Introduction
Preclinical models for cancers, including lung, are crucial for understanding biology and for the development and testing of conventional and novel therapeutic agents. As cited later, comprehensive reviews have been published recently of all of the three basic methods that form the pillars of preclinical models: Cell cultures, patient derived xenografts (PDXs) and genetically engineered mouse models (GEMMs). However, to the best of our knowledge, no comprehensive review of the entire subject has been published, although murine models are well covered in a recent review.1 Our aim was to provide such a review of preclinical models for lung cancers. In addition we discuss some recent novel approaches to potentially improve the basic models. As there is no perfect model, and probably never will be, we also discuss the advantages and disadvantages of each of the models. Because we are encompassing multiple models in one review, we cannot cover each model in as much detail as do reviews of individual models. However, by giving an overall review of the major models, a clearer picture of the field and the inter-relationships and uses of the different models can be obtained.
Because SCLC tumors are seldom resected, only sparse diagnostic materials are occasionally available for the study of biology and for the development and testing of innovative therapeutic approaches. Thus, in vitro models to study this “recalcitrant disease” are of crucial importance for this type of lung cancer.
The use of these models was first explored 30–40 years ago. Recently, new approaches have been proposed or implemented that may alter and improve our approach to the study of such models, and these are summarized at the end. The major strengths and limitations of these three basic preclinical model systems are summarized in the sections that follow. The current models, especially for GEMMs, represent major improvements and innovations over the earlier systems. We focus on the more recent ones, and also discuss newer concepts that may improve or alter our present models. We do not discuss the sparsely studied syngeneic and spontaneous mouse models. The three models allow experimental test of various therapeutic approaches, and the role various genetic and epigenetic changes in lung cancer pathogenesis and biology and the study of tumor heterogeneity and stem cells.
Methods
We searched the MEDLINE database via PubMed (PubMed.gov) using combinations of the Medical Subject Headings (MESH) terms as described in Table 1. In this review article we focus on and frequently cite review articles, as they give a broad overview of their respective topics, and reduce the number of references that we need to cite.
Table 1.
Citations to preclinical models for lung cancer. A MEDLINE search was conducted via PubMed (https://www.nlm.nih.gov/bsd/pmresources.html) on September 16, 2015 using the Major Medical Subject Heading (MeSH) term Lung neoplasms, and the other MeSH terms as indicated.
| MeSH terms used for MEDLINE search | Number of citations | |
|---|---|---|
| Cell line, tumor and human | 11,705 | |
| Mice, SCID or Mice nude | 3223 | |
| Animals and models, genetic | 923 | |
| Totals | 15,851 |
Results
A recent (September 18, 2015) search of the MEDLINE database (http://www.nlm.nih.gov/bsd/pmresources.html) using the Major Medical Subject Heading (MESH) term carcinoma, pulmonary, and other terms as indicated in Table 1, yielded over 15,800 citations, with the majority of them referring to lung cancer tumor cell lines. However, the other two major models were also well represented. Thus all of the three “pillars” of the preclinical models for lung cancer are well utilized and cited.
Tumor Cell Lines (TCL)
However, the relevance of cancer cell lines has remained controversial for many reasons beyond the scope of this article. Three recent articles have addressed these issues and indicated that carefully characterized cell lines are highly relevant for many but not all studies, and have to be evaluated for the specific purpose for which they were utilized.2–4 The pros and cons of cell lines are discussed in Table 2. Some of the same statements apply to all of the in vitro models. Of interest, a recent study found that newly established ovarian cancer cell lines faithfully reproduced the properties of their human tumor conterparts.5
Table 2.
Strengths and Limitations of cell lines for the study of lung cancer
Strengths
|
Weaknesses
|
Permanent lung cancer cell cultures were established in the 1970s. The first SCLC line was established in 1971,6 (although reported in 1973), precisely 20 years after George Gey established HeLa, the world’s first continuous human tumor cell line.7 Of interest, Gey also grew strains of lung adenocarcinomas,8 but apparently these could not be maintained as permanent cell lines. Compared to other types of human carcinomas, the number of lung cancer lines is much larger,2 and their study by hundreds of investigators worldwide has formed the basis of much of our knowledge of the biology of this disease, and, as previously noted, has generated many thousands of published reports.2 We maintain a database of the NCI-H- and HCC- series (initiated by AFG and JDM) and other readily available or cited lung cancer cell lines (n = 431, SCLC = 172, NSCLC = 259). Of those with known ethnicities, 84% are of Caucasian or Hispanic origin, 14% are of African American origin and only 2% are of East Asian origin. While the recent literature, in particular, cites many lung cancer lines initiated in Asian countries, unfortunately most of these are not readily available in Western countries, the EGFR mutant PC9 line being the most frequently cited one (149 citations). Much additional information about the cell lines is available from public databases:
Some of the most important findings regarding biology or therapeutic applications derived from the use of cell lines are cited in Table 3. A large amount of genetic information regarding gene expression, mutations and copy number variations about the NCI-H and HCC series of cell lines initiated by the authors (AFG and JDM) at the following locations:
Table 3.
Some highlight discoveries using lung cancer cell lines. With more than 11000 citations to human cell lines only a few of the important discoveries are mentioned, to demonstrate the impact of cell lines on lung cancer pathogenesis, biology and therapy.
Deletions of chromosome 3p are characteristic of both SCLC and NSCLC, and identification of multiple tumor suppressor genes in this region.57–61
|
Data from UT Southwestern is available from the Gene expression Omnibus (GEO) data sets maintained by the National Center for National Center for Biotechnology Information (NCBI): http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE32036
The Cancer Cell Line Encyclopedia (CCLE) maintained by the Broad Institute, http://www.broadinstitute.org/ccle/data/browseData?conversationPropagation=begin has copy number variation, gene expression and mutation data, as has the Catalogue of Somatic Mutations in Cancer (COSMIC) maintained by the Wellcome Trust Sanger institute): http://cancer.sanger.ac.uk/cell_lines/download
An examination of these databases provides an overview of the complexity of the mutational profile of lung cancer.9 The enormous mutational burden present in lung cancers, mainly as a result of tobacco exposure over many years, was first demonstrated when scientists at the Sanger Institute (in collaboration with AFG and JDM) first sequenced a SCLC cell line and its corresponding B lymphoblastoid line and found nearly 23,000 somatic substitutions.10 This study also vividly demonstrated the importance of having a corresponding source of constitutional DNA. Efforts to obtain DNA from the original tumor, whether fresh frozen of formalin fixed paraffin embedded (FFPE) are also of great value in interpreting somatic changes and the acquisition of additional changes during prolonged culture life. It is important to cryopreserve a large number of vials at early culture passage and utilize these rather than maintain cultures for indefinite passages.
A important problem with the use of cell lines is the absolute necessity to establish the true provenance of the cell lines, as mix-ups and misattributions remain a major problem with numerous reports based on spurious identification.11 Quality control and mycoplasma contamination are also major problems. The editorial policy of Journals should require evidence of provenance and identification of the original and direct sources of the cell lines used in submitted reports.
NSCLC lines
NSCLC lines were first initiated in the early 1970s, and included the widely used A549 cell line.12 Currently we are aware of over 200 cell lines, with perhaps 120 widely distributed and utilized by the scientific community. The widely distributed ones have been extensively characterized and utilized,2 although some of the information including complete genomic sequencing are not yet available to the scientific public. The cell lines are heavily biased towards adenocarcinomas, the commonest form of lung cancer. Although about 30 squamous carcinoma (SCC) cell lines have been reported, molecular classification indicates that many are misattributed or are poorly differentiated (authors unpublished data). Thus the number of bona fide well differentiated SCC lines needs to be increased. They will be of great importance in the near future as targeted therapies for SCC are tested and translated to the clinic. Full genomic classification of the frequently used NSCLC lines including molecular estimates of degree of differentiation and of tobacco induced damage will be published by the authors in the near future.
Although the clinical relevance of in vitro drug testing of cell lines remains controversial, genomic changes such as driver oncogene mutations and oncogene addiction are maintained. Thus cell lines accurately reflect the clinical responses to several targeted therapies including EGFR mutant cell line responses to tyrosine kinase inhibitors,13 and for crizotinib response in ALK-translocated cell lines.14 Thus NSCLC cell lines are useful preclinical models for evaluating targeted therapies and for investigating methods of therapy resistance. Because genomic changes, especially for driver mutations, are stable, these properties have been maintained in long cultured cell lines.
SCLC lines
The Japanese authors who grew the world’s first SCLC cell line noted that it grew as floating cell aggregates,6 in contrast to most adherent epithelial tumor cell cultures. Most SCLC lines grow as floating cultures and the few that grow adherent may not have arisen from true or typical SCLC. During the decade of the 1970s, investigators in Japan, Dartmouth Medical School15 and the NCI established panels of SCLC lines.16 Because of unique clinical resources then available at the NCI, a large series of cell lines was established by Drs. Gazdar, Desmond Carney and Minna (and collaborators) from both limited and extensive disease tumors, and even, in a few cases, from extra-pulmonary small cell carcinomas.17 In addition these lines were established before and after administration of cytotoxic therapy. Most lines retained the cytological and NE cell features of SCLC tumors. A recent unpublished characterization by the author has confirmed that vast majority of the NCI series of lines have retained these features even after 4 decades in culture. Some of the lines, especially those established after prior therapy and which had amplification of a MYC family gene, had atypical morphology and lacked some of the NE cell program. These were termed variant SCLC cell lines.18 The NCI series were deposited in the American Type Culture Collection and widely distributed to investigators worldwide. Because of the difficulty in obtaining human SCLC tumor materials, they remain the major resource for most of the biology studies performed in SCLC.2 Constitutional sources of DNA (mainly B lymphoblastoid cell lines) are available for some of the lines. A major shortcoming is lack of cell lines established from the putative precursor cell, the NE cells of the respiratory epithelium.
While they are an estimated 150 SCLC TCLs established worldwide, recent reports have been scarce. Two recent developments, discussed later, offered innovative new approaches to the establishment of SCLC cancer lines.
Patient Derived Xenografts (PDXs)
PDX tumors are generated by direct transfer of human tumor fragments or cell isolates from patient tumors to immune-deficient mice (or other rodent species). Established tumor cell lines may also be used as a source of xenografts, although they may represent a narrow range of subpopulations resulting from the “double” selection (i.e. during cell culture and during xenograft growth). A partial list of some of the cell line xenografts has been published.1 While PDXs were established over 40 years ago,19 the variety of immune-deficient rodent strains has increased considerably, with increased engraftment rates, although at considerably increased costs. As discussed below and in Table 4, PDXs may represent improved models for drug discovery and validation, they have also been utilized for biomarker discovery and validation, and they can provide relatively large numbers of tumor cells for a host of biological and other studies, and can be used to establish cell lines. In fact, when AFG and JDM were first attempting to establish SCLC cultures in the 1970s, initial efforts from human tumors failed while PDX cultures provided their first successful long term cell lines. Currently we are also using PDXs to boost the sparse number of well characterized human SCC cultures available worldwide. However, we have noticed that variable numbers of mouse stromal cells may persist in PDX derived cell lines for many years, and monitoring of the mouse:human cell ratio is important. In fact we, and others, have noted that some “PDXs” consist entirely of mouse derived sarcoma-like tumorigenic cells.
Table 4.
Strengths and Limitations of Patient Derived Xenografts (PDXs) for the study of lung cancer
Strengths
|
Weaknesses
|
Primary or metastatic tumors may be utilized, as well as malignant effusions. Sometimes the tumor cells are admixed with Matrigel or mesenchymal cells in efforts to increase engraftment rates. In general the more severe the immunodeficiency of the mouse host, the greater the take rates, and NOD/SCID or NSG mice are preferable, but more expensive. While the usual route of inoculation is subcutaneous in the dorsal region, orthotopic models for SCLC may increase metastatic potential and relevance for chemotherapy evaluation.20 However, as the usual reason for death from lung cancer is metastatic spread, this view is not universally held. Intracranial heterotransplantation of SCLC into the brain provides a model to study intracranial and leptomeningeal meatastases.21 Inoculation into vascular sites such as the subrenal capsule, may also increase take rates. One of the major advantages of the subcutaneous route is the ease of monitoring tumor growth.
At least during early serial passage, PDXs retain the genetic and morphological characteristics of the original human tumor, including histological features, gene expression profiles, copy number variations and chromosomal stability of PDX tumors (Table 4).19 In one recent study, the success rate for establishing PDXs from resected NSCLC was approximately 50%,22 although most studies report lower rates. Over 90% of the mutations identified in the primary tumors were also present in the corresponding PDXs. However, additional unique mutations were detected in the PDXs, suggesting genetic drift or heterogeneity in the original tumors. Potential shortcomings of PDX models include the gradual replacement of patient derived stroma with mouse derived stroma, lack of an intact immune system, lack of metastatic spread in most models and frequent engraftment in an unnatural (subcutaneous) setting. Another potential problem, contamination of the engrafted human cells with mouse xenotropic virus, is discussed below.
With the heightened interest in PDXs,23,24 especially for the testing of conventional and targeted therapies, it becomes important to establish large banks of the different tumor types, so as to capture the genetic diversity and heterogeneity of human tumors as well as the range of drug sensitivities. Because of the cost and effort of establishing such banks, they are best performed by consortiums that pool resources and make them available to the scientific public. One such effort is by the newly established European EurOPDX initiative.25
The mouse genome contains over 500,000 copies of integrated strains of mouse leukemia virus virus. Some strains are xenotropic and grow efficiently in human cells. Serial transplantation of PDXs, especially SCLC, is associated with a high frequency of xenotropic virus contamination,27 which poses potential health risks and may influence genetic, immune and metabolic analyses. Unfortunately, the tumors themselves cannot be directly monitored for leukemia virus contamination as the PDXs contain mouse derived cells having numerous copies of the integrated genomes. The only method currently available is for culture of the PDXs and to test for virus release into the supernatant fluids. Paradoxically, the mouse cells release low or absent levels of the virus, while the infected human cells release enormous amounts.27
Genetically engineered mouse models (GEMMs)
Earlier studies of mouse models focused on exposure to chemical carcinogens, including smoke exposure, and even on skin painting. However, most involved systemic or intratracheal administration of the carcinogen. These studies have been summarized recently, and are not discussed further.28
More comprehensive reviews of lung cancer GEMMs have been published,29–31 and we can only highlight certain aspects in this report. We start with a brief explanation of some of the important terms and techniques. The following definitions are from the Jackson Laboratory website (http://research.jax.org/grs/type/gemm/): Genetically engineered mice have induced mutations, including transgenes, targeted mutations (knockouts or knockins), and retroviral or proviral induced mutations. Transgenic mice carry a segment of foreign DNA incorporated into their genome via non-homologous recombination (e.g., pronuclear microinjection), infection with a retroviral vector, or homologous insertion. Targeted mutant mice are produced by first inducing gene disruptions, replacements, or duplications into embryonic stem (ES) cells via homologous recombination between the exogenous (targeting) DNA and the endogenous (target) gene. The genetically-modified ES cells are then microinjected into host embryos at the eight-cell blastocyst stage. These embryos are transferred to pseudopregnant host females, which then bear chimeric progeny. The chimeric progeny carrying the targeted mutation in their germ line are then bred to establish a line. If the newly established line has a disrupted or deleted gene, it is called a knockout; if it has a new or duplicated gene, it is called a knockin. Genetically modified mice are used extensively for in vitro studies, especially the knockout mouse, where the activity of one or more genes has been removed. Such models are of crucial importance in understanding the role that newly discovered lung cancer genes play in tumorigenesis, and for distinguishing passenger from driver oncogenes. They also play pivotal roles in understanding multistage pathogenesis, identification of tumor biomarkers, development and testing of newer therapeutic approaches, and for the understanding and overcoming of drug resistance (Table 5). We currently have the capacity to swiftly re-engineer complex genetic lesions present in lung cancers permits the study of the interplay between defined genetic combinations to tumor formation and metastasis. As such they are invaluable complements to cell culture systems, especially for studies requiring intact, immunocompetent animals. One example of an applied, clinically relevant application used multiple mouse models to identify blood proteomic signatures for lung ADC and neuroendocrine (NE) carcinomas.32
Table 5.
Strengths and Limitations of Genetically Engineered Mouse Models (GEMMs) for the study of lung cancer
Strengths
|
Weaknesses
|
GEMMs for ADCs of the lung
More GEMMs exist for ADC than for the other histologic types.31 The commonest models generated are for Kras or Egfr activation, but multiple other models involve Braf, Her2, Eml-Alk, Pik3ca or other genes.31 Conditional models, as for Egfr, may show complete tumor responses following removal of the driver gene, a dramatic demonstration of oncogene addiction. GEMMs for ADC are often stated to be peripherally arising cancers that faithfully reproduce the steps of human multistage pathogenesis.31 However, to lung cancer pathologists, there are some similarities and many differences. Most ADC GEMMs result in rapid and generalized hyperplasia of type 2 cells. This is said to resemble atypical adenomatous hyperplasia (AAH), an early preneoplastic stage in human lung cancer.33 However AAH lesions are usually discrete and small with some degree of inter-alveolar fibrosis, not generalized lesions involving much of the peripheral lung. The type 2 hyperplasia of ADC GEMMs may cause death or elective sacrifice from respiratory distress, before the lesions can progress. Mice that survive may develop adenoma formation, a minority of which may develop dysplasia and even later, invasive or metastatic carcinomas. However most mice die before tumors can develop. Adenoma formation is not part of human multistage pathogenesis. GEMMs for ADC have the least resemblance to the development of the human counterpart compared to GEMMs for SCC or NE carcinomas (see below). These differences do not diminish the overall importance of these models, and GEMMs for Egfr and Eml-Alk are excellent models to study therapies targeted towards these drivers.
It is becoming clear that KRAS driven lung cancers are not a single homogenous entity,34 and findings from GEMMs confirm the human observations.35 Of great interest, chemical carcinogen induced models and GEMMs may result in identical Kras activating mutations, although they have very different signatures and secondary alterations.35 Thus carcinogen models may play a role in understanding the multiple pathogenic pathways resulting from activation of a single oncogene.
GEMMs for NE carcinomas of the lung
A decade ago Berns and colleagues developed a GEMM for SCLC based on the finding that p53 and Rb1 were almost always inactivated in SCLC.36 This double knockout model closely recapitulated the histology and metastatic pattern of SCLC, but had a relatively long latent period. Several triple knockout variants of the basic model have been developed, specifically to reduce the long latent period, as recently reviewed.37 However, these variations often have more complex histologies, reflecting the spectrum of high grade NE carcinoma of the lung. The resultant histological phenotypes were influenced by the introduction of specific genetic alterations, by inactivation of one or both alleles of specific genes, by time from Cre activation and by targeting of lung epithelial cells in general or specific targeting of NE cell subpopulations. The lengthy latent time permitted observations of the preneoplastic and premalignant stages of SCLC development, which are seldom observed in human tumors because of the explosive growth of SCLC once it becomes invasive. The long latent period is caused by the development of secondary genetic changes required for tumor formation such as alterations of the PTEN and NFIB genes.38 A recent review37 concluded that GEMM models studied are representative for the entire spectrum of human high-grade NE carcinomas, including SCLC, large cell neuroendocrine carcinomas (LCNEC) and NSCLC with NE cell properties (NSCLC-NE) and are also useful for the study of multistage pathogenesis and the metastatic properties of these tumors. In some of the triple knockout models the LCNEC component was more dominant than the SCLC component. It also appeared as if these two components demonstrated plasticity and could alter from one to the other and were often closely intermixed. At early time points in situ lesions or in situ lesions with an invasive component can be observed. Because of the explosive growth of SCLC, and because SCLC tumors are seldom resected, preneoplastic and preinvasive lesions are seldom if ever observed in human tumors. Thus GEMMs for NE carcinomas provide the only opportunity to study the multistage pathogenesis of SCLC and other high grade NE carcinomas of the lung. Unlike GEMMs for NSCLC, explosive, widespread metastases often develop relatively early in the course of NE carcinoma GEMM development and mimic the patterns seen in SCLC – extensive vascular and lymphatic invasion, massive mediastinal nodal spread and multiple gross and microscopic hepatic metastases.37 Of interest, in tumors consisting of mixed SCLC and LCNEC histologies, the SCLC component was usually the predominant or sole histology in metastases, indicating that SCLC has more metastatic potential than LCNEC. They represent one of the most advanced forms of currently available GEMM models for the study of human cancer.37
GEMMs for lung squamous cell carcinomas (SCC)
There are relatively few mouse models for SCC. This may reflect the fact that there are no squamous cells in the normal respiratory epithelium, and SCC presumably arises from metaplastic squamous cells that appear after chronic irritation from exposure to cigarette smoke or other reasons including chronic inflammation, which is often a prominent feature in the lungs of smokers.
The metaplastic epithelium may then be subjected to progressive preneoplastic and preinvasive steps, ending in invasive SCC. These steps include squamous dysplasia of increasing degree and squamous in situ carcinoma. As lung chemoprevention trials have been largely performed using squamous dysplasia as an endpoint, mouse models are important for chemoprevention studies and for the development and testing of novel therapies for SCC. The cancers in these models arise after following the same multistage pathogenesis steps that precede the onset of human SCC.
Chemically induced and GEMM models for SCC exist, as recently reviewed.39 Three GEMM models for SCC also have recently been described. An important feature of the GEMM models for SCC is that they appear to follow a similar multistage pathogenesis as do human SCC. Simultaneous inactivation of Stk1 (Lkb1) and activation of mutant Kras result in a spectrum of histological types, including SCC, and have been utilized for chemoprevention studies.39 The shortcoming of this model is that Lkb1 and Kras inactivation are features more closely associated with ADC, and the mixture of the resultant tumor histologies. Another recent report found that kinase dead Ikkα knockin mice develop spontaneous SCC with down regulation of Ikkα and marked pulmonary inflammation.40 Ikkα is an integral component of Ikk, required for the maturation of squamous cells, and may play a role in tumor development. A third recent report found that loss of Lkb1 and Pten leads to purely SCC tumors with features of bronchial basal cells and other characteristics of human SCC.41 Of interest, the resultant tumors had elevated PDL1 expression. PDL1 expression is believed to facilitate the escape of tumor cells from immune surveillance, and is currently the intense focus of several clinical lung cancer trials.
Some recent applications for preclinical lung cancer models
“Conditionally Reprogrammed Cells”
Recently Richard Schlegel and colleagues from Georgetown University described the “Georgetown method” for the propagation of epithelial cells of non-malignant and malignant origin.42 The resultant “Conditionally Reprogrammed Cells” (CRC) had properties of epithelial stem cells. The success rates, even from small numbers of cells, were high. The method utilized an irradiated mouse 3T3 cell feed layer and the addition of a RHO kinase (ROCK) inhibitor. The irradiated cells provided growth factors while the ROCK inhibitor had multiple functions including suppression of CDKN2A. These findings were widely utilized to generate many new putative lung cancer TCLs, mainly of NSCLC origin, by multiple labs. Our extensive characterization (Boning Gao, AFG and JDM, unpublished) of CRC cells established from NSCLC specimens indicated robust growth of epithelial cells apparently free of fibroblast contamination. However, characterization of the cells indicated that they mostly had properties of respiratory epithelial basal stem cells derived from non-malignant cells, and were diploid and lacked mutations present in the corresponding tumors. However, some specimens contained a minority of tumor cells. These results suggest, at least for lung cancer specimens, that the CRC method preferentially grows the non-malignant epithelial stem cell component present in all lung cancer resections.
Using a modification of the original CRC method, substituting human feeder layer cells for mouse, and using recurrent tumors, mainly metastatic, a recent report claimed to have established several lung cancer cell lines that retained the original mutations present in the tumors.36 However it is was not possible to determine from the report whether the resultant cell lines consisted of tumor cells or mixtures of tumor and non malignant cells.
Circulating tumor cells – a new source for PDX formation
As previously discussed, SCLC tumor materials are hard to obtain for laboratory studies. It has been known for some time that SCLC is associated with high numbers of circulating tumor cells (CTCs). A resourceful study demonstrated that CTCs from SCLC patients could be utilized to form PDXs in immune-compromised mice (termed CDXs), and that the resultant CDXs mirrored the donor patient’s response to platinum and etoposide chemotherapy.43 Genomic analysis of isolated CTCs revealed considerable similarity to the corresponding CDX. These unique mouse models provide systems for therapy testing and for understanding drug resistance mechanisms. It remains to be determined if TCLs can be obtained from CTCs or whether CDXs could be used as a source for establishing new cell lines.
Are three better than two (dimensions)?
Several recent reports have suggested that three dimensional in vitro growth more closely resembles the natural growth characteristics of patient tumors, and may be more representative of drug response.44 Unlike two dimensional cultures, spheroid or 3D cultures may maintain polarity, differentiation, are enriched for stem cells and more closely resemble glandular organs.45 Additional advantages include non-uniform exposure to drugs/compounds, oxygen and nutrients, extracellular matrix-to-cell signaling, proliferation gradients, paracrine signaling and increased cell-to-cell interactions.46 Both cell lines and tumors may be adapted to spheroid culture, as has been shown for lung tumors.47 SCLC lines have the additional advantage of natural growth as spheroids or floating cell aggregates, and are enriched for stem cell populations.48
In vitro models for respiratory epithelium and for multistage pathogenesis
Non malignant lung adjacent to resected lung cancers is often used as “normal controls”. There are problems with this concept: a) there is little histologically normal lung in heavy smokers suffering from chronic inflammation and chronic obstructive pulmonary disease (COPD); b) field changes may be present throughout the lung from widespread exposure to tobacco smoke and other carcinogens, even in histologically “normal” lung; and c) the respiratory epithelial cells represent a small minority of the cells in peripheral lung. In an effort to develop better control cells, our group and others have immortalized respiratory cells from large and small airways without the use of viral oncogenes.49 Such cells from both sources have properties of basal stem cells of the bronchi, which are believed to be the progenitor cells of lung SCC. Sequential manipulations of genes (both oncogenes and tumor suppressor genes) involved in the pathogenesis of lung cancers results in a model for multistage pathogenesis of lung cancers, mainly SCC and large cell carcinomas.50 Inactivation of STK11 (in combination with other oncogenic manipulations) results in a wider range of morphologies including adenosquamous carcinomas.51
Mouse avatars for personalized therapy selection – a mighty mouse?
Until now we have discussed the role of preclinical models – but can they really lead to improved patient management? One potential direct clinical application from xenografts is the so-called mouse avatars26 (http://www.the-scientist.com/?articles.view/articleNo/42470/title/My-Mighty-Mouse/) – PDXs utilized for selection of personalized therapy selection. A commercial company is currently offering to personalize oncology drug selection using such a methodology. While of great theoretical interest, problems include the long latent time until therapy selection, the relatively high cost, and insufficient data to fully evaluate the usefulness of the method.
A CRISPR application
The CRISPR/Cas9 system of RNA guided genome editing is having a profound effect on many aspects of genetics research.52 CRISPR (Clustered Regularly Interspersed Short Palindromic Repeats) has applications to mouse models, including generation of null, conditional, precisely mutated, reporter or tagged alleles in mice.53 There are already several CRSPR applications to study mouse genomics and tumor biology52,54 and their use in lung cancer mouse models will surely follow in the near future.
Summary
Preclinical models have multiple specific and overlapping uses as well as limitations.1 Potential applications include preclinical development of novel targeted therapies in genetically defined tumors, an enhanced understanding of drug resistance and mechanisms to bypass this, identification of potential biomarkers and imaging techniques for early detection, testing drugs for cancer prevention, and mechanisms of invasion and metastasis, and to determine the effects of environmental factors on tumor progression. 30
Cell lines and PDXs represent the vast spectrum and heterogeneity of lung cancers. Cell lines, in particular are useful for studying biology, and are often used as the first screen for detection of conventional and targeted therapies. They may also be used for synthetic lethal screen identification of vulnerabilities in cancer cells.55 PDXs have the major advantage of having stromal cells and a vascular system (although of murine origin). They are often utilized as a secondary screen prior to moving therapeutic targets to the clinic. They may also be used for selection of personalized medicine (“mouse avatars”), although the lengthy lag time may mandate administration of non-personalized therapy during this period. The role of the tumor microenvironment and selection of the ideal inoculation site may vary with the application. In particular, does orthotopic transplantation offer concrete benefits over the more easily administered and monitored subcutaneous route? GEMM models are easily genetically modified, and may incorporate multiple genetic changes into a single model. As the GEMM models for lung cancer are not smoke induced, the genetic changes in the resultant tumors lack the characteristic mutational signatures of tobacco-associated malignancies. They are highly suitable for studying tumor initiation and progression including metastases.30,32,56 Their responses to targeted therapies may be dramatic. Despite these advances, clinical trials, especially of conventional therapies selected by testing preclinical models, have often yielded disappointing results. Thus much work remains to be performed on how to make the preclinical models more predictive of clinical response.
Despite their limitations, In vitro models remain the single most important source of knowledge about the non-clinical aspects of lung cancer and will likely remain so into the foreseeable future. Additional or improved recently developed approaches and methods may aid the usefulness of the models and provide greater versatility. Technical improvements have greatly aided the success rate of generating models as well as the ability to rapidly generate a wide variety of GEMMs of increasing molecular complexity. In order to capture the diversity and intra-tumor heterogeneity of human lung cancers, large banks of each model, preferably fully characterized, must be available to the scientific community. Because generation of such extensive, well characterized models is beyond the scope of most individual laboratories, close co-operation between investigators and sharing of pooled resources will be essential in order to maximize the usefulness of preclinical34 models. With the heightened interest in PDXs,23,24 especially for the testing of conventional and targeted therapies, it becomes important to establish large banks of the different tumor types, so as to capture the genetic diversity and heterogeneity of human tumors as well as the range of drug sensitivities. Cell line banks are operated by several commercial and not fro profit organizations as well as by the originators of large collections. Because of the cost and effort of establishing and maintaining PDX banks, they are best performed by consortiums that pool resources and make them available to the scientific public. One such effort is by the newly established European EurOPDX initiative.25 We are unaware of a central bank for the distribution of GEMMs.
The major preclinical models for lung cancer each have their individual strengths and weaknesses. Each model has to be carefully evaluated for its suitability for the proposed experimental approach.
Fig. 1.
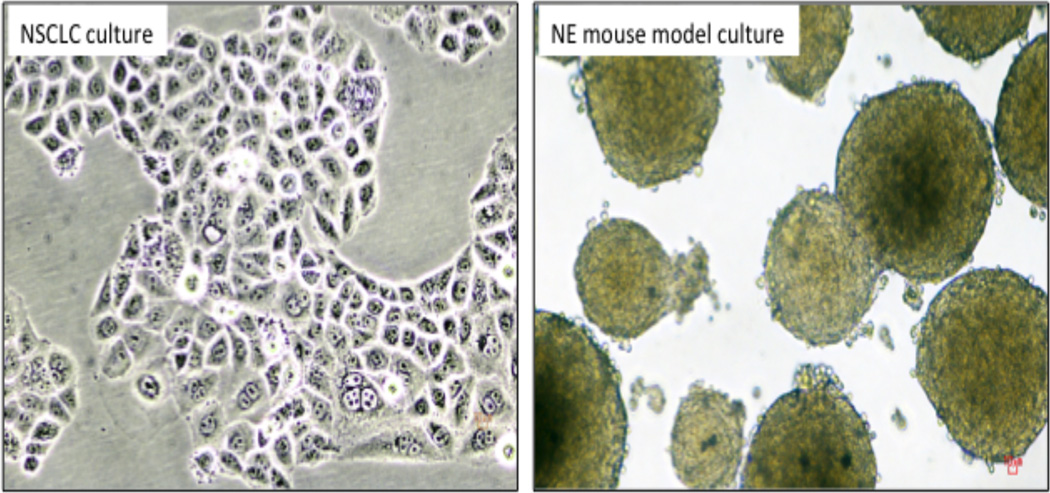
Lung cancer cell lines. Fig. 1A. NSCLC culture HCC1897, growing as flat adherent cells. Fig.1 B. SCLC culture, triple knockout mouse model. Both mouse and human SCLC cultures usually grow as floating aggregates or true spheroids (as illustrated). The larger spheroids frequently develop necrotic or hollow centers.
Fig 2.
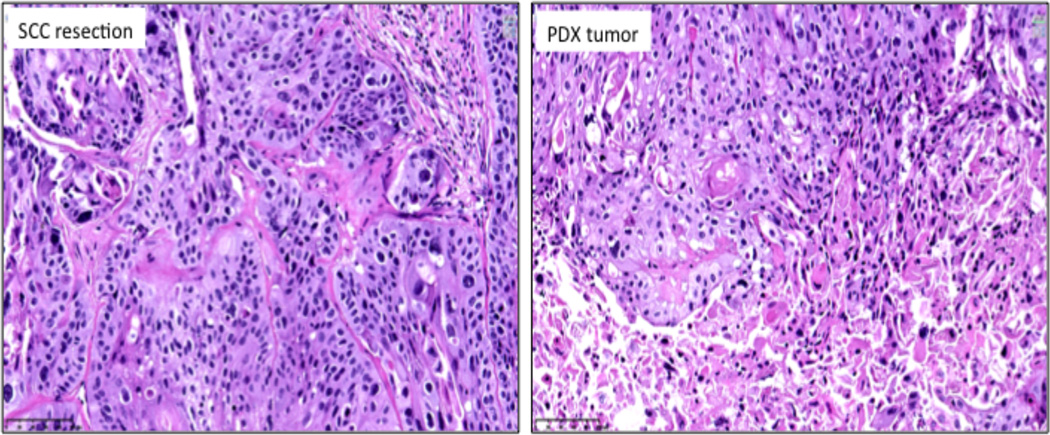
PDX of moderately differentiated SCC. The histological appearances of the PDX are similar to those of the original lung cancer.
Fig. 3.
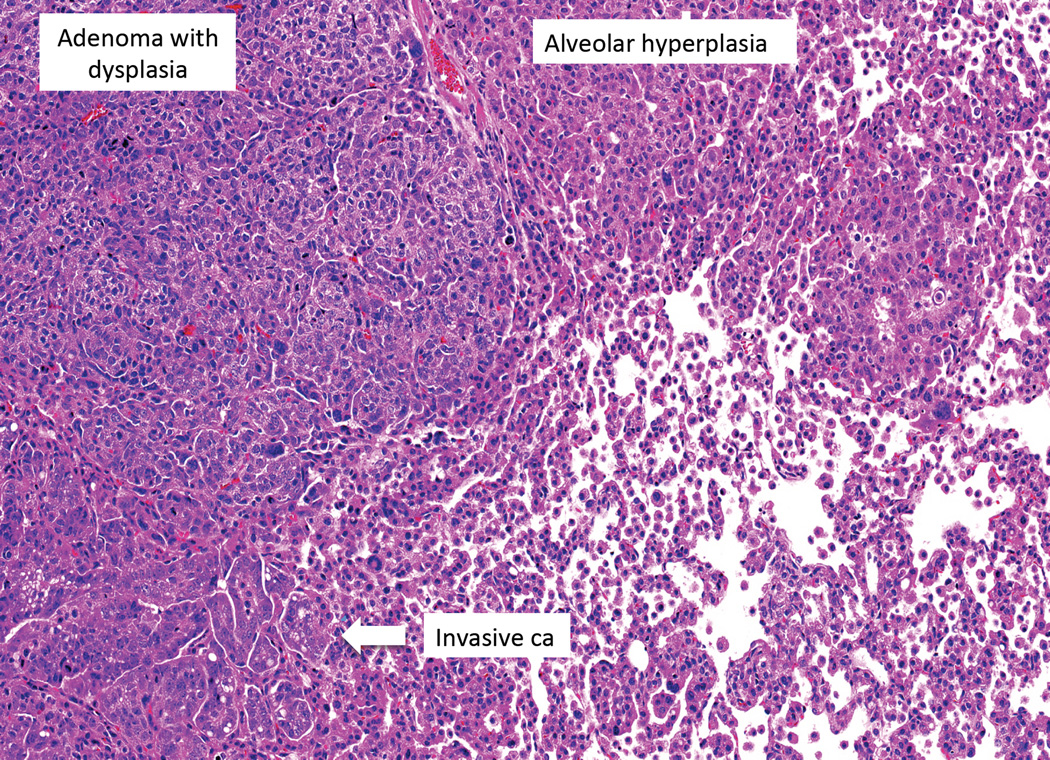
Typical Kras driven GEMM Model for lung adenocarcinoma. The most striking feature is the massive alveolar cell hyperplasia. If the mice do not die of respiratory failure, adenomas may develop which progress to adenomas with dysplasia, adenocarcinoma in situ and invasive carcinoma. Courtesy of James Kim
Fig 4.
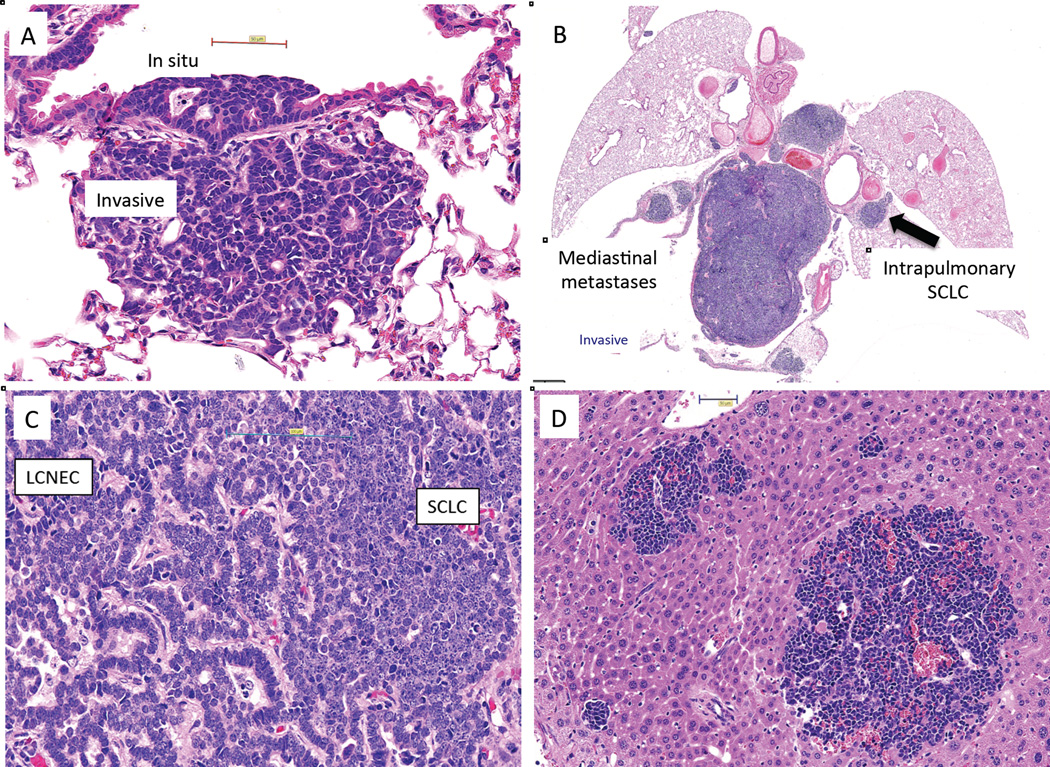
GEMM models for neuroendocrine lung cancers. Fig. 2a, Early central lesion arising in a large bronchus. Both the in situ and invasive components are characteristic of LCNEC with pseudoglandular formation. Fig. 2b. Massive mediastinal spread of SCLC component, even though corresponding intrapulmonary tumors are relatively small. Fig. 2C. Mixed tumor with LCNEC (on left) and SCLC (on right) components blending into each other. Fig 2D. Liver metastases having SCLC elements only, even though the primary tumor had both SCLC and LCNEC components. The SCLC component predominated in metastatic lesions, irrespective of the histology of the corresponding lung tumors. Triple knockout model (TP53-, RB1-, p130-) courtesy of Julien Sage, Trisha Savage and Jane Johnson.
Acknowledgments
Source of Funding: This work was supported by National Cancer Institute Specialized Program of Research Excellence in Lung Cancer Grant P50CA70907
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The Authors declare that they have no conflicts of Interest.
References
- 1.Kellar A, Egan C, Morris D. Preclinical murine models for lung cancer: Clinical trial applications. BioMed research international. 2015;2015 doi: 10.1155/2015/621324. Article ID 621324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gazdar AF, Girard L, Lockwood WW, et al. Lung cancer cell lines as tools for biomedical discovery and research. Journal of the National Cancer Institute. 2010;102:1310–1321. doi: 10.1093/jnci/djq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gazdar AF, Gao B, Minna JD. Lung cancer cell lines: Useless artifacts or invaluable tools for medical science? Lung cancer (Amsterdam, Netherlands) 2010;68:309–318. doi: 10.1016/j.lungcan.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillet JP, Varma S, Gottesman MM. The clinical relevance of cancer cell lines. Journal of the National Cancer Institute. 2013;105:452–458. doi: 10.1093/jnci/djt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ince TA, Sousa AD, Jones MA, et al. Characterization of twenty-five ovarian tumour cell lines that phenocopy primary tumours. Nature communications. 2015;6:7419. doi: 10.1038/ncomms8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oboshi S, Tsugawa S, Seido T, et al. A new floating cell line derived from human pulmonary carcinoma of oat cell type. Gan. 1971;62:505–514. [PubMed] [Google Scholar]
- 7.Scherer WF, Syverton JT, Gey GO. Studies on the propagation in vitro of poliomyelitis viruses IV Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J Exp Med. 1953;97:695–710. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed MV, Gey GO. Cultivation of normal and malignant human lung tissue I. The establishment of three adenocarcinoma cell strains. Lab Invest. 1962;11:638–652. [PubMed] [Google Scholar]
- 9.Blanco R, Iwakawa R, Tang M, et al. A gene-alteration profile of human lung cancer cell lines. Hum Mutat. 2009;30:1199–1206. doi: 10.1002/humu.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pleasance ED, Stephens PJ, O’Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463:184–190. doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman LP, Gibson MC, Ethier SP, et al. Reproducibility: changing the policies and culture of cell line authentication. Nat Methods. 2015;12:493–497. doi: 10.1038/nmeth.3403. [DOI] [PubMed] [Google Scholar]
- 12.Giard DJ, Aaronson SA, Todaro GJ, et al. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. Journal of the National Cancer Institute. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi J, Zhang J, Xie Y, et al. Alterations in genes of the EGFR signaling pathway and their relationship to EGFR tyrosine kinase inhibitor sensitivity in lung cancer cell lines. PLoS One. 2009;4:e4576. doi: 10.1371/journal.pone.0004576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sasaki T, Koivunen J, Ogino A, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer research. 2011;71:6051–6060. doi: 10.1158/0008-5472.CAN-11-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettengill OS, Sorenson GD, Wurster-Hill DH, et al. Isolation and growth characteristics of continuous cell lines from small-cell carcinoma of the lung. Cancer. 1980;45:906–918. doi: 10.1002/1097-0142(19800301)45:5<906::aid-cncr2820450513>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Gazdar AF, Carney DN, Russell EK, et al. Establishment of continuous, clonable cultures of small-cell carcinoma of lung which have amine precursor uptake and decarboxylation cell properties. Cancer Res. 1980;40:3502–3507. [PubMed] [Google Scholar]
- 17.Phelps RM, Johnson BE, Ihde DC, et al. NCI-Navy Medical Oncology Branch cell line data base. J Cell Biochem. 1996;(Suppl. 24):32–91. doi: 10.1002/jcb.240630505. [DOI] [PubMed] [Google Scholar]
- 18.Gazdar AF, Carney DN, Nau MM, et al. Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res. 1985;45:2924–2930. [PubMed] [Google Scholar]
- 19.Rosfjord E, Lucas J, Li G, et al. Advances in patient-derived tumor xenografts: from target identification to predicting clinical response rates in oncology. Biochem Pharmacol. 2014;91:135–143. doi: 10.1016/j.bcp.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Isobe T, Onn A, Morgensztern D, et al. Evaluation of novel orthotopic nude mouse models for human small-cell lung cancer. J Thorac Oncol. 2013;8:140–146. doi: 10.1097/JTO.0b013e3182725ff9. [DOI] [PubMed] [Google Scholar]
- 21.Gazdar AF, Carney DN, Sims HL, et al. Heterotransplantation of small-cell carcinoma of the lung into nude mice: comparison of intracranial and subcutaneous routes. Int J Cancer. 1981;28:777–783. doi: 10.1002/ijc.2910280617. [DOI] [PubMed] [Google Scholar]
- 22.Hao C, Wang L, Peng S, et al. Gene mutations in primary tumors and corresponding patient-derived xenografts derived from non-small cell lung cancer. Cancer Lett. 2015;357:179–185. doi: 10.1016/j.canlet.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stewart EL, Mascaux C, Pham NA, et al. Clinical Utility of Patient-Derived Xenografts to Determine Biomarkers of Prognosis and Map Resistance Pathways in EGFR-Mutant Lung Adenocarcinoma. J Clin Oncol. 2015;33:2472–2480. doi: 10.1200/JCO.2014.60.1492. [DOI] [PubMed] [Google Scholar]
- 24.Gandara DR, Lara PN, Jr, Mack PC. Patient-Derived Xenografts for Investigation of Acquired Resistance in Oncogene-Driven Cancers: Building a Better Mousetrap. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.61.9692. [DOI] [PubMed] [Google Scholar]
- 25.Hidalgo M, Amant F, Biankin AV, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4:998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malaney P, Nicosia SV, Dave V. One mouse, one patient paradigm: New avatars of personalized cancer therapy. Cancer Lett. 2014;344:1–12. doi: 10.1016/j.canlet.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang YA, Maitra A, Hsieh JT, et al. Frequent detection of infectious xenotropic murine leukemia virus (XMLV) in human cultures established from mouse xenografts. Cancer Biol Ther. 2011;12:617–628. doi: 10.4161/cbt.12.7.15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vikis HG, Rymaszewski AL, Tichelaar JW. Mouse models of chemically-induced lung carcinogenesis. Front Biosci (Elite Ed) 2013;5:939–946. doi: 10.2741/e673. [DOI] [PubMed] [Google Scholar]
- 29.Kwon MC, Berns A. Mouse models for lung cancer. Mol Oncol. 2013;7:165–177. doi: 10.1016/j.molonc.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Politi K, Pao W. How genetically engineered mouse tumor models provide insights into human cancers. J Clin Oncol. 2011;29:2273–2281. doi: 10.1200/JCO.2010.30.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farago AF, Snyder EL, Jacks T. SnapShot: Lung cancer models. Cell. 2012;149:246–246. e241. doi: 10.1016/j.cell.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Taguchi A, Politi K, Pitteri SJ, et al. Lung cancer signatures in plasma based on proteome profiling of mouse tumor models. Cancer cell. 2011;20:289–299. doi: 10.1016/j.ccr.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Travis W, Brambilla E, Noguchi M, et al. The new IASLC/ATS/ERS international multidisciplinary lung adenocarcinoma classification. Journal of Thoracic Oncology. 2009;4:244–285. [Google Scholar]
- 34.Skoulidis F, Byers LA, Diao L, et al. Co-occurring genomic alterations define major subsets of KRAS - mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015 doi: 10.1158/2159-8290.CD-14-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westcott PM, Halliwill KD, To MD, et al. The mutational landscapes of genetic and chemical models of Kras-driven lung cancer. Nature. 2015;517:489–492. doi: 10.1038/nature13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meuwissen R, Linn SC, Linnoila RI, et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer cell. 2003;4:181–189. doi: 10.1016/s1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 37.Gazdar AF, Savage TK, Johnson JE, et al. The comparative pathology of genetically engineered mouse models for neuroendocrine carcinomas of the lung. J Thorac Oncol. 2015;10:553–564. doi: 10.1097/JTO.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McFadden DG, Papagiannakopoulos T, Taylor-Weiner A, et al. Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell. 2014;156:1298–1311. doi: 10.1016/j.cell.2014.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You MS, Rouggly LC, You M, et al. Mouse models of lung squamous cell carcinomas. Cancer Metastasis Rev. 2013;32:77–82. doi: 10.1007/s10555-012-9406-4. [DOI] [PubMed] [Google Scholar]
- 40.Xiao Z, Jiang Q, Willette-Brown J, et al. The pivotal role of IKKalpha in the development of spontaneous lung squamous cell carcinomas. Cancer cell. 2013;23:527–540. doi: 10.1016/j.ccr.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu C, Fillmore CM, Koyama S, et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer cell. 2014;25:590–604. doi: 10.1016/j.ccr.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Ory V, Chapman S, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. The American journal of pathology. 2012;180:599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodgkinson CL, Morrow CJ, Li Y, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med. 2014;20:897–903. doi: 10.1038/nm.3600. [DOI] [PubMed] [Google Scholar]
- 44.Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today. 2013;18:240–249. doi: 10.1016/j.drudis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Vidi PA, Bissell MJ, Lelievre SA. Three-dimensional culture of human breast epithelial cells: the how and the why. Methods Mol Biol. 2013;945:193–219. doi: 10.1007/978-1-62703-125-7_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lovitt CJ, Shelper TB, Avery VM. Advanced cell culture techniques for cancer drug discovery. Biology (Basel) 2014;3:345–367. doi: 10.3390/biology3020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Endo H, Okami J, Okuyama H, et al. Spheroid culture of primary lung cancer cells with neuregulin 1/HER3 pathway activation. J Thorac Oncol. 2013;8:131–139. doi: 10.1097/JTO.0b013e3182779ccf. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan JP, Spinola M, Dodge M, et al. Aldehyde Dehydrogenase Activity Selects for Lung Adenocarcinoma Stem Cells Dependent on Notch Signaling. Cancer Res. 2010;70:9937–9948. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramirez RD, Sheridan S, Girard L, et al. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 2004;64:9027–9034. doi: 10.1158/0008-5472.CAN-04-3703. [DOI] [PubMed] [Google Scholar]
- 50.Sato M, Larsen JE, Lee W, et al. Human Lung Epithelial Cells Progressed to Malignancy through Specific Oncogenic Manipulations. Mol Cancer Res. 2013;11:638–650. doi: 10.1158/1541-7786.MCR-12-0634-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim HS, Mendiratta S, Kim J, et al. Systematic identification of molecular subtype-selective vulnerabilities in non-small-cell lung cancer. Cell. 2013;155:552–566. doi: 10.1016/j.cell.2013.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh P, Schimenti JC, Bolcun-Filas E. A mouse geneticist’s practical guide to CRISPR applications. Genetics. 2015;199:1–15. doi: 10.1534/genetics.114.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanchez-Rivera FJ, Jacks T. Applications of the CRISPR-Cas9 system in cancer biology. Nature Rev Cancer. 2015;15:387–395. doi: 10.1038/nrc3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen S, Sanjana NE, Zheng K, et al. Genome-wide CRISPR Screen in a Mouse Model of Tumor Growth and Metastasis. Cell. 2015;160:1246–1260. doi: 10.1016/j.cell.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitehurst AW, Bodemann BO, Cardenas J, et al. Synthetic lethal screen identification of chemosensitizer loci in cancer cells. Nature. 2007;446:815–819. doi: 10.1038/nature05697. [DOI] [PubMed] [Google Scholar]
- 56.Regales L, Gong Y, Shen R, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. The Journal of clinical investigation. 2009;119:3000–3010. doi: 10.1172/JCI38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whang-Peng J, Kao-Shan CS, Lee EC, et al. Specific chromosome defect associated with human small-cell lung cancer; deletion 3p(14–23) Science. 1982;215:181–182. doi: 10.1126/science.6274023. [DOI] [PubMed] [Google Scholar]
- 58.Whang-Peng J, Knutsen T, Gazdar A, et al. Nonrandom structural and numerical chromosome changes in non-small-cell lung cancer. Genes Chromosomes Cancer. 1991;3:168–188. doi: 10.1002/gcc.2870030303. [DOI] [PubMed] [Google Scholar]
- 59.Burbee DG, Forgacs E, Zochbauer-Muller S, et al. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. Journal of the National Cancer Institute. 2001;93:691–699. doi: 10.1093/jnci/93.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zabarovsky ER, Lerman MI, Minna JD. Tumor suppressor genes on chromosome 3p involved in the pathogenesis of lung and other cancers. Oncogene. 2002;21:6915–6935. doi: 10.1038/sj.onc.1205835. [DOI] [PubMed] [Google Scholar]
- 61.Dammann R, Li C, Yoon JH, et al. Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat Genet. 2000;25:315–319. doi: 10.1038/77083. [DOI] [PubMed] [Google Scholar]
- 62.Harbour JW, Lai SL, Whang-Peng J, et al. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988;241:353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takahashi T, Nau MM, Chiba I, et al. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989;246:491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- 64.Little CD, Nau MM, Carney DN, et al. Amplification and expression of the c-myc oncogene in human lung cancer cell lines. Nature. 1983;306:194–196. doi: 10.1038/306194a0. [DOI] [PubMed] [Google Scholar]
- 65.Nau MM, Brooks BJ, Battey J, et al. L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature. 1985;318:69–73. doi: 10.1038/318069a0. [DOI] [PubMed] [Google Scholar]
- 66.Johnson BE, Makuch RW, Simmons AD, et al. myc family DNA amplification in small cell lung cancer patients’ tumors and corresponding cell lines. Cancer Res. 1988;48:5163–5166. [PubMed] [Google Scholar]
- 67.Shigematsu H, Takahashi T, Nomura M, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer research. 2005;65:1642–1646. doi: 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- 68.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 69.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 70.Vikis H, Sato M, James M, et al. EGFR-T790M is a rare lung cancer susceptibility allele with enhanced kinase activity. Cancer Res. 2007;67:4665–4670. doi: 10.1158/0008-5472.CAN-07-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou W, Ercan D, Janne PA, et al. Discovery of selective irreversible inhibitors for EGFR-T790M. Bioorganic & medicinal chemistry letters. 2011;21:638–643. doi: 10.1016/j.bmcl.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hammerman PS, Sos ML, Ramos AH, et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov. 2011;1:78–89. doi: 10.1158/2159-8274.CD-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]