Abstract
Background:
Herpes simplex virus type 1 (HSV-1) is associated with orofacial infections and is transmitted by direct contact with infected secretions. Several efforts have been expended in the search for drugs to the treatment for herpes. Schinus terebinthifolius is used in several illnesses and among them, for the topical treatment of skin wounds, especially wounds of mucous membranes, whether infected or not.
Objective:
To evaluate the cytotoxicity and anti-HSV-1 activity of the crude hydroethanolic extract (CHE) from the stem bark of S. terebinthifolius, as well as its fractions and isolated compounds.
Materials and Methods:
The CHE was subjected to bioguided fractionation. The anti-HSV-1 activity and the cytotoxicity of the CHE, its fractions, and isolated compounds were evaluated in vitro by SRB method. A preliminar investigation of the action of CHE in the virus–host interaction was conducted by the same assay.
Results:
CHE presented flavan-3-ols and showed anti-HSV-1 activity, better than its fractions and isolated compounds. The class of substances found in CHE can bind to proteins to form unstable complexes and enveloped viruses, as HSV-1 may be vulnerable to this action. Our results suggest that the CHE interfered with virion envelope structures, masking viral receptors that are necessary for adsorption or entry into host cells.
Conclusion:
The plant investigated exhibited potential for future development treatment against HSV-1, but further tests are necessary, especially to elucidate the mechanism of action of CHE, as well as preclinical and clinical studies to confirm its safety and efficacy.
SUMMARY
Crude hydroethanolic extract (CHE) presents promising activity against herpes simplex virus type 1 (HSV 1), with selectivity index (SI) = 22.50
CHE has flavan-3-ols in its composition, such as catechin and gallocatechin
The fractions and isolated compounds obtained from CHE by bioguided fractionation are less active than the CHE against HSV-1
CHE interferes with viral entry process in the host cell and acts directly on the viral particle.
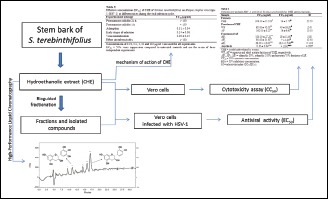
Abbreviations used: HSV: Herpes simplex virus, CHE: Crude hydroethanolic extract, WF: Water fraction, AF: Ethyl-acetate fraction, MPLC: Medium-performance liquid chromatography, TLC: Thin-layer chromatography, NMR: Nuclear magnetic resonance, ESI-MS: Electrospray ionization mass spectrometry, SRB: Sulforhodamine B, CPE: Cytopathic effect, CC50: 50% cytotoxic concentration, EC50: 50% effective concentration, PBS: Phosphate-buffered saline.
Keywords: Antiviral activity, Aroeira, cytotoxicity, herpes simplex virus, herpes simplex virus type 1, Schinus terebinthifolius Raddi
INTRODUCTION
Herpes simplex virus type 1 (HSV-1) is a common human pathogen associated with orofacial infections and encephalitis.[1] Antiviral agents currently licensed for the treatment of herpes virus infections include acyclovir and its derivatives, foscarnet and cidofovir.[2] The toxic side effects and the emergence of virus strains that are resistant to these drugs, enhance the need for new effective compounds against viral infectious diseases.[3,4]
A previous screening study evaluated the effects of plant extracts against HSV-1, in which the effects of Schinus terebinthifolius Raddi were notable.[5] S. terebinthifolius (Anacardiaceae), popularly known as “Aroeira,” “Aroeira da Praia,” and “Aroeira Pimenteira” is distributed from the Northeast to the South of the Brazilian coast.[6] Many studies have experimentally confirmed that this plant has antimicrobial, anti-inflammatory, and anti-ulcerogenic properties.[7,8,9,10] The extract of stem bark from S. terebinthifolius is widely used by the Northeastern Brazilian population for the topical treatment of skin wounds, especially wounds of mucous membranes, whether infected or not, in the cases of inflammation of the gums and throat in the form of gargle and mouthwash.[11] Several factors can lead to skin and mucous lesions, including infections by Candida albicans and HSV-1. Because of the difficulties with diagnoses and because sometimes it is the only treatment available, some people use this plant for any injury they sustain, even without knowing its cause. Despite the traditional uses and various biological studies, few phytochemical and antiviral studies of this plant have been performed.
MATERIALS AND METHODS
Plant material and extract preparation
Stem bark from S. terebinthifolius was collected at Universidade Estadual de Maringá (UEM) Campus, Maringá, Paraná, Brazil, of which voucher specimen had been deposited in the herbarium of the UEM (HUEM #22057) that was previously identified by Prof. Dr. Maria Auxiliadora Milaneze Gutierre.
The bark was dried at room temperature and then pulverized with a hammer mill (Tigre ASN-5). The crude hydroethanolic extract (CHE) was obtained by the turbo-extraction (Ultra-turrax, model UTC115KT, Wilmington, NC, USA) of 500 g of the powder barks with 70% ethanol (5000 ml) in water for 10 min, controlling the temperature so that it does not exceed 40°C.[12] The solvents were evaporated in a Rotavapor® (Büchi R-153, Switzerland) under reduced pressure and lyophilized to yield CHE (78.71 g; 15.7%).
Isolation and identification of compounds
The CHE was subjected to bioguided fractionation. The CHE (70 g) was resuspended in water (700 ml) and partitioned with ethyl acetate (700 ml) to obtain a water fraction (WF) and ethyl-acetate fraction (AF). The AF (4.50 g) was chromatographed on a Sephadex® LH-20 column (300 mm × 22 mm) using the sequence of the eluent system (v/v; 50% ethanol, 100% ethanol, and 70% acetone), yielding three fractions: AF1 (2.38 g), AF2 (0.29 g), and AF3 (0.64 g). Each fraction was assayed for cytotoxic and antiviral activity. Based on the biological results and yield, the active fraction AF1 (1.40 g) was subjected to medium-performance liquid chromatography (MPLC) using a reverse-phase C18 silica gel column (Bulk Media, Sepra™ C18-E, 50 µm, 65 Å, h = 340 mm, Ø =30 mm) coupled to a Waters 510 pump and eluted with methanol/water (40:60, 50:50, 60:40, 70:30, 80:20, 90:10, and 100:0; v/v). The obtained fractions were monitored by thin-layer chromatography (TLC), and similar fractions were combined, yielding 16 fractions (AF1-1 to AF1-16). Fraction AF1-1 was purified by MPLC (Sepra™ C18-E column, 420 mm × 10 mm) using the same conditions to obtain five fractions (AF1-1A, AF1-1B, AF1-1C, AF1-D, and AF1-1E). Fraction AF1-1C was chromatographed on silica gel 60 (230–400 mesh) with chloroform/methanol (80:20, 75:25, and 70:30; v/v) as the mobile phase. The fractions were monitored by TLC, and similar fractions were combined to yield two pure compounds: Gallocatechin (1) (15.00 mg) and catechin (2) (20.00 mg). The isolated compounds were identified by nuclear magnetic resonance (NMR) spectrometry (Varian Mercury Plus 300 [7.02T] operating at 75 MHz for 13C and 300 MHz for 1H and 2D NMR) and electrospray ionization mass spectrometry (Waters-Micromass® Quattro LC, Manchester, England), and the data were compared with the literature.
High-performance liquid chromatographic profile
CHE 10 μl was analyzed using an Thermo® HPLC system equipped with a photo diode array (PDA) spectrophotometric detector module (Model Finnigan Surveyor PDA Plus Detector), and a C18 Gemini Phenomenex column (250 mm × 4.6 mm), 5 µm particle size, and a guard column (Phenomenex) were used for separation throughout this study, with a flow rate of 0.6 ml/min. Mobile phases were water ultrapure (Milli-Q®, Millipore, USA) with 0.05% trifluoroacetic acid (TFA) (solvent A) and acetonitrile with 0.05% TFA (solvent B) with the detector set at 210 and 280 nm. The gradient system was 0 min (8% B) and 25 min (25% B), returns to 8% B at 27 min, and remains at 8% B for 5 min. The sample was prepared in a 500 µg/ml solution and filtered with a 0.22 µm Millipore® filter. A reference standard (catechin, Sigma, Lot #31K2512; gallocatechin, 95% purity obtained from Stryphnodendron adstringens [Mart.] Coville at the Palafito, UEM) was used to identify the isolated component in the crude extracts.
Cells and viruses
Vero (African green monkey kidney) cells (ATCC CCL-81) were cultured and maintained in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco) and 50 µg/ml gentamycin in an incubator set at 37°C, with 5% CO2 and 95% relative humidity. HSV-1 (clinical strain kindly provided by Virology Department of University State of Londrina) stocks were propagated in Vero cells and titrated. The virus was then stored at −20°C until use.
Cytotoxicity assay
Vero cells were seeded in 96-well tissue plates at a density of 2.5 × 105 cell/ml in 100 µl medium and incubated in a humid atmosphere with 5% CO2 at 37°C until a confluent monolayer formed. Different concentrations of samples (CHE, its fractions and isolated compounds, and acyclovir) were added to the wells. A control that used cells without the addition of any sample was also included. The plate was incubated in a humid chamber at 37°C with 5% CO2 for 72 h. Viable cells were detected using the sulforhodamine B (SRB) colorimetric method.[13] Data were used to calculate the 50% cytotoxic concentration (CC50) (the concentration that is able to destroy 50% of the cells).
Assays for antiviral activity
The Vero cells were seeded in 96-well tissue plates at a density of 2.5 × 105 cell/ml in 100 µl medium for 24 h. After, the cells were infected with 25 μl of viral suspension TCID 80 for 1 h at 37°C and various concentrations of the test samples (CHE, its fractions and isolated compounds, and acyclovir) were added per well. The cells were incubated for 72 h in a humid atmosphere with 5% CO2 at 37°C. Cell control and virus control were also performed. Acyclovir was used as positive control. The viable cells were detected using the SRB assay.[13] The percentage of inhibition of virus-induced cytopathic effect (CPE) is expressed as a percentage of the optical density of the test sample compared with untreated virus-infected cells.[14] The concentration that reduced 50% by CPE compared with the virus control was estimated from the data plots and defined as the 50% effective concentration (EC50). The tests were performed in triplicate in three independent experiments.
Mechanism of herpes simplex virus type 1 suppression induced by crude hydroethanolic extract
To investigate the steps of the virus multiplication cycle at which the CHE acts, the SRB assay was used to determine cell viability in all the experimental conditions described below. Activity was determined as a percentage of inhibition of virus-induced CPE and expressed as a percentage of the optical density of the test sample compared with untreated virus-infected cells.
The CHE was added to the cells at only certain times: Before the infection (for 1 h and 24 h), during the adsorption phase, during the early stages of infection (adsorption and penetration phases), and after infection.
The direct inactivation of HSV-1 by the CHE was also tested. For this, CHE at different concentrations (0.01, 0.10, 1.00, 10.00, and 100.00 µg/mL) were mixed with equal volumes of HSV-1 suspension (100-fold TCID80) and incubated for 1 h at 37°C. Thereafter, the mixtures were diluted 100-fold and used to infect confluent Vero cells for 1 h at 37°C. The cell monolayer was then washed with phosphate-buffered saline and further incubated in overlay medium for 72 h.
Statistical analysis
The results are presented as the mean values from three independent experiments. The values of EC50 and CC50 were obtained by dose-response curve and regression analysis of triplicate CC50, and EC50 values were analyzed by one-way ANOVA followed by Tukey test, considering P < 0.05 significant.
RESULTS
Structure elucidation
Fractionation of the crude extract from the stem bark of S. terebinthifolius yielded gallocatechin and catechin [Figure 1]. Their chemical structures were determined by comparing their NMR spectral and MS profiles with those in the literature.[15,16,17,18]
Figure 1.
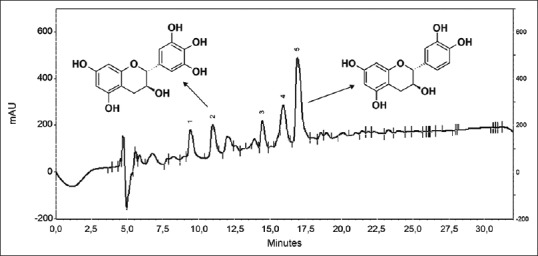
Chromatogram of crude hydroethanolic extract with gallocatechin (2; rt = 10.9 min) and catechin (5; rt = 16.9 min)
Evaluation of the CHE by HPLC-PDA showed 5 peaks that are well separated. The peaks numbered 2 (rt = 10.9 min.) and 5 (rt = 16.9 min) correspond to the substances gallocatechin and catechin, respectively [Figure 1]. The contents of gallocatechin and catechin in the CHE sample were determined as 6.96 and 55.20 mg/g. These were identified by co-injection of the sample plus their standards and quantified by adding known concentrations of the standards.
Cytotoxicity and antiviral activity
The CHE and its fractions, WF and AF, showed anti-HSV-1 activity at noncytotoxic concentrations (SI = 22.50, 2.40, and 10.00, respectively). The AF was chromatographed to yield three fractions (AF1, AF2, and AF3) and only AF1 and AF2 showed an antiviral effect (EC50 = 18.00 and 7.00 µg/ml, respectively) [Table 1].
Table 1.
Cytotoxicity and anti-HSV-1 activity of Schinus terebinthifolius CHE and its fractions
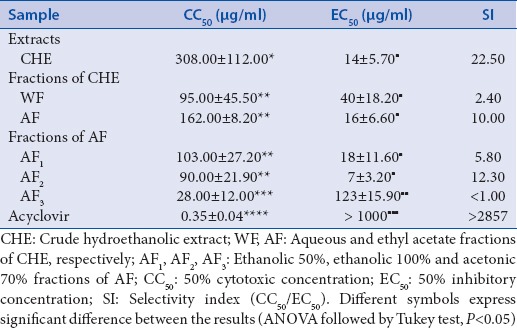
To continue the purification, the AF1 was chosen because it had a better yield than AF2. AF1 was chromatographed, yielding 10 fractions (AF1-1 to AF1-10). Of these, only AF1-1 (SI = 2.20) and AF1-3 (SI > 2.90) were active. From the AF1-1 fraction, five news fractions (AF1-1A to AF1-1E) were obtained, and only AF1-1B (SI = 3.70) and AF1-1C (SI = 3.00) were active. The AF1-1C fraction yielded the compounds gallocatechin (1) and catechin (2). Among them, only catechin exhibited anti-HSV-1 activity (EC50 = 6.50 µg/ml, SI = 3.60). Gallocatechin did not present anti-HSV-1 activity, even at the highest concentration tested.
The highest anti-herpes activity was detected for the CHE. For this reason, we decided to investigate the mechanism of anti-herpes action of this extract.
Mechanism of herpes simplex virus type 1 suppression induced by crude hydroethanolic extract
Herpes virus replication is characterized by a complex sequence of different steps with which antiviral agents might interfere. To investigate the inhibitory effects on HSV-1, the CHE was added at different stages of viral infection. Incubation of HSV-1 with the CHE in the adsorption experiment, adsorption and penetration stage experiments, and virucidal activity experiment caused a significant suppression of HSV-1 multiplication. In contrast, when the host cells were pretreated for 1 or 24 h prior to infection with CHE and when CHE was added after penetration of the virus into the host cells, the infectivity was not reduced [Table 2].
Table 2.
50% effective concentration EC50 of CHE of Schinus terebinthifolius on Herpes simplex virus type 1 (HSV-1) at different times during the viral infection cycle

DISCUSSION
The observation of popular knowledge is the most common strategy for selecting plant species that may be potentially used to treat diseases.[19] Botanical extracts exhibit a wide spectrum of biological and pharmacological properties, including cytoprotection, anticancer, anti-inflammatory, antimicrobial, and immunomodulation effects.[8,20,21,22] Such medicinal plants provide an important source for antiviral drug screening and development.[5,23,24,25] Several secondary metabolites, including flavonoids, saponins, and tannins, have been reported to present antiviral activity.[26,27]
Studies with S. terebinthifolius have shown the presence of several constituents, including terpens,[28] phenols,[29,30] tannins,[31] flavonoids,[32] anthraquinones, xanthones, and free steroids.[21] A solvent mixture (water, ethanol, and acetone) was optimized for extraction from the bark of S. terebinthifolius, and a UV-VIS spectrophotometric method was developed and validated for analysis of total polyphenols in the extract. The results demonstrated a total polyphenol content of 29.39% and an interesting antioxidant capacity (6.38 µl/ml).[24]
The present study reported the in vitro cytotoxicity and anti-HSV-1 activity of S. terebinthifolius extract, fractions, and pure compounds (gallocatechin and catechin) isolated from the stem bark of this plant used for medicinal purposes. Catechin and gallocatechin belong to the polyphenol class, which are a large and highly heterogeneous group of natural products, with the general characteristics of multiple hydroxylation of complex aromatic systems. We showed that catechin but not gallocatechin presented anti-HSV-1 activity. Despite the structural similarities between gallocatechin and catechin, the first one presents an additional hydroxyl group, which could influence its biological activity. The crude extract and one of the fractions rich in catechin obtained from coconut rusk fiber of Cocos nucifera L. showed inhibitory activity against acyclovir-resistant HSV-1.[25] These results correspond with the findings in the present study because the CHE showed to be rich in catechin, as noted by HPLC profile.
Methanolic extracts from dried Combretum micranthum G. Don (Combretaceae) leaves present antiviral activity against HSV-1 and HSV-2, and the precursors of the active compounds have been identified as condensed tannins.[33] The isolated compounds (gallocatechin and catechin) also belong to a class of condensed tannins. By analyzing the UV profile of the other peaks (data not shown), we can infer that these substances belong to the same class. Hence, we have an extract rich in tannins, confirming the findings of the literature that extracts rich in tannins present antiviral activity.[25,33]
Of the extracts, fractions and isolated compounds tested in the present study, the CHE showed the most promising results. The use of plant extracts that comprise highly complex mixtures of up to several hundred compounds in antiviral therapy is a part of multiple-target therapy. Different compounds may act against different molecular targets and inhibit viral infection more effectively than a single compound alone.[34] The results of this study suggest that the antiviral effect of the CHE is not caused by a single component. The biological effect seems to be dependent of the interaction among the different constituents.
Bioactivity-guided fractionation is important to isolate an active substance; however, this strategy may exclude compounds with relevant pharmacological activities because the effect cannot be caused by a single compound, but rather by a combination of compounds as a result of synergism or pharmacokinetic influences.[19] A propolis extract exhibited significantly higher anti-herpetic effects and higher SIs than single isolated constituents.[35] The SI of a Melissa officinalis extract against HSV was superior to the SIs of single constituents.[36] These results are in according with the findings in the present study.
To determine the mode of antiviral action of the CHE, time-of-addition experiments were performed at different steps in the herpes virus replication cycle. A significant decrease in viral infectivity was detected for HSV-1 when the viruses were treated with the CHE prior to infection and when the host cells were treated with the CHE during the adsorption and penetration phases.
Plant-derived polyphenols exhibit anti-HSV activity in many cases, mostly by influencing the early phases of virus infection.[34] Polyphenols bind to proteins to form unstable complexes[37] and enveloped viruses, among which HSVs may be vulnerable to the action of polyphenols because this class of naturally occurring substances can interact easily with the glycoproteins of the viral envelope.[38] Our results suggest that the CHE interfered with virion envelope structures, masking viral receptors that are necessary for adsorption or entry into host cells or interfering with cell structures that are related to the entry of the virus. Unknown is whether the inhibitory effect is attributable to the binding of some constituents of the extract to viral or cell proteins involved in host cell adsorption and penetration; or attributable to damage of the virions, possibly their envelopes, thereby impairing their ability to infect host cells.[39]
CONCLUSION
In summary, CHE of S. terebinthifolius presented flavan-3-ols and showed anti-HSV-1 activity, better than its fractions and isolated compounds. CHE seems to influence the viral entry process. Further experiments are required to determine the precise mechanism of action, and probably, the multiple targets of the CHE. In addition, preclinical and clinical investigations should be conducted to clarify the clinical potential of the CHE for therapeutic use in HSV-1 infections.
Financial support and sponsorship
We are grateful to National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel (CAPES), Funding Authority for Studies and Projects (FINEP), Araucaria Foundation, Central Complex for Research Support (COMCAP) and Post-graduate Program in Pharmaceutical Sciences, State University of Maringá, PR, Brazil, for financial support.
Conflicts of interest
The authors declare no conflicts of interest.
ABOUT AUTHOR

Tânia Ueda-Nakamura
Dr. Tânia Ueda-Nakamura, is an Associated Professor (Microbiology) at the Department of Health Basic Sciences, Maringá State University, Paraná, Brazil. Her research interests are the search of the antiviral and antiprotozoal activities of compounds, focused in showing the effectiveness and safety of natural products used in traditional medicine. She is also a Research Fellow of CNPq in the Pharmacy area.
Acknowledgments
The authors thank Viviane Fernandes da Silva and Ivânia Schuquel for helpful technical assistance in the biological and chemical experiments.
REFERENCES
- 1.Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–8. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 2.De Clercq E. Antiviral drugs in current clinical use. J Clin Virol. 2004;30:115–33. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Cassady KA, Whitley RJ. New therapeutic approaches to the alphaherpesvirus infections. J Antimicrob Chemother. 1997;39:119–28. doi: 10.1093/jac/39.2.119. [DOI] [PubMed] [Google Scholar]
- 4.Astani A, Reichiling J, Schnitzler P. Screening for antiviral activities of isolated compounds from essential oils. Evid Based Complement Alternat Med 2009. 2011:1–8. doi: 10.1093/ecam/nep187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moura-Costa GF, Nocchi SR, Ceole LF, de Mello JC, Nakamura CV, Dias Filho BP, et al. Antimicrobial activity of plants used as medicinals on an indigenous reserve in Rio das Cobras, Paraná, Brazil. J Ethnopharmacol. 2012;143:631–8. doi: 10.1016/j.jep.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Cavalher-Machado SC, Rosas EC, Brito Fde A, Heringe AP, de Oliveira RR, Kaplan MA, et al. The anti-allergic activity of the acetate fraction of Schinus terebinthifolius leaves in IgE induced mice paw edema and pleurisy. Int Immunopharmacol. 2008;8:1552–60. doi: 10.1016/j.intimp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Guerra MJ, Barreiro ML, Rodriguez ZM, Rubaiacaba Y. Actividad antimicrobiana de um extracto fluido al 80% de Schinus terebinthifolius Raddi (copal) Rev Cubana Plant Med. 2000;5:23–5. [Google Scholar]
- 8.Fedel Miyasato LES, Kassuya CAL, Auharek SA, Formagio ASN, Cardoso CAL, Mauro MO, et al. Evaluation of anti inflammatory, immunomodulatory, chemopreventive and wound healing potentials from Schinus terebinthifolius methanolic extract. Braz. J Pharmacog. 2014;24:565–75. [Google Scholar]
- 9.dos Santos OJ, Barros-Filho AK, Malafaia O, Ribas-Filho JM, Santos RH, Santos RA. Schinus terebinthifolius Raddi (Anacardiaceae) in the healing process of gastrorrhaphy in rats. Arq Bras Cir Dig. 2012;25:140–6. doi: 10.1590/s0102-67202012000300002. [DOI] [PubMed] [Google Scholar]
- 10.Alves LA, Freires Ide A, Pereira TM, de Souza A, Lima Ede O, de Castro RD. Effect of Schinus terebinthifolius on Candida albicans growth kinetics, cell wall formation and micromorphology. Acta Odontol Scand. 2013;71:965–71. doi: 10.3109/00016357.2012.741694. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzi H, Matos FJ. Plantas Medicinais no Brasil: Nativas e Exóticas. Nova Odessa: SP: Instituto Plantarum; 2002. [Google Scholar]
- 12.Voigt R. Berlin: Ullstein Mosby; 1993. Pharmazeutische Technologie. 7th ed; p. 540. [Google Scholar]
- 13.Camargo Filho I, Cortez DA, Ueda-Nakamura T, Nakamura CV, Dias Filho BP. Antiviral activity and mode of action of a peptide isolated from Sorghum bicolor. Phytomedicine. 2008;15:202–8. doi: 10.1016/j.phymed.2007.07.059. [DOI] [PubMed] [Google Scholar]
- 14.Semple SJ, Pyke SM, Reynolds GD, Flower RL. In vitro antiviral activity of the anthraquinone chrysophanic acid against poliovirus. Antiviral Res. 2001;49:169–78. doi: 10.1016/s0166-3542(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal PK, Bansal MC. Carbon-13 NMR of flavonoids. In: Agrawal PK, editor. Studies in Organic Chemistry Series. 39 Amsterdam: Elsevier Science Publishers; 1989. p. 192. [Google Scholar]
- 16.Harborne JB. London: Chapman and Hall; 19966. The Flavonoids: Advances in Research Since 1986. [Google Scholar]
- 17.Foo LY, Lu Y, McNabb WC, Waghorn G, Ulyatt MJ. Proanthocyanidins from Lotus pedunculatus. Phytochemistry. 1997;45:1689–96. [Google Scholar]
- 18.Antonelli-Ushirobira TA, Yamaguti E, Uemura LM, Nakamura CV, Dias-Filho BP, Pallazzo de Mello JC. Chemical and microbiological study of extract from seeds of Guaraná (Paullinia cupana var. sorbilis) Lat Am J Pharm. 2007;26:5–9. [Google Scholar]
- 19.Rates SM. Plants as source of drugs. Toxicon. 2001;39:603–13. doi: 10.1016/s0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 20.Patwardhan B, Gautam M. Botanical immunodrugs: Scope and opportunities. Drug Discov Today. 2005;10:495–502. doi: 10.1016/S1359-6446(04)03357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lima MR, Luna JS, Santos AF, Andrade MC, Sant’ana AE, Genet JP, et al. Anti-bacterial activity of some Brazilian medicinal plants. J Ethnopharmacol. 2006;105:137–47. doi: 10.1016/j.jep.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Gomes FS, Procópio TF, Napoleão TH, Coelho LC, Paiva PM. Antimicrobial lectin from Schinus terebinthifolius leaf. J Appl Microbiol. 2013;114:672–9. doi: 10.1111/jam.12086. [DOI] [PubMed] [Google Scholar]
- 23.Fritz D, Venturi CR, Cargnin S, Schripsema J, Roehe PM, Montanha JA, et al. Herpes virus inhibitory substances from Hypericum connatum Lam. a plant used in southern Brazil to treat oral lesions. J Ethnopharmacol. 2007;113:517–20. doi: 10.1016/j.jep.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 24.DiCiaula MC, Lopes GC, Scarminio IS, Mello JC. Optimization of solvent mixtures for extraction from bark of Schinus terebinthifolius by a statistical mixture-design technique and development of a UV-VIS spectrophotometric method for analysis of total polyphenols in the extract. Quíica Nova. 2014;37:158–63. [Google Scholar]
- 25.Esquenazi D, Wigg MD, Miranda MM, Rodrigues HM, Tostes JB, Rozental S, et al. Antimicrobial and antiviral activities of polyphenolics from Cocos nucifera Linn.(Palmae) husk fiber extract. Res Microbiol. 2002;153:647–52. doi: 10.1016/s0923-2508(02)01377-3. [DOI] [PubMed] [Google Scholar]
- 26.Khan MT, Ather A, Thompson KD, Gambari R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antiviral Res. 2005;67:107–19. doi: 10.1016/j.antiviral.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Chattopadhyay D, Khan MT. Ethnomedicines and ethnomedicinal phytophores against herpesviruses. Biotechnol Annu Rev. 2008;14:297–348. doi: 10.1016/S1387-2656(08)00012-4. [DOI] [PubMed] [Google Scholar]
- 28.Campelo J, Marsaioli AJ. Terebinthifolic acid and bauerenone: New triterpenoid ketones from Schinus terebinthifolius. Phytochemistry. 1975;14:2300–2. [Google Scholar]
- 29.Ceruks M, Romoff P, Fávero OA, Lago JH. Constituintes fenólicos polares de Schinus terebinthifolius Raddi (Anacardiaceae) Quíica Nova. 2007;30:597–9. [Google Scholar]
- 30.Wood CT, Schlindwein CC, Soares GL, Araujo PB. Feeding rates of Balloniscus sellowii (Crustacea, Isopoda, Oniscidea): The effect of leaf litter decomposition and its relation to the phenolic and flavonoid content. Zookeys. 2012;176:231–45. doi: 10.3897/zookeys.176.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorge LI, Markmann BE. Exame químico e microscópico de Schinus terebinthifolius Raddi (Aroeira) Rev Ciê Farm. 1996;17:139–45. [Google Scholar]
- 32.Kassem ME, El-Desoky SK, Sharaf M. Bifenyl esters and biflavonoids from the fruits of Schinus terebinthifolius. Chem Nat Compd. 2004;40:447–50. [Google Scholar]
- 33.Ferrea G, Canessa A, Sampietro F, Cruciani M, Romussi G, Bassetti D. In vitro activity of a Combretum micranthum extract against herpes simplex virus types 1 and 2. Antiviral Res. 1993;21:317–25. doi: 10.1016/0166-3542(93)90010-g. [DOI] [PubMed] [Google Scholar]
- 34.Gescher K, Hensel A, Hafezi W, Derksen A, Kühn J. Oligomeric proanthocyanidins from Rumex acetosa L. inhibit the attachment of herpes simplex virus type-1. Antiviral Res. 2011;89:9–18. doi: 10.1016/j.antiviral.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Schnitzler P, Neuner A, Nolkemper S, Zundel C, Nowack H, Sensch KH, et al. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother Res. 2010;24(Suppl 1):S20–8. doi: 10.1002/ptr.2868. [DOI] [PubMed] [Google Scholar]
- 36.Astani A, Reichling J, Schnitzler P. Melissa officinalis extract inhibits attachment of herpes simplex virus in vitro . Chemotherapy. 2012;58:70–7. doi: 10.1159/000335590. [DOI] [PubMed] [Google Scholar]
- 37.Haslam E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J Nat Prod. 1996;59:205–15. doi: 10.1021/np960040+. [DOI] [PubMed] [Google Scholar]
- 38.Serkedjieva J, Ivancheva S. Antiherpes virus activity of extracts from the medicinal plant Geranium sanguineum L. J Ethnopharmacol. 1999;64:59–68. doi: 10.1016/s0378-8741(98)00095-6. [DOI] [PubMed] [Google Scholar]
- 39.Nolkemper S, Reichling J, Sensch KH, Schnitzler P. Mechanism of herpes simplex virus type 2 suppression by propolis extracts. Phytomedicine. 2010;17:132–8. doi: 10.1016/j.phymed.2009.07.006. [DOI] [PubMed] [Google Scholar]


