Abstract
Background:
Regarding to the growing prevalence of nonalcoholic fatty liver disease (NAFLD), concentrating on various strategies to its prevention and management seems necessary. The aim of this study was to determine the effects of symbiotic on C-reactive protein (CRP), liver enzymes, and ultrasound findings in patients with NAFLD.
Methods:
Eighty NAFLD patients were enrolled in this randomized, double-blind, placebo-controlled clinical trial. Participants received symbiotic in form of a 500 mg capsule (containing seven species of probiotic bacteria and fructooligosaccharides) or a placebo capsule daily for 8 weeks. Ultrasound grading, CRP, and liver enzymes were evaluated at the baseline and the end of the study.
Results:
In the symbiotic group, ultrasound grade decreased significantly compared to baseline (P < 0.005) but symbiotic supplementation was not associated with changes in alanine aminotransferase (ALT) and aspartate transaminase (AST) levels. In the placebo group, there was no significant change in steatosis grade whereas ALT and AST levels were significantly increased (P = 0.002, P = 0.02, respectively). CRP values remained static in either group.
Conclusions:
Symbiotic supplementation improved steatosis in NAFLD patients and might be useful in the management of NAFLD or protective against its progression.
Keywords: Liver steatosis, nonalcoholic fatty liver disease, symbiotic
INTRODUCTION
Nowadays nonalcoholic fatty liver disease (NAFLD) known as the most common chronic liver disease worldwide and its prevalence continue to rise. Prevalence of NAFLD has been reported between 2.8% and 24% in the general population in different countries.[1,2,3,4,5] NAFLD is reported as the most common reason for elevated alanine aminotransferase (ALT) levels in Iranian population and its prevalence is estimated 7% for children and 35% for adults.[6,7]
Due to the lack of enough scientific evidence, the optimum approach for NAFLD treatment is unclear.[8] There are different therapeutic methods which all of them are based on the modification of underlying etiologic factors.[9,10,11] Increasing evidence shows the influence of the intestinal flora on liver pathology.[12] In the other, NAFLD is associated with increased intestinal permeability that is related to severity of hepatic steatosis[13] and intestinal bacterial overgrowth has been reported in 50% of these patients.[14] In addition, changes in intestinal bacterial flora due to stress or improper eating habits can play an important role in the pathogenesis or progression of NAFLD.[12] Fructooligosaccharids such as inulin, other oligosaccharides, lactulose, resistant starch and dietary fiber (prebiotics), and boost probiotics response. They can enhance the growth of bifidobacteria or lactobacilli and may be helpful in controlling or reduction of harmful bacteria growth.[15]
In several clinical trials, beneficial effects of probiotics have been observed on the animal and human intestinal microbial ecosystem. It seems that liver fat metabolism can be affected by bacteria and potentially by probiotics.[16,17,18,19,20,21] In addition, there is some evidence that shows probiotics have a protective effects on acute liver injury[14] and symbiotic can leading to improvement in both liver inflammation and fibrosis in animal model.[22] Food and Drug Administration identifies probiotics as generally recognized as safe.[23]
To our knowledge, there is no study, which investigated the effect of symbiotic in NAFLD patients. Hence with respect to the high prevalence of the fatty liver disease in our country and lack of accurate data on this patient's condition in Iran and neighboring countries, more researches on treatment of this patients seem necessary. Therefore, in present clinical trial we evaluated the effect of short-term symbiotic supplementation, as a simple, low cost and without side effect treatment component on liver enzymes, C-reactive protein (CRP) and steatosis grade in NAFLD patients.
METHODS
Study design and participants
The protocol of this randomized, double-blind, placebo-controlled clinical trial was approved by the Research Ethics Committee of Isfahan University of Medical Sciences and registered in Iranian Registry of Clinical Trial (IRCT2013122811763N15).
NAFLD volunteers (by ultrasound) were referred to Isfahan Endocrine and Metabolism Research Center and upon meeting the study criteria were enrolled into this study (from March to July 2014). The inclusion criteria included age 18–60 years, no other liver disease (such as hepatitis C, hepatitis B and autoimmune hepatitis; Wilson's disease), no organ transplantation, no inflammatory bowel disease, no self-reported specific disease and malignancies, no pregnancy and lactation, no corticosteroids, amiodarone, tamoxifen, cyclines, perhexiline, methotrexate, hydralazine, laxatives, and oral contraceptive pill medication, no vitamin-mineral, antioxidant, and omega-3 supplementation. Study volunteers were excluded for failure to follow the study's guidelines (<90% compliance, subject's compliance was evaluated by counting the remaining capsules at the end of the 4th and 8th week), and antibiotics therapy during the study.
Based on aspartate transaminase (AST) and ALT as main variables of the study, 34 patients were required in each group (power 80% and α =5%). Considering 20% sample loss, 80 patients were enrolled. Participants were randomly allocated to two numerically equal groups from a double-blind, 80-person list, using a table of random digits and given either symbiotic in form of a 500 mg capsule (Familact, produced by Zisttakhmir company) containing seven species of probiotic bacteria (Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum, Streptococcus thermophilus) and fructooligosaccharidesor a placebo capsule (containing 120 mg starch). Faculty of Pharmacy, Isfahan University of Medical Sciences, prepared placebo capsules, similar in shape and appearance as symbiotic capsules.
All study participants ingested the capsules (symbiotic or placebo) once daily for 8 weeks. Six participants were excluded during the study (because of unwillingness or failure to follow the study's guidelines), which left 38 volunteers in the symbiotic group and 36 in the placebo group [Figure 1].
Figure 1.
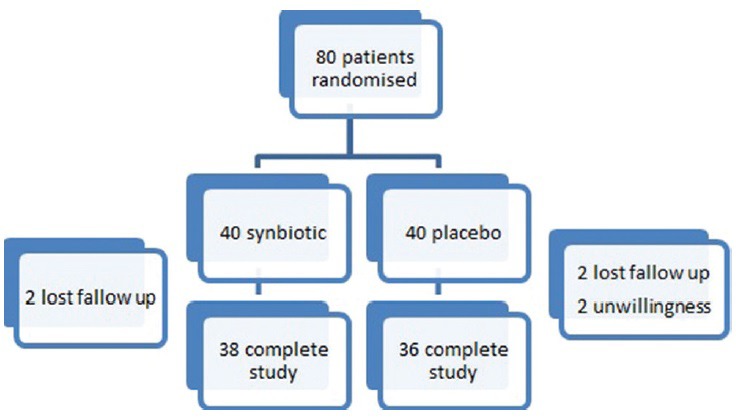
A flow chart of patients who entered the study
Procedures and variables assessments
Informed consent was obtained from all participants, at the beginning of the study. All data collection and measurements were performed by trained personnels. General information including age, sex, smoking, menopausal status, medical history, and medication were collected using interview. Weight and height were measured following standard procedures.[24] Body weight was measured to the nearest 0.1 kg with minimal clothing by means of a digital seca balance. Height was measured to the nearest 0.5 cm without shoes by means of a seca stadiometer. Body mass index (BMI) was calculated for each patient (BMI = weight in kg/height 2 in m). We metered waist and hip circumference on a horizontal plane at the level of the iliac crest by an Ergonomic Circumference Measuring Tape (model 201; Seca GmbH and Co, KG, Hamburg, Germany).
Venous blood samples were collected after a 12-h overnight fasting. The samples were centrifuged and serum samples were frozen and stored at −70°C. CRP and liver enzymes (ALT and AST) were measured. ALT and AST were measured with colorimetric method (using kits from Pars Azmoon Company, Tehran, Iran). The method of measurement of CRP was turbidimetry. Laboratory reference ranges of ALT, AST, and CRP were considered 5–31 (IU/L), 5–31 (IU/L) and up to 1.0 (mg/dl), respectively. Ultrasound grading to state liver steatosis was determined by means of an East Medical sonographic scanner equipped with a convex 3.5 MHz browser by a skilled radiologist. Nonalcoholic steatohepatitis (NASH) grade was assessed using the National Health and Nutrition Examination Survey III criteria: Grade 0 normal, grade 1 mild, grade 2 moderate, grade 3 severe.
The Compendium of Physical Activities[25] was used to standardize the assignment of metabolic equivalent of task intensities. Dietary intake was collected by food record (3-day food record in gram before, and the same after intervention). Dietary data were analyzed using Nutritionist IV software adjusted for Iranian foods (Version 4.1, First Databank Division, The Hearst Corporation, San Bruno, CA, USA). All study variables were measured at the beginning and the end of the intervention.
Statistical analysis
The data were analyzed using SPSS (version 20; SPSS Inc., Chicago, IL, USA). Descriptive statistics are presented as a mean ± standard deviation. Data were assessed for normality by the Shapiro–Wilk test. In case of normal distribution, Paired t-test and in case of nonnormal distribution, Wilcoxon signed rank test were used for comparing data within groups. Independent t-test (in case of normal distribution) or nonparametric statistical test, Mann–Whitney U-test (in case of nonnormal distribution), was used for comparing data between two groups. Analysis of covariance (ANCOVA) was used for evaluating between group differences based on quantitative data; adjustment was made for differences in baseline covariates. Within and between groups Differences based on qualitative variables were assessed using McNemar and Chi-square tests, respectively. All tests were two-sided, and P < 0.05 were considered statistically significant.
RESULTS
Patient characteristics at baseline
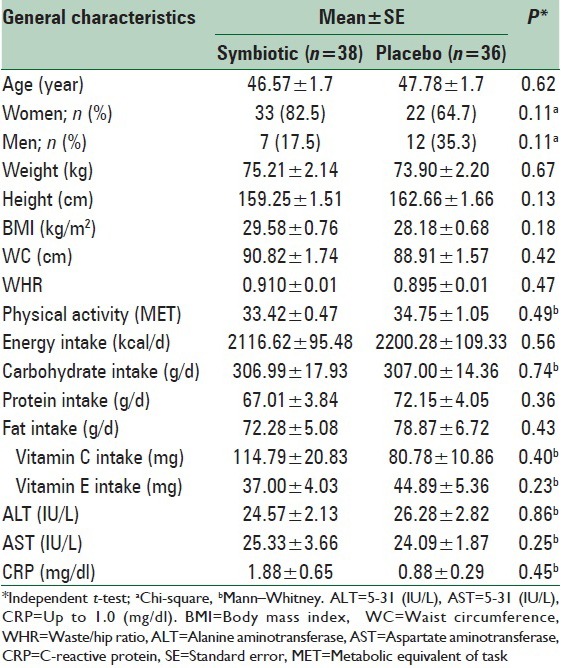
There were no differences in baseline characteristics between symbiotic and placebo groups [Table 1]. We observed no significant differences in age, anthropometric measures, CRP level, liver enzymes, dietary intake, and physical activity status between two groups.
Table 1.
Baseline characteristics of the study subjects who received symbiotic or placebo
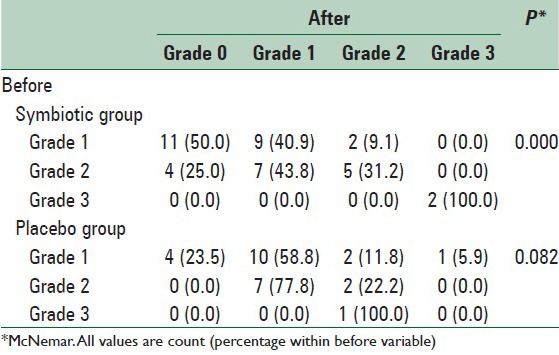
Symbiotic effect on the grade of hepatic steatosis
None of the participants had normal grade steatosis at baseline and comparison of severity of hepatic steatosis between two groups before (P = 0.59) and after the intervention (P = 0.18) through K2 test showed no significant difference. In symbiotic group within group changes assessment through McNemar test showed that 50% and 25% of patients with mild (grade 1) and moderate (grade 2) NAFLD became normal, respectively. In 43.8% of patients with moderate NAFLD, severity of hepatic steatosis reduced to grade 1and in the rest of subjects remained unchanged. Only in 9.1% of patients with mild NAFLD in symbiotic group steatosisseverity increased to grade 2. In the placebo group, 23.5% of patients with mild (grade 1) NAFLD recovered and in 11.8% and 5.9%, steatosis severity increased to grade 2 and 3, respectively. Severity of steatosis in a patient with grade 3 NAFLD in placebo group fell to grade 2. In general, changes in symbiotic group were significant and in placebo group were not significant [Table 2].
Table 2.
Comparison of steatosis grade before and after the intervention between symbiotic and placebo groups
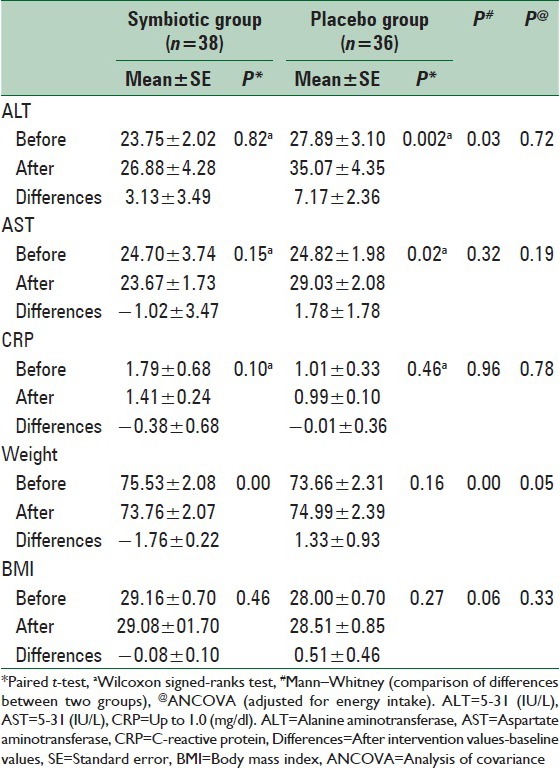
Symbiotic effect on inflammatory markers and hepatic function tests
CRP and liver enzymes levels at baseline and after intervention period were reported in Table 3. ALT and AST levels did not change within the intervention group but were significantly increased in the placebo group. No significant changes were observed in CRP in either group. ANCOVA detected no differences in inflammatory markers and hepatic function tests levels between two groups after adjustment for energy intake and baseline values.
Table 3.
CRP, liver enzymes and anthropometric measures of participants before and after the intervention
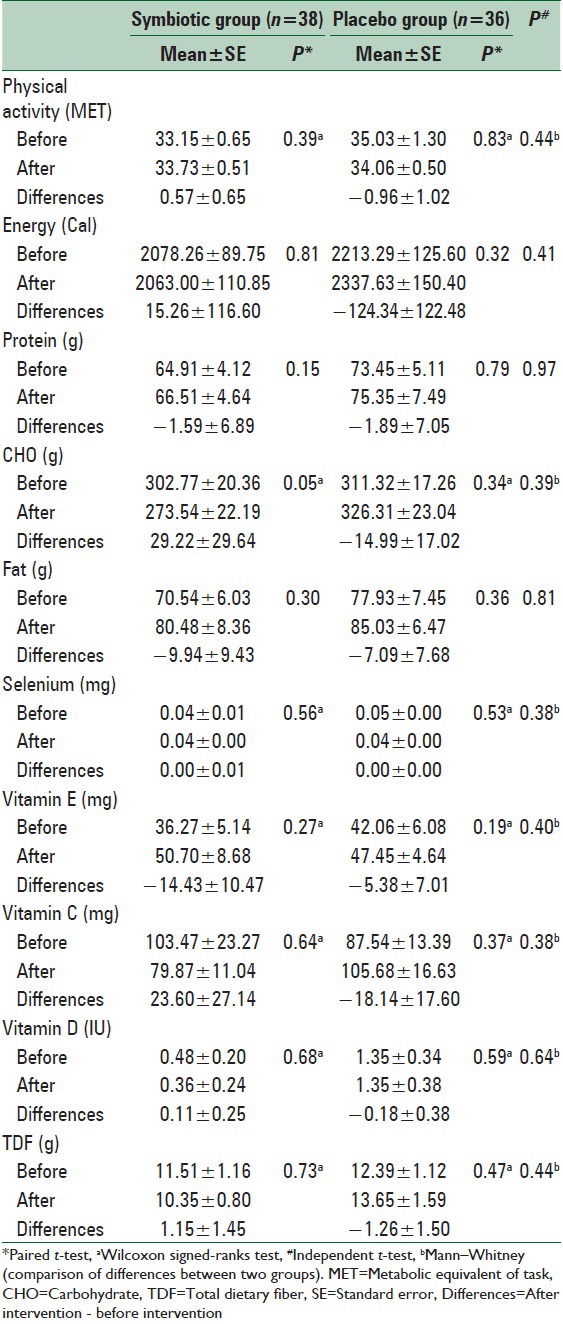
Physical activity level and dietary intake
Comparison of two groups at base line and the end of study showed no significant differences in dietary intake and physical activity level. Paired t-test and Wilcoxon signed ranks test also showed no significant within group changes in these variables [Table 4].
Table 4.
Physical activity and dietary intakes of participants before and after the intervention
DISCUSSION
The present study shows that an 8-week supplementation with a 500 mg symbiotic capsule (Familact, produced by Zisttakhmir company) containing seven species of probiotic bacteria (L. casei, L. acidophilus, L. rhamnosus, L. bulgaricus, B. breve, B. longum, S. thermophilus) and fructooligosaccharides in NAFLD patients does not change high-sensitivity-CRP, ALT and AST levels in comparison between two groups but is associated with steatosis grade improvement. Based on our knowledge, this is the first study that investigates the effect of symbiotic supplementation on liver enzymes, CRP and ultrasound findings in NAFLD patients. Nowadays, there is no registered drug for the treatment of NAFLD[26] and new data are coming. Lifestyle modification is often recommended but it is difficult to achieve.[27,28]
Our results showed that in symbiotic group aminotransferase levels remained static as a result of symbiotic supplementation while they were increased in placebo group. Consistent with the present study Velayudham et al., demonstrated that supplementation with VSL#3 (S. thermophilus and several species of Lactobacillus and Bifidobacteria) in rats for 9 weeks had no effect on ALT.[29] In Xu et al. study on Sprague-Dawley rats, probiotic supplementation for 12 weeks also showed no effect on ALT but attenuated hepatic fat accumulation.[21]
In contrast with our investigation Kelishadi et al. review of animal studies showed that the levels of liver enzymes reduced in 22% of cases as a result of treatment with probiotics.[30] A double-blind clinical trial by Aller et al., in 2011 on 30 patients with NAFLD showed that 6 months treatment with probiotics reduced ALT and AST levels.[16] Ma et al., in 2013 conducted a meta-analysis to investigate results of four randomized trials involving 134 NAFLD/NASH patients and concluded that probiotic therapy decreased ALT and AST, significantly.[26]
Most experimental and human studies in this field, in contrast with our results, had led to a reduction in liver enzymes.[18,31]
Despite observing some degrees of hepatic steatosis at baseline, but mean liver enzymes level was normal in the present study. Hence, detecting no change in ALT and AST levels seems reasonable. On the other hand, liver enzymes increase in the placebo group noted that a symbiotic can at least prevents progression of the disease and a longer period of intervention with a higher dosage of supplement in patients with elevated levels of liver enzymes might show significant effects on these markers.
In our study symbiotic supplementation resulted in improvement of liver steatosis. Previous studies in animals with alcoholic and NAFLD suggest that treatment with probiotics can reduce liver damage.[32,33,34] According to Wong et al., study in 2013, intra hepatic triglyceride content in patients with NASH was reduced as a result of 6 months intervention with probiotics.[35] Velayudham et al., study showed no effect on inflammation and liver steatosis[29] but their study duration was less than ours was and our supplements were containing probiotics and prebiotics that can amplify the effects.
Moreover, although weight in the symbiotic group significantly decreased, dietary intakes and physical activity levels (as the main factors contributing to the prevention and treatment of NAFLD patients) showed no significant differences at the baseline and the end of study between two groups. Therefore, it can be concluded that the observed changes in weight, liver enzymes and steatosis were not related to physical activity and dietary status. Moreover, as other studies showed, symbiotic supplementation can cause weight loss.[36,37] In addition, the observed results in liver enzymes and the degree of steatosis after adjustment for energy intake and baseline values remained unchanged, so we can conclude that the steatosis improvement and stability of transaminases levels in the symbiotic group (against their increase in the placebo group) might be a result of symbiotic supplementation.
It seems the beneficial effects of symbiotic can be related to reducing the impact of pathogenic bacteria on NAFLD development by exclusion or inhibition of invading bacteria, as well as by producing antimicrobial factors such as SCFA.[38] Furthermore, probiotics can improve epithelial barrier function.[39,40] Control of bacterial flora can lead to a reduction of endotoxins and other toxic compounds derived from bacteria, such as ethanol, phenol, and indole, which cause liver damage.[41] On the other side, they can inhibit urease activity of gut microflora bacteria, their ammonia production, and its diffusion into the portal system.[42]
It is said that CRP levels are significantly associated with fatty liver histological features (steatosis grade, necroinflammation, and fibrosis); this supports the earlier hypothesis that NAFLD is associated with low-grade systemic inflammation.[41] CRP in our study remain unchanged as results of Loguercio et al., study which showed 3 months of treatment with probiotic VSL#3 had no effect on proinflammatory cytokines in NAFLD patients.[18] Bhathena et al., study in 2013 also showed no effect for 12 weeks probiotic supplementation on CRP in Bio F1B Golden Syrian hamsters but decreased liver triglyceride and cholesterol concentrations.[43] Aller et al. study also had similar results.[16] Malaguarnera in a double-blind, placebo-controlled randomized control trial in 2011 on 66 NAFLD patient showed that probiotic with Fos and lifestyle modification (i.e. diet and exercise) for 24 weeks significantly reduced serum AST levels, tumor necrosis factor-alpha, and CRP.[44] Intervention duration of these studies was longer than present investigation and this can be cause of differences.
Symbiotic capsules in our study were containing fructooligosaccharides, which are now considered because of their prebiotic properties. Fructooligosaccharides can make Bifidobacteria the dominant species in the colon and may help to control or reduce the growth of harmful bacteria.[45]
In animal models, oligofructose increased the gut bifidobacterial content of high-fat diet fed mice which reduced oxidative stress and adipose tissue inflammation and resulted in an improvement of glucose tolerance.[46] In addition Daubioul et al. in a placebo-controlled pilot study evaluated the effect of oligofructose on NAFLD patients and observed oligo-fructose consumption resulted in reduced insulin and aminotransferase levels.[15]
To our search this is the first double-blind placebo controlled clinical trial that evaluated the effect of symbiotic as a low cost without side effect therapeutic component on steatosis grade and liver enzymes in Iranian NAFLD patients. Nevertheless, several limitations must be considered in the interpretation of our findings, including limited duration of the clinical trial and the sample size. Furthermore, it is well known that liver histology is the gold standard for NAFLD/NASH. Although ultrasonography is reasonably accurate, it cannot identify fatty infiltration of the liver below a threshold of 30%[26] but because of budget limitation we were not able to do the best.
CONCLUSIONS
Based on the present study results, it can be concluded that symbiotic supplementation for 8-week can improve steatosis in NAFLD patients and might be useful in management of NAFLD or be protective again progression of the disease. Further clinical trials with longer intervention period and higher doses of symbiotic may need for showing more clear results in this regard.
Financial support and sponsorship
Food Security Research Centre, School of Nutrition and Food Science, Isfahan University of Medical sciences, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This article was extracted from Msc. dissertation which was approved by the School of Nutrition and Food Sciences, Isfahan University of Medical Sciences with code 392326. Authors thank all the participants and Colleague of Endocrine and Metabolism Research Center and Department of Community Nutrition for their cooperation during the study.
REFERENCES
- 1.Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–7. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 2.Jamali R, Jamali A. Non-alcoholic fatty liver disease. Feyz J Kashan Univ Med Sci. 2010;14:12. [Google Scholar]
- 3.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 4.Nikroo H, Attarzade HS, Sima HR, Nematy M. The effect of diet and aerobic training onserum aminotransferases levels in patients with non-alcoholic steatohepatitis. J Daneshvar Med. 2011;18:10. [Google Scholar]
- 5.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–9. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 6.Alavian SM, Mohammad-Alizadeh AH, Esna-Ashari F, Ardalan G, Hajarizadeh B. Non-alcoholic fatty liver disease prevalence among school-aged children and adolescents in Iran and its association with biochemical and anthropometric measures. Liver Int. 2009;29:159–63. doi: 10.1111/j.1478-3231.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- 7.Jamali R, Khonsari M, Merat S, Khoshnia M, Jafari E, Bahram Kalhori A, et al. Persistent alanine aminotransferase elevation among the general Iranian population: Prevalence and causes. World J Gastroenterol. 2008;14:2867–71. doi: 10.3748/wjg.14.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: Implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007;86:285–300. doi: 10.1093/ajcn/86.2.285. [DOI] [PubMed] [Google Scholar]
- 9.Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006;82:315–22. doi: 10.1136/pgmj.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 11.Esposito E, Iacono A, Bianco G, Autore G, Cuzzocrea S, Vajro P, et al. Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J Nutr. 2009;139:905–11. doi: 10.3945/jn.108.101808. [DOI] [PubMed] [Google Scholar]
- 12.Iacono A, Raso GM, Canani RB, Calignano A, Meli R. Probiotics as an emerging therapeutic strategy to treat NAFLD: Focus on molecular and biochemical mechanisms. J Nutr Biochem. 2011;22:699–711. doi: 10.1016/j.jnutbio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–87. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 14.Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–11. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daubioul CA, Horsmans Y, Lambert P, Danse E, Delzenne NM. Effects of oligofructose on glucose and lipid metabolism in patients with nonalcoholic steatohepatitis: Results of a pilot study. Eur J Clin Nutr. 2005;59:723–6. doi: 10.1038/sj.ejcn.1602127. [DOI] [PubMed] [Google Scholar]
- 16.Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D, et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: A double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090–5. [PubMed] [Google Scholar]
- 17.Kekkonen RA, Sysi-Aho M, Seppanen-Laakso T, Julkunen I, Vapaatalo H, Oresic M, et al. Effect of probiotic Lactobacillus rhamnosus GG intervention on global serum lipidomic profiles in healthy adults. World J Gastroenterol. 2008;14:3188–94. doi: 10.3748/wjg.14.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loguercio C, Federico A, Tuccillo C, Terracciano F, D’Auria MV, De Simone C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–3. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 19.Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–30. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osman N, Adawi D, Ahrné S, Jeppsson B, Molin G. Endotoxin-and D-galactosamine-induced liver injury improved by the administration of Lactobacillus, Bifidobacterium and blueberry. Dig Liver Dis. 2007;39:849–56. doi: 10.1016/j.dld.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Xu RY, Wan YP, Fang QY, Lu W, Cai W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J Clin Biochem Nutr. 2012;50:72–7. doi: 10.3164/jcbn.11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Argenio G, Cariello R, Tuccillo C, Mazzone G, Federico A, Funaro A, et al. Symbiotic formulation in experimentally induced liver fibrosis in rats: Intestinal microbiota as a key point to treat liver damage? Liver Int. 2013;33:687–97. doi: 10.1111/liv.12117. [DOI] [PubMed] [Google Scholar]
- 23.Gratz SW, Mykkanen H, El-Nezami HS. Probiotics and gut health: A special focus on liver diseases. World J Gastroenterol. 2010;16:403–10. doi: 10.3748/wjg.v16.i4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohman TG, Roche AF, Martorell R. Champaign, Ill: Human Kinetics Books; 1988. Anthropometric Standardization Reference Manual. [Google Scholar]
- 25.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 26.Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: A meta-analysis. World J Gastroenterol. 2013;19:6911–8. doi: 10.3748/wjg.v19.i40.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark JM. Weight loss as a treatment for nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006;40(Suppl 1):S39–43. doi: 10.1097/01.mcg.0000168641.31321.fa. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-Hernández H, Cervantes-Huerta M, Rodríguez-Moran M, Guerrero-Romero F. Decrease of aminotransferase levels in obese women is related to body weight reduction, irrespective of type of diet. Ann Hepatol. 2011;10:486–92. [PubMed] [Google Scholar]
- 29.Velayudham A, Dolganiuc A, Ellis M, Petrasek J, Kodys K, Mandrekar P, et al. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989–97. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelishadi R, Farajian S, Mirlohi M. Probiotics as a novel treatment for non-alcoholic fatty liver disease; a systematic review on the current evidences. Hepat Mon. 2013;13:e7233. doi: 10.5812/hepatmon.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vajro P, Paolella G, Fasano A. Microbiota and gut-liver axis: Their influences on obesity and obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2013;56:461–8. doi: 10.1097/MPG.0b013e318284abb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–50. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 33.Loguercio C, De Simone T, Federico A, Terracciano F, Tuccillo C, Di Chicco M, et al. Gut-liver axis: A new point of attack to treat chronic liver damage? Am J Gastroenterol. 2002;97:2144–6. doi: 10.1111/j.1572-0241.2002.05942.x. [DOI] [PubMed] [Google Scholar]
- 34.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Exp Biol Med. 1994;205:243–7. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 35.Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, Li KC, et al. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2013;12:256–62. [PubMed] [Google Scholar]
- 36.Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol. 2014;20:16079–94. doi: 10.3748/wjg.v20.i43.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HY, Park JH, Seok SH, Baek MW, Kim DJ, Lee KE, et al. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta. 2006;1761:736–44. doi: 10.1016/j.bbalip.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Carr FJ, Chill D, Maida N. The lactic acid bacteria: A literature survey. Crit Rev Microbiol. 2002;28:281–370. doi: 10.1080/1040-840291046759. [DOI] [PubMed] [Google Scholar]
- 39.Roselli M, Finamore A, Britti MS, Mengheri E. Probiotic bacteria Bifidobacterium animalis MB5 and Lactobacillus rhamnosus GG protect intestinal Caco-2 cells from the inflammation-associated response induced by enterotoxigenic Escherichia coli K88. Br J Nutr. 2006;95:1177–84. doi: 10.1079/bjn20051681. [DOI] [PubMed] [Google Scholar]
- 40.Sherman PM, Johnson-Henry KC, Yeung HP, Ngo PS, Goulet J, Tompkins TA. Probiotics reduce enterohemorrhagic Escherichia coli O157:H7-and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect Immun. 2005;73:5183–8. doi: 10.1128/IAI.73.8.5183-5188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imani Fooladi AA, Mahmoodzadeh Hosseini H, Nourani MR, Khani S, Alavian SM. Probiotic as a novel treatment strategy against liver disease. Hepat Mon. 2013;13:e7521. doi: 10.5812/hepatmon.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clausen MR, Mortensen PB. Lactulose, disaccharides and colonic flora. Clinical consequences. Drugs. 1997;53:930–42. doi: 10.2165/00003495-199753060-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhathena J, Martoni C, Kulamarva A, Tomaro-Duchesneau C, Malhotra M, Paul A, et al. Oral probiotic microcapsule formulation ameliorates non-alcoholic fatty liver disease in Bio F1B Golden Syrian hamsters. PLoS One. 2013;8:e58394. doi: 10.1371/journal.pone.0058394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545–53. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]
- 45.Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975–82. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 46.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]


