Abstract
Background:
Dianthus superbus L. has been used in Chinese herbal medicine as a diuretic and anti-inflammatory agent.
Objective:
In this study, we isolated ten bioactive compounds from D. superbus and evaluated their neuroprotective activity against glutamate-induced cell death in the hippocampal neuronal HT22 cells.
Materials and Methods:
New compound, (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl) (1) and, nine known compounds, diosmetin-7-O (2’’,6’’-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside (2), 4-hydroxy-3-methoxy-pentyl ester benzenepropanoic acid (3), vanillic acid (4), 4-hydroxy-benzeneacetic acid (5), 4-methoxybenzeneacetic acid (6), (E)-4-methoxycinnamic acid (7), 3-methoxy-4-hydroxyphenylethanol (8), hydroferulic acid (9), and methyl hydroferulate (10), were isolated by bioactivity-guided separation. Structures of the isolated compounds were identified on the basis of 1H nuclear magnetic resonance (NMR), 13C NMR, and two-dimensional NMR spectra, while their neuroprotective properties were evaluated by performing the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay.
Results:
D. superbus extract had a neuroprotective effect and isolated 10 compounds. Among the compounds, compounds 5 and 6 effectively protected HT22 cells against glutamate toxicity.
Conclusion:
In conclusion, the extract of D. superbus and compounds isolated from it exhibited neuroprotective properties, suggesting therapeutic potential for applications in neurotoxic diseases.
SUMMARY
D. superbus extract significantly protected on glutamate-induced cell death in HT22 cells
New compound, (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl) (1) and, nine known compounds, diosmetin-7-O(2’’,6’’-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside (2), 4-hydroxy-3-methoxy-pentyl ester benzenepropanoic acid (3), vanillic acid (4), 4-hydroxy-benzeneacetic acid (5), 4-methoxybenzeneacetic acid (6), (E)-4-methoxycinnamic acid (7), 3-methoxy-4-hydroxyphenylethanol (8), hydroferulic acid (9), and methyl hydroferulate (10) were isolated from D. superbus extract
4-hydroxy-benzeneacetic acid and 4-methoxybenzeneacetic acid showed significant protective activity against glutamate-induced toxicity in HT22 cells.
Abbreviations used: CNS: Central nervous system, ROS: Reactive oxygen species, CHCl3: Chloroform, EtOAc: Ethyl acetate, BuOH: Butanol, HPLC: High performance liquid chromatography, TLC: Thin layer chromatography, MPLC: Middle performance liquid chromatography, MeOH: Methanol, OD: Optical density, COSY: Correlation spectroscopy, HMQC: Heteronuclear multiple-quantum correlation, HMBC: Heteronuclear multiple-bond correlation, HR-MS: High-resolution molecular spectroscopy, MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Keywords: Dianthus superbus, isolation, neuroprotective activity, nuclear magnetic resonance
INTRODUCTION
Glutamate is the main endogenous excitatory neurotransmitter in the central nervous system. However, high concentrations of glutamate are associated with neurotoxicity and may be involved in several neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, and stroke.[1,2,3] HT22, an immortalized mouse hippocampal cell line, has been mainly used for studying glutamate-induced nonreceptor-mediated neurotoxicity.[4] The major mechanisms of glutamate toxicity involve increased intracellular generation of reactive oxygen species (ROS). In a neuronal cell, elevated ROS levels cause lipid peroxidation, protein oxidation, and DNA damage, which can consequentially lead to cell death.[5] Moreover, high concentrations of glutamate lead to depletion of intracellular glutathione, a critical endogenous antioxidant.[6] Compounds with anti-oxidative properties, such as vitamin E, polyphenolic bioflavonoid, and melatonin can, therefore, prevent oxidative neuronal cell death.[7]
Dianthus superbus belongs to the Caryophyllaceae family and has been used in Chinese traditional medicine as a diuretic, a contraceptive, and an anti-inflammatory agent. Previous studies have revealed various biological activities of D. superbus, including antioxidant, antimicrobial, anticancer, and anti-inflammatory properties. In particular, an ethyl acetate (EtOAc) soluble fraction of the D. superbus extract was found to possess pronounced cytotoxic activity against cancer cells.[8,9,10] Triterpenoid saponins such as dianosides A to I are reported to be among the major bioactive compounds in D. superbus; compounds including dianthosaponins, dianthramide, flavonoid, coumarin, triterpenoid, pyran-type glycoside, and cyclic peptides have also been isolated from D. superbus.[11,12,13,14,15] However, no data on the neuroprotective activity of D. superbus and the compounds isolated from its extracts have yet been reported.
In this study, a new compound, terpene (1) together with one known flavonoid, (2) and eight known phenylpropanoid compounds, (3-10) were isolated from the EtOAc fraction of an 80% methanolic extract of D. superbus. In addition, we evaluated the neuroprotective effect of the isolated compounds against glutamate-induced cell death in HT22 cells by using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
MATERIALS AND METHODS
Plant material
The herbal samples of D. superbus L. (Caryophyllaceae) were obtained from Kyungdong traditional herbal market (Seoul, Korea) and authenticated by Dr. Young Bae Seo, a Professor of the College of Oriental Medicine, Daejeon University (Daejeon, Korea). A voucher specimen (No. CJ004M) has been deposited at the Kangwon National University (Chuncheon, Korea).
Extraction and partition
Dried D. superbus plants (9 kg) were extracted 3 times with 80% methanol (MeOH) at room temperature by ultrasonication-assisted extraction using a Branson 5510 (USA). Next, the MeOH extract was evaporated, and the residue was suspended. The residue suspended in distilled water and then partitioned with n-hexane, chloroform (CHCl3), EtOAc and butanol (BuOH) to yield n-hexane (42.52 g), CHCl3 (74.94 g), EtOAc (61.17 g), and BuOH (203.32 g) fractions, respectively.
Isolation from EtOAc fraction
The EtOAc fraction was subjected to a silica gel open column chromatography (90 cm × 10 cm, 70–230 mesh) using a gradient of n-hexane - EtOAc (100:1 → 0:1, v/v) as eluent. According to the thin layer chromatography profiles, 8 fractions (A-H) were obtained.
Fraction F7 was subjected to middle performance liquid chromatography (MPLC) (Biotage Isolera Specktra One Flash Purification system ISO-1SW) connected to an SANP cartridge KP-Sil 100 g (BiotageR) with a flow rate of 25 ml/min. Four sub-fractions were collected and then further purified with the Sephadex LH-20 eluted with MeOH to afford compound 1 (51.3 mg).
Fraction G was crystallized from MeOH. Its supernatant part was submitted to silica gel column (35.5 cm × 3.2 cm, CHCl3:MeOH 20:1 → 0:1, v/v) to give 7 sub-fractions (G1a-G1g). G1g sub-fraction was separated on MPLC (SANP cartridge KP-Sil 100 g, Biotage, CHCl3:MeOH - 90:10 → 0:100, v/v) to afford compound 2 (227.5 mg).
Fraction A was separated by a preparative high performance liquid chromatography (Gilson) on a C18 YMC hydrosphere column (S-5 µm, 20 mm I.D. × 250 mm) with acetonitrile/0.1% trifluoroacetic acid water (60:40 → 48:52, v/v) to afford compound 3 (2.9 mg). Ultraviolet wavelength was recorded a 207 nm and flow rate was set at 2.0 ml/min.
(E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl) (1) Yellow oil; 1H-nuclear magnetic resonance (NMR) (CD3OD 400 MHz): δ 0.94 (3H, s, H-11), δ 1.17–2.31 (10H, m, H-4, 5, 6, 7, 9), δ 2.40–2.77 (4H, m, H-2, 10), δ 3.73 (3H, s, H-16), δ 3.89 (1H, dd, J = 7.58, 2.85 Hz, H-12), δ 6.01 (1H, dd, J = 15.26, 5.43 Hz, H-13), δ 6.16 (1H, dd, J = 15.26, 1.06 Hz, H-14). 13C NMR (CD3OD 100 MHz) δ 18.20 (C-11), δ 23.01 (C-5), δ 23.77 (C-6), δ 29.00 (C-4), δ 29.14 (C-7), δ 33.77 (C-8), δ 36.85 (C-9), δ 42.28 (C-10), δ 50.99 (C-16), δ 52.27 (C-3), δ 52.95 (C-2), δ 77.40 (C-12), δ 139.67 (C-13), δ 124.67 (C-14), δ 174.99 (C-15), δ 210.25 (C-1).
Measurement of cell viability
The mouse hippocampal HT-22 cells, provided by Seoul National University (Korea), were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 1% antibiotics (penicillin/streptomycin) and were incubated at 37°C in a humidified incubator with 5% CO2. Cells were sub-cultured once every 2 day.
Cell viability was determined using MTT assays as preciously described by Mosmann (1983). HT-22 cells were seeded at a density of 6.7 × 104 cells/well and incubated for 24 h. The isolated compounds with various concentrations and Trolox, positive control, were treated and then 2 mM glutamate was treated after 1 h. After 24 h, MTT solution was added into each well and incubated for 3 h. The media was removed, and the dark blue formazan was dissolved in dimethyl sulfoxide. Optical density (OD) value was measured at 570 nm using microplate reader (TECAN A-5002). Neuroprotective activity was determined by relative protection (%). Relative protection (%) was calculated using the following equation:
Relative protection = (OD of glutamate-treated with sample − OD of only glutamate-treated)/(OD of control − OD of only glutamate-treated) × 100.
RESULTS AND DISCUSSION
Compound 1 was obtained as a yellow oil and exhibited a pseudomolecular ion peak at m/z 279 [M-H]− under high-resolution molecular spectroscopy (HR-MS); its molecular formula was C16H24O4. The 1H NMR spectrum showed a cycloalkane signal at δ 1.17–2.31 (10H, m, H-4, 5, 6, 7, 9) and δ 2.40–2.77 (4H, m, H-2, 10), a methyl group at δ 0.95 (3H, s, H-11), two olefinic protons at δ 6.01 (1H, d, J = 15.26 Hz, H-14) and δ 6.16 (1H, d, J = 15.46 Hz, H-13), and one methoxy group at δ 3.73 (3H, s, -COOCH3). The 13C NMR spectrum included ten cycloalkane structure carbons (δ 23.01, 23.77, 23.80, 29.00, 29.14, 33.77, 36.85, 42.28, 52.27, and 52.95), one methyl group (δ 18.20), two olefinic carbons (δ 124.67 and 139.67), and two carbonyls (δ 175.00 and 210.25). The assignment and configuration of proton and carbon signal were determined by correlation spectroscopy (COSY), heteronuclear multiple-quantum correlation and heteronuclear multiple bond correlation (HMBC) spectra. The COSY spectrum showed the coupling of two olefinic protons at δ 6.01 (H-14) and δ 6.16 (H-13). In HMBC spectra, methyl proton H-11 showed interaction with carbon C-7 (δ 29.14), 8 (δ 33.77), and 9 (36.85) and also showed the long-range coupling of the proton signal H-2 and H-10 δ 2.21–2.89 with the carbonyl carbon signal (C = O) at δ 210.25. The HMBC correlation of two protons at δ 6.01 and δ 6.16 with carbon signal δ 77.40, demonstrated olefinic methyl group. Two olefinic carbons were checked by carbon δ 124.67 (C-14), and 139.67 (C-13) showed interaction with proton H-13 (δ 6.16) and H-14 (δ 6.01). The long-range coupling of the proton signal H-16with the carbonyl carbon signal (C = O) at δ 174.99, indicated “-COOCH3.” Moreover, the nuclear overhauser effect (NOE) correlation between H-12, H-7, and H-9 was identified by NOE spectroscopy spectra, and it indicated β-orientation of C-3. The NMR data was determined that compound 1 was methyl-octahydronaphthalene substituted with one olefinic group and one acetate. Comparison with the structurally similar compound, 8a-(3-methoxycarbonyl-2(E)-propenyl-4a-methylocta-hydronaphthalen-2-one, did not reveal the H-11 proton signal (Hon et al., 2000). In addition, HR-MS data indicated a fragment ion at m/z 222 [M-COOCH3]− and at m/z 197 [M-C = CHCOOCH3]−. Compound 1 has therefore been identified as (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl), which has been isolated from the herb for the first time.
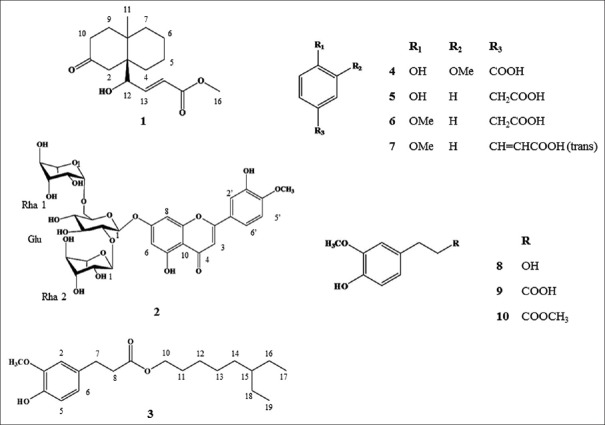
In addition to the one novel component, nine previously known compounds were isolated and identified as diosmetin-7-O (2’’,6’’-di-O-α-l-rhamnopyranosyl)-β-d-glucopyranoside, (2) 4-hydroxy-3-methoxy-pentyl ester benzenepropanoic acid, (3) vanillic acid, (4) 4-hydroxy-benzeneacetic acid, (5) 4-methoxybenzenacetic acid, (6) (E)-4-methoxycinnamic acid, (7) 3-methoxy-4-hydroxyphenylethanol, (8) hydroferulic acid, (9) and methyl hydroferulate, (10) by comparisons with previously published data.[16,17,18,19,20,21] All identified compounds were isolated from D. superbus for the first time. Chemical structures of isolated compounds are present in Figure 1.
Figure 1.
Structures of the 10 isolated compounds: (1) (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphthalen-4a-yl), (2) diosmetin-7-O (2’’,6’’-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside, (3) 6-ethyloctyl 3-(4-hydroxy-3-methoxyphenyl) propanoate, (4) vanillic acid, (5) 4-hydroxy-benzeneacetic acid, (6) 4-methoxy-benzeneacetic acid, (7) (E)-4-methoxycinnamic acid, (8) 3-methoxy-4-hydroxyphenylethanol, (9) hydroferulic acid, and (10) methyl hydroferulate
MTT assay was performed to investigate the neuroprotective activity of the ten compounds isolated from the D. superbus extract. Cell viability was used as an index of the protective effect against glutamate-induced oxidative cytotoxicity in the HT22 cells. Results of the MTT assay were expressed as the relative protection value (%). We investigated the neuroprotective effects of the partitioned fractions (n-hexane, CHCl3, EtOAc, and n-BuOH) and the isolated compounds (1-10). D. superbus extract has a significant neuroprotective effect (relation protection 92.13% of 10 µg/mL). Among the partitioned fractions, the EtOAc fraction significantly increased cell viability to 73.89% and 94.35% in HT22 cells treated with glutamate concentrations of 10 and 100 µg/mL, respectively, compared to the viability of the controls. Among the ten compounds isolated, 4-hydroxy-benzeneacetic acid (5) and 4-methoxybenzeneacetic acid (6) exhibited significant protective activity against glutamate-induced toxicity in HT22 cells, with relative ratios of 26.72% and 40.71% at 10 and 100 µM, respectively [Figure 2].
Figure 2.
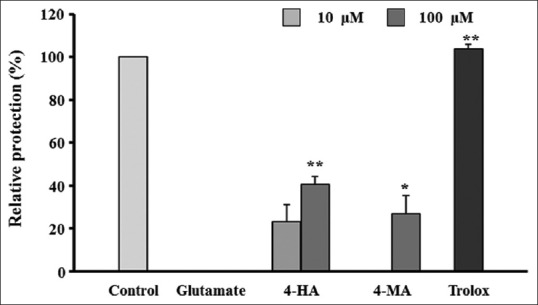
Neuroprotective activity of the isolated compounds against glutamate-induced oxidative stress in HT22 cells. HT22 cells were treated with 10 and 100 µM of compounds and 50 µM of Trolox (positive control) and then incubated for 24 h with glutamate (2 mM). Percentages of control cells are presented as means ± standard error of mean (n = 3). *P < 0.05, **P < 0.01 versus glutamate-treated cells (ANOVA) 4-HA: 4-hydroxy-benzeneacetic acid; 4-MA: 4-methoxy-benzeneacetic acid
Neuroprotective effects of partitioned fractions were evaluated on glutamate-induced cell death in HT22 cells and effect EtOAc fraction was higher than other fractions. Thus, we isolated compounds from EtOAc fraction.
One new compound, (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphtha-len-4a-yl) (1), and nine known compounds (3-10), diosmetin-7-O (2’’,6’’-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside, 4-hydroxy-3-methoxy-pentyl ester benzenepropanoic acid, vanillic acid, 4-hydroxy-benzeneacetic acid, 4-methoxybenzeneacetic acid, (E)-4-methoxycinnamic acid, 3-methoxy-4-hydroxy-phenylethanol, hydroferulic acid, and methyl hydroferulate EtOAc fraction were isolated and evaluated their neuroprotective properties. Of the 10 isolated compounds, 4-hydroxy-benzeneacetic acid (5) and 4-methoxybenzenacetic acid (6) exhibited neuroprotective activities against glutamate-induced oxidative stress in HT22 cells.
Oxidative stress has been implicated in neurodegenerative disorders including Alzheimer’s disease and Parkinson’s disease. Glutamate induces oxidative stress by stimulating ROS production and inhibiting cystine uptake into the cell through the cystine/glutamate transport system. ROS induce cell death by causing DNA damage and protein oxidation. Cystine plays an important role in the synthesis of glutathione, a key antioxidant in neuronal cells.[22] Phenylacetic acid derivatives, such as a 4-hydroxy-benzeneacetic acid (5) and 4-methoxybenzeneacetic acid (6) exhibit free radical scavenging activity and anti-oxidative effects. We found that cells treated with 4-hydroxy-benzeneacetic acid showed higher viability, reflecting the neuroprotective effect of 4-hydroxy-benzeneacetic acid, compared to those treated with 4-methoxybenzeneacetic acid. One possible explanation for this result is the presence of a phenolic ring with a hydroxyl group in the structure of 4-hydroxy-benzeneacetic acid.[23]
CONCLUSION
We isolated one new compound, (E)-methyl-4-hydroxy-4-(8a-methyl-3-oxodecahydronaphtha-len-4a-yl) (1), and nine known compounds (3-10), diosmetin-7-O (2’’,6’’-di-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside, 4-hydroxy-3-methoxy-pentyl ester benzenepropanoic acid, vanillic acid, 4-hydroxy-benzeneacetic acid, 4-methoxybenzeneacetic acid, (E)-4-methoxycinnamic acid, 3-methoxy-4-hydroxy-phenylethanol, hydroferulic acid, and methyl hydroferulate EtOAc fraction were isolated from D. superbus extract. Neuroprotective effects of ten compounds were evaluated against glutamate-induced cell death in HT22 cells. 4-hydroxy-benzeneacetic acid and 4-methoxybenzeneacetic acid showed neuroprotective effect.
Further study is warranted to elucidate the possible mechanisms underlying the neuroprotective effect of 4-hydroxy-benzeneacetic acid and 4-methoxybenzeneacetic acid.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors have declared that there is no conflicts of interest.
ABOUT AUTHOR

Choong Je Ma
Choong Je Ma, has completed his PhD at the age of 32 years from Seoul National University and postdoctoral studies from University of Michigan. He is the professor of Department of Medical Biomaterials Engineering, College of Biomedical science, Kangwon National University, Korea. He has published more than 20 papers in reputed journals.
Acknowledgment
This research was supported by a Basic Science Research Program grant from the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2010-0005149).
REFERENCES
- 1.Blandini F, Porter RH, Greenamyre JT. Glutamate and Parkinson’s disease. Mol Neurobiol. 1996;12:73–94. doi: 10.1007/BF02740748. [DOI] [PubMed] [Google Scholar]
- 2.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–95. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 3.Tan S, Wood M, Maher P. Oxidative stress induces a form of programmed cell death with characteristics of both apoptosis and necrosis in neuronal cells. J Neurochem. 1998;71:95–105. doi: 10.1046/j.1471-4159.1998.71010095.x. [DOI] [PubMed] [Google Scholar]
- 4.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–76. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 5.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–42. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behl C. Vitamin E protects neurons against oxidative cell death in vitro more effectively than 17-beta estradiol and induces the activity of the transcription factor NF-kappaB. J Neural Transm (Vienna) 2000;107:393–407. doi: 10.1007/s007020070082. [DOI] [PubMed] [Google Scholar]
- 7.Ding C, Zhang W, Li J, Lei J, Yu J. Cytotoxic constituents of ethyl acetate fraction from Dianthus superbus. Nat Prod Res. 2013;27:1691–4. doi: 10.1080/14786419.2012.763127. [DOI] [PubMed] [Google Scholar]
- 8.Shin IS, Lee MY, Ha H, Jeon WY, Seo CS, Shin HK. Dianthus superbus fructus suppresses airway inflammation by downregulating of inducible nitric oxide synthase in an ovalbumin-induced murine model of asthma. J Inflamm (Lond) 2012;9:41. doi: 10.1186/1476-9255-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu JO, Liao ZX, Lei JC, Hu XM. Antioxidant and cytotoxic activities of various fractions of ethanol extract of Dianthus superbus. Food Chem. 2007;104:1215–9. [Google Scholar]
- 10.Wang YC, Tan NH, Zhou J, Wu HM. Cyclopeptides from Dianthus superbus. Phytochemistry. 1998;49:1453–6. [Google Scholar]
- 11.Luo JG, Chen X, Kong LY. Three new triterpenoid saponins from Dianthus superbus. Chem Pharm Bull (Tokyo) 2011;59:518–21. doi: 10.1248/cpb.59.518. [DOI] [PubMed] [Google Scholar]
- 12.Hsieh PW, Chang FR, Wu CC, Wu KY, Li CM, Chen SL, et al. New cytotoxic cyclic peptides and dianthramide from Dianthus superbus. J Nat Prod. 2004;67:1522–7. doi: 10.1021/np040036v. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Luo JG, Kong LY. Two new triterpenoid saponins from Dianthus superbus L. J Asian Nat Prod Res. 2010;12:458–63. doi: 10.1080/10286020.2010.493326. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu M, Hayashi T, Shimizu K, Morita N. A pyran-type glycoside from Dianthus superbus var. Longicalycinus. Phytochemistry. 1982;21:245–7. [Google Scholar]
- 15.Hon YS, Lu L, Chang RC, Lin SW, Sun PP, Lee CF. Syntheses of α, β-unsaturated carbonyl compounds from the reactions of monosubstituted ozonides with stable phosphonium ylides. Tetrahedron. 2000;56:9269–79. [Google Scholar]
- 16.Emam AM, Elias R, Moussa AM, Faure R, Debrauwer L, Balansard G. Two flavonoid triglycosides from Buddleja madagascariensis. Phytochemistry. 1998;48:739–42. doi: 10.1016/s0031-9422(97)01043-1. [DOI] [PubMed] [Google Scholar]
- 17.Huang Z, Dostal L, Rosazza JP. Microbial transformations of ferulic acid by Saccharomyces cerevisiae and Pseudomonas fluorescens. Appl Environ Microbiol. 1993;59:2244–50. doi: 10.1128/aem.59.7.2244-2250.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehnert W, Hunkler D. Possibilities of selective screening for inborn errors of metabolism using high-resolution 1H-FT-NMR spectrometry. Eur J Pediatr. 1986;145:260–6. doi: 10.1007/BF00439397. [DOI] [PubMed] [Google Scholar]
- 19.Rosecke J, Konig WA. Odorous compounds from the fungus Gloeophyllum odoratum. Flavour Fragr J. 2000;15:315–9. [Google Scholar]
- 20.Yu J, Whitney PS, Spencer JB. Direct comparison between the mechanism of hydrometalation and β-elimination in heterogeneous and homogeneous hydrogenation. J Mol Catal A Chem. 1999;146:199–210. [Google Scholar]
- 21.Shimoji Y, Tamura Y, Nakamura Y, Nanda K, Nishidai S, Nishikawa Y, et al. Isolation and identification of DPPH radical scavenging compounds in Kurosu (Japanese unpolished rice vinegar) J Agric Food Chem. 2002;50:6501–3. doi: 10.1021/jf020458f. [DOI] [PubMed] [Google Scholar]
- 22.Satoh T, Enokido Y, Kubo T, Yamada M, Hatanaka H. Oxygen toxicity induces apoptosis in neuronal cells. Cell Mol Neurobiol. 1998;18:649–66. doi: 10.1023/a:1020633919115. [DOI] [PubMed] [Google Scholar]
- 23.Głód BK, Grieb P. Estimation of antioxidative properties of phenylacetic acids using ion-exclusion chromatography. Acta Chromatogr. 2005;15:258–68. [Google Scholar]