Abstract
Objective
This prospective study aimed to identify antibody profiles characterising mothers with fetuses developing congenital heart block (CHB) by comparing their antibody frequencies and levels with those in unaffected mothers.
Methods
Eighty-one consecutive pregnant patients positive to anti-Ro±anti-La antibodies, at high risk of developing fetal CHB were prospectively studied. The 16 patients with fetal CHB outcome were considered the study population and the 65 patients with normal pregnancy outcomes were considered the control cohort. Anti-Ro52, anti-Ro60, anti-p200 and anti-La antibodies were assayed using home-made ELISA assays.
Results
The prevalence of anti-p200 antibodies was significantly higher in the fetal CHB affected patients than in the controls (p=0.03). Combinations of anti-p200 with anti-Ro52 and anti-Ro60 antibodies were significantly more frequent in the women with fetuses developing CHB than in the controls (p=0.03 for all combinations). The women with fetal CHB had significantly higher mean anti-Ro52, anti-Ro60 and anti-p200 levels than the controls (p=0.003, p=0.0001 and p=0.04, respectively); mean anti-La/SSB level was not significantly different in the two cohorts (p=0.25).
Conclusions
Since anti-p200, anti-Ro52 and anti-Ro60 antibodies, especially at high level, seem to identify patients at increased risk of developing fetal CHB, their detection could recognise anti-Ro/La positive women at risk for having an infant with this rare, potentially dangerous disorder.
Keywords: Autoantibodies, Autoimmunity, Autoimmune Diseases
Introduction
Pregnant women with Ro52, Ro60 and La ribonucleoprotein antibodies have been found to be at risk of having infants with isolated congenital heart block (CHB).1 Antibodies reactive to Ro and/or La antigens seem to cross the placenta, enter the fetal circulation via trophoblast receptors, and presumably injure fetal heart, most often between the 18th and 24th gestational week. There is accumulating evidence that anti-Ro52 antibodies play a predominant role in the development of CHB by mediating the initial heart damage.2 More specifically, antibody response against the p200 epitope spanning Ro52 amino acids 200–239 has been shown to bind the cell surface receptors of cardiomyocytes inducing dysregulation of intracellular calcium movements and eventually causing death.3 Some authors have reported that intracellular Ro/La antigens translocate to the surface of cardiomyocytes undergoing apoptosis and subsequently promote proinflammatory and profibrotic responses.4
Pinpointing specific antibody profiles characterising a CHB outcome could be an important step in identifying mothers at risk of developing an infant with this disorder. While the clinical relevance of anti-Ro52/Ro60 and anti-La antibody detection in CHB mothers is well known,1 2 the association of anti-p200 antibodies with CHB occurrence is currently under debate.5–9 At the same time, while some investigators have reported higher anti-p200 antibody levels in all CHB affected mothers with respect to unaffected control women,5 6 9 others claim that the frequency and levels of those antibodies are not significantly different in the two groups.7 8
There are some investigations about the role of antibody titres and CHB development. Dörner et al10 found significantly higher anti-Ro52 and anti-La antibody levels in CHB affected women, and Tunks et al11 reported significantly higher anti-La antibody levels in CHB pregnancies. Jaeggi et al12 described a significant correlation between anti-Ro levels and risk of cardiac complications, and Anami et al13 defined anti-Ro antibody titres an independent risk factor for CHB.
The aim of this study was then to evaluate what maternal antibody profiles characterise CHB outcome by comparing anti-Ro52, anti-Ro60, anti-p200 and anti-La antibody frequencies and levels in affected (CHB+) and unaffected (CHB−) mothers.
Method
Study group
Eighty-one consecutive pregnant patients positive to anti-Ro±anti-La antibodies, at high risk of developing fetal CHB and attending the outpatient clinic of the Rheumatology Unit of the University of Padova Medical Center were prospectively enrolled in the study between January 2009 and December 2014. The patients were diagnosed in accordance with the current guidelines for systemic autoimmune diseases. The women whose fetuses later developed CHB were considered the study population (CHB+) and the women with normal pregnancy outcomes (CHB−) were considered the control population. All patients underwent a physical examination, fetal ultrasound studies and routine biochemistry testing every month from the beginning of the pregnancy to the time of delivery. Fetal echocardiographies were performed weekly by the same paediatric cardiologist from the 18th to the 26th week of gestation and then fortnightly until the time of delivery. The institutional review board for the University-Hospital of Padua approved the study design, and the study was carried out in accordance with the original Declaration of Helsinki and its later amendments. Once the patients were adequately informed about the study's aims and methodology they were asked to sign informed consent forms.
Antibody detection
Maternal serum samples were collected at the beginning of pregnancy or at the time CHB was first detected. Serum samples were stored at −80°C until anti-Ro52, anti-Ro60 and anti-La IgG antibodies could be assayed using a home-made ELISA, as described elsewhere.14 Anti-p200 antibodies were detected by ELISA according to the method proposed by Salomonsson et al5 with minor modifications. Briefly, synthetic p200 antigen was purchased from Thermo Biosciences (Ulm, Germany). High-binding 96-well plates (Nunc, Odense, Denmark) were coated (1 μg/well) overnight with p200 diluted in carbonate buffer (pH 9.6). Plates were blocked with 200 μL phosphate buffered saline (PBS)/0.05% Tween/5% milk powder and sera were tested at 1:500 dilution in PBS/0.05% Tween/1% milk powder. Bound antibodies were detected by affinity-purified alkaline phosphatase-conjugated antihuman IgG antibodies (Sigma, St Louis, Missouri, USA) at 1:2000 dilution. Phosphatase substrate tablets (Sigma) dissolved in Mg–carbonate buffer (pH 9.8) were added. Absorbance was measured at 405 nm and the optical density (OD) was arbitrarily converted into bound units (BU) using a high positive serum to build a seven dilution point calibration curve. If the sample's OD was greater than the last point of the standard curve, further dilutions were made and the BU were multiplied by the dilution factor.
The cut-off values were calculated as the 99th percentile of values obtained by testing the sera of 100 healthy women matched for age and sex with the study women. The cut-offs for positive tests were 7.7 BU for anti-Ro52, 6.1 BU for anti-Ro60, 2.0 BU for anti-La and 0.8 BU for anti-p200 antibodies.
Statistical analysis
The clinical and laboratory parameters of the two groups were compared using χ2 and Mann–Whitney U tests. A p value <0.05 was considered significant.
Results
CHB was detected between the 20th and the 29th gestational weeks in 16 (19.7%) of the women; the remaining 65 (80.2%) had normal pregnancy outcomes. Fourteen of CHB+ mothers had previous pregnancies and one of them had a previous fetal CHB. The clinical characteristics of the two groups are outlined in table 1. Only the absence of any rheumatic disease (asymptomatic women) was significantly prevalent in the CHB+ mothers (p=0.02). Since 2009 all mothers are currently followed at our outpatient clinics. During the follow-up period four of them developed a systemic autoimmune disease: a Sjögren’s syndrome and an undifferentiated connective tissue disease both in CHB+ and CHB− groups.
Table 1.
Clinical characteristic of the patients studied
| Clinical characteristics | CHB+ study cohort (n=16) | CHB− control cohort (n=65) | p Value |
|---|---|---|---|
| Mean age±SD | 32.7±5.2 | 34.4±4.52 | 0.35 |
| Diseases | |||
| Sjögren’s syndrome | 5 | 26 | 0.72 |
| Undifferentiated connective tissue disease | 3 | 20 | 0.52 |
| Systemic lupus erythematosus | 0 | 3 | 0.89 |
| Rheumatoid arthritis | 0 | 1 | 0.44 |
| Discoid lupus | 0 | 1 | 0.44 |
| Antiphospholipid syndrome | 0 | 2 | 0.85 |
| Asymptomatic women | 8 | 12 | 0.02* |
| Thyroiditis† | 4 | 8 | 0.37 |
*Statistically significant difference.
†Alone or associated.
CHB+, congenital heart block outcome; CHB−, normal pregnancy outcome.
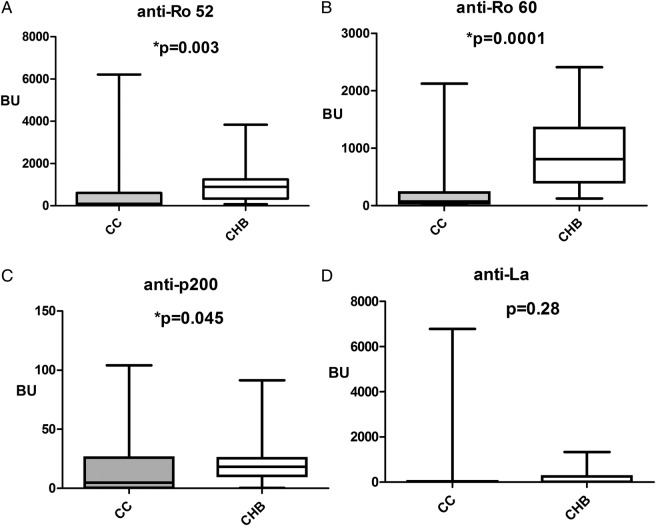
Both anti-Ro52 and anti-Ro60 antibodies were present in all the CHB+ patients; anti-La and anti-p200 antibodies were detected, respectively, in 13 (81.3%) and 15 (93.8%) of these. Anti-Ro52, anti-Ro60, anti-La and anti-p200 were present, respectively, in 54 (83.1%), 64 (98.5%), 48 (73.8%) and 43 (66.2%) of the CHB− patients. Only the prevalence of anti-p200 was significantly higher in the CHB+ than in the CHB− patients (p=0.03). The antibody combinations detected in the two groups are outlined in table 2. The anti-p200 antibody combination with anti-Ro52 and anti-Ro60 antibodies was significantly higher in the CHB+ group than in the control cohort. Mean maternal antibody levels are outlined in figure 1. No correlation between anti-p200 maternal antibody levels and severity of CHB was found. All children were alive at birth and during a follow-up time of 37.6 months±19.6 SD (range 20–70). During that period permanent pacemakers were implanted in three of the infants at 5, 6 and 10 months, respectively. A combined therapeutic protocol previously described15 16 has been administered in all cases, although the therapy was administered in different gestational age and in various types of CHB.
Table 2.
The autoantibody profiles of the patients studied
| Autoantibodies | CHB+ study cohort (n=16) | CHB− control cohort (n=65) | p Value |
|---|---|---|---|
| Autoantibodies n (%) | |||
| Ro52+Ro60 | 16 (100) | 53 (81.5) | 0.11 |
| Ro52+La | 13 (81.3) | 44 (67.7) | 0.37 |
| Ro60+La | 13 (81.3) | 48 (73.8) | 0.75 |
| Ro52+Ro60+La | 13 (81.3) | 44 (67.7) | 0.37 |
| p200+Ro52+Ro60 | 15 (93.8) | 42 (64.6) | 0.03* |
| p200+La | 13 (81.3) | 40 (61.5) | 0.24 |
| p200+Ro52+Ro60+La | 13 (81.3) | 39 (60) | 0.15 |
*Statistically significant difference.
CHB+, congenital heart block outcome; CHB−, normal pregnancy outcome.
Figure 1.
Antibody levels to Ro52, Ro60, p200 and La antigens in control and congenital heart block (CHB) cohort were measured as bound units (BU). (A) Anti-Ro52, (B) anti-Ro60, (C) anti-p200 and (D) anti-La antibodies. Women belonging to the CHB+ cohort (CHB) had significantly (*) higher median anti-52 kD, anti-Ro60, anti-p200 antibody levels than did the control cohort (CC); median anti-La antibody levels were not significantly different in the two cohorts.
Discussion
This study uncovered a significant prevalence of some antibody profiles in pregnant women affected with autoimmune CHB; more specifically, analysis of study data found that the women testing positive for anti-p200+anti-Ro52+anti-Ro60 antibodies were more susceptible to CHB than those with other antibody profiles. These results can probably be explained by the presence of anti-p200 antibodies whose frequency was significantly higher in the CHB+ than in the CHB− women. In accordance with the reports of other authors,6–9 anti-p200 antibodies were never found alone in our study population but were always detected in combination with other autoantibodies. The novelty of our investigation lies in the finding that when associated with anti-p200, anti-Ro52 and anti-Ro60 antibodies, antibodies can be considered predictive markers of autoimmune CHB. The results were obtained by prospectively following our patient's cohort, evaluating antibody profiles during ongoing pregnancies.
In accordance with other studies,6 8 10–13 ours demonstrated that anti-p200, anti-Ro52, anti-Ro60 antibody levels were significantly higher in the CHB+ with respect to the CHB− patients, but no differences were found between the two groups with regard to the anti-La levels. High titres of anti-p200, anti-Ro52 and anti-Ro60 antibodies can, therefore, be considered the antibody profile predicting risk for CHB, but given the current lack of reference standard sera, it is impossible at present to set an antibody cut-off level of the risk for CHB. The absence of correlation between anti-p200 maternal antibody levels and severity of CHB may be due to the use of treatment in different gestational age and in various types of CHB.
According to Brito-Zeròn et al17 report our study showed a statistically significant higher prevalence of asymptomatic women in the CHB+ with respect to the CHB− group. It will be interesting to see if this finding will be confirmed by larger studies. For the time being, the antibody profiles identified here could be taken into consideration by physicians monitoring their anti-Ro/La positive patients. The high incidence of CHB in our study population, which included only one patient having a previous CHB, could be considered a misleading finding. In fact, the expected incidence of CHB is certainly lower than 19.7%. The high CHB incidence found in our patients may have been due to the fact that the majority of the pregnant women with CHB (81.3%) were referred to our centre by different rheumatologic centres located in Italy. However, it is important to remember that the aim of our study was not to evaluate the epidemiology of CHB, but to investigate what antibody profile is associated to patients with CHB.
To conclude then, since anti-p200, anti-Ro52, anti-Ro60 antibodies, especially at high levels, seem to identify patients at increased risk of developing CHB, their detection in anti-Ro/La positive women could identify those at risk for having an infant with this rare, potentially dangerous disorder.
Due to the small number of examined patients, the present data on anti-p200 antibodies may be considered as preliminary results. Whether they will be confirmed by future studies on larger cohorts of anti-SSA/Ro positive patients, could be a useful tool for selecting women at high risk for developing fetal CHB. So these patients might be strictly monitored by fetal echocardiogram and, if necessary, treated early.
Footnotes
Contributors: MT and AR contributed to the conception and design of the article. All authors contributed to acquisition and/or analysis of the data and editing of manuscript before submission.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The institutional review board for the University-Hospital of Padua.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Buyon JP, Hiebert R, Copel J et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol 1998;31:1658–66. doi:10.1016/S0735-1097(98)00161-2 [DOI] [PubMed] [Google Scholar]
- 2.Ambrosi A, Sonesson SE, Wahren-Herlenius M. Molecular mechanisms of congenital heart block. Exp Cell Res 2014;325:2–9. doi:10.1016/j.yexcr.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 3.Salomonsson S, Sonesson SE, Ottosson L et al. Ro/SSA antibodies directly bind cardiomyocyties, disturb calcium homeostasis and mediate congenital heart block. J Exp Med 2005;201:11–17. doi:10.1084/jem.20041859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reed JH, Sim S, Wolin SL et al. Ro60 requires Y3 RNA for cell surface exposure and inflammation associated with cardiac manifestations of neonatal lupus. J Immunol 2013;191:110–16. doi:10.4049/jimmunol.1202849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salomonsson S, Doner T, Theander E et al. A serological marker for foetal risk of congenital heart block. Arthritis Rheum 2002;46:1233–41. doi:10.1002/art.10232 [DOI] [PubMed] [Google Scholar]
- 6.Strandberg L, Winqvist O, Sonesson SE et al. Antibodies to amino acid 200-232 (p200) of Ro52 as serological markers for the risk of developing congenital heart block. Clin Exp Immunol 2008;154:30–7. doi:10.1111/j.1365-2249.2008.03732.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clancy RM, Buyon JP, Ikeda K et al. Maternal antibody responses to the 52-kd SSA/Ro p200 peptide and the development of fetal conduction defects. Arthritis Rheum 2005;52:3079–86. doi:10.1002/art.21289 [DOI] [PubMed] [Google Scholar]
- 8.Reed JH, Clancy RM, Lee KH et al. Umbilical cord blood levels of maternal antibodies reactive with p200 and full-length Ro 52 in the assessment of risk for cardiac manifestations of neonatal lupus. Arthritis Care Res (Hoboken) 2012;64:1373–81. doi:10.1002/acr.21704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarsi M, Radice A, Pregnolato F et al. Anti-Ro/SSA-p200 antibodies in the prediction of congenital heart block. An Italian multicentre cross-sectional study on behalf of the ‘Forum Interdisciplinare per la Ricerca nelle Malattie Autoimmuni (FIRMA) Group’. Clin Exp Rheumatol 2014;32:848–54. [PubMed] [Google Scholar]
- 10.Dörner T, Chaoui R, Feist E et al. Significantly increased maternal and fetal IgG autoantibody levels to 52kD Ro(SS-A) and La(SS-B) in complete congenital heart block. J. Autoimmunity 1995;8:675–84. doi:10.1006/jaut.1995.0050 [DOI] [PubMed] [Google Scholar]
- 11.Tunks RD, Clowse ME, Miller SG et al. Maternal autoantibody levels in congenital heart block and potential prophylaxis with antiinflammatory agents. Am J Obstet Gynecol 2013;208:64.e1–7. doi:10.1016/j.ajog.2012.09.020 [DOI] [PubMed] [Google Scholar]
- 12.Jaeggi E, Laskin C, Hamilton R et al. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus. J Am Coll Cardiol 2010;55:2778–84. doi:10.1016/j.jacc.2010.02.042 [DOI] [PubMed] [Google Scholar]
- 13.Anami A, Fukushima K, Takasaki Y et al. The predictive value of anti-SSA antibodies titration in pregnant women with fetal congenital heart block. Mod Rheumatol 2013;23:653–8. doi:10.1007/s10165-012-0704-z [DOI] [PubMed] [Google Scholar]
- 14.Tonello M, Ruffatti A, Marson P et al. Plasma exchange effectively removes 52 and 60kd anti-Ro/SSA and anti-La/SSB antibodies in pregnant women with congenital heart block. Transfusion 2015;55:1782–6. doi:10.1111/trf.13046 [DOI] [PubMed] [Google Scholar]
- 15.Ruffatti A, Milanesi O. Chiandetti L et al. A combination therapy to treat second-degree anti Ro/La-related congenital heart block: a strategy to avoid stable third-degree heart block? Lupus 2012;21:666–71. doi:10.1177/0961203311430969 [DOI] [PubMed] [Google Scholar]
- 16.Ruffatti A, Marson P, Svaluto-Moreolo G et al. A combination therapy protocol of plasmapheresis, intravenous immunoglobulins and betametasone to treat anti-Ro/La-related congenital atrioventricular block. A case series and review of literature . Autoimmun Rev 2013;12:768–73. doi:10.1016/j.autrev.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 17.Brito-Zeròn P, Izmirly PM, Ramos-Casals M et al. The clinical spectrum of autoimmune congenital heart block. Nat Rev Rheumatol 2015;11:301–12. doi:10.1038/nrrheum.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]