), or LckW97A (W97A) were treated with GA for 0, 1, 2, or 3 h. Cell lysates were blotted for Lck. The W97A mutation affects substrate binding of the SH3 domain. (C) COS-7 cells were transfected either with HA-ubiquitin on its own (−) or together with the indicated Lck constructs. Lck immunoprecipitates (IP) were immunoblotted with antiubiquitin antibodies to detect ubiquitinated Lck (upper gels), and cell lysates were blotted for Lck directly (lower gels). The positions of the 56-kDa, 59-kDa, and ubiquitinated form of Lck (Ub-Lck) as well as that of the immunoglobulin heavy chain (Ig HC) are indicated.
FIG. 7.
PP2 binding stabilizes Lck and prevents its ubiquitination. (A) COS-7 cells were transfected with LckY505F (Y505F) or LckW97A (W97A). Cells either were treated with GA alone or were pretreated with PP2 for 1 h followed by incubation with both PP2 and GA for 0, 1, 2, or 3 h. Cells were lysed and immunoblotted for Lck. (B) 293T cells were transfected with LckY505F (Y505F) and were either left untreated (−), treated with GA for 1.5 h, or treated with PP2 for 15 h. Lck was immunoprecipitated by using MAb 3A5, and immunoprecipitates (IP) were immunoblotted for Hsp90 (top) or Lck (bottom). Untransfected (−) cells were not treated (−) and were used as a control. (C) COS-7 cells were transfected with LckY505F together with HA-ubiquitin and then were left untreated or were treated with PP2 for 15 h. Lck immunoprecipitates were immunoblotted with antiubiquitin (upper gel) and with anti-Lck antibodies (lower gel).
FIG. 5.
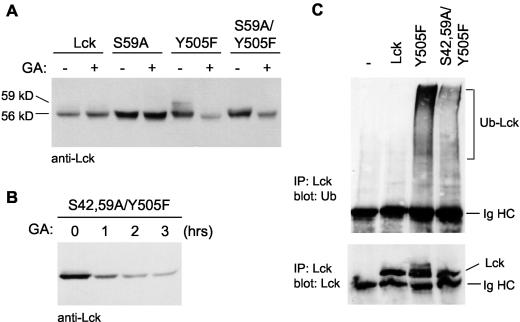
Phosphorylation of Ser42 and Ser59 is not required for Hsp90 monitoring and ubiquitination of Lck. (A) COS-7 cells transfected with Lck or Lck mutants were left untreated (−) or treated (+) with GA for 2 h, lysed, and blotted for Lck. Phosphorylation of active LckY505F (Y505F) on serine 59 is detected as a shift in molecular size from 56 to 59 kDa. Note the absence of the 59-kDa Lck form in Lck S59A/Y505F (S59A/Y505F). (B) COS-7 cells transfected with LckS42,59A/Y505F (S42,59A/Y505F) were treated with GA for 0, 1, 2, or 3 h. Cells were lysed and immunoblotted for Lck. (C) COS-7 cells were transfected with Lck constructs together with HA-ubiquitin. For the control (−), HA-ubiquitin was transfected on its own. Lck immunoprecipitates (IP) were immunoblotted with antiubiquitin (upper gel) or with anti-Lck antibodies (lower gel). The positions of the 56-kDa, 59-kDa, and ubiquitinated form of Lck (Ub-Lck) as well as that of the immunoglobulin heavy chain (Ig HC) are indicated.
FIG. 6.
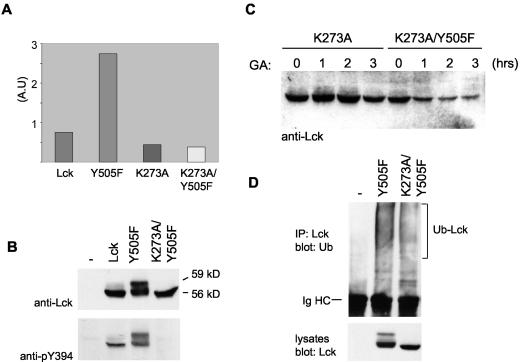
Lck kinase activity is not required for Hsp90 monitoring and ubiquitination. (A) COS-7 cells were transfected with the indicated Lck constructs, cells were lysed, and kinase activity was measured in an enzyme-linked immunosorbent assay-based assay with a peptide substrate. The assay was done in duplicate, and mean values are plotted (Lck, 0.754 and 0.742; Y505F, 2.783 and 2.699; K273A, 0.432 and 0.444; K273A/Y505F, 0.376 and 0.374). A.U, arbitrary units. (B) COS-7 cells were transfected with Lck constructs as indicated, and cell lysates were immunoblotted with anti-Lck (upper gel) or with anti-pY394 (lower gel) to detect Lck phosphorylated on tyrosine 394, the autophosphorylation site that is essential for kinase activity. (C) COS-7 cells transfected with LckK273A (K273A) or LckK273A/Y505F (K273A/Y505F) were treated with GA for 0, 1, 2, or 3 h. Cells were lysed and immunoblotted for Lck. The K273A mutation in the ATP-binding site abolishes Lck kinase activity. (D) COS-7 cells were transfected with LckY505F (Y505F) or LckK273A/Y505F (K273A/Y505F) together with HA-ubiquitin. For the control (−), HA-ubiquitin was transfected on its own. Lck immunoprecipitates (IP) were immunoblotted with antiubiquitin (top), and cell lysates were immunoblotted with anti-Lck (bottom). The positions of the 56-kDa, 59-kDa, and ubiquitinated form of Lck (Ub-Lck) as well as that of the immunoglobulin heavy chain (Ig HC) are indicated.
The construct LckW97A contains a point mutation in the substrate-binding site of the SH3 domain, which abolishes the inhibitory interaction between the SH3 domain and the SH2-SH1 linker (Fig. 1) (9, 11). Expression of LckW97A, like that of LckY505F, leads to an increase in total cellular phosphotyrosine levels (data not shown), confirming the enhanced activity of this mutant compared to that of wild-type Lck. The presence of the 59-kDa form further indicates that LckW97A activates and is phosphorylated by Erk1 and Erk2 (Fig. 3B). Like LckY505F, LckW97A was unstable in the presence of GA (Fig. 3B). In addition, LckW97A was ubiquitinated to a greater extent than wild-type Lck (Fig. 3C). Thus, the release of either the SH2 or the SH3 domain inhibitory interaction leads to an enhanced dependence on Hsp90 for stability as well as to enhanced ubiquitination of Lck.
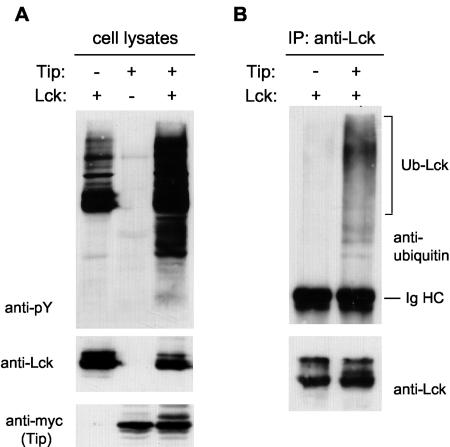
To investigate whether ubiquitination occurs not only for active Lck mutants but also for active forms of the wild-type protein, Lck was cotransfected with the simian herpesvirus Saimiri Tip protein into COS-7 cells. Tip activates Lck via binding of two separate motifs to the SH3 and the kinase domain of Lck, respectively (16, 50). Activation of Lck in the presence of Tip was verified by an increase in total cellular phosphotyrosine levels (Fig. 4A, anti-pY). Concurrent with this activation, an increase in Lck ubiquitination was observed in cells that coexpress the Tip protein (Fig. 4B). Together these results show that active Lck is stabilized by Hsp90 on the one hand and targeted for degradation by ubiquitination on the other hand. The levels of active Lck are therefore dependent on the balance of these opposing processes.
FIG. 4.
Activation by Saimiri virus Tip protein leads to ubiquitination of Lck. (A) COS-7 cells were transfected with Lck and myc-tagged Tip as indicated. Cell lysates were blotted with anti-Lck and anti-myc antibodies to verify expression of Lck and Tip and with MAb 4G10 to detect total cellular phosphotyrosine (anti-pY). (B) COS-7 cells were transfected with HA-ubiquitin together with Lck and Tip as indicated. Lck immunoprecipitates were immunoblotted with anti-ubiquitin antibodies to detect ubiquitinated Lck (upper gels) as well as Lck (lower gels). The positions of ubiquitinated Lck (Ub-Lck) and immunoglobulin heavy chain (Ig HC) are indicated.
Serine phosphorylation is not required for Hsp90 monitoring and ubiquitination of Lck.
We next examined which factors determine the enhanced Hsp90 monitoring and ubiquitination of active compared to inactive Lck. Because the ubiquitination of some proteins is reliant on preceding phosphorylation and because phosphorylation on serine 59 is only observed with active Lck, we investigated whether this modification plays a role in ubiquitination and/or Hsp90 monitoring. Therefore, serine 59 was changed to an alanine in LckY505F. The absence of a 59-kDa form of this mutant, LckS59A/Y505F, confirmed that phosphorylation of this residue was abolished (Fig. 5A). Despite this lack of serine 59 phosphorylation, LckS59A/Y505F was still degraded in the presence of GA (Fig. 5A). To ensure that the S59A mutation on its own did not compromise Lck stability, we also made this mutation in the wild-type background. This mutant, LckS59A, was indeed stable in the presence of GA (Fig. 5A). Whereas serine 59 phosphorylation is responsible for the molecular mass shift from 56 to 59 kDa, phosphorylation of serine 42 by either protein kinase A or protein kinase C has also been reported (52). A mutation of serine 42 to alanine was therefore introduced in LckS59A/Y505F. Again, the resulting mutant, LckS42,59A/Y505F, was degraded in the presence of GA (Fig. 5B). In addition, this mutant was ubiquitinated to a greater extent than wild-type Lck (Fig. 5C). It can therefore be concluded that phosphorylation of serines 42 and 59 does not play a role in the ubiquitination and Hsp90 monitoring of active Lck.
Kinase activity is not required for Hsp90 monitoring and ubiquitination of Lck.
We then analyzed whether the kinase activity itself is required for enhanced Lck ubiquitination and Hsp90 monitoring. To this end, lysine 273 in the ATP-binding site was mutated to an alanine in LckY505F, which eliminates the ability of the kinase to transfer phosphate from ATP to substrates (8). The absence of kinase activity was verified for LckK273A/Y505F in a kinase assay using a peptide substrate (Fig. 6A). The lack of tyrosine phosphorylation at the autophosphorylation site (Y394) (Fig. 6B), which is essential for kinase activity, also established that this is a kinase-dead mutant. Despite this lack of activity, LckK273A/Y505F was degraded in the presence of GA (Fig. 6C). Additionally, LckK273A/Y505F showed enhanced ubiquitination compared to that of wild-type Lck (Fig. 6D). A construct that contains the K273A but not the Y505F mutation was stable, showing that K273A by itself does not decrease the stability of Lck (Fig. 6C, LckK273A). We conclude that the enhanced ubiquitination and Hsp90 monitoring of Lck are induced by the release of one inhibitory intramolecular interaction but do not depend on the kinase activity. This indicates that both processes do not require the phosphorylation and/or activation of Lck substrates. The results also show that ubiquitination and Hsp90 monitoring are not determined by Y394 phosphorylation.
Binding of PP2 prevents ubiquitination and degradation of active Lck.
In the absence of a role for phosphorylation and kinase activity, it seems likely that conformational changes that accompany activation are responsible for inducing Hsp90 monitoring as well as ubiquitination. The crystal structure of the Lck kinase domain has been determined in the active autophosphorylated conformation (56) as well as in a form bound to the inhibitor PP2 (61). Moreover, the crystal structure of the complete Hck protein complexed to PP1, another pyrazolo pyrimidine-type inhibitor, has also been elucidated (41). The major differences between the active and inhibited forms of these structures are primarily found in the position of the small lobe relative to the large lobe, the position of an alpha helix (the alpha C helix) in the small lobe, and in the conformation of the activation loop.
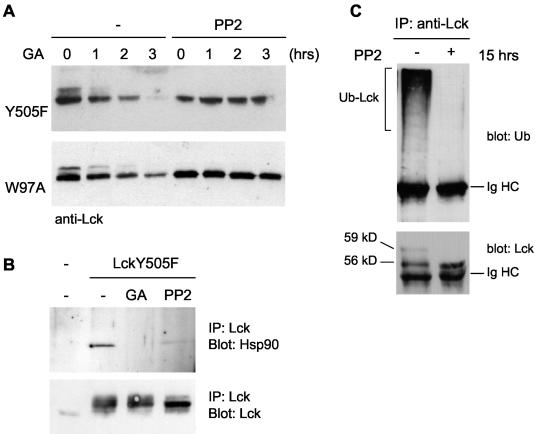
We investigated the effect of the inhibitor PP2, which binds to the ATP-binding site and is a potent selective inhibitor of Src kinases, on the stability and ubiquitination of Lck mutants. Cells were pretreated with PP2 for 1 h, followed by incubation with PP2 and GA together for up to 3 h. The results show that the presence of PP2 completely abolished the GA-induced degradation of LckY505F and LckW97A (Fig. 7A). To analyze this further, the association of Lck with Hsp90 in the presence of PP2 was studied. When expressed at high levels in 293T cells, an interaction between LckY505F and Hsp90 was detected by coimmunoprecipitation (Fig. 7B, upper gel). This interaction is completely abolished in the presence of GA. Moreover, treatment with PP2 also resulted in a considerable reduction in the amount of Hsp90 associated with LckY505F. Thus, PP2 stabilizes active Lck and overcomes the requirement for Hsp90 monitoring. In addition, ubiquitination of LckY505F was no longer detected following treatment with PP2 (Fig. 7B), supporting the notion that the inactive conformation of Lck is not recognized for ubiquitination.
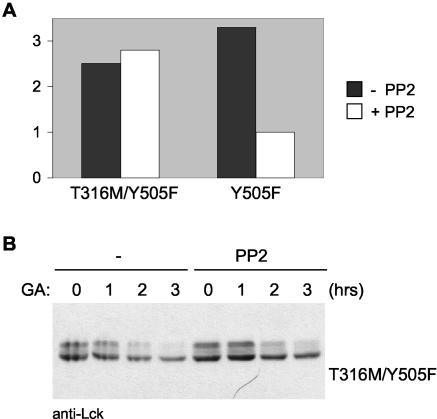
The absence of an interaction between LckY505F and Hsp90 in the presence of PP2 suggests that PP2 acts by binding to Lck rather than by inhibiting other targets. To confirm this, we mutated threonine 316, one of the residues that interacts with PP2 (61), to a methionine. A methionine is present at the equivalent position in the tyrosine kinase Zap-70, which is insensitive to inhibition by PP2 (14). Substitution of T316 does not interfere with kinase activity (10) (Fig. 8A) but renders LckT316MY505F refractory to inhibition by PP2 (Fig. 8A). This indicates that LckT316MY505F is unable to bind PP2. LckT316MY505F was degraded in the presence of GA, but in contrast to LckY505F this mutant was not rescued by PP2 from degradation (Fig. 8B). Thus, PP2 overcomes the requirement for Hsp90 monitoring and prevents ubiquitination by binding to the ATP-binding site of Lck.
FIG. 8.
(A) COS-7 cells transfected with LckY505F (Y505F) or LckT316 M/Y505F (T316 M/Y505F) were either left untreated or were incubated with PP2 for 2.5 h. Cells were lysed, and equivalent amounts of protein were used in an enzyme-linked immunosorbent assay-based kinase assay. Expression levels of Lck were comparable between transfectants as determined by Western blotting. To determine background kinase activity levels, untransfected COS-7 cells were measured in the same assay. The plotted assay is representative of two separate experiments. Mean values of duplicates are plotted after subtraction of the background activity. (B) COS-7 cells transfected with T316 M/Y505F were treated with GA and PP2 as described for Fig. 7A. Cells were lysed and immunoblotted for Lck.
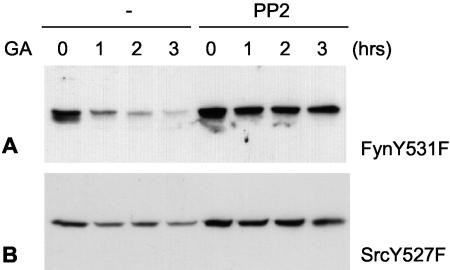
Whereas Src family proteins differ considerably at the amino acid level, the proteins have similar domain organizations and structures. We therefore studied the effects of GA and PP2 on two other members of the Src family, c-Src and Fyn. Similar to the results obtained for Lck, constitutively active forms of these proteins were degraded in the presence of GA (Fig. 9). Moreover, these proteins were rescued from GA-induced degradation in the presence of PP2. Thus, the regulation of Lck by Hsp90 monitoring and ubiquitination is likely to be shared by other members of the Src family.
FIG. 9.
(A) COS-7 cells were transfected with FynY531F, which is constitutively active because of a point mutation at the regulatory tail tyrosine (Y531). Cells were either treated with GA only or were pretreated with PP2 for 1 h followed by incubation with both PP2 and GA for 0, 1, 2, or 3 h. Cells were lysed and immunoblotted for Fyn. (B) Cells were transfected with a constitutively active form of c-Src, SrcY527F, and were treated as described for panel A. Cells were lysed and immunoblotted for Src.
DISCUSSION
Ligand recognition by T cells can lead to many different outcomes, ranging from apoptosis to positive selection of developing T cells and from nonresponsiveness to activation of mature T cells. Lck plays a crucial role in all of these processes, and the extent of its activity is important for the outcome of TCR stimulation. Recent studies have shown that the active forms of Src family kinases are selectively polyubiquitinated and degraded. In agreement with this, it was previously shown that constitutively active, but not inactive, Lck is degraded when Hsp90 function is inhibited. Now we have established (i) a role for Hsp90 during T-cell activation, (ii) the relationship between Hsp90 monitoring and ubiquitination, and (iii) the Lck determinants that trigger both processes.
We showed here that Hsp90 contributes to the regulation of Lck activity by protecting it from proteasomal degradation after T-cell activation. To assess the role of Hsp90 we used the inhibitor GA, which, like its derivatives, is a potential chemotherapeutic agent (32). Hsp90 functions in a large multiprotein complex that contains other chaperones and cofactors, including Hsp70, Hsp40, p23, Cdc37, Hip, and Hop. GA binds with high specificity to the ATP-binding site of Hsp90 but not to that of other cytosolic chaperones (39, 44, 49). Binding of GA interferes with substrate recognition, either by an effect on Hsp90 itself or by the release of p23 from the heterocomplex. A stable interaction between Hsp90 and the oncogenic viral form of Src, v-Src, has been demonstrated previously (7). However, an association has been more difficult to detect for c-Src and its cellular family members, even though these proteins require Hsp90 during synthesis. This difference between v-Src and c-Src is consistent with enhanced monitoring of the active forms of mature Src tyrosine kinases by Hsp90. For Lck, an interaction with Hsp90 has been demonstrated during in vitro translation in rabbit reticulocyte lysates (18) and in murine T cells that overexpress Lck by 40-fold (17). In the experiments presented here, an interaction with Hsp90 was detected when constitutively active LckY505F was highly overexpressed. Importantly, treatment with GA completely abrogated this interaction (Fig. 7). Therefore, the effects of GA in our experiments can be ascribed to the disruption of a direct association between the Hsp90 complex and Lck.
The effects of GA and the related ansamycin, herbimycin, on T cells has been studied before, showing that long exposure (16 h) to these drugs inhibits T-cell function (25, 58). Because Hsp90 is essential for the synthesis of Src kinases, this outcome might be primarily due to the loss of newly synthesized Lck. In addition, other Hsp90 client proteins that are important for T-cell function, such as c-Raf, are also likely to be affected by prolonged GA treatment (59). In our experiments, T cells were treated with GA for a maximum of 3 h, when the effect on newly synthesized Lck is negligible. The functions of Lck and c-Raf also were not compromised at this time, illustrated by the normal downstream activation of Erk kinases. Therefore, the degradation of Lck we observe when T cells are activated in the presence of GA (Fig. 2) indicates that the stability of mature active Lck is dependent on Hsp90 monitoring.
Whereas we found that T-cell activation leads to Hsp90 monitoring, Rao et al. demonstrated that this process leads to Lck ubiquitination (36). This suggests that the fate of active Lck is determined by two conflicting regulatory influences, targeting for degradation by ubiquitination and rescue from degradation by Hsp90 monitoring. Our other results also point towards such a relationship between the occurrence of Hsp90 monitoring and ubiquitination of Lck. For instance, Lck mutants that are degraded in the presence of GA, such as LckY505F and LckW97A, are also highly ubiquitinated (Fig. 3). Conversely, wild-type Lck that is resistant to GA-induced degradation is not ubiquitinated. Furthermore, treatment with PP2 reduces the Hsp90 requirement as well as the ubiquitination of Lck (Fig. 7). Given this correlation, Hsp90 monitoring and ubiquitination are expected to be reliant on overlapping molecular features of Lck.
We found that Hsp90 monitoring and ubiquitination are enhanced when Lck is active as a result of the release of either the SH2 or SH3 intramolecular interaction (Fig. 3), yet these processes do not require Lck kinase activity (Fig. 6). Based on the observation that binding of the inhibitor PP2 to the ATP-binding site of Lck inhibits both processes (Fig. 7), we conclude that the conformation of the active kinase domain is important for Hsp90 monitoring and ubiquitination. Although little is known about substrate recognition by Hsp90, other studies on tyrosine kinases also suggest a role for the kinase domains in this process. For instance, during in vitro synthesis in reticulocyte lysates, the folding of the kinase but not the SH2 domain of Lck requires Hsp90 activity (19). The receptor tyrosine kinase ErbB2 is another Hsp90 client protein affected by GA treatment. The effect of GA on ErbB2 requires the presence, but not the activity, of the kinase domain (55). Moreover, mutations in the kinase domain of ErbB2 that abolish the sensitivity to GA do not affect its kinase activity (46).
Ubiquitination is mediated by three classes of enzymes, comprised of an E1 that activates ubiquitin and transfers it to an E2-conjugating enzyme, which in turn transfers it to the substrate of an E3 ligase (21). The E3 ligases comprise a large and very diverse group of proteins that provide selectivity to the ubiquitination reaction by interacting with specific substrates. The E3 ligase Cbl, a negative regulator of many signaling pathways (27, 35), associates with Lck, and when overexpressed it results in enhanced Lck ubiquitination (36). The interaction between Lck and Cbl is independent of both T-cell activation and activation status of Lck (36, 37). Possibly, Lck ubiquitination in our experiments is mediated by Cbl, but other E3 ligases that selectively interact with active Lck might also be involved.
Although the Hsp90 monitoring and ubiquitination of Lck coincide, the GA-induced and constitutive ubiquitination of active Lck might be mediated by different E3 ligases. Hsp90, which can exist in a heterocomplex that promotes either protein folding or degradation, was recently found to interact with an E3 ligase, CHIP (4). GA favors the formation of a degradation-promoting Hsp90 complex (2, 59), and the degradation of ErbB2 in the presence of GA was shown to involve ubiquitination by CHIP (54, 60). We are presently investigating the roles of Cbl, CHIP, and other E3 ligases in the GA-induced as well as the constitutive ubiquitination of active Lck.
In conclusion, the work presented here suggests a novel regulatory mechanism for Lck where, after activation, the protein is subjected to two opposing forces, Hsp90 monitoring and ubiquitination. It will be interesting to determine how both processes are regulated during T-cell activation and differentiation. For instance, Hsp90 monitoring could be under the control of changes in the expression, recruitment, and activity of associated cochaperones and cofactors. Likewise, ubiquitination could be regulated by changes in the expression and activity of E3 ligases or deubiquitinating enzymes. Because of the similarities between the Src-related tyrosine kinases, we expect that the findings described here are also relevant for other members of this family of kinases.
Acknowledgments
We are grateful to B. Sefton, M. Treier, and R. Kypta for cDNA constructs, to A. Hayday, M. Marsh, D. Pennington, and B. Silva-Santos for critically reading the manuscript, and to S. John for helpful suggestions.
This work was supported by a Career Development Award from the Medical Research Council.
REFERENCES
- 1.Abraham, K. M., S. D. Levin, J. D. Marth, K. A. Forbush, and R. M. Perlmutter. 1991. Thymic tumorigenesis induced by overexpression of p56lck. Proc. Natl. Acad. Sci. USA 88:3977-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An, W. G., T. W. Schulte, and L. M. Neckers. 2000. The heat shock protein 90 antagonist geldanamycin alters chaperone association with p210bcr-abl and v-src proteins before their degradation by the proteasome. Cell Growth Differ. 11:355-360. [PubMed] [Google Scholar]
- 3.Andoniou, C. E., N. L. Lill, C. B. Thien, M. L. Lupher, Jr., S. Ota, D. D. Bowtell, R. M. Scaife, W. Y. Langdon, and H. Band. 2000. The Cbl proto-oncogene product negatively regulates the Src-family tyrosine kinase Fyn by enhancing its degradation. Mol. Cell. Biol. 20:851-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballinger, C. A., P. Connell, Y. Wu, Z. Hu, L. J. Thompson, L. Y. Yin, and C. Patterson. 1999. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 19:4535-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bijlmakers, M. J., and M. Marsh. 2000. Hsp90 is essential for the synthesis and subsequent membrane association, but not the maintenance, of the Src-kinase p56(lck). Mol. Biol. Cell. 11:1585-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bromley, S. K., W. R. Burack, K. G. Johnson, K. Somersalo, T. N. Sims, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 2001. The immunological synapse. Annu. Rev. Immunol. 19:375-396. [DOI] [PubMed] [Google Scholar]
- 7.Brugge, J. S. 1986. Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr. Top. Microbiol. Immunol. 123:1-22. [DOI] [PubMed] [Google Scholar]
- 8.Carrera, A. C., K. Alexandrov, and T. M. Roberts. 1993. The conserved lysine of the catalytic domain of protein kinases is actively involved in the phosphotransfer reaction and not required for anchoring ATP. Proc. Natl. Acad. Sci. USA 90:442-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denny, M. F., H. C. Kaufman, A. C. Chan, and D. B. Straus. 1999. The lck SH3 domain is required for activation of the mitogen-activated protein kinase pathway but not the initiation of T-cell antigen receptor signaling. J. Biol. Chem. 274:5146-5152. [DOI] [PubMed] [Google Scholar]
- 10.Denzel, A., K. J. Hare, C. Zhang, K. Shokat, E. J. Jenkinson, G. Anderson, and A. Hayday. 2003. Cutting edge: a chemical genetic system for the analysis of kinases regulating T cell development. J. Immunol. 171:519-523. [DOI] [PubMed] [Google Scholar]
- 11.Erpel, T., G. Superti-Furga, and S. A. Courtneidge. 1995. Mutational analysis of the Src SH3 domain: the same residues of the ligand binding surface are important for intra- and intermolecular interactions. EMBO J. 14:963-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonfloni, S., J. C. Williams, K. Hattula, A. Weijland, R. K. Wierenga, and G. Superti-Furga. 1997. The role of the linker between the SH2 domain and catalytic domain in the regulation and function of Src. EMBO J. 16:7261-7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakak, Y., and G. S. Martin. 1999. Ubiquitin-dependent degradation of active Src. Curr. Biol. 9:1039-1042. [DOI] [PubMed] [Google Scholar]
- 14.Hanke, J. H., J. P. Gardner, R. L. Dow, P. S. Changelian, W. H. Brissette, E. J. Weringer, B. A. Pollok, and P. A. Connelly. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271:695-701. [DOI] [PubMed] [Google Scholar]
- 15.Harris, K. F., I. Shoji, E. M. Cooper, S. Kumar, H. Oda, and P. M. Howley. 1999. Ubiquitin-mediated degradation of active Src tyrosine kinase. Proc. Natl. Acad. Sci. USA 96:13738-13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartley, D. A., K. Amdjadi, T. R. Hurley, T. C. Lund, P. G. Medveczky, and B. M. Sefton. 2000. Activation of the Lck tyrosine protein kinase by the herpesvirus Saimiri Tip protein involves two binding interactions. Virology 276:339-348. [DOI] [PubMed] [Google Scholar]
- 17.Hartson, S. D., D. J. Barrett, P. Burn, and R. L. Matts. 1996. Hsp90-mediated folding of the lymphoid cell kinase p56lck. Biochemistry 35:13451-13459. [DOI] [PubMed] [Google Scholar]
- 18.Hartson, S. D., and R. L. Matts. 1994. Association of Hsp90 with cellular Src-family kinases in a cell-free system correlates with altered kinase structure and function. Biochemistry 33:8912-8920. [DOI] [PubMed] [Google Scholar]
- 19.Hartson, S. D., E. A. Ottinger, W. Huang, G. Barany, P. Burn, and R. L. Matts. 1998. Modular folding and evidence for phosphorylation-induced stabilization of an hsp90-dependent kinase. J. Biol. Chem. 273:8475-8482. [DOI] [PubMed] [Google Scholar]
- 20.Hermiston, M. L., Z. Xu, R. Majeti, and A. Weiss. 2002. Reciprocal regulation of lymphocyte activation by tyrosine kinases and phosphatases. J. Clin. Investig. 109:9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 22.Hubbard, S. R. 1999. Src autoinhibition: let us count the ways. Nat. Struct. Biol. 6:711-714. [DOI] [PubMed] [Google Scholar]
- 23.Janes, P. W., S. C. Ley, A. I. Magee, and P. S. Kabouridis. 2000. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin. Immunol. 12:23-34. [DOI] [PubMed] [Google Scholar]
- 24.Joazeiro, C. A., S. S. Wing, H. Huang, J. D. Leverson, T. Hunter, and Y. C. Liu. 1999. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286:309-312. [DOI] [PubMed] [Google Scholar]
- 25.June, C. H., M. C. Fletcher, J. A. Ledbetter, G. L. Schieven, J. N. Siegel, A. F. Phillips, and L. E. Samelson. 1990. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc. Natl. Acad. Sci. USA 87:7722-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kane, L. P., J. Lin, and A. Weiss. 2000. Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12:242-249. [DOI] [PubMed] [Google Scholar]
- 27.Liu, Y. C., and H. Gu. 2002. Cbl and Cbl-b in T-cell regulation. Trends Immunol. 23:140-143. [DOI] [PubMed] [Google Scholar]
- 28.Marth, J. D., J. A. Cooper, C. S. King, S. F. Ziegler, D. A. Tinker, R. W. Overell, E. G. Krebs, and R. M. Perlmutter. 1988. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck). Mol. Cell. Biol. 8:540-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melander, F., T. Andersson, and K. Dib. 2003. Fgr but not Syk tyrosine kinase is a target for beta 2 integrin-induced c-Cbl-mediated ubiquitination in adherent human neutrophils. Biochem. J. 370:687-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moarefi, I., M. LaFevre-Bernt, F. Sicheri, M. Huse, C. H. Lee, J. Kuriyan, and W. T. Miller. 1997. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature 385:650-653. [DOI] [PubMed] [Google Scholar]
- 31.Molina, T. J., K. Kishihara, D. P. Siderovski, W. van Ewijk, A. Narendran, E. Timms, A. Wakeham, C. J. Paige, K. U. Hartmann, A. Veillette, et al. 1992. Profound block in thymocyte development in mice lacking p56lck. Nature 357:161-164. [DOI] [PubMed] [Google Scholar]
- 32.Neckers, L. 2002. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol. Med. 8:S55-S61. [DOI] [PubMed] [Google Scholar]
- 33.Oda, H., S. Kumar, and P. M. Howley. 1999. Regulation of the Src family tyrosine kinase Blk through E6AP-mediated ubiquitination. Proc. Natl. Acad. Sci. USA 96:9557-9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porter, M., T. Schindler, J. Kuriyan, and W. T. Miller. 2000. Reciprocal regulation of Hck activity by phosphorylation of Tyr(527) and Tyr(416). Effect of introducing a high affinity intramolecular SH2 ligand. J. Biol. Chem. 275:2721-2726. [DOI] [PubMed] [Google Scholar]
- 35.Rao, N., I. Dodge, and H. Band. 2002. The Cbl family of ubiquitin ligases: critical negative regulators of tyrosine kinase signaling in the immune system. J. Leukoc. Biol. 71:753-763. [PubMed] [Google Scholar]
- 36.Rao, N., S. Miyake, A. L. Reddi, P. Douillard, A. K. Ghosh, I. L. Dodge, P. Zhou, N. D. Fernandes, and H. Band. 2002. Negative regulation of Lck by Cbl ubiquitin ligase. Proc. Natl. Acad. Sci. USA 99:3794-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reedquist, K. A., T. Fukazawa, B. Druker, G. Panchamoorthy, S. E. Shoelson, and H. Band. 1994. Rapid T-cell receptor-mediated tyrosine phosphorylation of p120, an Fyn/Lck Src homology 3 domain-binding protein. Proc. Natl. Acad. Sci. USA 91:4135-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Resh, M. D. 1994. Myristylation and palmitylation of Src family members: the fats of the matter. Cell 76:411-413. [DOI] [PubMed] [Google Scholar]
- 39.Roe, S. M., C. Prodromou, R. O'Brien, J. E. Ladbury, P. W. Piper, and L. H. Pearl. 1999. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 42:260-266. [DOI] [PubMed] [Google Scholar]
- 40.Rudd, C. E., J. M. Trevillyan, J. D. Dasgupta, L. L. Wong, and S. F. Schlossman. 1988. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc. Natl. Acad. Sci. USA 85:5190-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindler, T., F. Sicheri, A. Pico, A. Gazit, A. Levitzki, and J. Kuriyan. 1999. Crystal structure of Hck in complex with a Src family-selective tyrosine kinase inhibitor. Mol. Cell 3:639-648. [DOI] [PubMed] [Google Scholar]
- 42.Schroder, A. J., P. Quehl, J. Muller, and Y. Samstag. 2000. Conversion of p56(lck) to p60(lck) in human peripheral blood T lymphocytes is dependent on co-stimulation through accessory receptors: involvement of phospholipase C, protein kinase C and MAP-kinases in vivo. Eur. J. Immunol. 30:635-643. [DOI] [PubMed] [Google Scholar]
- 43.Sicheri, F., I. Moarefi, and J. Kuriyan. 1997. Crystal structure of the Src family tyrosine kinase Hck. Nature 385:602-609. [DOI] [PubMed] [Google Scholar]
- 44.Stebbins, C. E., A. A. Russo, C. Schneider, N. Rosen, F. U. Hartl, and N. P. Pavletich. 1997. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell 89:239-250. [DOI] [PubMed] [Google Scholar]
- 45.Straus, D. B., and A. Weiss. 1992. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell 70:585-593. [DOI] [PubMed] [Google Scholar]
- 46.Tikhomirov, O., and G. Carpenter. 2003. Identification of ErbB-2 kinase domain motifs required for geldanamycin-induced degradation. Cancer Res. 63:39-43. [PubMed] [Google Scholar]
- 47.Veillette, A., M. A. Bookman, E. M. Horak, and J. B. Bolen. 1988. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell 55:301-308. [DOI] [PubMed] [Google Scholar]
- 48.Watts, J. D., J. S. Sanghera, S. L. Pelech, and R. Aebersold. 1993. Phosphorylation of serine 59 of p56lck in activated T cells. J. Biol. Chem. 268:23275-23282. [PubMed] [Google Scholar]
- 49.Whitesell, L., E. G. Mimnaugh, B. De Costa, C. E. Myers, and L. M. Neckers. 1994. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. USA 91:8324-8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiese, N., A. Y. Tsygankov, U. Klauenberg, J. B. Bolen, B. Fleischer, and B. M. Broker. 1996. Selective activation of T cell kinase p56lck by Herpesvirus Saimiri protein tip. J. Biol. Chem. 271:847-852. [DOI] [PubMed] [Google Scholar]
- 51.Williams, J. C., R. K. Wierenga, and M. Saraste. 1998. Insights into Src kinase functions: structural comparisons. Trends Biochem. Sci. 23:179-184. [DOI] [PubMed] [Google Scholar]
- 52.Winkler, D. G., I. Park, T. Kim, N. S. Payne, C. T. Walsh, J. L. Strominger, and J. Shin. 1993. Phosphorylation of Ser-42 and Ser-59 in the N-terminal region of the tyrosine kinase p56lck. Proc. Natl. Acad. Sci. USA 90:5176-5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu, W., S. C. Harrison, and M. J. Eck. 1997. Three-dimensional structure of the tyrosine kinase c-Src. Nature 385:595-602. [DOI] [PubMed] [Google Scholar]
- 54.Xu, W., M. Marcu, X. Yuan, E. Mimnaugh, C. Patterson, and L. Neckers. 2002. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc. Natl. Acad. Sci USA 99:12847-12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu, W., E. Mimnaugh, M. F. Rosser, C. Nicchitta, M. Marcu, Y. Yarden, and L. Neckers. 2001. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J. Biol. Chem. 276:3702-3708. [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi, H., and W. A. Hendrickson. 1996. Structural basis for activation of human lymphocyte kinase Lck upon tyrosine phosphorylation. Nature 384:484-489. [DOI] [PubMed] [Google Scholar]
- 57.Yokouchi, M., T. Kondo, A. Sanjay, A. Houghton, A. Yoshimura, S. Komiya, H. Zhang, and R. Baron. 2001. Src-catalyzed phosphorylation of c-Cbl leads to the interdependent ubiquitination of both proteins. J. Biol. Chem. 276:35185-35193. [DOI] [PubMed] [Google Scholar]
- 58.Yorgin, P. D., S. D. Hartson, A. M. Fellah, B. T. Scroggins, W. Huang, E. Katsanis, J. M. Couchman, R. L. Matts, and L. Whitesell. 2000. Effects of geldanamycin, a heat-shock protein 90-binding agent, on T cell function and T cell nonreceptor protein tyrosine kinases. J. Immunol. 164:2915-2923. [DOI] [PubMed] [Google Scholar]
- 59.Young, J. C., I. Moarefi, and F. U. Hartl. 2001. Hsp90: a specialized but essential protein-folding tool. J. Cell. Biol. 154:267-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou, P., N. Fernandes, I. L. Dodge, A. L. Reddi, N. Rao, H. Safran, T. A. DiPetrillo, D. E. Wazer, V. Band, and H. Band. 2003. ErbB2 degradation mediated by the co-chaperone protein CHIP. J. Biol. Chem. 278:13829-13837. [DOI] [PubMed] [Google Scholar]
- 61.Zhu, X., J. L. Kim, J. R. Newcomb, P. E. Rose, D. R. Stover, L. M. Toledo, H. Zhao, and K. A. Morgenstern. 1999. Structural analysis of the lymphocyte-specific kinase Lck in complex with non-selective and Src family selective kinase inhibitors. Structure Fold. Des. 7:651-661. [DOI] [PubMed] [Google Scholar]