Abstract
Background:
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system. Etiology of the disease is not well understood; however, it is more common in women than in men and occurs mainly during reproductive age. The aim of this study was to evaluate some risk factors in women of childbearing age with MS in Isfahan Province.
Methods:
This analytic case–control study was conducted in MS Clinic in Isfahan, 2014. The study was done on 200 patients with MS and 200 nonpatients (matched controls) that were randomly selected for inclusion in the study. The data collection tool was a researcher-designed questionnaire consisting of three parts: Demographics, disease characteristics, and some risk factors related to reproductive age. Data were analyzed by SPSS version 20, using descriptive and inferential statistics.
Results:
The results showed that risk of MS had a significant relationship with age at menarche (P < 0.001), prior use of oral contraceptives (OCs) (P = 0.002), duration of use of OCs (P = 0.008), and number of pregnancies (P < 0.001). However, there was no significant relationship between age of onset of use of OCs (P = 0.80) and age at the first pregnancy (P = 0.45) with the risk of MS.
Conclusions:
Results of this research determined that the following risk factors were associated with developing MS, age at menarche, history, and duration of use of OCs and number of pregnancies.
Keywords: Menarche, multiple sclerosis, oral contraceptive, pregnancy, reproductive age, women
INTRODUCTION
A type of frequent neurological disability caused by a chronic inflammatory and progressive demyelination of the central nervous system (CNS) is called multiple sclerosis (MS),[1] afflicting over 2.5 million young adults worldwide.[2] In the past, Iran was considered a region with low incidence of MS, but the number of patients has increased over recent decades.[3] At the 10th International Congress of MS, the number of MS cases was reportedly 37,655 people (49.2/100,000) with the highest prevalence in Isfahan, of about
85.5 people/100,000.[4] According to these results, Isfahan ranks first in terms of prevalence and incidence of MS in Asia.[5] MS is a complex neurological disease that has a strong tendency to affect women.[6] The women:men ratio of people affected by MS is 10:3.[7] The most common time for emergence of the disease is during the second and third decade of a person's life between the ages of 25-30 years.[7] Only rarely is MS contracted before puberty.[8,9] Incidence of the disease increases dramatically after puberty,[8,9,10,11,12] and the sex ratio also increases; when onset of the disease occurs between the ages of 13-18 years old, the female: male ratio reaches 2.2:1.[8,9,10] Complex reactions between genetic and environmental factors are involved in the etiology of MS.[13,14,15] The question that guided this research was: Do events at childbearing age (including puberty), such as using contraceptive drugs, pregnancy at an older age, and multiple pregnancies make women more susceptible to MS? Puberty is a complex biological phenomenon influenced by genetic, nutritional, environmental, economic, and socioeconomic factors.[16] Puberty also involves significant changes in the brain.[17] During puberty, the volume of white matter in the brain increases linearly. There are also changes in gray matter volume, first an increase and then a decrease over time.[18] Gender differences are triggered by sex hormones producing basal immune responses, and thus their important roles in the immune system are corroborated. Higher antibody responses to antigens, especially those resulted from more intense activities of T-cells, are caused by higher immunoglobulin levels in women.[19] Studies show an increased risk of MS in cases of lower age at menarche and appearance of the first signs of puberty at a younger age.[20,21,22,23] Regarding the use of oral contraceptives (OCs), no significant effect was determined on the risk of MS.[24] There was no definitive clinical data to demonstrate the beneficial effect of OC use on the course and progression of MS; however, possible delay in the onset of clinical symptoms of MS has been observed.[25] Regarding the effects of childbirth and pregnancy, the risk of MS among women in their first pregnancy has been reported as upper than in women who have had multiple pregnancies.[26] Even though, many studies have reported no association between the risk of MS and pregnancy or childbirth.[24,26,27] However, childbirth postpones MS to a later age, and this suggests that it has a protective effect.[28] Furthermore, pregnancy in patients with MS is associated with decreased neurological symptoms and improved quality of life; these changes are more obvious during the third trimester compared with the first trimester of pregnancy.[29] Research has shown an increased risk of MS 6 months after delivery.[27,29,30] In general, the relationship between reproductive risk factors and MS has not been studied extensively. However, the frequency of infertility is higher in women with MS than among the general population.[26] This may be associated with postponed pregnancy or concerns about the risk of disease transmission.[31] On the assumption that childbearing incidents such as puberty, pregnancy at later age, number of pregnancies, and OC use in women are related to the risk of developing MS, and given that no research has focused on impacts of childbearing age variables such as puberty, use of OCs, pregnancy at later age, and number of pregnancies on incidence of MS in women. Given the importance of the high prevalence of MS in Isfahan, the aim of this study was to assess correlations of some childbearing age variables with risk factor for MS in patients referred to the MS Clinic of Isfahan.
METHODS
Study design and participants
This analytic case–control study was approved by School of Health, Isfahan University of Medical Sciences, under project number 393260 and was conducted in Al-Zahra Hospital in 2014 on 400 women (200 patients and 200 controls). Patients who were referred to the MS clinic of the hospital whose disease had been confirmed by diagnostic studies such as magnetic resonance imaging and approved by the Neurology Committee of the Hospital using standard MS[32] were considered for inclusion in the study, and women who were referred to different wards of the hospital (other than psychiatry, obstetrics, and gynecology to avoid confounding factors) were selected as the control group. It is known that climate affects the onset of puberty.[34] Subjects in the control group were matched with patients according to area of residence. The province was divided into cold and tropical climates, and sampling was conducted from both types. Criteria common to both groups in the study were age range of women 15–50 years; all were residents of Isfahan Province, had consented to give their information, and lack of gynecologic disease.
Inclusion criteria for patients were as follows: Absence of acute conditions, full consciousness, member of Isfahan's Multiple Sclerosis Society and having medical records in this society, and duration of at least 1 year from the time of diagnosis.
Inclusion criteria for the control group were as follows: Lack of MS or any other neurologic/mental disease.
Study instrument and variables assessment
Information collected in this study included demographic characteristics such as age, marital status, place of residence, disease-related variables, and variables affecting women of childbearing age such as early onset of puberty, use of OCs prior to developing MS, age at the first pregnancy, number of pregnancies prior to MS, family history of MS, other autoimmune disease, and history of a viral disease in childhood. Demographic variables were collected from medical records and other variables related to diseases and reproductive age were recorded during interviews with patients. Participants were first questioned face-to-face in the presence of their parents who had access to patient information. Each questionnaire was completed by the researcher. Assessment of age of onset of puberty was made according to Tanner classification (age at breast growth and pubic hair growth) and age at menarche (first menstruation).[35] Early puberty was thus considered age at menarche in this study. Criterion for age of start of OC use was age of onset of using OCs before developing MS. Duration of OC use was recorded in months and determined by the amount of time that a participant had used OCs before the onset of MS.
Statistical analysis
After collecting information, the data analysis was performed by descriptive analysis and logistic regression tests using (PASW statistics for windows Chicago: SPSS Inc). Logistic regression was used for calculating the odds ratio (OR) and confidence interval (CI) was considered at 95%.
RESULTS
A total of 400 women were enrolled for participation in this study (200 patients and 200 controls). Sixty-eight percent of participants in the patient group and 77% in the control group were married. The mean age of patients was 31.76 ± 8.13 years and in the control group, it was 31.53 ± 8.97 years. The most common type of MS in patients was relapsing/remitting (RR) subtype with the highest frequency was 90%. The most common signs of MS presented in patients were impaired vision (46%) and numbness (38.5%), and mean age of onset of symptoms in the patient group was 26 ± 7.77 years. Seventeen percent of patients and 9.5% of the control group had a positive family history of MS; there was a significant association between family history of MS with developing the disease (P = 0.027). Seventy-five percent of patients had a history of viral disease in childhood that was significantly different between the two groups (P = 0.048). Table 1 shows the distribution of demographic characteristics of patient and control groups. Table 2 shows distribution of reproductive age risk factors including mean age, age at menarche, age at the first pregnancy, history, age and duration of use of OCs, and number of pregnancies in the two groups. Based on results obtained by the independent t-test, a significant correlation was observed between the age at menarche (P < 0.001), prior use of OC before developing MS (P = 0.004), history duration of contraceptive use before developing MS (P = 0.002), and number of pregnancies before MS (P < 0.001) [Table 2].
Table 1.
Frequency (%) of some demographic and health condition characteristics among cases and controls
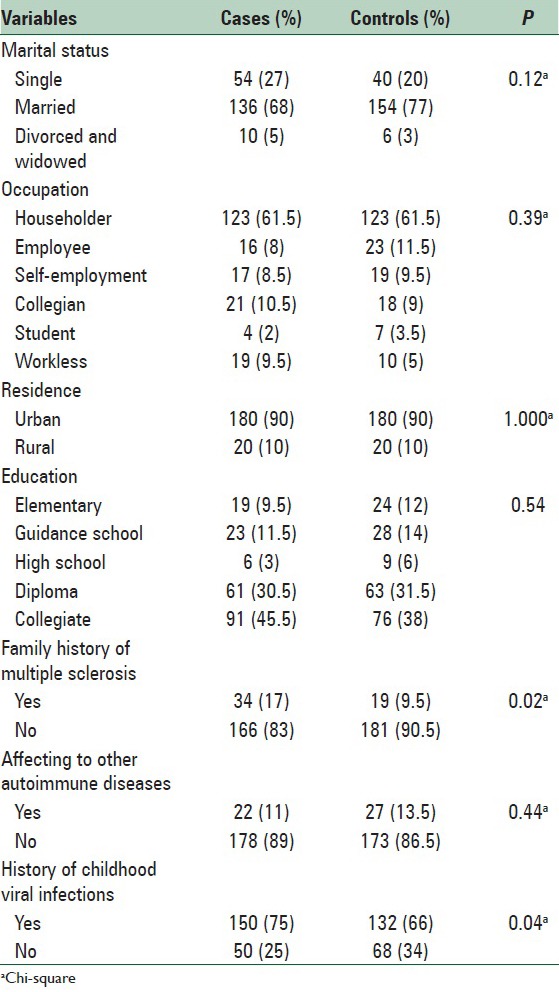
Table 2.
Frequencies of MS risk factors among cases and controls

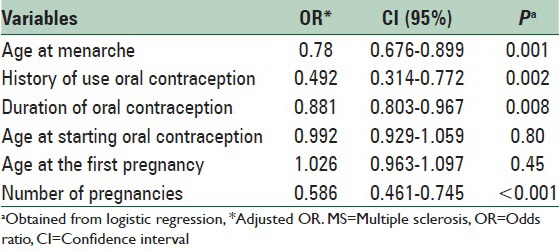
To determine the association between reproductive age, variables such as age at menarche, age of onset of OC use prior to developing MS, duration of use of OCs prior to MS, age at first pregnancy, number of pregnancies before MS and developing MS in women, 6 logistic regression models were separately fitted. The effects of these variables on the risk of MS were adjusted by entering variables such as age, marital status, place of residence, family history of MS, developing other autoimmune diseases, and history of viral diseases in childhood in these models. The results indicate a significant relationship between age of menarche onset and incidence of MS (OR = 0.780, CI = 0.676–0.899) that show an inverse relationship regarding OR. The relationship between use of OCs (OR = 0.492, CI = 0.314–0.772) and duration of use of OCs were statistically significant with the risk of MS (OR = 0.881, CI = 0.803–0.967). This means that consumption and higher duration of using OCs reduced the risk of MS. The relationship between the number of pregnancies and MS was also statistically significant (OR = 0.586, CI = 0.461–0.745), but there was no significant relationship the age at onset of using OCs (P = 0.80) and age at the first pregnancy (P = 0.45) with MS; the results are shown in Table 3.
Table 3.
Summary of MS risk factors in woman
DISCUSSION
The aim of this study was to determine the association between risk factors of reproductive age and the prevalence of MS. Results show that age at menarche was associated with risk of developing MS. Consistent with the results of this research, several other studies have considered age at menarche as an effective risk factor for development of MS.[20,21,22,23] The association of lower age at menarche with an increased risk of MS is possibly due to earlier exposure to hormonal changes during puberty that may affect the onset and course of the disease.[36,37] A higher age at menarche may lead to longer preteenage stage of development and thereby an increased volume of gray matter that increases the storage performance capacity against axonal degeneration levels of estrogen. This hormone has specific effects on the development of CNS.[17] It also affects function of the immune system.[19] Long exposure to this hormone increases the risk of developing an autoimmune disease such as MS.[23,38] A wide age range for onset of puberty is seen among people that may be affected by factors such as genetics, race, diet, environmental exposure, and lifestyle.[39] A meta-analysis of data from Europe suggests that age at menarche has become dramatically lower and this trend has been evident since the late 1800s and continued until 1950, but it seems to have leveled from 1960 to 2010.[40] Therefore, a decrease in age at onset of puberty during the past 100 years, especially in women, might be effective in increasing incidence and gender difference in MS.[37] This issue supports the hypothesis of an association between age at menarche and risk of MS. Potential biological mechanisms for these effects need further investigation.
The effect of OC use on MS has been assessed in the current study among patients and control groups in terms of the history of use, duration of OC use, and age of onset of OC use. According to the logistic regression model, the relationship between prior use of OCs with MS (CI = 0.314–0.772, OR = 0.492) and duration of use of OCs were statistically significant with the risk of MS (CI = 0.803–0.967, OR = 0.881). This means that consumption and higher duration of use of OCs reduced the risk of MS. Inconsistent with the results of this study, research by Alonso et al. reported no effect of OCs as a source of exogenous sex hormones on the risk of MS in many women.[25] Furthermore, inconsistent with the results of this research were those reported by Hernan et al. that suggest an increased risk of developing MS among OC users in the long-term.[27] Consistent with results of this study were those reported by Holmqvist et al. that determined an association between OC use before the onset of MS at a later age that indicated a protective effect against developing MS.[28] Exposure to female hormones at a younger age may have little influence on hormonal balance of androgens is in CNS by interacting with hormone receptors or with hormone synthesis or metabolism.[41] Currently, no clinical data exists to demonstrate a beneficial effect of OC use on the course and progression of MS, except a possible delay in the onset of clinical signs in cases of MS relapse. These findings are contrary to those of other research that report a protective effect of sex steroids on neurons and OC in the field of autoimmune diseases.[24] However, no significant relationship between age at onset of use of OCs was found (OR = 0.99, CI = 0.929–1.059).
In this study, the relationship between the number of pregnancies and MS was statistically significant by logistic regression model (OR = 0.586, CI = 0.461–0.745,), which means that the risk of MS decreased in relation to a greater number of pregnancies. While the results of a study by Runmarker and Andersen show a upper risk of MS in the first childbirth compared with women who had had multiple pregnancies.[26] This was consistent or Aligned to the results of our research. Some studies have reported no association between the risk of MS and pregnancy or childbirth.[24,26,29] Holmqvist et al. study showed an association of number of deliveries with delay in age of onset of MS.[28] Furthermore, Ponsonby et al. study showed a significant reduction in the risk of developing the first demyelination among women that had given birth to more children.[42] This indicates a protective effect of MS and is consistent with the results of this study. In this study, no relationship was determined between woman's age at the first pregnancy (OR = 1.02, CI = 0.963–1.097) with MS, but it should be noted that young women may decide to prevent being pregnant with the onset of the disease and this may have contributed to results in line with those of this current research. According to the available information, a beneficial effect of pregnancy on the risk of inflammatory demyelination may persist, which is probably indicative of hormonal changes.[41] However, experiments have shown that there is a reconstruction of white matter during pregnancy and that supports the results of this study.[43] Although women are considered at higher risk of developing MS than men, women are at lower risk of progression in disease onset, at least during their reproductive age.[41] As far as pathogenesis of progressive degeneration of axons in MS is not well-known, its possible mechanisms are speculative.
The strengths of this research include lack of a similar study in Iran that makes a direct assessment of the relationship between risk factors of reproductive age and MS. The limitations of this study include recall bias, as data of risk factors of reproductive age were collected by a self-reported method. Given the case–control design of the current study, it is logical that it is associated with recall bias and considering that the age at menarche is related to many years before, information may not be reliable, but it should be considered that menstruation is a unique indicator of the process of puberty and studies have demonstrated a strong relationship (r = 0.67–0.79) between remembering the age at menarche in middle age and original childhood data,[44,45] which supports observations that show a real association and not influenced by error. Moreover, this study was conducted in a limited area and with a limited number of patients that thus reduces generalizability of the results; it is therefore suggested that a similar study be done on a larger sample size and in other provinces.
CONCLUSIONS
The results obtained from this study suggest that among the studied reproductive age risk factors, age at menarche, use of OCs, duration of use of OCs, and number of pregnancies can increase the risk of MS. Hence, the results of this research can help to identify causes of the disease and disclose important new insights to prevention of the disease for our community, especially through identification of those at risk. However, it is recommended that further studies be done to evaluate the counter-effect of exposure with risk factors and biological changes around puberty to identify preventive actions against the development and progression of MS.
Financial support and sponsorship
Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This article was extracted from MSc thesis approved by School of Health, Isfahan University of Medical Sciences, under project No. 393260 and it financed by the center. Hereby, authors would like to express special thanks to the Research Council of Isfahan University of Medical Sciences and staff of Isfahan MS Clinic for their help and cooperation. In the end, the authors wish to thank participants who generously participated in this study.
REFERENCES
- 1.Rose AM, Bell LC. Epistasis and immunity: The role of genetic interactions in autoimmune diseases. Immunology. 2012;137:131–8. doi: 10.1111/j.1365-2567.2012.03623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yousem DM, Grossman RI. Mosby: Elsevier Health Sciences; 2010. Neuroradiology: The Requisites. [Google Scholar]
- 3.Elhami SR, Mohammad K, Sahraian MA, Eftekhar H. A 20-year incidence trend (1989-2008) and point prevalence (March 20, 2009) of multiple sclerosis in Tehran, Iran: A population-based study. Neuroepidemiology. 2011;36:141–7. doi: 10.1159/000324708. [DOI] [PubMed] [Google Scholar]
- 4.Etemadifar M, Abtahi SH, Akbari M, Murray RT, Ramagopalan SV, Fereidan-Esfahani M. Multiple sclerosis in Isfahan, Iran: An update. Mult Scler. 2013;20:1145–7. doi: 10.1177/1352458513516531. [DOI] [PubMed] [Google Scholar]
- 5.Etemadifar M, Maghzi AH. Sharp increase in the incidence and prevalence of multiple sclerosis in Isfahan, Iran. Mult Scler. 2011;17:1022–7. doi: 10.1177/1352458511401460. [DOI] [PubMed] [Google Scholar]
- 6.Orton SM, Herrera BM, Yee IM, Valdar W, Ramagopalan SV, Sadovnick AD, et al. Sex ratio of multiple sclerosis in Canada: A longitudinal study. Lancet Neurol. 2006;5:932–6. doi: 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]
- 7.Nedjat S, Montazeri A, Mohammad K, Majdzadeh R, Nabavi N, Nedjat F, et al. Quality of life in multiple sclerosis compared to the healthy population in Tehran. Iran J Epidemiol. 2006;2:19–24. [Google Scholar]
- 8.Banwell B, Kennedy J, Sadovnick D, Arnold DL, Magalhaes S, Wambera K, et al. Incidence of acquired demyelination of the CNS in Canadian children. Neurology. 2009;72:232–9. doi: 10.1212/01.wnl.0000339482.84392.bd. [DOI] [PubMed] [Google Scholar]
- 9.Pohl D, Hennemuth I, von Kries R, Hanefeld F. Paediatric multiple sclerosis and acute disseminated encephalomyelitis in Germany: Results of a nationwide survey. Eur J Pediatr. 2007;166:405–12. doi: 10.1007/s00431-006-0249-2. [DOI] [PubMed] [Google Scholar]
- 10.Ghezzi A, Deplano V, Faroni J, Grasso MG, Liguori M, Marrosu G, et al. Multiple sclerosis in childhood: Clinical features of 149 cases. Mult Scler. 1997;3:43–6. doi: 10.1177/135245859700300105. [DOI] [PubMed] [Google Scholar]
- 11.Pluchinotta FR, Schiavo B, Vittadello F, Martini G, Perilongo G, Zulian F. Distinctive clinical features of pediatric systemic lupus erythematosus in three different age classes. Lupus. 2007;16:550–5. doi: 10.1177/0961203307080636. [DOI] [PubMed] [Google Scholar]
- 12.Descloux E, Durieu I, Cochat P, Vital-Durand D, Ninet J, Fabien N, et al. Influence of age at disease onset in the outcome of paediatric systemic lupus erythematosus. Rheumatology (Oxford) 2009;48:779–84. doi: 10.1093/rheumatology/kep067. [DOI] [PubMed] [Google Scholar]
- 13.Taylor BV. The major cause of multiple sclerosis is environmental: Genetics has a minor role – Yes. Mult Scler. 2011;17:1171–3. doi: 10.1177/1352458511421105. [DOI] [PubMed] [Google Scholar]
- 14.Hutchinson M. Truly benign multiple sclerosis is rare: Let's stop fooling ourselves – Commentary. Mult Scler. 2012;18:15. doi: 10.1177/1352458511431716. [DOI] [PubMed] [Google Scholar]
- 15.Sawcer S. The major cause of multiple sclerosis is environmental: Genetics has a minor role – No. Mult Scler. 2011;17:1174–5. doi: 10.1177/1352458511421106. [DOI] [PubMed] [Google Scholar]
- 16.de Vries L, Kauschansky A, Shohat M, Phillip M. Familial central precocious puberty suggests autosomal dominant inheritance. J Clin Endocrinol Metab. 2004;89:1794–800. doi: 10.1210/jc.2003-030361. [DOI] [PubMed] [Google Scholar]
- 17.Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369:1130–9. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- 18.Giedd JN, Lenroot RK, Shaw P, Lalonde F, Celano M, White S, et al. Trajectories of anatomic brain development as a phenotype. Novartis Found Symp. 2008;289:101–12. doi: 10.1002/9780470751251.ch9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitacre CC. New York, USA: Nature America Inc; 2001. Sex differences in autoimmune disease. [DOI] [PubMed] [Google Scholar]
- 20.Ramagopalan SV, Valdar W, Criscuoli M, DeLuca GC, Dyment DA, Orton SM, et al. Age of puberty and the risk of multiple sclerosis: A population based study. Eur J Neurol. 2009;16:342–7. doi: 10.1111/j.1468-1331.2008.02431.x. [DOI] [PubMed] [Google Scholar]
- 21.Sloka JS, Pryse-Phillips WE, Stefanelli M. The relation between menarche and the age of first symptoms in a multiple sclerosis cohort. Mult Scler. 2006;12:333–9. doi: 10.1191/135248506ms1267oa. [DOI] [PubMed] [Google Scholar]
- 22.Sahebalzamani M, Mehri S, Altafi D. Study of important risk factors for the development of multiple sclerosis in patients admitted to Alavi Hospital of Ardabil. J Ardabil Univ Med Sci. 2012;12:426–36. [Google Scholar]
- 23.Ahn JJ, O’Mahony J, Moshkova M, Hanwell HE, Singh H, Zhang MA, et al. Puberty in females enhances the risk of an outcome of multiple sclerosis in children and the development of central nervous system autoimmunity in mice. Mult Scler. 2015;21:735–48. doi: 10.1177/1352458514551453. [DOI] [PubMed] [Google Scholar]
- 24.El-Etr M, Ghoumari A, Sitruk-Ware R, Schumacher M. Hormonal influences in multiple sclerosis: New therapeutic benefits for steroids. Maturitas. 2011;68:47–51. doi: 10.1016/j.maturitas.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Alonso A, Clark CJ. Oral contraceptives and the risk of multiple sclerosis: A review of the epidemiologic evidence. J Neurol Sci. 2009;286:73–5. doi: 10.1016/j.jns.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 26.Runmarker B, Andersen O. Pregnancy is associated with a lower risk of onset and a better prognosis in multiple sclerosis. Brain. 1995;118(Pt 1):253–61. doi: 10.1093/brain/118.1.253. [DOI] [PubMed] [Google Scholar]
- 27.Hernán MA, Hohol MJ, Olek MJ, Spiegelman D, Ascherio A. Oral contraceptives and the incidence of multiple sclerosis. Neurology. 2000;55:848–54. doi: 10.1212/wnl.55.6.848. [DOI] [PubMed] [Google Scholar]
- 28.Holmqvist P, Hammar M, Landtblom AM, Brynhildsen J. Age at onset of multiple sclerosis is correlated to use of combined oral contraceptives and childbirth before diagnosis. Fertil Steril. 2010;94:2835–7. doi: 10.1016/j.fertnstert.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 29.Alonso A, Jick SS, Olek MJ, Ascherio A, Jick H, Hernán MA. Recent use of oral contraceptives and the risk of multiple sclerosis. Arch Neurol. 2005;62:1362–5. doi: 10.1001/archneur.62.9.1362. [DOI] [PubMed] [Google Scholar]
- 30.Neuteboom RF, Janssens AC, Siepman TA, Hoppenbrouwers IA, Ketelslegers IA, Jafari N, et al. Pregnancy in multiple sclerosis: Clinical and self-report scales. J Neurol. 2012;259:311–7. doi: 10.1007/s00415-011-6186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poser S, Poser W. Multiple sclerosis and gestation. Neurology. 1983;33:1422–7. doi: 10.1212/wnl.33.11.1422. [DOI] [PubMed] [Google Scholar]
- 32.Cavalla P, Rovei V, Masera S, Vercellino M, Massobrio M, Mutani R, et al. Fertility in patients with multiple sclerosis: Current knowledge and future perspectives. Neurol Sci. 2006;27:231–9. doi: 10.1007/s10072-006-0676-x. [DOI] [PubMed] [Google Scholar]
- 33.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 34.Speroff L, Fritz MA. 7th edition. philadelphia, PA 19106; USA: Lippincott Williams and Wilkins; 2005. Clinical Gynecologic Endocrinology and Infertility. [Google Scholar]
- 35.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’hooghe MB, Haentjens P, Nagels G, D’Hooghe T, De Keyser J. Menarche, oral contraceptives, pregnancy and progression of disability in relapsing onset and progressive onset multiple sclerosis. J Neurol. 2012;259:855–61. doi: 10.1007/s00415-011-6267-7. [DOI] [PubMed] [Google Scholar]
- 37.Chitnis T. Role of puberty in multiple sclerosis risk and course. Clin Immunol. 2013;149:192–200. doi: 10.1016/j.clim.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Buck Louis GM, Gray LE, Jr, Marcus M, Ojeda SR, Pescovitz OH, Witchel SF, et al. Environmental factors and puberty timing: Expert panel research needs. Pediatrics. 2008;121(Suppl 3):S192–207. doi: 10.1542/peds.1813E. [DOI] [PubMed] [Google Scholar]
- 39.Dooley MA, Hogan SL. Environmental epidemiology and risk factors for autoimmune disease. Curr Opin Rheumatol. 2003;15:99–103. doi: 10.1097/00002281-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Sørensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A. Recent secular trends in pubertal timing: Implications for evaluation and diagnosis of precocious puberty. Horm Res Paediatr. 2012;77:137–45. doi: 10.1159/000336325. [DOI] [PubMed] [Google Scholar]
- 41.D’hooghe MB, D’Hooghe T, De Keyser J. Female gender and reproductive factors affecting risk, relapses and progression in multiple sclerosis. Gynecol Obstet Invest. 2013;75:73–84. doi: 10.1159/000346319. [DOI] [PubMed] [Google Scholar]
- 42.Ponsonby AL, Lucas RM, van der Mei IA, Dear K, Valery PC, Pender MP, et al. Offspring number, pregnancy, and risk of a first clinical demyelinating event: The AusImmune Study. Neurology. 2012;78:867–74. doi: 10.1212/WNL.0b013e31824c4648. [DOI] [PubMed] [Google Scholar]
- 43.Gregg C, Shikar V, Larsen P, Mak G, Chojnacki A, Yong VW, et al. White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci. 2007;27:1812–23. doi: 10.1523/JNEUROSCI.4441-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Must A, Phillips SM, Naumova EN, Blum M, Harris S, Dawson-Hughes B, et al. Recall of early menstrual history and menarcheal body size: After 30 years, how well do women remember? Am J Epidemiol. 2002;155:672–9. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]
- 45.Casey VA, Dwyer JT, Coleman KA, Krall EA, Gardner J, Valadian I. Accuracy of recall by middle-aged participants in a longitudinal study of their body size and indices of maturation earlier in life. Ann Hum Biol. 1991;18:155–66. doi: 10.1080/03014469100001492. [DOI] [PubMed] [Google Scholar]


