Abstract
Introduction
Correct inhaler technique and device preference are positively correlated with improved adherence and clinical outcomes. This study was designed to investigate inhaler technique mastery and device preference for three different dry powder inhalers, Spiromax, Easyhaler and Turbuhaler.
Methods
This was a single site, single visit, crossover study assessing device mastery, handling errors and preference using empty Spiromax, Easyhaler and Turbuhaler devices in healthy adult Finnish volunteers. Inhaler naïve adult participants were observed by healthcare professionals (HCPs) to evaluate the proportion of participants achieving device mastery (defined as an absence of HCP observed errors) using a three-step approach: (1) intuitive use (with no instructions), (2) after reading the patient information leaflet and (3) after HCP instruction. HCPs monitored and recorded errors based on device-specific handling error checklists. At the end of the study, participants completed a device preference questionnaire and rated their satisfaction with the three devices.
Results
Spiromax was correctly used by 37.5% and 93.3% of participants in steps 1 and 2, respectively, compared with 0% and 58.3% with Easyhaler, and 9.2% and 76.7% with Turbuhaler. All three devices showed high mastery (>95%) in step 3. The most common error reported with Spiromax was related to the orientation of the device. Not shaking the device was the most common error with Easyhaler. Errors in priming the device were the most common with Turbuhaler. Spiromax, Easyhaler and Turbuhaler were rated as the ‘easiest device to use’ by 73.1%, 12.6% and 14.3% of participants, respectively. The HCP instructions clearly improved the use of all devices.
Conclusion
Higher levels of device mastery, including intuitive/ease of use, were reported by naïve users when using Spiromax compared with Easyhaler and Turbuhaler.
Keywords: Asthma, Equipment Evaluations, Inhaler devices
Key messages.
This study evaluated inhaler technique mastery, ease of use and device preference between three different dry powder inhaler devices, Spiromax, Easyhaler and Turbuhaler.
In this study, more participants achieved device mastery with Spiromax compared with Easyhaler and Turbuhaler.
A lower proportion of participants showed device handling errors after intuitive use (without reading the patient information leaflet (PIL) or other instructions) and after reading the PIL with Spiromax compared with Easyhaler or Turbuhaler.
The majority of participants reported that they found Spiromax to be the easiest inhaler device to use and indicated that they would prefer Spiromax over Easyhaler or Turbuhaler if they were prescribed an inhaler.
Nearly all participants were able to achieve short-term device mastery after healthcare professional instruction, demonstrating the importance of face-to-face interaction during inhaler training.
Introduction
Disorders of the airways are prevalent worldwide and have major adverse effects on the quality of life of affected individuals; also, the economic costs associated with these diseases are considerable.1–3 Patient-related factors, including smoking, poor treatment adherence and device handling errors, have a negative impact on treatment control.4 The therapeutic efficacy of inhalation therapy requires the drug(s) to reach the targeted areas of the lower lung.5 As such, suboptimal adherence to pharmacological treatment, for example, of asthma, can have serious adverse effects on disease control.2 There is an increasing body of evidence to suggest that correct use of the inhalation device is critical for optimal drug delivery.6
Studies have shown that inhaler use is often highly suboptimal. Research indicates that between 20% and 82% of patients (depending on the type of inhaler and method of assessment) do not use dry powder inhalers (DPIs) correctly.4 7–9 It has also been reported that as many as 25% of patients have never received sufficient training for using their inhaler device,5 and more than 50% of patients have not had their inhaler technique checked within the past year.10 Therapeutic success is not only dependent on educating and training the patient but also on patients’ perception of the inhaler device. An ‘easy to use’ inhaler device that is also preferred by patients may facilitate the correct handling of the inhaler, potentially improving adherence and ultimately maintaining symptom control.5 Choosing the most appropriate inhaler device for the individual patient is among the key factors for successful asthma management,2 5 given that poor inhalation technique is associated with increased unscheduled healthcare resource use and poor clinical control.7
The combination therapy involving an inhaled corticosteroid (ICS) and a long-acting β2 agonist (LABA) is recommended for patients with inadequately controlled asthma. The fixed dose combination of the ICS/LABA budesonide/formoterol (BF) has shown greater improvement in pulmonary function and overall asthma control compared with either individual compound alone.11 12 Currently in Europe, BF is available via three different DPIs: Bufomix (BF) Easyhaler (Orion Pharma Oy), Symbicort (BF) Turbuhaler (AstraZeneca AB) and DuoResp (BF) Spiromax (Teva Pharmaceutical Industries Ltd).
Easyhaler is a multidose DPI that shares similarities in its handling steps and shape with a pressurised metered-dose inhaler (pMDI).13 14 It consists of seven plastic components, a metering cylinder spring and plastic components. The drug dose is delivered by pressing/pushing down once on the overcap of the inhaler.14 Easyhaler dose preparation requires vigorous shaking with the inhaler kept in the upright position.15 Turbuhaler is a multidose DPI designed to deliver predetermined doses of the drug (located within the inhaler in the form of a soft aggregate pellet). It consists of a mouthpiece with spiral-shaped channels, rotating disk, drug reservoir, turning grip and a protective cover. Dose preparation requires holding the device in the upright position and a full twist/rotation (forward and back) of the grip at the base of the device.14 Spiromax is a multidose DPI designed to provide high-dose consistency with maximal ease-of-use for the patients. Similar to Easyhaler, Spiromax has a design/look similar to a pMDI; however, the internal configurations of the inhaler are different (the devices use a novel X-ACT technology).16 Dose preparation consists of fully opening the cap until a click is heard while holding the device in the upright position.
While the therapeutic efficacy and clinical significance of BF combination in the treatment of adults with asthma is well established, the effectiveness of this therapy is dependent on patients using their inhaler correctly. Limited information is available on how the existing inhalers differ in their ease of handling, ease of use/intuitiveness, steps needed to achieve technique mastery (absence of inhalation errors) and whether patients have any preference on the type of inhaler they are prescribed.
This study was designed to evaluate the differences in device mastery between Spiromax, Easyhaler and Turbuhaler, using empty versions of the inhaler devices, in healthy adult Finnish volunteers. This study also assessed the participants’ preference and satisfaction with each of the inhaler devices used.
Methods
Study design
This was a single site, single visit, crossover study assessing device mastery, handling errors and device preference with three DPIs, Spiromax, Easyhaler and Turbuhaler. The study was conducted at the Åbo Akademi University, Turku, Finland (in April/May 2015). The study participants were provided with an identification number via email and randomly assigned to one of the six assessment groups (figure 1). All participants completed a background questionnaire at the study entry and provided signed informed consent. When enrolling via email or phone, the individuals who took part in the study provided their names; however, they remained anonymous to the results. Participants were compensated with a €20 gift card. The participants were healthy volunteers. In our opinion, the sample is appropriate although consisting of relatively highly educated persons. The recruitments were made using announcements that mainly reached students and staff at the campus area, and local companies.
Figure 1.
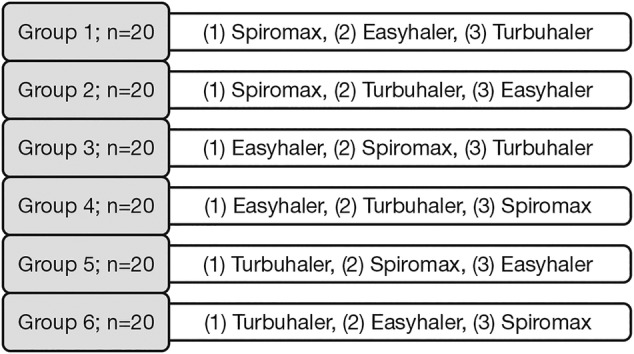
Study design.
Empty devices without active substance or excipients were used (figure 2). Each device was tested in three steps: (1) intuitive use (with no instructions), (2) after reading the Finnish or Swedish language patient information leaflet (PIL) and (3) after healthcare professionals (HCPs) provided instructions for use. The device use was monitored by one of the research coordinators in each step and the errors were reported according to the Device-Specific Handling Error Checklist (DSHEC, see online supplementary file 1), based on the approved PIL for each of the devices. The study participants were allowed to progress to the next device when no errors were observed with the device they were being assessed on. At the end of the study, the participants were asked to complete the Device Preference Questionnaire (DPQ, see online supplementary file 2) and rate their satisfaction with each of the devices used.
Figure 2.

Empty devices used in the study. From left to right: Easyhaler, Spiromax and Turbuhaler.
bmjresp-2015-000119supp_appendix1.pdf (56.1KB, pdf)
bmjresp-2015-000119supp_appendix2.pdf (78.7KB, pdf)
Inclusion criteria
The study subjects were adult (≥18 years old) Finnish individuals, who had not used any DPI (as confirmed via email during the screening process prior to enrolling in the study) during the past 18 months, and they were not trained in the use of/demonstration of any inhaler for the treatment of asthma, chronic obstructive pulmonary disease or other diseases.
Study end points
Primary end points
The primary end point of this study was the proportion of participants achieving device mastery when using Spiromax compared with the proportion of participants achieving device mastery when using Easyhaler, in step 2 (after reading the PIL). Device mastery was defined as absence of errors observed by HCPs in step 2.
Secondary end points
The secondary end points of this study included: the proportion of participants achieving device mastery in step 1 (intuitive use), step 2 and step 3 (after receiving HCP instructions); the number, type and characteristic of device handling errors; and device preference, ease of use and satisfaction when using Spiromax, Easyhaler or Turbuhaler. Turbuhaler was primarily included as a comparator device to validate the study.
Statistical analysis and study assessments
Recorded data from the background questionnaire, DSHEC and DPQ were transferred by the monitoring research coordinator to a Microsoft Excel spreadsheet. Descriptive and exploratory analyses were performed to assess the end point variables.
Device mastery for steps 1–3 for Spiromax, Easyhaler or Turbuhaler were defined as the percentage of participants with absence of observed errors listed in the DSHEC. McNemar's test for dependent categorical observations was used for comparisons. Percentages of errors observed using each of the devices were calculated using the Within-Subjects Analysis of Variance.
Spiromax, Easyhaler or Turbuhaler device handling errors were defined as the proportion of study participants with handling errors during preparation, during inhalation or after inhalation in steps 1, 2, or 3. The characterisation of device handing errors consisted of a list of the individual handling errors observed (for each of the devices used) by the monitoring HCPs.
Participants were asked to rate their satisfaction with the inhaler device (on a scale from 1 (unsatisfactory) to 5 (excellent)) for the following features: overall ease of use, quality of PIL instructions, preparing the dose, inhaling procedure and clarity of dose counter.
Results
Study population
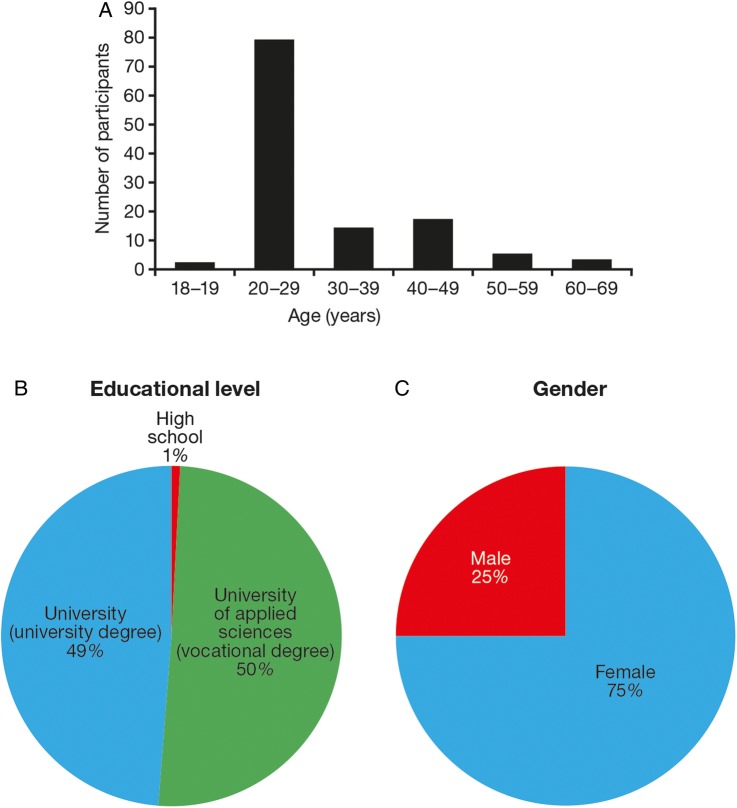
A total of 120 participants were included in the crossover study (n=20 for each of the six arms; figure 1). Participants’ demographics and educational level are shown in figure 3. The majority of study participants (n=79; 66%) were aged 20–29 years (figure 3A), almost all participants (99%) were educated to university level (figure 3B) and 75% were female (figure 3C). One should therefore be careful in generalisation of data to cover any type of population or age groups.
Figure 3.
Participants’ demographics and education level. Age (A), educational level (B), gender (C).
Device mastery
Device mastery is defined as absence of healthcare professional-observed errors during each of the three steps. During step 2 (after reading the PIL), the percentage of participants who used the device without error was higher with Spiromax (93.3%) compared with Easyhaler (58.3%) or Turbuhaler (76.7%; table 1). This difference was significant for all comparator groups (p<0.001 (Spiromax vs Easyhaler and Spiromax vs Turbuhaler); table 1). Similarly, in step 1, Spiromax was associated with a greater level of device mastery compared with Easyhaler or Turbuhaler; 37.5% of participants were able to use Spiromax with no errors in step 1 (intuitive use) compared with 0% with Easyhaler (p<0.001) and 9.2% with Turbuhaler (p<0.001; table 1). The device mastery level was high (>95%) with all three devices during step 3 (after receiving HCP instructions). The number of participants observed making errors at each step varied between the three devices. The mean proportion of observed errors during steps 1 and 2 was also lower with Spiromax (12.4% and 0.8%) compared with Easyhaler (18.7% and 5.0%; both p<0.001) or Turbuhaler (17.6% (p<0.002) and 2.8% (p<0.001)); the mean proportion of observed errors was similar between the devices in step 3 (0.1%, 0.5% and 0.1% for Spiromax, Easyhaler and Turbuhaler, respectively).
Table 1.
Proportion (%) of participants who were able to use Spiromax, Easyhaler or Turbuhaler without observed errors in steps 1–3
| Spiromax | Easyhaler | Turbuhaler | Spiromax vs Easyhaler, p value | Spiromax vs Turbuhaler, p value | Easyhaler vs Turbuhaler, p value | |
|---|---|---|---|---|---|---|
| Step 1 | 37.5 | 0 | 9.2 | <0.001* | <0.001 | <0.006* |
| Step 2 | 93.3 | 58.3 | 76.7 | <0.001 | <0.001 | <0.004 |
| Step 3 | 99.2 | 95.8 | 99.2 | ns | ns | ns |
*One observation was added to the Easyhaler group in order to perform the statistical analysis. As such, these values might be an underestimate of the actual differences.
ns, not significant.
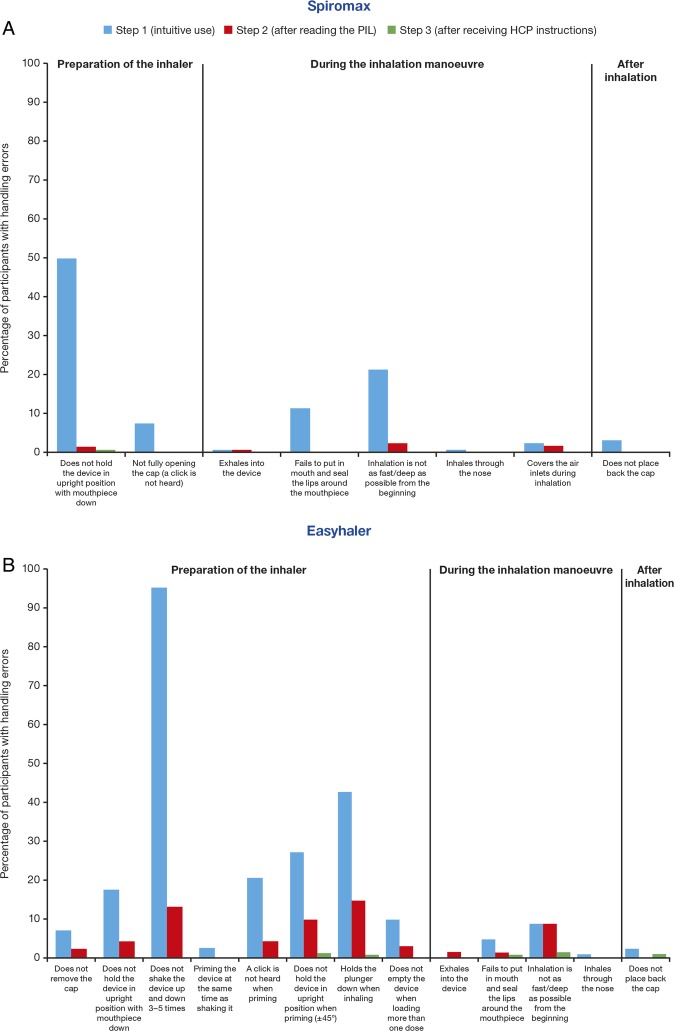
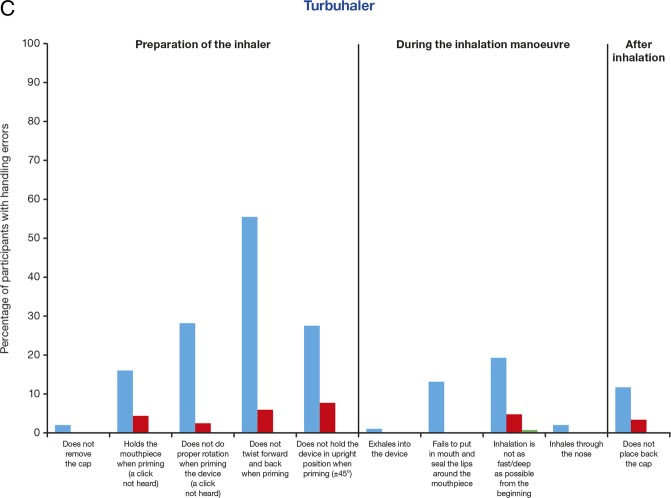
Since the types of device handling manoeuvres differ between Spiromax, Easyhaler and Turbuhaler, the percentages of handling errors were evaluated during preparation, during inhalation and after inhalation in steps 1–3 for each device, and for each of the device-specific handling manoeuvres (figure 4). Spiromax was associated with a smaller number of participants observed making errors during the preparation of the device in steps 1 and 2 (57.5% and 1.7%) compared with Easyhaler (100% and 39.2%) and Turbuhaler (86.7% and 16.7%). Easyhaler was associated with the lowest number of participants observed making errors during the ‘inhalation phase’ (which is similar for all DPIs and requires that the patients inhale as fast and as deeply as possible from the start of the inhalation manoeuvre) in step 1 (12.5%) compared with Spiromax (22.5%) and Turbuhaler (19.2%). All devices had a low number of errors during step 3. The most common error reported when using Spiromax was ‘not holding the device in the upright position with mouthpiece’ (50.8% of participants in step 1; figure 4A). Two of the participants inhaled with the Spiromax device in a horizontal position. The most common handling errors reported when using Easyhaler included: ‘not properly shaking the device when preparing it’ (95.8% and 13.3% of participants; steps 1 and 2, respectively) and ‘holding the plunger down when inhaling’ (43.3% and 15% of participants; steps 1 and 2, respectively; figure 4B). A large proportion of participants (41.7%) had handling errors with Easyhaler after reading the PIL. The most common errors with Turbuhaler were related to priming the device in step 1 and included: the ‘lack of twisting forward and back’ (55.8% of participants) and ‘not holding the device in the upright position’ (27.5% of participants; figure 4C). In general, age, educational level and gender were unrelated to device mastery (data not shown; the educational level and age were similar for all participants (only 1% not educated to university level, the majority of participants were 20–29 years of age)).
Figure 4.
Device handling errors by manoeuvre type with (A) Spiromax, (B) Easyhaler, (C) Turbuhaler, during preparation and inhalation, and after inhalation.
Figure 4.
Continued
Device preference and satisfaction
In addition to the device mastery observations, the participants also responded to a number of questions that assessed their perception of the three inhaler devices. Of the 119 participants who responded (one participant did not provide valid information), 73.1% rated Spiromax as the easiest inhaler to use and 71.4% reported that, if prescribed an inhaler, they would prefer Spiromax. In comparison, Easyhaler was rated as the easiest inhaler to use by 12.6% of the participants and 16.8% would prefer Easyhaler if prescribed an inhaler product. Turbuhaler was rated easiest to use by 14.3% of participants and 11.8% indicated that they would prefer Turbuhaler if prescribed an inhaler.
Participants were also asked to rate their satisfaction with five features for the three devices: ‘overall ease of use’, ‘quality of PIL instructions’, ‘preparing the dose’, ‘inhaling procedure’ and ‘clarity of dose counter’. With the exception of ‘inhaling procedure’, participants rated Spiromax significantly higher compared with both Easyhaler and Turbuhaler (p<0.001 for all comparisons). The difference was not significant for satisfaction with the ‘inhaling procedure’ between the three devices. Turbuhaler was rated significantly higher than Easyhaler for the ‘quality of PIL instructions’ (p<0.003) and Easyhaler was rated significantly higher than Turbuhaler for the ‘clarity of the dose counter’ (p<0.015). No significant differences were reported between Easyhaler and Turbuhaler for the remainder of the features.
Discussion
This study shows that more participants achieved device mastery using the empty Spiromax inhaler compared with empty Easyhaler or Turbuhaler inhalers. In step 1, 37.5% of participants were able to use Spiromax correctly (intuitive use), whereas only 9.2% of participants used Turbuhaler correctly and none of the participants were able to use Easyhaler without handling errors. Information provided in the PIL was beneficial in instructing participants how to use the inhaler device properly. Fewer errors were reported for all three devices in step 2; respectively, 93.3%, 58.3% and 76.7% of participants were able to use Spiromax, Easyhaler and Turbuhaler correctly. The superiority in device mastery with Spiromax over Easyhaler and Turbuhaler was maintained in step 2 (p<0.001). Inhaler technique mastery was better using Turbuhaler compared with Easyhaler in steps 1 and 2. In step 3, when participants were trained on how to use each of the inhaler devices by an HCP, almost all participants (>95%) were able to use the inhaler devices correctly. No significant differences between the three devices were reported in step 3. Two independent studies are currently investigating device mastery for Spiromax compared with Turbuhaler among HCPs (in Australia) and patients with asthma (in the UK). Outcomes from the study presented here are similar to results reported in the device mastery study among Australian HCPs. More HCP participants achieved device mastery with Spiromax prior to training or after reading the PIL compared with Turbuhaler.17
The improvement of device mastery in response to training highlights the importance of face-to-face training of participants at clinical visits. Inhaler technique is now an integral part of the Global Initiative for Asthma (GINA) management strategy.18 GINA guidelines recommend training patients in the use of inhalers as a fundamental and essential component of good clinical practice.18 Although training patients in inhaler use is of particular importance, and it is not recommended that patients are left to educate themselves, at least 25% of asthma patients have never received training.5 In some situations, it might not be possible or practical to provide training to a particular patient, which highlights the importance of an intuitive inhaler device. An innovative inhaler that is easy to use ‘out-of-box’ could potentially minimise the risk of poor asthma control by ensuring that patients are able to use the inhaler correctly even if they have not been verbally educated by an HCP in inhaler use or have not read the PIL, which is also of importance in instances where a switch/automatic substitution of inhaler devices postprescription takes place. Although an intuitive device might minimise the risk of error in the event of a switch, it should be noted that a switch without instruction and training is not recommended and is considered to be against good clinical practice. In this study, Spiromax was associated with fewer errors compared with Easyhaler and Turbuhaler when participants used the devices intuitively (step 1) or after reading instructions available in the PIL (step 2). Spiromax was also associated with the lowest number of errors during the ‘preparation’ of the device in step 1 and step 2. Such innovation in inhaler design could contribute to improving patient inhaler technique and achieving disease control leading to better allocation of healthcare resources.19
Optimal inhaler technique differs between devices.20 Manoeuvres while preparing the device and after inhalation are device-specific. This study further investigated the proportion of errors committed for each of the device-specific manoeuvres for Spiromax, Easyhaler and Turbuhaler.
The most common error reported for Spiromax was linked to the orientation of the device. Participants held the device upside down, both when preparing the device and during inhalation, which accounted for 51.3% of errors committed with Spiromax in step 1. Also, two of the participants held the device in a horizontal position. Per the inclusion criteria, participants were healthy individuals, inhaler-naïve and inhaler training-naïve. As such, this error could be related to participants not being familiar with the shapes of inhalers, whereas patients would probably be less likely to hold an inhaler in the horizontal position. Of note, during the device mastery observations, participants commented on the lack of text on the Spiromax device, which potentially could have contributed to holding the device in a wrong position. In contrast, the empty versions of the Easyhaler and Turbuhaler devices included text (figure 2). Given that the marketed Spiromax device will have a label with text on, it is likely this error would be less frequently seen in clinical practice. Although Spiromax needs to be held in the upright position during the preparation manoeuvre (while opening the cap of the inhaler device for dose preparation), the finding from a recent study that assessed the dose consistency delivered with Spiromax when the device is held at different orientations revealed that dose consistency is maintained when the inhaler is held at a + or −90° orientation.16 As such, the orientation error reported in this study with Spiromax might not be considered a critical error/error that could affect drug dose delivery to the lungs. Patients holding Spiromax in a different orientation than the recommended upright position during the inhalation manoeuvre might still be receiving the appropriate drug dose.
The most common error reported with Easyhaler was also during the preparation of the device. Participants did not shake the device prior to inhalation in step 1 (95.8%) and step 2 (13.3%). A surprisingly large proportion of participants (41.7%) were observed making handling errors even after reading the PIL with Easyhaler (compared with <10% of participants when using Spiromax or Turbuhaler). The high number of errors in step 2 with Easyhaler might be linked to some ambiguities in the Swedish language of the PIL. For example, one of the Swedish PIL instructions is: “skaka upp och ned”, which translates to: “shake the device up and down”, and the following instruction is: “se till att du skakar den upp och ner”, which, coming straight after the first instruction, could translate as: “make sure you shake the device holding it upside down”. This is different to the English version of the PIL in which the instructions are: “shake the Easyhaler vigorously up and down three to five times, to allow proper powder flow and a correct dose. After shaking, hold the Easyhaler in the upright position”.16
The most common handling errors with the Turbuhaler were also during the preparation of the device. Incorrect priming of the device, especially the lack of twisting forward and back, which would result in dose loading errors if an active device were being used (little or low medication would reach the lungs),21 was reported for 55.8% of the participants in step 1. Interestingly, in step 1, Easyhaler performed better than Turbuhaler and Spiromax during the ‘inhalation’ manoeuvres. This could be attributed to some participants (12 with Spiromax, 1 with Easyhaler and 6 with Turbuhaler) requesting instructions before inhaling through the device; inhalation manoeuvres were recoded as ‘errors’ in those instances. Of note, in general, errors during the ‘inhalation’ manoeuvre are not considered device specific. All DPIs require that patients inhale as deeply and as fast as possible from the start.
It is recognised that the selection of the most appropriate inhaler requires consideration of the patient’s ability to use the device correctly, preference and satisfaction with the device.13 22 When participants were asked to rate Spiromax, Easyhaler and Turbuhaler for device preference (using the DPQ), the majority of participants (73.1%) indicated that they found Spiromax the easiest device to use and 74.1% indicated that if they were prescribed an inhaler they would prefer Spiromax over Easyhaler or Turbuhaler. Similar findings were reported in a recent study that assessed device preference comparing Spiromax with Turbuhaler in patients with asthma. In this study, device preference was measured using the Patient Satisfaction and Preference. Preference for budesonide-formoterol (BF) Spiromax, which is easy to use, intuitive and preferred by patients using asthma inhalers—versus BF Turbuhaler—could ultimately improve adherence.19 Good medication adherence is an essential requirement for optimal clinical outcome and would also help to reduce treatment costs.19 Compared with Easyhaler and Turbuhaler, Spiromax was the ‘preferred’ inhaler by the participants of this study. Choosing the most appropriate inhaler for the patient has been shown to enhance adherence to therapy and, consequently, improve clinical outcomes.22–24 These results suggest that Spiromax use could have positive clinical implications on asthma management.
Among the strengths of this study is the crossover study design, which reduces bias that could potentially result from variations between study groups. The inclusion of participants who were inhaler naïve is another strength of this study. Randomised controlled trials have previously reported no differences in efficacy between inhaler devices.25 In most of these trials, patients enrolled had received training and demonstrate good inhaler technique. In the real world, most patients do not use their inhalers correctly and some have not received any training. As such, this study allows the evaluation of device handling and mastery without the influence of prior experience or knowledge of inhalers confounding the results.
One of the limitations of this study is that almost all participants were educated to university level (99%). Literacy and education play an important part in inhaler technique. A greater number of inhalation errors are committed by patients who are illiterate and educated to primary level compared with patients educated to a higher level and university graduates.26 It would be interesting to assess whether the superiority of Spiromax over Easyhaler and Turbuhaler is maintained if a similar study is conducted in naïve participants with low education and literacy levels. Ultimately, it would be most informative if a similar study is conducted in a ‘real world’ context, in a heterogeneous group of patients with asthma.
In conclusion, outcomes from this study indicate that more participants achieved device mastery with Spiromax compared with Easyhaler or Turbuhaler. Spiromax was associated with a lower number of errors after intuitive use (step 1) and after reading the PIL (step 2). More participants felt that Spiromax was easier to use than Easyhaler or Turbuhaler and reported that they would prefer Spiromax if they were prescribed an inhaler. The higher levels of device mastery achieved with Spiromax, combined with the ease of use of the device, could potentially improve adherence, leading to improved asthma control and potentially reducing treatment costs. In general, it is clear that face-to-face instruction is essential when prescribing inhalation therapy; however, an optimally designed device can play a role in maximising patient compliance and device mastery.
Footnotes
Contributors: NS, PS, AS and ST were responsible for the study design. JH and DL were responsible for acquisition of data. PS was responsible for analysis of the data and was assisted by all the authors for interpretation of the data. All the authors were responsible for development of manuscript, critical review and approval of final version of the manuscript.
Funding: The study was commissioned by Teva Pharmaceutical (Saku Torvinen). Medical writing support was provided by Nadia Korfali of GeoMed, an Ashfield company, part of UDG Healthcare plc, funded by Teva Pharmaceutical Inc. Teva Pharmaceutical Inc provided a full review of the article.
Competing interests: NS reports grants from MedEngine Ltd, during the conduct of the study. ST and AS are employees of Teva Pharmaceutical Inc. DL was a part-time employee of Teva Pharmaceutical Inc during the study but her duties did not relate to this work.
Ethics approval: The study was approved by the Ethical Committee of Åbo Akademi University. Participants gave informed consent to take part in the study.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Masoli M, Fabian D, Holt S et al. , Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59:469–78. doi:10.1111/j.1398-9995.2004.00526.x [DOI] [PubMed] [Google Scholar]
- 2.Mäkelä MJ, Backer V, Hedegaard M et al. . Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med 2013;107:1481–90. doi:10.1016/j.rmed.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 3.Jantunen J. Allergian ja astman kustannukset Suomessa vuonna 2011. Sosiaali-ja terveysturvan selosteita 85/2014.
- 4.Molimard M, Le Gros V. Impact of patient-related factors on asthma control. J Asthma 2008;45:109–13. doi:10.1080/02770900701815727 [DOI] [PubMed] [Google Scholar]
- 5.Lavorini F, Magnan A, Dubus JC et al. . Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med 2008;102:593–604. doi:10.1016/j.rmed.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 6.Papi A, Haughney J, Virchow JC et al. . Inhaler devices for asthma: a call for action in a neglected field. Eur Respir J 2011;37:982–5. doi:10.1183/09031936.00150910 [DOI] [PubMed] [Google Scholar]
- 7.Melani AS, Bonavia M, Cilenti V et al. , Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 2011;105:930–8. doi:10.1016/j.rmed.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 8.Al-Jahdali H, Ahmed A, Al-Harbi A et al. . Improper inhaler technique is associated with poor asthma control and frequent emergency department visits. Allergy Asthma Clin Immunol 2013;9:8–9. doi:10.1186/1710-1492-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arora P, Kumar L, Vohra V et al. . Evaluating the technique of using inhalation device in COPD and bronchial asthma patients. Respir Med 2014;108:992–8. doi:10.1016/j.rmed.2014.04.021 [DOI] [PubMed] [Google Scholar]
- 10.Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med 2014;24:14009 doi:10.1038/npjpcrm.2014.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calverley PM, Boonsawat W, Cseke Z et al. . Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J 2003;22:912–19. doi:10.1183/09031936.03.00027003 [DOI] [PubMed] [Google Scholar]
- 12.Rabe KF, Atienza T, Magyar P et al. . Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised, controlled, double-blind study. Lancet 2006;368:744–53. doi:10.1016/S0140-6736(06)69284-2 [DOI] [PubMed] [Google Scholar]
- 13.Chrystyn H. The Diskus: a review of its position among dry powder inhaler devices. Int J Clin Pract 2007;61:1022–36. doi:10.1111/j.1742-1241.2007.01382.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy SC, Swain K, Shivakumar HG et al. . Past and present trends of dry powder inhaler devices: a review. J Drug Deliv Ther 2014;4:97–107. [Google Scholar]
- 15.Easyhaler® Instructions for Use in English. http://www.orionpharma.se/OrionPharmaSE_Global/ATTACHMENTS_Sweden/Order_Asthma/Engelska.pdf
- 16.Canonica GW, Arp J, Keegstra JR et al. . Spiromax, a new dry powder inhaler: dose consistency under simulated real-world conditions. J Aerosol Med Pulm Drug Deliv 2015;28:309–19. doi:10.1089/jamp.2015.1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosnic-Anticevich S, Lim D, Steel J et al. . Investigating the maintenance of inhaler device mastery of healthcare professionals’. Abstract and Poster Presentation at EAACI; 2015. [Google Scholar]
- 18.Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention 2014. http://www.ginasthma.org
- 19.Virchow J, Akdis C, Darba J et al. . A review of the value of innovation in inhalers for COPD and asthma. J Mark Access Health Policy 2015. http://www.jmahp.net/index.php/jmahp/article/view/28760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orion Corporation. Easyhaler Budesonide Patient Information Leaflet. 2014. https://www.medicines.org.uk/emc/medicine/21563
- 21.Basheti IA, Qunaibi E, Bosnic-Anticevich SZ et al. . User error with Diskus and Turbuhaler by asthma patients and pharmacists in Jordan and Australia. Respir Care 2011;56:1916–23. doi:10.4187/respcare.01205 [DOI] [PubMed] [Google Scholar]
- 22.Anderson P. Patient preference for and satisfaction with inhaler devices. Eur Respir Rev 2005;14:109–16. doi:10.1183/09059180.05.00009606 [Google Scholar]
- 23.Kozma CM, Slaton TL, Monz BU et al. . Development and validation of a patient satisfaction and preference questionnaire for inhalation devices. Treat Respir Med 2005;4:41–52. doi:10.2165/00151829-200504010-00005 [DOI] [PubMed] [Google Scholar]
- 24.Lavorini F, Fontana GA. Inhaler technique and patient's preference for dry powder inhaler devices. Expert Opin Drug Deliv 2014;11:1–3. doi:10.1517/17425247.2014.846907 [DOI] [PubMed] [Google Scholar]
- 25.Haughney J, Price D, Barnes NC et al. . Choosing inhaler devices for people with asthma: current knowledge and outstanding research needs. Respir Med 2010;104:1237–45. [DOI] [PubMed] [Google Scholar]
- 26.Saugat R, Bera R, Gujrani M et al. . Evaluation of techniques of inhalation devices among patient of COPD and bronchial asthma. Eur J Pharm Med Res 2015;2:376–91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2015-000119supp_appendix1.pdf (56.1KB, pdf)
bmjresp-2015-000119supp_appendix2.pdf (78.7KB, pdf)