Abstract
Ubiquitin-dependent proteolysis makes a major contribution to decreasing the levels of p27. Ubiquitin-dependent proteolysis of p27kip1 is growth and cell cycle regulated in two ways: first, skp2, a component of the E3-ubiquitin ligase, is growth regulated, and second, a kinase must phosphorylate the threonine-187 position on p27 so that it can be recognized by skp2. In vitro, p27 is phosphorylated by cyclin E- and cyclin A-associated cdk2 as well as by cyclin B1-cdk1. Having analyzed the effect of different cyclin-cyclin-dependent kinase complexes on ubiquitination of p27 in a reconstitution assay system, we now report a noncatalytic requirement for cyclin A-cdk2. Multiparameter flow cytometric analysis also indicates that p27 turnover correlates best with the onset of S phase, once the levels of cyclin A become nearly maximal. Finally, increasing the amount of both cyclin E-cdk2 and skp2 was less efficient at promoting p27 ubiquitination than was increasing the amount of cyclin A-cdk2 alone in extracts prepared from cultures of >93%-purified G1 cells. Together these lines of evidence suggest that cyclin A-cdk2 plays an ancillary noncatalytic role in the ubiquitination of p27 by the SCFskp2 complex.
The cdk2 inhibitor p27kip1 plays an important role in the cellular response to anti-mitogenic differentiation-inducing signals (6, 45). The abundance of p27 is regulated at the level of gene transcription (8, 20, 44), mRNA translation (1, 10, 22, 23), and proteolysis (2). Each level of regulation has cell cycle- and growth phase-dependent features. For example the rate of p27 synthesis increases in differentiated HL-60 promyelocytes (23), platelet-derived growth factor-starved BALB/c 3T3 fibroblasts (1), and lovastatin-treated MDA-468 breast epithelial cells (10, 22). In epithelial cells, rho-family GTPases affect the translation of p27 mRNA (42). Ubiquitin-dependent proteolytic regulation was first described in poor-prognosis colon tumors (30) and later in S- and G2-phase cells (23, 27).
Ubiquitin-dependent proteolysis is an essential feature of many cell cycle transitions (13). Ubiquitination involves a highly ordered series of enzymatic reactions (11). First, ubiquitin is activated by the formation of a carboxyl-adenylate intermediate and conjugated to the E1 enzyme through a thioester bond. Next it is trans-esterified to a ubiquitin-conjugating enzyme, E2. Although some E2s can directly conjugate ubiquitin onto substrates in vitro, most require another class of enzymes, the ubiquitin ligases or E3. One family of E3 enzymes, termed the SCF (3, 36), has emerged as an important component of the G1/S transition (17, 31, 41). The SCF is a multiprotein complex containing cullin, skp1, roc1, and F-box proteins. The structure of a heterotetrameric SCFskp2 E3 complex has been solved (47). In this structure cul1 serves as a rigid scaffold upon which the skp1-skp2 and roc1 subunits assemble. skp2 is an F-box protein that binds to the substrate. It is recruited to cul1 by interaction with skp1. roc1 interacts with cul1 and enhances ubiquitination, likely by facilitating the interaction of the SCFskp2 complex with an E2 enzyme (29). roc1 and skp2 are separated by approximately 100 Å in this structure, and mutations eliminating the rigidity of cul1 eliminate the activity of this complex (47).
Two groups independently reconstituted the ubiquitination of p27 using recombinant proteins (9, 38). Genetic evidence overwhelmingly supports a role for skp2 and cks1 in the E3 enzyme (26, 38). The E3 enzyme consists of skp2, skp1, cul1, and roc1, and when supplemented with cks1, cyclin A-cdk2, E1, cdc34, and ubiquitin it can ubiquitinate p27 (47). cks1 enhances the interaction of T187-phosphorylated p27 with skp2 (9, 24, 35).
Phosphorylation of p27 facilitates its interaction with SCFskp2. Cyclin A-cdk2, cyclin B1-cdk1, and cyclin E-cdk2 phosphorylate the threonine-187 position on p27. All three kinases support ubiquitination of p27 in vitro, but they are not considered part of the E3 complex. Nevertheless, two of the E3 proteins, skp2 and skp1, were cloned as cyclin A-cdk2-interacting proteins (46); cul1 coprecipitates with cyclin A-cdk2 (21); and, when normalized for substrate phosphorylation, in crude cell extracts, ubiquitination reaction mixtures containing cyclin A-cdk2 yield more product than those containing either cyclin E-cdk2 or cyclin B1-cdk1 (25).
Here we report a noncatalytic requirement for cyclin A-cdk2, not shared by cyclin E-cdk2, in an in vitro-reconstituted SCFskp2-dependent p27 ubiquitination system. A role for cyclin A-cdk2 specifically in p27 turnover is further suggested by the timing of cyclin A, cyclin E, and p27 accumulation in single cells and the finding that added cyclin A-cdk2 is better at inducing p27 turnover in G1 extracts than are added cyclin E-cdk2 and skp2/skp1. Together these lines of evidence are consistent with the hypothesis that cyclin A-cdk2 plays an ancillary noncatalytic role in the ubiquitination of p27 by the SCFskp2 complex.
MATERIALS AND METHODS
Cell culture, cell extracts, and flow cytometric analysis.
HeLa S3 cells were maintained at a density of 2 × 105 and 5 × 105 cells/ml in a minimal essential medium spinner supplemented with 10% enriched calf serum. HL-60 and TK6 cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, and antibiotics at densities between 2 × 105 and 5 × 105 cells/ml. Elutriation was performed as described previously (16). Cell extracts were prepared as described previously (4, 27).
For flow cytometric analysis, cells were washed in Hanks' balanced salt solution and fixed in 70% ice-cold ethanol. After fixation, cells were rehydrated in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and aliquots containing 0.5 × 106 to 1 × 106 cells were resuspended in 100 μl of PBS-BSA. The abundance of cyclin E and cyclin A or cyclin A and p27 was determined after immunofluorescence staining. We identified cells in G1 and G2 phase by DNA content and divided the intervening region into 10 to 12 equal increments to allow the calculation of mean protein-associated fluorescence and mean DNA content. The exact same analysis was performed on the appropriate aliquot stained with nonspecific immunoglobulin. Protein fluorescence was determined by subtracting the mean from the nonspecific samples. Cells were stained with mouse anti-human monoclonal antibodies to cyclin E (1:100; clone HE67), cyclin A (1:50; clone BF683), and p27 (1:50; clone 57) or a nonspecific immunoglobulin (DAKO) overnight. Samples were washed in PBS-BSA and stained with a fluorescein isothiocyanate-labeled goat anti-mouse secondary antibody (1:30; DAKO) for 30 min in the dark. Cells were washed and resuspended in PBS containing 10 μg of propidium iodide/ml and 100 μg of RNase A/ml and stained for 30 min in the dark.
Recombinant proteins.
skp2/skp1 and cul1/roc1 were purified from infected Sf9 cells as described previously (5).
Cyclin A mutants were constructed with the Gene Editor in vitro site-directed mutagenesis system (Promega). The HPM cyclin A mutant was provided by Brenda Shulman. Cyclin A mutants were purified from infected Sf9 cells in parallel with wild-type cyclin A. Cyclins and cyclin-dependent kinases (cdk's) were expressed individually and then combined for final purification of the complex as previously described for cyclin B and cdk1 (7) or cyclin A and cdk2 (R. Fisher, unpublished data) by affinity chromatography followed by ATP affinity chromatography. Purification was assessed by Coomassie blue staining, with results indicating about 90% pure cyclin-cdk. Cak was generously provided by Robert Fisher (Memorial Sloan-Kettering Cancer Center [MSKCC]). Recombinant cks1 was prepared as a glutathione S-transferase (GST) fusion protein and purified on glutathione agarose supports, and cks1 was released by cleavage with thrombin as described previously (47).
Bacterially produced purified GST-skp2/skp1 and Δskp2/skp1 were provided by Nikola Pavletich (MSKCC). E1 and cdc34 were purchased (Affiniti).
p27, p27T187A, and p27ck(−) substrates were produced by in vitro translation in rabbit reticulocyte lysates as previously described (27). These lysates contain endogenous cul1, roc1, skp1, p27, cdk2, and cdc2 but no detectable cyclin A, cyclin B1, cyclin E, or skp2 (data not shown).
In vitro degradation assay.
The in vitro degradation assay was done exactly as described previously (27). Unless specified, 150 μg of extract was supplemented with ATP and ATP regeneration system (1 mM ATP, 25 mM phosphocreatine, 10 μg of creatine phosphokinase/ml), 1.2 μl of rabbit reticulocyte lysate, and 0.1 μl of 35S-p27 (equivalent to 0.3 fmol). The reaction mixture was incubated at 30°C for 2.5 h. In some reactions, aliquots were removed at 30-min intervals. Sodium dodecyl sulfate (SDS) sample buffer was added to stop the reaction, and products were resolved by SDS-polyacrylamide gel electrophoresis (PAGE). Gels were fixed in 10% acetic acid and 10% methanol for 30 min and then immersed in 1 M salicylic acid for 30 min, dried, and exposed to film. To monitor endogenous p27 or skp2 degradation, the same reaction was performed as described above except that the rabbit reticulocyte lysate was unprogrammed and one-fourth of the reaction mixture was resolved by SDS-PAGE.
Ubiquitination assay.
p27 substrate was first incubated at 30°C for 30 min with cyclin-cdk complexes in 40 mM Tris-HCl (pH 7.6)-1 mM ATP-10 mM MgCl2-1 mM dithiothreitol-1 μM okadaic acid. Phosphorylated p27 was added to the ubiquitination reaction mixture, which contains 10 mM creatine phosphate, 0.1 mg of creatine kinase/ml, 1 μM ubiquitin aldehyde, 1 mg of methylated ubiquitin/ml, 0.2 μg of E1, 2 μg of E2, 1 μg of His-cul1/roc1, 0.6 μg of His-skp1/skp2, and 0.05 μg of cks1. All substrates and E3 components were supplied in the linear range when 50 ng of cyclin A-cdk2 was used as the kinase activity. Following incubation at 30°C for 90 min, the samples were resolved and subjected to autoradiography for analysis.
For the experiments with addition of the noncatalytic cdk subunit (cdk2D145N), staurosporine was added to 5 μM and roscovitine was added to 0.4 mM. Substrates were phosphorylated with either 50 ng of cyclin A-cdk2 or 200 ng of cyclin B1-cdk1. Fifty nanograms of cyclin-cdk2D145N complex was added.
We added 0.6 μg of bacterially produced skp2/skp1 or the N-terminal truncation mutant when measuring the ability to bind to cyclin A and promote ubiquitination.
Western blotting and kinase assays.
Immunoblotting was performed as described previously (37). Antibodies were used at the following concentrations: 1:1,000 for skp2 (Santa Cruz N-19), cyclin A (Santa Cruz H432), cyclin E (Santa Cruz HE12), cyclin B1 (Santa Cruz GNS1), p27 (Santa Cruz C19), cul1 (Zymed), roc1 (Neomarkers), skp2 (Santa Cruz H435), skp1 (Santa Cruz H-163), cdk1 (Santa Cruz C19), cdk2 (Santa Cruz M2), and p27-phospho-T187 (Zymed); 1:200 for cks1 (Santa Cruz FL79); and 1:2,500 for cdc34 (Signal Transduction). Twenty-five micrograms of protein extract was resolved to detect p27, cyclin A, skp2, skp1, cul1, or cdc34. Eighty micrograms of protein extract was resolved to detect roc1, cks1, or cyclin E.
The immunoprecipitation kinase assay was done as described previously (27) with the following antibodies: cyclin A (Santa Cruz H432), cyclin E antiserum (15), cdk2 (Santa Cruz 163), skp2 (Santa Cruz H435), and RαM (Zymed). Immunodepletion was carried out with hemagglutinin antibody (Santa Cruz Y11) and cdk2 antibody (Santa Cruz M2).
In vitro binding.
Full-length skp2 protein or a 100-amino-acid N-terminal truncation was fused to GST and copurified with skp1 on glutathione agarose. These proteins were subsequently released by proteolytic cleavage and incubated in rabbit reticulocyte lysates programmed with cyclin A mRNA. Immunoprecipitation was carried out with antibodies raised against the full-length skp2 protein, and cyclin A was detected by autoradiography.
RESULTS
SCFskp2 ubiquitination activity is increased by cyclin A-cdk2.
We were interested in determining whether the cdk's that can phosphorylate threonine-187 on p27 were equivalent in their abilities to induce SCFskp2-dependent p27 ubiquitination. To accomplish this, we measured p27 ubiquitination activity in the reconstituted SCFskp2-dependent assay developed by Pagano and his colleagues (9). With the use of cyclin A-cdk2 to phosphorylate p27, the assay was shown to be linear with respect to each SCF component and the addition of no single component (cks1, skp2/skp1, or cul1/roc1) increased ubiquitination. In vitro-translated p27 was used as the substrate because bacterially produced p27T187A was ubiquitinated under these conditions, consistent with the possibility of an alternative degradation pathway superimposed on the cdk2-dependent, T187-dependent pathway (19). The substrate was not limiting.
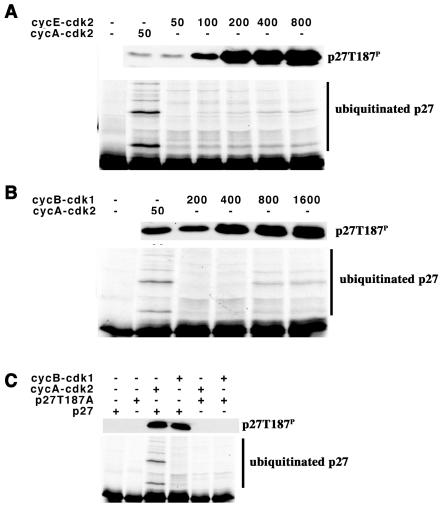
Cyclin A-cdk2, cyclin E-cdk2, or cyclin B1-cdk1 can all phosphorylate T187 (Fig. 1). In order to compare the abilities of these cyclin-cdk complexes to support ubiquitination, we first needed to establish the amount of each complex that would phosphorylate T187 to an equivalent level, in the milieu of the ubiquitination reaction. Cyclin E-cdk2 (Fig. 1A) or cyclin B1-cdk1 (Fig. 1B) was titrated into the reaction mixture, and 90 min later, at the end of the reaction, the samples were split, the extent of T187 phosphorylation was determined by immunoblotting, and p27 ubiquitination was determined by autoradiography. Under these conditions the majority of the product migrated in two discrete bands with a smaller amount of higher-molecular-weight species being detected.
FIG. 1.
Cyclin-cdk dependence for p27 ubiquitination. (A) Comparison of cyclin A-cdk2 with cyclin E-cdk2. The amount of added cyclin-cdk complex (nanograms) is indicated above the respective lanes. Phosphorylation of T187 was determined by immunoblotting, and ubiquitination was determined by autoradiography. Ubiquitinated products are indicated to the right of the autoradiograms. (B) Comparison of cyclin A-cdk2 with cyclin B1-cdk1. This is essentially identical to that described for panel A, except that cyclin B1-cdk1 was used instead of cyclin E-cdk2. (C) Ubiquitination depends on T187. The reactions are essentially as described for panel A. The cyclin-cdk complex used and the in vitro-translated substrates are indicated above their respective lanes.
In a comparison of the amount of phosphorylated p27 with ubiquitination of p27, it was clear that cyclin A-cdk2 was better at promoting ubiquitination than was either cyclin E-cdk2 or cyclin B1-cdk1. Increasing the amount of cyclin E-cdk2 eightfold (Fig. 1A) or cyclin B1-cdk1 16-fold (Fig. 1B) increased both phosphorylation of T187 and ubiquitination. Ubiquitination was blocked by mutation of T187 to alanine (T187A) (Fig. 1C). We interpreted this to suggest that cyclin A-cdk2 might have an additional function in p27 ubiquitination, not shared with cyclin E-cdk2 or cyclin B1-cdk1.
Cyclin A-cdk2 enhances ubiquitination activity of SCFskp2 in a noncatalytic manner.
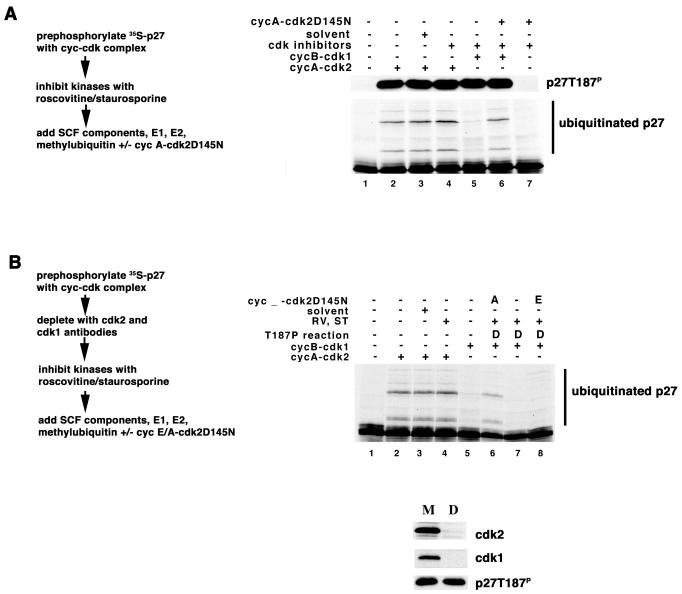
Cyclin E-cdk2, cyclin A-cdk2, and cyclin B1-cdk1 share the ability to phosphorylate threonine-187 on p27. We wanted to determine if the enhanced ability of cyclin A-cdk2 to promote ubiquitination was associated with a novel noncatalytic function of the cyclin A-cdk2 complex. We phosphorylated the substrate by first incubating it with either cyclin A-cdk2 or cyclin B1-cdk1 and then inactivated the kinase by adding staurosporine and roscovitine. The prephosphorylated substrate was subsequently added directly to the remaining components of the ubiquitination reaction mixture. Ninety minutes later, at the end of the reaction, samples were split, the extent of phosphorylation was compared by immunoblotting, and ubiquitination was determined by autoradiography. Phosphorylation of the substrate was equivalent in all reactions (Fig. 2A). Staurosporine and roscovitine do not interfere with the ubiquitination reaction carried out in this manner (Fig. 2A, compare lanes 2 to 4). Reactions in which p27 was prephosphorylated by cyclin A-cdk2 were more capable of ubiquitinating p27 than were those in which cyclin B1-cdk1 was used (Fig. 2A, compare lanes 4 and 5).
FIG. 2.
Cyclin A-cdk2 facilitates ubiquitination of p27 in a noncatalytic manner. (A) Cyclin A-cdk2D145N supports ubiquitination of cyclin B1-cdk1-phosphorylated p27. Ubiquitination reaction mixtures were assembled in a stepwise manner indicated to the left of the autoradiogram. The reaction components are indicated above their respective lanes. Phosphorylation of threonine-187 and ubiquitination of in vitro-translated p27 are presented essentially as described in the legend to Fig. 1. (B) Cyclin A-cdk2D145N is better than cyclin E-cdk2D145N at supporting ubiquitination of cyclin B1-cdk1-phosphorylated p27. The upper panel is essentially as described above. The use of a mock-depleted (M) or cdk2/cdk1-depleted (D) extract is indicated above the respective lanes. Numbers under each lane are for reference in the text. Immunodepletion is shown in the lower panel. Antibodies indicated to the right of each panel were used to judge the efficiency of the depletion reaction.
We then asked whether a catalytically inactive mutant of the cyclin A-cdk2 kinase, cdk2D145N, would support the ubiquitination of substrate prephosphorylated by cyclin B1-cdk1. This was carried out alongside the experiment above, with the only difference being that the substrate was added directly to the remaining components of the ubiquitination reaction that also contained cyclin A-cdk2D145N. Alone cyclin A-cdk2D145N did not phosphorylate p27 and did not promote ubiquitination of p27 (Fig. 2A, lane 7); however, it was able to promote ubiquitination of the substrate prephosphorylated by cyclin B1-cdk1 (Fig. 2A, lane 6).
To rule out the possibility of cdk subunit exchange during the incubation, we next asked if cyclin A-cdk2D145N would promote ubiquitination in reaction mixtures depleted of endogenous cdk2 and cdk1. To accomplish this, first substrate was phosphorylated by cyclin B1-cdk1 and then immunoadsorption was used to deplete cdk2 and cdk1. We depleted approximately 95% of the endogenous cdk2 and cdk1, and the amount of phosphorylated p27 remaining was equivalent in the mock-depleted and cdk2/cdk1-depleted samples (Fig. 2B, lower panel). Staurosporine and roscovitine were added to inhibit any residual kinase activity that might be present. The prephosphorylated substrate depleted of cdk2 and cdk1 was subsequently added to the remaining components of the ubiquitination reaction mixture with and without added cyclin A-cdk2D145N (Fig. 2B, lanes 6 and 7, respectively). Ubiquitination was enhanced in reaction mixtures that contained cyclin A-cdk2D145N. We simultaneously measured the ability of cyclin E-cdk2D145N to support ubiquitination in this assay. Ubiquitination was not enhanced by including cyclin E-cdk2D145N (Fig. 2B, lane 8), suggesting a cyclin-specific noncatalytic role for cyclin A-cdk2 in p27 ubiquitination.
cdk2 and/or skp2/skp1 binding is required for the ability of cyclin A to promote p27 ubiquitination.
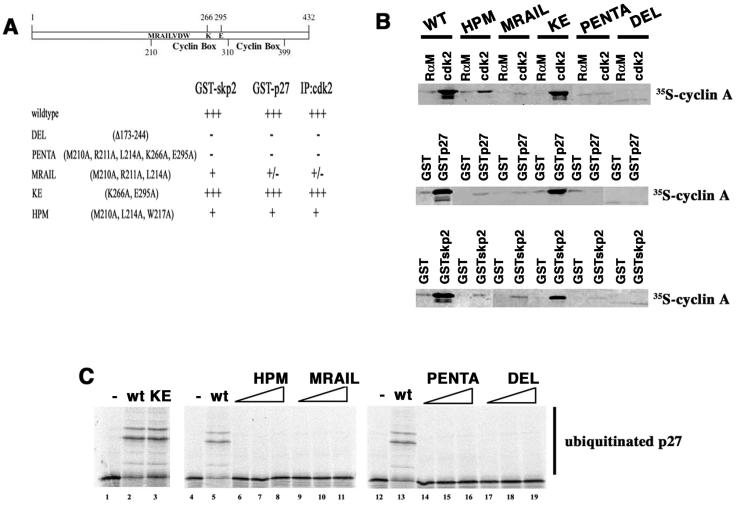
We next wanted to identify whether the ability of cyclin A to bind to cdk2 was required for this noncatalytic function in p27 ubiquitination. Because rabbit reticulocyte lysate contains a number of cdk-like proteins that bind cyclin A but do not phosphorylate histone H1 (unpublished data), we generated a set of five cyclin A mutants expected to alter cdk binding based on prior mutagenesis and structural studies by Harlow and colleagues (18, 34), Hunt and colleagues (14), and Pavletich and colleagues (12, 32). We made a deletion mutant that removes sequences in the first cyclin box (DEL), two mutants that target residues in and around the MRAIL alpha-helix (HPM [34] and MRAIL), and another mutant altering two residues predicted to be important for cyclin-cdk interaction (KE) which lie outside the MRAIL domain. Finally, to make a mutation more subtle than the deletion but expected to eliminate all cdk2 binding, we generated PENTA, a 5-amino-acid substitution mutant that combines both the MRAIL and KE mutations. The abilities of these mutants to bind to cdk2, skp2, and p27 are summarized in Fig. 3A.
FIG. 3.
Cyclin A mutants. (A) Schematic and summary of mutations used and their interactions with skp2, p27, and cdk2. Cyclin A is represented by the open bar with the cyclin box shown spanning amino acids 210 to 310. A second, more degenerate cyclin-box-like motif from 310 to 399 is also indicated. Amino acid sequences pertinent to the mutations are shown inside the box and numbered for orientation. The abilities of the mutants to bind p27, cdk2, and skp2/skp1 are indicated. The nomenclature of the mutants follows standard conventions, and names are indicated to the left. (B) Interaction of the cyclin A mutants with cdk2, skp2/skp1, and p27. Mutants are indicated above each pair of lanes. Precipitated in vitro-translated cyclin A was measured by autoradiography. In the top panel immunoprecipitations were carried out with either rabbit anti-mouse antibody (RαM) or antibodies to cdk2 (cdk2). For the middle and bottom panels GST, GST-skp2/skp1, or GST-p27 was used as the affinity reagent and collected on glutathione agarose. These data are representative of at least three different experiments for each condition. (C) Ubiquitination reactions. Reactions are presented essentially as described in the legend to Fig. 2. Inclusion of the wild-type or mutant versions of cyclin A is indicated above the respective lanes. Cyclin A was titrated as follows: 50 ng (lanes 2, 3, 5, 6, 9, 13, 14, and 17), 200 ng (lanes 7, 10, 15, and 18), or 400 ng (lanes 8, 11, 16, and 19). Ubiquitinated products are indicated to the left of the autoradiograms. wt, wild type.
To determine if these mutations affected cdk2 binding, p27 binding or skp2 binding mutants were expressed from in vitro transcription-translation vectors in rabbit reticulocyte lysates and 35S-labeled proteins were detected either in cdk2 immunoprecipitates or by glutathione-agarose pull-down assays with GST-skp2/skp1 or GST-p27 as bait (Fig. 3B). cdk2 antibodies coprecipitated both wild-type cyclin A and the KE mutant but not the deletion mutant or the PENTA mutant under these conditions (Fig. 3B, top panel). Weaker association was detected with the HPM mutant, and still-weaker association was detected with the MRAIL mutant. Both wild-type cyclin A and the KE mutant were able to bind to p27 and skp2/skp1 (Fig. 3B, middle and bottom panels, respectively), but the DEL mutant and the PENTA mutant were not. The HPM mutant and MRAIL mutant were weakly associated.
We then asked if these mutants would support ubiquitination in the reconstituted system. The mutants and the wild-type protein were expressed from baculoviral vectors in insect cells, and the proteins were purified by nickel-affinity chromatography. Substrates were phosphorylated with cyclin B1-cdk1, and staurosporine and roscovitine were added to inhibit residual kinase activity. Prephosphorylated substrate was subsequently added directly to the remaining components of the ubiquitination reaction mixture, this time in the presence of either cyclin A or one of the mutants (Fig. 3C). Under these conditions, cyclin A can associate with endogenous cdk2 in the rabbit reticulocyte lysate (28). p27 was ubiquitinated in reactions in which cyclin A or the KE mutant was included (Fig. 3C, lanes 2 and 3). Reactions in which the other mutants were added were not capable of ubiquitinating p27 (Fig. 3C, lanes 6 to 19). We interpreted this to suggest that cdk2 binding and the association of cyclin A-cdk2 with skp2/skp1 and/or p27 were important for the function of cyclin A in the ubiquitination reaction.
Mutants of skp2 and p27 that do not bind cyclin A-cdk2 do not promote p27 ubiquitination.
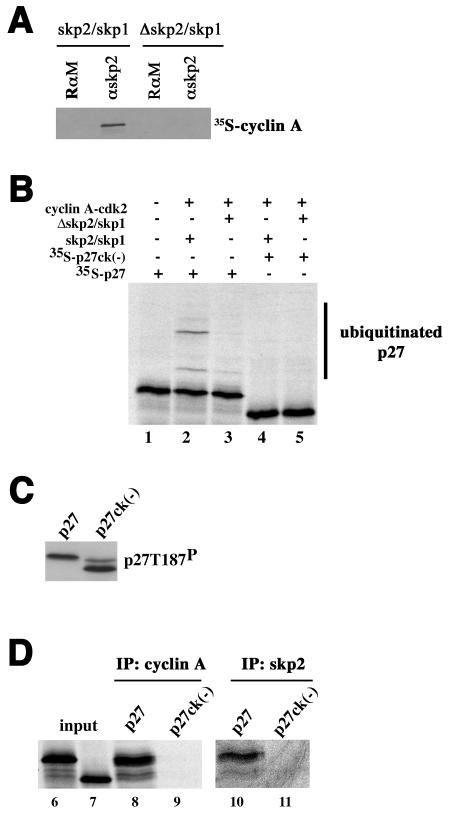
Since in using cyclin A mutants that disrupt cdk2 binding we were unable to differentiate between a role in skp2/skp1 binding and that in p27 binding, we asked whether mutations in skp2 or p27 that diminish its association with cyclin A would be informative. To ask if association of skp2 with cyclin A-cdk2 was important, we compared the abilities of full-length skp2 and a 100-amino-acid N-terminal deletion of skp2 to support ubiquitination. These proteins were kindly provided by Nikola Pavletich as part of a GST-skp2/skp1 complex purified from bacteria. The N-terminal truncation mutant was used in crystallizing the skp2/skp1 complex (33) and the complete SCFskp2 complex (47). The ability of bacterially produced full-length skp2 protein to support p27 ubiquitination was also demonstrated (47). After proteolytic removal of the GST tag, we incubated the proteins with in vitro-translated [35S]methionine-labeled cyclin A and subsequently immunoprecipitated skp2 and looked for cyclin A in the immunoprecipitate. Deleting the N-terminal 100 amino acids of skp2 reduces its ability to bind to cyclin A-cdk2 (Fig. 4A). We were thus able to ask if removing the cyclin A-cdk2 interaction domain from skp2 affected p27 ubiquitination activity by replacing the complex produced in insect cells with the bacterially produced GST-skp2/skp1 complexes in the in vitro reconstitution assay. Deletion of the 100 amino acids at the N terminus of skp2 reduced ubiquitination activity (Fig. 4A, lanes 2 and 3), suggesting that its ability to interact with cyclin A-cdk2 may facilitate p27 ubiquitination.
FIG. 4.
The association of p27 with cyclin A-cdk2 promotes its interaction with skp2. (A) The N terminus of skp2 is required for efficient cyclin A-cdk2 interaction. Following incubation of either full-length or amino-terminally deleted skp2/skp1 complexes with in vitro-translated cyclin A, skp2 was immunoprecipitated and the amount of associated cyclin A was determined by autoradiography. The antibodies are indicated above each lane (RαM [rabbit anti-mouse antibody] and αskp2). (B) Ubiquitination. This is essentially as described in the legend to Fig. 2, except that 0.6 μg of skp2/skp1 or a mutant lacking the amino-terminal 100 acids (Δskp2/Δskp1) was added with either metabolically labeled p27 or the ck(−) mutant as indicated above each lane. (C) Phosphorylation. Reaction mixtures for reactions carried out in lanes 2 and 4 of panel B were divided in half, and the extent of T187 phosphorylation was determined by immunoblotting with p27 phosphospecific T187 antibody. Substrates are indicated above the respective lanes. (D) The binding of p27 to cyclin A-cdk2 and skp2 is compromised by the ck(−) mutation. Three ubiquitination reaction mixtures containing either p27 or p27ck(−) as a substrate (indicated above each lane) were pooled, stopped by the addition of 0.5% NP-40-radioimmunoprecipitation assay buffer, divided, and immunoprecipitated with either cyclin A- or skp2-specific antibodies, as indicated above each pair of lanes. The reactions are numbered below each lane. Input represents 10% of the amount of material in the reaction.
In the same experiments, we also asked whether a p27 mutant that does not bind to cyclin A-cdk2 would be ubiquitinated. p27ck(−) contains four mutations (R30A, L32A, F62A, and F64A) preventing it from inhibiting cyclin-cdk complexes, as described previously (43). When immunoblotting in vitro translation reaction mixtures containing p27ck(−) mRNA with T187-phosphospecific antibodies, we detected two bands (Fig. 4C). A slower band that comigrates with in vitro-translated p27 reflects the presence of endogenous p27 in rabbit reticulocyte lysate, and the faster-migrating band is the in vitro-translated product. Why this mutation alters p27 mobility is not clear, but it could reflect changes in the structure of the protein. In vitro-translated p27 and p27ck(−) proteins were equivalently phosphorylated (Fig. 4C). p27ck(−) was not ubiquitinated (Fig. 4B, compare lanes 2 and 4).
To ask if the ck(−) mutation affected the association of p27 with skp2 in the context of a ubiquitination reaction, we also looked at whether p27ck(−)was able to interact with full-length skp2/skp1. In the milieu of a ubiquitination reaction, p27 was coprecipitated with both cyclin A and skp2 antibodies (Fig. 4D, lanes 8 and 10). In contrast, p27ck(−) was not coprecipitated with either cyclin A or skp2 antibodies (Fig. 4D, lanes 9 and 11). The skp2 antibody precipitates less of the input p27 than the cyclin A antibody does, but this may be due to the relative affinities of the antibodies used. Together, we interpreted these data to suggest that cyclin A-cdk2 makes contacts with skp2/skp1 and with p27, both of which may contribute to its ability to promote p27 ubiquitination.
p27 turnover correlates with the accumulation of cyclin A.
The data above suggest a noncatalytic role for cyclin A-cdk2 in SCFskp2-dependent p27 ubiquitination. However, other groups have presented biochemical data suggesting that any cyclin-cdk is sufficient for p27 ubiquitination. Because each SCF component is dispersed among a number of different complexes, we cannot ever know the absolute amount of SCFskp2 available for p27 ubiquitination in a cell. Thus, we recognized that the noncatalytic contribution of cyclin A-cdk2 defined here might be a biochemical peculiarity of our using a limiting amount of SCFskp2 in the reconstitution assay. To begin to address this, we felt that it was appropriate to ask if there was a correlation between the expression of cyclin A and p27 turnover in unmanipulated cycling cells, not those synchronized by drugs or serum starvation.
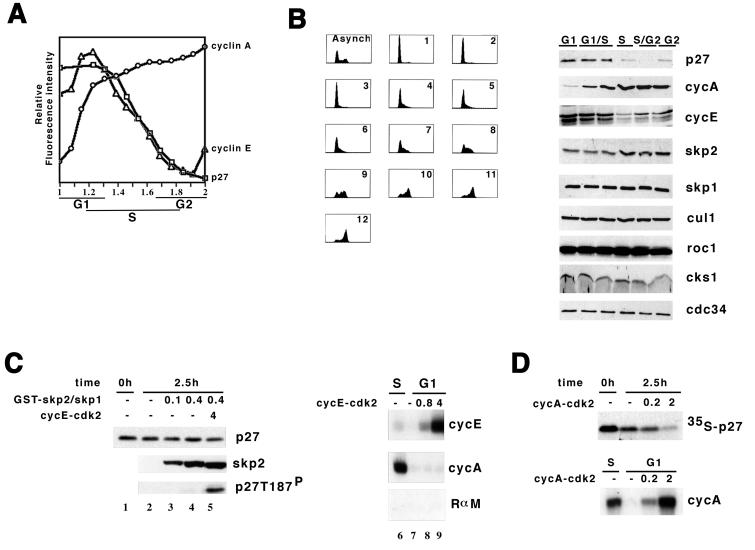
In order to determine the timing of cyclin expression with p27 turnover during the unperturbed mitotic cell cycle, we measured the accumulation of cyclin E, cyclin A, and p27 in HL-60 cells by a multiparametric flow cytometric approach. This technique allows us to examine the accumulation of multiple proteins in individual cells, as opposed to immunoblotting, which determines the accumulation of proteins in a large, diverse cell population. Furthermore, the flow cytometric approach allows us to correlate this value with respect to the cell's position in the cycle by measuring DNA content. Multiple analyses can be carried out in the same cell with different fluorescent markers. Using fluorescence-specific antibody probes and propidium iodide staining of the DNA, we could superimpose analysis of cyclin A/p27/DNA with cyclin E/DNA and with individual cyclin A/DNA and p27/DNA staining conditions. Both cyclin E and p27 levels decreased once cyclin A accumulated to levels seen in late-G1/early-S-phase cells (Fig. 5A). This is consistent with the observations of a number of groups that p27 turnover increases as cells enter S phase or decreases as cells become quiescent.
FIG. 5.
Accumulation of cyclin A correlates with p27 ubiquitination activity and p27 turnover in cells and extracts. (A) Abundance of cyclin E, cyclin A, and p27 in individual cells. Relative staining intensity (on the ordinate) is plotted against DNA content (abscissa) determined by propidium iodide staining (cyclin A, ○; cyclin E, ▵; p27, □). Individual cyclin or p27 staining was carried out at least three times, and a combined triple analysis for cyclin A/p27/DNA was carried out once. The figure is a composite of these analyses. (B) Cell cycle-associated changes in the abundance of proteins involved in ubiquitination of p27. Asynchronously growing HeLa cells were fractionated into cell cycle phase-enriched populations by centrifugal elutriation. In the left panel, DNA content was measured in each of the 12 fractions by propidium iodide staining. Fractions were pooled into representative G1 (pool fractions 1 to 3), G1/S (two pools, fractions 4 to 5 and 6 to 7), S (pool fractions 8 to 9), S/G2 (pool fractions 10 to 11), and G2/M (fraction 12) populations. In the right panel, proteins were extracted from the pooled populations, resolved on SDS-polyacrylamide gels, and blotted to membranes and these were probed with an antibody specific to the protein indicated to the right. Above each lane is indicated the representative cell cycle phase. (C) Addition of skp2/skp1 with and without cyclin E-cdk2 failed to induce p27 turnover in G1 extract. Reaction mixtures were assembled with GST-skp2/skp1 (micrograms) and cyclin E-cdk2 complex (pmol) as indicted above their respective lanes. Following incubation, the amounts of p27, skp2, and T187-phosphorylated p27 were determined by immunoblotting with antibodies indicated to the right of the respective panels. In the right panel, the amount of recombinant cyclin E-cdk2 (picomoles) added to the extracts is indicated above the respective lanes, and the activity of the cyclin E-associated or cyclin A-associated histone H1 kinase after incubation was measured by immunoprecipitation with antibodies indicated to the right of each autoradiogram (RαM, rabbit anti-mouse antibody). The numbers below each lane are for reference in the text. (D) Cyclin A-cdk2 promotes p27 turnover in G1 extract. This is arranged similarly to panel C.
Another way to compare the p27 ubiquitination activity and cyclin expression was to measure the amount of each protein following centrifugal elutriation of either asynchronously growing HeLa or TK6 cells into phase-enriched populations (Fig. 5B). As in the flow cytometric assay, cyclin E was highest in the G1 fractions and decreased as cells progressed in the cell cycle. Reduction of p27 correlated well with an increase to near-maximal levels of cyclin A. skp2 was only modestly regulated, being lowest but detectable in G1 cells and increasing as cells progressed through the cell cycle. The amounts of skp1, cul1, roc1, cks1, and cdc34 were constant during the cell cycle. Thus, we concluded that cell cycle phase-specific reduction in p27 was best correlated with early S phase, once cyclin A accumulation had begun to plateau.
Elutriation of cells also allowed us to generate G1 extracts that had only a minimal amount of cyclin A contamination, unlike those that we had prepared from cells treated with nocodazole and released from the drug (27). We subsequently prepared extracts from elutriated G1 cells and measured p27 turnover as we previously described (27). p27 was stable in extracts prepared from elutriated G1 cells (half-life [t1/2] of >4 h) and unstable in extract prepared from elutriated S-phase cells (t1/2 = 1 h). p27 turnover was blocked by two cdk inhibitors (staurosporine and roscovitine), K48R mutant ubiquitin, or two proteasome inhibitors (MG132 and LLnL), indicating that elutriation did not affect the cdk2-dependent, ubiquitin-dependent, and proteasome-dependent turnover process (data not shown).
It was reported that p27 turnover occurs in quiescent cells infected with vectors expressing skp2, even in the presence of mimosine, which prevents S-phase entry (39). We then asked if increasing the amount of skp2 would be sufficient to induce turnover in G1 extracts. In order to determine if skp2 alone would be sufficient to induce turnover in the extracts prepared from elutriated G1 cells, we measured turnover of p27 by either immunoblotting the endogenous protein (Fig. 5C, lanes 1 to 5) or monitoring metabolically labeled in vitro-translated protein (data not shown). Addition of recombinant GST-skp2/skp1, purified from bacteria, was unable to stimulate p27 turnover; however, phosphorylated p27 did not accumulate under these conditions (Fig. 5C, lanes 3 and 4).
We asked if adding skp2 and increasing the amount of cdk activity would be sufficient. To correct for extract-dependent changes in activity, the activity of the supplemental kinase was measured following incubation in the extracts. Adding 4 pmol of cyclin E-cdk2 with recombinant GST-skp2/skp1 was able to induce threonine-187 phosphorylation, but p27 turnover was modest (Fig. 5C, lane 5). Two picomoles of recombinant cyclin E-cdk2 has an amount of activity equivalent to the amount of cyclin A-associated kinase activity detected in an S-phase extract (Fig. 5C, lanes 6 to 9). Increasing cyclin E-cdk2 to 20 pmol induced p27 turnover, albeit the rate (t1/2 = 2 h) was still lower than that observed in S-phase extract (t1/2 = 1 h). This is consistent with previous reports that cyclin E-cdk2 activity induces p27 turnover in extracts or with reconstituted proteins; however, the amount of cyclin E activity required was much greater than that normally reached inside a G1 cell.
Finally, we wanted to determine if cyclin A was sufficient to trigger p27 turnover in G1 extracts. Adding 2 pmol of cyclin A-cdk2 to the G1 extract induced p27 turnover (Fig. 5D) at rates comparable to that observed in S-phase extract (t1/2 = 1 h). In this experiment we monitored the turnover of in vitro-translated substrate. Two picomoles of cyclin A-cdk2 produces approximately twice as much cyclin A-associated kinase activity in an S-phase extract (Fig. 5D). p27 turnover was prevented by addition of K48R-ubiquitin or the proteasome inhibitor MG132 or by mutating T187 to alanine (data not shown). This indicated that p27 turnover was dependent on cdk2, T187, ubiquitin, and proteasome and was induced by the addition of cyclin A-cdk2.
DISCUSSION
A completely reconstituted system has been established for the study of p27 ubiquitination (9, 38). A key component in this system is the SCFskp2 E3-ubiquitin ligase consisting of cul1, roc1, skp2, skp1, and cks1. Each of the core proteins in the E3 is necessary for ubiquitination of p27, and the role of each component in this complex is well defined. roc1 interacts with cul1 and enhances ubiquitination of p27, likely by facilitating the interaction of the SCFskp2 complex with the E2 enzyme (29). skp2 provides the interface for p27 binding, and cks1 association enhances this interaction (5, 24, 35, 40). skp1 connects skp2 to cul1. The skp2/skp1 heterodimer and the SCFskp2 heterotetramer have been crystallized (33, 47). skp2 is at a distance along the cul1 backbone from roc1. Rigidity of the cul1 backbone is important for the function of the SCFskp2 complex (47), suggesting that it might either orient the substrate and the E2 enzyme or provide a binding interface for other proteins. However, the E3 might be decorated by additional proteins which either modulate its activity or specify its substrates.
skp2 and skp1 were cloned as cyclin A-cdk2-interacting proteins (46), and cul1 coprecipitates with cyclin A-cdk2 (21). Pagano and his colleagues showed that, when normalized for substrate phosphorylation, cyclin A-cdk2 presents p27 to the ubiquitination apparatus better than either cyclin E-cdk2 or cyclin B1-cdk1 does in crude cell extracts (25). Additionally, other experiments anecdotally suggest that cyclin A-cdk2 may be involved in p27 ubiquitination. For example, when expressing skp2 in quiescent fibroblasts there is increased p27 turnover; however, cyclin A accumulates in these cells as well (39). Our biochemical data and the cellular data looking at the timing of p27 turnover and cyclin accumulation suggest that increases in cyclin A-cdk2 may contribute to the turnover of p27. Interestingly, this contribution may not require kinase activity. One possibility is that cyclin A-cdk2 presents p27 to the SCFskp2 complex. This is consistent with the finding that p27ck(−) mutants are less capable of being ubiquitinated in vivo (43) or in vitro. Furthermore, the fact that cyclin E-cdk2 does not promote ubiquitination as well as cyclin A-cdk2 in the reconstituted system or in extracts suggests that sequences in the cyclin may also be required for its interaction with the SCFskp2 complex as well. We speculate that cyclin A-cdk2 interacting with both skp2 and p27 may fit into the extended cul1 backbone, helping to orient the substrate. Future genetic studies identifying cyclin A mutants that discriminate among cdk2 binding, p27 binding, and skp2 binding and structural studies focusing on cocrystallizing the SCFskp2 complex with cyclin A-cdk2 and p27 substrate may provide further insight into this possibility.
Acknowledgments
We thank Robert Fisher (MSKCC), Brenda Schulman (St. Jude Children's Research Hospital, Memphis, Tenn.), Nikola Pavletich (MSKCC), and Michele Pagano (NYU) for providing reagents and Pengbo Zhou (Cornell), Jim Roberts (FHCRC), and John Petrini (MSKCC) for comments.
This work was supported by grant GM52597 (NIH), The Irma T. Hirschl Trust, and the Golfers Against Cancer Foundation to Andrew Koff.
REFERENCES
- 1.Agrawal, D., P. Hauser, F. McPherson, F. Dong, A. Garcia, and W. J. Pledger. 1996. Repression of p27kip1 synthesis by platelet-derived growth factor in BALB/c 3T3 cells. Mol. Cell. Biol. 16:4327-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessandrini, A., D. S. Chiaur, and M. Pagano. 1997. Regulation of the cyclin-dependent kinase inhibitor p27 by degradation and phosphorylation. Leukemia 11:342-345. [DOI] [PubMed] [Google Scholar]
- 3.Bai, C., P. Sen, K. Hofmann, L. Ma, M. Goebl, J. W. Harper, and S. J. Elledge. 1996. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86:263-274. [DOI] [PubMed] [Google Scholar]
- 4.Brandeis, M., and T. Hunt. 1996. The proteolysis of mitotic cyclins in mammalian cells persists from the end of mitosis until the onset of S phase. EMBO J. 15:5280-5289. [PMC free article] [PubMed] [Google Scholar]
- 5.Carrano, A. C., E. Eytan, A. Hershko, and M. Pagano. 1999. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1:193-199. [DOI] [PubMed] [Google Scholar]
- 6.Chellappan, S. P., A. Giordano, and P. B. Fisher. 1998. Role of cyclin-dependent kinases and their inhibitors in cellular differentiation and development. Curr. Top. Microbiol. Immunol. 227:57-103. [DOI] [PubMed] [Google Scholar]
- 7.Desai, D., Y. Gu, and D. O. Morgan. 1992. Activation of human cyclin-dependent kinases in vitro. Mol. Biol. Cell 3:571-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkers, P. F., R. H. Medema, C. Pals, L. Banerji, N. S. Thomas, E. W. Lam, B. M. Burgering, J. A. Raaijmakers, J. W. Lammers, L. Koenderman, and P. J. Coffer. 2000. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27KIP1. Mol. Cell. Biol. 20:9138-9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganoth, D., G. Bornstein, T. K. Ko, B. Larsen, M. Tyers, M. Pagano, and A. Hershko. 2001. The cell-cycle regulatory protein Cks1 is required for SCFSkp2-mediated ubiquitinylation of p27. Nat. Cell Biol. 3:321-324. [DOI] [PubMed] [Google Scholar]
- 10.Hengst, L., and S. I. Reed. 1996. Translational control of p27Kip1 accumulation during the cell cycle. Science 271:1861-1864. [DOI] [PubMed] [Google Scholar]
- 11.Hochstrasser, M. 1996. Ubiquitin-dependent protein degradation. Annu. Rev. Genet. 30:405-439. [DOI] [PubMed] [Google Scholar]
- 12.Jeffrey, P. D., A. A. Russo, K. Polyak, E. Gibbs, J. Hurwitz, J. Massague, and N. P. Pavletich. 1995. Mechanism of CDK activation revealed by the structure of a cyclin A-CDK2 complex. Nature 376:313-320. [DOI] [PubMed] [Google Scholar]
- 13.King, R. W., R. J. Deshaies, J. M. Peters, and M. W. Kirschner. 1996. How proteolysis drives the cell cycle. Science 274:1652-1659. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi, H., E. Stewart, R. Poon, J. P. Adamczewski, J. Gannon, and T. Hunt. 1992. Identification of the domains in cyclin A required for binding to, and activation of, p34cdc2 and p32cdk2 protein kinase subunits. Mol. Biol. Cell 3:1279-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koff, A., F. Cross, A. Fisher, J. Schumacher, K. Leguellec, M. Philippe, and J. M. Roberts. 1991. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell 66:1217-1228. [DOI] [PubMed] [Google Scholar]
- 16.Koff, A., A. Giordano, D. Desai, K. Yamashita, J. W. Harper, S. Elledge, T. Nishimoto, D. O. Morgan, B. R. Franza, and J. M. Roberts. 1992. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science 257:1689-1694. [DOI] [PubMed] [Google Scholar]
- 17.Krek, W. 1998. Proteolysis and the G1-S transition: the SCF connection. Curr. Opin. Genet. Dev. 8:36-42. [DOI] [PubMed] [Google Scholar]
- 18.Lees, E. M., and E. Harlow. 1993. Sequences within the conserved cyclin box of human cyclin A are sufficient for binding to and activation of cdc2 kinase. Mol. Cell. Biol. 13:1194-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malek, N. P., H. Sundberg, S. McGrew, K. Nakayama, T. R. Kyriakides, J. M. Roberts, and T. R. Kyriakidis. 2001. A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature 413:323-327. [DOI] [PubMed] [Google Scholar]
- 20.Medema, R. H., G. J. Kops, J. L. Bos, and B. M. Burgering. 2000. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404:782-787. [DOI] [PubMed] [Google Scholar]
- 21.Michel, J. J., and Y. Xiong. 1998. Human CUL-1, but not other cullin family members, selectively interacts with SKP1 to form a complex with SKP2 and cyclin A. Cell Growth Differ. 9:435-449. [PubMed] [Google Scholar]
- 22.Millard, S. S., A. Vidal, M. Markus, and A. Koff. 2000. A U-rich element in the 5′ untranslated region is necessary for the translation of p27 mRNA. Mol. Cell. Biol. 20:5947-5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millard, S. S., J. S. Yan, H. Nguyen, M. Pagano, H. Kiyokawa, and A. Koff. 1997. Enhanced ribosomal association of p27Kip1 mRNA is a mechanism contributing to accumulation during growth arrest. J. Biol. Chem. 272:7093-7098. [DOI] [PubMed] [Google Scholar]
- 24.Mongay, L., S. Plaza, E. Vigorito, C. Serra-Pages, and J. Vives. 2001. Association of the cell cycle regulatory proteins p45SKP2 and CksHs1. Functional effect on CDK2 complex formation and kinase activity. J. Biol. Chem. 276:25030-25036. [DOI] [PubMed] [Google Scholar]
- 25.Montagnoli, A., F. Fiore, E. Eytan, A. C. Carrano, G. F. Draetta, A. Hershko, and M. Pagano. 1999. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 13:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakayama, K., H. Nagahama, Y. A. Minamishima, M. Matsumoto, I. Nakamichi, K. Kitagawa, M. Shirane, R. Tsunematsu, T. Tsukiyama, N. Ishida, M. Kitagawa, and S. Hatakeyama. 2000. Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J. 19:2069-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen, H., D. M. Gitig, and A. Koff. 1999. Cell-free degradation of p27kip1, a G1 cyclin-dependent kinase inhibitor, is dependent on CDK2 activity and the proteasome. Mol. Cell. Biol. 19:1190-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen, T. B., K. Manova, P. Capodieci, C. Lindon, S. Bottega, X. Y. Wang, J. Refik-Rogers, J. Pines, D. J. Wolgemuth, and A. Koff. 2002. Characterization and expression of mammalian cyclin b3, a prepachytene meiotic cyclin. J. Biol. Chem. 277:41960-41969. [DOI] [PubMed] [Google Scholar]
- 29.Ohta, T., J. J. Michel, A. J. Schottelius, and Y. Xiong. 1999. ROC1, a homolog of APC11, represents a family of cullin partners with an associated ubiquitin ligase activity. Mol. Cell 3:535-541. [DOI] [PubMed] [Google Scholar]
- 30.Pagano, M., S. W. Tam, A. M. Theodoras, P. Beer-Romero, G. Del Sal, V. Chau, P. R. Yew, G. F. Draetta, and M. Rolfe. 1995. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269:682-685. [DOI] [PubMed] [Google Scholar]
- 31.Patton, E. E., A. R. Willems, and M. Tyers. 1998. Combinatorial control in ubiquitin-dependent proteolysis: don't Skp the F-box hypothesis. Trends Genet. 14:236-243. [DOI] [PubMed] [Google Scholar]
- 32.Pavletich, N. P. 1999. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol. 287:821-828. [DOI] [PubMed] [Google Scholar]
- 33.Schulman, B. A., A. C. Carrano, P. D. Jeffrey, Z. Bowen, E. R. Kinnucan, M. S. Finnin, S. J. Elledge, J. W. Harper, M. Pagano, and N. P. Pavletich. 2000. Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature 408:381-386. [DOI] [PubMed] [Google Scholar]
- 34.Schulman, B. A., D. L. Lindstrom, and E. Harlow. 1998. Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc. Natl. Acad. Sci. USA 95:10453-10458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sitry, D., M. A. Seeliger, T. K. Ko, D. Ganoth, S. E. Breward, L. S. Itzhaki, M. Pagano, and A. Hershko. 2002. Three different binding sites of Cks1 are required for p27-ubiquitin ligation. J. Biol. Chem. 277:42233-42240. [DOI] [PubMed] [Google Scholar]
- 36.Skowyra, D., K. L. Craig, M. Tyers, S. J. Elledge, and J. W. Harper. 1997. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91:209-219. [DOI] [PubMed] [Google Scholar]
- 37.Soos, T. J., H. Kiyokawa, J. S. Yan, M. S. Rubin, A. Giordano, A. DeBlasio, S. Bottega, B. Wong, J. Mendelsohn, and A. Koff. 1996. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 7:135-146. [PubMed] [Google Scholar]
- 38.Spruck, C., H. Strohmaier, M. Watson, A. P. Smith, A. Ryan, T. W. Krek, and S. I. Reed. 2001. A CDK-independent function of mammalian Cks1: targeting of SCFSkp2 to the CDK inhibitor p27Kip1. Mol. Cell 7:639-650. [DOI] [PubMed] [Google Scholar]
- 39.Sutterluty, H., E. Chatelain, A. Marti, C. Wirbelauer, M. Senften, U. Muller, and W. Krek. 1999. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 1:207-214. [DOI] [PubMed] [Google Scholar]
- 40.Tsvetkov, L. M., K. H. Yeh, S. J. Lee, H. Sun, and H. Zhang. 1999. p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Curr. Biol. 9:661-664. [DOI] [PubMed] [Google Scholar]
- 41.Tyers, M., and P. Jorgensen. 2000. Proteolysis and the cell cycle: with this RING I do thee destroy. Curr. Opin. Genet. Dev. 10:54-64. [DOI] [PubMed] [Google Scholar]
- 42.Vidal, A., S. S. Millard, J. P. Miller, and A. Koff. 2002. Rho activity can alter the translation of p27 mRNA and is important for RasV12-induced transformation in a manner dependent on p27 status. J. Biol. Chem. 277:16433-16440. [DOI] [PubMed] [Google Scholar]
- 43.Vlach, J., S. Hennecke, and B. Amati. 1997. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 16:5334-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang, W., J. Shen, M. Wu, M. Arsura, M. FitzGerald, Z. Suldan, D. W. Kim, C. S. Hofmann, S. Pianetti, R. Romieu-Mourez, L. P. Freedman, and G. E. Sonenshein. 2001. Repression of transcription of the p27Kip1 cyclin-dependent kinase inhibitor gene by c-Myc. Oncogene 20:1688-1702. [DOI] [PubMed] [Google Scholar]
- 45.Zavitz, K. H., and S. L. Zipursky. 1997. Controlling cell proliferation in differentiating tissues: genetic analysis of negative regulators of G1→S-phase progression. Curr. Opin. Cell Biol. 9:773-781. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, H., R. Kobayashi, K. Galaktionov, and D. Beach. 1995. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell 82:915-925. [DOI] [PubMed] [Google Scholar]
- 47.Zheng, N., B. A. Schulman, L. Song, J. J. Miller, P. D. Jeffrey, P. Wang, C. Chu, D. M. Koepp, S. J. Elledge, M. Pagano, R. C. Conaway, J. W. Conaway, J. W. Harper, and N. P. Pavletich. 2002. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature 416:703-709. [DOI] [PubMed] [Google Scholar]