Abstract
Background:
A great number of novel compounds with rich chemical diversity and significant bioactivity have been reported from Red Sea sponges.
Objective:
To isolate, identify, and evaluate the cytotoxic activity of the chemical constituents of a sponge belonging to genus Haliclona collected from the Eastern coast of the Red Sea.
Materials and Methods:
The total ethanolic extract of the titled sponge was subjected to intensive chromatographic fractionation and purification guided by cytotoxic bioassay toward various cancer cell lines. The structures of the isolated compounds were elucidated using spectroscopic techniques including one-dimension and two-dimension nuclear magnetic resonance, mass spectrometry, ultraviolet, and infrared data, as well as comparison with the reported spectral data for the known compounds. X-ray single-crystal structure determination was performed to determine the absolute configuration of compound 4. The screening of antiproliferative activity of the compounds was carried on three tumor cell lines, namely the human cervical cancer (HeLa), human hepatocellular carcinoma (HepG2), and human medulloblastoma (Daoy) cells using MTT assay.
Results:
This investigation resulted in the isolation of a new indole alkaloid, 1-(1H-indol-3-yloxy) propan-2-ol (1), with the previously synthesized pyrrolidine alkaloid, (2R, 3S, 4R, 5R) pyrrolidine-(1-hydroxyethyl)-3,4-diol hydrochloride (4), isolated here from a natural source for the first time. In addition, six known compounds tetillapyrone (2), nortetillapyrone (3), 2-methyl maleimide-5-oxime (5), maleimide-5-oxime (6), 5-(hydroxymethyl) dihydrofuran-2 (3H)-one (7), and ergosta-5,24 (28)-dien-3-ol (8) were also identified. Most of the isolated compounds exhibited weak cytotoxic activity against HepG-2, Daoy, and HeLa cancer cell lines.
Conclusion:
This is the first report of the occurrence of the indole and pyrrolidine alkaloids, 1-(1H-indol-2-yloxy) propan-2-ol (1), and the - (1-hydroxyethyl)-3,4-diol hydrochloride (4), in the Red Sea Haliclona sp.
SUMMARY
From the Red Sea Haliclona sp. two alkaloids with indole and pyrrolidine nuclei, 1-(1H-indol-2-yloxy) propan-2-ol-(1) and pyrrolidine-(1-hydroxyethyl)-3,4-diol hydrochloride (4) were isolated and fully characterized; in addition to six known compounds (2, 3, 5-8)
The absolute configuration and the three-dimension stereo-molecular structure of compound 4 were determined by X-ray crystallography
The different extracts and isolated compounds showed weak cytotoxic activity against HepG-2, Daoy, and HeLa cancer cell lines.
Keywords: 5-(hydroxymethyl) dihydrofuran-2 (3H)-one, Haliclona sp., indole alkaloid, maleimide-5-oxime, pyrrolidine alkaloid
INTRODUCTION
The Red Sea is characterized by a great diversity of living organisms.[1] Its coral reefs, which extend about 2000 km, sustain more than 200 species of sponge, yet only a few of them have been studied.[2] During the last two decades, a great number of novel compounds with rich chemical diversity and significant bioactivity have been reported from Red Sea sponges.[3] Previous chemical studies of marine sponges belonging to the genus Haliclona (family Chalinidae) led to the isolation of a variety of bioactive secondary metabolites including alkaloids,[4,5] macrolides,[6] polyacetylenes,[7] polyketides,[8] steroids,[9] peptides, and halogenated derivatives.[10,11,12] Numerous bioactivities were reported for these metabolites such as anticancer, anti-inflammatory, antifouling, antidiabetic, and antimicrobial activities.[5,10,13,14,15] Many of these interesting secondary metabolites became a target for chemical synthesis and the optimization of lead compounds.[6,16] For instance, the isoquinoline alkaloid mimosamycin, isolated from the Haliclona sponge, was found to be cytotoxic against melanoma and ovarian tumor cell lines in humans, with an IC50 of approximately 10 µg/mL.[4]
In the course of our ongoing research activities toward the isolation of biologically active compounds from marine and terrestrial sources, we had the opportunity to work on the ethanolic extract of a sponge belonging to the genus Haliclona collected from the Eastern coast of the Red Sea in Jeddah, Saudi Arabia. We herein present the isolation and structural elucidation of eight compounds, two of which are reported for the first time from a natural source. In addition, the antiproliferative activity of the extracts and the isolated compounds was also checked. Our data are a contribution to the exploration of the structural diversity of secondary metabolites from Red Sea organisms.
MATERIALS AND METHODS
General experimental procedures
Optical rotations (α)D25 were measured on a Perkin-Elmer Model 341 LC polarimeter (Perkin-Elmer, Waltham, MA, the USA). The ultraviolet (UV) and infrared (IR) spectra were recorded on Hitachi-UV-3200 and JASCO 320-A spectrometers. The 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded at the NMR Unit at the College of Pharmacy, Sattam Bin Abdulaziz University, on an UltraShield Plus 500 MHz (Bruker) spectrometer operating at 500 MHz for proton and 125 MHz for carbon, respectively. The chemical shift values are reported in δ (ppm) relative to the internal standard TMS or residual solvent peak; the coupling constants (J) are reported in Hertz (Hz). Two dimension-NMR experiments (correlation spectroscopy [COSY], heteronuclear single quantum coherence [HSQC], heteronuclear multiple bond correlation [HMBC], and nuclear overhauser effect spectroscopy) were obtained using a standard Bruker program. A high-resolution mass spectrophotometer, Jeol JMS-700, was used for accurate mass determination. Electron impact mode of ionization was used, keeping ionization energy at 70eV. Resolution was set up to 10 K direct probe was used with temperature ramp setting, initial temperature 50°C rise with rate of 32°C/min and final temperature set up to 350°C. X-ray was measured using a Bruker APEX-II D8 Venture diffractometer at 100 K. Data collection was performed on a Bruker Smart Apex II D8 Venture system, using Mo Kα radiation, with a graphite monochromator, fine-focus micro tube. Thin-layer chromatography (TLC) was performed on precoated silica gel F254 plates (E. Merck, Darmstadt, Germany); detection was done at 254 nm and by spraying with p-anisaldehyde/H2SO4 reagent. Centrifugal preparative TLC (CPTLC) was performed on a chromatotron (Harrison Research, Palo Alto, CA, the USA). Plates coated with 2 mm of silica gel 60, 0.04–0.06 mm, were used. All chemicals were purchased from Sigma Chemical Company (St. Louis, MO, USA). The absorbance at 549 nm was read on a microplate reader (ELX 800, Bio-Tek Instruments, Winooski, VT, the USA).
Animal material
The marine sponge Haliclona sp. was collected, in 2012, by scuba divers from SharmObhur, Jeddah, on the Saudi Arabian Red Sea coast, and was identified by Dr. Yahia Folos, Faculty of Marine Sciences, King Abdulaziz University, Jeddah, Saudi Arabia. The sponge was deep-frozen immediately after collection and then freeze-dried to provide the dry material.
Extraction and isolation
The freeze-dried sponge (350 g) was extracted with 70% ethanol (3 × 1 L) at room temperature. The combined alcohol extract was filtered and evaporated under reduced pressure using a rotatory evaporator at 38°C to produce 25 g of the alcohol extract. The residue was suspended in water (200 mL) and successively partitioned with n-hexane (3 × 300 mL), dichloromethane (3 × 400 mL), ethyl acetate (3 × 300 mL), and n-butanol (3 × 200 mL). The n-hexane and dichloromethane fractions, combined due to the similarity on TLC (1.9 g), were subjected to chromatographic purification using a chromatotron with an n-hexane/EtOAc gradient, followed by EtOAc/methanol up to 20% to give 84 fractions of 5 ml each. Fractions were monitored by TLC silica gel, and similar fractions were pooled together to obtain four main fractions, designated as fractions A – D. Fraction A (10–23, 25 mg), eluted with 10% EtOAc/n-hexane, was further purified by CPTLC (1% MeOH/CHCl3) to afford 5 mg of 8. Fraction B (26–65, 15 mg), eluted by 50% EtOAc/n-hexane, was further purified using CPTLC (5% MeOH/CHCl3) to afford 1 (3 mg). A similar treatment of fractions C (76–78, 20 mg) and D (79–84, 50 mg) afforded 7 (10 mg) from C and 5 (20 mg) and 6 (19 mg) from D, using MeOH/CHCl3 (0.5 and 2%, respectively).
The combined ethyl acetate and n-butanol fractions (2.2 g) were applied onto the top of a silica gel packed column, eluted with CHCl3/MeOH in gradient elution mode to obtain three main fractions (E – G). Fraction E (75 mg), eluted with 5% MeOH/CHCl3, was subjected to CPTLC to afford 5 and 6 from fractions 16 to 21. Fraction 31 (19 mg) was subjected to CPTLC purification (5% MeOH/CHCl3) to afford 10 mg of 3. Conversely, fraction F, eluted with 15% MeOH/CHCl3, afforded two main sub-fractions after CPTLC purification. CPTLC chromatography of the subfractions 32–33 (14 mg), using 5% MeOH/CHCl3, afforded 5 mg of 2. Subfractions 46–49 (47 mg) were subjected to Sephadex LH-20 CC eluted with 10% H2O/MeOH to give 30 mg of 4.
Crystal structure determination
Slow evaporation of chloroform solution of pure compound 4 yielded colorless crystals. A crystal of dimensions, 0.47 mm × 0.35 mm × 0.14 mm was selected for X-ray diffraction analysis. Data collection of 4 was performed on a Bruker Smart Apex II D8 Venture system, using Mo Kα radiation from a fine focus microtube equipped with a graphite monochromator. The selected crystal was kept at 100 K under a stream of cooled nitrogen gas from a KRYO-FLEX low-temperature device. Data collection, indexing, and initial cell refinements were all carried out using APEX II software (Inc.: Analytical X-ray systems, In APEX II, B. A., Ed. 5465 East Cheryl Parkway, Madison WI 53711-5373, 2005). Frame integration and final cell refinements were done using SAINT software (SAINT, I., Analytical X-ray Systems. In version 6.45A; Bruker AXS, Ed. 5465 East Cheryl Parkway, Madison WI 53711-5373, 2003). Structure solution, refinement, graphics, and generation of publication materials were performed using SHELXTL software (SHELXTL, Bruker AXS, 5 In Systems, I. A. X-r., Ed. 465 East Cheryl Parkway, Madison WI 53711-5373, 2002).[17]
Cytotoxic activity
Three tumor cell lines were utilized in this study, namely the human cervical cancer (HeLa), human hepatocellular carcinoma (HCC HepG2), and human medulloblastoma (Daoy) cells. The cervical cancer cell line HeLa was obtained from American Type Culture Collection (Rockville, MD, USA). The HCC HepG2 cells were a kind gift from Dr. Ayman El-Kadi (University of Alberta, Edmonton, Alberta, Canada). The medulloblastoma cell line Daoy was a kind gift from Dr. Abdelilah Aboussekhra (Department of Molecular Oncology, King Faisal Specialist Hospital and Research Center, Riyadh, Kingdom of Saudi Arabia). HeLa and HepG2 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM)/high glucose supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, and 1% penicillin/streptomycin. Daoy was cultured in DMEM/F12 supplemented with 10% FBS, 2 mM L-glutamine, and 1% penicillin/streptomycin.
Screening of antiproliferative activity by MTT assay
The isolated compounds were evaluated at the Cell Culture Laboratory, College of Pharmacy, King Saud University, in a primary three-cell line-one concentration (50 µg/ml) anticancer assay against the previously mentioned cell lines. The cytotoxicity was assessed by testing the capacity of the reducing enzymes present in viable cells to convert MTT to formazan crystals as described by Al-Salahi et al.[18]
The cytotoxic activity of the anticancer drug dasatinib, a potent, multitargeted kinase inhibitor of BCR-ABL and SRC family kinases,[19] against the three cell lines was measured at the same concentrations of tested compounds and utilized as a standard for comparative purposes.
RESULTS AND DISCUSSION
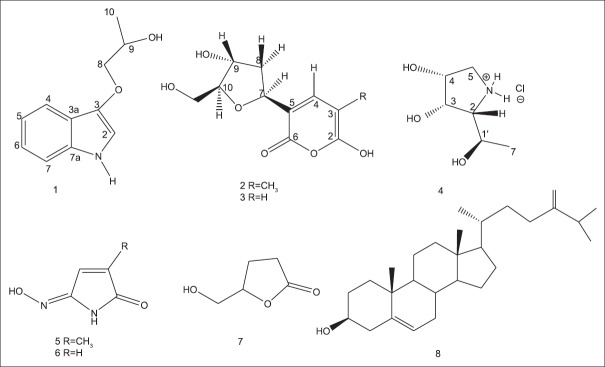
A combination of different chromatographic techniques as CPTLC and gel filtration of the ethanolic extract of the marine sponge Haliclona sp. afforded eight compounds (1–8) [Figure 1], from which two were identified from a natural source for the 1st time (1, 4).
Figure 1.
Structures of isolated compounds from Haliclona sp.
Structure elucidation of the new compounds (1, 4)
1-(1H-indol-2-yloxy) propan-3-ol (1) was isolated as a viscous liquid. Its high-resolution electron impact mass spectrometry (HREI-MS) showed an odd molecular ion peak at m/z 191.0946 [M]+, calculated for C11H13NO2 with six degrees of unsaturation. A broad absorption band at 3435 cm−1 with intense bands at 1595, 1500, and 1220 cm−1 in the IR spectrum indicated the presence of OH and/or NH in addition to an aromatic ring. The 13C spectrum of 1 [Table 1] revealed the presence of 11 carbon signals, which were assigned, with the aid of DEPT experiments, to one methyl, one sp3 oxygenated methylene, one sp3 oxygenated methine, five sp2 methines, and three sp2 quaternary carbons. The 1H NMR spectrum showed the resonance of four coupled aromatic protons at δH 8.13 (1H, brd, J = 7.8 Hz), 7.45 (1H, brd, J = 7.6 Hz), 7.20 (1H, dt,J = 7.6, 1.3 Hz), and 7.16 (1H, dt, J = 7.6, 1.3 Hz), assigned for H-4, H-7, H-6, and H-5, respectively. Moreover, a broad singlet at δH 7.95 (H-2) was observed, indicating an ABCD aromatic spin system of an indole derivative with a C-3 substitution.[20] The 1H NMR spectrum also showed the presence of a methyl doublet at δH 1.15 (J = 6.4 Hz), an oxygenated methine at δH 3.80, and an oxygenated methylene at δH 3.44, diagnostic for an alkyl moiety. Its identity was deduced from the clear 1H- 1H COSY correlation signals between the oxygenated proton at δH 3.80 (1H, m, H-9) and both proton signals at δH 3.44 (2H, m, H-8), as well as the methyl doublet at δH 1.15 (3H, d, J = 6.4, H3-10).
Table 1.
1H and 13C-NMR data of compounds (1) and (4) (500 MHz for 1H- and 13C-NMR, (1) in CD3OD and (4) in DMSO-d6)
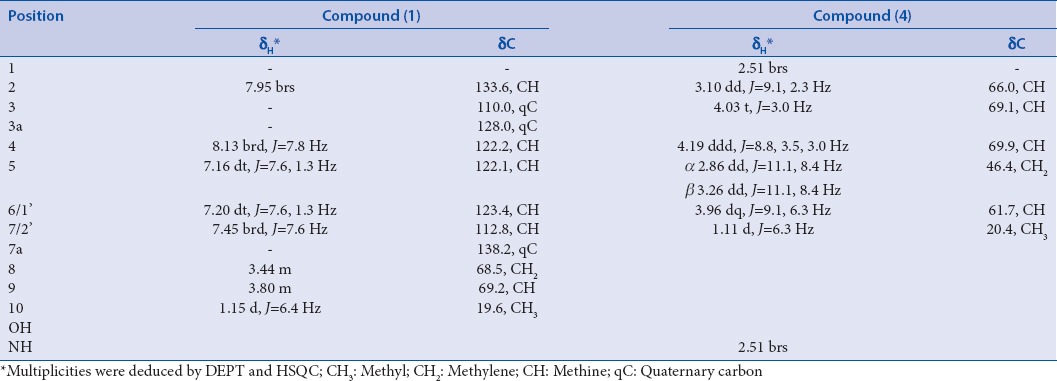
In addition, 2J and 3J HMBC correlations between the methyl protons at δH 1.15 (H3-10) and the carbon signals at δC 69.2 (C-9) and 68.5 (C-8) confirmed the side chain identity as 2-hydroxy propane. The attachment of the 2-hydroxy propane moiety was confirmed to be at position 3 and not 2 by the appearance of two significant cross-peak correlations in the HMBC spectrum between both protons at δH 8.13 (brd, H-4) and 3.44 (2H, m, H-8) and the quaternary carbon at δC 132.5 (C-3) [Figure 2]. Based on the above discussion, compound 1 was proved to be 1-(1H-indol-3-yloxy) propan-2-ol, isolated here for the 1st time from a natural source. Several indole alkaloid derivatives have been reported from different sponges, including Hyrtios, Sarcotragus, Tedania, and Dragmacidon.[20,21,22,23] Lately, the indole series of alkaloids have become an attractive research field for the development of new pharmacological lead compounds due to their interesting and diverse biological activities.[24]
Figure 2.
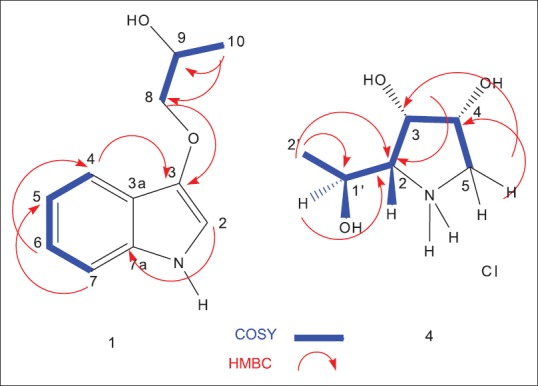
Key correlation spectroscopy and heteronuclear multiple bond correlation correlations of 1 and 4
(2R, 3S, 4R, 5R) pyrrolidine-(1-hydroxyethyl)-3,4-diol hydrochloride (4) was obtained as white needle crystals. The HREI-MS showed an odd M+ at m/z 147.0895, calculated for C6H13NO3 with one degree of unsaturation. A monocyclic structure was suggested by the absence of a double bond or carbonyl carbon signals in the 13C-NMR. The IR spectrum showed absorption bands at 3392 cm−1 (OH and/or NH) and 1640 cm−1 (N-H bending). The 13C-NMR and DEPT experiment of 4 showed a total of six protonated carbons ascribed as one methyl, one methylene, and four downfield methine carbons. The 1H-NMR spectrum showed a methyl doublet at δH 1.11 and a broad singlet at δH 2.51, assigned for NH [Table 1]. The two protons at δH 2.86 and 3.26 and correlated to a carbon signal at δC 46.4 (CH2) in the HSQC spectrum were assigned for CH2-5. COSY correlations of protons enabled the assignment of the initial structure of 4 as shown in Figure 2. The HMBC experiment was useful to prove the final structure of 4, where cross-peak correlations were observed from the methyl protons at δH 1.10 to C-1’ (δC 61.7) and C-2 (δC 66.0); from the proton signal at δH 3.96 (H-1’) to carbon C-2 (δC 66.0); from the proton signal at δH 4.03 (H-3) to C-2 (δC 66.0); from H-5 β at δH 3.26 to C-3 at δC 69.1; and from the proton signal at 2.86 (H-5α) to carbon at δC 69.9 (C-4). The unambiguous orientation of the hydroxyl groups at C-1’, C-3, and C-4 and the configuration at the asymmetric centers of positions 1’, 2, 3, and 4 were confirmed by X-ray diffraction. Fortunately, quality crystals for X-ray diffraction were obtained by slow evaporation of a solution of 4 in CHCl3, confirming the proposed structure and relative configuration [Figure 3]. The absolute configuration of all four chiral centers was established as 2R, 3S, 4R, and 5R. The Flack absolute configuration parameter was 0.04,[25] where a value close or equal to zero represents the correct structure.
Figure 3.
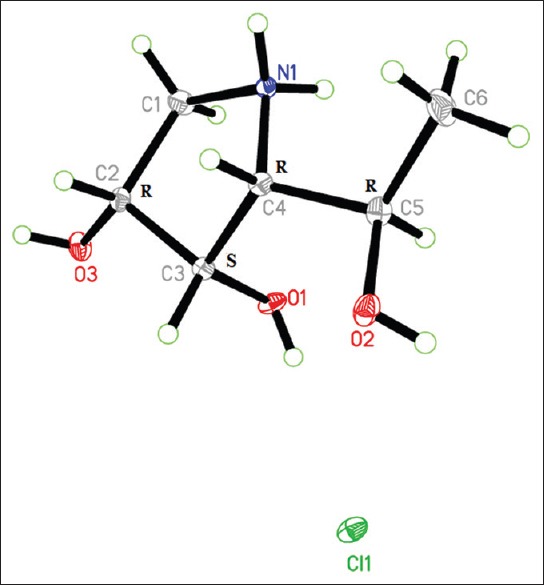
ORTEP projection of 4, with the displacement ellipsoids drawn at the 50% probability level
The polyhydroxylated pyrrolidine derivative 4 is apparently an isomer of the synthetic compound 6-deoxy-1,4-dideoxy-1,4-imino-D-mannitol (DIM).[26] DIM and 6-deoxy-DIM are known as powerful amino-sugar glycosidase inhibitors, which act by mimicking the natural substrates. They are used for studying the mechanism of action of these enzymes and tested as potential drugs to treat a variety of carbohydrate-mediated diseases, such as diabetes mellitus.[27] In addition, several isomers of 4 were previously synthesized, along with some pyrrolidine analogues of galactofuranose, from carbohydrate lactones and reported to be the first known inhibitor of Escherichia coli K12 UDP-Gal mutase and mycobacterial galactan biosynthesis.[28]
Through comparison with data from previous literature, the structures of the known compounds were determined to be tetillapyrone (2), nortetillapyrone[3,29,30] 2-methyl maleimide-5-oxime (5),[31] Maleimide-5-oxime (6),[29] 5-(hydroxymethyl) dihydrofuran-2 (3H)-one (7),[32,33] and Ergosta-5,24 (28)-dien-3-ol (8), also known as chalinasterol or ostreasterol.[34] It is worth noting that compound 5 is reported here for the 1st time from the genus Haliclona, while compound 7 is not yet been reported as occurring in marine organisms.
Spectral data of the known compounds (2, 3, 5-8)
Tetillapyrone (2)
1H NMR (CD3OD, 500 MHz): δH 7.83 (1H, s, H-4), 6.30 (1H, t, 6.7 Hz, H-7), 2.20 (1H, m, H-8a), 2.25 (1H, m, H-8b), 4.42 (1H, m, H-9), 3.93 (1H, q, 4.0 Hz, H-10), 3.83 (1H, ddd, 12, 5.0, 3.5 Hz, H-11a), 3.81 (1H, ddd, 12, 5.0, 3.5 Hz, H-11b), 1.90 (3H, br s, CH3).
13C NMR (CD3OD, 125 MHz): δC 152.4 (C-2), 111.6 (C-3), 138.2 (CH-4), 111.6 (C-5), 166.5 (C-6), 86.3 (CH-7), 41.2 (CH2-8), 72.2 (C-9), 88.8 (C-10), 62.7 (C-11), 12.55 (CH3).
Nortetillapyrone (3)
1H NMR (CD3OD, 500 MHz): δH 5.73 (1H, d, 8.1 Hz, H-3), 7.99 (1H, d, 8.1 Hz, H-4), 6.28 (1H, t, 6.6 Hz, H-7), 2.31 (1H, m, H-8a), 2.22 (1H, m, H-8b), 4.41 (1H, dq, 4.0, 3.5 Hz, H-9), 3.94 (1H, q, 3.5 Hz, H-10), 3.85 (1H, m, H-11a), 3.80 (1H, m, H-11b).
13C NMR (CD3OD, 125 MHz): δC 152.3 (C-2), 102.8 (CH-3), 142.6 (CH-4), 102.8 (C-5), 166.3 (C-6), 86.7 (CH-7), 41.3 (CH2-8), 72.3 (C-9), 89.0 (C-10), 62.9 (C-11).
2-Methyl maleimide-5-oxime (5)
1H NMR (DMSO-d6, 500 MHz): δH 11.00 brs (-NH), 10.59 brs (=N-OH), 7.24 s (1H, H-4),
1.72 (3H, CH3); 13CNMR (DMSO-d6, 125 MHz): δC 164.93 (CO-2), 151.47 (C-5), 137.70 (CH-4), 107.67 (C-3), 11.75 (CH3).
Maleimide-5-oxime (6)
1H NMR (DMSO-d6, 500 MHz): δH 11.00 brs (-NH), 10.59 brs (=N-OH), 7.24 s (H-4), 1.72 (CH3); 13CNMR (DMSO-d6, 125 MHz): δC 164.93 (CO-2), 151.47 (C-5), 137.70 (CH-4), 107.67 (C-3).
Dihydrofuran-2 (3H)-one (7)
1H NMR (CDCl3, 500 MHz): δH 2.47 (2H, H-3), 2.16 (1H, H-3a), 1.95 (2H, H-3b), 4.52 (1H, H-5), 3.58 (2H, H-6), 5.06 (OH).
13C NMR (CDCl3, 125 MHz): δC 177.5 (C-2), 28.1 (CH2-3), 23.0 (CH2-4), 80.6 (CH2-5), 62.7 (CH2-6).
Ergosta-5,24 (28)-dien-3-ol (8)
1H NMR (CDCl3, 500 MHz): δH 1.80 (2H, d, 5.1 Hz, H-1), 1.51 (2H, H-2), 3.53 (1H, m, H-3), 2.27 (2H, m, H-4), 5.35 (1H, brd, 5.7, H-6), 1.95 (2H, m, H-7), 0.66 (3H, s, H-18), 1.00 (3H, s, H-19), 0.95 (2H, d, 6.6 Hz, H-21), 1.03 (3H, d, 2.3 Hz, H-26), 1.02 (3H, d, 2.3 Hz, H-27), 4.66 (1H, s, H-28), 4.71 (1H, s, H-28).
13C NMR (CDCl3, 125 MHz): δC 37.3 (CH2-1), 31.7 (CH2-2), 71.8 (CH-3), 42.3 (CH2-4), 140.8 (C-5), 121.7 (CH-6), 31.0 (CH2-7), 31.9 (CH-8), 50.1 (CH-9), 36.6 (C-10), 21.1 (CH2-11), 39.8 (CH2-12), 42.3 (C-13), 56.8 (CH-14), 24.3 (CH-15), 28.2 (CH2-16), 56.0 (CH-17), 11.9 (CH3-18), 19.4 (CH3-19), 35.8 (CH-20), 18.7 (CH3-21), 23.9 (CH2-22), 34.7 (CH2-23), 156.9 (C-24), 33.8 (CH-25), 22.0 (CH3-26), 28.1 (CH3-27), 105.9 (C-28).
Crystal data for compound4
The three-dimension (3D) stereo-molecular structure presented herein was elucidated from a single colorless crystal suitable to X-ray diffraction, obtained from a batch of crystals obtained by slow evaporation of chloroform solvent. Data were collected using a Bruker APEX-II D8 Venture diffractometer at 100 K. Crystal data of compound 1: C6H13NO3.HCl, Mr = 183.63, colorless needle, 0.71 mm × 0.12 mm × 0.06 mm, orthorhombic, space group P212121, a = 5.1645 (3) Å, b = 12.4218 (7) Å, c = 13.0547 (7) Å, V = 837.49 (8) Å3, Z = 4, rcalcd = 1.456 Mg m−3, F (000) =392, Mo Kα radiation, λ =0.71073 Å, θmax = 30.6, −7≤h≤7, −17≤k≤17, −18≤l≤18, 22465 measured reflections, 2563 independent, R (int) =0.084, completeness to θ max = 99.9%, µ =0.42 mm−1, 156 parameters were refined against all reflections, R (F2 > 2 σ [F2]) =0.034, wR (F2) =0.075, Flack parameter = 0.04 (3), Δρmax = 0.38 e Å−3, Δρmin= −0.32 e Å−3, GOF = 1.02 based on F2. After extensive refinement and software calculations using likelihood methods, the compound is proven to be 100% enantiopure as (2R, 3S, 4R, 5R) [Figure 3].
Crystallographic data reported in this paper, have been deposited with The Cambridge Crystallographic Data Centre, deposit No. CCDC 684583. Copies of these data can be obtained, free of charge on application to the Director, CCDC 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 1223 336033; or E-mail: deposit@ccdc.cam.uk.
Cytotoxic activity
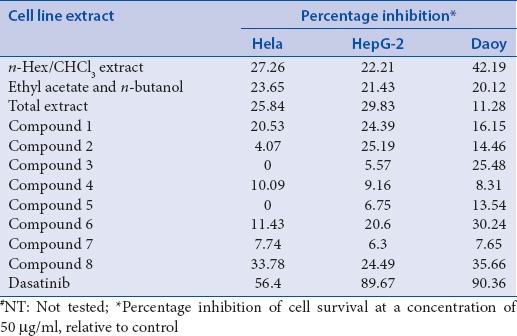
The cytotoxicity was tested against the three cancer cell lines HepG-2 (human liver cancer cell line), Daoy (human medulloblastoma), and HeLa (human cervical cancer cell line) using MTT assay,[18] with dasatinib as a reference drug. As shown in Table 2, the n-hexane/CHCl3 extract displayed the highest cytotoxic activity toward Daoy cell line (42.19% inhibition), while the two other extracts were weakly cytotoxic against HepG-2, Daoy, and HeLa cancer cell lines (11.28–29.83% inhibition). All isolated compounds showed weak cytotoxic activities against the tested cell lines. The strongest cytotoxic activity was exhibited by compound 8 against Daoy and HeLa cells (35.66 and 33.78% inhibition, respectively), followed by compound 6 (30.24% inhibition against Daoy cells). All compounds displayed very weak activity against the HeLa cell line, with inhibition percentages from 0% to 20%. In fact, the present findings for cytotoxic activity of compound 8 are consistent with results demonstrated for similar steroidal compounds obtained from various marine natural sources.[35,36] A previous study had shown that ergosta-5,24 (28)-dien-3-ol (8), reported from several Haliclona sponges, possess moderate cytotoxicity against the human foreskin fibroblast cell line (Hs27 cells) with an IC50 value of 58 μM.[34] On the other hand, a recent study revealed that alkaloids, isolated from roots of Zanthoxylum nitidum, with mannopyranoside indole nucleus, possessed significant cytotoxic activities against selected tumor cell lines with IC50 of <30 μm. Structural variations of attached functional groups might explain the lack of activity in compound 1.[37] On the basis of these results, further lead optimization and structure-activity relationship studies are recommended to synthesize derivatives with potent antitumor activities.
Table 2.
Cytotoxic activities of Haliclona sp. extracts and isolated compounds
CONCLUSION
Two alkaloids from the Red Sea Haliclona sp. with indole and pyrrolidine nuclei, 1-(1H-indol-2-yloxy) propan-2-ol (1) and pyrrolidine-(1-hydroxyethyl)-3,4-diol hydrochloride (4), were isolated and fully characterized; in addition to six known compounds (2, 3, 5-8). Furthermore, the absolute configuration and the 3D stereo-molecular structure of compound 4 were determined by X-ray crystallography. The different extracts and isolated compounds showed weak cytotoxic activity against HepG-2, Daoy, and HeLa cancer cell lines.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Shaza Mohamed Al-Massarani
Shaza Mohamed Al-Massarani is an Assistant Professor at the Pharmacognosy Department, College of Pharmacy, King Saud University. She got her PhD in 2011. Her experience in the area of Pharmacognosy and Chemistry of Natural Products, working mainly in analysis of medicinal plants and marine organisms, isolation and identification of pure compounds through chromatographic techniques and spectroscopic analysis (NMR, IR, MS). She has published many scientific papers, and she Shaza Mohamed Al-Massarani is a member in a research group.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding the work through the Research Group Project no. RGP-VPP-326.
REFERENCES
- 1.Lira NS, Montes RC, Tavares JF, da Silva MS, da Cunha EV, de Athayde-Filho PF, et al. Brominated compounds from marine sponges of the genus Aplysina and a compilation of their 13 C NMR spectral data. Mar Drugs. 2011;9:2316–68. doi: 10.3390/md9112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian PY, Wang Y, Lee OO, Lau SC, Yang J, Lafi FF, et al. Vertical stratification of microbial communities in the Red Sea revealed by 16S rDNA pyrosequencing. ISME J. 2011;5:507–18. doi: 10.1038/ismej.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blunt JW, Copp BR, Munro MH, Northcote PT, Prinsep MR. Marine natural products. Nat Prod Rep. 2005;22:15–61. doi: 10.1039/b415080p. [DOI] [PubMed] [Google Scholar]
- 4.Rashid MA, Gustafson KR, Boyd MR. A new isoquinoline alkaloid from the marine sponge Haliclona species. J Nat Prod. 2001;64:1249–50. doi: 10.1021/np0102004. [DOI] [PubMed] [Google Scholar]
- 5.Mol VP, Raveendran TV, Parameswaran PS. Antifouling activity exhibited by secondary metabolites of the marine sponge, Haliclona exigua (Kirkpatrick) Int Biodeterior Biodegradation. 2009;63:67–72. [Google Scholar]
- 6.Liu YH, Wang B, Liu DY, Li LD, Fei LN. Chemical and biological activities of marine sponge genus Haliclona. JTO. 2008;27:70–82. [Google Scholar]
- 7.Alarif WM, Abdel-Lateff A, Al-Lihaibi SS, Ayyad SE, Badria FA. A new cytotoxic brominated acetylenic hydrocarbon from the marine sponge Haliclona sp. with a selective effect against human breast cancer. Z Naturforsch C. 2013;68:70–5. [PubMed] [Google Scholar]
- 8.Kennedy J, Codling CE, Jones BV, Dobson AD, Marchesi JR. Diversity of microbes associated with the marine sponge, Haliclona simulans, isolated from Irish waters and identification of polyketide synthase genes from the sponge metagenome. Environ Microbiol. 2008;10:1888–902. doi: 10.1111/j.1462-2920.2008.01614.x. [DOI] [PubMed] [Google Scholar]
- 9.Santalova EA, Makarieva TN, Ponomarenko LP, Denisenko VA, Krasokhin VB, Mollo E, et al. Sterols and related metabolites from five species of sponges. Biochem Syst Ecol. 2007;35:439–46. [Google Scholar]
- 10.Randazzo A, Bifulco G, Giannini C, Bucci M, Debitus C, Cirino G, et al. Halipeptins A and B: two novel potent anti-inflammatory cyclic depsipeptides from the Vanuatu marine sponge Haliclona species. J Am Chem Soc. 2001;123:10870–6. doi: 10.1021/ja010015c. [DOI] [PubMed] [Google Scholar]
- 11.Faulkner DJ. Marine natural products. Nat Prod Rep. 2001;18:1–49. doi: 10.1039/b006897g. [DOI] [PubMed] [Google Scholar]
- 12.Rashid MA, Gustafson KR, Boswell JL, Boyd MR. Haligramides A and B, two new cytotoxic hexapeptides from the marine sponge Haliclona nigra. J Nat Prod. 2000;63:956–9. doi: 10.1021/np000051+. [DOI] [PubMed] [Google Scholar]
- 13.Charan RD, Garson MJ, Brereton IM, Willis AC, Hooper JN. Haliclonacyclamines A and B, cytotoxic alkaloids from the tropical marine sponge Haliclona sp. Tetrahedron. 1996;52:9111–220. [Google Scholar]
- 14.Trianto A, Hermawan I, de Voogd NJ, Tanaka J. Halioxepine, a new meroditerpene from an Indonesian sponge Haliclona sp. Chem Pharm Bull (Tokyo) 2011;59:1311–3. doi: 10.1248/cpb.59.1311. [DOI] [PubMed] [Google Scholar]
- 15.Nuzzo G, Ciavatta ML, Villani G, Manzo E, Zanfardino A, Varcamonti M, et al. Fulvynes, antimicrobial polyoxygenated acetylenes from the Mediterranean sponge Haliclona fulva. Tetrahedron. 2012;68:754–60. [Google Scholar]
- 16.Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619–38. doi: 10.3390/md8102619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheldrick GM. A short history of SHELX. Acta Crystallogr A. 2008;64:112–22. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 18.Al-Salahi R, Marzouk M, Ashour AE, Alswaidan I. Synthesis and antitumor activity of 1,2,4-triazolo[1,5-a] quinazolines. Asian J Chem. 2014;26:2173–6. [Google Scholar]
- 19.Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino) thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–61. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 20.Ashour MA, Ehab S, Elkhayat ES, Ebel R, Edrada R, Proksch P. Indole alkaloid from the red sea sponge Hyrtios erectus. Arcivoc. 2007;xv:225–31. [Google Scholar]
- 21.Dillman RL, Cardellina JH. Aromatic secondary metabolites from the sponge Tedania ignis. J Nat Prod. 1991;54:1056–61. [Google Scholar]
- 22.Mancini I, Guella G, Pietra F, Debitus C, Waikedre J. From inactive nortopsentin D, a novel bis (indole) alkaloid isolated from the Axinellid sponge Dragmacidon sp. from deep waters south of new Caledonia, to a strongly cytotoxic derivative. Helv Chim Acta. 1996;79:2075–82. [Google Scholar]
- 23.Liu Y, Jung JH, Zhang S. Indole alkaloids from a sponge Sarcotragus species. Biochem Syst Ecol. 2006;34:453–6. [Google Scholar]
- 24.Kochanowska-Karamyan AJ, Hamann MT. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem Rev. 2010;110:4489–97. doi: 10.1021/cr900211p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flack HD. On enantiomorph-polarity estimation. Acta Cryst. 1983;39:876–81. [Google Scholar]
- 26.Bashyal BP, Fleet GW, Gough MJ, Smith PW. Synthesis of the α-mannosidase inhibitors swainsonine [(1S, 2R, 8R, 8aR)-1,2,8-trihydroxyoctahydroindolizine] and 1,4-dideoxy-1,4-imino-d-mannitol from mannose. Tetrahedron. 1987;43:3083–93. [Google Scholar]
- 27.Winchester B, al Daher S, Carpenter NC, Cenci di Bello I, Choi SS, Fairbanks AJ, et al. The structural basis of the inhibition of human alpha-mannosidases by azafuranose analogues of mannose. Biochem J. 1993;290:743–9. doi: 10.1042/bj2900743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee RE, Smith MD, Nash RJ, Griffiths RC, McNeil M, Grewal RK, et al. Inhibition of UDP-gal mutase and mycobacterial galactan biosynthesis by pyrrolidine analogues of galactofuranose. Tetrahedron Lett. 1997;38:6733–6. [Google Scholar]
- 29.Wattanadilok R, Sawangwong P, Rodrigues C, Cidade H, Pinto M, Pinto E, et al. Antifungal activity evaluation of the constituents of Haliclona baeri and Haliclona cymaeformis, collected from the Gulf of Thailand. Mar Drugs. 2007;5:40–51. doi: 10.3390/md502040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanadilok R, Sonchaeng P, Kijjoa A, Damas AM, Gales L, Silva AM, et al. Tetillapyrone and nortetillapyrone, two unusual hydroxypyran-2-ones from the marine sponge Tetilla japonica. J Nat Prod. 2001;64:1056–8. doi: 10.1021/np0100690. [DOI] [PubMed] [Google Scholar]
- 31.Kijjoa A, Bessa J, Wattanadilok R, Sawangwong P, Nascimento MS, Pedro M, et al. Dibromotyrosine derivatives, a maleimide, aplysamine-2 and other constituents of the marine sponge Pseudoceratina purpurea. Z Naturforsch. 2005;60b:904–8. [Google Scholar]
- 32.Hamamoto H, Suzuki Y, Takahashi H, Ikegami S. A new solid-phase reaction system utilizing a temperature- responsive catalyst: Oxidative cyclization with hydrogen peroxide. Adv Synth Catal. 2007;349:2685–9. [Google Scholar]
- 33.Yu Z, Liu J, Gong Y, Fan W, Ma J, Zhou N, et al. Chemical constituents of non-saponin from Pulsatilla cernua. Zhongcaoyao. 2013;44:3264–9. [Google Scholar]
- 34.Viegelmann C, Parker J, Ooi T, Clements C, Abbott G, Young L, et al. Isolation and identification of antitrypanosomal and antimycobacterial active steroids from the sponge Haliclona simulans. Mar Drugs. 2014;12:2937–52. doi: 10.3390/md12052937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y, Tian L, Huang J, Li W, Pei YH. Cytotoxic sterols from marine-derived fungus Pennicillium sp. Nat Prod Res. 2006;20:381–4. doi: 10.1080/14786410600661229. [DOI] [PubMed] [Google Scholar]
- 36.Byju K, Anuradha V, Vasundhara G, Nair SM, Kumar NC. In vitro and in silico studies on the anticancer and apoptosis-inducing activities of the sterols identified from the soft coral, Subergorgia reticulate. Pharmacogn Mag. 2014;10(Suppl 1):S65–71. doi: 10.4103/0973-1296.127345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu J, Shi X, Mao X, Chen J, Li H. Cytotoxic mannopyranosides of indole alkaloids from Zanthoxylum nitidum. Chem Biodivers. 2014;11:970–4. doi: 10.1002/cbdv.201300381. [DOI] [PubMed] [Google Scholar]