Abstract
Background:
Gallic acid (GA) is an endogenous plant phenol known to have antioxidant, free radical scavenging ability, anti-inflammatory, anti-cancer, and anti-fungal properties. The aim of this study was to assess the protective effect of GA on cyclophosphamide (CPA)-induced hepatotoxicity in male Wistar rats.
Methods:
Sixty rats were grouped into six groups of 10 rats per group. Group 1 received distilled water. Group 2 received CPA at 200 mg/kg single dose intraperitoneally on day 1. Groups 3 and 4 received a single dose of CPA (200 mg/kg) intraperitoneally on day 1 and then were treated with GA at 60 and 120 mg/kg body weight for 14 days, respectively. Rats in Groups 5 and 6 only received GA at 60 and 120 mg/kg body weight for 14 days, respectively. GA was administered orally.
Results:
CPA induced hepatic damage as indicated by significant elevation (P < 0.05) in aspartate aminotransferase, organ weight, and evidence by the histological study. CPA also induced hepatic oxidative stress as indicated by significant elevation (P < 0.05) in malondialdehyde content, hydrogen peroxide (H2O2) generation, nitrite level, and the level of glutathione (GSH) peroxidase crashed in the CPA-treated group. GA enhanced the antioxidant defense system as indicated by significant elevation (P < 0.05) in GSH level, catalase activity, and GSH-S-transferase activity.
Conclusions:
Taken together, the result of this present study shows that GA has a protective effect on CPA-induced hepatotoxicity.
Keywords: Antioxidant, cyclophosphamide, gallic acid, hepatotoxicity, oxidative stress
INTRODUCTION
Cyclophosphamide (CPA) is a cytotoxic alkylating agent that has been in clinical use for over 50 years and it is effective in the treatment of neoplastic diseases such as solid tumors and lymphomas as well as nonneoplastic diseases such as rheumatoid arthritis and systemic lupus erythematosus.[1] However, the clinical use of CPA has been limited due to its ability to damage normal tissues which usually resulted in multiple organ toxicity mainly in the heart, testes, and urinary bladder.[2] Hepatotoxicity is a major side effect of CPA as it is metabolized principally within the hepatocytes by hepatic microsomal cytochrome p450 mixed function oxidase system to produce its two active metabolite phosphoramide mustard and acrolein.[3,4] Phosphoramide is associated with its immunosuppressive and antineoplastic effect, while acrolein is associated with its toxic effect.[5,6] Studies have suggested that oxidative stress is associated with its hepatotoxic effect.[7] CPA toxicity results from acrolein binding to cellular antioxidant nucleophiles such as glutathione (GSH) resulting in the depletion of the antioxidant defense system and it also initiates lipid peroxidation (LPO).[8]
Gallic acid (GA) is an endogenous plant polyphenolic substance that is abundant in processed beverages such as red wines, green teas, grapes, different berries, and also a secondary metabolite of the plant.[9,10,11] Epidemiological studies have revealed that GA possess desirable health benefit beyond basic nutrition and that its presence in the diet is beneficial to human health. GA possesses anti-inflammatory, anti-allergic, anti-microbial, and antioxidant property.[12] Studies suggest that the binding of the gallate compounds to lipid membrane is a principal determining factor for its antioxidant property.[13]
Therefore, this study intends to examine the possible protective effects of GA on CPA- induced hepatotoxicity and determine whether this effect was modulated through antioxidant mechanisms in the liver.
Therefore, the present study was designed to examine the protective effects of GA on CPA-induced hepatotoxicity.
METHODS
Animal treatment
Sixty adult male rats weighing approximately (200–290 g) obtained from the Experimental Animal Unit of Faculty of Veterinary Medicine, University of Ibadan, Nigeria, were randomly divided into six groups of 10 animals per group. The animals were kept in wire mesh cages under controlled light cycle (12 h light/12 h dark) and fed with commercial rat chow ad libitum and liberally supplied with water.
Group 1 (Control) received saline. Group 2 received CPA at 200 mg/kg single dose intraperitoneally on day 1. Groups 3 and 4 received single dose of CPA (200 mg/kg) intraperitoneally on day 1 and were treated with GA at 60 and 120 mg/kg body weight for 14 days, respectively. Rats in groups 5 and 6 received GA at 60 and 120 mg/kg body weight for 14 days, respectively. GA was administered orally. Hepatotoxicity was induced as described.[14]
Care of animals
All the animals received humane care according to the criteria outline in the Guide for the Care and the Use of Laboratory Animals prepared by the National Academy of Science and published by the National Institute of Health. The ethics regulations were followed in accordance with national and institutional guidelines for the protection of the animals’ welfare during experiments.[15]
Chemicals
Potassium hydroxide, reduced GSH, trichloroacetic acid, sodium hydroxide, 1, 2-dichloro-4-nitrobenzene (1-chloro-2, 4-dinitrobenzene [CDNB]), thiobarbituric acid, xylenol orange, and hydrogen peroxide (H2O2), N-(1-naphthyl) ethylenediamine dihydrochloride, CPA, and 5, 5-dithiobis-(2-nitrobenzene) were purchased from Sigma (St. Louis, MO, USA). All other chemicals were of analytical grade.
Blood collection and preparation of erythrocyte
About 5 ml of blood was drawn from the retro-orbital venous plexus of the animals into vials without anticoagulant before they were sacrificed by cervical dislocation. The blood was centrifuged at 3,000 rpm for 15 min and the serum was aspirated using a Pasteur pipette and then stored at −4°C until the time of use.
Preparation of microsomal fraction from liver tissues
After euthanasia of the rats, the liver was removed, rinsed in 1.15% KCl, homogenized in aqueous potassium phosphate buffer (0.1 M, pH 7.4), and homogenates were centrifuged at 12,000 × g for 15 min to obtain the supernatant fraction. The supernatants obtained were stored at −4°C until the time of use.
Biochemical assays
The supernatants from the liver tissues were used for the following biochemical assays. Superoxide dismutase (SOD) was determined by measuring the inhibition of auto-oxidation of epinephrine at pH 10.2 at 30°C as described[16] with modification from our laboratory.[17,18] Catalase (CAT) activity was determined according to the method of Shinha.[19] The reduced GSH was determined at 412 nm using the method described by Jollow et al.[20] Glutathione-S-transferase (GST) was estimated by the method of Habig et al.[21] using CDNB as substrate. Protein concentration was determined by the method of Gornal et al.[22] The malondialdehyde (MDA) level was calculated according to the method of Farombi et al.[23] LPO in units/mg protein or gram tissue was computed with a molar extinction coefficient of 1.56 × 105 M−1 cm−1 according to the method of Farombi et al.[23] Glutathione peroxidase (GPx) activity was measured according to Beutler et al.[24] Hydrogen peroxide generation was determined as described.[25] The activities of aspartate aminotransferase (AST) was determined according to Reitman and Frankel[26] and was determined using the Randox kit (Random Laboratories Limited, UK).
Histopathology
Small pieces of liver tissues were collected in 10% formal saline buffer for proper fixation. These tissues were processed and embedded in paraffin wax. Sections of 5–6 μm in thickness were made and stained with hematoxylin and eosin for histopathological examination.[27]
Statistical analysis
All values are expressed as a mean ± standard deviation. The test of significance between two groups was estimated using Student's t-test. One-way ANOVA with Dunnett's posttest was also performed using GraphPad Prism version 4.00 (GraphPad Software, Inc., San Diego, California, USA.).
RESULTS
Hepatic markers of oxidative stress
The results obtained show that CPA administration significantly (P < 0.05) increased hepatic MDA content, H2O2 generation, and nitrite level [Figures 1 and 2]. However, posttreatment with GA (60 and 120 mg/kg body weight) significantly (P < 0.05) reduced the aforementioned markers of oxidative stress.
Figure 1.
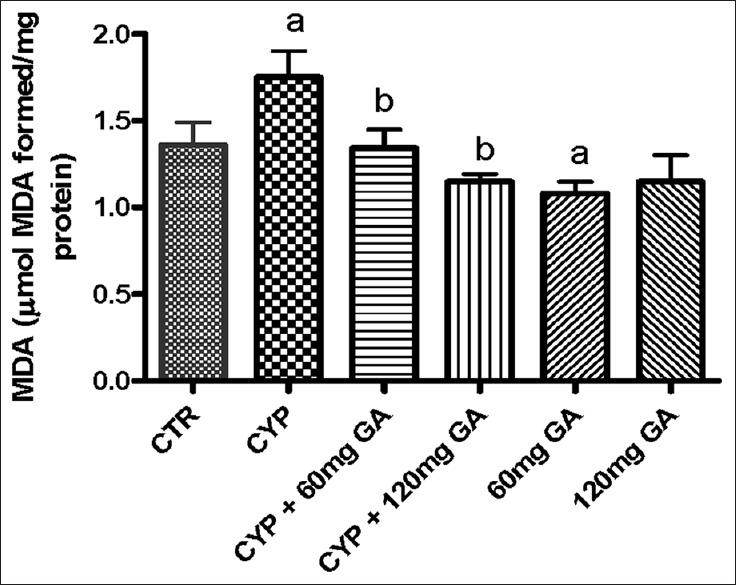
Effects of CYP on hepatic malondialdehyde and hydrogen peroxide (H2O2) contents. Superscript (a) indicates significant difference between CTR compared with CYP and GA, whereas superscript (b) indicates significant difference between CPY when compared with GA. P < 0.05 indicates significant difference and mean standard deviation (n = 10). CYP: Cyclophosphamide. CTR: Control, GA: Gallic acid
Figure 2.
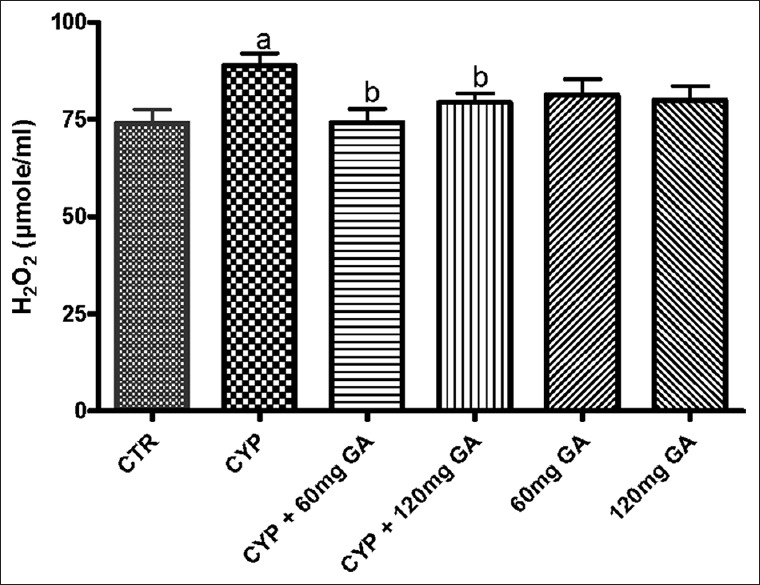
Effects of CYP on hepatic malondialdehyde and hydrogen peroxide (H2O2) contents. Superscript (a) indicates significant difference between CTR compared with CYP and GA, whereas superscript (b) indicates significant difference between CPY when compared with GA. P < 0.05 indicates significant difference and mean standard deviation (n = 10). CYP: Cyclophosphamide. CTR: Control, GA: Gallic acid
Hepatic nonenzymic and enzymic antioxidant defense system
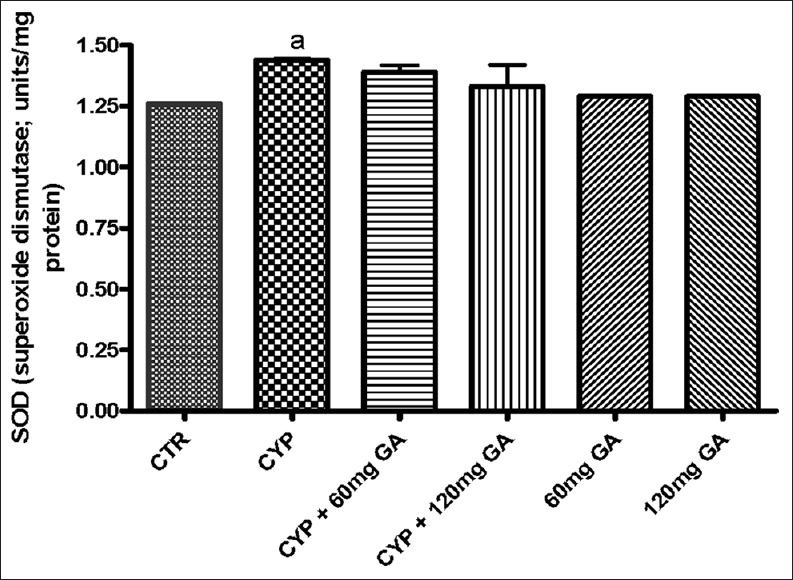
There was a significant (P < 0.05) reduction of the hepatic nonenzymic antioxidant (GSH) in and GPx in CPA-treated rats when compared with the control [Figures 3 and 4]. However, a significant (P < 0.05) increase of the hepatic GSH and GPx values in rats posttreated with GA when compared with the CPA-treated group was observed. However, the enzymatic antioxidant activities of hepatic CAT, SOD, and GST in CPA-treated rats were significantly increased compared to the control [Figures 5–7]. However, significant (P < 0.05) reductions in the activities of these enzymes were seen in rats posttreated with GA (60 and 120 mg/kg body weight). The increased activities of CAT, SOD, and GST in CPA-treated group reflect an innate adaptive response in the body of the rats to overcome the toxic challenge of CPA. There was a significant (P < 0.05) reduction in hepatic GPx activity in CPA-treated rats when compared with control [Figure 7]. Moreover, the activity of this antioxidant enzyme improved and increased significantly in the hepatic tissues of rats posttreated with GA (60 and 120 mg/kg body weight), respectively, as shown in Figure 7. Furthermore, CPA alone significantly increased nitrite content, whereas GA treatment attenuated aberration in nitrite content [Figure 8].
Figure 3.
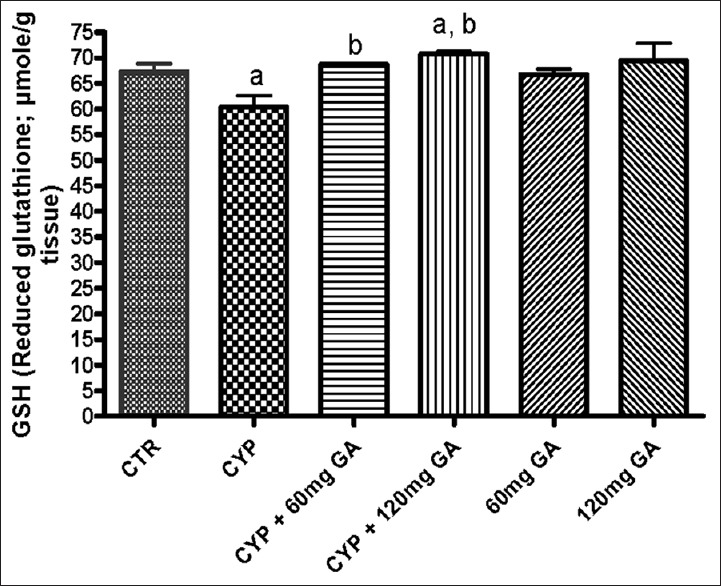
Effect of CYP on hepatic reduced glutathione and glutathione peroxidase activity. Superscript (a) indicates significant difference between CTR compared with CYP and GA whereas superscript (b) indicates significant difference between CPY when compared with GA. P < 0.05 indicates significant difference and mean standard deviation (n = 10). CYP: Cyclophosphamide, CTR: Control, GA: Gallic acid
Figure 4.
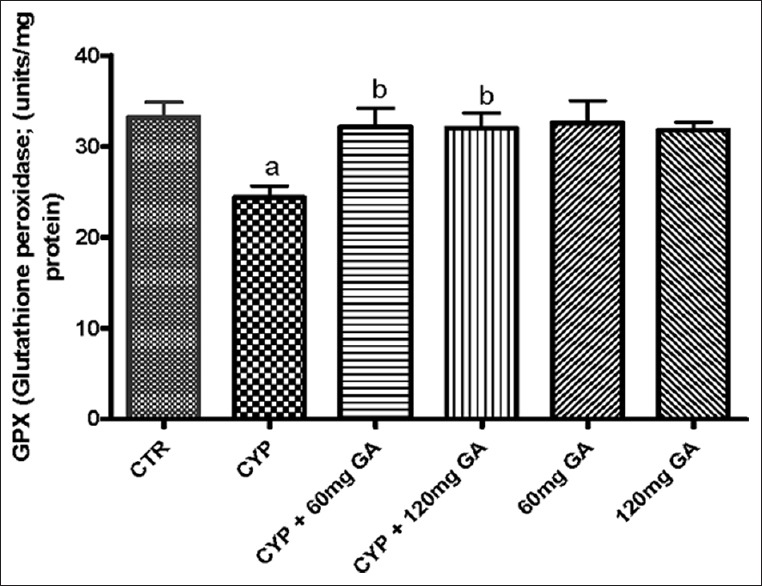
Effect of CYP on hepatic reduced glutathione and glutathione peroxidase activity. Superscript (a) indicates significant difference between CTR compared with CYP and GA, whereas superscript (b) indicates significant difference between CPY when compared with GA. P < 0.05 indicates significant difference and mean standard deviation (n = 10). CYP: Cyclophosphamide, CTR: Control, GA: Gallic acid
Figure 5.
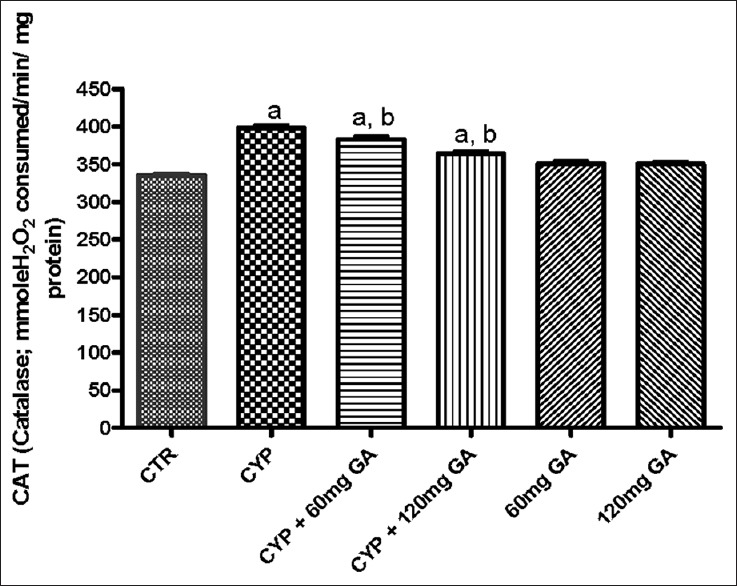
Effects of CYP on hepatic on catalase and glutathione-S-transferase activity. Superscript (a) indicates significant difference between CTR compared with CYP and GA whereas superscript (b) indicates significant difference between CPY when compared with GA. P < 0.05 indicates significant difference and mean standard deviation (n = 10). CYP: Cyclophosphamide. CTR: Control, GA: Gallic acid
Figure 7.
Effect of CYP on hepatic superoxide dismutase activity and nitrite serum nitrite content. Superscript (a) indicates significant difference between CTR compared with CYP and GA, whereas superscript (b) indicates significant difference between CPY when compared with GA. P < 0.05 indicates significant difference and mean standard deviation (n = 10). CYP: Cyclophosphamide, CTR: Control, GA: Gallic acid
Figure 8.
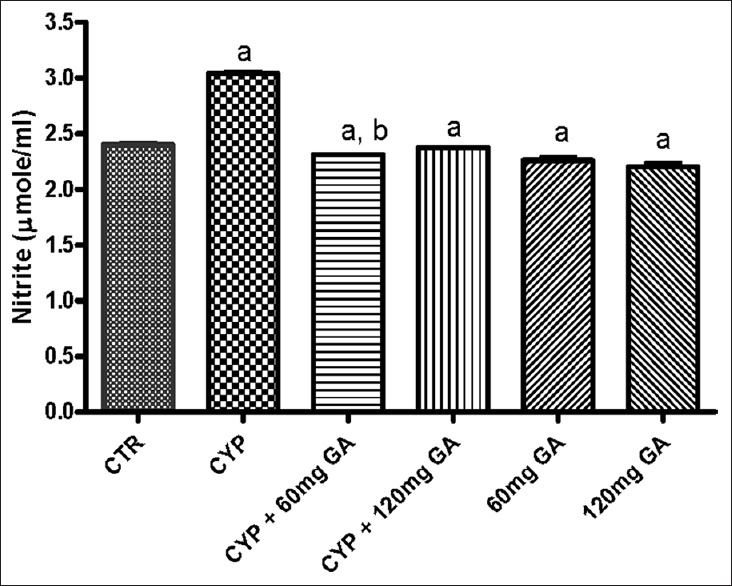
Effect of CYP on hepatic superoxide dismutase activity and nitrite serum nitrite content. Superscript (a) indicates significant difference between CTR compared with CYP and GA, whereas superscript (b) indicates significant difference between CPY when compared with GA. P < 0.05 indicates significant difference and mean standard deviation (n = 10). CYP: Cyclophosphamide, CTR: Control, GA: Gallic acid
Figure 6.
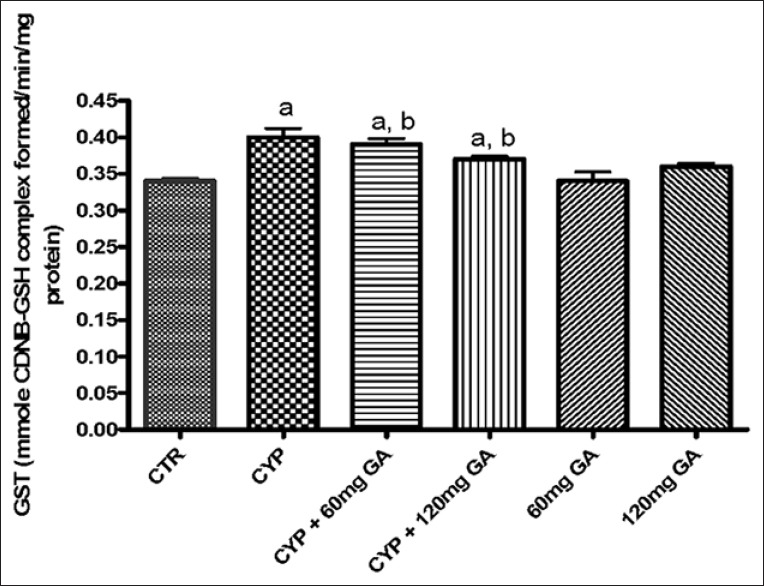
Effects of CYP on hepatic on catalase and glutathione-S-transferase activity. Superscript (a) indicates significant difference between CTR compared with CYP and GA, whereas superscript (b) indicates significant difference between CPY when compared with GA. P < 0.05 indicates significant difference and mean standard deviation (n = 10). CYP: Cyclophosphamide. CTR: Control, GA: Gallic acid
Serum marker of hepatic damage
The result obtained shows that CPA significantly (P < 0.05) increased serum alanine aminotransferase activity [Figure 9]. However, posttreatment with GA (60 and 120 mg/kg body weight) significantly (P < 0.05) reduced the activity of this enzyme in the serum.
Figure 9.
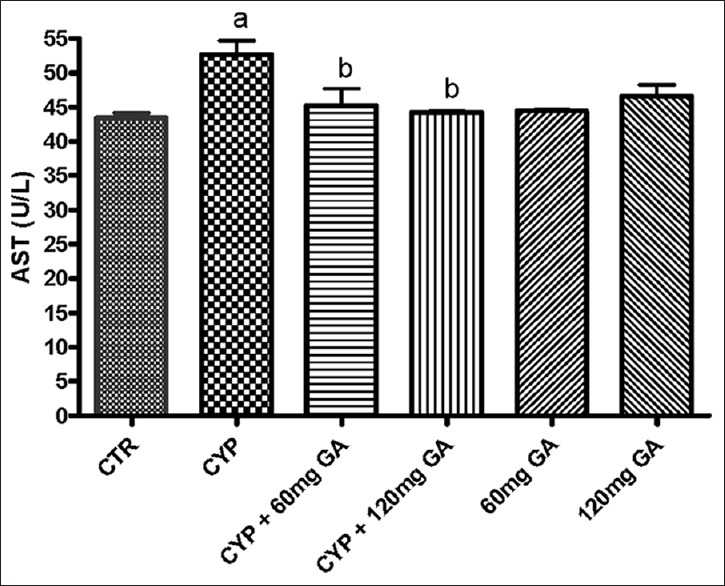
Effect of CYP on aminotransferase (aspartate aminotransferase) and liver weight. Superscript (a) indicates significant difference between CTR compared with CYP and GA, whereas superscript (b) indicates significant difference between CPY when compared with GA. P < 0.05 indicates significant difference and mean standard deviation (n = 10). CYP: Cyclophosphamide, CTR: Control, GA: Gallic acid
Hepatic weight
Figure 10 shows that there was significant (P < 0.05) increase in hepatic weight in CPA-treated rats when compared to the control. However, significant (P < 0.05) reduction in hepatic weight was seen in the rats posttreated with GA (60 and 120 mg/kg body weight) when compared with the CPA-treated rats.
Figure 10.
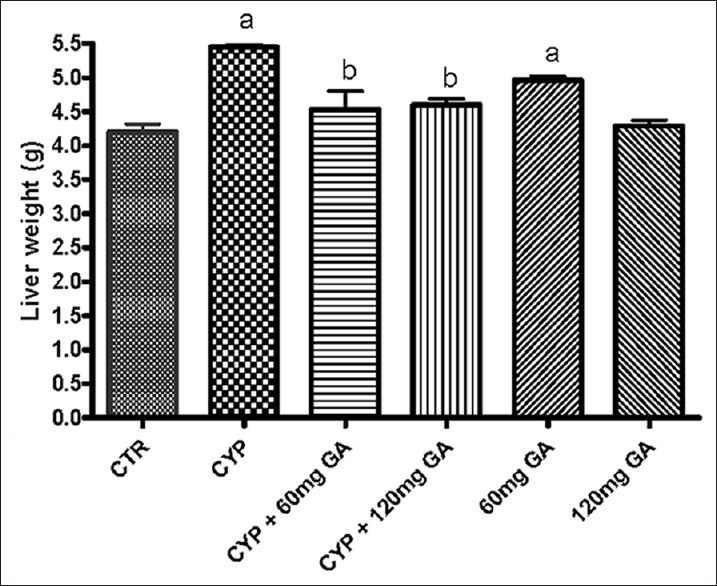
Effect of CYP on aminotransferase (aspartate aminotransferase) and liver weight. Superscript (a) indicates significant difference between CTR compared with CYP and GA, whereas superscript (b) indicates significant difference between CPY when compared with GA. P < 0.05 indicates significant difference and mean standard deviation (n = 10). CYP: Cyclophosphamide, CTR: Control and GA: Gallic acid
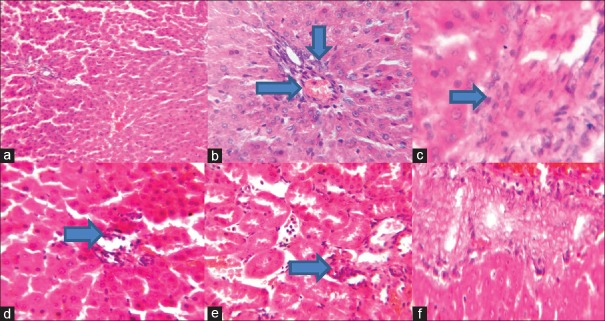
Histology
Figure 11 shows that CPA-treated rats have hepatic tissue periportal inflammation, hemorrhage, and congestion, while those treated with GA shows less lesion.
Figure 11.
Photomicrograph (a) shows no visible lesions, whereas rat treated with cyclophosphamide as indicated in photomicrograph (b) shows hepatic tissue periportal inflammation, hemorrhage, and congestion. Photomicrograph (c) of the hepatic tissue shows slight inflammation of the portal tract. Photomicrograph (d) shows the hepatic tissue with moderate inflammation of the portal triad and portal vein. Photomicrograph (e) of the hepatic tissue shows mild focal and periportal inflammation. No significant lesion seen in photomicrograph (f). Representative H and E, liver sections (×100 objectives)
DISCUSSION
CPA is an alkylating agent that is used in cancer chemotherapy and its use has been associated with several side effects. The toxic effect of CPA is associated with the production of acrolein, one of its metabolite.[28,29] Gave evidenced that antioxidants could protect normal cells against CPA toxicity. Bhatia et al.[30] and Rouissil and Kouidhi et al.[31] reported that hepatotoxicity observed with CPA treatment was associated with the induction of oxidative stress due to overproduction of ROS that eventually resulted in LPO of the cellular membrane. In this present study, we showed the protective effect of GA on CPA-induced hepatotoxicity. GA an endogenous plant phenol is known to have antioxidant, anti-cancer, anti-inflammatory, neuroprotective, and free radical scavenging activity.[12,32,33,34,35,36,37,38]
In this present study, administration of CPA resulted in significant elevation of MDA level, H2O2 generation, nitrite level, and significant reduction in the level of GSH, all these are indications of induction of oxidative stress by CPA. Studies have shown that oxygen-derived free radical plays an important role in the pathogenesis of injury to various tissues.[39]
One of the consequences of hepatic injury induced by CPA is the leaching out of markers of liver damage (alanine transaminase, AST, and alkaline phosphatase) from the hepatocyte resulting in increased enzyme activity in the systemic circulation.[40] This present study shows that CPA treatment resulted in hepatic injury seen from significantly increased activity of AST in the serum and also a significant increase in liver weight in CPA-treated group. Studies have shown that increased serum enzyme activity is a reflection of cellular damage and alteration of functional membrane integrity.[41] The present study also confirms the previous work that reported a significant increase in serum AST following induction of hepatotoxicity induced by CPA.[42,43] It has been[37,44] reported that low dose of CPA at 200 mg/kg body weight following intravenous administration can induce hepatotoxicity. Similarly, hepatoprotective effect of medicinal plants against CPA-induced toxicity has been documented.[7,42]
In this present study, administration of CPA resulted in significant elevation of MDA level, H2O2 generation, nitrite level, and significant reduction in the level of GSH, all these are an indication of induction of oxidative stress and nitrosative stress generated by the administration of CPA. Studies have shown that oxygen-derived free radicals play an important role in the pathogenesis of injury to various tissues.[39] However, GA treatment was able to mitigate hepatic damage associated with CPA treatment and this is attributed to its potent anti-inflammatory and antioxidant activity being able to protect the cellular membrane integrity and prevent inflammation.
MDA is one of the end products of LPO and its level within a tissue indicates the level of LPO. LPO is a major marker of oxidative stress, altered membrane structure, and enzyme inactivation.[45] From this study, the level of hepatic MDA was significantly increased in rats treated with CPA alone resulted in hepatic oxidative stress. However, treatment with GA resulted in significant reduction in hepatic MDA and this can be associated with its antioxidant potential and free radical scavenging activity. H2O2 is a reactive oxygen species and its level within the tissue can be used as a marker of oxidative stress. This present study revealed a high concentration of H2O2 in CPA-treated rats suggesting the induction of oxidative stress by CPA, whereas GA-treated rats showed significant reduction in H2O2 level, MDA content, and improvement in GSH levels, indicating antioxidant capacity of GA.
Nitrite, a metabolite of nitric oxide (NO) can be used to assess the overall formation of NO in vivo. NO is a short-lived and highly reactive free radical.[46] Our study shows a significant increase in hepatic nitrite level in CPA-treated rats. Moreover, treatment of experimental animals with GA significantly reduced hepatic nitrite level suggesting a protective role of the compound against nitrosative stress induced by CPA and its ultimate metabolites.
In this study, we also noticed the ability of CPA to alter the antioxidant defense system and detoxification status. Reduced GSH is a nonenzymatic antioxidant which functions as a direct free radical scavenger and a co-substrate for GPx. GSH plays an important role in protecting the cell against oxidative injury by scavenging free radicals and ROS and its involvement in the catalytic cycle of some antioxidant enzymes such as GPx, GSH reductase, and CAT are also very important.[47] There was a significant depletion of GSH following CPA treatment in this study and this reduction may be due to binding of CPA and its metabolites to GSH.[48] The depletion of GSH results in lowered cellular defense against free radical leading to cellular injury and cell death.[49] This study shows that GA prevented CPA hepatotoxicity. This may be due to its ability to prevent depletion of GSH; thus, preventing oxidative stress. Increased GSH level seen in GA-treated group may be due to its ability to enhance the activity of GSH synthesizing enzymes.
GPx is an antioxidant enzyme that breaks down H2O2 to water. Depletion of this enzyme is an indication of oxidative stress. In this study, there was depletion of this antioxidant enzyme (GPx) in rats administered with CPA and this contributed to the oxidative stress known to be associated with CPA administration. However, rats treated with GA showed significant increase activity of these enzymes comparable to those of control reflecting the antioxidant property of this compound and suggesting its ability to restore the activity of the antioxidant enzyme.
SOD, CAT, and GST are antioxidant enzymes that play a very important role in preventing the induction of oxidative stress. SOD is involved in the dismutation of superoxide anion to H2O2 and O2, this harmful H2O2 is further broken down to water by CAT.[50] GST, a family of cytosolic enzymes has an important role in detoxification of xenobiotics, drugs, and carcinogens and thus protects the cells against redox cycling and oxidative stress. Therefore, the activities of these antioxidant enzymes confer a vital protection against oxidative stress. This study revealed that there was a significant increase in the activity of these enzymes in CPA-treated rats. This increase should not lead to a misconception that there was no oxidative stress or hepatic damage but rather this increased enzyme activity may be due to the upregulation of the mRNA that codes for these enzymes in an attempt to overcome the toxic challenge induced following CPA treatment.
The hepatotoxic effect of CPA treatment was further ascertained by the assessment of histological alteration of the liver. Histological assessment of the hepatic tissue in CPA-treated rats showed periportal inflammation, hemorrhage, and congestion; these pathological changes may be associated with the ability of CPA to induce the generation of free radical and to deplete the antioxidant defense system. However, those treated with GA only showed hepatic tissue with mild to moderate inflammation further reflecting the anti-inflammatory activity of GA.
CONCLUSIONS
From the present study, CPA treatment resulted in oxidative stress and also disrupts the antioxidant defense system within the liver, and GA treatment reverses the oxidative stress by enhancing the antioxidant defense system and preventing LPO. We, therefore, speculate that the inherent antioxidant property of this compound is responsible for its hepatoprotective effect against CPA-induced toxicity. We also suggest that to minimize the several side effects of CPA during chemotherapy, food supplement containing GA can be used as an adjunct to chemotherapy.
ACKNOWLEDGEMENTS
The authors want to acknowledge the technical assistance of Mr. Agboola Olalekan Samson of the Department of Veterinary Physiology, Biochemistry and Pharmacology, Faculty of Veterinary Medicine, University of Ibadan, Nigeria.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lawson M, Vasilaras A, De Vries A, Mactaggart P, Nicol D. Urological implications of cyclophosphamide and ifosfamide. Scand J Urol Nephrol. 2008;42:309–17. doi: 10.1080/00365590701570953. [DOI] [PubMed] [Google Scholar]
- 2.Fraiser LH, Kanekal S, Kehrer JP. Cyclophosphamide toxicity. Characterising and avoiding the problem. Drugs. 1991;42:781–95. doi: 10.2165/00003495-199142050-00005. [DOI] [PubMed] [Google Scholar]
- 3.King PD, Perry MC. Hepatotoxicity of chemotherapy. Oncologist. 2001;6:162–76. doi: 10.1634/theoncologist.6-2-162. [DOI] [PubMed] [Google Scholar]
- 4.Ludeman SM. The chemistry of the metabolites of cyclophosphamide. Curr Pharm Des. 1999;5:627–43. [PubMed] [Google Scholar]
- 5.Kern JC, Kehrer JP. Acrolein-induced cell death: A caspase-influenced decision between apoptosis and oncosis/necrosis. Chem Biol Interact. 2002;139:79–95. doi: 10.1016/s0009-2797(01)00295-2. [DOI] [PubMed] [Google Scholar]
- 6.Angulo I, Jiménez-Díaz MB, García-Bustos JF, Gargallo D, de las Heras FG, Muñoz-Fernández MA, et al. Candida albicans infection enhances immunosuppression induced by cyclophosphamide by selective priming of suppressive myeloid progenitors for NO production. Cell Immunol. 2002;218:46–58. doi: 10.1016/s0008-8749(02)00521-x. [DOI] [PubMed] [Google Scholar]
- 7.Zarei M, Shivanandappa T. Amelioration of cyclophosphamide-induced hepatotoxicity by the root extract of Decalepis hamiltonii in mice. Food Chem Toxicol. 2013;57:179–84. doi: 10.1016/j.fct.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Adams JD, Jr, Klaidman LK. Acrolein-induced oxygen radical formation. Free Radic Biol Med. 1993;15:187–93. doi: 10.1016/0891-5849(93)90058-3. [DOI] [PubMed] [Google Scholar]
- 9.Ma J, Luo XD, Protiva P, Yang H, Ma C, Basile MJ, et al. Bioactive novel polyphenols from the fruit of Manilkara zapota (Sapodilla) J Nat Prod. 2003;66:983–6. doi: 10.1021/np020576x. [DOI] [PubMed] [Google Scholar]
- 10.Singh J, Rai GK, Upadhyay AK, Kumar R, Singh KP. Antioxidant phytochemicals in tomato (Lycopersicon esculentum) Indian J Agric Sci. 2004;74:3–5. [Google Scholar]
- 11.Abu-Amsha Caccetta R, Burke V, Mori TA, Beilin LJ, Puddey IB, Croft KD. Red wine polyphenols, in the absence of alcohol, reduce lipid peroxidative stress in smoking subjects. Free Radic Biol Med. 2001;30:636–42. doi: 10.1016/s0891-5849(00)00497-4. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Gu Q, Gao T, Wang X, Chue P, Wu Q, et al. Flavonols and derivatives of gallic acid from young leaves of Toona sinensis (A. Juss.) Roemer and evaluation of their anti-oxidant capacity by chemical methods. Pharmacogn Mag. 2014;10:185–90. doi: 10.4103/0973-1296.131034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahrzad S, Aoyagi K, Winter A, Koyama A, Bitsch I. Pharmacokinetics of gallic acid and its relative bioavailability from tea in healthy humans. J Nutr. 2001;131:1207–10. doi: 10.1093/jn/131.4.1207. [DOI] [PubMed] [Google Scholar]
- 14.Mahmoud AM. Hesperidin protects against cyclophosphamide-induced hepatotoxicity by upregulation of PPARg and abrogation of oxidative stress and inflammation. Can J Physiol Pharmacol. 2014;92:717–24. doi: 10.1139/cjpp-2014-0204. [DOI] [PubMed] [Google Scholar]
- 15.Washington, DC: U.S Department of Health and Human Services; 1996. PHS (Public Health Service). Public Health Service Policy on Humane Care and the Use of Laboratory Animals; pp. 99–158. [Google Scholar]
- 16.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 17.Omobowale TO, Oyagbemi AA, Akinrinde AS, Saba AB, Daramola OT, Ogunpolu BS, et al. Failure of recovery from lead induced hepatoxicity and disruption of erythrocyte antioxidant defence system in wistar rats. Environ Toxicol Pharmacol. 2014;37:1202–11. doi: 10.1016/j.etap.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Oyagbemi AA, Omobowale TO, Akinrinde AS, Saba AB, Ogunpolu BS, Daramola O. Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol. 2015;30:1235–43. doi: 10.1002/tox.21994. [DOI] [PubMed] [Google Scholar]
- 19.Shinha KA. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 20.Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Philadelphia, Pennsylvania, USA: JB lippinocott, USA JB lippinocott; 1994. Bromobenzene-induced liver necrosis; protective role of GSH and evidence for 3, 4 bromobenzene oxide as the hepatotoxic metabolite; pp. 108–44. [DOI] [PubMed] [Google Scholar]
- 21.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 22.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177:751–66. [PubMed] [Google Scholar]
- 23.Farombi EO, Tahnteng JG, Agboola AO, Nwankwo JO, Emerole GO. Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron – A Garcinia kola seed extract. Food Chem Toxicol. 2000;38:535–41. doi: 10.1016/s0278-6915(00)00039-9. [DOI] [PubMed] [Google Scholar]
- 24.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 25.Wolff SF. Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydrogen peroxides. Methods Enzymol. 1994;233:182–9. [Google Scholar]
- 26.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 27.Drury RA, Wallington EA, Cancerson R. 4th ed. Oxford, London, New York: Oxford University Press; 1976. Carlton's Histopathological Techniques. [Google Scholar]
- 28.Sheeja K, Kuttan G. Ameliorating effects of Andrographis paniculata extract against cyclophosphamide-induced toxicity in mice. Asian Pac J Cancer Prev. 2006;7:609–14. [PubMed] [Google Scholar]
- 29.Tripathi DN, Jena GB. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: Role of Nrf2, p53, p38 and phase-II enzymes. Mutat Res. 2010;696:69–80. doi: 10.1016/j.mrgentox.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Bhatia K, Ahmad F, Rashid H, Raisuddin S. Protective effect of S-allylcysteine against cyclophosphamide-induced bladder hemorrhagic cystitis in mice. Food Chem Toxicol. 2008;46:3368–74. doi: 10.1016/j.fct.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Rouissil K, Kouidhi S, Hamrita B, Ouerhani S, Cherif M, Elgaaied AB. Therapeutic effects of Aloe vera plant extract against cyclophosphamide and buthionine sulfoximine induced toxicities in the bladder. Biochem Pharmacol. 2012;1:101. [Google Scholar]
- 32.Kuo CL, Lai KC, Ma YS, Weng SW, Lin JP, Chung JG. Gallic acid inhibits migration and invasion of SCC-4 human oral cancer cells through actions of NF-kB, Ras and matrix metalloproteinase-2 and -9. Oncol Rep. 2014;32:355–61. doi: 10.3892/or.2014.3209. [DOI] [PubMed] [Google Scholar]
- 33.Asnaashari M, Farhoosh R, Sharif A. Antioxidant activity of gallic acid and methyl gallate in triacylglycerols of Kilka fish oil and its oil-in-water emulsion. Food Chem. 2014;159:439–44. doi: 10.1016/j.foodchem.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 34.Korani MS, Farbood Y, Sarkaki A, Fathi Moghaddam H, Taghi Mansouri M. Protective effects of gallic acid against chronic cerebral hypoperfusion-induced cognitive deficit and brain oxidative damage in rats. Eur J Pharmacol. 2014;733:62–7. doi: 10.1016/j.ejphar.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 35.Trevisan G, Rossato MF, Tonello R, Hoffmeister C, Klafke JZ, Rosa F, et al. Gallic acid functions as a TRPA1 antagonist with relevant antinociceptive and antiedematogenic effects in mice. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:679–89. doi: 10.1007/s00210-014-0978-0. [DOI] [PubMed] [Google Scholar]
- 36.Abarikwu SO, Akiri OF, Durojaiye MA, Alabi AF. Combined administration of curcumin and gallic acid inhibits gallic acid-induced suppression of steroidogenesis, sperm output, antioxidant defenses and inflammatory responsive genes. J Steroid Biochem Mol Biol. 2014;143:49–60. doi: 10.1016/j.jsbmb.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Subramanian V, Venkatesan B, Tumala A, Vellaichamy E. Topical application of gallic acid suppresses the 7,12-DMBA/croton oil induced two-step skin carcinogenesis by modulating anti-oxidants and MMP-2/MMP-9 in Swiss albino mice. Food Chem Toxicol. 2014;66:44–55. doi: 10.1016/j.fct.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Zhao B, Hu M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncol Lett. 2013;6:1749–55. doi: 10.3892/ol.2013.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santra A, Chowdhury A, Chaudhuri S, Das Gupta J, Banerjee PK, Mazumder DN. Oxidative stress in gastric mucosa in Helicobacter pylori infection. Indian J Gastroenterol. 2000;19:21–3. [PubMed] [Google Scholar]
- 40.Senthilkumar S, Devaki T, Manohar BM, Babu MS. Effect of squalene on cyclophosphamide-induced toxicity. Clin Chim Acta. 2006;364:335–42. doi: 10.1016/j.cca.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 41.Kumar G, Banu GS, Kannan V, Pandian MR. Antihepatotoxic effect of beta-carotene on paracetamol induced hepatic damage in rats. Indian J Exp Biol. 2005;43:351–5. [PubMed] [Google Scholar]
- 42.Nitharwal RK, Patel H, Karchuli MS, Ugale RR. Chemoprotective potential of Coccinia indica against cyclophosphamide-induced toxicity. Indian J Pharmacol. 2013;45:502–7. doi: 10.4103/0253-7613.117783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Germoush MO, Mahmoud AM. Berberine mitigates cyclophosphamide-induced hepatotoxicity by modulating antioxidant status and inflammatory cytokines. J Cancer Res Clin Oncol. 2014;140:1103–9. doi: 10.1007/s00432-014-1665-8. [DOI] [PubMed] [Google Scholar]
- 44.Akay H, Akay T, Secilmis S, Kocak Z, Donderici O. Hepatotoxicity after low-dose cyclophosphamide therapy. South Med J. 2006;99:1399–400. doi: 10.1097/01.smj.0000251467.62842.ad. [DOI] [PubMed] [Google Scholar]
- 45.Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol 2012. 2012 doi: 10.5402/2012/137289. 137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paula FB, Gouvêa CM, Alfredo PP, Salgado I. Protective action of a hexane crude extract of Pterodon emarginatus fruits against oxidative and nitrosative stress induced by acute exercise in rats. BMC Complement Altern Med. 2005;5:17. doi: 10.1186/1472-6882-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–55. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yousefipour Z, Ranganna K, Newaz MA, Milton SG. Mechanism of acrolein-induced vascular toxicity. J Physiol Pharmacol. 2005;56:337–53. [PubMed] [Google Scholar]
- 49.Anderstam B, Vaca C, Harms-Ringdahl M. Lipid peroxide levels in a murine adenocarcinoma exposed to hyperthermia: The role of glutathione depletion. Radiat Res. 1992;132:296–300. [PubMed] [Google Scholar]
- 50.Matés JM, Pérez-Gómez C, Núñez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]