Abstract
Objective
Rheumatoid arthritis (RA) is associated with low muscle mass and density. The objective of our study was to evaluate associations between 2 serum biomarkers [insulin-like growth factor 1 (IGF-1) and adiponectin] and skeletal muscle in RA.
Methods
Whole-body dual energy X-ray absorptiometry measures of the appendicular lean mass index (ALMI; kg/m2) and total fat mass index (kg/m2), as well as the peripheral quantitative computed tomography measures of the lower leg muscle and fat cross-sectional area (CSA; cm2) and muscle density (an index of fat infiltration) were obtained from 50 participants with RA, ages 18–70 years. Multivariable linear regression analyses evaluated associations between body composition and levels of adiponectin and IGF-1, adjusted for age, sex, and adiposity.
Results
Greater age was associated with higher adiponectin (p = 0.06) and lower IGF-1 (p = 0.004). Eight subjects had IGF-1 levels below the reference range for their age and sex. These subjects had significantly lower ALMI and muscle CSA in multivariable models. Lower IGF-1 levels were associated with greater clinical disease activity and severity, as well as low ALMI, muscle CSA, and muscle density (defined as 1 SD below normative mean). After adjusting for age and sex, greater adiponectin levels were associated with lower BMI (p = 0.02) as well as lower ALMI, and lower muscle CSA, independent of adiposity (p < 0.05). Only greater Health Assessment Questionnaire scores were significantly associated with lower adiponectin levels.
Conclusion
Low IGF-1 and greater adiponectin levels are associated with lower muscle mass in RA. Lower IGF-1 levels were seen in subjects with greater disease activity and severity.
Keywords: ADIPONECTIN, RHEUMATOID ARTHRITIS, CACHEXIA, LEAN MASS
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease associated with deficits in muscle mass and muscle quality1. In addition to the effects of decreased physical activity and use of corticosteroids among patients with RA, mechanisms for muscle loss likely include direct toxicity of the systemic inflammatory state and resulting dysregulation of normal homeostatic pathways regulating skeletal muscle2.
Signaling molecules such as insulin-like growth factor 1 (IGF-1) likely contribute to the development of cachexia in RA and other disease states. IGF-1, either by systemic or paracrine effect, is a primary promoter of increases in muscle mass acting through a phosphatidylinositol 3/AKT-mediated pathway to cause muscle hypertrophy and hyperplasia in response to growth hormone3,4,5. Systemic inflammation and energy imbalance related to severe RA are likely to result in resistance to growth hormone and low circulating IGF-1 levels. Several studies in RA have demonstrated growth hormone resistance resulting in low-circulating IGF-1 levels6,7,8,9. One previous study reported that IGF-1 was reduced in RA in association with low lean body mass9. Tumor necrosis factor-α (TNF-α), an important cytokine in RA pathogenesis, may also inhibit IGF-1 signaling10. The IGF-1 pathway is a potential therapeutic target because recombinant IGF-1 is available and approved for use in children. Two previous studies have demonstrated some improvements in either serum or muscle IGF-1 with exercise interventions in RA11,12.
Fat-derived cytokines, or adipokines, are also of increasing interest in RA and other inflammatory disease states. In studies, higher adiponectin levels were associated with greater radiographic progression in RA13,14, suggesting that this adipokine is associated with an aggressive disease phenotype. High adiponectin levels have been considered a biomarker of a catabolic state or cachectic state, such as that seen in starvation15,16. Therefore, high levels of adiponectin might be associated with poor prognosis in RA by identifying subjects with a severe phenotype also associated with a catabolic state, muscle wasting, and cachexia.
We therefore hypothesized that greater serum adiponectin levels and lower IGF-1 levels would be associated with low lean mass (i.e., rheumatoid cachexia) and low muscle density (i.e., myosteatosis) in RA. The objective of our study was to evaluate associations between adiponectin and IGF-1 and measures of skeletal muscle mass and muscle density using whole-body dual-energy X-ray absorptiometry (DEXA) and peripheral quantitative computed tomography (pQCT) in patients with RA, adjusted for age, sex, and measures of adiposity.
MATERIALS AND METHODS
Study sample
RA subjects, ages 18–70 years, who met the 2010 American College of Rheumatology criteria were recruited from the University of Pennsylvania rheumatology practices as previously described1. Subjects with juvenile idiopathic arthritis (or another inflammatory arthritis), active cancer, a history of a chronic disease known to affect bone health (e.g., chronic kidney disease, liver disease, malabsorption syndromes), or pregnancy were excluded. The protocol was approved by the Institutional Review Board at the University of Pennsylvania and informed consent was obtained from all participants.
Assessment of anthropometrics and race
Weight and height were measured using a digital scale (Scaltronix) and stadiometer (Holtain Ltd.), respectively. Body mass index (BMI) was calculated by dividing weight in kilograms by height in square meters. An RA-specific definition was used to define the prevalence of obesity17. Participants self-identified race according to the US National Institute of Health categories.
DEXA
Whole-body fat mass and lean mass (kg) were assessed by DEXA using a Hologic densitometer (Delphi Systems, Hologic Inc.). The measurements were performed in the array mode using standard positioning techniques. Quality control scans were performed daily. In our institution, the in vivo coefficient of variation (CV) in adults was < 1%18. Fat mass, lean body mass, and appendicular lean body mass were converted to the fat mass index (FMI; kg/m2), lean body mass index (LBMI; kg/m2), and appendicular lean mass index (ALMI; kg/m2) using height. Whole-body DEXA was not performed on 1 subject whose weight exceeded the 300-pound limit for the scanner.
pQCT
Muscle and fat measures in the left calf were obtained by pQCT (Stratec XCT2000 12-detector unit, Orthometrix Inc.) with a voxel size of 0.4 mm, slice thickness of 2.3 mm, and scan speed of 25 mm/s. All scans were analyzed with Stratec software version 6.00. A scout view was obtained to place the reference line at the distal endplate. Calf muscle and subcutaneous fat cross-sectional area (CSA; mm2) were assessed at a distance equivalent to 66% of tibia length proximal to the medial malleolus using threshold 40 mg/cm3 for fat/lean separation and 711 mg/cm3 for lean/bone separation. Quality control was monitored daily using a phantom. In our laboratory, the CV for short-term precision ranged from 0.5% to 1.6% for pQCT outcomes.
Clinical disease measures
Medication use was determined by the subjects’ self-report and verified in the electronic medical record. Disease activity and disability were measured in a standard fashion using the Disease Activity Score at 28 joints with C-reactive protein (DAS28-CRP) and using the Health Assessment Questionnaire (HAQ) Disability Index including adjustment for use of aids and devices19. Standard radiographs of the hands and feet were performed and Sharp/van der Heijde method (SvdH) scores were determined by a trained radiologist in a blinded fashion.
Serum testing: inflammatory markers, adipokines, IGF-1
Adiponectin was measured using a commercially available ELISA from R&D Systems (CV, 3–5%). IGF-1 levels were performed at Esoterix Laboratory Services Inc. using a Blocking Radio-Immunoassay (CV, 10%). Low IGF-1 levels were defined as a level below the lower limit of normal for age- and sex–specific published reference ranges20. Erythrocyte sedimentation rate was performed using the Westergren method. CRP levels were measured using a Fixed Point Immuno Rate Assay. TNF-α levels were measured using a commercially available ELISA (CV, 9%). Phlebotomy was unsuccessful in 1 participant after multiple attempts.
Statistical analysis
Statistical analyses were performed using Stata 11 software (StataCorp). Adiponectin levels were skewed; therefore, Spearman correlations and rank-sum tests were used to examine associations of demographic variables (age, sex, race, and BMI) with adiponectin levels. Adiponectin levels were natural log-transformed to fit a normal distribution for multivariable linear regression models.
Models evaluating associations with measures of muscle mass were adjusted for measures of adiposity because ALMI was positively associated with FMI (β 0.21, 95% CI 0.17–0.25, p < 0.001), and muscle CSA was positively associated with fat CSA (β 0.39, 95% CI 0.16–0.62, p = 0.001) after adjusting for age and sex. As described, there was no association between muscle density and FMI among RA subjects (p > 0.9)1. Regression models evaluated associations between IGF-1 and adiponectin levels and muscle outcomes (ALMI and pQCT muscle CSA) before and after adjusting for measures of adiposity (FMI and pQCT fat CSA, respectively). Analyses incorporating pQCT measures of muscle and fat CSA were further adjusted for tibia length to adjust for the effect of body size.
ALMI and FMI were converted to sex- and race-specific Z scores (SD scores) relative to age using the US Food and Drug Administration-approved reference curves developed from the National Health and Nutrition Examination Survey (NHANES), as described21,22. The reference curves were generated using the LMS method that accounts for the nonlinearity, heteroscedasticity, and skew of body composition data relative to age in adults. The Z score generated in RA participants represents the number of SD above or below the average result for NHANES subjects of the same age, sex, and race. For example, a Z score of 0 represents the 50th percentile for age, sex, and race, and a Z score of −1 represents the 16th percentile. National reference data are not available for pQCT measures; therefore, calf muscle and fat CSA outcomes were converted to sex- and race-specific Z scores relative to age using the LMS method in 467 healthy control participants scanned on the same pQCT device, as described1,21. These pQCT Z scores were further adjusted for tibia length23,24. Muscle density scores were also converted to sex- and race-specific Z scores relative to age based on healthy control distributions.
We hypothesized that subjects with deficits in muscle outcomes compared with normal have deficient levels of IGF-1. Therefore, we defined a low muscle outcome as a value greater than 1 SD below the expected value for age, sex, and race (Z score < −1), suggesting a value at the < 16th percentile for what would be expected for a given age, sex, and race. Similar results were seen when categorizing muscle outcomes according to the lowest quartile of the muscle outcome (not shown).
Age- and sex-adjusted associations between prehypothesized disease activity and disease severity measures with serum adiponectin and IGF-1 levels were evaluated. Data with non-normal distributions (TNF-α, swollen joint counts) were log-adjusted to fit a normal distribution (lnskew0 command in Stata). SvdH scores, HAQ scores, and CRP levels were more highly skewed, therefore these variables were dichotomized. SvdH scores were dichotomized at the median value for the study population21. Ten subjects had HAQ scores of 0 and 26 subjects had CRP levels < 0.5 mg/dl (lower limit for the test), therefore these variables were dichotomized at these cutoffs.
RESULTS
Fifty participants completed our study and their characteristics are shown in Table 1. RA subjects had a high prevalence of obesity (defined as a BMI > 28 kg/m2), median disease duration of about 10 years, and moderately high median disability scores. Disease activity was low on average in the setting of frequent use of methotrexate (MTX) and biologic therapies. Muscle deficits in this group have been previously described1,22. On average, ALMI and muscle CSA Z scores were modestly low (−0.24 and −0.42, respectively) in the setting of modestly elevated FMI and fat CSA Z scores (0.09 and 0.36, respectively).
Table 1.
RA participant characteristics. Data are presented as mean (SD) or median (IQR) unless otherwise specified.
| Characteristics | All Subjects |
|---|---|
| n | 50 |
| Age, yrs | 51.2 (13.3) |
| Female, n (%) | 39 (78) |
| Black, n (%) | 18 (36) |
| BMI, kg/m2 | 30.1 (8.5) |
| Obese, BMI ≥ 28 kg/m2, n (%) | 30 (60) |
| ALMI, kg/m2 | 7.5 (1.30) |
| ALMI Z score | −0.24 (1.05) |
| LBMI, kg/m2 | 17.3 (2.5) |
| LBMI Z score | −0.22 (1.07) |
| FMI, kg/m2 | 12.4 (5.6) |
| FMI Z score | 0.09 (1.20) |
| pQCT muscle CSA, cm2 | 64.4 (12.5) |
| Tibia-adjusted muscle CSA Z score | −0.42 (1.23) |
| pQCT fat CSA, cm2 | 32.7 (14.9) |
| Tibia-adjusted fat CSA Z score | 0.36 (1.13) |
| Muscle density, mg/cm3 | 73.3 (71.0, 75.0) |
| Muscle density Z score | −0.58 (0.82) |
| DAS28-CRP | 3.14 (1.17) |
| HAQ score | 0.775 (0.15–1.45) |
| CCP-positive, n (%) | 38 (76) |
| Disease duration, yrs | 10 (4–19) |
| SvdH score | 21 (2–53) |
| CRP, mg/dl | 0.5 (0.5–1.3) |
| TNF-α, pmol/l | 1.12 (0.83–4.89) |
| Adiponectin, ng/ml | 7790 (5430–12,230) |
| IGF-1, ng/ml | 138.5 (48.4) |
| Medications, n (%) | |
| Current MTX | 32 (64) |
| Current biologic therapy | 32 (64) |
| Current prednisone | 20 (40) |
| Ever MTX | 41 (87) |
| Ever biologic therapy | 38 (76) |
| Ever prednisone | 40 (80) |
RA: rheumatoid arthritis; IQR: interquartile range; BMI: body mass index; ALMI: appendicular lean mass index; LBMI: lean body mass index; FMI: fat mass index; pQCT: peripheral quantitative computed tomography; CSA: cross-sectional area; DAS28: Disease Activity Score in 28 joints; CRP: C-reactive protein; HAQ: Health Assessment Questionnaire; CCP: cyclic citrullinated peptide antibody; SvdH: Sharp/van der Heijde method; TNF-α: tumor necrosis factor-1; IGF-1: insulin-like growth factor 1; MTX: methotrexate.
IGF-1
Among RA subjects, mean (SD) IGF-1 levels were 138.5 ng/ml (48.4). On average, subjects had IGF-1 levels 0.93 SD (± 0.87) below the normative mean (p < 0.001). Eight of 49 participants (16%) had IGF-1 levels below the normal reference range for age and sex. IGF-1 levels were significantly lower in older participants (Spearman ρ −0.41, p = 0.004). Mean levels did not differ significantly between women and men [141.4 (46.1) vs 128.2 (57.0), p = 0.4], or according to race or BMI (both p > 0.5).
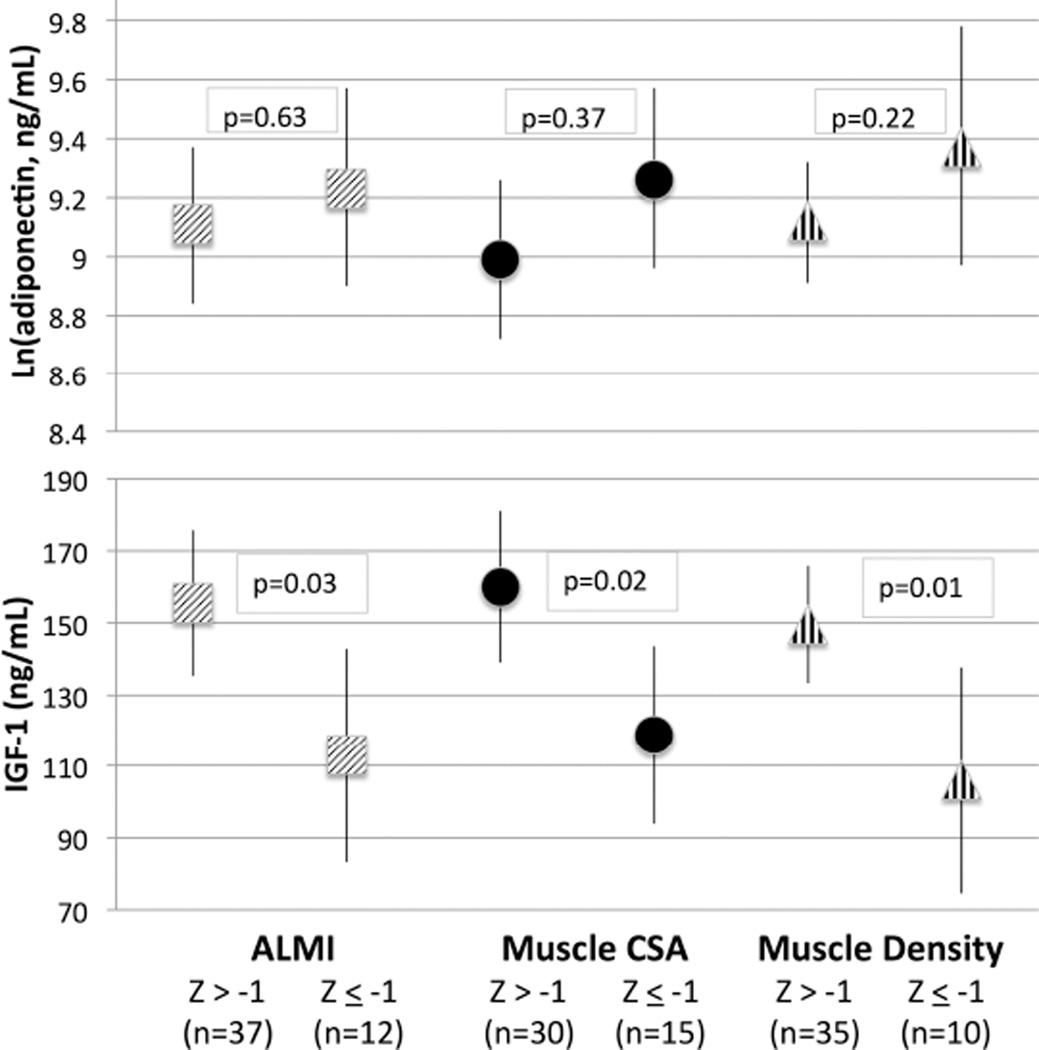
Greater IGF-1 levels were not associated with greater ALMI before or after adjustment for FMI (Table 2). However, the 8 subjects who had an IGF-1 level that was below the normal reference range had significantly lower ALMI after adjusting for age, sex, and FMI. Further, greater IGF-1 levels tended to be associated with greater muscle CSA on pQCT before adjusting for pQCT fat CSA (p = 0.056) and were significantly associated with muscle CSA after adjusting for fat CSA (Table 2). Similarly, the 8 subjects who had an IGF-1 level that was below the normal reference range for their age and sex had significantly lower muscle CSA before and after adjusting for fat CSA. There was no association between IGF-1 levels and muscle density. IGF-1 levels were significantly lower among subjects with ALMI, muscle CSA, and muscle density Z scores ≤ –1 after adjusting for FMI or fat CSA (Figure 1).
Table 2.
Multivariable linear regression models assessing independent associations between Ln serum adiponectin levels (ng/ml) and IGF-1 levels (ng/ml) with muscle outcomes before and after adjusting for adiposity. All models adjusted for age and sex.
| Variable | ALMI | ALMI, FMI-adjusted | ||
| β(95% CI) | p | β(95% CI) | p | |
| Adiponectin | ||||
| Ln(Adiponectin) | −0.87 (−1.51 – −0.24) | 0.008 | −0.40 (−0.78 – −0.010) | 0.04 |
| IGF-1 | ||||
| IGF-1, ng/ml | 0.0020 (0.0068–0.011) | 0.6 | 0.0033 (0.0013–0.0080) | 0.16 |
| Low IGF-1 | −0.49 (−1.50–0.52) | 0.3 | −0.55 (−1.08 – −0.018) | 0.04 |
| pQCT Muscle CSA* | pQCT Muscle CSA*, Fat CSA-adjusted | |||
| β(95% CI) | p | β(95% CI) | p | |
| Adiponectin | ||||
| Ln(Adiponectin) | −5.82 (−12.00–0.37) | 0.07 | −6.11 (−11.47 – −0.75) | 0.03 |
| IGF-1 | ||||
| IGF-1, ng/ml | 0.074 (−0.0020–0.15) | 0.056 | 0.086 (0.021–0.15) | 0.01 |
| Low IGF-1 | −11.08 (−20.18 – −1.99) | 0.02 | −10.55 (−18.48 – −2.62) | 0.01 |
| Ln(Muscle Density) | Ln(Muscle Density), FMI-adjusted | |||
| β(95% CI) | p | β(95% CI) | p | |
| Adiponectin | ||||
| Ln(Adiponectin) | 0.013 (−0.10–0.13) | 0.8 | 0.012 (−0.11–0.14) | 0.8 |
| IGF-1 | ||||
| IGF-1, ng/ml | −0.00062 (−0.0021–0.00082) | 0.4 | −0.00062 (−0.0021–0.00085) | 0.4 |
| Low IGF-1 | 0.11 (−0.064–0.28) | 0.2 | 0.11 (−0.052–0.28) | 0.2 |
Also adjusted for tibia length.
Ln: natural log-adjusted; IGF-1: insulin-like growth factor 1; ALMI: appendicular lean mass index; FMI: fat mass index; pQCT: peripheral quantitative computed tomography; CSA: cross-sectional area.
Figure 1.
Serum IGF-1 and log-adjusted adiponectin levels among subjects with low muscle mass and low muscle density defined as age-, sex-, and race-specific Z score < −1 (15.9th percentile), adjusting for age, sex, and adiposity (FMI Z score for ALMI or fat CSA Z score for muscle CSA). IGF-1: insulin-like growth factor 1; FMI: fat mass index; ALMI: appendicular lean mass index; CSA: cross-sectional area.
After adjusting for age and sex, IGF-1 levels were lower in the 26 subjects who had elevated CRP levels (> 0.5 mg/dl), in the 40 subjects with a HAQ score > 0, and in the 22 subjects with SvdH scores above the median value, and among subjects with greater swollen joint counts, greater TNF-α levels, and greater disease duration (all p < 0.05; Table 3). There was no association between IGF-1 levels and current prednisone use or cyclic citrullinated peptide antibody status. MTX use was associated with significantly higher IGF-1 levels (Table 3). In contrast, TNF inhibitor users had significantly lower IGF-1 levels (β −30.2, 95% CI −54.6 – −5.7, p = 0.02). This was substantially attenuated when adjusting for the significantly greater circulating TNF levels among TNF users (β −14.3, 95% CI −43.0–14.3, p = 0.32).
Table 3.
Associations between disease severity measures and lGF-1 levels and Ln adiponectin levels adjusted for age and sex.
| Variable | Ln(adiponectin) | IGF-1 | ||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| DAS28-CRP, per 1 unit | −0.081 (−0.22–0.054) | 0.2 | −9.46 (−20.16–1.24) | 0.08 |
| Abnormal HAQ, > 0 | −0.44 (−0.83 – −0.060) | 0.02 | −32.90 (−64.05 – −1.73) | 0.04 |
| CRP > 0.5 mg/dl | −0.18 (−0.52–0.15) | 0.3 | −28.39 (−54.44 – −2.34) | 0.03 |
| Ln(TNF-α) | 0.029 (−0.047–0.10) | 0.4 | −7.82 (−13.36 – −2.28) | 0.007 |
| Ln(SJC) | 0.074 (−0.15–0.30) | 0.5 | −18.55 (−35.59 – −1.50) | 0.03 |
| High SvdH, > 21 | 0.20 (−0.17–0.57) | 0.3 | −32.49 (−61.70 – −3.27) | 0.03 |
| Disease duration, per 1 yr | −0.0045 (−0.020–0.011) | 0.6 | −1.21 (−2.40 – −0.0094) | 0.048 |
| CCP-positive | 0.093 (−0.29–0.48) | 0.6 | 6.23 (−24.65–37.11) | 0.7 |
| Current prednisone | −0.30 (−0.62–0.017) | 0.06 | 1.52 (−24.99–28.03) | 0.9 |
| Current anti-TNF | −0.073 (−0.40 – −0.25) | 0.65 | −30.2 (−54.6 – −5.7) | 0.02 |
| Current MTX | −0.018 (−0.35–0.31) | 0.91 | 35.9 (11.5–60.3) | 0.005 |
IGF-1: insulin-like growth factor 1; Ln: natural log-adjusted; DAS28: Disease Activity Score in 28 joints; CRP: C-reactive protein; HAQ: Health Assessment Questionnaire; TNF-α: tumor necrosis factor-α; SJC: swollen joint count; SvdH: Sharp/van der Heijde method; CCP: cyclic citrullinated peptide antibody; MTX: methotrexate.
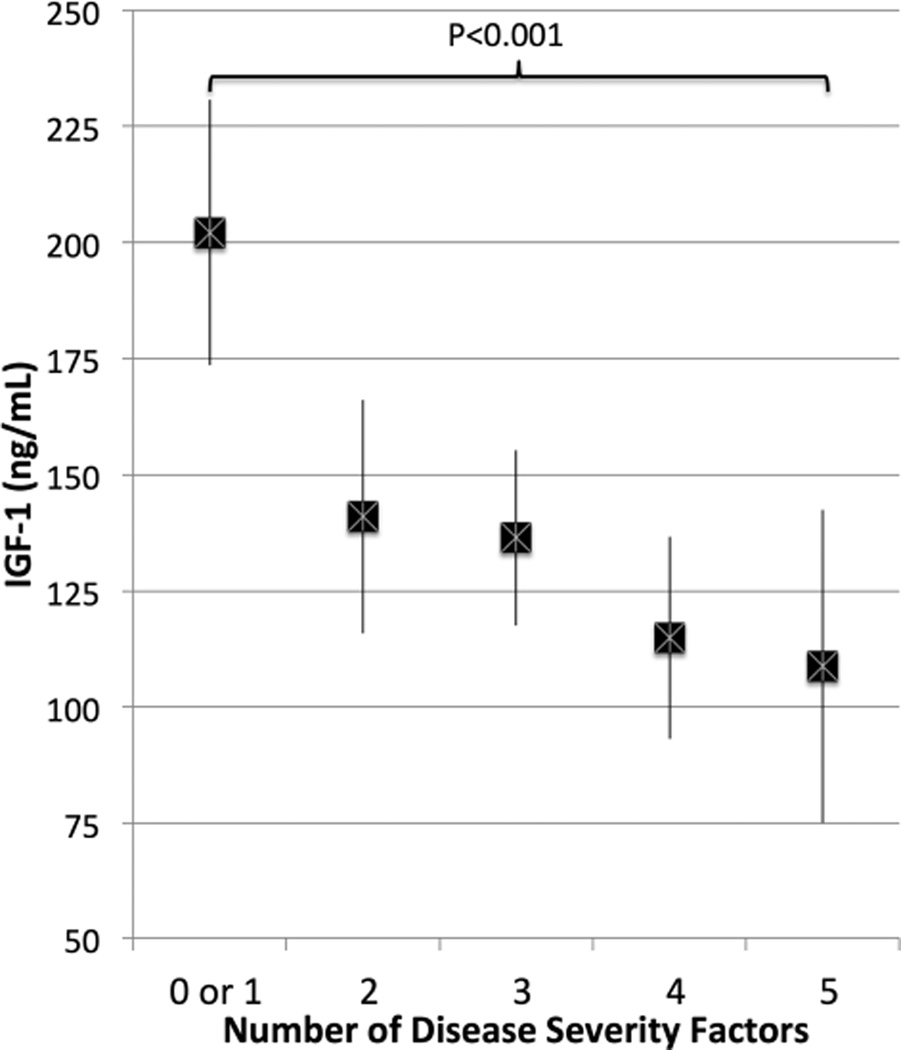
Figure 2 demonstrates that subjects with a greater number of disease severity measures (abnormal HAQ score, disease duration > 10 years, high SvdH score, swollen joint count > 1, and high CRP levels) had lower IGF-1 levels after adjusting for age and sex. For example, the 9 subjects who had 5 severity measures (CRP > 0.5 mg/dl, abnormal HAQ, disease duration > 10 yrs, more than 1 swollen joint, and a high SvdH score) had lower IGF-1 levels (β −87.7, 95% CI −133.4 – −42.1, p < 0.001) compared with the 11 subjects with 1 or fewer factors.
Figure 2.
Serum IGF-1 levels (mean and 95% CI) among subjects with increasing number of disease severity factors (CRP > 0.5 mg/dl, SvdH > 23, HAQ > 0, disease duration > 10 yrs, SJC > 1), adjusted for age and sex. IGF-1: insulin-like growth factor 1; CRP: C-reactive protein; SvdH: Sharp/van der Heijde method; HAQ: Health Assessment Questionnaire; SJC: swollen joint count.
Adiponectin
Adiponectin levels were marginally higher in older participants (Spearman ρ 0.27, p = 0.06). Median [interquartile range (IQR)] levels did not differ significantly between women and men (8260, IQR 5580–16,800 vs 7480, IQR 3760–9750, p = 0.2). Adiponectin levels did not differ according to race (p = 0.5). Greater BMI was associated with lower adiponectin levels (ρ −0.31, p = 0.03).
Lower BMI was associated with greater adiponectin levels in multivariable linear regression models after adjusting for age and sex (β −0.28, 95% CI −0.051 – −0.0049, p = 0.02). Lower ALMI was also associated with greater adiponectin levels, adjusted for age and sex, and the association remained significant with additional adjustment for FMI (Table 2). Similarly, lower muscle CSA on pQCT tended to be associated with greater adiponectin levels (p = 0.07), adjusted for age and sex, and was significant with additional adjustment for fat CSA (Table 2). There was no association between adiponectin and muscle density before or after adjusting for FMI. Adiponectin levels were not significantly different among subjects with ALMI or muscle CSA Z scores ≤ −1 (≤ 16th percentile) before or after adjusting for FMI or fat CSA Z score (Figure 1). In multivariable models adjusting for ALMI, age, and sex, there was not a significant independent association between FMI or fat CSA and adiponectin levels (p > 0.2). However, in subsequent models that included regional measures of fat composition (trunk fat and subcutaneous fat indices), trunk fat index was associated inversely with adiponectin (β −0.12, 95% CI −0.22 – −0.022, p = 0.02) while subcutaneous fat index was positively associated with adiponectin (β 0.18, 95% CI 0.06–0.29, p = 0.02). ALMI remained significantly associated with adiponectin in this model (β −0.21, 95% CI −0.42 – −0.013, p = 0.02).
Associations between adiponectin levels and measures of disease activity (DAS28-CRP, CRP, HAQ score) and disease severity (SvdH score, disease duration, seropositivity, current prednisone use) were also assessed (Table 3). Only current prednisone use (β −0.30, 95% CI −0.62–0.017, p = 0.06) and an elevated HAQ score (β −0.44, 95% CI −0.83 – −0.060, p = 0.02) suggested an association with lower adiponectin levels after adjustment for age and sex. There was no correlation between the number of years of prednisone use with adiponectin levels (ϱ 0.03, p = 0.82).
DISCUSSION
Our study evaluated 2 serum markers that were hypothesized to be associated with deficits in skeletal muscle in RA. These data demonstrate significant associations between these measures and the measures of skeletal muscle mass performed by 2 separate modalities — whole-body DEXA and pQCT. These observations are novel and important because the identification and treatment of muscle deficits in RA is of increasing interest; they may correlate with important outcomes, including physical functioning1,25,26.
Few studies have evaluated the potential involvement of IGF-1 dysregulation in the development of muscle deficits in RA. The finding in our study that low levels of IGF-1 were associated with low ALMI and muscle CSA in RA supports a previous report by Engvall, et al9. Our current study furthers previous observations by assessing multiple measures of muscle mass and the effect of adjustment for the confounding effect of adiposity. Our current study also demonstrated low levels of IGF-1 among subjects with deficits in muscle density. Overall, these findings suggest that IGF-1 dysregulation could be a mediator of muscle deficits in RA9.
Further, these data are the first to demonstrate associations between IGF-1 and greater disease duration, disease activity, disability, and joint destruction. IGF-1 levels were progressively lower with more severe and longstanding RA. This observation helps support the hypothesis that the disease process contributes to dysregulation of this signaling pathway in RA, likely because of the acquired resistance to growth hormone. IGF-1 has been demonstrated to be a negative acute-phase reactant in dogs treated with lipopolysaccharide27. In humans, resistance to growth hormone has been described in the setting of starvation and other inflammatory states3,28,29. This may represent a normal mechanism to limit growth in the setting of limited energy availability. Longitudinal studies are needed to further clarify the involvement of IGF-1 in the pathogenesis of cachexia (i.e., correlative vs causal).
The observation of low IGF-1 levels in subjects treated with TNF inhibitors is consistent with 2 previous small studies demonstrating a decrease in IGF-1 levels with treatment30,31. The underlying mechanism for these observations is unclear. Paradoxical increases in circulating TNF-α seen with TNF inhibitors could suppress IGF-132. This mechanistic explanation could explain why the association was attenuated by adjustment for circulating TNF-α in our study.
In the general population, adiponectin secretion by adipocytes is downregulated in obesity. While previous studies have demonstrated associations between low adiponectin levels and visceral adiposity in RA33, to our knowledge, our present study is the first to observe that adiponectin levels are inversely associated with lean mass. This remained true after adjusting for the positive and confounding association between muscle and fat34. In these models, regional fat indices, but not total adiposity were associated with adiponectin levels. We believe that these novel findings support previous literature suggesting that adiponectin is a marker of a distinct disease phenotype associated with muscle loss, cachexia, and other adverse longterm outcomes.
Because adiponectin has been described as a marker of cachexia and starvation, high adiponectin levels may simply represent a biological marker of a catabolic state in RA as in other populations15. Elevations in adiponectin have been seen in other diseases associated with cachexia, such as chronic kidney disease, heart failure, and anorexia35,36,37,38,39. High circulating adiponectin has been associated with low lean mass in patients with chronic heart failure40 and is a predictor of greater mortality, perhaps as a marker of cachexia and wasting41. Therefore, high circulating levels of adiponectin may identify those who have undergone and have ongoing catabolism, and therefore who are at risk for both cachexia and other adverse outcomes in RA. On the other hand, high adiponectin levels might be hypothesized to be involved in the development of muscle deficits in RA by direct involvement in inflammatory pathways and in tissue damage42.
Strengths of our study include the well-characterized body composition measures and clinical characteristics. The cross-sectional design and modest sample size may have limited the ability to detect associations between disease characteristics and biomarker levels, or to assess independent relationships in comprehensive multivariable models. However, we were sufficiently powered to assess the primary associations of interest. We did not comprehensively measure growth hormone levels to evaluate for the presence of growth hormone resistance. Study in this area could help focus future treatments aimed at this pathway. Our study identified an inverse association between adiponectin and trunk fat indices that confirms observations in a previous study in RA of an inverse association between visceral fat and serum adiponectin levels, using computed tomography33. However, our study was limited by the use of DEXA alone to quantify the extent of abdominal/visceral adiposity. Previous studies have shown differences in the correlation of intraabdominal and subcutaneous fat with adiponectin levels among nondiabetic adults43. Future studies should consider the implications of altered fat distribution on IGF-1 and adiponectin levels in this disease. Our study did not use a control group, and thus we were not specifically able to evaluate how associations within RA subjects compare with those seen in a healthy population. Finally, our study did not address other pathways that are likely involved in the development of cachexia in RA, including the contribution of myostatin, an inhibitor of the AKT signaling pathway and negative regulator of skeletal muscle mass.
RA subjects with deficits in skeletal muscle as measured by whole-body DEXA and pQCT have greater serum levels of adiponectin levels and lower levels of IGF-1 independent of age, sex, and measures of adiposity. IGF-1 levels are lower among subjects with RA who have greater disease activity and severity.
Acknowledgments
Supported by the University of Pennsylvania Clinical and Translational Research Center (UL1 RR024134). Dr. Baker is supported by a VA Clinical Science Research and Development Career Development Award (IK2 CX000955). Dr. Leonard is supported by a K24 award (K24 DK076808). Also supported by US National Institutes of Health grant R01 DK064966.
REFERENCES
- 1.Baker JF, Von Feldt J, Mostoufi-Moab S, Noaiseh G, Taratuta E, Kim W, et al. Deficits in muscle mass, muscle density, and modified associations with fat in rheumatoid arthritis. Arthritis Care Res. 2014;66:1612–1618. doi: 10.1002/acr.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsmith J, Roubenoff R. Cachexia in rheumatoid arthritis. Int J Cardiol. 2002;85:89–99. doi: 10.1016/s0167-5273(02)00237-1. [DOI] [PubMed] [Google Scholar]
- 3.Perrini S, Laviola L, Carreira MC, Cignarelli A, Natalicchio A, Giorgino F. The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205:201–210. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- 4.Song YH, Song JL, Delafontaine P, Godard MP. The therapeutic potential of IGF-I in skeletal muscle repair. Trends Endocrinol Metab. 2013;24:310–319. doi: 10.1016/j.tem.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shefer G, Benayahu D. The effect of exercise on IGF-I on muscle fibers and satellite cells. Front Biosci. 2012;4:230–239. doi: 10.2741/e372. [DOI] [PubMed] [Google Scholar]
- 6.Blackman MR, Muniyappa R, Wilson M, Moquin BE, Baldwin HL, Wong KA, et al. Diurnal secretion of growth hormone, cortisol, and dehydroepiandrosterone in pre- and perimenopausal women with active rheumatoid arthritis: a pilot case-control study. Arthritis Res Ther. 2007;9:R73. doi: 10.1186/ar2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denko CW, Malemud CJ. Role of the growth hormone/insulin-like growth factor-1 paracrine axis in rheumatic diseases. Semin Arthritis Rheum. 2005;35:24–34. doi: 10.1016/j.semarthrit.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Lemmey A, Maddison P, Breslin A, Cassar P, Hasso N, McCann R, et al. Association between insulin-like growth factor status and physical activity levels in rheumatoid arthritis. J Rheumatol. 2001;28:29–34. [PubMed] [Google Scholar]
- 9.Engvall IL, Elkan AC, Tengstrand B, Cederholm T, Brismar K, Hafstrom I. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin-like growth factor. Scand J Rheumatol. 2008;37:321–328. doi: 10.1080/03009740802055984. [DOI] [PubMed] [Google Scholar]
- 10.Anwar A, Zahid AA, Scheidegger KJ, Brink M, Delafontaine P. Tumor necrosis factor-alpha regulates insulin-like growth factor-1 and insulin-like growth factor binding protein-3 expression in vascular smooth muscle. Circulation. 2002;105:1220–1225. doi: 10.1161/hc1002.105187. [DOI] [PubMed] [Google Scholar]
- 11.Lemmey AB, Marcora SM, Chester K, Wilson S, Casanova F, Maddison PJ. Effects of high-intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum. 2009;61:1726–1734. doi: 10.1002/art.24891. [DOI] [PubMed] [Google Scholar]
- 12.Karatay S, Yildirim K, Melikoglu MA, Akcay F, Senel K. Effects of dynamic exercise on circulating IGF-1 and IGFBP-3 levels in patients with rheumatoid arthritis or ankylosing spondylitis. Clin Rheumatol. 2007;26:1635–1639. doi: 10.1007/s10067-007-0559-4. [DOI] [PubMed] [Google Scholar]
- 13.Klein-Wieringa IR, van der Linden MP, Knevel R, Kwekkeboom JC, van Beelen E, Huizinga TW, et al. Baseline serum adipokine levels predict radiographic progression in early rheumatoid arthritis. Arthritis Rheum. 2011;63:2567–2574. doi: 10.1002/art.30449. [DOI] [PubMed] [Google Scholar]
- 14.Giles JT, van der Heijde DM, Bathon JM. Association of circulating adiponectin levels with progression of radiographic joint destruction in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1562–1568. doi: 10.1136/ard.2011.150813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behre CJ. Adiponectin: saving the starved and the overfed. Med Hypotheses. 2007;69:1290–1292. doi: 10.1016/j.mehy.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 16.Szabó T, Scherbakov N, Sandek A, Kung T, von Haehling S, Lainscak M, et al. Plasma adiponectin in heart failure with and without cachexia: catabolic signal linking catabolism, symptomatic status, and prognosis. Nutr Metab Cardiovasc Dis. 2014;24:50–56. doi: 10.1016/j.numecd.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Stavropoulos-Kalinoglou A, Metsios GS, Koutedakis Y, Nevill AM, Douglas KM, Jamurtas A, et al. Redefining overweight and obesity in rheumatoid arthritis patients. Ann Rheum Dis. 2007;66:1316–1321. doi: 10.1136/ard.2006.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS. Interpretation of whole body dual energy X-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone. 2004;34:1044–1052. doi: 10.1016/j.bone.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe F, Michaud K, Pincus T. Development and validation of the health assessment questionnaire II: a revised version of the health assessment questionnaire. Arthritis Rheum. 2004;50:3296–3305. doi: 10.1002/art.20549. [DOI] [PubMed] [Google Scholar]
- 20.Friedrich N, Alte D, Völzke H, Spilcke-Liss E, Lüdemann J, Lerch MM, et al. Reference ranges of serum IGF-1 and IGFBP-3 levels in a general adult population: results of the Study of Health in Pomerania (SHIP) Growth Horm IGF Res. 2008;18:228–237. doi: 10.1016/j.ghir.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker JF, Long J, Ibrahim S, Leonard MB, Katz P. Are men at greater risk of lean mass deficits in rheumatoid arthritis? Arthritis Care Res. 2015;67:112–119. doi: 10.1002/acr.22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsampalieros A, Kalkwarf HJ, Wetzsteon RJ, Shults J, Zemel BS, Foster BJ, et al. Changes in bone structure and the muscle-bone unit in children with chronic kidney disease. Kidney Int. 2013;83:495–502. doi: 10.1038/ki.2012.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber DR, Moore RH, Leonard MB, Zemel BS. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr. 2013;98:49–56. doi: 10.3945/ajcn.112.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giles JT, Ling SM, Ferrucci L, Bartlett SJ, Andersen RE, Towns M, et al. Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum. 2008;59:807–815. doi: 10.1002/art.23719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer HR, Fontaine KR, Bathon JM, Giles JT. Muscle density in rheumatoid arthritis: associations with disease features and functional outcomes. Arthritis Rheum. 2012;64:2438–2450. doi: 10.1002/art.34464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tvarijonaviciute A, Eralp O, Kocaturk M, Yilmaz Z, Ceron JJ. Adiponectin and IGF-1 are negative acute phase proteins in a dog model of acute endotoxaemia. Vet Immunol Immunopathol. 2011;140:147–151. doi: 10.1016/j.vetimm.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Helaly GF, El-Afandy NM. Influence of HCV infection on insulin-like growth factor 1 and proinflammatory cytokines: association with risk for growth hormone resistance development. Egypt J Immunol. 2009;16:115–124. [PubMed] [Google Scholar]
- 29.Misra M, Klibanski A. Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol. 2014;2:581–592. doi: 10.1016/S2213-8587(13)70180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engvall IL, Tengstrand B, Brismar K, Hafström I. Infliximab therapy increases body fat mass in early rheumatoid arthritis independently of changes in disease activity and levels of leptin and adiponectin: a randomised study over 21 months. Arthritis Res Ther. 2010;12:R197. doi: 10.1186/ar3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarzi-Puttini P, Atzeni F, Scholmerich J, Cutolo M, Straub RH. Anti-TNF antibody treatment improves glucocorticoid induced insulin-like growth factor 1 (IGF1) resistance without influencing myoglobin and IGF1 binding proteins 1 and 3. Ann Rheum Dis. 2006;65:301–305. doi: 10.1136/ard.2005.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatia A, Kast RE. Tumor necrosis factor (TNF) can paradoxically increase on etanercept treatment, occasionally contributing to TNF-mediated disease. J Rheumatol. 2007;34:447–449. [PubMed] [Google Scholar]
- 33.Giles JT, Allison M, Bingham CO, 3rd, Scott WM, Jr, Bathon JM. Adiponectin is a mediator of the inverse association of adiposity with radiographic damage in rheumatoid arthritis. Arthritis Rheum. 2009;61:1248–1256. doi: 10.1002/art.24789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker JF, Davis M, Alexander R, Zemel BS, Mostoufi-Moab S, Shults J, et al. Associations between body composition and bone density and structure in men and women across the adult age spectrum. Bone. 2013;53:34–41. doi: 10.1016/j.bone.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamczak M, Chudek J, Wiecek A. Adiponectin in patients with chronic kidney disease. Semin Dial. 2009;22:391–395. doi: 10.1111/j.1525-139X.2009.00587.x. [DOI] [PubMed] [Google Scholar]
- 36.Iwahashi H, Funahashi T, Kurokawa N, Sayama K, Fukuda E, Okita K, et al. Plasma adiponectin levels in women with anorexia nervosa. Horm Metab Res. 2003;35:537–540. doi: 10.1055/s-2003-42655. [DOI] [PubMed] [Google Scholar]
- 37.Pannacciulli N, Vettor R, Milan G, Granzotto M, Catucci A, Federspil G, et al. Anorexia nervosa is characterized by increased adiponectin plasma levels and reduced nonoxidative glucose metabolism. J Clin Endocrinol Metab. 2003;88:1748–1752. doi: 10.1210/jc.2002-021215. [DOI] [PubMed] [Google Scholar]
- 38.Tagami T, Satoh N, Usui T, Yamada K, Shimatsu A, Kuzuya H. Adiponectin in anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2004;89:1833–1837. doi: 10.1210/jc.2003-031260. [DOI] [PubMed] [Google Scholar]
- 39.McEntegart MB, Awede B, Petrie MC, Sattar N, Dunn FG, MacFarlane NG, et al. Increase in serum adiponectin concentration in patients with heart failure and cachexia: relationship with leptin, other cytokines, and B-type natriuretic peptide. Eur Heart J. 2007;28:829–835. doi: 10.1093/eurheartj/ehm033. [DOI] [PubMed] [Google Scholar]
- 40.Loncar G, Bozic B, von Haehling S, Dungen HD, Prodanovic N, Lainscak M, et al. Association of adiponectin with peripheral muscle status in elderly patients with heart failure. Eur J Intern Med. 2013;24:818–823. doi: 10.1016/j.ejim.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 42.Ehling A, Schäffler A, Herfarth H, Tarner IH, Anders S, Distler O, et al. The potential of adiponectin in driving arthritis. J Immunol. 2006;176:4468–4478. doi: 10.4049/jimmunol.176.7.4468. [DOI] [PubMed] [Google Scholar]
- 43.Hsieh CJ, Wang PW, Chen TY. The relationship between regional abdominal fat distribution and both insulin resistance and subclinical chronic inflammation in non-diabetic adults. Diabetol Metab Syndr. 2014;6:49. doi: 10.1186/1758-5996-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]