Abstract
The signal transduction molecule, Stat1, is critical for the expression of type I and II interferon (IFN)-responsive genes in most cells; however, we previously showed that primary hippocampal mouse neurons express low basal Stat1, with delayed and attenuated expression of IFN-responsive genes. Moreover, IFNγ-dependent resolution of a neurotropic viral challenge in permissive mice is Stat1-independent. Here, we show that exogenous INFγ has no deleterious impact on neuronal viability, and staurosporine-induced apoptosis in neurons is significantly blunted by the addition of INFγ, suggesting that INFγ confers a pro-survival signal in neurons. To identify the pathways induced by INFγ in neurons, the activation of alternative signal transducers associated with INFγ signaling was assessed. Rapid and pronounced activation of extracellular signal regulated kinase (Erk1/2) was observed in neurons, compared to a modest response in fibroblasts. Moreover, the absence of Stat1 in primary fibroblasts led to enhanced Erk activation following IFNγ addition, implying that the cell-specific availability of signal transducers can diversify the cellular response following IFN engagement.
Keywords: Interferon-gamma, Erk-1/2, neuron, hippocampus, Stat1, astrocytes, inflammation, cytokines
Introduction
Cell type-intrinsic variations in the abundance or bioavailability of key signal transduction molecules could substantially skew the cellular response to a given extracellular ligand. For those cytokines that induce an anti-viral state, such cell-specific diversification may dictate whether a virus-infected cell will undergo apoptosis, initiate an anti-viral program, or induce pro-survival genes to maximize cell viability. We have focused on defining how central nervous system (CNS) neurons respond to extracellular interferons (specifically, interferon gamma; IFNγ) following viral infection, and how the relative availability of associated signaling molecules within the neuronal cytosol shapes survival and viral clearance (O'Donnell et al. 2012, Rose et al. 2007, Patterson et al. 2002). Here, we define a critical contribution of the IFNγ-induced Erk1/2 pathway in conferring protection to neurons.
IFNγ is the sole type II IFN within the IFN family, which includes type I and III IFNs as well. Unlike type I IFNs, which are expressed by most cells soon after infection, IFNγ is chiefly produced by activated immune cells, including natural killer cells and T cells (Biron et al. 1999, Kawanokuchi et al. 2006). The prevailing view is that IFNγ initiates a cellular response by binding to the IFNγ receptor complex (consisting of a hetero-tetramer of IFNγR1 and R2 subunits), triggering activation of receptor-associated Janus Kinases (Jak)-1/2, and subsequent tyrosine phosphorylation of the cytoplasmic tail of the IFNγR1 subunits. Signal Transducer and Activator of Transcription (Stat)-1 is recruited to the phosphorylated R1 subunit, followed by its phosphorylation, homodimerization, and translocation to the nucleus. Once in the nucleus, activated Stat1 binds to Gamma Activated Sequence (GAS) elements within the promoters of more than 250 IFNγ-responsive genes (ISGs) (Stark et al. 1998). Expression of these genes comprises the canonical antiviral program. While Stat1 is thought to be central to an IFNγ response, a number of studies have shown that Stat1-independent pathways also exist (Gil et al. 2001, Joshi et al. 2010, Kaur et al. 2008, Lin & Lin 2010, Mann et al. 2008, Ramana et al. 2001, Ramana et al. 2005, Shresta et al. 2005, Soler et al. 2003). For example, Stat1 plays a biphasic role in the control of systemic Dengue infection in mice: Stat1-dependent pathways are required for early viral control, but Stat1-independent pathways are required for eventual viral clearance (Shresta et al. 2005).
Though the brain has historically been considered an immune privileged site, the host immune response can successfully resolve many neurotropic viral and bacterial infections (Binder & Griffin 2001, Burdeinick-Kerr & Griffin 2005, Cantin et al. 1999, Fiette et al. 1995, Geiger et al. 1997, Jin et al. 2004, Pearce et al. 1994, Metcalf et al. 2013, Gomme et al. 2012, Shrestha et al. 2012, Brooke et al. 2012). However, distinct immune strategies may be employed, depending on the CNS cell type that is infected. For example, mouse hepatitis virus (MHV), which can infect astrocytes, microglia, and oligodendrocytes (Wang et al. 1992) is cleared from astrocytes and microglia by a perforin-dependent process, whereas IFNγ is sufficient for MHV control in oligodendrocytes (Bergmann et al. 2006, Parra et al. 1999). Noncytolytic viral control, mediated by IFNγ, occurs following infection by a number of other neurotropic viruses (Larena et al. 2013, Burdeinick-Kerr & Griffin 2005, Patterson et al. 2002, Finke et al. 1995, Stubblefield Park et al. 2011), but recent data has shown that the signaling pathways triggered by IFNγ may differ in infected neurons. For example, certain subsets of neural cells, such as neural precursors in the retina, preferentially utilize Stat3 instead of Stat1 in response to IFNγ (Zhang et al. 2005). Thus, while IFNγ is a crucial immune mediator in the brain, the role of Stat1, particularly in neurons, remains less well-defined.
Our previous work utilized a novel transgenic mouse system in which infection with a vaccine strain of measles (MV) virus is restricted to CNS neurons. In this model, the MV vaccine strain receptor, CD46, is transcriptionally restricted to neurons by the neuron-specific enolase (NSE) promoter (Rall et al. 1997). While all immunocompetent NSE-CD46 adults survive an intracerebral MV challenge, T and B cell deficient NSE-CD46/Rag2 KO mice succumb to unrestricted viral replication by 2–3 weeks post-challenge (O'Donnell et al. 2012, Patterson et al. 2002). Subsequent efforts to define the immunological components responsible for protection revealed a key role for IFNγ: NSE-CD46/IFNγ KO mice are as vulnerable to MV-induced neuropathology as NSE-CD46/Rag KO mice (Patterson et al. 2002). We subsequently demonstrated that the canonical IFNγ response is not induced in neurons, likely owing to delayed and attenuated activation of Stat1 (O'Donnell et al. 2012, Rose et al. 2007, Podolsky et al. 2012). These data indicate that IFNγ-triggered, but Stat1-independent, signaling pathways are likely operative in CNS neurons. Such cell-intrinsic differences between neurons and non-neuronal cells may be important in the diversification of the cellular response to a viral insult or to the potentially cytotoxic immune response.
One of our objectives is to define how the abundance or bioavailability of key signal transduction molecules within CNS neurons shapes survival and viral clearance. In this manuscript, we define a central role for an IFNγ-induced, Stat1-independent pathway that affords protection of neurons from cytotoxicity. We present these findings in the context of a “receptor occupancy” model, in which the bioavailability of cytosolic substrates defines the antiviral pathways that are preferentially activated upon IFN engagement.
Materials and Methods
Primary cells and transgenic mice
Primary hippocampal neurons were prepared from embryonic (E15-16) mice as described (O'Donnell et al. 2012, Rose et al. 2007, Banker 1998). Neurons were plated on 15mm diameter glass coverslips or in 12-well plates coated with poly-L-lysine (Sigma) at a density of 2.5×105 cells/well, unless noted. Neuron cultures were routinely >95% MAP2 positive. Neurons were plated and incubated at 37°C in a humidified incubator with 5% CO2 for 5d prior to IFNγ treatment to allow for differentiation.
Mixed glial cultures were prepared from E16 cortices as described (O'Donnell et al. 2012, Banker 1998) and were routinely >95% GFAP positive. After 7d in culture, monolayers were trypsinized and re-plated in poly-L-lysine coated 96-well plates (1×104 cells/well) and incubated for 48h before treatment. Primary mouse embryonic fibroblasts (MEF) were isolated from the same embryos and maintained in complete DMEM medium (supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 ng/ml streptomycin). Briefly, liver tissue was removed, and the remaining specimen was then dissociated in 0.4% trypsin, followed by trituration. The suspension was incubated for 10 min at 37°C; 5 ml fresh trypsin was then added and the suspension was incubated for an additional 10 min at 37°C. The suspension was transferred to a 15 ml conical tube, in which undigested tissue was allowed to settle for 2 min. The cell suspension was then mixed with complete DMEM medium and centrifuged at 400×g for 5 min. The resulting pellet was resuspended in complete DMEM medium and plated into culture flasks. All cells were maintained at 37°C, 5% CO2 in a humidified incubator.
Homozygous NSE-CD46 transgenic mice (line 18; H-2b) (Rall et al. 1997) and NSE-CD46+/Stat1-KO mice were maintained in the closed breeding colony of The Fox Chase Cancer Center. All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee. Homozygous NSE-CD46 and haplotype-matched homozygous immune knockout (KO) mice were intercrossed for three or more generations to obtain NSE-CD46 mice on the desired KO background. Stat1-KO mice were originally purchased from Taconic, and intercrossed to the NSE-CD46 line at Fox Chase Cancer Center. The genotypes of all strains of mice were confirmed by PCR analysis of tail biopsy DNA for each experiment.
IFNγ, staurosporine, and kinase inhibitor treatments
Primary mouse embryonic fibroblasts, primary cortical astrocytes, and primary hippocampal neurons from NSE-CD46 mice and NSE-CD46/Stat1-KO mice were treated with IFNγ at a final concentration of 1-1000U/ml, depending upon the experiment. Murine IFNγ (BD Transduction Labs) was prepared as a 10x working stock in B27-free Neurobasal Media, and diluted in conditioned culture media to the desired concentration.
For kinase inhibitor studies, all inhibitors (Tocris) were diluted to a stock of 100μM in 10% DMSO in PBS. Stocks were then directly added to cell culture media for a final concentration of 10μM. Inhibitors were incubated with the cells for 1 h prior to the addition of IFNγ at 37°C.
For some experiments, staurosporine (Tocris) was diluted to a stock solution of 10mM in DMSO, and diluted to a working solution of 10μM in neurobasal media. Staurosporine was diluted in the culture media at different concentrations (1-200nM) as noted. For experiments using an Erk-1/2 inhibitor and IFNγ, cells were treated with FR 180204 (10μM) for 1h, then with IFNγ for 30 min, and with staurosporine for 72h before assaying for MAP2 levels by In Cell western.
Bromodeoxyuridine (BrdU) and 7-amino-actinomycin D (7-AAD) labeling
Cell cycle progression was measured through BrdU and 7-AAD staining (BD Biosciences) according to manufacturer's instructions. Briefly, fibroblast or mixed glial cultures were incubated with IFNγ for 24 or 72h, and BrdU (25mM) was added for one hour prior to harvest. Cells were trypsinized, fixed and permeabilized using 1X cytofix/cytoperm solution (BD biosciences), and incubated with an anti-BrdU antibody labeled with fluorescein isothiocyanate (FITC; 30 min) and 7-AAD (25μM, 15 min). Labeled cells were assayed by flow cytometry (Accuri C6, BD Biosciences) with live cell gating.
Immunofluorescence Assay
Primary neurons plated on coverslips were fixed in a 1:1 solution of methanol:acetone for 5 min at −20°C, and rehydrated in DPBS. To prevent nonspecific antibody binding, cells were incubated in a 10% goat serum/20% fetal bovine serum solution in DPBS for 1 h at RT. Rabbit polyclonal anti-MAP2 (Chemicon; 1:250) was then added in blocking solution for 1h at RT. After three washes in DPBS, the cells were incubated in secondary antibody in blocking solution for 1h at RT (anti-rabbit AlexaFluor-555; 1:10,000; Invitrogen) with Hoechst 33342 nuclear stain (10 μM, Molecular Probes). After three washes in DPBS, coverslips were mounted onto glass slides using Citi-Fluor AF1 (Electron Microscopy Sciences, Inc). Images were captured using an Inverted TE2000 with Nikon C1 confocal scanhead (40X objective) in the Fox Chase Cancer Center Confocal Facility.
Immunoblots
Treated cells were collected at the indicated times in Cell Lysis Buffer with 1x protease inhibitor cocktail (Sigma), and immunoblots performed as previously described (O'Donnell et al. 2012). Antibodies used included: rabbit anti-Stat1 total (1:1000; BD Biosciences), mouse anti-phospho-specific Stat1 (pY701) (1:1000; BD Biosciences); rabbit anti-Erk1/2 total (1:1000; Cell Signaling Technologies), rabbit anti-phospho Erk1/2 (1:1000; Cell Signaling Technologies), rabbit anti-cleaved caspase-3 (1:500; Cell Signaling Technologies) and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:10,000; Chemicon). All were diluted in PBS-T containing 5% milk. After three washes in PBS-T (10 min each), the blots were incubated in secondary antibody solution for 1h at RT. Antibodies included: goat anti-rabbit horseradish peroxidase (HRP; 1:1000; Vector Laboratories Inc.) or goat anti-mouse HRP (1:2000; Santa Cruz) in PBS-T with 5% milk. For quantitative analysis of immunoblots, densitometric analysis was performed using NIH Image software (v.1.63). For phospho- and total Erk-1/2, the 44 and 42 kD bands were quantified together. When reprobing was necessary, blots were stripped in an acidic glycine/SDS solution for 2h at RT.
Cell Viability Assays
ATP levels were assessed with a modification of the luciferase-based Cell Titer Glo assay (25 μl Cell Titer Glo reagent in 50 μl media) as described previously (Posimo et al. 2013). Neurons, astrocytes, and MEFs were plated in poly-L-lysine coated plates as described above. Cells were treated with IFNγ for 72h, and 150 μl of media was removed from each well before addition of the Cell Titer Glo reagent. Luminescence was measured after a 30 min incubation at RT.
Cell viability was measured by methanethiosulfonate (MTS) One Solution colorimetric assay (Promega). Neurons were plated at in poly-L-lysine coated 96-well plates at 7×104 cells/well, and maintained for 5d in vitro before addition of pharmacological inhibitors and IFNγ for 72h. Each condition was repeated in triplicate on two separate plates. MTS One Reagent (40μl) was added to each well according to the manufacturer's instructions and incubated for 2h at 37°C. Plates were read on a 96-well plate reader (VICTOR3 1420 multi-label counter; PerkinElmer) at 490nm.
In Cell Western
Primary hippocampal neurons were plated at 70,000 cells/well in 96-well plates, and maintained for 5d in neurobasal media before treatment with FR 180204, IFNγ, and/or staurosporine. After treatments, cells were fixed in 4% paraformaldehyde/4% sucrose in PBS for 30 min and washed 2x in PBS. Cells were blocked in a 1:1 mix of Odyssey Blocking Buffer (Licor) and PBS for 30 min and incubated in primary antibody (rabbit anti-MAP2, 1:500; Millipore) in Odyssey Blocking Buffer:PBS at 4°C overnight. Cells were washed 3x with PBS and incubated in secondary antibody (anti-rabbit IgG-800CW, 1:5000; Licor) and DRAQ5 far-red DNA stain (0.5 μM, Biostatus) for 1h at room temp. Plates were washed 3x with PBS and imaged on a Licor Odyssey Imager.
Statistical Analysis
Statistical significance was determined by Student's t-test (GraphPad Prism, Version 6.0); p < 0.05 was considered significant. Cell viability assays were performed at least twice, using primary cells from different dissections. Western blot data was performed in at least three independent experiments. The number of replicates for each experiment is noted within the figure legends. For comparison of Erk1/2 activation across wildtype and knockout genotypes and different cell types, two-way ANOVA was used.
Results
Exogenous IFNγ does not impair neuronal viability
Previous work from our lab showed that IFNγ was required for control of a neuron-restricted MV infection in a transgenic mouse model (NSE-CD46), but indicated that Stat1, the primary signal transducer through which IFNγ signals, was dispensable for viral clearance (Patterson et al. 2002, O'Donnell et al. 2012). To define the Stat1-independent pathways that may be uniquely activated in neurons, we first compared the effects of IFNγ in primary neurons and primary, actively dividing cells. As neurons are generally nonrenewable, we hypothesized that the cytotoxicity seen in many mitotic cells following IFNγ treatment (Chin et al. 1997, Dai & Krantz 1999, Kano et al. 1997, Pedersen et al. 2004, Shin et al. 2001) may be dampened or absent in neurons.
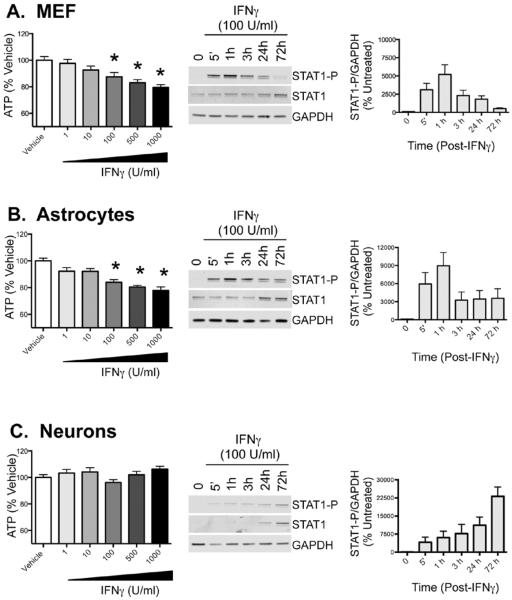
Explanted primary mouse hippocampal neurons, astrocytes and fibroblasts were treated with recombinant murine IFNγ at varying concentrations (1–1000 U/ml); 72h after treatment, cell viability was quantified using a CellTiter Glo assay, which measures cellular ATP production. Reduced ATP production may be indicative of a loss of cells due to cell death, inhibition of proliferation of dividing cells, or perturbations in cellular metabolism. As shown in Figure 1, IFNγ at 1000, 500, and 100 U/ml significantly reduced ATP levels in primary fibroblasts (Figure 1A) and astrocytes (Figure 1B), but produced no ATP loss in neurons (Figure 1C). Decreased ATP production in the presence of IFNγ was associated with a rapid, but transient induction of Stat1 phosphorylation in MEFs and astrocytes (Figure 1A and 1B). In contrast, activation of STAT1 was delayed in primary neurons, with peak Stat1 phosphorylation at 72h post-IFNγ treatment (Figure 1C). Stat1 phosphorylation in neurons also correlated with increased expression of total Stat1 protein, suggesting that low basal levels of Stat1 expression may limit the initial Stat1-dependent response to IFNγ in neurons.
Figure 1. IFNγ reduces ATP production in primary fibroblasts and astrocytes, but not in primary neurons.
Primary mouse embryonic fibroblasts (MEFs) (A), cortical astrocytes (B) and hippocampal neurons (C) were explanted from E16 mouse embryos. All cell types were treated with vehicle control (PBS) or IFNγ at indicated concentrations (1–1000U/ml). ATP levels were measured using CellTiter-Glo assay 72h post treatment. Fluorescence values were plotted as a percentage of PBS-treated controls. Shown are the mean + S.E.M. from 3–5 experiments. Western blots for phosphorylated Stat1 (Stat1-P), total Stat1, and GAPDH as a loading control were performed over the 72h time course. Representative blots are shown in the middle column, and quantitation of the western blots is shown in right column with Stat1-P signal normalized to GAPDH levels (n=3). Each graph includes cells from 3 biological replicates from independent dissections, and statistical analysis was applied by 1-way ANOVA with Bonferroni multiple comparison test (* p<0.05 vehicle control treated cells).
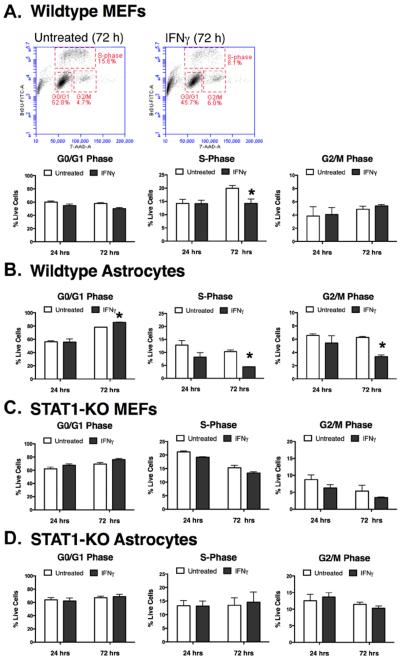
Reduced ATP levels, reflective of reduced cell number, in MEF and glial cultures could be due to cell death or to a blockade in cell cycle progression. To determine if IFNγ altered cell division, wildtype MEFs (Figure 2A) and astrocytes (Figure 2B) were treated with IFNγ (100 U/ml) for 24 or 72h. Cultures were assayed by flow cytometry for BrdU incorporation into newly-synthesized DNA and for 7-AAD staining to label for total DNA content. Cells were then gated into G0/G1 phase (resting), S phase (synthesis of DNA) or G2/M phases (cell division) based upon BrdU and 7-AAD levels (Figure 2A). In MEFs, IFNγ did not induce significant changes in the cell cycle at 24h post-IFNγ treatment. However, IFNγ led to a significant decrease in the percentage of MEFs in S-phase by 72h post-treatment, suggesting that IFNγ reduces the number of MEFs actively synthesizing DNA in preparation for cell division. Similarly, in astrocytes, IFNγ induced a decrease in S-phase cells by 72h, but also caused a decrease in cells in G2/M phase and an increase in the percentage of cells in the resting G0/G1 phase (Figure 2). Together, these results suggest that IFNγ induces a cytostatic effect in MEFs and astrocytes, with fewer cells actively progressing through the cell cycle.
Figure 2. IFNγ inhibits cell cycle progression in fibroblasts and astrocytes in a Stat1-dependent manner.
Primary wild type fibroblasts (A) and astrocytes (B) were treated with IFNγ (100 U/ml) for 24 or 72h. Stages of the cell cycle were measured by incorporation of BrdU into newly-synthesized DNA and by 7-AAD, which stained total DNA. Samples were then assayed by flow cytometry. Representative scatter plots and gates for fibroblasts (MEFs) at 72h post-IFNγ treatment are shown in (A). Stat1-KO fibroblasts (C) and astrocytes (D) were also assayed by the same approach. Experiments with cells from individual dissections were averaged (n=3–4) and plotted with SEM. Statistical significance was assessed via paired t-test (* p<0.01).
Both MEFs and astrocytes demonstrate rapid Stat1 activation in response to IFNγ, though this level of Stat1 phosphorylation was not maintained over a 72h period (Figure 1A and Figure 1B). Thus, we could not assume that the cytostatic effects caused by IFNγ at 72h were due to Stat1 activation. To determine if Stat1 was responsible for the cytostatic effects of IFNγ in these cultures over time, we treated MEFs (Figure 2C) and astrocytes (Figure 2D) from Stat1-KO mice with IFNγ. In the absence of functional STAT1, IFNγ did not affect cell cycle progression in MEFs or astrocytes at either time point. Thus, the cytostatic effects of IFNγ are Stat1-dependent in fibroblasts and astrocytes.
Erk-1/2 signaling confers a survival benefit from IFNγ
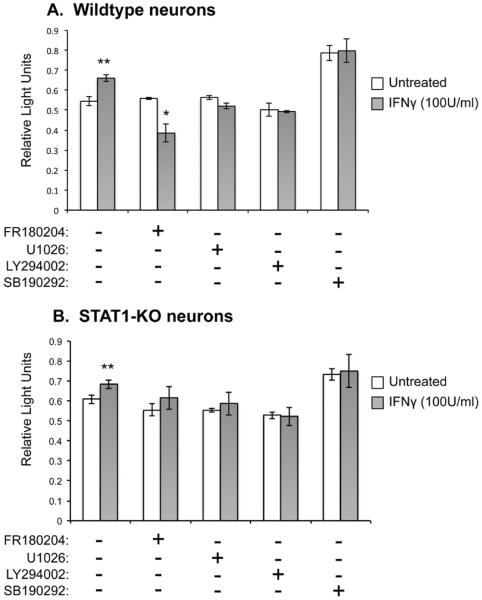
We previously showed that the response of terminally differentiated neurons to IFNγ was distinct from rapidly dividing cells: rather than the rapid and transient activation of Stat1 seen in fibroblasts and astrocytes, neurons do not produce appreciable activated Stat1 until ~72h after IFNγ addition, but this response is then sustained. Moreover, the results presented in Figures 1 and 2 show that IFNγ is not neurotoxic, even when Stat1 is activated in these cells. Thus, we asked whether cell survival pathways might be triggered in neurons. Hippocampal neurons from wildtype or Stat1-KO mice were cultured for 5d in vitro, and then treated with either an Erk1/2 inhibitor (FR180204), a p38-Mapk inhibitor (SB202190), a PI3-Kinase inhibitor (LY294002), or a Mek1/2 inhibitor (U1026) (added at 10μM for 1h; concentrations based on published effective doses) (Zhuang et al. 2004, O'Donnell et al. 2007, Ramirez et al. 2010). IFNγ (100U/ml) was then added to the cultures for 72h, and cell viability was measured by MTS assay (Figure 3), which is indicative of the number of viable cells. The effect of each inhibitor in the presence of IFNγ (gray bars) was compared to inhibitor-treated neurons that were not exposed to IFNγ (white bars). In untreated neurons, IFNγ confers modest (10%) neuronal protection, even in the absence of an acute viral insult, and, as predicted, Stat1 is not required for this enhanced protection (Figure 3A and 3B; left bars). Regardless of the presence of IFNγ, treatment with the PI3K inhibitor (LY294002) and the Mek1/2 inhibitor (U1026) did not affect neuron viability over baseline in the presence of IFNγ, suggesting that these kinases do not play an active role in IFNγ-mediated signaling. Inhibiting p38 with SB202190 increased cell viability overall, but in an IFNγ-independent manner, consistent with a role of p38-Mapk in cell death induction. Importantly, however, inhibition of Erk1/2 activation by FR180204, which blocks both Erk1 and Erk 2, significantly decreased cell viability in IFNγ-treated neurons (Figure 3A). In neurons lacking Stat1, the Erk1/2 inhibitor did not significantly impact neuronal survival in the presence of IFNγ (Figure 3B). This suggests that Erk1/2 signaling may counteract Stat1-dependent cell death during IFNγ exposure in neurons.
Figure 3. Blockade of Erk1/2 in neurons negates the IFNγ-mediated protection against Stat1-mediated cytotoxicity.
Primary wildtype (A) and Stat1-KO (B) hippocampal neurons were pre-treated with inhibitors FR180204 (Erk1/2 inhibitor), U1026 (Mek1/2 inhibitor), LY294002 (PI3-Kinase inhibitor), and SB190292 (p38-Mapk inhibitor) (10μM). After 1h, IFNγ (100 U/ml) was applied to the cultures in the presence of the inhibitors and incubated for 72h. Cultures were assayed by MTS assay as a measure of cell survival. Statistical analysis was applied by paired t-test (* p<0.05 vs. corresponding untreated; # p<0.01 vs. corresponding untreated; n=2).
IFNγ activates Erk1/2 in neurons
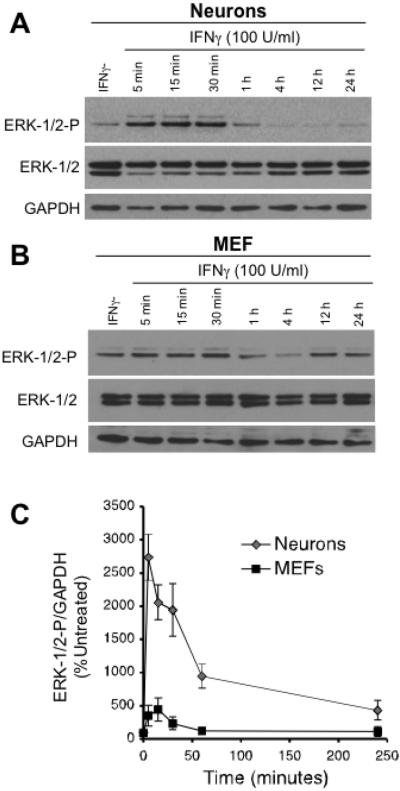
Based on the inhibitor results, we next determined the kinetics of Erk-1/2 phosphorylation in neurons as a downstream effector of IFNγ signaling (Figure 4). Immunoblots of cell lysates from hippocampal neurons and MEFs exposed to IFNγ were probed with antibodies against total Erk1/2 and activated (phosphorylated) Erk1/2. Erk1/2 activation in neurons is significantly induced (2700-fold over untreated neurons) as compared to MEFs (Figure 4A and 4B) after a brief (5 min) exposure to IFNγ. Erk1/2 phosphorylation is sustained for over an hour post-exposure, and remained above baseline for greater than 24h post treatment (Figure 4C). The immediate and robust activation of Erk in neurons post-IFNγ treatment stands in stark contrast to our previous work that demonstrated a muted activation of Stat1 in neurons that only reached detectable levels of phosphorylation after 24 h of IFNγ exposure (O'Donnell et al. 2012, Rose et al. 2007). Moreover, MEFs, which induce rapid activation of Stat1, demonstrate negligible Erk1/2 activation in response to IFNγ (Figure 4B). Other kinases that we tested were not significantly activated in neurons (Akt, p38-MAPK; data not shown) during IFNγ treatment, consistent with the inhibitor results (Figure 3). Based on these observations, we conclude that Erk1/2 signaling is an alternate IFNγ-mediated pathway in neurons.
Figure 4. IFNγ activates Erk1/2 in primary neurons but not in fibroblasts.
(A) and (B) Hippocampal neurons and fibroblasts (MEFs) explanted from mouse embryos were treated with IFNγ (100 U/ml). Cells were lysed at indicated times post treatment and subjected to Western blot for Erk1/2, phosphorylated Erk1/2 (Erk1/2-P), and GAPDH as a loading control. C. Western blots from A and B were quantified by densitometry using ImageJ software. Erk1/2 and p-Erk1/2 signal was normalized to GAPDH as a loading control. Statistical analysis was determined using two-way ANOVA (n = 3, p < 0.002).
IFNγ confers a survival advantage to neurons upon staurosporine exposure
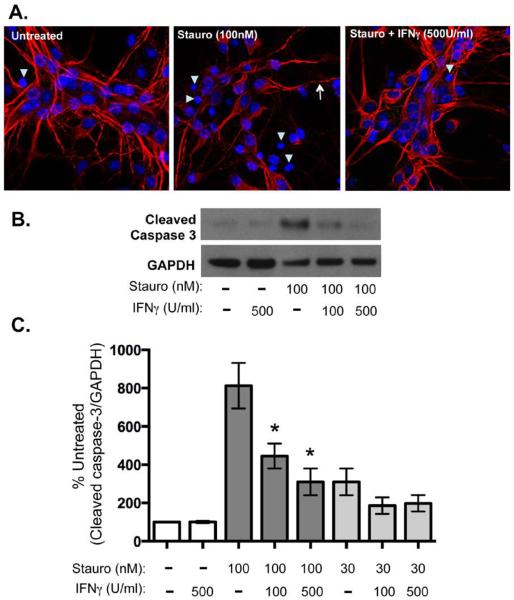
IFNγ appears to increase neuronal survival by the MTS assay, and activates Erk-1/2 in neurons but not in MEFs, cells in which IFNγ exerts a cytostatic effect. However, it is unclear how IFNγ would affect neuronal survival in the context of an apoptotic insult. To determine if IFNγ potentiated or inhibited apoptotic signaling in neurons, hippocampal neurons were treated with staurosporine (100 or 30 nM), an inducer of caspase-3 dependent apoptosis, in the presence or absence of IFNγ (500 or 100 U/ml) for 72h. Neurons treated with staurosporine alone showed classic indications of cell death, including dendritic beading and pyknotic nuclei with condensed chromatin (Figure 5A); these signs of cell damage were reduced by co-administration of IFNγ (Figure 5A). To determine if IFNγ inhibited caspase 3 activation, neuronal lysates were probed for cleaved (activated) caspase-3 by Western blot (Figure 5B) and quantified by normalization to untreated controls (Figure 5C). While high (100 nM) staurosporine treatment alone conferred an 8-fold increase in cleaved caspase 3 compared to untreated neurons, this increase was significantly dampened by concurrent treatment with both concentrations of IFNγ. Importantly, IFNγ treatment alone (500U/ml) did not increase caspase 3 activation in comparison to untreated neurons, confirming that IFNγ does not induce apoptotic signaling, which occurs in many mitotically-active cells.
Figure 5. IFNγ blocks apoptotic signaling in neurons upon staurosporine exposure.
Cultured hippocampal neurons were treated with IFNγ at the indicated concentrations (100 or 500 U/ml). After 30 min of pretreatment with IFNγ, staurosporine (30 or 100nM) was added to the cultures for 72 h. A. Cells were fixed and stained for MAP2 (red) as a marker for neurons and Hoechst (blue) for cell nuclei. Arrowheads indicate pyknotic nuclei and arrows indicate beaded dendrites. B. Cells were lysed in protein solubilization buffer and analyzed by Western blot with antibodies against cleaved caspase 3 and GAPDH as a loading control. C. Western blots were quantified by densitometry using ImageJ software. Cleaved caspase 3 signal was normalized to GAPDH as a loading control and statistical analysis was determined using a paired t test (n = 4, *p < 0.04 versus signal in untreated control).
IFNγ partially protects neurons from staurosporine-induced apoptosis in an Erk-1/2 dependent manner
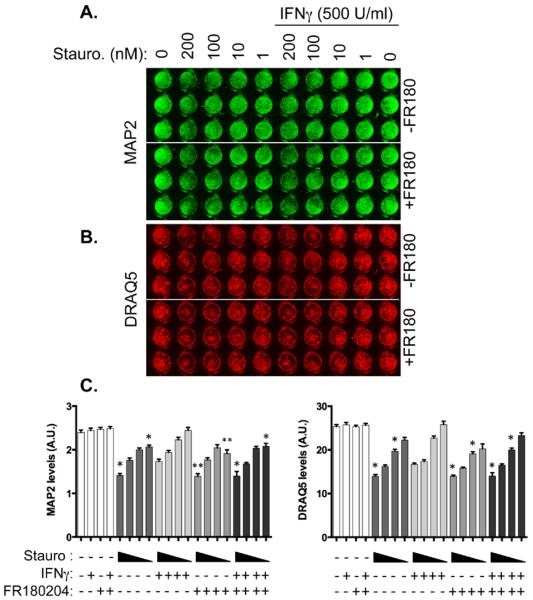
If Erk1/2 is a critical downstream mediator of IFNγ signaling, then ablation of Erk1/2 activation should diminish the ability of IFNγ to support neuronal survival. To test whether IFNγ-mediated Erk1/2 activation was protective, we pharmacologically inhibited Erk1/2 with FR180204 (10 μM) in the presence of IFNγ and staurosporine. Hippocampal neurons were treated with the Erk-1/2 inhibitor, FR180204, for 30 min after which IFNγ (500 U/ml) was added for 1h. Staurosporine (1-200nM) was then added to the cells for 72h to induce cell death. Neuronal loss was measured via In Cell western for MAP2 staining as a marker of viability (green, Figure 6A) and with the DNA label DRAQ5 as a marker for cell nuclei (red, Figure 6B). IFNγ provided modest but significant protection against 1 nM and 200 nM of staurosporine treatment as measured by MAP2 staining, and reduced loss of DRAQ5 staining against 10nM and 200nM staurosporine (Figure 6C). The protective effect of IFNγ was abrogated by pretreatment with the Erk1/2 inhibitor. These results suggest that IFNγ protects neurons against apoptotic insults in an Erk1/2-dependent manner.
Figure 6. Inhibition of Erk1/2 abrogates IFNγ-mediated protection against staurosporine.
Primary hippocampal cultures were treated with the Erk inhibitor FR180204 for 30 min prior to addition of IFNγ (500 U/ml). One hour after IFNγ treatment, cells were treated with a range of staurosporine concentrations (200, 100, 10, and 1 nM) for 72h. Cultures were stained for MAP2 as a neuronal marker (green; A) and DRAQ5 as a label for DNA (red, B). Results were quantified in C. Graphs show the average signal intensity from three biological replicates of independent dissections (n = 5 plates) with SEM. Triangles below the graph indicate decreasing concentrations of staurosporine (200–1 nM). Statistical analysis was applied via one-way ANOVA with multiple comparisons (* p<0.05 versus IFNγ + equivalent staurosporine concentration, ** p<0.01 versus IFNγ + equivalent staurosporine concentration.)
Enhanced Erk activation in Stat1-KO fibroblasts
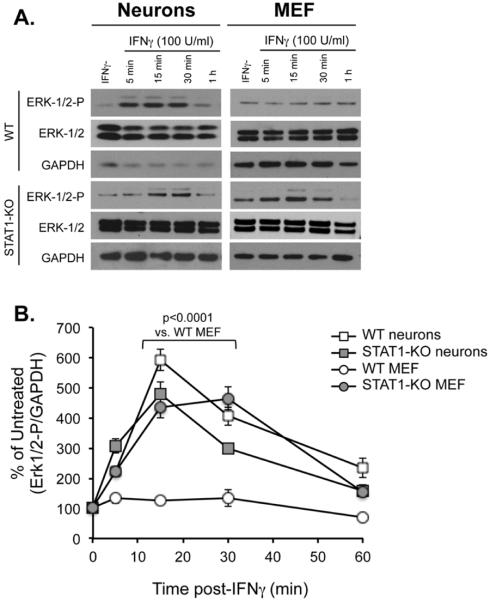
A hypothesis we considered throughout these studies was that cell type-specific utilization of particular signaling pathways is predicated on the balance and availability of critical intracellular signaling substrates. For example, Stat1-induced genes may predominate in MEFs due to an abundance of Stat1, whereas less bioavailable Stat1 in neurons may afford signaling through other potential pathways, including Erk1/2. If true, then genetic deletion of Stat1 in MEFs should mimic the natural paucity of Stat1 in neurons, and result in selective activation of other signaling kinases. To test this, hippocampal neurons and MEFs from wildtype and Stat1-KO mice were treated with IFNγ (100U/ml), and protein lysates were collected at early time points after IFNγ exposure (5, 15, 30, or 60 min). Stat1-deficient MEFs showed a correspondingly appreciable and rapid activation of Erk1/2, similar to both wild type and Stat1-deficient neurons. This was in stark contrast to the more modest activation of Erk seen in MEFs derived from wild type mice (Figure 7A and 7B). These data suggest that the availability and balance of signaling substrates in the cell define the IFNγ-dependent pathways that are activated. Consequently, natural differences in the abundance (or cytoplasmic availability) of these molecules could skew the cellular response to a given cytokine ligand.
Figure 7. Enhanced Erk1/2 activation in Stat1 KO fibroblasts.
Wildtype and Stat1-KO hippocampal neurons and MEFs were treated with IFNγ (100 U/ml). Whole-cell lysates were harvested post treatment at times indicated and subjected to Western blot analysis for phospho-Erk1/2 (Erk-1/2/-P), total Erk-1/2, and GPADH as a loading control (A). The signal of both Erk bands was analyzed together by densitometry on ImageJ software, and p-Erk1/2 signal was normalized to GAPDH as a loading control (B). Statistical analysis was applied using two-way ANOVA with Tukey's post hoc test (n=3).
Discussion
Mounting evidence suggests that IFNγ can activate distinct signaling pathways and gene expression profiles in a cell type-specific manner, with subsequent effects on cell physiology, function, and survival (Huynh & Dorovini-Zis 1993, Natarajan et al. 2014, van Boxel-Dezaire & Stark 2007, Zhang et al. 2013, Vidal et al. 2012). In neural cells of the CNS, a variety of IFNγ signaling signatures are seen, often involving Jak/Stat1 activation in combination with other Stat family members, other transcription factors, or kinases (Lin et al. 2012, Burdeinick-Kerr & Griffin 2005, Walter et al. 2012, Kim et al. 2007). Previous work from our laboratory has shown that primary hippocampal neurons express low basal levels of Stat1 in comparison to primary fibroblasts and astrocytes, leading to a delay in Stat1 activation and reduced expression of canonical Stat1-responsive genes in primary neurons (O'Donnell et al. 2012, Rose et al. 2007). Here, we demonstrate that IFNγ confers neuroprotection against apoptotic insults via an Erk1/2 dependent mechanism, in contrast to astrocytes and fibroblasts, in which IFNγ induces a cytostatic effect. Moreover, IFNγ induces Erk1/2 activation in other cell types that express low levels of basal Stat1 protein, including Stat1 knockout fibroblasts and neurons.
Erk1/2 signaling has been associated with neuronal survival in other models of neurodegeneration as well (Head et al. 2008, Hetman et al. 1999, Li et al. 2008, Lin et al. 2008, Rossler et al. 2004, Troadec et al. 2002, Wruck et al. 2008). For example, neurotrophic growth factors (e.g. nerve growth factor and brain-derived neurotrophic factor) promote cell survival in an Erk1/2-dependent manner (Anderson & Tolkovsky 1999, Hughes et al. 2011, Liu et al. 1999, Hetman et al. 1999), and Erk1/2-mediated activation of cell survival proteins (Elk1, Rsk90) is protective against a variety of apoptotic insults (Groot et al. 2000, Xifro et al. 2011, Mebratu & Tesfaigzi 2009). However, evidence also exists for pro-apoptotic effects of Erk1/2 signaling in some neural cell types, including proliferating oligodendrocyte progenitor cells treated with IFNγ (Horiuchi et al. 2006), brain capillary endothelial cells (Lecht et al. 2010), and rat cerebellar granule neurons treated with N-methyl-D-aspartate (Amadoro et al. 2006). Thus, we could not assume that Erk1/2 activation in primary neurons would be protective. A variety of factors can influence the activation of Erk1/2, including cytokines, growth factors, and, importantly, viral infection. Specifically, viruses such as Visna (Barber et al. 2002) and RSV induce Erk1/2 in the infected cell (Monick et al. 2001), and Erk1/2 activation is critical for the host response to avian influenza virus (H9N2) (Xing et al. 2010) and double stranded RNA (Maggi et al. 2003).
In our experiments, pharmacological inhibition of Erk1/2 signaling resulted in IFNγ-dependent toxicity in primary neurons in the presence of delayed but sustained Stat1 activation. Thus, we hypothesize that Erk1/2 signaling may be a buffer to protect neurons from the cytotoxic pathways that can be activated by Stat1, which are induced in neurons hours following IFNγ engagement (12–72 h), well after Erk1/2 activation (Rose et al. 2007). Furthermore, the protective effects of IFNγ may be more pronounced in neurons than in other cell types owing to the relatively low level of basal Stat1 expression and delayed activation. Stat1 activity is associated with cell cycle inhibition and cytotoxicity in many cell types (Kato et al. 2003, Handy & Patel 2013, Bromberg et al. 1996, Dimco et al. 2010, Stout et al. 2007, Shin et al. 2014, Kim & Lee 2005) and with neuronal death in a model of ischemic brain injury (Takagi et al. 2002). Our data shows that primary mouse fibroblasts and cortical astrocytes, both of which rapidly and robustly activate Stat1 in response to IFNγ, produce less ATP when treated with IFNγ in comparison to untreated controls, suggesting that IFNγ is either impeding cellular metabolism or preventing cell division (Figure 2). In contrast, neurons show no signs of cellular damage following IFNγ exposure, with unchanged levels of ATP production and an increase in viability as measured by the MTS assay during IFNγ treatment. Moreover, IFNγ treatment blunted caspase 3 activation in neurons during exposure to staurosporine, a potent inducer of caspase-dependent apoptosis (Deshmukh & Johnson 2000) and preserved MAP2 expression and nuclear staining in staurosporine-treated neurons.
Wildtype neurons were sensitized to IFNγ-mediated toxicity when Erk1/2 was blocked, but Stat1-KO neurons remained resistant to IFNγ. Pharmacological inhibition of Erk1/2, but not Mek1/2 (Mapk kinase), revealed Stat1-dependent toxicity in the presence of IFNγ. Mek1/2 often links upstream Ras/Raf signaling to Erk activation as part of the Mapk pathway. However, Mek-independent activation of Erk1/2 has been noted in a number of cell types, including fibroblasts and breast cancer cell lines. When Erk-1/2 activation occurs independently of Mek1/2, other kinases such as PI3-Kinase, Protein Kinase C (PKC) isoforms, and PBK/TOPK, have been implicated in linking receptor engagement with Erk-1/2 activation (Bapat et al. 2001, Grammer & Blenis 1997, Aksamitiene et al. 2010). While IFNγ has been to shown to activate PI3-Kinase, PKCs, and Phospholipase C (PLC), as well as Erk1/2 in other cell types, the intracellular signaling molecules that link the IFNGR subunits with alternative signaling pathways remain largely undefined. In neurons, identifying the upstream signaling components that link the IFNγ receptor complex to Erk1/2 may prove important for understanding how IFNγ triggers protective pathways in neurons.
We wish to emphasize that Erk-1/2 is one, but likely not the only, alternative signaling molecule induced in neurons following IFNγ stimulation. While we have excluded some kinases from being activated (e.g., Akt and p38), we have not conducted an exhaustive kinase assessment, and fully expect that other signal tranduction pathways may also influence the post-IFNγ transcriptional profile. Nevertheless, these data link “non-canonical” signaling pathways with unique cellular effects, and serve as further support for the evolving view that cytokine/interferon ligands may result in distinct cellular responses, depending on the availability of cytoplasmic signal transducers.
In mitotically active cells, deletion of Stat1 is often compensated by Stat3 activation (Qing & Stark 2004). Since Stat3 activation is often associated with cell survival and proliferation, one prediction is that Stat3 signaling dominates in cells with low Stat1 expression such as neurons, leading to a protective effect in neurons upon IFNγ stimulation. However, primary hippocampal neurons, which express low levels of Stat1 and demonstrate limited Stat1 activation immediately after IFNγ stimulation, do not activate Stat3 as a compensatory mechanism for reduced Stat1 expression (Rose et al. 2007). Moreover, Stat3 expression in the primary neurons was also negligible, implicating other signaling pathways as mediators of the phenotypic effects of IFNγ in CNS neurons. While we present evidence for IFNγ-mediated Erk1/2 activation in primary neurons, and we were unable to detect significant activation of other cell survival kinases, such as Akt, other mitogen-activated kinases, such as p38, or Stat2 (data not shown), this does not rule out the involvement of yet more kinases in governing the neuronal response to IFNγ. Erk1/2 is often activated in parallel with Akt in models of neuroprotection, which may partially explain while we see only modest protection with IFNγ in primary hippocampal neurons. Thus, one advantage of stimulating alternative signal transduction pathways may be to protect the neuron from the potentially cytotoxic effects of “classical” anti-viral gene products via Stat1 activation. An additional possibility is that rapid Erk1/2 activation contributes to subsequent Stat1 phosphorylation. Studies in macrophages demonstrate that complete activation of Stat1 is dependent on Erk1/2 activity, suggesting crosstalk between Stat1 and Erk1/2 signaling in some cell types (Li et al. 2010). Ongoing studies are focused on identifying the immediate downstream targets of Erk1/2 during IFNγ treatment, and defining how neuroprotection is conferred. Such cell type-specific roles for IFNγ signaling would permit induction of immunity in the CNS while also protecting non-renewable neurons during the inflammatory cascade.
Acknowledgements
This work was supported by the following sources: R01 NS40500 (GFR), 5T32 CA 9035-38 (LAO'D, KMH), F32 NS062519-01 (LAO'D), a grant from Autism Speaks (GFR), a gift from the F. M. Kirby Foundation (GFR), and by P30CA006927 from the National Cancer Institute.
Abbreviations
- INFγ
Interferon-gamma
- Jak
Janus activated kinase
- Stat
Signal Transducers and Activators of Transcription
- Mapk
Mitogen-activated protein kinases
- Erk1/2
Extracellular regulated kinase 1/2
- PI3-Kinase
Phosphoinositide 3-kinase
References
- Aksamitiene E, Kholodenko BN, Kolch W, Hoek JB, Kiyatkin A. PI3K/Akt-sensitive MEK-independent compensatory circuit of ERK activation in ER-positive PI3K-mutant T47D breast cancer cells. Cell Signal. 2010;22:1369–1378. doi: 10.1016/j.cellsig.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2892–2897. doi: 10.1073/pnas.0511065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CN, Tolkovsky AM. A role for MAPK/ERK in sympathetic neuron survival: protection against a p53-dependent, JNK-independent induction of apoptosis by cytosine arabinoside. J Neurosci. 1999;19:664–673. doi: 10.1523/JNEUROSCI.19-02-00664.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G. a. G., K. Culturing Nerve Cells. A Bradford Book; 1998. [Google Scholar]
- Bapat S, Verkleij A, Post JA. Peroxynitrite activates mitogen-activated protein kinase (MAPK) via a MEK-independent pathway: a role for protein kinase C. FEBS letters. 2001;499:21–26. doi: 10.1016/s0014-5793(01)02511-x. [DOI] [PubMed] [Google Scholar]
- Barber SA, Bruett L, Douglass BR, Herbst DS, Zink MC, Clements JE. Visna virus-induced activation of MAPK is required for virus replication and correlates with virus-induced neuropathology. Journal of virology. 2002;76:817–828. doi: 10.1128/JVI.76.2.817-828.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nature reviews. Microbiology. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder GK, Griffin DE. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science. 2001;293:303–306. doi: 10.1126/science.1059742. [DOI] [PubMed] [Google Scholar]
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Bromberg JF, Horvath CM, Wen Z, Schreiber RD, Darnell JE., Jr. Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:7673–7678. doi: 10.1073/pnas.93.15.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke CB, Schafer A, Matsushima GK, White LJ, Johnston RE. Early activation of the host complement system is required to restrict central nervous system invasion and limit neuropathology during Venezuelan equine encephalitis virus infection. The Journal of general virology. 2012;93:797–806. doi: 10.1099/vir.0.038281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdeinick-Kerr R, Griffin DE. Gamma interferon-dependent, noncytolytic clearance of sindbis virus infection from neurons in vitro. Journal of virology. 2005;79:5374–5385. doi: 10.1128/JVI.79.9.5374-5385.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin E, Tanamachi B, Openshaw H. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. Journal of virology. 1999;73:3418–3423. doi: 10.1128/jvi.73.4.3418-3423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla-Sarkar M, Lindner DJ, Liu YF, Williams BR, Sen GC, Silverman RH, Borden EC. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis : an international journal on programmed cell death. 2003;8:237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- Chin YE, Kitagawa M, Kuida K, Flavell RA, Fu XY. Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Molecular and cellular biology. 1997;17:5328–5337. doi: 10.1128/mcb.17.9.5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Krantz SB. Interferon gamma induces upregulation and activation of caspases 1, 3, and 8 to produce apoptosis in human erythroid progenitor cells. Blood. 1999;93:3309–3316. [PubMed] [Google Scholar]
- Deshmukh M, Johnson EM., Jr. Staurosporine-induced neuronal death: multiple mechanisms and methodological implications. Cell Death Differ. 2000;7:250–261. doi: 10.1038/sj.cdd.4400641. [DOI] [PubMed] [Google Scholar]
- Dimco G, Knight RA, Latchman DS, Stephanou A. STAT1 interacts directly with cyclin D1/Cdk4 and mediates cell cycle arrest. Cell cycle. 2010;9:4638–4649. doi: 10.4161/cc.9.23.13955. [DOI] [PubMed] [Google Scholar]
- Fiette L, Aubert C, Muller U, Huang S, Aguet M, Brahic M, Bureau JF. Theiler's virus infection of 129Sv mice that lack the interferon alpha/beta or interferon gamma receptors. J Exp Med. 1995;181:2069–2076. doi: 10.1084/jem.181.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke D, Brinckmann UG, ter Meulen V, Liebert UG. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. Journal of virology. 1995;69:5469–5474. doi: 10.1128/jvi.69.9.5469-5474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger KD, Nash TC, Sawyer S, et al. Interferon-gamma protects against herpes simplex virus type 1-mediated neuronal death. Virology. 1997;238:189–197. doi: 10.1006/viro.1997.8841. [DOI] [PubMed] [Google Scholar]
- Gil MP, Bohn E, O'Guin AK, Ramana CV, Levine B, Stark GR, Virgin HW, Schreiber RD. Biologic consequences of Stat1-independent IFN signaling. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6680–6685. doi: 10.1073/pnas.111163898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomme EA, Wirblich C, Addya S, Rall GF, Schnell MJ. Immune clearance of attenuated rabies virus results in neuronal survival with altered gene expression. PLoS pathogens. 2012;8:e1002971. doi: 10.1371/journal.ppat.1002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammer TC, Blenis J. Evidence for MEK-independent pathways regulating the prolonged activation of the ERK-MAP kinases. Oncogene. 1997;14:1635–1642. doi: 10.1038/sj.onc.1201000. [DOI] [PubMed] [Google Scholar]
- Groot M, Boxer LM, Thiel G. Nerve growth factor- and epidermal growth factor-regulated gene transcription in PC12 pheochromocytoma and INS-1 insulinoma cells. European journal of cell biology. 2000;79:924–935. doi: 10.1078/0171-9335-00126. [DOI] [PubMed] [Google Scholar]
- Handy I, Patel RC. STAT1 requirement for PKR-induced cell cycle arrest in vascular smooth muscle cells in response to heparin. Gene. 2013;524:15–21. doi: 10.1016/j.gene.2013.03.124. [DOI] [PubMed] [Google Scholar]
- Head BP, Patel HH, Tsutsumi YM, et al. Caveolin-1 expression is essential for N-methyl-D-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:828–840. doi: 10.1096/fj.07-9299com. [DOI] [PubMed] [Google Scholar]
- Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- Horiuchi M, Itoh A, Pleasure D, Itoh T. MEK-ERK signaling is involved in interferon-gamma-induced death of oligodendroglial progenitor cells. J Biol Chem. 2006;281:20095–20106. doi: 10.1074/jbc.M603179200. [DOI] [PubMed] [Google Scholar]
- Hughes R, Gilley J, Kristiansen M, Ham J. The MEK-ERK pathway negatively regulates bim expression through the 3' UTR in sympathetic neurons. BMC neuroscience. 2011;12:69. doi: 10.1186/1471-2202-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh HK, Dorovini-Zis K. Effects of interferon-gamma on primary cultures of human brain microvessel endothelial cells. The American journal of pathology. 1993;142:1265–1278. [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Lundkvist G, Dons L, Kristensson K, Rottenberg ME. Interferon-gamma mediates neuronal killing of intracellular bacteria. Scand J Immunol. 2004;60:437–448. doi: 10.1111/j.0300-9475.2004.01500.x. [DOI] [PubMed] [Google Scholar]
- Joshi S, Kaur S, Kroczynska B, Platanias LC. Mechanisms of mRNA translation of interferon stimulated genes. Cytokine. 2010;52:123–127. doi: 10.1016/j.cyto.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Kano A, Watanabe Y, Takeda N, Aizawa S, Akaike T. Analysis of IFN-gamma-induced cell cycle arrest and cell death in hepatocytes. Journal of biochemistry. 1997;121:677–683. doi: 10.1093/oxfordjournals.jbchem.a021639. [DOI] [PubMed] [Google Scholar]
- Kato K, Kamezaki K, Shimoda K, et al. Intracellular signal transduction of interferon on the suppression of haematopoietic progenitor cell growth. British journal of haematology. 2003;123:528–535. doi: 10.1046/j.1365-2141.2003.04650.x. [DOI] [PubMed] [Google Scholar]
- Kaur S, Sassano A, Joseph AM, Majchrzak-Kita B, Eklund EA, Verma A, Brachmann SM, Fish EN, Platanias LC. Dual regulatory roles of phosphatidylinositol 3-kinase in IFN signaling. J Immunol. 2008;181:7316–7323. doi: 10.4049/jimmunol.181.10.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanokuchi J, Mizuno T, Takeuchi H, Kato H, Wang J, Mitsuma N, Suzumura A. Production of interferon-gamma by microglia. Mult Scler. 2006;12:558–564. doi: 10.1177/1352458506070763. [DOI] [PubMed] [Google Scholar]
- Kim HS, Lee MS. Essential role of STAT1 in caspase-independent cell death of activated macrophages through the p38 mitogen-activated protein kinase/STAT1/reactive oxygen species pathway. Molecular and cellular biology. 2005;25:6821–6833. doi: 10.1128/MCB.25.15.6821-6833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Son TG, Kim K, Park HR, Mattson MP, Lee J. Interferon-gamma promotes differentiation of neural progenitor cells via the JNK pathway. Neurochemical research. 2007;32:1399–1406. doi: 10.1007/s11064-007-9323-z. [DOI] [PubMed] [Google Scholar]
- Larena M, Regner M, Lobigs M. Cytolytic effector pathways and IFN-gamma help protect against Japanese encephalitis. European journal of immunology. 2013;43:1789–1798. doi: 10.1002/eji.201243152. [DOI] [PubMed] [Google Scholar]
- Lecht S, Arien-Zakay H, Marcinkiewicz C, Lelkes PI, Lazarovici P. Nerve growth factor-induced protection of brain capillary endothelial cells exposed to oxygen-glucose deprivation involves attenuation of Erk phosphorylation. Journal of molecular neuroscience : MN. 2010;41:183–192. doi: 10.1007/s12031-009-9318-0. [DOI] [PubMed] [Google Scholar]
- Lee J, Kornfeld H. Interferon-gamma Regulates the Death of M. tuberculosis-Infected Macrophages. Journal of cell death. 2010;3:1–11. doi: 10.4137/jcd.s2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, McLaren JE, Michael DR, Clement M, Fielding CA, Ramji DP. ERK is integral to the IFN-gamma-mediated activation of STAT1, the expression of key genes implicated in atherosclerosis, and the uptake of modified lipoproteins by human macrophages. J Immunol. 2010;185:3041–3048. doi: 10.4049/jimmunol.1000993. [DOI] [PubMed] [Google Scholar]
- Li P, Du Q, Cao Z, et al. Interferon-gamma induces autophagy with growth inhibition and cell death in human hepatocellular carcinoma (HCC) cells through interferon-regulatory factor-1 (IRF-1) Cancer letters. 2012;314:213–222. doi: 10.1016/j.canlet.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QF, Zhu YS, Jiang H. Isoflurane preconditioning activates HIF-1alpha, iNOS and Erk1/2 and protects against oxygen-glucose deprivation neuronal injury. Brain research. 2008;1245:26–35. doi: 10.1016/j.brainres.2008.09.069. [DOI] [PubMed] [Google Scholar]
- Lin E, Cavanaugh JE, Leak RK, Perez RG, Zigmond MJ. Rapid activation of ERK by 6-hydroxydopamine promotes survival of dopaminergic cells. Journal of neuroscience research. 2008;86:108–117. doi: 10.1002/jnr.21478. [DOI] [PubMed] [Google Scholar]
- Lin W, Lin Y. Interferon-gamma inhibits central nervous system myelination through both STAT1-dependent and STAT1-independent pathways. Journal of neuroscience research. 2010;88:2569–2577. doi: 10.1002/jnr.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Jamison S, Lin W. Interferon-gamma activates nuclear factor-kappa B in oligodendrocytes through a process mediated by the unfolded protein response. PloS one. 2012;7:e36408. doi: 10.1371/journal.pone.0036408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YZ, Boxer LM, Latchman DS. Activation of the Bcl-2 promoter by nerve growth factor is mediated by the p42/p44 MAPK cascade. Nucleic acids research. 1999;27:2086–2090. doi: 10.1093/nar/27.10.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi LB, Jr., Moran JM, Buller RM, Corbett JA. ERK activation is required for double-stranded RNA- and virus-induced interleukin-1 expression by macrophages. J Biol Chem. 2003;278:16683–16689. doi: 10.1074/jbc.M211744200. [DOI] [PubMed] [Google Scholar]
- Mann BA, Huang JH, Li P, et al. Vaccinia virus blocks Stat1-dependent and Stat1-independent gene expression induced by type I and type II interferons. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2008;28:367–380. doi: 10.1089/jir.2007.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf TU, Baxter VK, Nilaratanakul V, Griffin DE. Recruitment and retention of B cells in the central nervous system in response to alphavirus encephalomyelitis. Journal of virology. 2013;87:2420–2429. doi: 10.1128/JVI.01769-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monick M, Staber J, Thomas K, Hunninghake G. Respiratory syncytial virus infection results in activation of multiple protein kinase C isoforms leading to activation of mitogen-activated protein kinase. J Immunol. 2001;166:2681–2687. doi: 10.4049/jimmunol.166.4.2681. [DOI] [PubMed] [Google Scholar]
- Natarajan VT, Ganju P, Singh A, et al. IFN-gamma signaling maintains skin pigmentation homeostasis through regulation of melanosome maturation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2301–2306. doi: 10.1073/pnas.1304988111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell LA, Agrawal A, Sabnekar P, Dichter MA, Lynch DR, Kolson DL. Apelin, an endogenous neuronal peptide, protects hippocampal neurons against excitotoxic injury. J Neurochem. 2007;102:1905–1917. doi: 10.1111/j.1471-4159.2007.04645.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell LA, Conway S, Rose RW, Nicolas E, Slifker M, Balachandran S, Rall GF. STAT1-independent control of a neurotropic measles virus challenge in primary neurons and infected mice. J Immunol. 2012;188:1915–1923. doi: 10.4049/jimmunol.1101356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Seol JW, Lee YJ, et al. IFN-gamma enhances TRAIL-induced apoptosis through IRF-1. Eur J Biochem. 2004;271:4222–4228. doi: 10.1111/j.1432-1033.2004.04362.x. [DOI] [PubMed] [Google Scholar]
- Parra B, Hinton DR, Marten NW, Bergmann CC, Lin MT, Yang CS, Stohlman SA. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J Immunol. 1999;162:1641–1647. [PubMed] [Google Scholar]
- Patterson CE, Lawrence DM, Echols LA, Rall GF. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. Journal of virology. 2002;76:4497–4506. doi: 10.1128/JVI.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce BD, Hobbs MV, McGraw TS, Buchmeier MJ. Cytokine induction during T-cell-mediated clearance of mouse hepatitis virus from neurons in vivo. Journal of virology. 1994;68:5483–5495. doi: 10.1128/jvi.68.9.5483-5495.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen KB, Andersen K, Fodstad O, Maelandsmo GM. Sensitization of interferon-gamma induced apoptosis in human osteosarcoma cells by extracellular S100A4. BMC cancer. 2004;4:52. doi: 10.1186/1471-2407-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky MA, Solomos AC, Durso LC, Evans SM, Rall GF, Rose RW. Extended JAK activation and delayed STAT1 dephosphorylation contribute to the distinct signaling profile of CNS neurons exposed to interferon-gamma. Journal of neuroimmunology. 2012;251:33–38. doi: 10.1016/j.jneuroim.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta C, Hadj-Slimane R, Nejmeddine M, Pampin M, Tovey MG, Espert L, Alvarez S, Chelbi-Alix MK. Interferons alpha and gamma induce p53-dependent and p53-independent apoptosis, respectively. Oncogene. 2005;24:605–615. doi: 10.1038/sj.onc.1208204. [DOI] [PubMed] [Google Scholar]
- Posimo JM, Titler AM, Choi HJ, Unnithan AS, Leak RK. Neocortex and allocortex respond differentially to cellular stress in vitro and aging in vivo. PloS one. 2013;8:e58596. doi: 10.1371/journal.pone.0058596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing Y, Stark GR. Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J Biol Chem. 2004;279:41679–41685. doi: 10.1074/jbc.M406413200. [DOI] [PubMed] [Google Scholar]
- Rall GF, Manchester M, Daniels LR, Callahan EM, Belman AR, Oldstone MB. A transgenic mouse model for measles virus infection of the brain. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4659–4663. doi: 10.1073/pnas.94.9.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramana CV, Gil MP, Han Y, Ransohoff RM, Schreiber RD, Stark GR. Stat1-independent regulation of gene expression in response to IFN-gamma. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6674–6679. doi: 10.1073/pnas.111164198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramana CV, Kumar A, Enelow R. Stat1-independent induction of SOCS-3 by interferon-gamma is mediated by sustained activation of Stat3 in mouse embryonic fibroblasts. Biochemical and biophysical research communications. 2005;327:727–733. doi: 10.1016/j.bbrc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Ramirez SH, Fan S, Dykstra H, Reichenbach N, Del Valle L, Potula R, Phipps RP, Maggirwar SB, Persidsky Y. Dyad of CD40/CD40 ligand fosters neuroinflammation at the blood-brain barrier and is regulated via JNK signaling: implications for HIV-1 encephalitis. J Neurosci. 2010;30:9454–9464. doi: 10.1523/JNEUROSCI.5796-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RW, Vorobyeva AG, Skipworth JD, Nicolas E, Rall GF. Altered levels of STAT1 and STAT3 influence the neuronal response to interferon gamma. Journal of neuroimmunology. 2007;192:145–156. doi: 10.1016/j.jneuroim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossler OG, Giehl KM, Thiel G. Neuroprotection of immortalized hippocampal neurones by brain-derived neurotrophic factor and Raf-1 protein kinase: role of extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase. J Neurochem. 2004;88:1240–1252. doi: 10.1046/j.1471-4159.2003.02255.x. [DOI] [PubMed] [Google Scholar]
- Shin EC, Ahn JM, Kim CH, Choi Y, Ahn YS, Kim H, Kim SJ, Park JH. IFN-gamma induces cell death in human hepatoma cells through a TRAIL/death receptor-mediated apoptotic pathway. International journal of cancer. Journal international du cancer. 2001;93:262–268. doi: 10.1002/ijc.1310. [DOI] [PubMed] [Google Scholar]
- Shin ES, Huang Q, Gurel Z, Palenski TL, Zaitoun I, Sorenson CM, Sheibani N. STAT1-mediated Bim expression promotes the apoptosis of retinal pericytes under high glucose conditions. Cell death & disease. 2014;5:e986. doi: 10.1038/cddis.2013.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shresta S, Sharar KL, Prigozhin DM, Snider HM, Beatty PR, Harris E. Critical roles for both STAT1-dependent and STAT1-independent pathways in the control of primary dengue virus infection in mice. J Immunol. 2005;175:3946–3954. doi: 10.4049/jimmunol.175.6.3946. [DOI] [PubMed] [Google Scholar]
- Shrestha B, Pinto AK, Green S, Bosch I, Diamond MS. CD8+ T cells use TRAIL to restrict West Nile virus pathogenesis by controlling infection in neurons. Journal of virology. 2012;86:8937–8948. doi: 10.1128/JVI.00673-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler C, Felipe A, Garcia-Manteiga J, et al. Interferon-gamma regulates nucleoside transport systems in macrophages through signal transduction and activator of transduction factor 1 (STAT1)-dependent and -independent signalling pathways. The Biochemical journal. 2003;375:777–783. doi: 10.1042/BJ20030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Stout BA, Melendez K, Seagrave J, Holtzman MJ, Wilson B, Xiang J, Tesfaigzi Y. STAT1 activation causes translocation of Bax to the endoplasmic reticulum during the resolution of airway mucous cell hyperplasia by IFN-gamma. J Immunol. 2007;178:8107–8116. doi: 10.4049/jimmunol.178.12.8107. [DOI] [PubMed] [Google Scholar]
- Stubblefield Park SR, Widness M, Levine AD, Patterson CE. T cell-, interleukin-12-, and gamma interferon-driven viral clearance in measles virus-infected brain tissue. Journal of virology. 2011;85:3664–3676. doi: 10.1128/JVI.01496-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Harada J, Chiarugi A, Moskowitz MA. STAT1 is activated in neurons after ischemia and contributes to ischemic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22:1311–1318. doi: 10.1097/01.WCB.0000034148.72481.F4. [DOI] [PubMed] [Google Scholar]
- Troadec JD, Marien M, Mourlevat S, Debeir T, Ruberg M, Colpaert F, Michel PP. Activation of the mitogen-activated protein kinase (ERK(1/2)) signaling pathway by cyclic AMP potentiates the neuroprotective effect of the neurotransmitter noradrenaline on dopaminergic neurons. Molecular pharmacology. 2002;62:1043–1052. doi: 10.1124/mol.62.5.1043. [DOI] [PubMed] [Google Scholar]
- van Boxel-Dezaire AH, Stark GR. Cell type-specific signaling in response to interferon-gamma. Current topics in microbiology and immunology. 2007;316:119–154. doi: 10.1007/978-3-540-71329-6_7. [DOI] [PubMed] [Google Scholar]
- Vidal C, Bermeo S, Li W, Huang D, Kremer R, Duque G. Interferon gamma inhibits adipogenesis in vitro and prevents marrow fat infiltration in oophorectomized mice. Stem cells. 2012;30:1042–1048. doi: 10.1002/stem.1063. [DOI] [PubMed] [Google Scholar]
- Wall L, Burke F, Smyth JF, Balkwill F. The anti-proliferative activity of interferon-gamma on ovarian cancer: in vitro and in vivo. Gynecologic oncology. 2003;88:S149–151. doi: 10.1006/gyno.2002.6707. [DOI] [PubMed] [Google Scholar]
- Walter J, Hartung HP, Dihne M. Interferon gamma and sonic hedgehog signaling are required to dysregulate murine neural stem/precursor cells. PloS one. 2012;7:e43338. doi: 10.1371/journal.pone.0043338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FI, Hinton DR, Gilmore W, Trousdale MD, Fleming JO. Sequential infection of glial cells by the murine hepatitis virus JHM strain (MHV-4) leads to a characteristic distribution of demyelination. Laboratory investigation; a journal of technical methods and pathology. 1992;66:744–754. [PubMed] [Google Scholar]
- Wruck CJ, Gotz ME, Herdegen T, Varoga D, Brandenburg LO, Pufe T. Kavalactones protect neural cells against amyloid beta peptide-induced neurotoxicity via extracellular signal-regulated kinase 1/2-dependent nuclear factor erythroid 2-related factor 2 activation. Molecular pharmacology. 2008;73:1785–1795. doi: 10.1124/mol.107.042499. [DOI] [PubMed] [Google Scholar]
- Xifro X, Anglada-Huguet M, Rue L, Saavedra A, Perez-Navarro E, Alberch J. Increased 90-kDa ribosomal S6 kinase (Rsk) activity is protective against mutant huntingtin toxicity. Molecular neurodegeneration. 2011;6:74. doi: 10.1186/1750-1326-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Cardona CJ, Anunciacion J, Adams S, Dao N. Roles of the ERK MAPK in the regulation of proinflammatory and apoptotic responses in chicken macrophages infected with H9N2 avian influenza virus. The Journal of general virology. 2010;91:343–351. doi: 10.1099/vir.0.015578-0. [DOI] [PubMed] [Google Scholar]
- Zhang FF, Morioka N, Nakashima-Hisaoka K, Nakata Y. Spinal astrocytes stimulated by tumor necrosis factor-alpha and/or interferon-gamma attenuate connexin 43-gap junction via c-jun terminal kinase activity. Journal of neuroscience research. 2013;91:745–756. doi: 10.1002/jnr.23213. [DOI] [PubMed] [Google Scholar]
- Zhang SS, Liu MG, Kano A, Zhang C, Fu XY, Barnstable CJ. STAT3 activation in response to growth factors or cytokines participates in retina precursor proliferation. Experimental eye research. 2005;81:103–115. doi: 10.1016/j.exer.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Xu H, Clapham DE, Ji RR. Phosphatidylinositol 3-kinase activates ERK in primary sensory neurons and mediates inflammatory heat hyperalgesia through TRPV1 sensitization. J Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]