Abstract
We investigated the relationship between the tumor suppressor p53 and the hypoxia-inducible factor-1 (HIF-1)-dependent expression of the hypoxia marker, carbonic anhydrase IX (CAIX). MCF-7 (wt p53) and Saos-2 (p53-null) cells displayed similar induction of CAIX expression and CA9 promoter activity under hypoxic conditions. Activation of p53 by the DNA damaging agent mitomycin C (MC) was accompanied by a potent repression of CAIX expression and the CA9 promoter in MCF-7 but not in Saos-2 cells. The activated p53 mediated increased proteasomal degradation of HIF-1α protein, resulting in considerably lower steady-state levels of HIF-1α protein in hypoxic MCF-7 cells but not in Saos-2 cells. Overexpression of HIF-1α relieved the MC-induced repression in MCF-7 cells, confirming regulation at the HIF-1α level. Similarly, CA9 promoter activity was downregulated by MC in HCT 116 p53+/+ but not the isogenic p53−/− cells. Activated p53 decreased HIF-1α protein levels by accelerated proteasome-dependent degradation without affecting significantly HIF-1α transcription. In summary, our results demonstrate that the presence of wtp53 under hypoxic conditions has an insignificant effect on the stabilization of HIF-1α protein and HIF-1-dependent expression of CAIX. However, upon activation by DNA damage, wt p53 mediates an accelerated degradation of HIF-1α protein, resulting in reduced activation of CA9 transcription and, correspondingly, decreased levels of CAIX protein. A model outlining the quantitative relationship between p53, HIF-1α, and CAIX is presented.
Tumor progression toward a more aggressive and metastatic potential is a fundamental process, but the factors that are required for this progression are poorly characterized. It has been recognized for some time that the specific tumor microenvironment is implicated not only in the malignant progression but also in the outcome of therapy (45). Hypoxia develops in most solid tumors as a result of inefficient vascular development and/or abnormal vascular architecture (8). Cellular response to reduced oxygen levels is mediated by the hypoxia-inducible transcription factor 1 (HIF-1) (47). HIF-1 is a heterodimer that consists of the regulated HIF-1α and the constitutively expressed HIF-1β (also known as aryl hydrocarbon receptor nuclear translocator) subunits (47). In the presence of oxygen, HIF-1α becomes hydroxylated in the oxygen-dependent degradation domain by a multimeric prolyl hydroxylase (22, 25). Binding of the tumor suppressor von Hippel-Lindau protein to the hydroxylated form of HIF-1α initiates ubiquitinylation, targeting to the proteasome, and rapid degradation of HIF-1α (37). In the absence of oxygen, degradation of HIF-1α does not occur and HIF-1 binds to hypoxia-response elements (HRE), thereby activating the expression of hypoxia-response genes (19, 42, 47).
The mutual relationship between hypoxia and the tumor suppressor p53 has been the subject of several studies, but the underlying mechanisms remain ill defined (for reviews, see references 14 and 19). Although hypoxia-induced accumulation of p53 arrests replication in the absence of any detectable DNA-damage (33), this p53 is transcriptionally impaired (5, 33). It has been speculated that the more aggressive nature of hypoxic tumors and the frequent occurrence of p53 mutations in advanced stages of tumor development are the consequence of a selective pressure exerted by hypoxia. According to this theory, hypoxia induces p53-dependent apoptosis and will therefore counterselect cells with wild-type p53, thereby facilitating the clonal expansion of cells with mutant or otherwise-compromised p53 protein function (17).
The relationship between p53 and HIF-1 function was the subject of several earlier studies. It was reported that the loss of p53 in the colon carcinoma cell line HCT 116 enhances HIF-1α levels and augments HIF-1-dependent transcriptional activation in response to hypoxia (40). HIF-1-dependent transcription in a panel of prostate cell lines increased from low in normal epithelial to high in highly metastatic cells, and this observation was related to decreasing p53 activity in the same direction (41). In overexpression experiments, p53 was observed to downregulate HIF-stimulated transcription via competition for p300 coactivator (7). On the other hand, using the same isogenic HCT 116 p53+/+ and p53−/− cells used in reference 40 no differences in hypoxic induction of hexokinase 1, adrenomedulin, and a number of other genes were found (18). Similarly, HIF-1α protein levels were comparable in hypoxic HCT 116 p53+/+ and p53−/− cells (1). Furthermore, the presence of hypoxia-stabilized p53 did not have any effect on HIF-1α stabilization in RKO cells (18). Recent reviews of HIF-1α biology have drawn attention to these somewhat contradictory observations (19, 42).
Carbonic anhydrase IX (CAIX) is one of the emerging markers of tumor hypoxia (3, 23, 36). CAIX expression was found to correlate with lowered O2 tension in tumors (36) and may have prognostic significance in a variety of cancers (9, 15). Previously, CAIX (also known as MN) was identified in a large number of carcinomas but not in the corresponding healthy tissues (48, 50; also see reference 23 and references therein). Although its exact role in carcinogenesis is not known, it was suggested that tumor-associated transmembrane CA isozymes (CAIX and CAXII) may facilitate acidification of the extracellular milieu surrounding the cancer cells and in this way promote tumor growth and spread (23, 24).
We and others have shown that expression of CAIX is positively regulated by low O2 tension via the HRE in the CA9 promoter, immediately upstream of the transcription start (28, 48).
HIF(s) plays a crucial role in cellular adaptation to conditions of lowered oxygen supply (19, 42). In principle, by perturbing HIF activity p53 could lower the viability of hypoxic cells by not allowing them to accumulate sufficient levels of HIF-dependent effector molecules. The previous observations that there is an accumulation of transcriptionally impaired p53 under hypoxic conditions (5, 33) raise the question of whether this p53 is capable of regulating HIF-1-dependent transcription. Because of the number of apparently contradictory reports on the interplay between p53 and HIF-1 function (1, 7, 18, 40, 41), we wished to investigate the effects of the tumor suppressor p53 on expression of the endogenous hypoxia marker CAIX and CA9 promoter function. We asked the following questions. Is DNA damage required in addition to hypoxic conditions for p53 activation? Will p53 activation have any inhibitory effect on the activation of HIF-1-target genes? If there is an effect, is it at the level of transcriptional regulation or protein degradation? To address these questions we studied endogenous CAIX expression and CA9 promoter activity in wild-type (wt) p53 or p53-null cell lines in relation to p53 and HIF-1α functions under conditions of hypoxia (0.5%-1.0% O2) or a hypoxia-mimicking agent in the absence or presence of a DNA-damaging agent.
MATERIALS AND METHODS
Sequences are written in the 5′-3′ direction and numbers in brackets indicate each position relative to the CA9 transcription start or the position in the appropriate database entry. Kits, enzymes, antibodies and reagents were used according to the manufacturers' recommendations.
Plasmid constructions.
The [−173,+31] and [−46,+14] CA9 promoter fragments were cloned in pGL2 basic vector (Promega). 3×HRE-Luc and 3×PR1-Luc constructs were generated by cloning three copies of the CAAGACATACGTGCTGTCTCA HRE and TGGGTGGGGGAGGAGCAAGCC PR1 (27) sequences, respectively, into pLuc-MCS vector (Stratagene). The PG13-Luc construct contains a multimerized p53 consensus binding sequence (32). The pCEP-HIF-1α construct contains a HIF-1α cDNA inserted in the pCEP4 vector (43). wt p53 plus V143A, N247I, R273P, and L22Q/W23S mutant p53 cDNAs were expressed in the pCEP4 vector. The pRL-tk vector was obtained from Promega.
Cell lines and culture.
Human breast carcinoma MCF-7, containing wt p53 (7), and p53-null osteosarcoma Saos-2 (11) cell lines were grown in Dulbecco's modified Eagle's medium (BioWhittaker), supplemented with 10% fetal calf serum (Life Technologies), 102 U of penicillin (Sigma) per ml, 102 μg of streptomycin (ICN) per ml, and 125 ng of amphotericin B (Sigma) per ml. The human colon cancer cell line HCT 116 p53+/+, containing wt p53, and its derivative HCT 116 p53−/−, which has both TP53 alleles disrupted (10), were cultured in McCoy's 5A medium supplemented with 10% fetal calf serum and penicillin-streptomycin. All cell lines were regularly tested for microbial contamination (44) and were uniformly negative. The effect of the DNA-damaging drug mitomycin C (MC) (Sigma) at 5 or 10 μg/ml (concentrations routinely used in a number of other studies; e.g., see reference 6) on endogenous CAIX expression was tested on cells that had been seeded at 10,000/cm2 and grown for 3 days. The cells were plated at 40,000/cm2, pretreated with MC for 2 h, and exposed to a 1% O2 environment in a PROOX in vitro chamber (BioSpherix), controlled by the PROOX instrument (model 110; BioSpherix), for 24 h in the presence of MC. Alternatively, following pretreatment with MC, CAIX expression was induced with 100 μM hypoxia-mimicking desferrioxamine mesylate (DFO) (Sigma) for 24 h. For cell density-dependent CAIX induction, the cells were plated at 160,000/cm2 and incubated in the presence of MC for 24 h. The mechanism of HIF-1α degradation in MC-treated cells was studied in MCF-7 cells pretreated with the proteasome inhibitor LLnV (Sigma) and MC for 90 min and then exposed to normoxic or hypoxic (1% O2) conditions for 18 h in the presence of both reagents.
MTT assay.
MCF-7 and Saos-2 cells (20,000/100 μl) were transferred onto 96-well microtiter plates, incubated overnight, pretreated with MC for 2 h, and exposed to 1% O2 for 24 h in the presence of MC. Each control and MC concentration was run in triplicate. Cells were rinsed with phosphate-buffered saline, 100 μl of medium containing 25 μl of a 5-mg/ml stock solution of 3-(4,5-dimethylthiazolyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma) was added to each well, and the plates were incubated 4 h at 37°C. The cells were then lysed with 100 μl of lysis buffer (2% [wt/vol] sodium dodecyl sulfate, 50% [vol/vol] N,N-dimethylformamide, and 0.4% [vol/vol] glacial acetic acid) for 1 h at room temperature. The color development was read at 595 nm in a SpectraMax 340 Microplate Reader (Molecular Devices), and the data were expressed as the percent of the normoxic control.
Western blot analysis.
Western blot analysis of CAIX and β-actin expression was performed as described previously (28). Additional antibodies used were: total p53 DO-1 (Santa Cruz Biotechnology), p-S15 and p-S20 phospho-p53 (Cell Signaling Technology), and HIF-1α (BD Bioscience). For these antibodies, the cells were lysed in lysis buffer I (20 mM Tris-Cl [pH 7.5], 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, leupeptin [1 μg/ml], aprotinin [1 μg/ml], 25 mM NaF, 1 mM phenylmethylsulfonyl fluoride) for 20 min on ice. Lysates were centrifuged (13,000 × g, 10 min, 4°C), and the protein concentration in supernatant was measured with a BCA protein assay kit (Pierce). The insoluble pellet remaining after the lysis in lysis buffer I was solubilized in lysis buffer II (62.5 mM Tris-Cl [pH 6.8], 2% sodium dodecyl sulfate, 10% glycerol, 50 mM dithiothreitol, 0.01% [wt/vol] bromophenol blue) by passing it through a 27-guage needle at least five times.
Transient-transfection assay.
Cells were cotransfected with a CA9-driven firefly luciferase reporter construct and pRL-tk expressing Renilla luciferase (internal control for transfection efficiency) as described previously (28). Effects of cotransfected wt and mutant p53 (10 ng/12-well plate) on CA9 promoter activity were tested in HCT 116 p53−/− cells. After exposure to the transfection mixture for 24 h, the cells were trypsinized, transferred to plates at a concentration of 40,000 cells/cm2, and allowed to adhere for 3 h. The cells were then pretreated with MC at 5 or 10 μg/ml for 2 h and exposed to 0.5% O2 or 100 μM DFO for 24 h in the presence of MC. Reporter assays were performed as described previously (28). Promoter activities were expressed as the average ratio of firefly to Renilla luciferase activities (± standard deviations [SD]) from at least three independent experiments.
Reverse transcription (RT)- and real-time PCR.
MCF-7 cells (6 × 105 plated at 40,000 cells/cm2) were pretreated with MC (10 μg/ml) for 2 h, followed by exposure to 1% O2 or 100 μM DFO for 16 h in the presence of MC. Total RNA was isolated with an RNeasy Mini Kit (QIAGEN), and cDNA was synthesized with ProtoScript first strand cDNA synthesis kit (New England Biolabs). cDNA fragments were amplified with the following primer pairs: HIF-1α (accession no. U22431), sense, GCAGCCAGATCTCGGCGAAG [101 to 120]; antisense, CTGTGTCCAGTTAGTTCAAACTG [420 to 398]; CA9 (accession no. NM_001216), sense, CTGTCACTGCTGCTTCTGAT [121 to 140]; antisense, TCCTCTCCAGGTAGATCCTC [321 to 301]; β-actin (accession no. NM_001101), sense, ACAACGGCTCCGGCATGTGCAA [105 to 126]; antisense, CGGTTGGCCTTGGGGTTCAG [420 to 402]. PCRs were performed in GeneAmp PCR System 9700 (PE Applied Biosystems) for 30 cycles: 94°C for 30 s, 56°C for 30 s, and 72°C for 30 s. Products were analyzed on a 1.5% agarose gel.
Real-time PCR analysis was performed with the iCycler iQ Multicolor Real-Time PCR Detection System using SYBR Green Supermix (both Bio-Rad). Primers for HIF-1α were GCCGAGGAAGAACTATGAAC [558 to 577] (sense) and ATATTTGATGGGTGAGGAATGG [726 to 704] (antisense). The same β-actin primers described above were used. cDNAs were amplified with the following profile: 95°C for 4 min and 40 cycles of 94°C for 10 s and 56°C for 30 s. The relative HIF-1α transcription for each sample was expressed as the ratio of HIF-1α and β-actin values.
RESULTS
Induction of CAIX expression is not affected by the presence of normal wt p53.
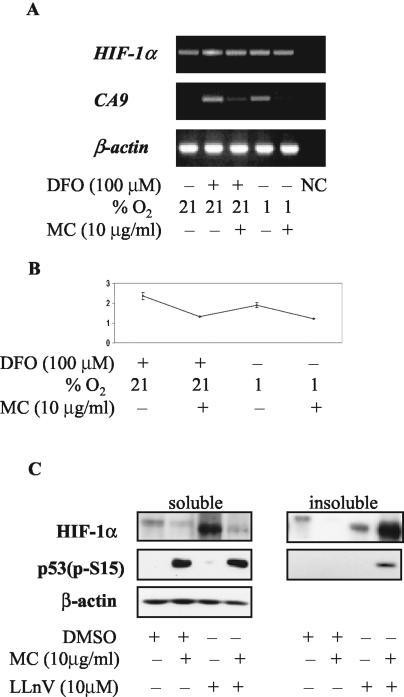
Initially, we tested the effect of p53 status on CAIX inducibility in the frequently used isogenic cell lines HCT 116 p53+/+ and HCT 116 p53−/− that differ only in respect to the presence of p53 (10). Regardless of exposure to hypoxia (0.5 to 1.0% O2) or the hypoxia-mimicking DFO, we were unable to detect CAIX expression in either of the cell lines. Subsequent analysis showed that the CA9 promoter was highly methylated in these cells (I. Kuzmin, personal communication). Therefore, we chose the genetically more diverse MCF-7 (wt p53) and Saos-2 (p53-null) cell lines for further comparative studies. We asked whether presence or absence of wt p53 would have any effect on CAIX expression, induced by various treatments. In both cell lines tested, the basal CAIX level was undetectable by Western blotting (Fig. 1A). CAIX expression was readily stimulated by all tested treatments, without appreciable differences in magnitude of induction (Fig. 1A). Similar observations were made with a number of other cell lines with different p53 status (HeLa, wt p53, but functionally null due to human papillomavirus E6-mediated degradation; HT1080 6TG, two mutant p53 alleles; PC-3, p53 null; data not shown). With the exception of DFO treatment, where we observed a slight stabilization of p53 with accompanying phosphorylation at S15 and S20 (Fig. 1A), the other two treatments had no effect on p53 stability or phosphorylation at these serine residues in MCF-7 cells (Fig. 1A). We therefore conclude that wt p53 is not activated under these conditions of hypoxia and does not significantly affect induction of CAIX expression under these conditions. The lack of activation of p53 at this degree of hypoxia is consistent with the observations of Koumenis et al. (33).
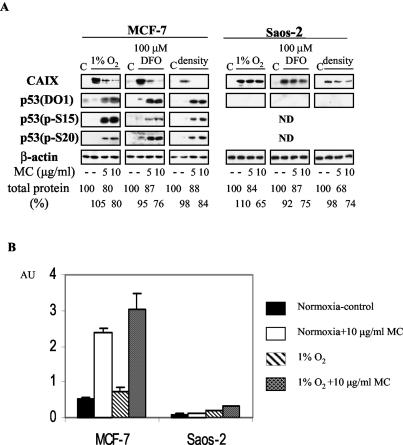
FIG. 1.
(A) Effect of MC treatment on CAIX expression in wt p53 MCF-7 and p53-null Saos-2 cells. Cells were seeded at 40,000/cm2 (1% O2 and DFO) or 160,000/cm2 (density), allowed to attach for 5 h, pretreated with MC for 2 h, and exposed to 1% O2 or 100 μM DFO for 24 h in the presence of MC. Total protein lysates (40 μg) were tested for CAIX, total p53 (DO1), phospho p53 (S15 and S20), and β-actin by Western blotting. Total protein yield is expressed as percentages of control. (B) p53 transactivation effects in MCF-7 and Saos-2 cells. Cells were cotransfected with the PG13 (containing 13 copies of the p53 response element and the firefly luciferase gene) and pRL-tk (expressing Renilla luciferase) plasmids. After 16 h the transfectants were trypsinized, seeded at 40,000 cells/cm2, and treated as in panel A. p53 activity is expressed as the ratio of firefly activity to Renilla activity, and each of the bars represents the mean value (x ± SD) from at least three individual experiments.
Effect of MC-inflicted DNA damage on CAIX expression in wt and p53-null cell lines.
Control, hypoxic, and overconfluent (dense [160,000 cells/cm2]) MCF-7 cells contain almost undetectable levels of p53 protein. Hypoxia does not stabilize or activate the p53 with the exception of the hypoxia-mimic DFO where a slight increase in phospho-p53 (S15 and S20) is seen (Fig. 1A). Therefore, we asked whether activation of p53, which is accomplished by exposing cells to the DNA-damaging agent MC, would have any effect on CAIX expression. As can be seen in Fig. 1A, there is a significant inverse relation between p53 activation and CAIX expression in hypoxic MCF-7 cells. MC treatment stimulates a considerable accumulation of p53 that is phosphorylated at S15 and S20 (Fig. 1A). This activated p53 is transcriptionally competent, as verified with the PG13 reporter construct, containing multimerized p53 response elements (Fig. 1B) and by detection of WAF-1 protein in MC-treated cells by Western blotting (data not shown). We also tested the effects of MC treatment on CAIX expression in the p53-null Saos-2 cells. In contrast to MCF-7 cells, high levels of CAIX (only slightly lower than those in the control hypoxic cells) were detected in the MC-treated Saos-2 cells (Fig. 1A). Following exposure to MC at 5 and 10 μg/ml for 24 h, only moderate decreases in the total protein yields were observed (Fig. 1A). In addition, to assess the cell viability under these conditions, we performed an MTT assay. The viability of treated cells was expressed as the percentages of viability of control cells under normoxia (100%). For 1% O2, 1% O2 plus MC at 5 μg/ml, and 1% O2 plus MC at 10 μg/ml the viabilities were 91% ± 3.6%, 91% ± 6.6%, and 86% ± 8.6%, respectively, for MCF-7 cells and 90% ± 4.7%, 90% ± 7.6%, and 78% ± 3.5%, respectively, for Saos-2 cells. This confirms that the MC treatment at either concentration was not selectively toxic for MCF-7 cells. We conclude that, despite some marginal p53-independent downregulation of CAIX observed in p53-null Saos-2 cells, activation of wt p53 is associated with a strong inhibitory effect on CAIX expression.
Effect of MC-inflicted DNA damage on CA9 promoter activity in wt and p53-null cell lines.
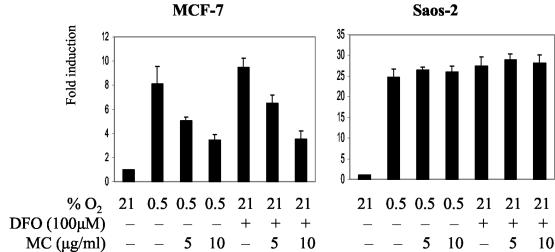
Next, we studied whether wt p53 exerts its inhibitory effect on CAIX expression at the transcriptional level. To this end, we probed the effects of MC treatment on activity of the luciferase reporter construct, driven by the [−46, +14] CA9 promoter fragment. In addition to the transcription start site, this CA9 fragment contains only two characterized regulatory elements: HRE (48) and the SP1/SP3-binding PR1 domain (31). Transient-transfection experiments confirmed induction of CA9 promoter activity by hypoxia (0.5% O2) and DFO in both MCF-7 and Saos-2 cells. A slightly higher magnitude of induction was observed in Saos-2 cells (Fig. 2). MC inhibited promoter activity in a dose-dependent manner in MCF-7 cells, whereas no inhibitory effect was seen in Saos-2 cells. Therefore, MC exerts its inhibitory effect on CAIX expression in a p53-dependent way by interfering with CA9 promoter inducibility, presumably by targeting transcription factors that are critical for CA9 promoter function.
FIG. 2.
The effect of MC treatment on CA9 promoter activity in MCF-7 and Saos-2 cells. Cells were cotransfected with the [−46, +14] CA9 reporter construct (containing firefly luciferase gene) and pRL-tk for 16 h, trypsinized, seeded at 40,000/cm2, allowed to attach for 5 h, pretreated with MC for 2 h, and exposed to 0.5% O2 or 100 μM DFO for 24 h in the presence of MC. CA9 promoter activity was expressed as the ratio of firefly activity to Renilla activity and set as 1 in control cells (21% O2). Activities under various treatments are expressed as the level of induction relative to the control, and each of the bars represents the mean value (x ± SD) from at least three individual experiments.
Identification of regulatory elements within the CA9 promoter whose function is inhibited by activated wt p53.
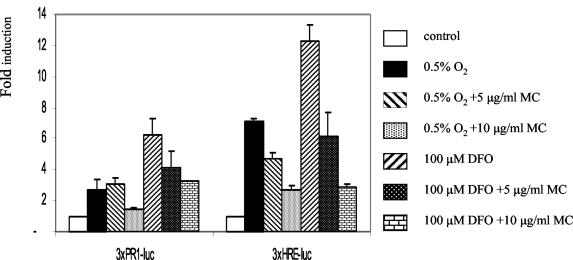
Previous deletion and mutational analysis identified the HRE (29, 48) and SP1/SP3 binding PR1 sites (29, 31) as the most critical regulatory elements in the CA9 promoter, and inhibition of either is sufficient to downregulate the CA9 promoter function (29). We wished to identify which of these regulatory elements is targeted by activated p53 in MCF-7 cells. We found that activity of the reporter constructs with a single copy of HRE or PR1 in front of a heterologous promoter was not significantly increased in response to hypoxic treatment (data not shown). Therefore, we tested effects of MC on activity of reporter constructs driven by multiple copies of these elements in front of a minimal TATA-box-containing promoter. The activities of both constructs were induced significantly by exposure to hypoxia or DFO (Fig. 3). Although both constructs showed a lower level of induction following MC treatment, the HRE-driven construct was significantly more affected (Fig. 3). The inhibition profile of the HRE-driven construct (Fig. 3) strongly resembles that of the CA9 promoter (Fig. 2), suggesting that, at least in the cells tested, HIF-1 may be the most important target for the inhibitory effect of p53 on CA9 promoter transactivation.
FIG. 3.
The effect of MC treatment on PR1 and HRE activity in MCF-7 cells. Cells were cotransfected with 3 × PR1-Luc or 3 × HRE-Luc and pRL-tk as in Fig. 2. Promoter activities are expressed as in Fig. 2.
Activated p53 downregulates HIF-1α levels in MCF-7 cells.
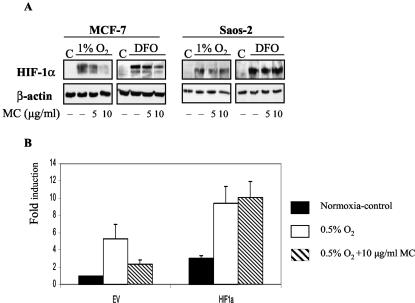
Having identified HRE as the primary target for the p53-mediated inhibitory effect on CA9 promoter activity, we wished to address the corresponding mechanism. In principle, HRE-dependent transcription can be modulated by two different mechanisms: (i) through availability of HIF-1α, the regulatable component of HIF-1 (47), or (ii) through regulation of p300/CBP that acts as an essential cofactor for HIF-1 (4, 7). To investigate these possibilities, we initially measured the amounts of HIF-1α protein in MC-treated MCF-7 cells by Western blotting. As expected, HIF-1α is undetectable in control cells, but it is readily stabilized in hypoxic cells (Fig. 4A). Regardless of the mode of induction, MC treatment decreased amounts of the HIF-1α protein in a dose-dependent manner in MCF-7 cells (Fig. 4A). Conversely, in p53-null Saos-2 cells MC treatment had no effect on the amount of stable HIF-1α protein (Fig. 4A). The conclusion that downregulation of HIF-1α is the primary cause of the inhibitory effect of activated wt p53 on the CA9 promoter was further strengthened by cotransfection experiments with HIF-1α cDNA. Overexpression of HIF-1α counteracts the inhibitory effect of MC treatment and confirms that the observed decreased CA9 promoter activity is due to decreased availability of HIF-1α (Fig. 4B). These results suggest that p53 downregulates HIF-1α protein levels either by inhibiting transcription of the HIF 1α gene and/or increasing the degradation rate of HIF-1α protein.
FIG. 4.
(A) The effect of MC treatment on HIF-1α levels in MCF-7 and Saos-2 cells. Cells were seeded at 40,000/cm2 and treated as described in Fig. 1A. Total protein lysates (40 μg) were tested for HIF-1α and β-actin by Western blotting. (B) Cotransfected HIF-1α rescues CA9 promoter activation in MC-treated MCF-7 cells. Cells were cotransfected with the [−173, +31] CA9 reporter construct, pRL-tk, and 500 ng of pCEP4 (EV) or pCEP-HIF-1α (HIF-1α) as in Fig. 2. CA9 promoter activity in the presence of EV in control cells (21% O2) was set as 1, and the rest is expressed as the level of induction as in Fig. 2.
MC-activated p53 stimulates degradation of HIF-1α by the proteasome pathway without interfering with HIF-1α transcription.
Next, we investigated whether transcriptionally competent p53, induced by MC treatment, downregulates HIF-1α through repressing its transcription. RT-PCR revealed no differences in HIF-1α mRNA levels in control and MC-treated MCF-7 cells (Fig. 5A). This observation was further supported by the results of real-time PCR (Fig. 5B). HIF-1α transcription, normalized to β-actin transcription and expressed in arbitrary units, was 2.36 ± 0.18 and 1.32 ± 0.2 for DFO- and DFO-MC-treated cells, respectively, and 1.89 ± 0.13 and 1.22 ± 0.1 for 1% O2- and 1% O2-MC-treated cells, respectively. Although real-time PCR indicated a decrease in HIF-1α transcription in MC-treated cells (1.79-fold for DFO and 1.55-fold for 1% O2), this decrease is not sufficient to account for the decreased overall HIF-1α protein levels under these conditions. In fact, the real-time PCR results indicate a greater (albeit modest) decrease in transcription in the DFO-MC cells than 1% O2-MC cells. However, the levels of HIF-1α protein (Fig. 4A) in the 1% O2-MC cells are significantly less than those in the DFO-MC cells.
FIG. 5.
(A) RT-PCR analysis of HIF-1α and CA9 transcription in MC-treated MCF-7 cells. Cells were seeded and treated as in Fig. 1A and were harvested 16 h later, and total RNA was isolated, reverse-transcribed, and amplified. NC, negative control. (B) Real-time PCR analysis of HIF-1α transcription in MC-treated MCF-7 cells. The relative HIF-1α transcription for each sample was expressed in arbitrary units as the ratio of HIF-1α and β-actin values. (C) MC-activated p53 accelerates proteasome-dependent degradation of HIF-1α in MCF-7 cells. Cells were pretreated with MC and LLnV for 90 min, exposed to 1% hypoxia for 18 h in the presence of both reagents, and lysed in lysis buffer I. Insoluble pellet was solubilized in lysis buffer II, and both fractions were tested for HIF-1α, phospho-p53 (S15), and β-actin by Western blotting.
RT-PCR also confirmed a tight control of CA9 transcription, corresponding to the observed CAIX expression pattern. The CA9 signal is below detectable levels in control cells, induced considerably by hypoxia or hypoxia-mimicking treatment, and downregulated in the presence of MC (Fig. 5A). We next studied the involvement of the proteasome pathway in downregulation of HIF-1α by activated p53. Pretreatment with the proteasome inhibitor LLnV for 90 min followed by 1% hypoxia for 18 h resulted in a significant increase in the level of the HIF-1α protein (Fig. 5C). Surprisingly, HIF-1α levels detected in cells pretreated with LLnV and MC were significantly lower (Fig. 5C). This unexpected observation was elucidated when we found that under these conditions almost all of the HIF-1α protein was insoluble in lysis buffer I. Solubilization of the remaining insoluble fraction in lysis buffer II revealed the presence of considerable amounts of HIF-1α protein (Fig. 5C). The total amount of HIF-1α in the soluble and insoluble fractions was approximately the same, suggesting that proteasome function is required for p53-dependent downregulation of HIF-1α. Therefore, we conclude that acceleration of proteasome-mediated degradation is responsible for downregulation of HIF-1α by MC-activated p53.
The effect of MC-treatment on CA9 promoter activity in HCT 116 p53+/+ and p53−/− cells.
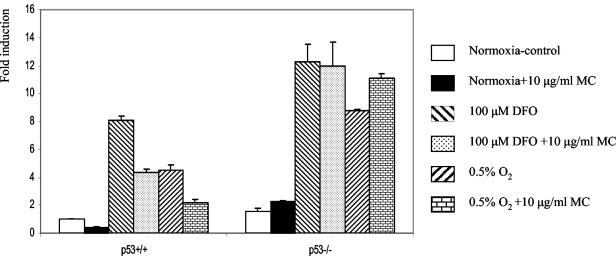
Finally, we sought to strengthen our conclusions about the relationship between p53, HIF-1α, and CAIX in the pair of isogenic p53+/+ and p53−/− HCT 116 cells. Despite the fact that neither cell line expressed endogenous CAIX, preliminary experiments indicated that an exogenous, transfected CA9 promoter activity was induced by hypoxic treatment in these cells (data not shown). Therefore, we studied CA9 promoter activation and the effect of MC treatment in parallel in transiently transfected HCT 116 p53+/+ and p53−/− cells. Our results, illustrated in Fig. 6, show that under normoxic conditions the basal activity in the p53−/− cells was approximately 1.5-fold higher than that in p53+/+ cells. Following the MC treatment, this basal activity was suppressed markedly in p53+/+ cells and unchanged in p53−/− cells. In response to hypoxia (0.5% O2) or exposure to the hypoxia-mimicking DFO, strong induction (8-fold) of CA9 promoter activity was observed that was again higher (1.5-fold) in p53−/− cells. Notably, MC elicited inhibition of promoter activity in the p53+/+ cells but not in the p53−/− cells. Thus, the requirement for activated p53 for MC-induced inhibition of CA9 promoter observed in the paired p53+/+ and p53−/− isogenic HCT 116 cells confirms our previous observation in the more genetically diverse MCF-7 and Saos-2 cells.
FIG. 6.
Effect of MC treatment on CA9 promoter activity in HCT 116 p53+/+ and p53−/− cells. Cells were cotransfected with the [−173, +31] CA9 reporter construct and pRL-tk as in Fig. 2. CA9 promoter activity in p53+/+ cells under normoxic conditions was set as 1, and the rest is expressed as the level of induction as in Fig. 2.
MC-induced degradation of HIF-1α is dependent on p53 activation in HCT 116 cells.
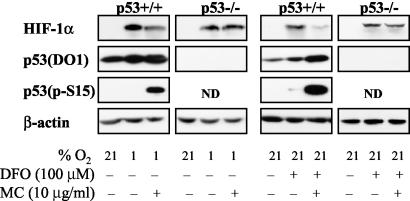
Next we investigated the profile of HIF-1α response to MC treatment in HCT 116 p53+/+ and p53−/− cells. MC decreased hypoxia- and DFO-induced HIF-1α levels in p53+/+ but not in p53−/− HCT 116 cells (Fig. 7). Unlike in MCF-7 cells, total p53 protein levels were detected in control, hypoxic, and DFO-treated p53+/+ cells (Fig. 7). However, S15 phosphorylation on p53 (an indicator of p53 activation) occurred only following MC treatment (Fig. 7), thus confirming the correlation between activated p53 and decreased HIF-1α levels previously observed in MCF-7.
FIG. 7.
Effect of MC treatment on HIF-1 α levels in HCT 116 p53+/+ and p53−/− cells. Total protein lysates were prepared as in Fig. 4A and tested for HIF-1α, total p53 (DO1), phospho-p53 (S15), and β-actin by Western blotting.
Effect of ectopically expressed wt and mutant p53 on the CA9 promoter in HCT 116 p53−/− cells.
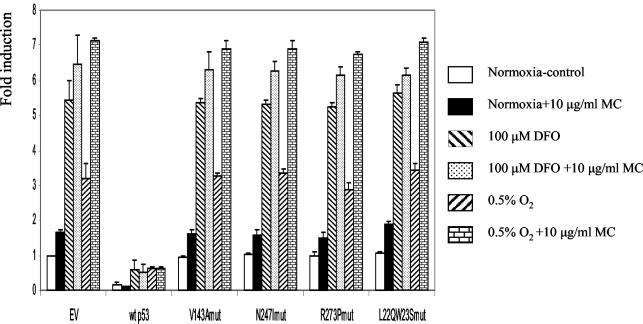
Previously it was reported that the transcriptionally inactive mutant p53, mutated in its DNA binding domain, retained the ability to inhibit HIF-1 transcriptional activity, whereas a double mutant in the p53 transactivation domain lost this inhibitory function (7). To test the inhibitory effect of various p53 mutants on CA9 promoter activity, we coexpressed wt or mutant p53 with the [−173,+31] CA9 promoter fragment in HCT 116 p53−/− cells and measured the reporter activity. As can be seen in Fig. 8, wt p53 strongly suppressed the basal and hypoxia-induced reporter activity, whereas the 143A, 247I, 273P, and L22Q/W23S mutants failed to do so. The apparent activation of the CA9 promoter in the presence of MC is due to the effect of MC on the internal control pRL-tk. In the HCT 116 p53−/− cell line, MC treatment downregulated activity of the thymidine kinase promoter in a p53-independent manner. Thus, downregulation of the internal control, not transactivation of the CA9 promoter construct, increased the ratio of firefly activity to Renilla activity in the presence of MC. Unlike the previous report (7), we observed the loss of repressing activity on CA9 promoter activity with all transcriptionally impaired p53 mutants, not only the double mutant in the transactivation domain. These results with ectopically expressed p53 confirm that wt p53 indeed confers a strong negative regulation on the CA9 promoter. Transcriptionally impaired mutant p53s were neutral in respect to CA9 promoter function, indicating that transcriptional transactivational activity of p53 is required for the inhibitory effect of p53 on HIF-1-driven transcription. However, as noted above, this is not a direct effect on transcriptional activation of the HIF-1α promoter itself.
FIG. 8.
The effect of cotransfected wt and mutant p53 on CA9 promoter activity in HCT 116 p53−/− cells. Cells were cotransfected with the [−173, +31] CA9 reporter construct, pRL-tk, and 10 ng of pCEP4 (EV) or a construct expressing wt or mutant p53 as in Fig. 2. CA9 promoter activity in the presence of EV under normoxic conditions was set as 1, and the rest is expressed as the level of induction relative to the control.
DISCUSSION
The fact that the tumor suppressor p53 is the most commonly mutated tumor suppressor gene in human cancers underscores the importance of impairing its function in tumorigenesis (20). Before developing their full potential, tumor cells have to escape p53-imposed controls at the level of cell cycle checkpoints and apoptosis (46). The p53 network is normally in an “off” mode and it becomes activated only when cells are damaged or stressed (46). Thus, p53 can be activated by DNA damage, aberrant growth signals from oncogenes, and chemotherapeutic drugs (46). p53 activation was implicated in hypoxia-induced apoptosis (17), but its inadequate transcriptional activation led to separating transcriptional repression and apoptotic function from p53-dependent transactivation (33). However, a more recent report concludes that induction of functionally impaired p53 is not necessarily a hypoxia-specific event but rather the consequence of inhibition of DNA replication (18). These studies, together with the other recently published reports that differ with respect to the effect of p53 status on HIF-1 activity, as well as expression of hypoxia-inducible genes (1, 7, 18, 19, 40, 41), prompted us to seek further elucidation of the role p53 plays in regulation of HIF-1-dependent transcription. To this end, we investigated expression patterns of the hypoxia marker CAIX in cell lines differing in p53 status.
We found that hypoxia-induced CAIX expression was independent of p53 status, as no difference was observed between MCF-7 (wt p53) and Saos-2 (p53-null) cells. The [−46, +14] CA9 promoter construct was also readily inducible by hypoxia in both cell lines. To verify whether the higher inducibility of the CA9 promoter observed in Saos-2 cells is indeed due to the absence of p53 in these cells or some other cell-type-specific differences, we used the pair of isogenic cell lines HCT 116 p53+/+ and HCT 116 p53−/− (10). Although neither of the cell lines shows endogenous CAIX expression, exogenous CA9 promoter activity via transfection was inducible by hypoxia in both cell lines, and in p53-null cells it was 1.5-fold higher than in the wt-p53-containing counterparts. Based on these results, it appears that hypoxia activates CAIX expression regardless of p53 status, the only difference being a slightly higher activation of the CA9 promoter in p53-null cells.
In addition to other stimuli of p53 activation, it has been proposed that hypoxia stabilizes p53 (14, 18, 33). Although phosphorylation of p53 at S15 and S37 was observed in RKO and 293T cells exposed to severe hypoxic conditions (0.02% O2) or DFO (18), it was also shown that hypoxia-induced phosphorylated p53 is transcriptionally impaired (5, 33). Even under conditions where p53 was stabilized and phosphorylated, it did not have any effect on HIF-1α stability (18). In our studies, although we did observe some increase in stabilization of the p53 protein and phosphorylation at S15 and S20 in DFO-treated MCF-7 cells, this was approximately 20-fold less than in MC-induced cells. Compared to control cells that have no activated p53 (46), p53 activation in cells exposed to hypoxia or hypoxia mimic is barely detectable and appears insufficient for interfering with activation of hypoxia-dependent transcription. However, activation of p53 by MC inflicted DNA damage potently repressed hypoxia-induced CAIX expression in wt p53 MCF-7 cells but not in p53-null Saos-2 cells. MC treatment in MCF-7 cells elicited considerable stabilization and phosphorylation of wt p53 at S15 and S20.
The HRE within the CA9 promoter was identified as the primary target of MC-mediated inhibition of CA9 expression. Furthermore, we showed that MC treatment decreased the steady-state levels of HIF-1α protein in MCF-7 but not in Saos-2 cells. The decreased level of HIF-1α protein appears to be responsible for downregulation of CA9 transcription following MC-inflicted DNA damage as this can be relieved by HIF-1α overexpression. The mechanism responsible for decreased HIF-1α levels in cells with activated p53 is accelerated targeting of HIF-1α protein for proteasome-mediated degradation, as this decrease was not observed in the presence of the proteasome inhibitor LLnV. The combination of the proteasome inhibitor LLnV, MC, and hypoxic conditions resulted in accumulation of HIF-1α protein in the insoluble fraction, but the total amounts in soluble and insoluble fractions combined, both in the presence of LLnV alone and LLnV and MC, were approximately the same. Accumulation of HIF-1 α protein in the detergent-insoluble fraction has been described previously under the conditions of a combination of proteasome and Hsp90 inhibition (21). This report, together with our observation, may indicate a general tendency of HIF-1 α to aggregate into detergent-insoluble complexes due to proteasome inhibition and the resulting higher HIF-1 α concentration. This tendency may be further aggravated in the presence of MC that may influence the insolubility of HIF-1 α under these conditions.
Observations made in MCF-7 and Saos-2 cells were again confirmed in hypoxic HCT 116 p53+/+ and HCT 116 p53−/− cells, where the inhibitory effect of MC treatment on CA9 promoter activity and steady-state levels of HIF-1 α were also dependent on sufficient activation of p53. Together, these results demonstrate that, upon stabilization and activation, wt p53 is capable of interfering with hypoxic induction of CAIX via inhibiting HIF-1 activity due to increased proteasome-mediated proteolytic degradation of the HIF-1α protein. Thus, these results suggest the existence of another mechanism of regulating HIF-1α protein levels via proteasomal degradation. Another intriguing aspect of these results is the localization of the HIF-1α protein to the insoluble fraction following DNA damage, in the presence of proteasome inhibitors. The functional importance of the observed insolubility of the HIF-1α protein is currently under investigation.
Our observations are at variance with the previously published conclusions about regulation of tumor angiogenesis by p53 in the same model of HCT 116 p53+/+ and p53−/− cells (40). The authors of that study observed decreased levels of HIF-1 α protein in p53+/+ (compared to p53−/−) cells in which p53 was not activated (40). However, no differences in HIF-1α levels between HCT 116 p53+/+ and p53−/− cells were observed in another recent report (1). The reason for these discrepancies is not clear; even though the control HCT 116 cells contain relatively high levels of p53, this p53 is not activated as concluded from the absence of phosphorylation at S15. In all cell lines tested in this study, p53 had to be activated by the DNA damaging agent MC before its inhibitory effect on HIF-1α could be manifested.
The authors of another study came to the conclusion that p53 inhibits hypoxia-inducible transcription because of competition for the transcriptional coactivator p300, despite the fact that overexpression of p300 did not relieve the inhibition (7). However, regulation at the HIF-1 level was not considered and HIF-1α levels (mRNA, protein) were not measured. We also tested the possible role of p300 in p53-mediated inhibition of hypoxia-inducible transcription. We found that activation of p53 had no effect on p300 levels in MCF-7 cells, and overexpression of p300 did not overcome the inhibitory effect of p53 on transcription from the CA9 promoter (data not shown). This suggests that, at least in MCF-7 cells, p300 function is not inhibited by p53 and the inhibitory effect of p53 is exerted at the level of degradation of HIF-1α protein. These authors also reported that the 273H p53 mutant still repressed transcription driven by the erythropoietin HRE and only the L22Q/W23S double mutant was defective (7). In our study, neither of the transcriptionally incapacitated p53 mutants (mutated in the DNA binding or transactivation domain) was able to interfere with hypoxic induction of the CA9 promoter.
To date, no systematic typing of the p53 status of CAIX-expressing tumors has been performed. Available data on CAIX expression in various cell lines do not support the notion that CAIX expression associates with a particular p53 status. In one study the most efficient CAIX-expressing cell lines (U373MG, HCT-15, and HT-29) were deficient in p53 function (23), whereas in another study a number of wt-p53-harboring cell lines (A549, U2-OS, and HBL-100) readily expressed CAIX (48). Very little difference in CAIX expression level was also observed in the breast cancer cell lines MDA-MB-231 (mutant p53) and ZR-75.1 (wt p53) (49).
A number of conflicting studies on the effects of p53 on the transcriptional activation of another hypoxia-regulated gene, coding for the vascular endothelial growth factor (VEGF), have been published. In an early report, wt p53 was shown not to repress hypoxia-induced transcription of VEGF (2). On the other hand, a link between an impaired p53/MDM-2 pathway and increased VEGF expression was observed in angiosarcomas (52). The loss of p53 function in tumor cells was postulated to enhance HIF-1 α levels and augment HIF-1-dependent transcriptional activation of VEGF (40). At least two other reports showed that wt p53 can repress VEGF expression by modulating the transcriptional activity of the SP1 factor (38, 51), suggesting that HRE/HIF-1 may not always be the primary p53 target in VEGF transcriptional regulation and pointing out alternative mechanisms for p53-mediated downregulation of VEGF.
Our preliminary results with HREs from the lactate dehydrogenase A and erythropoietin genes (data not shown) suggest that the p53-dependent inhibitory effect observed with the CA9 HRE is general and, therefore, MC treatment will result in downregulation of activation of other hypoxia-inducible genes in a wt-p53-dependent manner.
The importance of elucidating the relationship between p53 and CAIX is obvious. In this and earlier studies (30, 35) we examined whether p53 can modulate induction of CAIX expression. Even though we did not find an inhibitory relationship between the nonactivated p53 and CAIX expression, we did observe a considerable inhibitory effect of activated wt p53 on CAIX expression. Modalities used for cancer therapy frequently employ DNA-damaging agents (e.g., chemotherapy or ionizing radiation) that generally induce p53. This means that the efficacy of CAIX (and possibly other HIF-1-dependent gene products)-targeted immunotherapy of tumors (13, 26) or HRE-driven expression constructs for cancer gene therapy (39) would be seriously compromised in wt-p53-containing tumors treated with DNA-damaging agents. Given the emerging consensus on the critical role of HIF-1 in tumor progression, new therapeutic approaches selectively targeting HIF-1 activity are being developed (19, 42). On the basis of observations presented in this report, it seems reasonable to speculate that the inhibitory effect of activated p53 on HIF-1 activity could be, at least in part, responsible for the well-documented antitumor effects of DNA-damaging agents.
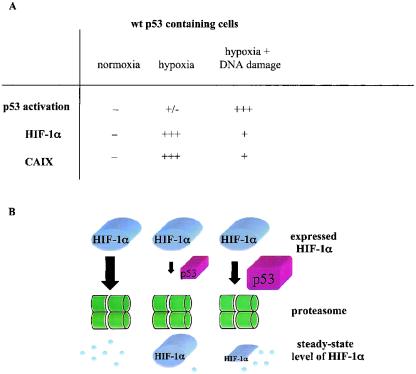
Our understanding of the p53/HIF-1α/CAIX relationship is outlined in Fig. 9. Under normoxic conditions, both HIF-1α and p53 are rapidly degraded and there is no CAIX expression. Hypoxic conditions (0.5 to 1.0% O2) stabilize HIF-1α and activate HIF-1-dependent expression of CAIX. Under these conditions, p53 levels and activation are insufficient to exert an inhibitory effect on this process. Hypoxic cells treated with a DNA-damaging agent have high levels of activated p53, and consequently this p53 accelerates proteasome-mediated degradation of HIF-1α. These cells, therefore, have much lower steady-state levels of HIF-1α and CAIX. It should be noted that more extreme levels of hypoxia (≤0.02% O2) in the absence of DNA damage stabilize p53 but it is transcriptionally impaired and does not affect HIF-1α levels (5, 33).
FIG. 9.
(A) Quantitative relationship between wt p53, HIF-1α, and CAIX under various conditions. The symbols + and − represent relative amounts of protein. (B) Outline of the proposed mechanism responsible for downregulation of HIF-1α protein by activated wt p53. Final steady-state levels of HIF-1 α protein reflect relative efficiency of the proteasome-mediated degradation of HIF-1α under various conditions. Under normoxia, there is no p53 stabilization and activation; HIF-1α becomes hydroxylated, ubiqitylated, and rapidly degraded. Hypoxia inhibits hydroxylation, and therefore large amounts of HIF-1α escape degradation. A small amount of activated p53, induced by strong hypoxia, is unable to interfere significantly with stabilization of HIF-1α and function of HIF-1. Under hypoxic conditions in the presence of DNA-damaging agent high levels of stabilized and activated p53 are induced. This p53 is capable of promoting proteasome-mediated degradation of HIF-1α that results in lower steady-state levels of HIF-1α and lowered HIF-1 activity.
Our data are consistent with the notion that even extreme conditions of hypoxia, which regionally exist in solid tumors, are likely to result only in stabilization of transcriptionally impaired p53. Although this may play some role in apoptosis induction (17), it is unlikely to affect the stabilization of HIF-1α protein and HIF-1-dependent activation of target genes, such as CA9 and VEGF, etc. Our studies indicate that the critical element involved in downregulation of HIF-1α under hypoxic conditions is activation of p53, by DNA damage or some other stress-related events. The mechanism by which this p53-mediated degradation occurs is currently under investigation. An attractive candidate for this mechanism is Mdm2, an E3 ubiquitin ligase that has been shown to target p53 (34) and other proteins (16) for proteasomal degradation. Furthermore, HIF-1α and Mdm2 proteins have been reported to directly interact (12). In preliminary studies where we tested the involvement of Mdm2 in proteasomal degradation of HIF-1α by overexpressing dominant-negative forms of Mdm2 in MCF-7 cells in the presence of MC we saw no obvious effects (data not shown). Thus, the mechanism of p53-mediated proteasomal degradation of HIF-1α remains unknown and is the subject of further studies.
Acknowledgments
This study was supported by a grant from the California Cancer Research Program (00-00789V-20240) and The Avon Foundation. M.I.L. was supported by funds from the National Cancer Institute under contract N01-CO-56 000.
We thank J. Pastorek and S. Pastorekova for the CAIX antibody. We also thank B. Vogelstein for the PG13-Luc construct and for the human colon cancer cell lines HCT 116 p53+/+ and HCT 116 p53−/−. The L22Q/W23S mutant p53 cDNA was a kind gift from J. K. Nyborg, and the pCEP-HIF-1α construct was kindly provided by D. Theodorescu. We also acknowledge K. Flick for assistance with real-time PCR.
REFERENCES
- 1.Achison, M., and T. R. Hupp. 2003. Hypoxia attenuates the p53 response to cellular damage. Oncogene 22:3431-3440. [DOI] [PubMed] [Google Scholar]
- 2.Agani, F., D. G. Kirsch, S. L. Friedman, M. B. Kastan, and G. L. Semenza. 1997. p53 does not repress hypoxia-induced transcription of the vascular endothelial growth factor gene. Cancer Res. 57:4474-4477. [PubMed] [Google Scholar]
- 3.Airley, R. E., J. Loncaster, J. A. Raleigh, A. L. Harris, S. E. Davidson, R. D. Hunter, C. M. L. West, and I. J. Stratford. 2003. Glut-1 and CAIX as intrinsic markers of hypoxia in carcinoma of the cervix: relationship to pimonidazole binding. Int. J. Cancer 104:85-91. [DOI] [PubMed] [Google Scholar]
- 4.Arany, Z., L. E. Huang, R. Eckner, S. Bhattacharya, C. Jiang, M. A. Goldberg, H. F. Bunn, and D. M. Livingston. 1996. An essential role for p300/CBP in the cellular response to hypoxia. Proc. Natl. Acad. Sci. USA 93:12969-12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashcroft, M., Y. Taya, and K. H. Vousden. 2000. Stress signals utilize multiple pathways to stabilize p53. Mol. Cell. Biol. 20:3224-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai, J., and A. I. Cederbaum. 2003. Catalase protects HepG2 cells from apoptosis induced by DNA-damaging agents by accelerating the degradation of p53. J. Biol. Chem. 278:4660-4667. [DOI] [PubMed] [Google Scholar]
- 7.Blagosklonny, M. V., W. G. An, L. Y. Romanova, J. Trepel, T. Fojo, and L. Neckers. 1998. p53 inhibits hypoxia-inducible factor-stimulated transcription. J. Biol. Chem. 273:1995-11998. [DOI] [PubMed] [Google Scholar]
- 8.Brown, J. M., and A. J. Giaccia. 1998. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 58:1408-1416. [PubMed] [Google Scholar]
- 9.Bui, M. H. T., D. Seligson, K. Han, A. J. Pantuck, F. J. Dorey, Y. Huang, S. Horvath, B. C. Leibovich, S. Chopra, S.-Y. Liao, E. Stanbridge, M. I. Lerman, A. Palotie, R. A. Figlin, and A. S. Belldegrun. 2003. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin. Cancer Res. 9:802-811. [PubMed] [Google Scholar]
- 10.Bunz, F., A. Dutriaux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy, K. W. Kinzler, and B. Vogelstein. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science (Washington, D.C.) 282:1497-1501. [DOI] [PubMed] [Google Scholar]
- 11.Chen, X., L. J. Ko, L. Jayaraman, and C. Prives. 1996. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 10:2438-2451. [DOI] [PubMed] [Google Scholar]
- 12.Chen, D., M. Li, J. Luo., and W. Gu. 2003. Direct interactions between HIF-1α and mdm2 modulate p53 function. J. Biol. Chem. 278:13595-13598. [DOI] [PubMed] [Google Scholar]
- 13.Divgi, C. R., N. H. Bander, A. M. Scott, J. A. O'Donoghue, G. Sgouros, S. Welt, R. D. Finn, F. Morrissey, P. Capitelli, J. M. Williams, D. Deland, A. Nakhre, E. Oosterwijk, S. Gulec, M. C. Graham, S. M. Larson, and L. J. Old. 1998. Phase I/II radioimmunotherapy trial with iodine-131-labeled monoclonal antibody G250 in metastatic renal carcinoma. Clin. Cancer Res. 4:2729-2739. [PubMed] [Google Scholar]
- 14.Giaccia, A. J., and M. B. Kastan. 1998. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 12:2973-2983. [DOI] [PubMed] [Google Scholar]
- 15.Giatromanolaki, A., M. I. Koukourakis, E. Sivridis, J. Pastorek, C. C. Wykoff, K. C. Gatter, and A. L. Harris. 2001. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 61:7992-7998. [PubMed] [Google Scholar]
- 16.Girnita, L., A. Girnita, and O. Larsson. 2003. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc. Natl. Acad. Sci. USA 100:8247-8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graeber, T. G., C. Osmanian, T. Jacks, D. E. Housman, C. J. Koch, S. W. Lowe, and A. J. Giaccia. 1996. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumors. Nature 379:88-91. [DOI] [PubMed] [Google Scholar]
- 18.Hammond, E. M., N. C. Denko, M. J. Dorie, R. T. Abraham, and A. J. Giaccia. 2002. Hypoxia links ATR and p53 through replication arrest. Mol. Cell. Biol. 22:1834-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris, A. L. 2002. Hypoxia-a key regulatory factor in tumor growth. Nat. Rev. Cancer 2:38-47. [DOI] [PubMed] [Google Scholar]
- 20.Hollstein, M., D. Sidransky, B. Vogelstein, and C. C. Harris. 1991. p53 mutations in human cancers. Science 253:49-53. [DOI] [PubMed] [Google Scholar]
- 21.Isaacs, J. S., Y.-J. Jung, E. G. Mimnaugh, A. Martinez, F. Cuttita, and L. M. Neckers. 2002. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1α-degradative pathway. J. Biol. Chem. 277:29936-29944. [DOI] [PubMed] [Google Scholar]
- 22.Ivan, M., K. Kondo, H. Yang, W. Kim, J. Valiando, M. Ohh, A. Salic, J. M. Asara, W. S. Lane, and W. G. Kaelin. 2001. HIF α targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464-468. [DOI] [PubMed] [Google Scholar]
- 23.Ivanov, S., S.-Y., Liao, A. Ivanova, A. Danilkovitch-Miagkova, N. Tarasova, G. Weirich, M. J. Merril, M. A. Proescholdt, E. H. Oldfield, J. Lee, J. Závada, A. Waheed, W. Sly, M. I. Lerman, and E. J. Stanbridge. 2001. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am. J. Pathol. 158:905-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivanov, S. V., I. Kuzmin, M.-H. Wei, S. Pack, L. Geil, B. E. Johnson, E. J. Stanbridge, and M. I. Lerman. 1998. Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc. Natl. Acad. Sci. USA 95:12596-12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaakkola, P., D. R. Mole, Y.-M. Tian, M. I. Wilson, J. Gielbert, S. J. Gaskell, A. von Kriegsheim, H. F. Hebestreit, M. Mukherji, C. J. Schofield, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. 2001. Targeting of HIF α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468-472. [DOI] [PubMed] [Google Scholar]
- 26.Jongmans, W., K. van den Oudenalder, D. M. Tiemessen, J. Molkenboer, R. Willemsen, P. F. A. Mulders, and E. Oosterwijk. 2003. Targeting of adenovirus to human renal cell carcinoma cells. Urology 62:559-565. [DOI] [PubMed] [Google Scholar]
- 27.Kaluz, S., M. Kaluzová, R. Opavský, S. Pastoreková, A. Gibadulinová, F. Dequiedt, R. Kettmann, and J. Pastorek. 1999. Transcriptional regulation of the MN/CA 9 gene coding for the tumor-associated carbonic anhydrase IX. Identification and characterization of a proximal silencer element. J. Biol. Chem. 274:32588-32595. [DOI] [PubMed] [Google Scholar]
- 28.Kaluz, S., M. Kaluzová, A. Chrastina, P. L. Olive, S. Pastoreková, J. Pastorek, M. I. Lerman, and E. J. Stanbridge. 2002. Lowered oxygen tension induces expression of the hypoxia marker MN/carbonic anhydrase IX in the absence of hypoxia-inducible factor 1α stabilization: a role for phosphatidylinositol 3′-kinase. Cancer Res. 62:4469-4477. [PubMed] [Google Scholar]
- 29.Kaluz, S., M. Kaluzová, and E. J. Stanbridge. 2003. Expression of the hypoxia marker carbonic anhydrase IX is critically dependent on SP1 activity. Identification of a novel type of hypoxia-responsive enhancer. Cancer Res. 63:917-922. [PubMed] [Google Scholar]
- 30.Kaluzová, M., S. Pastoreková, J. Pastorek, and S. Kaluz. 2000. P53 tumour suppressor modulates transcription of the TATA-less gene coding for the tumour-associated carbonic anhydrase MN/CAIX in MaTu cells. Biochim. Biophys. Acta 1491:20-26. [DOI] [PubMed] [Google Scholar]
- 31.Kaluzová, M., S. Pastoreková, E. Svastová, J. Pastorek, E. J. Stanbridge, and S. Kaluz. 2001. Characterization of the MN/CA 9 promoter proximal region-a role for SP and AP1 factors. Biochem. J. 359:669-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kern, S. E., J. A. Pietenpol, S. Thiagalingam, A. Seymour, K. W. Kinzler, B. Vogelstein. 1992. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science 256:827-830. [DOI] [PubMed] [Google Scholar]
- 33.Koumenis, C., R. Alarcon, E. Hammond, P. Suthpin, W. Hoffman, M. Murphy, J. Derr, Y. Taya, S. W. Lowe, M. Kastan, and A. J. Giaccia. 2001. Regulation of p53 by hypoxia: dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol. Cell. Biol. 21:1297-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubbutat, M. H., S. N. Jones, and K. H. Vousden. 1997. Regulation of p53 stability by Mdm2. Nature 387:299-303. [DOI] [PubMed] [Google Scholar]
- 35.Lieskovská, J., M. Kaluzová, R. Opavský, S. Kaluz, J. Pastorek, R. Kettmann, and S. Pastoreková. 1998. Up-regulation of p53 by antisense expression of HPV 18 E6 oncogene does not influence the level of MN/CA IX tumor-associated protein in HeLa cervical carcinoma cells. Int. J. Oncol. 13:1081-1086. [DOI] [PubMed] [Google Scholar]
- 36.Loncaster, J. A., A. L. Harris, S. E. Davidson, J. P. Logue, R. D. Hunter, C. C. Wykoff, J. Pastorek, P. J. Ratcliffe, I. J. Stratford, and C. M. L. West. 2001. Carbonic anhydrase (CAIX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 61:6394-6399. [PubMed] [Google Scholar]
- 37.Maxwell, P. H., M. Wiesener, G-W. Chang, S. Clifford, E. Vaux, M. Cockman, C. C. Wykoff, C. Pugh, E. Maher, and P. J. Ratcliffe. 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature (London) 399:271-275. [DOI] [PubMed] [Google Scholar]
- 38.Pal, S., K. Datta, and D. Mukhopadhyay. 2001. Central role of p53 on regulation of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in mammary carcinoma. Cancer Res. 61:6952-6957. [PubMed] [Google Scholar]
- 39.Post, D. E., and E. G. van Meir. 2001. Generation of bi-directional hypoxia/HIF-responsive expression vectors to target gene expression to hypoxic cells. Gene Ther. 8:1801-1807. [DOI] [PubMed] [Google Scholar]
- 40.Ravi, R., B. Mookerjee, Z. M. Bhujwalla, C. H. Sutter, D. Artemov, Q. Zeng, L. E. Dillehay, A. Madan, G. L. Semenza, and A. Bedi. 2000. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev. 14:34-44. [PMC free article] [PubMed] [Google Scholar]
- 41.Salnikow, K., M. Costa, W. D. Figg, and M. V. Blagosklonny. 2000. Hyperinducibility of hypoxia-responsive genes without p53/p21-dependent checkpoint in aggressive prostate cancer. Cancer Res. 60:5630-5634. [PubMed] [Google Scholar]
- 42.Semenza, G. L. 2003. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer. 3:721-732. [DOI] [PubMed] [Google Scholar]
- 43.Sheta, E. A., H. Trout, J. J. Gildea, M. A. Harding, and D. Theodorescu. 2001. Cell density mediated pericellular hypoxia leads to induction of HIF-1α via nitric oxide and Ras/MAP kinase mediated signaling pathways. Oncogene 20:7624-7634. [DOI] [PubMed] [Google Scholar]
- 44.Stanbridge, E. J. 1981. Mycoplasma detection-an obligation to scientific accuracy. Isr. J. Med. Sci. 17:563-568. [PubMed] [Google Scholar]
- 45.Thomlinson, R., and L. Gray. 1955. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br. J. Cancer. 9:539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogelstein, B., D. Lane, and A. J. Levine. 2000. Surfing the p53 network. Nature 408:307-310. [DOI] [PubMed] [Google Scholar]
- 47.Wang, G. L., B. H. Jiang, E. A. Rue, and G. L. Semenza. 1995. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92:5510-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wykoff, C. C., N. J. Beasley, P. H. Watson, K. J. Turner, J. Pastorek, A. Sibtain, G. D. Wilson, H. Turley, K. L. Talks, P. H. Maxwell, C. W. Pugh, P. J. Ratcliffe, and A. L. Harris. 2000. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 60:7075-7083. [PubMed] [Google Scholar]
- 49.Wykoff, C. C., N. J. Beasley, P. H. Watson, L. Campo, S. K. Chia, R. English, J. Pastorek, W. S. Sly, P. J. Ratcliffe, and A. L. Harris. 2001. Expression of the hypoxia-inducible and tumor-associated carbonic anhydrases in ductal carcinoma in situ of the breast. Am. J. Pathol. 158:1011-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Závada, J., Z. Závadová, S. Pastoreková, F. Ciampor, J. Pastorek, and V. Zelník. 1993. Expression of MaTu-MN protein in human tumor cultures and in clinical specimens. Int. J. Cancer. 54:268-274. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, L., D. Yu, M. Hu, S. Xiong, A. Lang, L. M. Ellis, and R. E. Pollock. 2000. Wild-type p53 suppresses angiogenesis in human leiomyosarcoma and synovial sarcoma by transcriptional suppression of vascular endothelial growth factor expression. Cancer Res. 60:3655-3661. [PubMed] [Google Scholar]
- 52.Zietz, C., M. Rossle, C. Haas, A. Sendelhofert, A. Hirschmann, M. Sturzl, and U. Lohrs. 1998. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am. J. Pathol. 153:1425-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]