Abstract
Background:
Oxidative stress (OS) has been regarded as one of the major pathogeneses of ulcerative colitis (UC) through damaging colon. It has been shown that Scutellariae radix (SR) extract has a beneficial effect for the prevention and treatment of UC.
Objective:
The aim of this study was to investigate whether SR had a potential capacity on oxidant damage for colon injury both in vivo and in vitro.
Materials and Methods:
The 2,4,6-trinitrobenzene sulfonic acid (TNBS) was used to induce UC rats model while 1 μg/ml lipopolysaccharide (LPS) was for RAW264.7 cell damage. Disease activity index (DAI) was determined to response the severity of colitis. The myeloperoxidase (MPO) activity in rat colon was also estimated. The 2,2’-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid assay was performed to evaluate the total antioxidant capacity of SR. Furthermore, the activity of glutathione peroxidase (GSH-PX), catalase (CAT), superoxide dismutase (SOD), and lipid peroxidation malondialdehyde (MDA) in cell supernatant and rat serum were detected by appropriate kits. In addition, an immunohistochemical assay was applied to examine transforming growth factor beta 1 (TGF-β1) protein expression in colon tissue.
Results:
The treatment with SR could significantly increase the activity of GSH-PX, CAT, and SOD associated with OS in LPS-induced RAW264.7 cell damage and TNBS-induced UC rats. However, the level of MDA was markedly reduced both in vitro and in vivo. Furthermore, SR significantly decreased DAI and reversed the increased MPO activity. Thus, SR could decrease the severity of acute TNBS-induced colitis in rats. Immunohistochemical assay showed that SR significantly downregulated TGF-β1 protein expression in colon tissue.
Conclusion:
Our data provided evidence to support this fact that SR attenuated OS in LPS-induced RAW264.7 cell and also in TNBS-induced UC rats. Thus, SR may be an interesting candidate drug for the management of UC.
SUMMARY
Scutellariae radix (SR) could significantly increase the activity of glutathione peroxidase, catalase, and superoxide dismutase associated with OS in lipopolysaccharide-induced RAW264.7 cell damage and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced ulcerative colitis rats
The level of malondialdehyde was markedly reduced by SR both in vitro and in vivo
SR could decrease the severity of acute TNBS-induced colitis in rats
SR could significantly downregulate the expression of transforming growth factor beta 1 protein in colon tissue.
Abbreviations used: OS: Oxidative stress, UC: Ulcerative colitis, SR: Scutellariae radix, TNBS: 2,4,6-trinitrobenzene sulfonic acid, DAI: Disease activity index, MPO: Myeloperoxidase, GSH-PX: Glutathione peroxidase, CAT: Catalase, SOD: Superoxide dismutase, MDA: Malondialdehyde, TGF-β1: Transforming growth factor beta 1, OD: Optical density, ROS: Reactive oxygen species.
Keywords: Oxidative stress, reactive oxygen species, Scutellariae radix, transforming growth factor beta 1, ulcerative colitis
INTRODUCTION
Ulcerative colitis (UC), a chronic inflammatory condition of the large intestine, mainly affects the population in the western countries. Due to the adoption of Western lifestyle, UC has become common in the whole word.[1] Patients suffering from UC have a higher risk of developing colorectal cancer which is the third most common malignancy generally observed in humans.[2] The exact etiology of UC has not been clearly known; however, a growing body of evidence showed that oxidative stress (OS) played a crucial role in the development and progression of UC.[3,4] Damage at the local site could extend beyond the site of inflammation and may affect other organs globally, in which OS has a major role to play.[5] Therefore, it is critical to use appropriate antioxidants targeting OS to treat UC.
It was reported that transforming growth factor beta family (TGF-β) had a great relationship with OS via the regulation of protein levels of antioxidant enzymes such as glutathione peroxidase (GSH-PX), catalase (CAT), superoxide dismutase (SOD), and others.[6] In addition, a growing body of evidence suggested that enhanced expression of TGF-β message was found in the cells of the lamina propria in UC patients.[7,8]
The dried root of Scutellariae radix (SR, common name Huangqin) has been used for treating disease in China and other Asian countries for a long time. It has been shown to have a protective effect against various tumors, pyrexia, inflammatory diseases, hepatitis, diarrhea, and other diseases.[9,10] It is worthy of note that SR has an inhibitory effect on the production of reactive oxygen species (ROS).[11] However, there are little reports referring to the effect of SR on lipopolysaccharide (LPS)-induced OS in vitro and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced oxidant damage in vivo. In the present study, we have investigated the attenuation of SR on LPS-induced OS for colon injury in RAW264.7 cell in vitro and TNBS-induced UC rats in vivo. Our findings in this study may provide a better insight for understanding the potential therapeutic effect of SR on the development of UC.
MATERIALS AND METHODS
Chemicals and reagents
SR, derived from the root of Scutellaria baicalensis Georgi (common name Huangqin), came from Anhui Huqiao Chinese Medicine Technology Co., Ltd. (Tongling, Anhui Province, China), was identified by Prof. Dekang Wu in Nanjing University of Chinese Medicine.
Preparation of Scutellariae radix
SR (100 g) was refluxed in 500 ml 60% (v/v) ethanol for 1 h twice. All the extracts were combined together to recycle ethanol. After removing ethanol under reduced pressure, the concentrated extract was diluted to 10 mg/ml and stored at 4°C until needed.
Cell culture
The murine macrophage-like cell line, RAW264.7, was maintained in low-glucose Dulbecco’s modified eagle medium (Gibco) supplemented with 10% fetal bovine serum (Gibco) and 80 units/ml of penicillin/streptomycin. Cells were grown in cell culture dishes and incubated at 37°C and in 5% CO2/95% air. All media should be replaced every day. When generating the 80–90% confluent layer, the cells were used for the experiments. RAW264.7 cells were stimulated for 48 h by 1 μg/ml LPS in the presence or absence of SR.
Animal model
Male Sprague-Dawley rats (250 ± 20 g of body weight) were obtained from Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). Rats used in the studies were housed in wire-mesh-bottomed cages in a 12 h light/dark cycle and kept in a room at a constant temperature of 22–24°C and a relative humidity of 60%. Food and water were provided ad libitum. The experimental procedures used in this study were in accordance with national and international laws for the use and care of laboratory animals. UC model was induced according to a previously published method.[12] A day before the experiment, the rats fasted overnight but were provided deionized water. Rats were anesthetized lightly with ether following a 24 h fast. A single intracolonic injection of 0.2 ml 5% TNBS (Sigma) in 0.25 mL of 50% ethanol was administered to rats in the model group and each treatment group through a 2 mm diameter rubber hose connected to a 1 ml syringe that was placed 8 cm proximal to the anus. Following instillation of the TNBS solution, rats were maintained in a head-down position for a few minutes to prevent leakage of the intracolonic instillation.
The rats with normal diet were used as control group. UC rats were randomly divided into five groups (n = 15/group): Model group (UC), positive control group (treated with mesalazine, 100 mg/kg),[13] high dose of SR group (200 mg/kg), medium dose of SR group (100 mg/kg), and low dose of SR group (50 mg/kg). All rats were administered daily for 15 days. On the 16th day, after 24 h fasting, plasma samples obtained via the orbital sinus were centrifuged at 3000 g, 4°C for 10 min, and the supernatants were stored at −70°C. Then, the rats were sacrificed by cervical dislocation. Distal colonic specimens were frozen in liquid nitrogen or fixed immediately in a 10% (w/v) formalin solution for further analyses.
Disease activity index
A disease activity index (DAI) was determined by scoring the extent of body weight loss, stool hemoccult positive or gross bleeding, and stool consistency at sacrifice in accordance with the method described,[14] which could response the severity of colitis.
Measurement of colonic myeloperoxidase activity
Myeloperoxidase (MPO), a marker of polymorphonuclear leukocyte accumulation and general inflammation occurring in colonic tissues, was determined as previously described.[15,16] On the 16th day, rats were sacrificed and then the colon tissue segments were homogenized in 5% EDTA/NaCl buffer (pH 4.7), centrifuged at 6000 g for 20 min at 4°C. The pellets were then resuspended in 0.5% hexadecyl trimethyl ammonium bromide buffer (pH 5.4). The suspensions were freeze–thawed for three times, heated for 2 h at 60°C to increase MPO recovery, and finally centrifuged at 6000 g for 20 min at 4°C to separate the supernatants for MPO assay. The level of MPO in the supernatant was measured using o-Dianisidine. MPO activity was assayed by measuring the change in optical density (OD) for 5 min at 690 nm, and the results were expressed as changed in OD per milligram tissue.
Immunocytochemistry analysis
For immunohistochemical analysis, rat colonic samples were removed according to the previous studies.[17] Then, the tissues were blocked in paraffin and cut to 5 μm thickness. To retrieve antigens, the sections were heated for 20 min in 10 mM sodium citrate buffer (pH 6.0). According to endogenous peroxidase, slides were incubated in hydrogen peroxide in methanol to reduce nonspecific background staining. Sequentially, tissues were boiled in citrate buffer solution for 10 min. They were cooled and then washed with phosphate buffered saline (PBS) before the application of blocking serum. Primary antibody anti-TGF-β1 (1:500) was diluted for application at the incubation of tissues, followed by the secondary antibody. An Elivison two-step method was performed for the immunohistochemical staining and photographs of slides were taken using a DM2500 optical microscope.
Total Antioxidant Capacity Assay Kit with 2,2’-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid method
The 2,2’-azino-bis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) method was applied to determine the total antioxidant capacity of SR according to the manufacturer’s protocols. ABTS working solution and SR of different concentrations (0.05, 0.1, 0.2, 0.4, 0.6, 0.8, and 1.0 mg/ml) were added into 96-well plate. The OD value was determined at 734 nm wavelength in a microplate reader after solution being incubated at room temperature for 2–6 min. Then, the standard curve was obtained, and the total antioxidant capacity of SR could be calculated conveniently.
Reactive oxygen species content assay
To evaluate intracellular ROS generation, RAW264.7 cells were probed with the redox sensitive dye 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) in a dark humidified chamber for 20 min at 37°C. At the end of the incubation, PBS was performed to wash away the free DCFH-DA molecules. The fluorescence was then quantified using an FLx800 fluorescence reader (Bio-Tek Instruments, USA) with an excitation wavelength of 485 nm and an emission wavelength of 520 nm.
Determination of catalase and glutathione peroxidase
The CAT enzymatic activity in RAW264.7 cells and the serum of UC rats was measured as previously described method.[18] The supernatant was mixed with 50 mmol/L phosphate buffer (pH 7.0) and 20 mmol/L H2O2. The enzymatic activity CAT was determined at 405 nm and expressed in terms of units/ml. The level of GSH in this study was performed by GSH-PX kit according to the manufacturer’s protocols. The absorbance of samples was determined at 412 nm at the end of reaction on a microplate reader.
Determination of superoxide dismutase and malondialdehyde
The enzymatic activity of the antioxidant enzyme SOD in cell supernatant and rat serum was measured by Kono[19] method. Superoxide anions generated hydroxylamine hydrochloride oxidation mediate nitrobluetetrazolium reduction to a blue formazan, which was then measured at 560 nm in a microplate reader. SOD inhibits nitrobluetetrazolium reduction. The extent of the inhibition was taken as a measure of SOD activity. Measurement of malondialdehyde (MDA) content by TBA reactivity was the most widely used method to assess lipid peroxidation.[20] The principle of the method is based on measurement of the absorbance of the pink color produced by the interaction of TBA with MDA at 530 nm. Values were expressed as nmol/ml.
Statistical analysis
All data in this study are expressed as means ± standard deviation from individual experiments. One-way analysis of variance was used to compare the statistical significance with the Statistical Package for the Social Sciences 13.0 (SPSS Inc, Chicago, IL) software. Statistical significance was indicated by the P value which was >0.05.
RESULTS
Effect of Scutellariae radix on 2,4,6-trinitrobenzene sulfonic acid-induced disease activity index
The DAI of UC rats was significantly increased compared to the control group. Severe rectal bleeding was observed in the model group. The treatment of high-dose SR could significantly reduce UC-induced increase in DAI of animals [Figure 1]. Rats with UC in high-dose SR group did not show any significant difference in the body weight as compared with the control group, and no rectal bleeding was observed at the end of the experiment.
Figure 1.
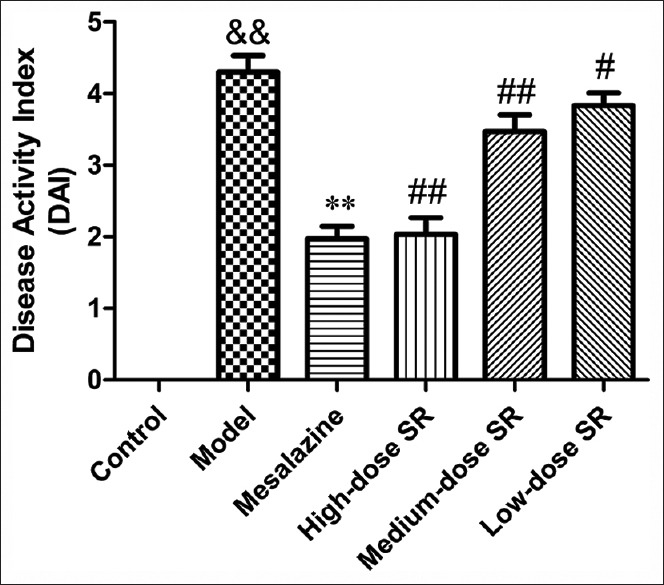
Effect of Scutellariae radix on 2,4,6-trinitrobenzene sulfonic acid-induced disease activity index in ulcerative colitis rats. Data from individual experiments are presented as means ± standard deviation (n = 6). &&P < 0.01, 2,4,6-trinitrobenzene sulfonic acid versus control group; **P < 0.01, mesalazine versus 2,4,6-trinitrobenzene sulfonic acid group; ##P < 0.01, #P < 0.05 Scutellariae radix versus 2,4,6-trinitrobenzene sulfonic acid group
Myeloperoxidase activity
The MPO activity of the colonic tissues increased in the TNBS group compared with the control blank group [Figure 2]. As expected, SR treatments caused a significant and dose-dependent inhibition of MPO activity (P < 0.05 for SR 50 mg/kg and P < 0.001 for SR 100 and 200 mg/kg vs. model rats), indicating that SR was able to control immune cells infiltration in rat colonic tissues.
Scutellariae radix downregulated transforming growth factor beta 1 protein expression in colon tissue
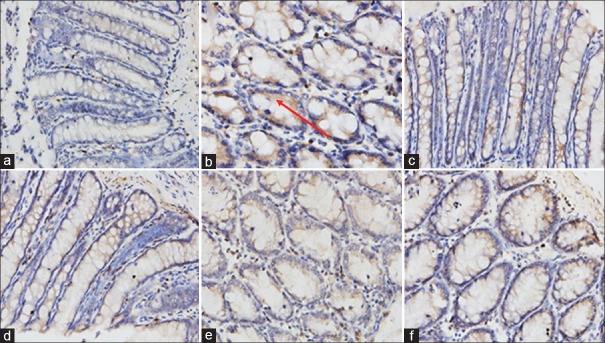
As shown in [Figure 3], the protein expression of TGF-β1 in UC model rats was dramatically elevated compared to the control group. However, after the treatment with SR or positive drug mesalazine for 15 days, the overexpression of TGF-β1 protein illustrated as brown staining was attenuated significantly in a dose-dependent manner compared with model group. The current results suggested that SR could ameliorate colonic damage by downregulating the protein expression of TGF-β1.
Figure 2.
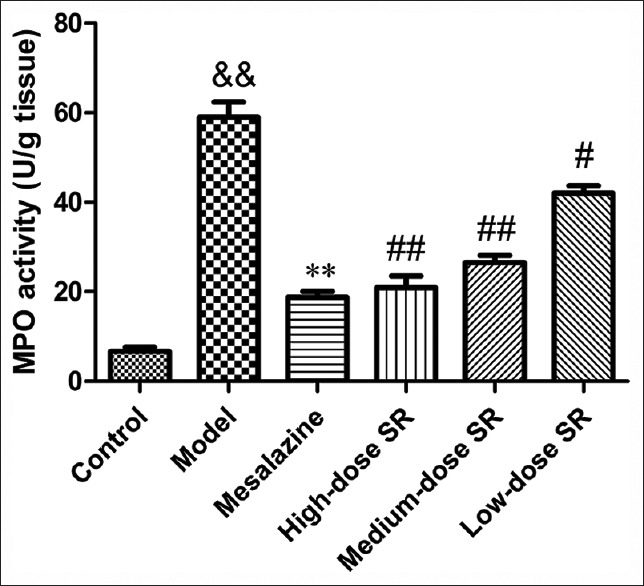
The myeloperoxidase activity in the colon tissue. Data from individual experiments are presented as means ± standard deviation (n = 6). &&P < 0.01, 2,4,6-trinitrobenzene sulfonic acid versus control group; **P < 0.01, mesalazine versus 2,4,6-trinitrobenzene sulfonic acid group; ##P < 0.01, #P < 0.05 Scutellariae radix versus 2,4,6-trinitrobenzene sulfonic acid group
Total antioxidant capacity of Scutellariae radix
The total antioxidant capacity of SR was tested using total antioxidant capacity assay kit with ABTS method to explore its antioxidant capacity. As shown in [Figure 4], the inhibitory effects of SR at the concentrations of 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, and 2.0 mg/ml were 20.01 ± 3.61%, 23.67 ± 4.16%, 46.33 ± 4.51%, 57.45 ± 5.51%, 70.13 ± 6.24, 75.33 ± 5.54, and 80.62 ± 5.69%, respectively. The IC50 of SR on inhibition of ABTS + generation was 0.258 mg/ml. These findings demonstrated that SR had a great antioxidant capacity.
Figure 3.
The downregulation of Scutellariae radix on 2,4,6-trinitrobenzene sulfonic acid-induced transforming growth factor beta 1 protein expression in colonic tissues. (a) Control group; (b) model group (2,4,6-trinitrobenzene sulfonic acid); (c) mesalazine group; (d) Scutellariae radix (high-dose, 200 mg/kg); (e) Scutellariae radix (medium-dose, 100 mg/kg); (f) Scutellariae radix (low-dose, 50 mg/kg)
Scutellariae radix attenuated lipopolysaccharide-induced reactive oxygen species generation in RAW264.7 cell
ROS were the most important biomarkers of OS. Increased concentrations of oxidizing agents (typically ROS) provided evidence of OS. As depicted in Figure 5, the fluorescence intensity in RAW264.7 cell was significantly enhanced after the exposure to LPS compared to bovine serum albumin (BSA). However, the over generation of ROS was reduced markedly by the treatment with SR. Our findings demonstrated that SR could attenuate LPS-induced intracellular ROS over generation in RAW264.7 cell.
Figure 4.
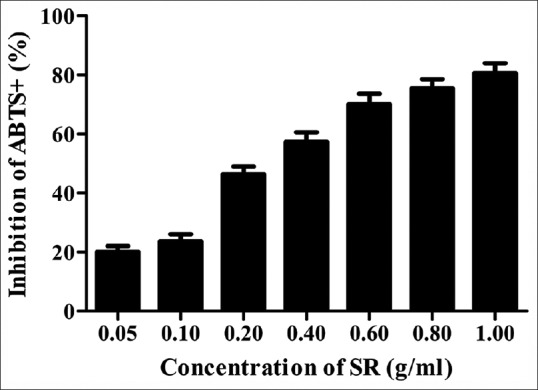
The inhibition of Scutellariae radix on 2,2’-azino-bis- 3-ethylbenzthiazoline-6-sulfonic acid + generation. Data from individual experiments are presented as means ± standard deviation (n = 3)
Scutellariae radix increased catalase and glutathione activity in RAW264.7 cell and the serum of ulcerative colitis rats
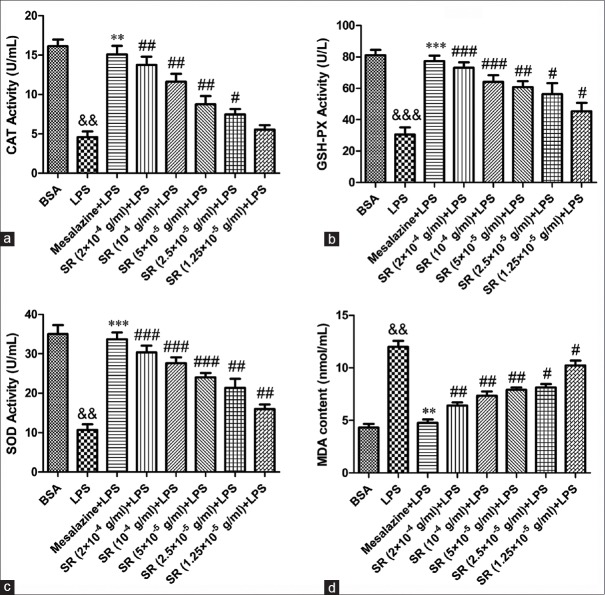
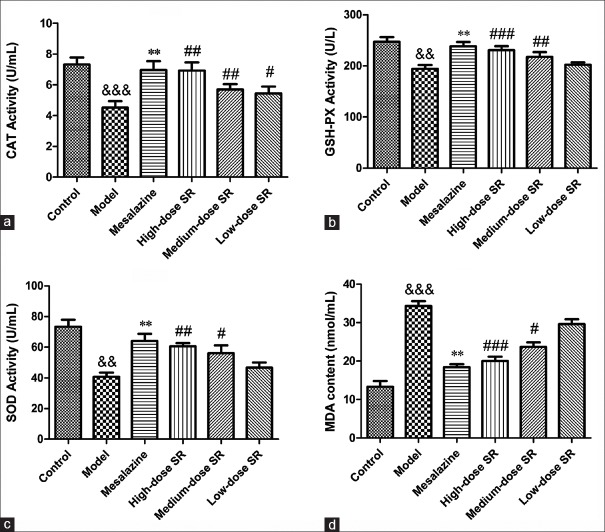
The antioxidant activity of SR on OS for colon injury was evaluated by the levels of CAT and GSH-PX in RAW264.7 cell and the serum of UC rats. As shown in Figures 6a and b, 7a and b, the activities of CAT and GSH-PX in cells or rats serum were decreased markedly by exposure to LPS or TNBS (P < 0.01, P < 0.001). However, positive drug mesalazine (100 mg/kg) could increase the activity of antioxidant enzymes. As expected, the treatment with SR (2 × 10−4 g/ml, 1.0 × 10−4 g/ml, 5.0 × 10−5 g/ml, 2.5 × 10−5 g/ml, and 1.25 × 10−5 g/ml for cell while 50 mg/kg, 100 mg/kg, and 200 mg/kg for rats) significantly increased CAT and GSH-PX activities compared with model group. These data suggested that SR protected RAW264.7 cell and UC rats from colon injury by attenuating oxidative damage.
Figure 5.
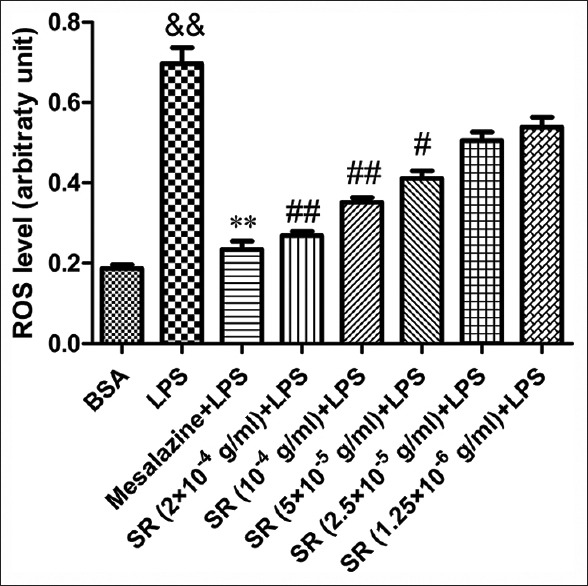
Attenuation of Scutellariae radix on lipopolysaccharide-induced reactive oxygen species generation in RAW264.7 cells. Data from individual experiments are presented as means ± standard deviation (n = 3). &&P < 0.01, lipopolysaccharide versus bovine serum albumin; **P < 0.01, mesalazine versus lipopolysaccharide group; ##P < 0.01, #P < 0.05 Scutellariae radix versus lipopolysaccharide group
Figure 6.
Effect of Scutellariae radix on lipopolysaccharide-induced oxidant stress in RAW264.7 cells. (a) The effect of Scutellariae radix on lipopolysaccharide-induced catalase activity; (b) the effect of Scutellariae radix on lipopolysaccharide-induced glutathione peroxidase activity; (c) the effect of Scutellariae radix on lipopolysaccharide-induced superoxide dismutase activity; (d) the effect of Scutellariae radix on lipopolysaccharide-induced malondialdehyde content. &&&P < 0.001 and &&P < 0.01, lipopolysaccharide versus bovine serum albumin; ***P < 0.001 and **P < 0.01, mesalazine versus lipopolysaccharide group; ###P < 0.001, ##P < 0.01 and #P < 0.05 Scutellariae radix versus lipopolysaccharide group. Data from individual experiments are presented as means ± standard deviation (n = 3)
Figure 7.
Effect of Scutellariae radix on 2,4,6-trinitrobenzene sulfonic acid-induced oxidant stress in ulcerative colitis rats. (a) The effect of Scutellariae radix on 2,4,6-trinitrobenzene sulfonic acid-induced catalase activity; (b) the effect of Scutellariae radix on 2,4,6-trinitrobenzene sulfonic acid-induced glutathione peroxidase activity; (c) the effect of Scutellariae radix on 2,4,6-trinitrobenzene sulfonic acid-induced superoxide dismutase activity; (d) the effect of Scutellariae radix on 2,4,6-trinitrobenzene sulfonic acid-induced malondialdehyde content. &&&P < 0.001 and &&P < 0.01, model group versus control group; **P < 0.01, mesalazine versus model group; ###P < 0.001, ##P < 0.01, and #P < 0.05 Scutellariae radix versus model group. Data from individual experiments are presented as means ± standard deviation (n = 6)
Scutellariae radix increased superoxide dismutase activity and decreased malondialdehyde level in RAW264.7 cell and the serum of ulcerative colitis rats
In the present study, the SOD activity and MDA level in cell supernatant and the serum of UC rats were evaluated. As shown in Figures 6c and d, 7c and d, the SOD activity was decreased significantly by 1 µg/ml LPS in vitro or 0.2 ml 5% TNBS in vivo while MDA content was increased (P < 0.01, vs. BSA; P < 0.001, vs. control). However, the treatment with SR could significantly enhance SOD activity while markedly reduce MDA level in RAW264.7 cell as well as in the serum of UC rats in a concentration-dependent manner. The results indicated that SR had a potential capacity on attenuating LPS- or TNBS-induced oxidant damage for colon injury both in vivo and in vitro.
DISCUSSION
It has been well known that TNBS might break the antioxidant defense system through regulating the activity of antioxidant enzymes, such as CAT and GSH-PX. In fact, CAT and GSH-PX, two important antioxidant enzymes, played a vital role in the antioxidant defense system in UC. Many studies have shown that CAT and GSH-PX are able to enhance the oxidation resistance as the main biochemical target.[3,20,21,22] They could also maintain the low steady-state concentration of ROS.[23] In the current study, CAT and GSH-PX activity in RAW264.7 cell and the serum of UC rats were conducted to assess the antioxidant activity of SR on OS for colon injury. The results demonstrated that SR could increase CAT and GSH-PX activities not only in RAW264.7 cells but also in serum of UC rats. SR had a potential capacity on attenuating LPS- or TNBS-induced oxidant damage for colon injury both in vivo and in vitro. These data suggested that SR protected RAW264.7 cell and UC rats from colon injury by attenuating oxidative damage.
Increased levels of free radicals were found in colonic tissue specimens of patients with UC.[24,25] SOD, the cytoprotective antioxidant enzyme, played a major role in the organism defense against excess free radicals generated under disease conditions.[18] The overexpression of SOD exclusively appeared at the accumulation of LPS-induced ROS.[26] The SOD activity in RAW264.7 cell supernatant and the serum of UC rats were evaluated in the current study. The results showed that the treatment with SR could attenuate LPS-induced oxidative damage in RAW264.7 cell and TNBS-induced oxidative damage in UC rats through increasing SOD activity. MDA was a typical biomarker used in the evaluation of injury due to lipid peroxidation, which represented the most frequent injury resulting from the activation of ROS.[27,28] Contradictory results were present in literature with respect to plasma lipid peroxidation in UC patients. A study by Bhaskar et al.[29] showed that the MDA levels in plasma were similar between UC patients and controls. Durak et al.[30] had demonstrated that MDA levels in UC patients were significantly lower compared to controls, showing that mucosa was not under OS and that the defense mechanism was not reduced. However, we observed in the present study that the OS generated in LPS-induced RAW264.7 cells and TNBS-induced UC rats produced oxidative damage as demonstrated by an increase in lipid peroxidation (MDA formation). The protective effect of SR on pathological changes of colon injury in UC might be associated with its function on oxidant damage.
OS induces the expression and secretion of TGF-β (containing TGF-β1, TGF-β2, etc.).[31] TGF-β, a biological switch, has been found to affect adhesion, differentiation and OS of cells, and cell cycle.[32] Growing evidence indicated that TGF-β was highly expressed in patients or rats of UC, and it usually played a considerable role in the modulation of the intestinal immune system.[33] The reduction of TGF-β expression could significantly regulate OS. In the current study, we choose to study the expression of TGF-β1 on UC model rats. Finally, we could observe that the regulation of SR on OS had a protective effect on colon injury in UC through TNBS-induced UC in vivo. Our experimental results suggested that SR could ameliorate colon damage via downregulating the protein expression of TGF-β1.
CONCLUSION
Overall, the extract of SR could protect the colon via decreasing DAI, MPO activity, and fibrosis-related factor TGF-β1 expression in UC rats. Moreover, SR could attenuate OS for colon injury in LPS-induced RAW264.7 cells and TNBS-induced UC rats through increasing the activities of CAT, GSH-PX, and SOD and decreasing the level of MDA. Our present study demonstrated that the protective effect of SR on colon injury in UC was associated with its antioxidant activity. Thus, SR might be a beneficial agent for the prevention and treatment of colon injury.
Financial support and sponsorship
This project was funded by the Science and Technology Program of Liaoning Province (2013225303).
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Changqing Zheng
Changqing Zheng, is a professor at the department of gastroenterology, Shengjing Hospital affiliated to China Medical University, Shenyang city, Liaoning Province. His main research field is the pathogenesis and treatment of inflammatory bowel disease. In addition, he is also interested in the area of gastrointestinal hormone, colorectal cancer.
REFERENCES
- 1.Sartor RB. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 2.Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11:9–20. doi: 10.1038/nri2891. [DOI] [PubMed] [Google Scholar]
- 3.Ghatule RR, Gautam MK, Goel S, Singh A, Joshi VK, Goel RK. Protective effects of Aegle marmelos fruit pulp on 2,4,6-trinitrobenzene sulfonic acid-induced experimental colitis. Pharmacogn Mag. 2014;10(Suppl 1):S147–52. doi: 10.4103/0973-1296.127366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou CL, Zhang J, Liu XT, Liu H, Zeng XF, Qiao SY. Superoxide dismutase recombinant Lactobacillus fermentum ameliorates intestinal oxidative stress through inhibiting NF-jB activation in a trinitrobenzene sulphonic acid-induced colitis mouse model. J Appl Microbiol. 2014;116:1621–31. doi: 10.1111/jam.12461. [DOI] [PubMed] [Google Scholar]
- 5.Jena G, Trivedi PP, Sandala B. Oxidative stress in ulcerative colitis: An old concept but a new concern. Free Radic Res. 2012;46:1339–45. doi: 10.3109/10715762.2012.717692. [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Palacián A, del Castillo G, Suárez-Causado A, García-Álvaro M, de Morena-Frutos D, Fernández M, et al. Mouse hepatic oval cells require met-dependent PI3K to impair TGF-β-induced oxidative stress and apoptosis. PLoS One. 2013;8:e53108. doi: 10.1371/journal.pone.0053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swidsinski A, Ladhoff A, Pernthaler A, Swidsinski S, Loening-Baucke V, Ortner M, et al. Mucosal flora in inflammatory bowel disease. Gastroenterology. 2002;122:44–54. doi: 10.1053/gast.2002.30294. [DOI] [PubMed] [Google Scholar]
- 8.Ardizzone S, Bollani S, Manzionna G, Bianchi Porro G. Inflammatory bowel disease approaching the 3rd millennium: Pathogenesis and therapeutic implications? Eur J Gastroenterol Hepatol. 1999;11:27–32. doi: 10.1097/00042737-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Wojdyło A, Oszmiański J. Influence of polyphenols isolated from Scutellaria baicalensis Georgi and Crataegus oxyacantha on the oxidative stability of cholesterol in butter stored in various conditions. Eur Food Res Technol. 2007;224:635–42. [Google Scholar]
- 10.Wang H, Hui KM, Xu S, Chen Y, Wong JT, Xue H. Two flavones from Scutellaria baicalensis Georgi and their binding affinities to the benzodiazepine site of the GABAA receptor complex. Pharmazie. 2002;57:857–8. [PubMed] [Google Scholar]
- 11.Shao ZH, Vanden Hoek TL, Li CQ, Schumacker PT, Becker LB, Chan KC, et al. Synergistic effect of Scutellaria baicalensis and grape seed proanthocyanidins on scavenging reactive oxygen species in vitro . Am J Chin Med. 2004;32:89–95. doi: 10.1142/S0192415X04001722. [DOI] [PubMed] [Google Scholar]
- 12.Cui L, Feng L, Zhang ZH, Jia XB. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-κB pathway activation. Int Immunopharmacol. 2014;23:294–303. doi: 10.1016/j.intimp.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Deng X, Tolstanova G, Khomenko T, Chen L, Tarnawski A, Szabo S, et al. Mesalamine restores angiogenic balance in experimental ulcerative colitis by reducing expression of endostatin and angiostatin: Novel molecular mechanism for therapeutic action of mesalamine. J Pharmacol Exp Ther. 2009;331:1071–8. doi: 10.1124/jpet.109.158022. [DOI] [PubMed] [Google Scholar]
- 14.Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–34. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 15.Bento AF, Leite DF, Claudino RF, Hara DB, Leal PC, Calixto JB. The selective nonpeptide CXCR2 antagonist SB225002 ameliorates acute experimental colitis in mice. J Leukoc Biol. 2008;84:1213–21. doi: 10.1189/jlb.0408231. [DOI] [PubMed] [Google Scholar]
- 16.Schierwagen C, Bylund-Fellenius AC, Lundberg C. Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Methods. 1990;23:179–86. doi: 10.1016/0160-5402(90)90061-o. [DOI] [PubMed] [Google Scholar]
- 17.Fan H, Liu XX, Zhang LJ, Hu H, Tang Q, Duan XY, et al. Intervention effects of QRZSLXF, a Chinese medicinal herb recipe, on the DOR-ß-arrestin1-Bcl2 signal transduction pathway in a rat model of ulcerative colitis. J Ethnopharmacol. 2014;154:88–97. doi: 10.1016/j.jep.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Aleisa AM, Al-Rejaie SS, Abuohashish HM, Ola MS, Parmar MY, Ahmed MM. Pretreatment of Gymnema sylvestre revealed the protection against acetic acid-induced ulcerative colitis in rats. BMC Complement Altern Med. 2014;14:49. doi: 10.1186/1472-6882-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 20.Akcan A, Muhtaroglu S, Akgun H, Akyildiz H, Kucuk C, Sozuer E, et al. Ameliorative effects of bombesin and neurotensin on trinitrobenzene sulphonic acid-induced colitis, oxidative damage and apoptosis in rats. World J Gastroenterol. 2008;14:1222–30. doi: 10.3748/wjg.14.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ek RO, Serter M, Ergin K, Cecen S, Unsal C, Yildiz Y, et al. Protective effects of citicoline on TNBS-induced experimental colitis in rats. Int J Clin Exp Med. 2014;7:989–97. [PMC free article] [PubMed] [Google Scholar]
- 22.Arab HH, Al-Shorbagy MY, Abdallah DM, Nassar NN. Telmisartan attenuates colon inflammation, oxidative perturbations and apoptosis in a rat model of experimental inflammatory bowel disease. PLoS One. 2014;9:e97193. doi: 10.1371/journal.pone.0097193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zamocky M, Furtmüller PG, Obinger C. Evolution of catalases from bacteria to humans. Antioxid Redox Signal. 2008;10:1527–48. doi: 10.1089/ars.2008.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bitiren M, Karakilcik AZ, Zerin M, Ozardali I, Selek S, Nazligül Y, et al. Protective effects of selenium and Vitamin E combination on experimental colitis in blood plasma and colon of rats. Biol Trace Elem Res. 2010;136:87–95. doi: 10.1007/s12011-009-8518-3. [DOI] [PubMed] [Google Scholar]
- 25.Ademoglu E, Erbil Y, Tam B, Barbaros U, Ilhan E, Olgac V, et al. Do Vitamin E and selenium have beneficial effects on trinitrobenzenesulfonic acid-induced experimental colitis. Dig Dis Sci. 2004;49:102–8. doi: 10.1023/b:ddas.0000011610.47179.0b. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Fang L, Cai R, Zong C, Chen X, Lu J, et al. Shuang-Huang-Lian exerts anti-inflammatory and anti-oxidative activities in lipopolysaccharide-stimulated murine alveolar macrophages. Phytomedicine. 2014;21:461–9. doi: 10.1016/j.phymed.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Aikemu A, Yusup A, Umar A, Berké B, Moore N, Upur H. The impact of the Uighur medicine abnormal savda munziq on antitumor and antioxidant activity in a S180 and Ehrlich ascites carcinoma mouse tumor model. Pharmacogn Mag. 2012;8:141–8. doi: 10.4103/0973-1296.96568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia H, Liu JW, Ufur H, He GS, Liqian H, Chen P. The antihypertensive effect of ethyl acetate extract from red raspberry fruit in hypertensive rats. Pharmacogn Mag. 2011;7:19–24. doi: 10.4103/0973-1296.75885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhaskar L, Ramakrishna BS, Balasubramanian KA. Colonic mucosal antioxidant enzymes and lipid peroxide levels in normal subjects and patients with ulcerative colitis. J Gastroenterol Hepatol. 1995;10:140–3. doi: 10.1111/j.1440-1746.1995.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 30.Durak I, Yasa MH, Bektas A, Kaçmaz M, Cimen MY, Oztürk HS. Mucosal antioxidant defense is not impaired in ulcerative colitis. Hepatogastroenterology. 2000;47:1015–7. [PubMed] [Google Scholar]
- 31.Montorfano I, Becerra A, Cerro R, Echeverría C, Sáez E, Morales MG, et al. Oxidative stress mediates the conversion of endothelial cells into myofibroblasts via a TGF-ß1 and TGF-ß2-dependent pathway. Lab Invest. 2014;94:1068–82. doi: 10.1038/labinvest.2014.100. [DOI] [PubMed] [Google Scholar]
- 32.Liu RM, Gaston Pravia KA. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic Biol Med. 2010;48:1–15. doi: 10.1016/j.freeradbiomed.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko JK, Auyeung KK. Inflammatory bowel disease: Etiology, pathogenesis and current therapy. Curr Pharm Des. 2014;20:1082–96. doi: 10.2174/13816128113199990416. [DOI] [PubMed] [Google Scholar]