At the Fourth Annual Meeting of the American Society of Gene (and Cell) Therapy, which was held in Seattle, Washington, in 2001, Jeffrey Bluestone, PhD, currently A.W. and Mary Margaret Clausen Distinguished Professor at the University of California, San Francisco, was the final speaker at the Presidential Symposium on Friday, June 1, 2001, where he concluded his lecture on Immunity with the following statement: “I know you are all very smart, but you will never be able to outsmart the immune system.”
He was clearly referring to the use of viruses as viral vectors, and those words proved to be prophetic. In the first adeno-associated virus serotype 2 (AAV2) vector-mediated, liver-directed gene therapy trial for hemophilia B reported in 2006,1 a significant immune response to AAV capsid proteins led to a complete loss of therapeutic levels of factor IX (F.IX) in one subject.
Preamble to the Problems
There is little doubt that viruses did not evolve for the purposes of delivery of therapeutic genes. Thus viral vectors, although used successfully to achieve the main objective, are unlikely to reach their full potential because of the host immune response. The use of AAV, in particular, is complicated by the following two facts: (1) the naked icosahedral capsid, which is subject to ready modification(s) by host cell enzymes; and (2) the single-stranded nature of the viral DNA, which is transcriptionally inactive.
Solution to the Capsid Puzzle
Having worked with stalwarts such as Kenneth I. Berns, MD, PhD, Nicholas Muzyczka, PhD, William W. Hauswirth, PhD, and R. Jude Samulski, PhD, on AAV2 as a virus since 1980, and subsequently as vectors, it eventually became obvious to me that the naturally occurring AAV as a virus could not be used optimally as a vector. In other words, the virus had to be modified in some way, shape, or form, such that the delivered vector was not seen by the host immune system as a virus. But how, or in what way?
Fortunately for me, this question was soon answered when my long-time colleague, Li Zhong, MD, and I, as well as others, reported in 20072 that after infection, the AAV2 capsid protein becomes phosphorylated at surface-exposed tyrosine residues by the cellular epidermal growth factor receptor protein tyrosine kinase. Tyrosine phosphorylation, which leads to ubiquitination, followed by proteasomal degradation of the vectors in the cytoplasm (elegantly demonstrated by Duan and colleagues),3 provided one possible clue to circumvent this barrier. Another long-term collaborator, Mavis Agbandje-McKenna, PhD, the “structural guru” of AAV, and colleagues quickly identified the seven surface-exposed tyrosine residues on the AAV2 capsid, which were mutagenized to develop tyrosine-mutant, next-generation (“NextGen”) AAV2 vectors in 2008,4 which circumvented the problems associated with the first-generation AAV2 vectors. Later, several of these mutations were combined to generate a “tyrosine triple-mutant,” which was not only significantly more efficient5 but also less immunogenic.6 A herculean effort by yet another long-term colleague, George Aslanidi, PhD, and colleagues, who mutagenized all 17 surface-exposed serines, all 15 surface-exposed threonines, and various permutations and combinations thereof, led to the identification of the most efficient AAV2 vectors to date.7 In addition, because ubiquitination occurs on lysines, all seven surface-exposed lysine residues on the AAV2 capsid were also mutagenized.8
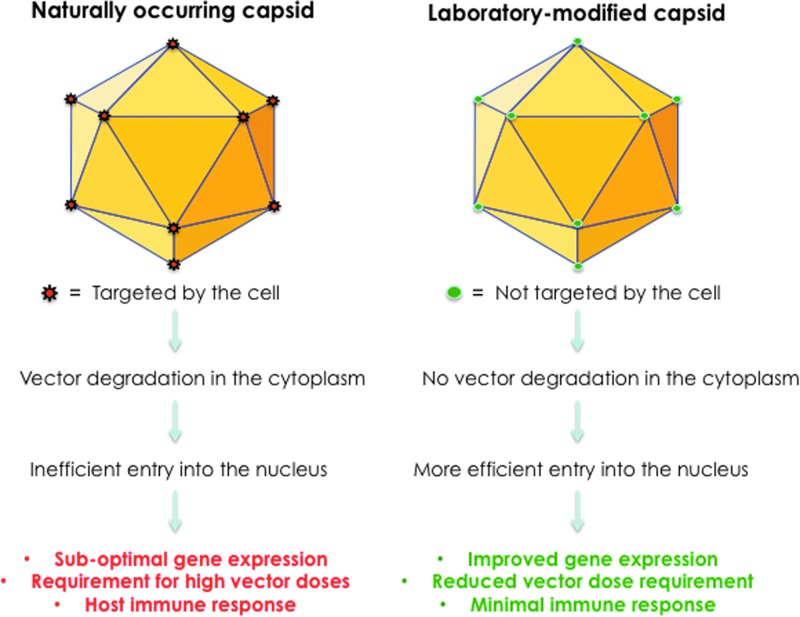
Remarkably, most, if not all, of the surface-exposed tyrosine, serine, threonine, and lysine residues are highly conserved among all 10 commonly used AAV serotype vectors. However, it has proven difficult to predict which specific combination of mutations for a given AAV serotype vector would be optimal, depending on the cell type and the host species to be transduced. It has, nonetheless, become abundantly clear, as schematically illustrated in Fig. 1, that the use of capsid-modified NextGen AAV vectors is likely to overcome some of the limitations associated with the conventional AAV vectors. Indeed, three phase I clinical trials with the NextGen AAV2 vectors are currently underway9–11 and, it is hoped, more will follow in the not-too-distant a future.
Figure 1.
Development of NextGen AAV vectors. Surface-exposed, specific tyrosine, serine, and threonine residues on AAV capsids can be phosphorylated, which is a signal for ubiquitination. Similarly, surface-exposed, specific lysine residues on AAV capsids can be ubiquitinated, and subsequently degraded by the host cell proteasome machinery. Site-directed mutagenesis of these residues led to the generation of NextGen AAV vectors that circumvent these problems, and are more efficient at reduced vector doses, thus minimizing host immune responses.
Solution to the Genome Puzzle
The AAV genome is composed of single-stranded DNA, and although both [+] and [–] polarity strands are encapsidated in separate mature virions with equal frequency, it is now a well-established fact that the viral second-strand DNA synthesis, rather than DNA strand-annealing, is the predominant mechanism that mediates transgene expression.12–15 In retrospect, it is remarkable that a therapeutic level of F.IX expression, albeit transiently, was achieved at all in the first hemophilia B trial,1 given the well-known propensity of a vast percentage of hepatocytes to prevent viral second-strand DNA synthesis.16–18 Double-stranded, self-complementary AAV (scAAV) vectors, generated by the Samulski group,19 were easily able to overcome this limitation. Indeed, the subsequent landmark successful clinical trial for hemophilia B by Nathwani and colleagues20,21 with scAAV8 vectors is a clear testament to the fact that the use of scAAV vectors, rather than the AAV8 serotype, was largely responsible for the successful outcome, because compared with mouse hepatocytes, AAV8 vectors transduce human hepatocytes much less efficiently.22–24
With the exception of the second hemophilia B trial with scAAV8 vectors,20,21 all clinical trials reported thus far with AAV vectors have been carried out with single-stranded AAV (ssAAV) vectors. In spite of that, clinical efficacy has been achieved in the potential gene therapy of four additional human diseases, including Leber's congenital amaurosis,25–28 lipoprotein lipase deficiency,29 aromatic l-amino acid decarboxylase deficiency,30 and choroideremia.31 Logic would suggest that the use of scAAV vectors would perhaps be more efficacious, provided the expression cassettes of the therapeutic genes are within the limited packaging constraint of ∼2.5 kb for scAAV vectors.
Because not all therapeutic genes can be encapsidated in scAAV vectors, additional strategies are needed to modify the single-stranded DNA genome in some way, shape, or form, such that the delivered therapeutic gene is transcriptionally active. But how, or in what way?
Once again, fortunately for me, this question was answered when yet another long-time colleague, Keyun Qing, MD, and I, as well as others, reported in 199732 that a host cell protein, FKBP52, in its phosphorylated form, binds specifically to the single-stranded D sequence within the AAV inverted terminal repeat (ITR) at the 3′ end of the viral genome and strongly inhibits viral second-strand DNA synthesis, and consequently, negatively impacts transgene expression. Over the ensuing years, a number of strategies were developed that circumvented this obstacle.33–38 Another long-term colleague, Xu-Shan Wang, MD, and others attempted to simply delete the D sequences from the viral genome, but quickly learned that the D-sequence deletion was incompatible with genome encapsidation, thereby identifying the D sequence as the “packaging signal” for the AAV genome.39–41 Another colleague, Giridhara Rao Jayandharan, PhD, and others identified the presence of a putative negative regulatory element in the D sequence in the 5′ ITR, to which the NF-κB-repressing factor binds and inhibits transcription.42 Yet another long-term colleague, Chen Ling, PhD, and others eventually developed one D sequence-deleted genome that led to the generation of the generation X (“GenX”) AAV2 vectors in 2015,43 and documented significantly enhanced transgene expression in human cell lines in vitro as well as in murine hepatocytes in vivo.
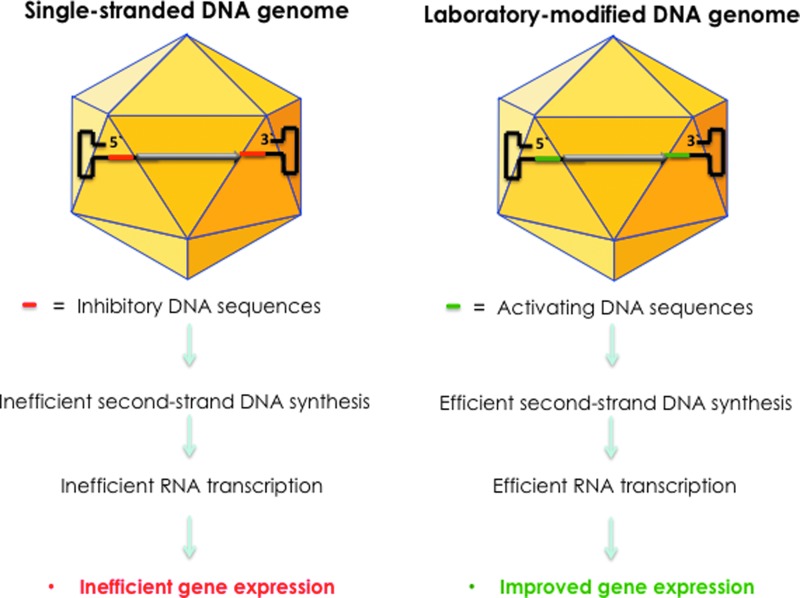
Thus, it is clear, as schematically illustrated in Fig. 2, that the use of genome-modified GenX AAV vectors is likely to overcome the limitations associated with the conventional ssAAV vectors, once again, it is hoped, in the not-too-distant future.
Figure 2.
Development of GenX AAV vectors. The D sequence at the 3′ end in the viral inverted terminal repeat (ITR) contains the binding site for a cellular protein, FKBP52, phosphorylated forms of which strongly inhibit viral second-strand DNA synthesis. The D sequence at the 5′ end in the ITR contains the binding site for NF-κB-repressing factor, a negative regulator of transcription. Removal of these sequences led to the generation of GenX AAV vectors that circumvent these problems, and led to more efficient transgene expression.
Future Prospects
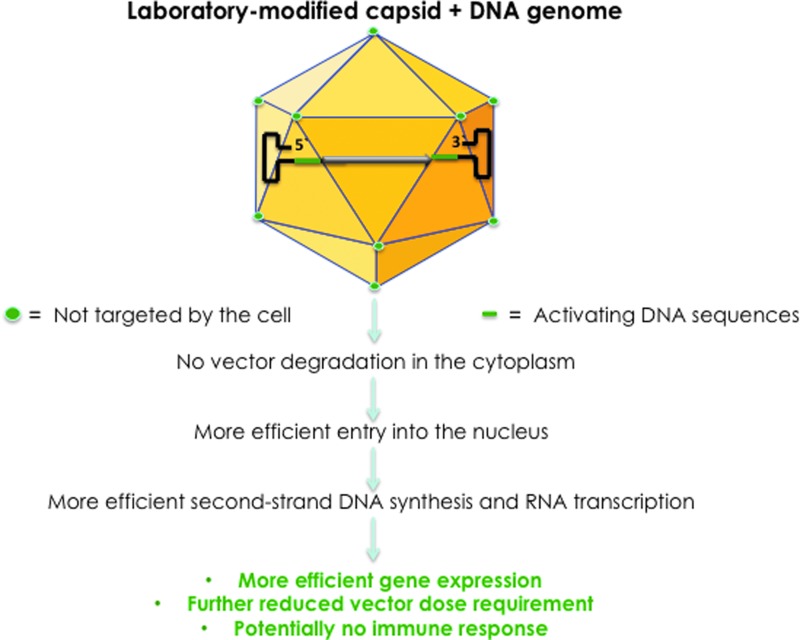
Although it appears that both major problems—the naked capsid, subject to enzymatic modifications and degradation, and the transcriptionally inactive, single-stranded DNA genome—associated with the conventional AAV vectors have been resolved for the most part, there is still room for further improvements. The reason for this optimism is twofold. First, it is relatively straightforward to conceive of a scenario where a GenX genome is encapsidated into the NextGen capsid to generate the optimized (“Opt”) AAV vector. Such an OptAAV vector is schematically depicted in Fig. 3. Indeed, in our more recent studies, we have observed a significant increase in the transduction efficiency of both NextGen AAV2 and NextGen AAV3 vectors containing GenX AAV2 genomes both in human cell lines in vitro and in a murine xenograft model in vivo (our unpublished results).
Figure 3.
Development of OptAAV vectors. Encapsidation of GenX viral genomes into NextGen capsids led to the generation of OptAAV vectors that circumvent the problems associated with the first-generation AAV vectors, and are more efficient at further reduced vector doses, thus potentially further minimizing host immune responses.
Additional future possibilities to further increase the overall safety and efficacy of NextGen, GenX, and OptAAV vectors include the following:
• Development of vectors that can overcome the preexisting immunity
• Development of vectors with increased bioavailability in vivo
• Development of tissue-specific vectors
• Development of vectors capable of site-specific integration
Overall Summary
As stated previously, viruses did not evolve for the purposes of delivery of therapeutic genes. Thus, gene therapy with viral vectors should be pursued only after they have been modified such that they are not targeted by, or are capable of evading, the host immune system. One review article supports the “revitalization of gene therapy,”44 but I would suggest that the full potential of this approach is unlikely to be achieved by the first generation of viral vectors in general, and by AAV vectors in particular. Because capsid-modified NextGen AAV serotype vectors transduce cells and tissues more efficiently at lower doses, and genome-modified GenX vectors transduce cells and tissues more efficiently, combining the two to generate the OptAAV serotype vectors offers the potential advantages of being more efficient, less immunogenic, and more cost-effective. Thus, all future clinical trials in humans should be performed with NextGen, GenX, or OptAAV vectors.
To summarize, viruses did not evolve for the purposes of delivery of therapeutic genes. Thus, gene therapy with viral vectors should be pursued only after they have been modified such that they are not targeted by, or are capable of evading, the host immune system. I concur with Dr. Bluestone that we should never attempt to outsmart the immune system when it comes to viruses, but with utmost humility, I submit that we are smart enough to find ways to transiently evade the immune system when it comes to viral vectors in general, and AAV vectors in particular.
Note Added in Proof
While this article was being reviewed, the initial results of the efficacy of NextGen AAV2 vectors in the first clinical trial (Ref. 9) were reported in an article entitled: “Gene Therapy for Leber Hereditary Optic Neuropathy: Initial Results”, by Feuer and colleagues, which was published online in Opthalmology (DOI: http://dx.doi.org/10.1016/j.ophtha.2015.10.025, November 19, 2015).
Author Disclosure
No competing financial interests exist.
References
- 1.Manno CS, Pierce GF, Arruda VR, et al. . Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347 [DOI] [PubMed] [Google Scholar]
- 2.Zhong L, Zhao W, Wu J, et al. . A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol Ther 2007;15:1323–1330 [DOI] [PubMed] [Google Scholar]
- 3.Duan D, Yue Y, Yan Z, et al. . Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest 2000;105:1573–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong L, Li B, Mah CS, et al. . Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A 2008;105:7827–7832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markusic DM, Herzog RW, Aslanidi GV, et al. . High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther 2010;18:2048–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martino AT, Basner-Tschakarjan E, Markusic DM, et al. . Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood 2013;121:2224–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslanidi GV, Rivers AE, Ortiz L, et al. . Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: the final threshold? PLoS One 2013;8:e59142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li B, Ma W, Ling C, et al. . Site-directed mutagenesis of surface-exposed lysine residues leads to improved transduction by AAV2, but not AAV8, vectors in murine hepatocytes in vivo. Hum Gene Ther Methods 2015;26:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guy J; National Eye Institute. Safety study of an adeno-associated virus vector for gene therapy of Leber's hereditary optic neuropathy (LHON). In: ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine; 2014. – [cited 2015 Dec 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT02161380 [NLM Identifier: NCT02161380] [Google Scholar]
- 10.Applied Genetic Technologies Corp. Safety and efficacy of rAAV-hRS1 in patients with X-linked retinoschisis (XLRS). In: ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine; 2015. – [cited 2015 Dec 16]. Available from: https://clinicaltrials.gov/ct2/show/NCT02416622 [NLM Identifier: NCT02416622] [Google Scholar]
- 11.Applied Genetic Technologies Corp. Safety and efficacy trial of AAV gene therapy in patients with CNGB3 achromatopsia. In: ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine; 2015- [cited 2015 Nov 5]. Available from: https://clinicaltrials.gov/cL2/show/NCT02599922 [NLM Identifier: NCT02599922] [Google Scholar]
- 12.Fisher KJ, Gao GP, Weitzman MD, et al. . Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol 1996;70:520–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari FK, Samulski T, Shenk T, et al. . Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol 1996;70:3227–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong L, Zhou X, Li Y, et al. . Single-polarity recombinant adeno-associated virus 2 vector-mediated transgene expression in vitro and in vivo: mechanism of transduction. Mol Ther 2008;16:290–295 [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, Zeng X, Fan Z, et al. . Adeno-associated virus of a single-polarity DNA genome is capable of transduction in vivo. Mol Ther 2008;16:494–499 [DOI] [PubMed] [Google Scholar]
- 16.Ponnazhagan S, Mukherjee P, Yoder MC, et al. . Adeno-associated virus 2-mediated gene transfer in vivo: organ-tropism and expression of transduced sequences in mice. Gene 1997;190:203–210 [DOI] [PubMed] [Google Scholar]
- 17.Snyder RO, Miao CH, Patijn GA, et al. . Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet 1997;16:270–276 [DOI] [PubMed] [Google Scholar]
- 18.Qing K, Khuntirat B, Mah C, et al. . Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J Virol 1998;72:1593–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarty DM, Monahan PE, and Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther 2001;8:1248–1254 [DOI] [PubMed] [Google Scholar]
- 20.Nathwani AC, Tuddenham EG, Rangarajan S, et al. . Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathwani AC, Reiss UM, Tuddenham EG, et al. . Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014;371:1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lisowski L, Dane AP, Chu K, et al. . Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 2014;506:382–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Ling C, Zhong L, et al. . Efficient and targeted transduction of nonhuman primate liver with systemically delivered optimized AAV3B vectors. Mol Ther 2015;23:1867–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Bell P, Somanathan S, et al. . Comparative study of liver gene transfer with AAV vectors based on natural and engineered AAV capsids. Mol Ther 2015;23:1877–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bainbridge JW, Smith AJ, Barker SS, et al. . Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med 2008;358:2231–2239 [DOI] [PubMed] [Google Scholar]
- 26.Maguire AM, Simonelli F, Pierce EA, et al. . Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med 2008;358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cideciyan AV, Aleman TS, Boye SL, et al. . Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A 2008;105:15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauswirth WW, Aleman TS, Kaushal S, et al. . Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther 2008;19:979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroes ES, Nierman MC, Meulenberg JJ, et al. . Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler Thromb Vasc Biol 2008;28:2303–2304 [DOI] [PubMed] [Google Scholar]
- 30.Hwu WL, Muramatsu S, Tseng SH, et al. . Gene therapy for aromatic l-amino acid decarboxylase deficiency. Sci Transl Med 2012;4:134ra61. [DOI] [PubMed] [Google Scholar]
- 31.MacLaren RE, Groppe M, Barnard AR, et al. . Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet 2014;383:1129–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qing K, Wang XS, Kube DM, et al. . Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc Natl Acad Sci U S A 1997;94:10879–10884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mah C, Qing K, Khuntirat B, et al. . Adeno-associated virus type 2-mediated gene transfer: role of epidermal growth factor receptor protein tyrosine kinase in transgene expression. J Virol 1998;72:9835–9843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qing K, Li W, Zhong L, et al. . Adeno-associated virus type 2-mediated gene transfer: role of cellular T-cell protein tyrosine phosphatase in transgene expression in established cell lines in vitro and transgenic mice in vivo. J Virol 2003;77:2741–2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong L, Li W, Yang Z, et al. . Improved transduction of primary murine hepatocytes by recombinant adeno-associated virus 2 vectors in vivo. Gene Ther 2004;11:1165–1169 [DOI] [PubMed] [Google Scholar]
- 36.Zhong L, Chen L, Li Y, et al. . Self-complementary adeno-associated virus 2 (AAV)-T cell protein tyrosine phosphatase vectors as helper viruses to improve transduction efficiency of conventional single-stranded AAV vectors in vitro and in vivo. Mol Ther 2004;10:950–957 [DOI] [PubMed] [Google Scholar]
- 37.Jayandharan GR, Zhong L, Li B, et al. . Strategies for improving the transduction efficiency of single-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther 2008;15:1287–1293 [DOI] [PubMed] [Google Scholar]
- 38.Jayandharan GR, Zhong L, Sack BK, et al. . Optimized adeno-associated virus (AAV)-protein phosphatase-5 helper viruses for efficient liver transduction by single-stranded AAV vectors: therapeutic expression of factor IX at reduced vector doses. Hum Gene Ther 2010;21:271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang XS, Ponnazhagan S, and Srivastava A. Rescue and replication signals of the adeno-associated virus 2 genome. J Mol Biol 1995;250:573–580 [DOI] [PubMed] [Google Scholar]
- 40.Wang XS, Ponnazhagan S, and Srivastava A. Rescue and replication of adeno-associated virus type 2 as well as vector DNA sequences from recombinant plasmids containing deletions in the viral inverted terminal repeats: selective encapsidation of viral genomes in progeny virions. J Virol 1996;70:1668–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ling C, Wang Y, Lu Y, et al. . The adeno-associated virus genome packaging puzzle. J Mol Genet Med 2015;9: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jayandharan G, Aslanidi G, Martino AT, et al. . Activation of the NF-κB pathway by adeno-associated virus (AAV) vectors and its implications in immune response and gene therapy. Proc Natl Acad Sci U S A 2011;108:3743–3748 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Ling C, Wang Y, Lu Y, et al. . Enhanced transgene expression from recombinant single-stranded D-sequence-substituted adeno-associated virus vectors in human cell lines in vitro and in murine hepatocytes in vivo. J Virol 2015;89:952–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naldini L. Gene therapy returns to centre stage. Nature 2015;526:351–360 [DOI] [PubMed] [Google Scholar]