Abstract
Background:
Pregnancy is associated with unfavorable metabolic profile, which might in turn result in adverse pregnancy outcomes. The current study was designed to evaluate the effects of calcium plus Vitamin D administration on metabolic status and pregnancy outcomes in healthy pregnant women.
Methods:
This randomized double-blind placebo-controlled clinical trial was performed among 42 pregnant women aged 18–40 years who were at week 25 of gestation. Subjects were randomly allocated to consume either 500 mg calcium-200 IU cholecalciferol supplements (n = 21) or placebo (n = 21) for 9 weeks. Blood samples were obtained at the onset of the study and after 9-week trial to determine related markers. Post-delivery, the newborn's weight, length, and head circumference were measured during the first 24 h after birth.
Results:
Consumption of calcium-Vitamin D co-supplements resulted in a significant reduction of serum high-sensitivity C-reactive protein levels compared with placebo (−1856.8 ± 2657.7 vs. 707.1 ± 3139.4 μg/mL, P = 0.006). We also found a significant elevation of plasma total antioxidant capacity (89.3 ± 118.0 vs. −9.4 ± 164.9 mmol/L, P = 0.03), serum 25-hydroxyvitamin D (2.5 ± 3.5 vs. −1.7 ± 1.7 ng/mL, P < 0.0001), and calcium levels (0.6 ± 0.6 vs. −0.1 ± 0.4 mg/dL, P < 0.0001). The supplementation led to a significant decrease in diastolic blood pressure (−1.9 ± 8.3 vs. 3.1 ± 5.2 mmHg, P = 0.02) compared with placebo. No significant effect of calcium-Vitamin D co-supplements was seen on other metabolic profiles. We saw no significant change of the co-supplementation on pregnancy outcomes as well.
Conclusions:
Although calcium-Vitamin D co-supplementation for 9 weeks in pregnant women resulted in improved metabolic profiles, it did not affect pregnancy outcomes.
Keywords: Calcium-Vitamin D supplementation, high sensitivity C-reactive protein, insulin resistance, oxidative stress, pregnancy outcome
INTRODUCTION
Pregnant women are at an increased risk of insulin resistance, elevated inflammation, and oxidative stress, in particular during the third trimester.[1,2] These conditions might result from micronutrient deficiency,[3,4] increased maternal adipose tissue, and production of hormones by the placenta.[5,6] It is assumed that pregnant women with increased serum levels of insulin and inflammatory biomarkers are at elevated risk of preeclampsia,[7] adverse pregnancy outcome,[8] premature delivery, and gestational diabetes mellitus (GDM).[6,9,10] Preeclampsia is estimated to occur in 2–7% of all pregnancies and causes maternal and perinatal mortality worldwide, particularly in developing countries.[11,12,13] It is responsible for about 60,000 maternal deaths worldwide.[14]
To reduce maternal and fetal complications resulting from unfavorable metabolic profile, various strategies have been proposed such as the consumption of antioxidants and Vitamins E and C.[14,15,16,17] Recently, few studies have shown that Vitamin D supplementation during pregnancy might affect pregnancy outcomes[18] through influencing insulin resistance,[19] systemic inflammation, and oxidative stress.[20] In addition, improved levels of systemic inflammation[21] as well as favorable pregnancy outcomes[22,23] were seen with taking calcium supplements during pregnancy. Among nonpregnant women, calcium supplementation has resulted in decreased insulin resistance.[24] Despite these positive documents, findings from a meta-analysis revealed that calcium supplementation without Vitamin D might increase the risk of cardiovascular (CV) events.[25] Therefore, the role of calcium supplements in the management of chronic conditions, in particular during pregnancy, needs further assessment. Although some studies have shown that Vitamin D supplements alone had similar effects on serum Vitamin D levels, compared to combined calcium-Vitamin D supplementation, others believe that the efficacy of a single supplementation with calcium or Vitamin D would be improved with joint supplementation. Calcium-Vitamin D co-supplementation might affect insulin resistance, inflammation, and oxidative stress through their effects on the regulation of cell cycle,[26] activation of antioxidant enzymes,[27] and inhibition of the synthesis of inflammatory cytokines.[28] These favorable effects might mediate the effect of the co-supplementation on pregnancy outcomes. We are aware of no study examining the effects of calcium-Vitamin D co-supplementation on insulin resistance, high-sensitivity C-reactive protein (hs-CRP), and oxidative stress among pregnant women as well as on pregnancy outcomes. We hypothesized that calcium-Vitamin D co-supplementation might help pregnant women to control their metabolic profiles and pregnancy outcomes. Therefore, the aim of this study was to assess the effects of joint calcium-Vitamin D administration on markers of insulin resistance, inflammation, and oxidative stress as well as pregnancy outcomes in Iranian pregnant women.
METHODS
Participants
The current clinical trial was done in Kashan, Iran, from March 2012 to September 2012. Based on suggested formula for clinical trials, we needed 20 persons in each group (α =0.05; β =0.20; and plasma hs-CRP as the main variable).[29] We enrolled women aged 18–40 years carrying singleton pregnancy at week 25 of their pregnancy, who were attended to at the maternity clinics in Kashan. Affiliated to Kashan University of Medical Sciences, Kashan, Iran. Last menstrual period was considered as the basis for assessment of gestational age.[30] Sixty pregnant women were screened for the current study; of them, 46 met the inclusion criteria. We excluded those with preeclampsia, placenta abruption, and GDM. A total of 46 pregnant women were recruited in the study and were randomly allocated to take either placebo (n = 23) or calcium-Vitamin D (n = 23) for 9 weeks. We used random numbers, taken from a computer program to do random assignment. This study was conducted based on the guidelines laid down in the Declaration of Helsinki. The Ethical Committee of Kashan University of Medical Sciences approved the study and informed written consent was obtained from all participants.
Study design
At study baseline (25 weeks of gestation), pregnant women were randomly allocated to consume either the placebo or calcium-Vitamin D supplements (containing 500 mg calcium carbonate plus 200 IU Vitamin D3 or cholecalciferol) once daily for 9 weeks. As pregnant women are at increased risk of insulin resistance, elevated inflammation, and oxidative stress, in particular at the third trimester,[1,2] we decided to start the intervention at week 25 of gestation. The intervention lasted for 9 weeks because this duration of intervention seems enough for the influence of calcium-Vitamin D supplementation on dependent variables in the current study. In addition, we were afraid of high dropouts in case the intervention continued to the last weeks of gestation. Subjects were requested not to change their usual physical activity or diets and not to take any other supplements containing cholecalciferol or calcium other than the one provided to them by the investigators. The calcium-Vitamin D supplements as well as the placebo were provided by Shahre Daru Co., Tehran, Iran. Placebo pills contained microcrystalline cellulose and were similar in terms of shape and color to the supplements. Both calcium and cholecalciferol supplements were assessed for quality in the laboratory of the Food and Drug Administration in Tehran, Iran, by enzymatic and high-performance liquid chromatography method. Based on these tests, the amount of calcium and cholecalciferol in the prescribed tablets were 475–600 mg and 190–240 IU, respectively. Subjects were also consuming 400 μg folic acid every day, from the beginning of the pregnancy and 50 mg ferrous sulfate from the second trimester. Before use, we kept all supplements in cool temperature Compliance with the intake of supplements was done by the use of 3-day dietary records completed throughout the study. To obtain daily macro- and micro-nutrient intakes of subjects, we used Nutritionist IV software (First DataBank, San Bruno, CA, USA) modified for Iranian foods.
Primary and secondary outcomes
In the current study, primary outcomes were including fasting plasma glucose (FPG), insulin metabolism parameters, hs-CRP, biomarkers of oxidative stress, serum calcium, Vitamin D levels, and blood pressure. Secondary outcome measures included birth size, gestational age, and mode of delivery.
Assessment of variables
Anthropometric measurements were recorded at the onset of the study and after a 9-week trial. Body weight was measured in an overnight fasting status, without shoes, and with minimal clothing using a digital scale (Seca, Hamburg, Germany) to the nearest 0.1 kg. Height was measured using a nonstretched tape measure (Seca, Hamburg, Germany) to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Data on the prepregnancy weight and BMI were obtained from the available information on the patients’ records. After delivery, the newborn's length and weight were determined by the use of standard methods (Seca 155 Scale, Hamburg, Germany). Newborn's head circumference was quantified with a girth measuring tape. Maternal systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured via a sphygmomanometer (ALPK2, Zhejiang, China). Maternal DBP was considered as the fifth Korotkoff sound. Fasting blood samples (10 mL) were taken at baseline and after 9-week intervention at the Kashan reference laboratory in the early morning after an overnight fast. The serum and plasma samples were separated from whole blood by centrifugation at 3500 rpm for 10 min (Hettich D-78532, Tuttlingen, Germany) and stored at −70°C until analyzed at the reference laboratory. FPG levels were quantified by the use of glucose oxidase/peroxidase method with commercially available kits (Pars Azmun, Tehran, Iran) by automatic biochemistry analyzer (BT 3000, Monsano, Italy). Serum insulin levels were assayed by enzyme-linked immunoassay kits (DiaMetra, Milano, Italy). The homeostatic model assessment for insulin resistance (HOMA-IR) and b-cell function (HOMA-B) and the quantitative insulin sensitivity check index (QUICKI) were calculated based on suggested formulas.[31] Serum hs-CRP concentration was quantified by ELISA kit (LDN, Nordhorn, Germany). Plasma total antioxidant capacity (TAC) was assessed by the use of ferric reducing antioxidant power method developed by Benzie and Strain.[32] The plasma total glutathione (GSH) was measured by the method of Beutler et al.[33] Serum 25-hydroxyvitamin D was determined by ELISA (Awareness Stat Fax 2100, Bohemia, USA) using available kits (IDS, Boldon, UK). Serum calcium and magnesium concentrations were assayed using mentioned kits (Pars Azmun, Tehran, Iran). The ELISA intra-assay variation was evaluated by the CV of the duplicate measurements. A CV <5% was detected for insulin, hs-CRP, and Vitamin D measurements. Measurements of 25-hydroxyvitamin D, glucose, insulin, hs-CRP, TAC, GSH, calcium, and magnesium were performed in a blinded fashion.
Statistical analysis
To assess if the variables in the study were normally distributed or not, we used histogram and Kolmogorov–Smirnov test. Log transformation was conducted for nonnormally distributed variables. Independent samples Student's t-test was applied to compared data on general features of study participants as well as their dietary intakes across the groups. To examine the distribution of study subjects, we used Chi-square test. To determine the effects of calcium-Vitamin D supplements on insulin resistance, hs-CRP, and biomarkers of oxidative stress, we used one-way repeated measures analysis of variance, where the effect of time, treatment, and time-treatment interactions were tested. In this analysis, the treatment (calcium-Vitamin D vs. placebo) was regarded as between-subject factor and time with two time-points (baseline and week 9 of intervention) considered as within-subject factor. P < 0.05 was considered as statistically significant. All statistical analyses were done using the Statistical Package for Social Science version 17 (SPSS Inc., Chicago, Illinois, USA).
RESULTS
Among individuals in the placebo group, two women (severe preeclampsia [n = 1] and placenta abruption [n = 1]) were excluded. The exclusions in the calcium-Vitamin D group were also two persons (preterm delivery [n = 1] and hospitalization [n = 1]). Finally, 42 participants (placebo [n = 21] and calcium-Vitamin D [n = 21]) completed the trial [Figure 1].
Figure 1.
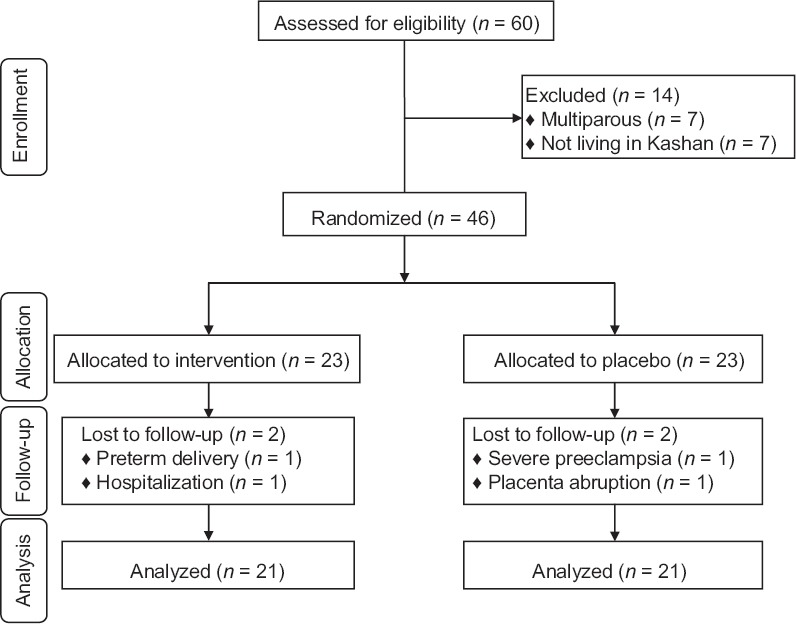
Consort 2010 flow diagram of subject enrollment, allocation, follow-up, and analysis
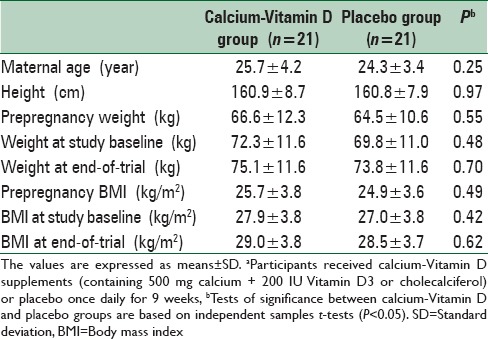
Mean age of study participants was not statistically different between the two groups, calcium-Vitamin D and placebo. Baseline prepregnancy weight and BMI as well as their means before and after intervention were not significantly different between the two groups [Table 1].
Table 1.
General characteristics of the study participantsa
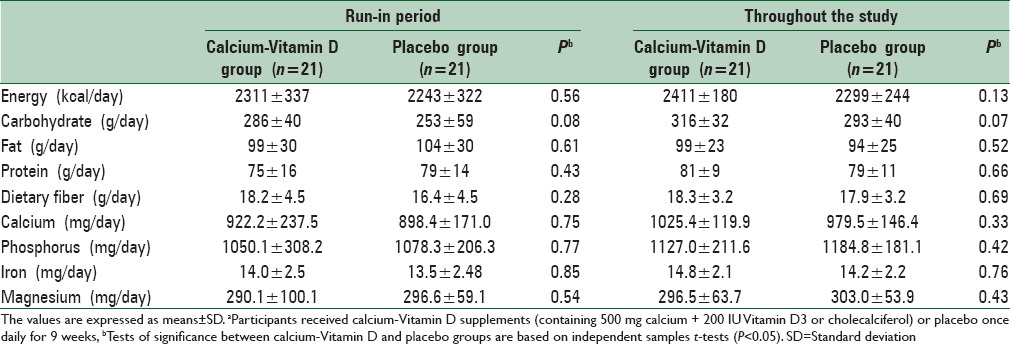
We found no statistically significant difference between the two groups in terms of dietary intakes of energy, carbohydrate, fat, protein, dietary fiber, calcium, phosphorus, iron, and magnesium [Table 2].
Table 2.
Dietary intakes of study participants at run-in period and throughout the studya
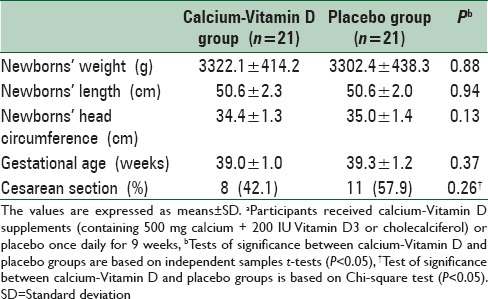
On average, the rate of compliance in our study was relatively high; such that more than 90% of pills were taken throughout the study in both groups. Calcium-Vitamin D supplementation did not affect birth size, gestational age, and mode of delivery compared with the placebo [Table 3].
Table 3.
The effect of calcium-Vitamin D supplementation on pregnancy outcomesa
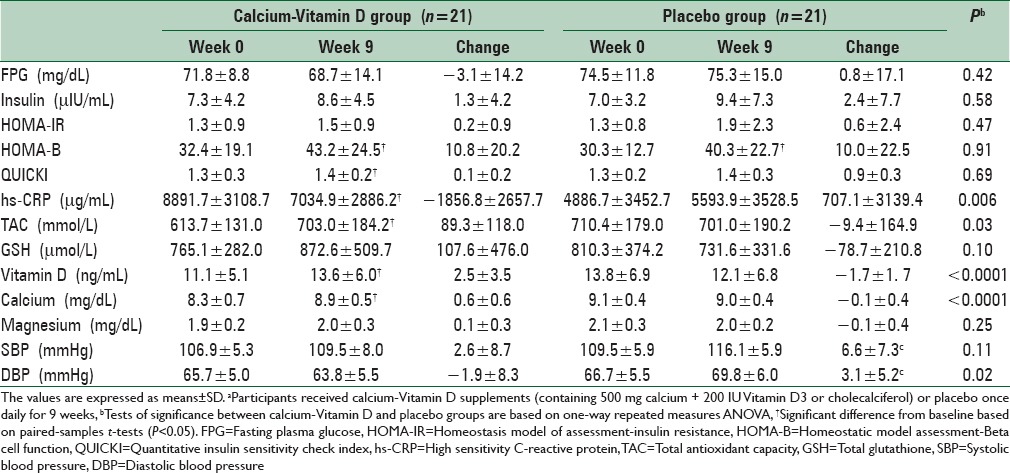
Consumption of calcium-Vitamin D supplements resulted in a significant reduction of serum hs-CRP levels compared with placebo (−1856.7 ± 2657.7 vs. 707.1 ± 3139.4 μg/mL, P = 0.006) [Table 4]. We found a significant elevation of plasma TAC (89.3 ± 118.0 vs. −9.4 ± 164.9 mmol/L, P = 0.030), serum 25-hydroxyvitamin D (2.5 ± 3.5 vs. −1.7 ± 1.7 ng/mL, P < 0.0001), and calcium levels (0.6 ± 0.6 vs. −0.1 ± 0.4 mg/dL, P < 0.0001) following consumption of calcium-Vitamin D supplements. Compared to placebo, the calcium-Vitamin D supplementation led to a significant decrease in DBP (−1.9 ± 8.3 vs. 3.1 ± 5.2 mmHg, P = 0.02). No significant effect of calcium-Vitamin D supplementation was seen on SBP, FPG, serum insulin levels, indicators of insulin action (HOMA-IR, HOMA-B, QUICKI index) as well as plasma total GSH. Compared with study baseline, a significant rise in serum calcium (P < 0.001) and 25-hydroxyvitamin D (P = 0.004) levels was seen in the calcium-Vitamin D group.
Table 4.
Insulin resistance, hs-CRP and biomarkers of oxidative stress at baseline and after the 9-week supplementation period in calcium-Vitamin D and placebo groupsa
DISCUSSION
We found that 9 weeks calcium-Vitamin D co-supplementation among pregnant women resulted in a significant decrease in serum hs-CRP and DBP and a significant increase in serum 25-hydroxyvitamin D, calcium, and plasma TAC levels compared to the placebo group. Therefore, some part of the study hypothesis was accepted. However, no significant effect of supplementation was observed on pregnancy outcomes, maternal FPG, serum insulin levels, and insulin action as well as plasma total GSH. This means that some other parts of our hypothesis were rejected.
It must be kept in mind that the change from baseline to 9 weeks follow-up may have led to statistically significant differences in 25OHD concentrations, the differences might be clinically not significant. The inter-assay variability for 25OHD measured by ELISA is >1.7 ng/mL. Both groups meet the institute of medicine definition of significant Vitamin D deficiency and remain in this category despite 200 IU Vitamin D/day. This dose then does not result in a clinically significant change in 25OHD. The recommended dose for neonates and infants is 400 IU Vitamin D/day. 200 IU Vitamin D on a per kilogram basis assuming a woman weighs 65 kg results in about 3 IU/kg whereas for an infant who weighs 3 kg at birth this represents 67 IU/kg, so it is not surprising then that the concentrations changed little during the 9 weeks of supplementation.
Pregnant women are susceptible to insulin resistance, systemic inflammation, and oxidative stress which could in turn lead to several complications in maternal and fetal life.[6] Our data revealed that administration of calcium-Vitamin D supplements for 9 weeks among pregnant women could not affect indicators of glucose homeostasis. In line with our study, 1-year supplementation with 20,000 or 40,000 IU/week Vitamin D3 could not improve glucose metabolism in overweight or obese Caucasian subjects.[34] Injection of 100,000 IU Vitamin D3 did not improve fasting glucose or insulin sensitivity in Caucasian adults with Vitamin D deficiency (serum 25(OH) D <50 nmol/L).[35] The same finding was also seen with supplementation of 1000 mg calcium and 400 IU Vitamin D3 in healthy women after 7 years.[36] In contrast, Vitamin D supplementation (2500 IU/day) resulted in a significant increase in insulin secretion among Vitamin D deficient women with polycystic ovary syndrome after 2 months.[37] Others have shown that the use of Vitamin D3 supplements significantly reduced HOMA-IR index, serum insulin, and glucose concentrations in elderly people with impaired fasting glucose.[38] Among type 2 diabetic patients, Vitamin D supplementation resulted in increased insulin secretion.[39] Heterogeneous findings in different studies might be explained by the discrepancy in dosage and formulation of calcium-Vitamin D supplements, duration of intervention as well as different groups of study populations.
Our study showed that calcium-Vitamin D supplementation for 9 weeks during pregnancy resulted in a significant decrease in serum hs-CRP levels. In agreement with our findings, Vitamin D3 supplementation in patients with colorectal adenoma led to decreased hs-CRP levels in men after 6 months.[29] Eleftheriadis et al.[40] showed a significant association between serum 25-hydroxyvitamin D levels and markers of inflammation including serum hs-CRP and IL-6. In contrast, consumption of fortified milk containing Vitamin D in healthy men did not influence serum hs-CRP levels.[41] Lack of a significant effect of Vitamin D-calcium supplements on serum hs-CRP levels has also been reported in overweight and obese adults[42] as well as in bedridden older patients.[43] The beneficial effects of calcium-Vitamin D supplements on serum hs-CRP in the current study might be explained by the high levels of this biomarker in pregnancy. Some studies have shown that the active Vitamin D receptor (VDR) agonists can decrease the production of inflammatory cytokines after various inflammatory stimuli.[44,45] Administration of an active VDR agonist has also decreased CRP production independent of its effects on hemodynamics or parathyroid hormone suppression in patients with kidney failure.[46]
The current study showed that taking calcium-Vitamin D supplements for 9 weeks among pregnant women resulted in a significant elevation in plasma TAC levels, but did not affect plasma total GSH. In a study by Ekici et al.[47] increased GSH activity was seen with Vitamin D3 + docosahexaenoic acid supplementation in both cortex and corpus striatum in rats. The use of calcium and Vitamin D3 supplements decreased oxidative DNA damage in the normal human colorectal mucosa.[48] Calcium along with Vitamin D might have a lesser effect on oxidative stress and a higher effect on antioxidants than either calcium or Vitamin D alone.[49]
We found that the use of calcium-Vitamin D supplements for 9 weeks among pregnant women resulted in a significant reduction of DBP but could not affect SBP. In a study by Pfeifer et al.,[50] an 8-week supplementation with 1200 mg calcium plus 800 IU Vitamin D3 compared with 1200 mg calcium/day in elderly women resulted in a decrease in SBP but did not affect DBP. Similar findings have also been observed with Vitamin D intake in hypertensive patients for 3 months.[51] Several mechanisms may result in the favorable effects of calcium-Vitamin D supplementation on blood pressure. It seems that Vitamin D has a critical role in the regulation of renin-angiotensin system.[51,52] Furthermore, calcium may act as a regulatory factor of renin-angiotensin system and may result in blood pressure regulation via altering cellular concentrations of sodium and calcium ions.[51]
We did not see any significant effect of calcium-Vitamin D intake on pregnancy outcome. Our findings are consistent with previous studies indicating that 1500 mg calcium intake from week 20 of pregnancy among women with low calcium intake did not influence infant growth during the 1st year of life.[53] Similar findings have also been reached in other studies.[22] In relation to Vitamin D status, Mehta et al.[54] showed no association between maternal Vitamin D status in HIV-infected pregnant women and adverse pregnancy outcomes. Consumption of 25 mg/day ergocalciferol in pregnant women did not influence mean birth weight in other studies.[55,56] Others have also failed to find a significant effect of Vitamin D effect on pregnancy outcomes.[57,58] In contrast, some studies have indicated that either one oral dose of 1500 μg Vitamin D3 or two doses of 3000 μg Vitamin D3 in the second and the third trimesters has led to an increased birth size.[59] Discrepancies between our study and others might be explained by the different doses of calcium and cholecalciferol used as well as the different duration of supplementations.
Our study had some limitations. Due to limited funding, we did not evaluate the effect of calcium-Vitamin D co-administration on other biomarkers of inflammation and oxidative stress. We could not assess if the baseline deficiency of cholecalciferol can explain the effects of supplementation or not. This was because of having small sample size in the investigation. Further investigations are needed to shed light on this issue. In addition, the used dosage of cholecalciferol supplements in our study was low. High-dose supplementation might result in greater changes. We calculated the sample size based on hs-CRP. However, other variables including variables of pregnancy outcome were not taken into account in the sample size calculation. Therefore, interpretation of our findings for these variables should be done cautiously. In addition, sample size was low in the current study. Therefore, further large-scale studies with higher sample size are needed to examine the effect of calcium-Vitamin D co-supplementation on pregnancy outcome. It must also be kept in mind that the beneficial effect seen in the current study by calcium-Vitamin D co-supplementation might be explained by the fact that the mean serum 25(OH) D concentrations at baseline were low in both groups. Calcium and Vitamin D has been hypothesized to act jointly rather than independently. Previous studies have shown that calcium combined with Vitamin D3 resulted in improved metabolic profiles and circulating inflammatory biomarkers than does either calcium or Vitamin D alone.[60,61]
CONCLUSIONS
Consumption of calcium-Vitamin D supplements for 9 weeks among pregnant women resulted in a significant decrease in serum hs-CRP and DBP and a significant increase in serum Vitamin D, calcium, and plasma TAC levels, but had no effect on pregnancy outcomes, maternal FPG, serum insulin levels, and insulin action as well as plasma total GSH.
Financial support and sponsorship
The present study was supported by a grant from the Vice-chancellor for Research, Kashan University of Medical Sciences, Kashan, Iran.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The present study was supported by a grant from the Vice-chancellor for Research, Kashan University of Medical Sciences, Kashan, Iran.
REFERENCES
- 1.Ghojazadeh M, Azami-Aghdash S, Mohammadi M, Vosoogh S, Mohammadi S, Naghavi-Behzad M. Prognostic risk factors for early diagnosing of preeclampsia in nulliparas. Niger Med J. 2013;54:344–8. doi: 10.4103/0300-1652.122368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asemi Z, Karamali M, Esmaillzadeh A. Effects of calcium-Vitamin D co-supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: A randomised placebo-controlled trial. Diabetologia. 2014;57:1798–806. doi: 10.1007/s00125-014-3293-x. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Ferre N, Torrejon MJ, Fuentes M, Fernandez MD, Ramos A, Bordiu E, et al. Association of low serum 25-hydroxyvitamin D levels in pregnancy with glucose homeostasis and obstetric and newborn outcomes. Endocr Pract. 2012;18:676–84. doi: 10.4158/EP12025.OR. [DOI] [PubMed] [Google Scholar]
- 4.Asemi Z, Taghizadeh M, Sarahroodi S, Jazayeri S, Tabasi Z, Seyyedi F. Assessment of the relationship of Vitamin D with serum antioxidant Vitamins E and A and their deficiencies in Iranian pregnant women. Saudi Med J. 2010;31:1119–23. [PubMed] [Google Scholar]
- 5.Jahromi AS, Zareian P, Madani A. Association of insulin resistance with serum interleukin-6 and TNF-a levels during normal pregnancy. Biomark Insights. 2011;6:1–6. doi: 10.4137/BMI.S6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–13. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 7.Walsh SW. Plasma from preeclamptic women stimulates transendothelial migration of neutrophils. Reprod Sci. 2009;16:320–5. doi: 10.1177/1933719108327594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min J, Park H, Park B, Kim YJ, Park J, Lee H, et al. Paraoxonase gene polymorphism and vitamin levels during pregnancy: Relationship with maternal oxidative stress and neonatal birthweights. Reprod Toxicol. 2006;22:418–24. doi: 10.1016/j.reprotox.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Szarka A, Rigó J, Jr, Lázár L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010;11:59. doi: 10.1186/1471-2172-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasim T. Gestational diabetes mellitus: Maternal and perinatal outcomes in 220 saudi women. Oman Med J. 2012;27:140–4. doi: 10.5001/omj.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broughton Pipkin F. Risk factors for preeclampsia. N Engl J Med. 2001;344:925–6. doi: 10.1056/NEJM200103223441209. [DOI] [PubMed] [Google Scholar]
- 12.Mistry HD, Wilson V, Ramsay MM, Symonds ME, Broughton Pipkin F. Reduced selenium concentrations and glutathione peroxidase activity in preeclamptic pregnancies. Hypertension. 2008;52:881–8. doi: 10.1161/HYPERTENSIONAHA.108.116103. [DOI] [PubMed] [Google Scholar]
- 13.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 14.Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamins in Pre-eclampsia (VIP) trial consortium. Vitamin C and Vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): Randomised placebo-controlled trial. Lancet. 2006;367:1145–54. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- 15.Polyzos NP, Mauri D, Tsappi M, Tzioras S, Kamposioras K, Cortinovis I, et al. Combined Vitamin C and E supplementation during pregnancy for preeclampsia prevention: A systematic review. Obstet Gynecol Surv. 2007;62:202–6. doi: 10.1097/01.ogx.0000256787.04807.da. [DOI] [PubMed] [Google Scholar]
- 16.Rumbold A, Crowther CA. Vitamin E supplementation in pregnancy. Cochrane Database Syst Rev. 2005;2:CD004069. doi: 10.1002/14651858.CD004069.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Kamiya K, Wang M, Uchida S, Amano S, Oshika T, Sakuragawa N, et al. Topical application of culture supernatant from human amniotic epithelial cells suppresses inflammatory reactions in cornea. Exp Eye Res. 2005;80:671–9. doi: 10.1016/j.exer.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Wagner CL, McNeil R, Hamilton SA, Winkler J, Rodriguez Cook C, Warner G, et al. A randomized trial of Vitamin D supplementation in 2 community health center networks in South Carolina. Am J Obstet Gynecol. 2013;208:137.e1–13. doi: 10.1016/j.ajog.2012.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soheilykhah S, Mojibian M, Moghadam MJ, Shojaoddiny-Ardekani A. The effect of different doses of Vitamin D supplementation on insulin resistance during pregnancy. Gynecol Endocrinol. 2013;29:396–9. doi: 10.3109/09513590.2012.752456. [DOI] [PubMed] [Google Scholar]
- 20.Zhang QL, Zhou XJ, Hong JG. Effect of Vitamin D supplementation in early life on the expression of interleukin-10 and intercellular adhesion molecule-1 in rat asthma model. Zhonghua Er Ke Za Zhi. 2009;47:735–9. [PubMed] [Google Scholar]
- 21.López-Jaramillo P. Calcium, nitric oxide, and preeclampsia. Semin Perinatol. 2000;24:33–6. doi: 10.1016/s0146-0005(00)80052-x. [DOI] [PubMed] [Google Scholar]
- 22.Buppasiri P, Lumbiganon P, Thinkhamrop J, Ngamjarus C, Laopaiboon M. Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes. Cochrane Database Syst Rev. 2011;10:CD007079. doi: 10.1002/14651858.CD007079.pub2. doi: 10.1002/14651858.CD007079.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Imdad A, Bhutta ZA. Effects of calcium supplementation during pregnancy on maternal, fetal and birth outcomes. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):138–52. doi: 10.1111/j.1365-3016.2012.01274.x. [DOI] [PubMed] [Google Scholar]
- 24.Shalileh M, Shidfar F, Haghani H, Eghtesadi S, Heydari I. The influence of calcium supplement on body composition, weight loss and insulin resistance in obese adults receiving low calorie diet. J Res Med Sci. 2010;15:191–201. [PMC free article] [PubMed] [Google Scholar]
- 25.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without Vitamin D and risk of cardiovascular events: Reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040. doi: 10.1136/bmj.d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nair-Shalliker V, Armstrong BK, Fenech M. Does Vitamin D protect against DNA damage? Mutat Res. 2012;733:50–7. doi: 10.1016/j.mrfmmm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Hajsadeghi S, Hejrati M, Moghadami S, Rismantab S, Namiranian P. Dilated cardiomyopathy in two patients with xeroderma pigmentosum disease: A case report. Acta Med Iran. 2012;50:147–50. [PubMed] [Google Scholar]
- 28.Sandhu MS, Casale TB. The role of Vitamin D in asthma. Ann Allergy Asthma Immunol. 2010;105:191–9. doi: 10.1016/j.anai.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins MH, Owen J, Ahearn T, Fedirko V, Flanders WD, Jones DP, et al. Effects of supplemental Vitamin D and calcium on biomarkers of inflammation in colorectal adenoma patients: A randomized, controlled clinical trial. Cancer Prev Res (Phila) 2011;4:1645–54. doi: 10.1158/1940-6207.CAPR-11-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jehan I, Zaidi S, Rizvi S, Mobeen N, McClure EM, Munoz B, et al. Dating gestational age by last menstrual period, symphysis-fundal height, and ultrasound in urban Pakistan. Int J Gynaecol Obstet. 2010;110:231–4. doi: 10.1016/j.ijgo.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pisprasert V, Ingram KH, Lopez-Davila MF, Munoz AJ, Garvey WT. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: Impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care. 2013;36:845–53. doi: 10.2337/dc12-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 33.Beutler E, Gelbart T. Plasma glutathione in health and in patients with malignant disease. J Lab Clin Med. 1985;105:581–4. [PubMed] [Google Scholar]
- 34.Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med. 2010;267:462–72. doi: 10.1111/j.1365-2796.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 35.Tai K, Need AG, Horowitz M, Chapman IM. Glucose tolerance and vitamin D: Effects of treating Vitamin D deficiency. Nutrition. 2008;24:950–6. doi: 10.1016/j.nut.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 36.de Boer IH, Tinker LF, Connelly S, Curb JD, Howard BV, Kestenbaum B, et al. Calcium plus Vitamin D supplementation and the risk of incident diabetes in the Women's Health Initiative. Diabetes Care. 2008;31:701–7. doi: 10.2337/dc07-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ardabili HR, Gargari BP, Farzadi L. Vitamin D supplementation has no effect on insulin resistance assessment in women with polycystic ovary syndrome and Vitamin D deficiency. Nutr Res. 2012;32:195–201. doi: 10.1016/j.nutres.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Naharci I, Bozoglu E, Kocak N, Doganci S, Doruk H, Serdar M. Effect of Vitamin D on insulin sensitivity in elderly patients with impaired fasting glucose. Geriatr Gerontol Int. 2012;12:454–60. doi: 10.1111/j.1447-0594.2011.00791.x. [DOI] [PubMed] [Google Scholar]
- 39.Eftekhari MH, Akbarzadeh M, Dabbaghmanesh MH, Hasanzadeh J. Impact of treatment with oral calcitriol on glucose indices in type 2 diabetes mellitus patients. Asia Pac J Clin Nutr. 2011;20:521–6. [PubMed] [Google Scholar]
- 40.Eleftheriadis T, Antoniadi G, Liakopoulos V, Stefanidis I, Galaktidou G. Inverse association of serum 25-hydroxyvitamin D with markers of inflammation and suppression of osteoclastic activity in hemodialysis patients. Iran J Kidney Dis. 2012;6:129–35. [PubMed] [Google Scholar]
- 41.Peake JM, Kukuljan S, Nowson CA, Sanders K, Daly RM. Inflammatory cytokine responses to progressive resistance training and supplementation with fortified milk in men aged 50+years: An 18-month randomized controlled trial. Eur J Appl Physiol. 2011;111:3079–88. doi: 10.1007/s00421-011-1942-z. [DOI] [PubMed] [Google Scholar]
- 42.Carrillo AE, Flynn MG, Pinkston C, Markofski MM, Jiang Y, Donkin SS, et al. Vitamin D supplementation during exercise training does not alter inflammatory biomarkers in overweight and obese subjects. Eur J Appl Physiol. 2012;112:3045–52. doi: 10.1007/s00421-011-2279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bjorkman MP, Sorva AJ, Tilvis RS. C-reactive protein and fibrinogen of bedridden older patients in a six-month Vitamin D supplementation trial. J Nutr Health Aging. 2009;13:435–9. doi: 10.1007/s12603-009-0080-3. [DOI] [PubMed] [Google Scholar]
- 44.Müller K, Haahr PM, Diamant M, Rieneck K, Kharazmi A, Bendtzen K. 1,25-Dihydroxyvitamin D3 inhibits cytokine production by human blood monocytes at the post-transcriptional level. Cytokine. 1992;4:506–12. doi: 10.1016/1043-4666(92)90012-g. [DOI] [PubMed] [Google Scholar]
- 45.Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I, Galaktidou G. Paricalcitol reduces basal and lipopolysaccharide-induced (LPS) TNF-alpha and IL-8 production by human peripheral blood mononuclear cells. Int Urol Nephrol. 2010;42:181–5. doi: 10.1007/s11255-009-9541-1. [DOI] [PubMed] [Google Scholar]
- 46.Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: A randomized double-blind pilot trial. Hypertension. 2008;52:249–55. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- 47.Ekici F, Ozyurt B, Erdogan H. The combination of Vitamin D3 and dehydroascorbic acid administration attenuates brain damage in focal ischemia. Neurol Sci. 2009;30:207–12. doi: 10.1007/s10072-009-0038-6. [DOI] [PubMed] [Google Scholar]
- 48.Fedirko V, Bostick RM, Long Q, Flanders WD, McCullough ML, Sidelnikov E, et al. Effects of supplemental Vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: A randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2010;19:280–91. doi: 10.1158/1055-9965.EPI-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kállay E, Bareis P, Bajna E, Kriwanek S, Bonner E, Toyokuni S, et al. Vitamin D receptor activity and prevention of colonic hyperproliferation and oxidative stress. Food Chem Toxicol. 2002;40:1191–6. doi: 10.1016/s0278-6915(02)00030-3. [DOI] [PubMed] [Google Scholar]
- 50.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term Vitamin D (3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 51.Goel RK, Lal H. Role of Vitamin D supplementation in hypertension. Indian J Clin Biochem. 2011;26:88–90. doi: 10.1007/s12291-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89-90:387–92. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Abdel-Aleem H, Merialdi M, Elsnosy ED, Elsedfy GO, Abdel-Aleem MA, Villar J. The effect of calcium supplementation during pregnancy on fetal and infant growth: A nested randomized controlled trial within WHO calcium supplementation trial. J Matern Fetal Neonatal Med. 2009;22:94–100. doi: 10.1080/14767050802464569. [DOI] [PubMed] [Google Scholar]
- 54.Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, et al. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal Vitamin D status in Tanzania. J Infect Dis. 2009;200:1022–30. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delvin EE, Salle BL, Glorieux FH, Adeleine P, David LS. Vitamin D supplementation during pregnancy: Effect on neonatal calcium homeostasis. J Pediatr. 1986;109:328–34. doi: 10.1016/s0022-3476(86)80396-1. [DOI] [PubMed] [Google Scholar]
- 56.Mallet E, Gügi B, Brunelle P, Hénocq A, Basuyau JP, Lemeur H. Vitamin D supplementation in pregnancy: A controlled trial of two methods. Obstet Gynecol. 1986;68:300–4. doi: 10.1097/00006250-198609000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Biesalski HK. Vitamin E requirements in parenteral nutrition. Gastroenterology. 2009;137(5 Suppl):S92–104. doi: 10.1053/j.gastro.2009.07.073. [DOI] [PubMed] [Google Scholar]
- 58.Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab. 2006;91:906–12. doi: 10.1210/jc.2005-1479. [DOI] [PubMed] [Google Scholar]
- 59.Kalra P, Das V, Agarwal A, Kumar M, Ramesh V, Bhatia E, et al. Effect of Vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr. 2012;108:1052–8. doi: 10.1017/S0007114511006246. [DOI] [PubMed] [Google Scholar]
- 60.Asemi Z, Foroozanfard F, Hashemi T, Bahmani F, Jamilian M, Esmaillzadeh A. Calcium plus Vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese Vitamin D deficient women with polycystic ovary syndrome. Clin Nutr. 2015;34:586–92. doi: 10.1016/j.clnu.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 61.Tabesh M, Azadbakht L, Faghihimani E, Esmaillzadeh A. Calcium-Vitamin D co-supplementation influences circulating inflammatory biomarkers and adipocytokines in Vitamin D insufficient diabetics: A randomized controlled clinical trial. J Clin Endocrinol Metab. 2014;99:E2485–93. doi: 10.1210/jc.2014-1977. [DOI] [PubMed] [Google Scholar]


