Abstract
The focus of this paper is to explore better strategies for optimising bone strength and reducing risk of fracture, while at the same time decreasing risk of cardiovascular disease. The majority of Americans do not consume the current recommended dietary allowance for calcium, and the lifetime risk of osteoporosis is about 50%. However, traditional mononutrient calcium supplements may not be ideal. We comprehensively and systematically reviewed the scientific literature in order to determine the optimal dietary strategies and nutritional supplements for long-term skeletal health and cardiovascular health. To summarise, the following steps may be helpful for building strong bones while maintaining soft and supple arteries: (1) calcium is best obtained from dietary sources rather than supplements; (2) ensure that adequate animal protein intake is coupled with calcium intake of 1000 mg/day; (3) maintain vitamin D levels in the normal range; (4) increase intake of fruits and vegetables to alkalinise the system and promote bone health; (5) concomitantly increase potassium consumption while reducing sodium intake; (6) consider increasing the intake of foods rich in vitamins K1 and K2; (7) consider including bones in the diet; they are a rich source of calcium-hydroxyapatite and many other nutrients needed for building bone.
Keywords: QUALITY OF CARE AND OUTCOMES, CV RISK, CALCIUM, VITAMIN D
Key questions.
What is already known about this subject?
The lifetime risk of osteoporosis is approximately 50%. Most people do not consume the Recommended Daily Allowance of calcium. Traditional mononutrient calcium supplements may not be ideal for promoting long-term cardiovascular and skeletal health.
What does this study add?
Calcium is ideally obtained from dietary sources. The form of calcium in bones and bone meal is calcium-hydroxyapatite, which may be particularly effective for building bone.
How might this impact on clinical practice?
Increased consumption of calcium-rich foods such as bones, fermented dairy products (e.g. yogurt, kefir, cheese), leafy greens, almonds, and chia seeds may be effective for improving both skeletal and cardiovascular health.
Introduction
Calcium: general physiology and epidemiology
Calcium is the most ubiquitous mineral in the human body. An average-sized adult body contains approximately 1000 to 1200 g of calcium, which is predominately incorporated into bones and teeth in the form of calcium-hydroxyapatite (Ca10(PO4)6(OH)2) crystals. The remainder circulates throughout the blood and soft tissues, and plays fundamental roles in cell conduction, muscle function, hormone regulation, vitamin (Vit) K-dependent pathways, and cardiac and blood vessel function.1
Some studies indicate only 30% of the US population consumes the Recommended Dietary Allowance of calcium, which is 1000–1200 mg daily.1 Furthermore, humans absorb only about 30% of calcium from foods depending on the specific source.1 The body will demineralise its own skeletal system to maintain serum calcium levels in situations where dietary calcium is insufficient and/or absorption is decreased, and/or excretion is increased.2
Osteopenia/osteoporosis: an epidemic
Starting at about age 50 years, postmenopausal women lose about 0.7–2% of their bone mass each year, while men over age 50 years lose 0.5–0.7% yearly. Between ages 45 and 75 years, women, on average, lose 30% of their bone mass, whereas men lose 15%.
According to the US Surgeon General's Report, 1 in 2 Americans over age 50 years is expected to have or to be at risk of developing osteoporosis.3 Osteoporosis causes 8.9 million fractures annually, with an estimated cumulative cost of incident fractures predicted at US$474 billion over the next 20 years in the USA.3–6 Among adult women over age 45 years, osteoporosis accounts for more days spent in hospital than many other diseases such as diabetes, myocardial infarction (MI), chronic obstructive airway disease and breast cancer.3 Fragility fractures are the primary cause of hospitalisation and/or death for US adults ≥ age 65 years and older; and 44% of nursing home admissions are due to fractures.3
A Mayo Clinic study reported that compared to 30 years ago, forearm fractures have risen more than 32% in boys and 56% in girls. The authors concluded that dietary changes, including insufficient calcium and excess phosphate, were significantly associated with increased fractures.7 Public health approaches are crucial to prevent symptomatic bone disease, but widespread pharmacological prophylaxis is prohibitively expensive and carries potential serious adverse effects.
Cardiovascular disease and bone mineral disease: a calcium nexus
Strong epidemiological associations exist between decreased bone mineral density (BMD) and increased risk of both cardiovascular (CV) disease and CV death.8 For example, individuals with osteoporosis have a higher risk of coronary artery disease, and vice versa. This problem will be magnified if the therapies for osteoporosis (eg, calcium supplements) independently increase risk of MI.
Issues with dairy as primary source of calcium
Dairy foods and beverages account for about 70% of all dietary calcium intake among Americans. Dozens of epidemiological and randomised controlled trials in adults and children have used dairy products as the principal source of calcium, and have credited dairy intake with preventive benefits on study end points including bone mass, fractures and osteoporosis. A recent meta-analysis of over 270 000 people showed a strong trend for dairy intake protecting against hip fracture; the relative risk (RR) of hip fracture per daily glass of milk was 0.91, 95% CI 0.81 to 1.01.9
In many industrialised nations, milk is often the most cost-effective strategy for achieving recommended levels of calcium intake at a population level. Yet, legitimate concerns exist regarding potential deleterious effects of chronic dairy intake on health.10–16 Dairy foods, on an evolutionary time scale, are relative ‘new-comers’ to the hominin diet.17 Domestication of cattle, sheep and goats first occurred approximately 11 000–10 000 years Before Present.17 Furthermore, it appears that an estimated 65% of the worldwide population expresses the phenotype of lactase non-persistence.18
Consumption of cow's milk has been inconsistently associated with cataracts, ovarian and prostate cancers, and Parkinson's disease, and it has been implicated in certain autoimmune diseases, such as type 1 diabetes and multiple sclerosis. Overall, the evidence for dairy-induced human disease appears to be most consistent for prostate cancer and for type 1 diabetes.19
A recent study of over 106 000 adults followed for 20 years showed that drinking three or more glasses of milk per day was associated with increased risks for bone fracture and higher mortality rates compared with drinking not more than one glass of milk per day.20 By contrast, for the women in that study, each daily serving of cheese and/or other fermented milk products such as yogurt was associated with a 10–15% decrease in the rates of mortality and hip fractures (p<0.001). However, this was an observational study with inherent limitations such as residual confounding and reverse causation, and thus, firm conclusions cannot be drawn from the data.
The sugar in milk, lactose, is broken down in the gastrointestinal tract to d-galactose and d-glucose. d-Galactose has been found to increase inflammation and oxidation in adult humans, and in adult animals this sugar triggers accelerated ageing, neurodegeneration, and a shortened life span.20
Thus, cow's milk, though rich in many nutrients, including calcium, has issues that render it less than ideal as a dietary staple for many adults. On the contrary, fermented dairy foods, such as yogurt and cheese, appear to be safer than milk, possibly because the most or all of d-galactose has been metabolised by bacteria.20
Plant-based dietary sources of calcium and protein: effects on bone health
Most vegetarians, especially vegans, appear to absorb less calcium because of the oxalic and phytic acid contained in many plant, grain and legume products.1 Indeed, several studies have reported that risks of bone fracture are higher in vegans—likely due, at least in part, to their lower dietary calcium intake, and/or poor absorption of this key mineral (table 1).21
Table 1.
Calcium-rich foods
| Food | Serving size | Calcium (mg) |
|---|---|---|
| Yogurt | 6 oz | 300 |
| Sardines | 3.5 oz with bones | 250 |
| Salmon | 3.5 oz with bones | 240 |
| Spinach | 1 cup cooked | 240 |
| Chia seeds | 1 oz | 200 |
| Kale | 1 cup chopped | 100 |
| Orange | 1 medium size | 80 |
| Almonds | Raw, ¼ cup (1 oz) | 80 |
| Broccoli | 1 cup cooked | 60 |
| Brussels sprouts | 1 cup cooked | 60 |
| Brazil nuts | 1 oz | 50 |
Dietary protein, calcium and bone health
Evolutionary evidence suggests that preagricultural diets were net base-yielding, and contributed to the robust bone health generally seen among hunter-gatherers.10 17 By contrast, processed foods displace base-yielding fruits and vegetables, thereby shifting to a net acid-yielding diet.2 22–24
Increased protein intake can raise levels of insulin-like growth factor 1, which is anabolic, and contributes to bone building. Experts currently agree that diets moderate in protein (≈1.0–1.5 g/kg/day) are associated with normal calcium metabolism, and do not adversely alter bone metabolism; however, at lower protein intakes (<0.8 g/kg/day), intestinal calcium absorption is reduced and levels of parathyroid hormone rise, causing the mobilisation of calcium from bone.25 26
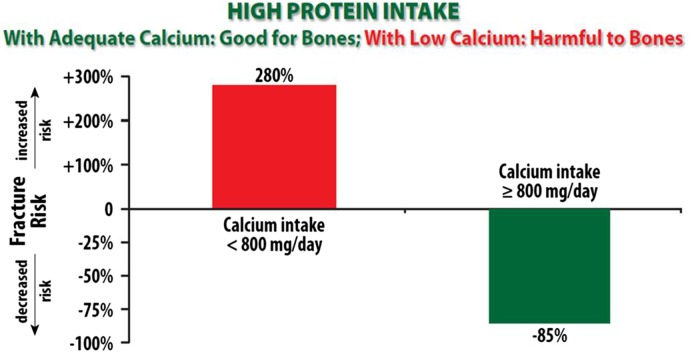
A growing body of evidence indicates that diets higher in animal protein associate with greater bone mass and fewer fractures, particularly if the calcium intake is also sufficient (approximately 1000 mg of calcium/day) (figure 1).26–28 Thus, a diet providing ample dietary calcium, along with alkalising nutrients, such as fruits and vegetables, and possibly also alkaline mineral waters, may create a milieu where moderate intake of animal protein contributes favourably to bone health. Additionally, intake of protein plus calcium with Vit D may reduce fracture rates through mechanisms independent of bone density.29
Figure 1.
A diet that contains moderate amounts of fresh, lean, animal protein, when combined with adequate calcium intake, promotes bone strength and reduces fracture risk. In contrast, high protein diet with inadequate calcium intake increases risk of fracture.28
Magnesium
Maintaining replete magnesium status may reduce risk for the metabolic syndrome, diabetes, hypertension and MI.30 Circumstantial and experimental evidence has also implicated magnesium deficiency in osteoporosis.31–34 Optimal dietary magnesium intake is about 7–10 mg/kg/day, preferably in the context of a net base-yielding diet, since a net acid-yielding diet increases excretion of both magnesium and calcium (table 2).
Table 2.
Magnesium dietary sources
| Food | Serving size | Magnesium (mg) |
|---|---|---|
| Pumpkin seeds | One-third cup | 400 |
| Spinach | 1 cup cooked | 160 |
| Mixed nuts | One-third cup | 160 |
| Beans and lentils | 1 cup cooked | 150 |
| Fish | 4 oz | 100 |
| Dark chocolate | 1 square (about 1 oz) | 100 |
| Brown rice | 1 cup cooked | 90 |
| Yogurt (non-fat, plain) | 1 cup | 50 |
| Banana | 1 medium sized | 40 |
| Avocado | One-half medium sized | 30 |
Potassium/sodium ratio affects calcium metabolism
A potassium/sodium ratio of 1.0 or higher is associated with a 50% lower risk of CVD and total mortality compared with a ratio under 1.0.35 Reducing excessive sodium intake is also associated with resultant decreased urinary calcium excretion, which may help to prevent against bone demineralisation.36 The average potassium content (about 2600 mg/day) of the typical US diet is substantially lower than its sodium content (about 3300 mg/day).35 Approximately 77% of dietary sodium chloride is consumed in the form of processed foods. By contrast, potassium is naturally abundant in many unprocessed foods, especially vegetables, fruits, tubers, nuts, legumes, fish and seafood. In fact, a high potassium/sodium ratio is a reliable marker for high intake of plant foods and lower intake of processed foods.35 High dietary sodium intake has been associated with endothelial damage, arterial stiffness, decreased nitric oxide production and increased levels of transforming growth factor β; whereas, high potassium dietary intake can counteract these effects.35 36
Evidence indicates that the lowest CV event rates occur in the moderate sodium excretion and high potassium excretion groups.37 Thus, it appears that a moderate sodium diet (2800–3300 mg/day) in conjunction with a high potassium intake (>3000 mg/day) might confer the optimal CV benefits for the general population.37
Vit K and bone health
Emerging evidence suggests that Vit K may confer protective effects for both the skeletal and CV systems. Vit K operates in the context of other fat-soluble vitamins, such as A and D, all of which are involved in maintenance of serum calcium concentration, along with the manipulation of materials leading to bone morphogenesis and maintenance of bone tissue.38 Specifically, the oxidation of Vit K results in activation/carboxylation of matrix Gla protein (MGP) which is partially responsible for mineralising bone.39
Also, Vit K is required for the activation (γ-carboxylation) of osteocalcin; the inactivated form, or per cent of undercaboxylated-osteocalcin (%ucOC), has been found to be a sensitive indicator of Vit K nutrition status.38 In cross-sectional and prospective analyses, elevated %ucOC, which occurs when Vit K status is low, is a marker of increased risk for hip fracture in the elderly.38
Several large observational studies appear to support the benefits of Vit K on bone health.38 A meta-analysis concluded that while supplementation with phytonadione (Vit K1) improved bone health, Vit K2 was even more effective in this regard.40 This large and statistically rigorous meta-analysis concluded that high Vit K2 levels were associated with reduced vertebral fractures by approximately 60% (95% CI 0.25% to 0.65%), hip fractures by 77% (95% CI 0.12% to 0.47%), and all non-vertebral fractures by approximately 81% (95% CI 0.11% to 0.35%). Moreover, the benefit of Vit K on bone may not be due to its ability to increase BMD, but rather to its effects at increasing bone strength.41
Vit K benefits in CV health
Mounting evidence suggests vascular calcification whether in the coronary or peripheral arteries is a powerful predictor of CV morbidity and all-cause mortality.42 Prevention of vascular calcification is therefore important as an early intervention to potentially improve long-term CV prognosis.
A major calcification inhibitory factor, is a Vit K-dependent protein synthesised by vascular smooth muscle cells.42 Increased Vit K2 intake has been associated with decreased arterial calcium deposition and the ability to reverse vascular calcification in animal models. Vit K2 prevents pathological calcification in soft tissues via the carboxylation of protective MGP. The undercarboxylated (inactive) species of MGP is formed during inadequate Vit K status, or as a result of Vit K antagonists.42 Low Vit K status is associated with increased vascular calcifications, and can be improved by effective Vit K supplementation (table 3).43 44 In two different randomised, double-blind controlled trials, supplemental Vit K has been shown to significantly delay both the development of coronary artery calcification and the deterioration of arterial elasticity.45 46
Table 3.
Vitamin K1 dietary sources
| Food | Serving size | Vitamin K1 (µg) |
|---|---|---|
| Kale | 1 cup cooked | 1000 |
| Spinach | 1 cup cooked | 500 |
| Brussels spouts | 1 cup cooked | 220 |
| Broccoli | 1 cup cooked | 220 |
| Cabbage | 1 cup cooked | 170 |
| Green onions (scallions) | 3 small onions | 100 |
| Dill pickle | One medium sized | 50 |
| Asparagus | 4 spears | 30 |
| Fresh basil | 3 leaves | 20 |
| Olive oil | One tablespoon | 8 |
Dietary Vit K exists as two major forms: phylloquinone (K1) and menaquinones (MK-n). K1, the predominant dietary form of Vit K, is abundant in dark-green leafy vegetables and seeds. The main dietary sources for MK-n in Western populations are fermented foods, especially natto, cheese and curds (mainly MK-8 and MK-9).47
Calcium supplementation and bone health
A recent large meta-analysis of 26 randomised controlled trials reported that calcium supplements lowered the risk of any fracture by a modest but statistically significant 11% (n=58 573; RR 0.89, 95% CI 0.81 to 0.96).48 Even so, the authors concluded that the evidence for calcium supplements on bone health was weak and inconsistent.
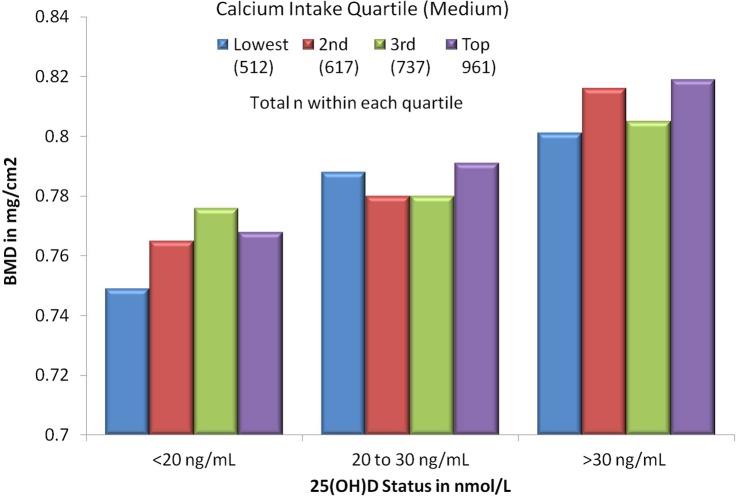
Other large meta-analyses found that calcium supplementation was most effective for preventing hip fractures when it was combined with Vit D.49–51 Indeed Vit D plays a major role in intestinal calcium absorption and bone health (figure 2).52 Additionally, calcium absorption is, in part, dependent on adequate stomach acid, and both these parameters tend to decrease with age. Drugs that markedly reduce stomach acid, such as proton pump inhibitors, have been shown to reduce calcium absorption and increase risk of osteoporosis and fractures.53
Figure 2.
Total hipbone mineral density by quartiles of calcium intake, stratified by 25(OH) vitamin D. Vitamin D status strongly modifies the bone building effects of oral calcium intake.71
A large meta-analysis focusing on calcium intake and fracture risk found that in women (seven prospective cohort studies=170 991 women, 2954 hip fractures), there was no association between total calcium intake and hip fracture risk (pooled RR per 300 mg total=1.01; 95% CI 0.97 to 1.05).50 In men (five prospective cohort studies= 68 606 men, 214 hip fractures), the pooled RR per 300 mg of calcium daily was 0.92 (95% CI 0.82 to 1.03).
Monosupplementation with calcium, especially using the most commonly prescribed formulations (calcium carbonate and calcium citrate) might drive down the absorption of phosphate, thereby contributing to bone demineralisation secondary to abnormal calcium to phosphate ratios.54 The recently updated US Preventive Services Task Force (USPSTF) has stated that there is insufficient evidence that calcium and Vit D prevent fractures in premenopausal women or in men who have not experienced a prior fracture. Indeed, the USPSTF now recommends against daily calcium supplementation for primary prevention of fragility fractures; stating, ‘the balance of benefits and harms cannot be determined’.55
Calcium supplementation and arterial health
The Women's Health Initiative, a 7-year, placebo-controlled randomised trial involving 36 282 participants, found that calcium supplementation with Vit D (1000 mg/400 IU daily) had a neutral effect on coronary risk and cerebrovascular risk.56 By contrast, some subsequent publications have reported data challenging the CV safety of calcium supplementation.57–60
One meta-analysis of placebo-controlled trials involving 28 000 participants reported that a daily calcium supplement was associated with an increased risk of MI (HR 1.24, 95% CI 1.07 to 1.45, p=0.004).58 A prospective study of 388 229 men and women with a 12-year follow-up showed that calcium supplementation was associated with elevated risk of heart disease death in men, but not in women.61 Yet, only one randomised controlled trial of calcium supplementation using adverse cardiac events as the primary end point has been published. In that study, daily supplementation using 1200 mg of calcium carbonate did not increase the risk of CV death or hospitalisation for 1460 women (mean age 75 years).62
In a prospective cohort study with a mean follow-up of 19 years, both high and low dietary calcium intakes were associated with increased CV disease and higher all-cause mortality (figure 3).51 Importantly, a low dietary calcium intake with or without calcium supplementation is also associated with higher CV morbidity and mortality rates.51
Figure 3.
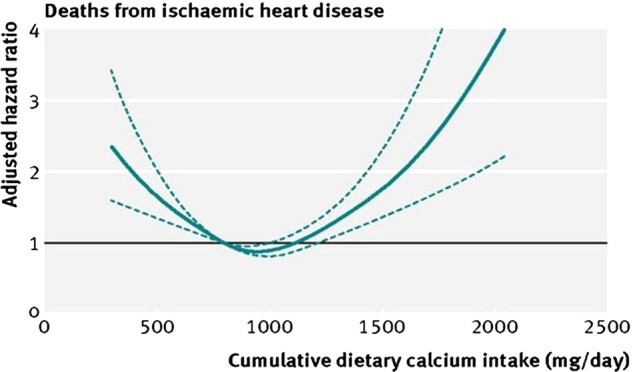
Relationship of daily calcium intake to risk of CV mortality during follow-up. Data were fully adjusted for confounding variables. The calcium intake for optimising CV longevity is about 1000 mg/day, with higher and lower calcium intakes associated with increased CV mortality.51
Elevated serum calcium concentrations are associated with carotid artery plaque thickness, arterial and aortic calcification, and incidence of MI.57 58 Transient elevations in serum calcium levels have been noted following ingestion of 500–1000 mg of calcium supplements.63 64 By contrast, calcium from dietary sources or bone (calcium hydroxyapatite) ingestion results in much smaller changes in circulating calcium levels.65
Other possible mechanisms that have linked calcium supplements with CV disease include coronary artery calcification, impaired vasodilation, increased arterial stiffness, and hypercoagulability.51 66
Food as the ideal source of calcium
The traditional focus in nutrition based on supplementation of single isolated nutrients may be especially misguided in the case of calcium and bone health. A diet supplemented with calcium as a mononutrient pill is not ideal for promoting bone health, and may instead accelerate arterial plaque growth and vascular calcification, and increase risk of MI. Food-based solutions place evidence-based emphasis on finding the admixture of foods that balance the acid–base status of the body, and that most favourably impact the body's calcium metabolism and bone health.
A plant-rich, grain-free diet alters the acid–base status so as to be slightly alkaline, which is conducive for bone health. However, plants are relatively poor sources of calcium compared to animal sources such as dairy products and animal bones. We suspect that milk, though an excellent source of bioavailable calcium, has potential adverse health effects for some individuals. Additionally, 65% of the world's population show some decrease in lactase activity during adulthood. Importantly, fermented dairy has been linked to favourable outcomes for bone health and mortality risk.
Benefits of consuming bones or bone meal
Ethnographic and anthropological studies indicate that adult human hunter-gatherers consumed most of their calcium in the form of bones from animals, such as small and large mammals, birds, fish and reptiles.67 68 Indeed through millions of years of evolution, we are genetically adapted to consume a large proportion of our dietary calcium from bones, where calcium is absorbed along with a matrix of nutrients including magnesium, phosphorus, strontium, zinc, iron, copper, collagen protein, aminoglycans and osteocalcin—all of which also support robust bone formation.68 69 Theoretically, including animal bones (sardines, salmon, soft chicken bones, bone broths, etc) may be an effective dietary strategy to ensure adequate calcium intake and to optimise long-term bone health.
Mineral supplements made from bone meal, when taken with food, theoretically might provide a more practical means to ensure adequate calcium intake without predisposing to CVD risk. Ingestion of microcrystalline hydroxyapatite (the form of calcium found in bone) produces less of an acute spike in blood calcium levels compared to soluble calcium salts typically used in standard supplements, and thus may be less likely to increase vascular calcification and coronary risk.65 Hydroxyapatite also stimulates bone osteoblast cells and contains virtually all the essential building blocks needed to construct bone tissue. In a small placebo-controlled randomised trial, women who took 1000 mg of calcium in the form of hydroxyapatite in conjunction with oral Vit D showed a significant increase in bone thickness, whereas those who took 1000 mg of a standard calcium carbonate supplement did not (figure 4).70 Another double-blind placebo-controlled study found that supplementing with hydroxyapatite and Vit D3 significantly improved serological markers of bone health.15
Figure 4.
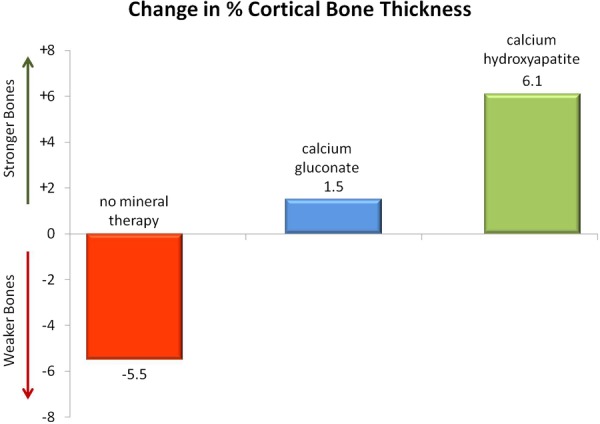
Change in cortical bone thickness among 64 women with osteopenia randomised to 14-month treatment with no mineral therapy (red bar), calcium gluconate (blue bar), or calcium hydroxyapatite (green bar). The control group showed bone loss (p<0.01 compared to baseline), while the hydroxyapatite group showed bone gain (p<0.01 compared to baseline). The calcium gluconate showed no significant change.70
In theory, the addition of Vit K2 and magnesium to an organic bone meal supplement might further enhance its effectiveness and reduce the risk of soft tissue calcification. However, the quantity and quality of the experimental data testing the effects of Vit D and calcium on bone health dwarfs the data for bone meal supplementation. Much larger randomised trials will be needed to firmly establish the safety and effectiveness of bone meal as well as Vit K and magnesium as supplements for building bone without increasing vascular calcification.
Conclusion
It is becoming increasingly clear that the fundamental unit for nutrition is the food (eg, milk, nuts, eggs), not the nutrient (eg, calcium, saturated fat, cholesterol). A nutrient perceived as beneficial, such as calcium, may be unhealthy if the parent food, say milk, contains other nutrients, such as galactose, that on the balance might stimulate adverse effects in the body. In theory, consuming calcium-rich foods such as bones, fermented dairy (eg, unsweetened yogurt, kefir, cheese), leafy greens, almonds, and chia seeds may be an effective strategy for improving both calcium intake and long-term health.
Footnotes
Twitter: Follow Maelán Fontes-Villalba at @maelanfontes
Contributors: NB, PC-B and MF-V assisted with the gathering and review of the data; JD, LC and JHO reviewed the data; NB, PC-B, MF-V, JD, LC and JHO assisted in the concept and design of the manuscript. JHO, NB and PC-B wrote, rewrote and finalised the manuscript.
Funding: This manuscript received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. This paper was not commissioned.
Competing interests: JHO is Chief Medical Officer and has an ownership interest in CardioTabs, a nutraceutical company that markets products containing vitamins and minerals.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Ross AC, Taylor CL, Yaktine AL, del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press, 2011:349 http://www.ncbi.nlm.nih.gov/books/NBK56070/ [PubMed] [Google Scholar]
- 2.Frassetto L, Morris RC Jr, Sellmeyer DE et al. Diet, evolution and aging—the pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. Eur J Nutr 2001;40:200–13. [DOI] [PubMed] [Google Scholar]
- 3.Surgeons AAoO. The burden of musculoskeletal diseases in the United States: prevalence, societal and economic cost. Rosemont, IL: Amer Academy of Orthopaedic, 2008. [Google Scholar]
- 4.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17:1726–33. 10.1007/s00198-006-0172-4 [DOI] [PubMed] [Google Scholar]
- 5.Facts and Statistics. http://www.iofbonehealth.org/facts-statistics. Secondary Facts and Statistics. http://www.iofbonehealth.org/facts-statistics 2013. http://www.iofbonehealth.org/facts-statistics
- 6.Burge R, Dawson-Hughes B, Solomon DH et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 2007;22:465–75. 10.1359/jbmr.061113 [DOI] [PubMed] [Google Scholar]
- 7.Khosla S, Melton LJ III, Dekutoski MB et al. Incidence of childhood distal forearm fractures over 30 years: a population-based study. JAMA 2003;290:1479–85. 10.1001/jama.290.11.1479 [DOI] [PubMed] [Google Scholar]
- 8.Choi SH, An JH, Lim S et al. Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and postmenopausal women. Clin Endocrinol 2009;71:644–51. [DOI] [PubMed] [Google Scholar]
- 9.Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA et al. Milk intake and risk of hip fracture in men and women: a meta-analysis of prospective cohort studies. J Bone Miner Res 2011;26:833–9. 10.1002/jbmr.279 [DOI] [PubMed] [Google Scholar]
- 10.Carrera-Bastos P, Fontes-Villaba M, O'Keefe JH et al. The western diet and lifestyle and diseases of civilization. Res Rep Clin Cardio 2011;2011:15–35. [Google Scholar]
- 11.Winer S, Astsaturov I, Cheung RK et al. T cells of multiple sclerosis patients target a common environmental peptide that causes encephalitis in mice. J Immunol 2001;166:4751–6. [DOI] [PubMed] [Google Scholar]
- 12.Artaud-Wild SM, Connor SL, Sexton G et al. Differences in coronary mortality can be explained by differences in cholesterol and saturated fat intakes in 40 countries but not in France and Finland. A paradox. Circulation 1993;88:2771–9. [DOI] [PubMed] [Google Scholar]
- 13.Segall JJ. Plausibility of dietary lactose as a coronary risk factor. J Nutr Enviro Med 2002;12:217–29. [Google Scholar]
- 14.Cordain L, Toohey L, Smith MJ et al. Modulation of immune function by dietary lectins in rheumatoid arthritis. Br J Nutr 2000;83:207–17. [DOI] [PubMed] [Google Scholar]
- 15.Disilvestro RA, Crawford B, Zhang W et al. Effects of micronutrient supplementation plus resistance exercise training on bone metabolism markers in young adult woman. J Nutr Enviro Med 2007;16:16–25. [Google Scholar]
- 16.Sandler RB, Slemenda CW, LaPorte RE et al. Postmenopausal bone density and milk consumption in childhood and adolescence. Am J Clin Nutr 1985;42:270–4. [DOI] [PubMed] [Google Scholar]
- 17.Cordain L, Eaton SB, Sebastian A et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 2005;81:341–54. [DOI] [PubMed] [Google Scholar]
- 18.Ingram CJ, Mulcare CA, Itan Y et al. Lactose digestion and the evolutionary genetics of lactase persistence. Hum Genet 2009;124:579–91. 10.1007/s00439-008-0593-6 [DOI] [PubMed] [Google Scholar]
- 19.Melnik BC, John SM, Carrera-Bastos P et al. The impact of cow's milk-mediated mTORC1-signaling in the initiation and progression of prostate cancer. Nutr Metab 2012;9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michaelsson K, Wolk A, Langenskiold S et al. Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ 2014;349:g6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appleby P, Roddam A, Allen N et al. Comparative fracture risk in vegetarians and nonvegetarians in EPIC-Oxford. Eur J Clin Nutr 2007;61:1400–6. 10.1038/sj.ejcn.1602659 [DOI] [PubMed] [Google Scholar]
- 22.Sebastian A, Harris ST, Ottaway JH et al. Improved mineral balance and skeletal metabolism in postmenopausal women treated with potassium bicarbonate. N Engl J Med 1994;330:1776–81. 10.1056/NEJM199406233302502 [DOI] [PubMed] [Google Scholar]
- 23.Bushinsky DA. Metabolic alkalosis decreases bone calcium efflux by suppressing osteoclasts and stimulating osteoblasts. Am J Physiol 1996;271(1 Pt 2):F216–22. [DOI] [PubMed] [Google Scholar]
- 24.Sebastian A, Frassetto LA, Sellmeyer DE et al. Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am J Clin Nutr 2002;76:1308–16. [DOI] [PubMed] [Google Scholar]
- 25.Kerstetter JE, O'Brien KO, Insogna KL. Dietary protein, calcium metabolism, and skeletal homeostasis revisited. Am J Clin Nutr 2003;78(3 Suppl):584S–92S. [DOI] [PubMed] [Google Scholar]
- 26.Heaney RP, Layman DK. Amount and type of protein influences bone health. Am J Clin Nutr 2008;87:1567S–70S. [DOI] [PubMed] [Google Scholar]
- 27.Hannan MT, Tucker KL, Dawson-Hughes B et al. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 2000;15:2504–12. 10.1359/jbmr.2000.15.12.2504 [DOI] [PubMed] [Google Scholar]
- 28.Sahni S, Cupples LA, McLean RR et al. Protective effect of high protein and calcium intake on the risk of hip fracture in the Framingham offspring cohort. J Bone Miner Res 2010;25: 2770–6. 10.1002/jbmr.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabenda V, Bruyere O, Reginster JY. Relationship between bone mineral density changes and risk of fractures among patients receiving calcium with or without vitamin D supplementation: a meta-regression. Osteoporos Int 2011;22:893–901. 10.1007/s00198-010-1469-x [DOI] [PubMed] [Google Scholar]
- 30.He K, Liu K, Daviglus ML et al. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 2006;113:1675–82. 10.1161/CIRCULATIONAHA.105.588327 [DOI] [PubMed] [Google Scholar]
- 31.Lakshmanan FL, Rao RB, Kim WW et al. Magnesium intakes, balances, and blood levels of adults consuming self-selected diets. Am J Clin Nutr 1984;40(6 Suppl):1380–9. [DOI] [PubMed] [Google Scholar]
- 32.Greger JL, Baligar P, Abernathy RP et al. Calcium, magnesium, phosphorus, copper, and manganese balance in adolescent females. Am J Clin Nutr 1978;31:117–21. [DOI] [PubMed] [Google Scholar]
- 33.Gullestad L, Nes M, Ronneberg R et al. Magnesium status in healthy free-living elderly Norwegians. J Am Coll Nutr 1994;13:45–50. [DOI] [PubMed] [Google Scholar]
- 34.Sojka JE, Weaver CM. Magnesium supplementation and osteoporosis. Nutr Rev 1995;53:71–4. [DOI] [PubMed] [Google Scholar]
- 35.Yang Q, Liu T, Kuklina EV et al. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2011;171:1183–91. 10.1001/archinternmed.2011.257 [DOI] [PubMed] [Google Scholar]
- 36.Lin PH, Ginty F, Appel LJ et al. The DASH diet and sodium reduction improve markers of bone turnover and calcium metabolism in adults. J Nutr 2003;133:3130–6. [DOI] [PubMed] [Google Scholar]
- 37.O'Donnell MJ, Yusuf S, Mente A et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA 2011;306:2229–38. [DOI] [PubMed] [Google Scholar]
- 38.Booth SL. Roles for vitamin K beyond coagulation. Annu Rev Nutr 2009;29:89–110. 10.1146/annurev-nutr-080508-141217 [DOI] [PubMed] [Google Scholar]
- 39.Kanellakis S, Moschonis G, Tenta R et al. Changes in parameters of bone metabolism in postmenopausal women following a 12-month intervention period using dairy products enriched with calcium, vitamin D, and phylloquinone (vitamin K(1)) or menaquinone-7 (vitamin K (2)): the Postmenopausal Health Study II. Calcif Tissue Int 2012;90:251–62. 10.1007/s00223-012-9571-z [DOI] [PubMed] [Google Scholar]
- 40.Cockayne S, Adamson J, Lanham-New S et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med 2006;166:1256–61. 10.1001/archinte.166.12.1256 [DOI] [PubMed] [Google Scholar]
- 41.Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int 2007;18:963–72. 10.1007/s00198-007-0337-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beulens JW, Bots ML, Atsma F et al. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis 2009;203:489–93. 10.1016/j.atherosclerosis.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 43.Rennenberg RJ, de Leeuw PW, Kessels AG et al. Calcium scores and matrix Gla protein levels: association with vitamin K status. Eur J Clin Invest 2010;40:344–9. 10.1111/j.1365-2362.2010.02275.x [DOI] [PubMed] [Google Scholar]
- 44.Schurgers LJ, Barreto DV, Barreto FC et al. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol 2010;5:568–75. 10.2215/CJN.07081009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shea MK, O'Donnell CJ, Hoffmann U et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr 2009;89:1799–807. 10.3945/ajcn.2008.27338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braam LA, Hoeks AP, Brouns F et al. Beneficial effects of vitamins D and K on the elastic properties of the vessel wall in postmenopausal women: a follow-up study. Thromb Haemost 2004;91:373–80. 10.1160/TH03-07-0423 [DOI] [PubMed] [Google Scholar]
- 47.McCann JC, Ames BN. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am J Clin Nutr 2009;90:889–907. 10.3945/ajcn.2009.27930 [DOI] [PubMed] [Google Scholar]
- 48.Bolland MJ, Leung W, Tai V et al. Calcium intake and risk of fracture: systematic review. BMJ 2015;351:h4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang BM, Eslick GD, Nowson C et al. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 2007;370:657–66. 10.1016/S0140-6736(07)61342-7 [DOI] [PubMed] [Google Scholar]
- 50.Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA et al. Calcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr 2007;86:1780–90. [DOI] [PubMed] [Google Scholar]
- 51.Michaelsson K, Melhus H, Warensjo Lemming E et al. Long term calcium intake and rates of all cause and cardiovascular mortality: community based prospective longitudinal cohort study. BMJ 2013;346:f228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christakos S. Recent advances in our understanding of 1,25-dihydroxyvitamin D(3) regulation of intestinal calcium absorption. Arch Biochem Biophys 2012;523:73–6. 10.1016/j.abb.2011.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khalili H, Huang ES, Jacobson BC et al. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ 2012;344:e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heaney RP, Nordin BE. Calcium effects on phosphorus absorption: implications for the prevention and co-therapy of osteoporosis. J Am Coll Nutr 2002;21:239–44. [DOI] [PubMed] [Google Scholar]
- 55.Moyer VA. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013;158:691–6. 10.7326/0003-4819-158-9-201305070-00603 [DOI] [PubMed] [Google Scholar]
- 56.Hsia J, Heiss G, Ren H et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007;115:846–54. 10.1161/CIRCULATIONAHA.106.673491 [DOI] [PubMed] [Google Scholar]
- 57.Bolland MJ, Barber PA, Doughty RN et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008;336:262–6. 10.1136/bmj.39440.525752.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bolland MJ, Wang TK, van Pelt NC et al. Abdominal aortic calcification on vertebral morphometry images predicts incident myocardial infarction. J Bone Miner Res 2010;25:505–12. 10.1359/jbmr.091005 [DOI] [PubMed] [Google Scholar]
- 59.Reid IR, Bolland MJ, Grey A. Does calcium supplementation increase cardiovascular risk? Clin Endocrinol 2010;73:689–95. [DOI] [PubMed] [Google Scholar]
- 60.Pentti K, Tuppurainen MT, Honkanen R et al. Use of calcium supplements and the risk of coronary heart disease in 52–62-year-old women: The Kuopio Osteoporosis Risk Factor and Prevention Study. Maturitas 2009;63:73–8. 10.1016/j.maturitas.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 61.Xiao Q, Murphy RA, Houston DK et al. Dietary and supplemental calcium intake and cardiovascular disease mortality: The National Institutes of Health-AARP diet and health study. JAMA Intern Med 2013;173:639–46. 10.1001/jamainternmed.2013.3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewis JR, Calver J, Zhu K et al. Calcium supplementation and the risks of atherosclerotic vascular disease in older women: results of a 5-year RCT and a 4.5-year follow-up. J Bone Miner Res 2011;26:35–41. 10.1002/jbmr.176 [DOI] [PubMed] [Google Scholar]
- 63.Reid IR, Bolland MJ, Avenell A et al. Cardiovascular effects of calcium supplementation. Osteoporos Int 2011;22:1649–58. 10.1007/s00198-011-1599-9 [DOI] [PubMed] [Google Scholar]
- 64.Karp HJ, Ketola ME, Lamberg-Allardt CJ. Acute effects of calcium carbonate, calcium citrate and potassium citrate on markers of calcium and bone metabolism in young women. Br J Nutr 2009;102:1341–7. 10.1017/S0007114509990195 [DOI] [PubMed] [Google Scholar]
- 65.Tucker LA, Nokes N, Adams T. Effect of a dietary supplement on hip and spine BMD: a randomized, double-blind, placebo-controlled trial: 1515: board #5 May 30 2:00 PM—3:30 PM. Med Sci Sports Exer 2007;39:S230. [Google Scholar]
- 66.West SL, Swan VJ, Jamal SA. Effects of calcium on cardiovascular events in patients with kidney disease and in a healthy population. Clin J Am Soc Nephrol 2010;5(Suppl 1):S41–7. 10.2215/CJN.05860809 [DOI] [PubMed] [Google Scholar]
- 67.Reinhard KJ, Ambler JR, Szuter CR. Hunter-gatherer use of small animal food resources: coprolite evidence. J Osteoarch 2007;17:416–28. [Google Scholar]
- 68.Vieugue J, Salanova L, Regert M et al. The consumption of bone powder in the early neolithic societies of Southeastern Europe: evidence of a diet stress? Cambridge Archaeological J 2015;02:495–511. [Google Scholar]
- 69.Schulman RC, Weiss AJ, Mechanick JI. Nutrition, bone, and aging: an integrative physiology approach. Curr Osteoporos Rep 2011;9:184–95. 10.1007/s11914-011-0079-7 [DOI] [PubMed] [Google Scholar]
- 70.Epstein O, Kato Y, Dick R et al. Vitamin D, hydroxyapatite, and calcium gluconate in treatment of cortical bone thinning in postmenopausal women with primary biliary cirrhosis. Am J Clin Nutr 1982;36:426–30. [DOI] [PubMed] [Google Scholar]
- 71.Bischoff-Ferrari HA, Kiel DP, Dawson-Hughes B et al. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J Bone Miner Res 2009;24:935–42. 10.1359/jbmr.081242 [DOI] [PMC free article] [PubMed] [Google Scholar]