Abstract
The mammalian Nrf/CNC proteins (Nrf1, Nrf2, Nrf3, p45 NF-E2) perform a wide range of cellular protective and maintenance functions. The most thoroughly described of these proteins, Nrf2, is best known as a regulator of antioxidant and xenobiotic defense, but more recently has been implicated in additional functions that include proteostasis and metabolic regulation. In the nematode Caenorhabditis elegans, which offers many advantages for genetic analyses, the Nrf/CNC proteins are represented by their ortholog SKN-1. Although SKN-1 has diverged in aspects of how it binds DNA, it exhibits remarkable functional conservation with Nrf/CNC proteins in other species and regulates many of the same target gene families. C. elegans may therefore have considerable predictive value as a discovery model for understanding how mammalian Nrf/CNC proteins function and are regulated in vivo. Work in C. elegans indicates that SKN-1 regulation is surprisingly complex and is influenced by numerous growth, nutrient, and metabolic signals. SKN-1 is also involved in a wide range of homeostatic functions that extend well beyond the canonical Nrf2 function in responses to acute stress. Importantly, SKN-1 plays a central role in diverse genetic and pharmacologic interventions that promote C. elegans longevity, suggesting that mechanisms regulated by SKN-1 may be of conserved importance in aging. These C. elegans studies predict that mammalian Nrf/CNC protein functions and regulation may be similarly complex and that the proteins and processes that they regulate are likely to have a major influence on mammalian life- and healthspan.
Keywords: Aging, C. elegans, Detoxification, Metabolism, Proteasome, SKN-1/Nrf, Stress response
1. Background and overview
The Nrf (NF-E2-related factor)/CNC family of transcription regulators are named after their founding member, p45 NF-E2, and are defined by the presence of the CNC (Cap’n’ collar) domain and adjacent basic region (BR), which are located within their DNA-binding domain (Fig. 1A) [1–4]. The Nrf/CNC proteins are unrelated to the nuclear respiratory factor (NRF) transcription factors, which regulate nuclearly encoded mitochondrial genes. Of the mammalian Nrf/CNC proteins (Nrf1, Nrf2, Nrf3, p45 NF-E2), Nrf2 is by far the most extensively studied. As is described below, Nrf2 is well known to function in stress responses, but along with other Nrf/CNC family members, it is also emerging as a central regulator of various aspects of metabolism and other functions [1,3–5].
Fig. 1.
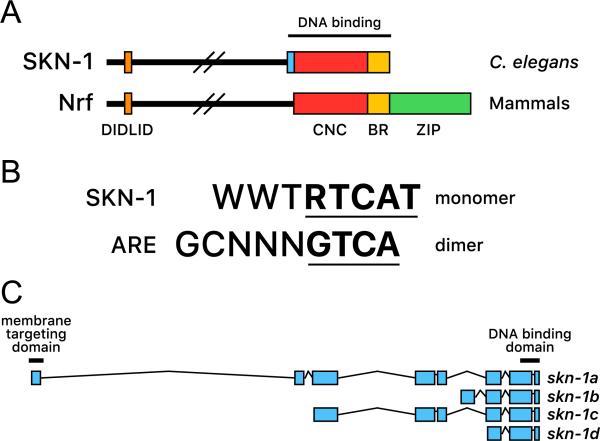
SKN-1 is the C. elegans ortholog of mammalian Nrf/CNC proteins. (A) Similarities between SKN-1 and Nrf/CNC proteins. SKN-1 and Nrf/CNC proteins are most closely related within the DIDLID and DNA-binding regions [6,17]. SKN-1 interacts directly with specific DNA sequences through its BR, which is highly similar to that of Nrf/CNC proteins, and a short element that binds in the minor groove (light blue) [11]. The CNC domain stabilizes these interactions. (B) Comparison of SKN-1 binding site with the ARE. SKN-1 and Nrf-Maf dimers each bind to sequences that include a consensus bZIP protein half-site (underlined), but diverge within the reminder of the binding site, where SKN-1 binds the consensus WWT in the minor groove [11]. (C) Simple diagram of SKN-1 isoforms. Expression of SKN-1d is predicted but has not been confirmed in vivo. skn-1 exons are drawn approximately to scale (WormBase Web site, skn-1, available at http://www.wormbase.org/db/get?name=skn-1:class=Gene).
In the nematode Caenorhabditis elegans, the simplest major multicellular model organism, the Nrf/CNC proteins are represented by their sequence and functional ortholog SKN-1 [6,7]. C. elegans is a highly advantageous organism for genetic and other in vivo analyses [8]. SKN-1 has diverged considerably from mammalian Nrf/CNC proteins with respect to its mode of DNA binding (Figs. 1A and B). However, as we describe below, the degree of functional conservation between SKN-1 and these proteins is remarkable. These similarities suggest that C. elegans SKN-1 provides a powerful model for investigating how Nrf2 and other Nrf/CNC proteins are regulated, and how they influence the development and functions of normal tissues in vivo. Importantly, in C. elegans, SKN-1 plays a central role in many regulatory pathways and interventions that extend lifespan. This suggests that Nrf/CNC proteins are likely to be important in aging across the evolutionary spectrum, a model for which support continues to build.
Nrf/CNC proteins are basic-leucine zipper (ZIP) transcription factors that require their Maf dimerization partner to bind DNA stably [9], but SKN-1 lacks the ZIP dimerization module (Fig. 1A) [10,11]. SKN-1 has been endowed by evolution with a clever solution to this problem, however. As it turns out, the SKN-1 DNA binding region is built around a “souped-up” version of the CNC domain that forms a relatively stable helical structure, along with an additional peptide motif with which SKN-1 recognizes bases in the DNA minor groove (Fig. 1A) [11–15]. SKN-1 therefore binds to its cognate sites on its own with affinity comparable to that of a bZIP dimer [11,12]. Amazingly, although the SKN-1 recognition preference has diverged from the antioxidant response element recognized by mammalian Nrf/CNC-Maf dimers (Fig. 1B), SKN-1 directly regulates many of the very same genes that are known to be Nrf/CNC targets (described below). Regulation of these many diverse targets must have been preserved as SKN-1 and the other Nrf/CNC proteins diverged from a common precursor.
Outside their DNA-binding region, the homology between SKN-1 and Nrf/CNC proteins is limited except for a highly conserved 14-residue motif called DIDLID, which is a unique signature of the Nrf/CNC protein family [6,16] (Fig. 1A). This conservation suggests a critical function, and in SKN-1 the DIDLID element is important for transcription activation [6] and interaction with the protein WDR-23, which targets SKN-1 for proteasomal degradation (described below) [17]. However, we still do not understand why the function of the DIDLID element is important enough to have warranted this degree of conservation. Other SKN-1 regions appear to activate transcription by binding to the p300/CBP acyltransferases [6], another parallel between SKN-1 and Nrf2 [18]. Four major predicted isoforms are encoded by the skn-1 gene, three of which have been shown to be expressed in vivo (SKN-1a-c; Fig. 1C) [7,19,20]. Genetic studies have yielded considerable information about requirements for skn-1, which may represent the entire group of mammalian Nrf/CNC proteins, but we still understand relatively little about the functions of the individual SKN-1 isoforms.
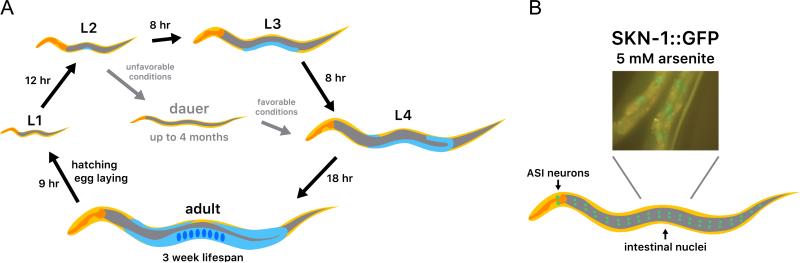
In C. elegans, protein expression in individual tissues is most commonly examined through translational fusion to green fluorescent protein (GFP) or a similar tag [21]. Using this assay, SKN-1 expression has been detected throughout the C. elegans life cycle (Fig. 2A). During embryogenesis, zygotically expressed SKN-1 is present in nuclei in precursors of the intestine, the worm counterpart to the mammalian digestive system (liver, adipose tissue, pancreas, and gut) [7]. In contrast, during larval and adult stages, intestinal SKN-1 is predominantly cytoplasmic, but accumulates in nuclei in response to stress (Fig. 2B) [7,22]. This intestinal SKN-1 expression seems to be derived predominantly from the SKN-1a and SKN-1c isoforms [7,19,20]. SKN-1a includes an N-terminal transmembrane segment (Fig. 1C) and has been detected in mitochondrial and endoplasmic reticulum (ER) preparations [20,23,24]. This suggests that SKN-1a may correspond to Nrf1, which is inserted in the ER membrane and must be cleaved in order to be released [25,26]. This transmembrane segment is absent from SKN-1c (Fig. 1C), which may correspond to Nrf2. The C. elegans adult “brain” and nervous system consist of a network of 302 neurons [27]. During all postembryonic stages, the short isoform SKN-1b is highly prominent in the nuclei of the two ASI chemosensory neurons, which sense food and regulate metabolism, and may correspond to a rudimentary hypothalamus (Fig. 2B) [19]. In developing larvae, some additional head neurons express the SKN-1a isoform [20].
Fig. 2.
Analysis of SKN-1 expression in vivo. (A) C. elegans life cycle stages. The hermaphrodite life cycle is shown [8]. The majority of C. elegans produced are hermaphrodites, which are typically studied in the laboratory. A small proportion of males are produced within each generation of approximately 300 animals. The pharynx, a pumping organ used for feeding, is shown in orange, the intestine in grey, the gonad in light blue, and embryos in dark blue. (B) SKN-1 protein expression. The expression pattern of a transgene that encodes the SKN-1b and SKN-1c isoforms is shown under stress conditions [7]. The SKN-1b isoform is expressed constitutively in the nuclei of the ASI neurons, and SKN-1c accumulates in intestinal nuclei in response to certain stresses [7,19].
Given that the intestine not only mediates digestion but also is the major detoxification and biosynthetic tissue, it is not surprising that many of the functions of SKN-1 have been linked to its intestinal expression [7,19,28–30]. Accordingly, analyses in which GFP is expressed from SKN-1 target gene promoters suggest that SKN-1 prominently influences gene expression in that tissue. Other evidence indicates that SKN-1 has important functions in neurons [19,31,32]. Through an unknown mechanism, SKN-1b that is expressed in the ASI neurons appears to promote oxidative metabolism in the setting of dietary restriction [19]. SKN-1 functioning in cholinergic neurons enhances synaptic function and stress resistance [31,32]. SKN-1-induced detoxification gene expression has also been detected in the hypodermis (skin), in which the SKN-1::GFP fusion protein is not readily detectable [23], indicating that SKN-1 is active in some tissues in which it is present at low levels.
While most recent studies of SKN-1 address its postembryonic functions, it is important to note that SKN-1 was initially discovered based upon an essential developmental role. In 1992, Bruce Bowerman and James Priess identified skn-1 as a gene that is required for tissue specification during the earliest stages of embryonic development [10]. At the four-cell embryonic stage, when zygotic transcription begins, SKN-1 is expressed from mRNAs that are provided maternally to the oocyte [10,33]. This SKN-1 protein initiates development of the endoderm and associated mesodermal tissues, including the entire digestive system (pharynx and intestine). In mice, Nrf1 is required for completion of embryonic development, but it is not clear whether any overlapping combination of Nrf/CNC proteins might have as fundamental a developmental role as SKN-1 [34–36]. It is intriguing, however, that many detoxification and metabolic functions of mammalian Nrf/CNC proteins are associated with the liver, just as SKN-1 specifies development of the C. elegans intestine. One speculative possibility is that during evolution, a precursor to SKN-1 was co-opted for this early developmental role based upon its preexisting detoxification and metabolic functions. Other examples have been identified in which development of individual organs is specified by transcription factors that control the major downstream functions of those organs [37,38], but in this case SKN-1 specifies development of an entire organ system that is critical for metabolism, biosynthesis, and detoxification.
2. SKN-1 functions in stress responses and homeostasis
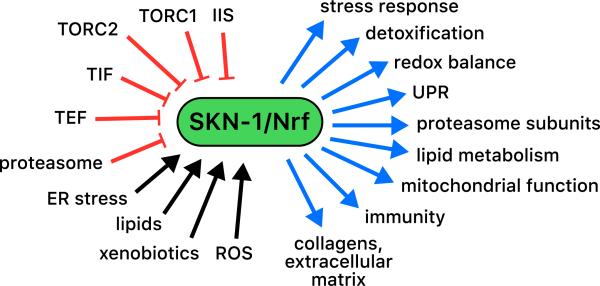
With respect to the trajectory on which its downstream functions have been uncovered, analyses of SKN-1 have paralleled studies of mammalian Nrf/CNC proteins. The initial finding that SKN-1 controls specific aspects of oxidative stress defense has been followed by an accumulating body of evidence implicating it in a wide range of detoxification processes, as well as in immunity, proteostasis, and metabolism (Fig. 3). In addition, the idea that intestinal SKN-1 inducibly responds to acute stress has given way to a more nuanced view, in which SKN-1 also functions continuously to maintain homeostasis of these various processes.
Fig. 3.
Complexity of SKN-1 functions. SKN-1 directly or indirectly controls genes involved in a wide variety of biological processes (blue arrows), overlapping subsets of which may be up-regulated by different stresses. SKN-1 is activated by stress signals (black arrows) and senses the activity of multiple cellular processes, some of which are shown here in red. TEF and TIF refer to translation elongation and initiation, disruption of which appears to activate SKN-1 through different mechanisms [50,78].
The first insights into the postembryonic functions of SKN-1 came with the finding that, like Nrf2, SKN-1 regulates Phase II detoxification genes and is needed to defend against acute oxidative stress [7]. In C. elegans, as in other eukaryotes, small molecule detoxification occurs in three phases [39,40]. In Phase I, lipophilic xenobiotics or endobiotics are solubilized by enzymes such as cytochrome P450s (CYPs) and short-chain dehydrogenases (SDHs). Reactive products generated by these or other processes are detoxified by the Phase II system, a broad category encompassing enzymes that synthesize the reducing agent glutathione (GSH), metabolize reactive molecules, or conjugate reactive groups (glutathione-S-transferases (GSTs) and UDP-glucuronosyl/glucosyl transferases (UGTs)). These reactive molecules include oxygen free radicals that arise from mitochondrial respiration or other sources. Finally, conjugated toxins are exported from cells by the Phase III proteins, including ATP-binding cassette (ABC) and other transporters. When C. elegans is exposed to acute oxidative stress, SKN-1 accumulates in intestinal nuclei (Fig. 2B) and activates Phase II detoxification genes that correspond to canonical Nrf2 targets [7]. Transcriptome profiling and further candidate gene analyses later revealed that SKN-1 induces expression of a wide variety of Phase I, II, and III detoxification proteins, including CYP, SDH, GSH biosynthetic, GST, and UGT enzymes and ABC transporters [23,30,31,41–43]. The presence of predicted SKN-1 binding sites, together with chromatin immunoprecipitation (ChIP) analyses, suggests that most of these detoxification genes are regulated directly by SKN-1 [24,29,30,41,43,44]. Thus, like Nrf2, SKN-1 controls many critical detoxification processes directly. As would be predicted, skn-1 mutants are considerably less resistant than wild-type (WT) animals to oxidative or xenobiotic stress exposure [7,24,41,45].
SKN-1 also regulates numerous genes in the absence of exogenous stress. Expression profiling has identified approximately 300 genes as being up- or down-regulated by SKN-1 in intact animals under nonstressed conditions [41]. These SKN-1-up-regulated genes include many from the expected categories of canonical detoxification target genes, but also other genes that suggest intriguing “new” SKN-1 functions. For example, many of these genes encode C-type lectins or other proteins that localize to the intestinal cell surface and are thought to have antimicrobial properties [41,46,47]. Accordingly, SKN-1 activation is required for resistance to infection from Pseudomonas aeruginosa [48,49]. It will be interesting to determine whether these little-understood cell surface proteins might have additional functions in the C. elegans gut, and whether mammalian Nrf/CNC proteins might also influence innate immunity directly. While most SKN-1-down-regulated genes appear to be controlled by SKN-1 indirectly, some are flanked by SKN-1 binding sites, suggesting the exciting but as yet unexplored idea that SKN-1 might function as either an activator or a repressor of transcription [41].
A relatively new area of importance is the role of SKN-1 in supporting proteostasis, a blanket term that covers maintenance of protein folding and clearing of damaged or aggregated proteins. In the intestine, SKN-1 maintains activity of the proteasome [50], a multisubunit structure that degrades proteins that are marked by ubiquitylation for decay [51,52]. SKN-1 increases expression of most proteasome subunit genes [41,50,53], apparently by targeting them directly [30]. If the proteasome is inhibited genetically or pharmacologically, SKN-1 up-regulates proteasome genes in a compensatory response [50]. Accordingly, proteasome inhibition is differentially toxic to skn-1 mutants [50]. Similarly, in mammals, proteasome genes are activated by Nrf1 in response to proteasome inhibition [54–56] or by Nrf2 after oxidative stress [53,57]. SKN-1 appears to be degraded by ubiquitin-mediated proteolysis [28], making it possible that it simply accumulates to higher levels when the proteasome is inhibited. However, the complexity of other aspects of SKN-1 regulation (described below) suggests that additional mechanisms are likely to be involved. For example, C. elegans responds to a loss of germ cells by activating SKN-1, thereby increasing proteasome activity, stress resistance, and lifespan (see below) [30]. An exciting possibility is that the genetic tools available in C. elegans may facilitate exploration of mechanisms through which Nrf/CNC proteins control proteasome activity, an interaction that could be important in the setting of cancer or other diseases that are treated through proteasome inhibition [52].
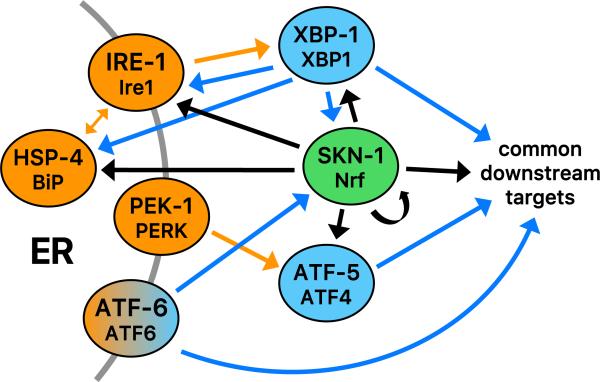
In C. elegans, SKN-1 also plays an essential role in the unfolded protein response (UPR) [24], a complex gene expression network that is activated by accumulation of unfolded proteins in the ER (ER stress) [58,59] (Fig. 4). SKN-1 directly activates expression of many of the core regulators that sense ER stress and control downstream UPR target genes, and skn-1 expression is in turn dependent upon the UPR transcription factors XBP-1 and ATF-6 (Fig. 4). SKN-1 also cooperates with those factors to control downstream targets directly and is important for resistance to ER stress [24]. Under ER stress conditions, SKN-1 activates a set of genes that differs from those activated by its response to oxidative stress. This finding builds upon a theme developed from other observations, that under different stress or metabolic conditions SKN-1 activates overlapping but distinct sets of genes that appear to be customized for the situation at hand (Fig. 3) [23,29,30,41,43,50]. The role of SKN-1 in the UPR provides a paradigm that might explain this flexibility, because it suggests that the actions of SKN-1 can be tailored through its cooperation with other transcription factors, such as XBP-1 and ATF-6 [24].
Fig. 4.
Integration of SKN-1 into the UPR. Unfolded proteins that accumulate in the ER (ER stress) are sensed by the transmembrane proteins ATF-6, PEK-1 (PERK), and IRE-1, acting in conjunction with the ER chaperone HSP-4 (BiP) [59,60]. Under ER stress conditions, the transcription factor ATF-6 is released by processing in the Golgi, and the PEK-1 (PERK) kinase inhibits translation by phosphorylating the initiation factor eIF-2α. Lower levels of translation reduce the secretory load, but also result in preferential translation of the transcription factor ATF-5 (ATF4). ER stress also induces IRE-1 to splice the mRNA encoding the transcription factor XBP-1, leading to synthesis of an active XBP-1 form. XBP-1 controls the greatest proportion of the UPR. Genetic, gene expression, and ChIP studies indicate that SKN-1 directly activates transcription of ire-1, xbp-1, hsp-4, atf-5, and itself, and cooperates with the UPR transcription factors to induce expression of downstream UPR target genes [24]. In turn, skn-1 expression is up-regulated by ATF-6 and XBP-1. The UPR encompasses activation of ER function, chaperone, and stress response genes [59] and overlaps with but is distinct from the SKN-1-mediated oxidative stress response [24]. Unfolded protein sensors or chaperones are shown in orange, and canonical UPR transcription factors in blue. Functional interactions in the canonical UPR are shown with orange arrows [59,60]. Direct transcriptional effects of canonical UPR transcription factors and SKN-1 are diagrammed with blue and black arrows, respectively [24].
The question of how SKN-1 is regulated by ER stress is also intriguing. Like Nrf1, SKN-1a includes a transmembrane domain (Fig. 1A), but it is unknown whether ER stress induces cleavage of these proteins. ER stress increases SKN-1 levels through transcription and preferential translation (discussed below) and increases direct binding of SKN-1 to downstream target genes [24]. Interestingly, however, this is not accompanied by a detectable increase in overall nuclear SKN-1 levels, as would be predicted from the standard paradigm. Apparently SKN-1 is active in the nucleus even when it is not present there at high levels, as predicted by evidence that SKN-1 regulates expression of numerous genes under nonstressed conditions [41]. Under ER stress conditions, SKN-1 that is present in the nucleus might be captured to bind target promoters through cooperative interactions with XBP-1 or other co-regulators. It has been reported that the PERK kinase phosphorylates and activates Nrf2 [60,61], but it is otherwise unexplored whether Nrf/CNC proteins are involved in the mammalian UPR. Our understanding of SKN-1 functions in the C. elegans UPR predicts that even if mammalian Nrf/CNC proteins are not activated by ER stress through the standard pathway of increased nuclear accumulation, they may nevertheless play a critical role under ER stress conditions through interactions with other UPR transcription regulators. In addition to its regulation of UPR and proteasome genes, SKN-1 also controls genes that encode chaperones, lysosomal proteases, and autophagy proteins [41], suggesting that it may promote proteostasis at many levels.
A surprising new area of interest is that SKN-1 regulates key aspects of lipid metabolism. SKN-1 mediates transcriptional responses to starvation, and under particular starvation or dietary conditions it up-regulates lipid metabolism genes and seems to induce fat mobilization [23,62]. SKN-1 also controls many lipid metabolism genes under normal conditions and responds to elevated lipid levels by activating genes involved in β-oxidation, lipolysis, fatty acid desaturation, elongation, and transport, and stress defense, many of which seem to be direct SKN-1 targets [30]. Accordingly, skn-1 mutants store excess amounts of fat [30]. Mammalian Nrf proteins affect lipid metabolism gene expression [4,23,62,63], and hepatic knockout of Nrf1 predisposes to nonalcoholic fatty liver disease (NAFLD) that progresses to nonalcoholic steatohepatitis (NASH) [63–65]. When fed a high-fat diet, Nrf2−/− mice also develop NASH [66]. Given that they store excess fat in their intestine (gut/liver), skn-1 mutants may provide a C. elegans model for studying NAFLD development [30]. Reduced Nrf/CNC protein function is thought to trigger NASH by increasing hepatic stress [64,67], but the recent data on SKN-1 suggest that Nrf/CNC proteins are likely to play a more direct role in maintaining lipid homeostasis.
SKN-1 also controls other aspects of metabolism. Expression profiling indicates that SKN-1 regulates genes involved in carbohydrate and amino acid metabolism, as well as mitochondrial function [41]. More recently, SKN-1 was shown to be important for mitochondrial biogenesis and health [68]. SKN-1 responds to mitochondrial stress and activates mitochondrial biogenesis and mitophagy genes. A lack of skn-1 function results in impaired mitochondrial network morphology, function, and mitophagy and reduced mitochondrial DNA content [68]. Interestingly, mammalian Nrf2 has been implicated directly in metabolic reprogramming that occurs under conditions of cell proliferation and cancer [3,69,70]. Further elucidation of how SKN-1 and mammalian Nrf/CNC proteins influence mitochondrial function and energy metabolism is likely to be a fruitful area of further investigation.
In summary, despite its divergent mode of target gene recognition, C. elegans SKN-1 fulfills the canonical functions associated with Nrf2 (oxidative and xenobiotic stress detoxification) and Nrf1 (proteasome expression). SKN-1 is also important in additional key processes such as protein synthesis in the ER and diverse aspects of metabolism, and maintains homeostasis under normal conditions, not just in response to acute stresses or insults. Continued parallel C. elegans and mammalian studies are likely to be mutually beneficial with respect to elucidating additional functions of these very interesting regulators, and understanding how they interface with other mechanisms that maintain metabolic and proteostatic homeostasis and defend against stresses.
3. Regulation of SKN-1 in vivo
Nrf/CNC proteins are subject to negative regulation that prevents them from activating target genes constitutively, and positive regulation that releases or overcomes this inhibition [2–4]. It is essential to elucidate how these regulatory mechanisms operate, in order to understand how Nrf/CNC proteins and their functions might be influenced by disease states or potential therapies. Elegant biochemical and molecular studies have yielded detailed mechanistic insights into how Nrf2 is inhibited by the ubiquitin ligase component Keap1 and how electrophilic compounds that bind specific Cys residues in Keap1 activate Nrf2 by relieving it from degradation [3,4]. We do not yet understand SKN-1 regulation at such a deep biochemical level, but in vivo and genetic analyses in C. elegans have identified multiple phosphorylation signals and other processes that regulate SKN-1 in vivo (Fig. 5). It appears likely that many of these mechanisms may also be relevant to Nrf/CNC protein regulation.
Fig. 5.
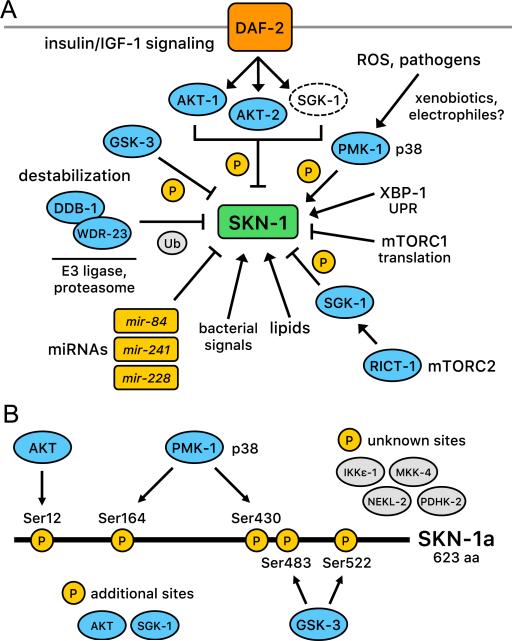
(A) Mechanisms that regulate SKN-1 and are described in the text. (B) Posttranslational regulation of SKN-1 through phosphorylation. AKT [20], PMK-1/p38 [45], and GSK-3 [72] have been found to regulate SKN-1 directly at the positions indicated. SGK-1 also phosphorylates and inhibits SKN-1 [20]. Of these kinases, PMK-1/p38 activates SKN-1 and the other kinases are inhibitory. The kinases shown as white ovals also inhibit SKN-1, but it is unknown whether they act directly or indirectly [22]. Numbering is according to positions within SKN-1 a isoform.
Although C. elegans lacks a true Keap1 ortholog, like Nrf2, SKN-1 appears to be regulated through ubiquitin-mediated proteolysis [28]. The E3 ubiquitin ligase substrate adaptor WDR-23 binds simultaneously to SKN-1 and the cullin4 (CUL4) ortholog DDB-1, an interaction that would be predicted to trigger SKN-1 ubiquitylation and subsequent degradation (Fig. 5A) [28]. Accordingly, knockdown of wdr-23 by RNAi increases SKN-1 protein abundance, accumulation of SKN-1 in intestinal nuclei, and SKN-1 target antioxidant gene expression, and the effects of wdr-23 RNAi on development and growth are restored by loss of SKN-1 [16,28]. One of the two WDR-23 isoforms (WDR-23a) has been implicated in SKN-1 inhibition and is bound to the mitochondrial outer membrane [31]. The idea that SKN-1 might be regulated by WDR-23 at mitochondria could be analogous to Keap1 and Nrf2 being tethered to mitochondria by the mitochondrial outer membrane protein PGAM5 [71]. SKN-1 also interacts directly with PGAM-5, which presumably facilitates its interactions with mitochondria, a location that could be opportune for sensing mitochondrial function and mitochondrially produced ROS [23]. However, it is important to note that although WDR-23 contains 17 Cys residues, it is still unknown whether these might be targeted by ROS, xenobiotics, or other reactive molecules, as has been demonstrated for Keap1 [28]. WDR-23 not only marks SKN-1 for degradation, but also directly inhibits SKN-1-DNA binding, providing a second level of regulation [17]. Interestingly, SKN-1 activates the wdr-23 gene, establishing a feedback loop that may limit the duration of SKN-1-mediated detoxification responses [16]. While WDR-23 might represent a functional counterpart to Keap1, a highly conserved WDR-23 ortholog is present in mammals, raising the intriguing possibility that mammalian Nrf/CNC proteins might also be regulated by WDR23 [28].
Another mechanism that inhibits SKN-1 is phosphorylation by glycogen synthase kinase (GSK-3) (Fig. 5A and B) [72]. Specific GSK-3 phosphorylation sites within SKN-1 have been identified, mutation of which results in accumulation of SKN-1 in intestinal nuclei. Knockdown of gsk-3 increases SKN-1 nuclear localization and antioxidant gene expression, supporting the importance of GSK-3 for SKN-1 regulation in vivo. GSK-3 phosphorylation targets mammalian Nrf2 for binding by the E3 ubiquitin ligase β-TrCP, and proteasomal degradation [73,74]. Interestingly, knockdown of the functional C. elegans β-TrCP ortholog lin-23 results in intestinal abnormalities that are largely skn-1-dependent [75]. This suggests that GSK-3- and possibly β-TrCP-mediated regulation of Nrf/CNC proteins may be evolutionarily conserved. The biological rationale for GSK-3 regulation of SKN-1 is still unknown. In mammals, GSK-3-mediated inhibition of Nrf2 may mediate regulation by AKT, which phosphorylates and inhibits GSK-3 [76], but C. elegans GSK-3 lacks a predicted AKT phosphorylation site. GSK-3 recognizes targets that have been phosphorylated by a priming kinase, and mutation of a predicted priming site in SKN-1 results in its constitutive nuclear localization [72]. Regulation by GSK-3 therefore allows SKN-1 and Nrf2 to be inhibited by signals from a priming kinase(s), the identity of which remains unknown.
A striking aspect of SKN-1 regulation is that its activity in the intestine is typically dependent upon signaling through the p38 mitogen-activated protein kinase (MAPK) pathway (Fig. 5) [45]. Treatment with sodium arsenite or other oxidative stressors leads to activation of the p38 kinase through phosphorylation in C. elegans [45], as is seen in mammalian cells [77]. Genetic ablation of the C. elegans p38 pathway prevents SKN-1 nuclear accumulation and target gene activation in response to oxidative stress and severely impairs oxidative stress resistance [45]. The C. elegans p38 kinase PMK-1 phosphorylates SKN-1 directly at two residues in response to stress (Fig. 5A), and mutation of these residues largely abolishes SKN-1 activity [45]. p38 signaling is required for all biological stimuli examined so far to activate SKN-1 in the intestine, although certain genetic manipulations induce SKN-1-dependent gene expression independently of this pathway, through mechanisms that are not understood [20,28,45,48,78]. Interestingly, the ER unfolded protein sensor IRE-1 (Fig. 4) appears to be required for arsenite-induced oxidative stress to trigger this p38 signal [24], suggesting a possible regulatory link between the ER and SKN-1-dependent oxidative stress responses.
It has been reported that the response of mammalian Nrf2 to oxidative stress is blocked by chemical inhibition of the p38 pathway [79,80], suggesting conservation of this regulatory mechanism. However, p38 phosphorylation sites within Nrf2 that were identified under nonstressed conditions do not appear to be required for its function [81], although the possible significance of stress-induced Nrf2 phosphorylation by p38 has not been examined. The profound importance of p38 signaling for SKN-1 activity in C. elegans suggests that further exploration of this question in mammals may be warranted.
Genetic and molecular studies have implicated a variety of additional mechanisms in regulation of SKN-1 (Fig. 5A). Signaling through the ERK-MAPK pathway promotes SKN-1 activity directly, through phosphorylation at the same residues targeted by p38 [82]. RNAi screening has revealed that SKN-1 nuclear localization and target gene expression in the intestine are inhibited by the cell cycle kinase nekl-2, the IκB-kinase ortholog ikke-1, the neuron-specific kinase mkk-4, and the pyruvate dehydrogenase kinase pdhk-2 (Fig. 5B), although it is unclear which of these signals affect SKN-1 directly or indirectly [22]. SKN-1 expression is inhibited by the microRNAs (miRNAs) mir-228, mir-84, and mir-241, each of which affects SKN-1 functions in vivo [83,84], and SKN-1 is inhibited by its interaction with the conserved transcriptional cofactor HCF-1 (host cell factor) [85]. Finally, as will be discussed in the next section, SKN-1 activity is modulated by growth, nutrient, metabolic, and dietary signals in vivo (Fig. 5A). The breadth of these regulatory interactions supports the notion that SKN-1 monitors many cellular processes, and has important homeostatic functions in the absence of stress.
4. Importance of SKN-1 for longevity
C. elegans is a powerful system for studying aging because of its advantages for genetic analysis, and its short mean adult lifespan of approximately three weeks (Fig. 2A) [8,86,87]. It was first shown in this organism that lifespan can be profoundly influenced by single-gene mutations that affect regulatory pathways [86,88,89]. This finding suggested that the rate of aging is neither random nor fixed, but instead is determined by particular biological processes. Subsequent work strengthened this idea by identifying numerous regulatory and downstream effector mechanisms that may be harnessed to increase longevity and the duration of healthy life (healthspan) [87,90–94]. An accumulating body of evidence in C. elegans implicates SKN-1 in regulating many of these mechanisms and indicates that it is a major modulator of aging.
SKN-1 promotes longevity in otherwise WT animals. Loss-of-function skn-1 mutants have a shortened lifespan [7], and while high-level transgenic overexpression of SKN-1 is harmful, lifespan is extended significantly by more modest SKN-1 overexpression [20] and by a gain-of-function mutation in SKN-1 that impairs interaction with the inhibitor WDR-23 (Fig. 6A) [17]. The aging process also affects SKN-1: during aging the constitutive expression of many SKN-1-regulated genes declines progressively [43], and the responsiveness of SKN-1 target genes to acute oxidative stress is lost [95]. In Drosophila, a similar decline in Nrf2 stress responsiveness occurs with aging [96].
Fig. 6.
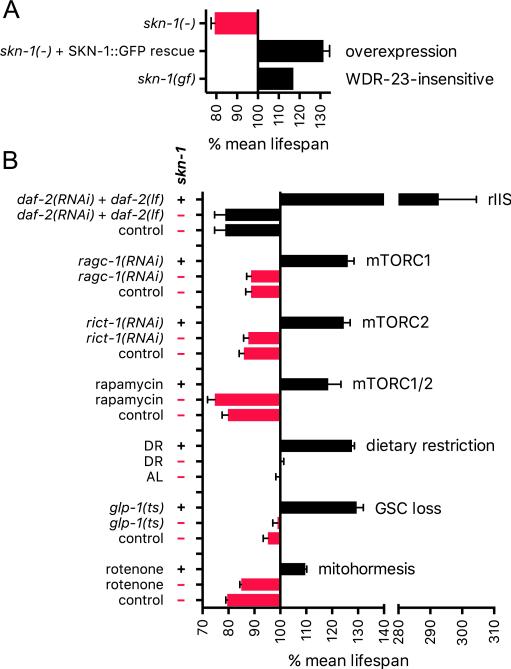
SKN-1 and longevity. (A) SKN-1 promotes longevity in otherwise WT animals. Lifespan was extended when an skn-1 mutant was rescued through overexpression from a transgene that encodes SKN-1b and SKN-1c in which the GSK-3 site (Fig. 5B) is mutated, resulting in constitutively nuclear localization in the intestine [20]. Smaller effects were observed in the WT background. Lifespan was also increased by an skn-1 gain-of-function mutation that disrupts inhibition by WDR-23 [17]. Relative median lifespan is graphed for this experiment, with means shown for all others. (B) skn-1-dependent longevity interventions. The reduced IIS (rIIS) experiment shown was performed at 15 °C [43]. skn-1 was also required for lifespan extension by daf-2 RNAi alone at 15 °C, 20 °C, and 25 °C. In each case of skn-1-dependent rIIS lifespan extension, dauer-related processes appeared to be inactive in adults [43]. Analyses of ragc-1, rict-1, and rapamycin are described in [29]. RAGC-1 is needed to transduce amino acid signals to mTORC1. DR results shown are from [19]. skn-1 was also largely required for lifespan extension in a different liquid DR protocol that gave greater lifespan extension [127]. AL refers to ad libitum feeding. For unknown reasons, skn-1 mutation does not reduce lifespan under AL conditions in these liquid culture protocols. Germline stem cell (GSC) loss increases lifespan in an skn-1-dependent manner [30]. Mitohormesis refers to stress resistance and longevity that arise from mitochondrial ROS production, here induced by rotenone treatment [128].
Many mechanisms that promote C. elegans longevity increase SKN-1 activity and require skn-1 for lifespan extension, the best-described example of which is the insulin/IGF-1 (insulin-like growth factor) signaling (IIS) pathway (Fig. 5A). Reduced IIS is associated with longevity in C. elegans, Drosophila, mammals, and possibly humans [87,90,97]. In C. elegans, multiple insulin/IGF-1-like factors signal through a single receptor tyrosine kinase (DAF-2, Fig. 5A) to regulate a conserved IIS pathway, in which DAF-2 signals through phosphatidylinositol-3 (PI3) kinase to activate the AKT kinase. AKT phosphorylates and inhibits nuclear localization of the FOXO ortholog DAF-16, which is required for lifespan to be extended by reduced IIS. DAF-16 (FOXO) is the canonical transcriptional target of IIS [87,90], but in C. elegans this pathway also inhibits SKN-1 directly [20]. When IIS signaling is reduced in C. elegans, for example by knockdown or mutation of daf-2, SKN-1 accumulates in intestinal nuclei in parallel to DAF-16 and activates downstream target genes [20,43,98]. Furthermore, SKN-1 is phosphorylated by AKT at multiple positions in vitro and localizes to intestinal nuclei constitutively after mutation of a Ser that both is phosphorylated by AKT, and is predicted at high stringency to be an AKT target [20]. Other evidence indicates that reducing IIS leads to production of ROS that promote SKN-1 activity [98].
Interestingly, SKN-1 is essential for the increase in oxidative stress resistance that results from reduced IIS, but its requirement for lifespan extension in this context is conditional [20,43,98]. IIS promotes growth but also inhibits development into the dauer larva (Fig. 2A), a diapause state that is highly resistant to harsh environmental conditions and is controlled by DAF-16 [8]. When IIS is reduced in a way that allows some dauer-related processes to be activated during adulthood, as indicated by presence of mild dauer-like traits, the requirement for SKN-1 for lifespan extension is relieved by DAF-16 [43]. By contrast, when IIS is reduced under conditions in which the dauer program is inactive, SKN-1 and DAF-16 are both fully essential for lifespan extension [43]. Under the latter conditions of reduced IIS, even extreme lifespan extensions (~3-fold greater mean lifespan than WT, Fig. 6B) are accompanied by a corresponding increase in healthspan [43], illustrating the potency with which SKN-1 can promote longevity when acting in concert with DAF-16 and possibly other factors.
SKN-1 is also essential in lifespan extensions that occur in response to reduced activity of another mechanism that promotes growth, mechanistic Target of Rapamycin (mTOR) signaling (Figs. 5A and 6B) [29,99]. mTOR is a conserved serine/threonine protein kinase that acts as a master regulator of cellular growth and metabolism in response to nutrient and hormonal cues [100–102]. mTOR functions in two distinct complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 has anabolic functions that include promoting protein synthesis and inhibiting autophagy and is activated by amino acid, oxygen, and growth factor signals. mTORC2 is less well understood, but increases activity of AKT, SGK, and related kinases, promotes growth, and requires interaction with the ribosome for its activity [102–106]. mTOR signaling is of intense interest in the aging field because inhibition of mTORC1 has been shown to extend lifespan in many model organisms and has been implicated in the lifespan-extending effects of dietary restriction [94,107,108]. The mTOR inhibitor rapamycin is a clinically approved drug that extends lifespan in diverse model organisms, including mice [109–111], and a different mTOR inhibitor (RAD001) enhances immune function in elderly humans [112].
When mTORC1 activity is reduced through RNAi knockdown of components in this signaling pathway, lifespan is extended in a manner that requires both SKN-1 and DAF-16 and involves activation of target genes of each of these transcription factors (Fig. 6B) [29]. Several parallels suggest that these effects are mediated at least in part through the reductions in translation initiation that result from mTORC1 inhibition. For example, when translation initiation is reduced by knockdown or mutation of specific initiation factors, SKN-1 and DAF-16 target genes are activated, and lifespan is extended dependent upon SKN-1 [78]. Most analyses indicate that DAF-16 is also required for longevity arising from translational suppression [78,113–116], as is the case for mTORC1 inhibition [29]. Interestingly, inhibiting translation initiation or mTORC1 does not detectably increase the levels of SKN-1 and DAF-16 in nuclei, except for a single DAF-16 isoform (DAF-16f) [29,78]. ChIP assays nevertheless show that genetic mTORC1 inhibition increases binding of SKN-1 to target genes [29]. Perhaps, when translation is reduced, an increased proportion of nuclear SKN-1 binds to target promoters through interactions with co-regulators, as is seen under ER stress conditions [24]. Under conditions of globally decreased translation, many proteins involved in protective mechanisms are translated preferentially in C. elegans and other species [107,116–118]. When overall protein synthesis is reduced, ribosomal loading of skn-1 mRNA is increased [116] and SKN-1 translation seems to be enhanced [24]. Preferential translation of SKN-1 therefore provides a mechanism by which its levels are increased under conditions of translational suppression and possibly stress.
The effects of mTORC2 on lifespan and SKN-1 are even more complex. When C. elegans is grown on the standard E. coli food OP50, inhibition of mTORC2 activity by mutation of its subunit gene rict-1 (Rictor) decreases lifespan [99,104,119]. This seems to be because neuronal mTORC2 is required in a little-understood skn-1-independent pathway that promotes longevity at moderate and lower temperatures [99,120]. In contrast, with feeding of certain other bacterial strains (such as HT115), the net effect of mTORC2 reduction on lifespan is positive [29,99,119], because under these conditions mTORC2 inhibition increases SKN-1 nuclear accumulation and target gene activation (Figs. 5A and 6B) [29,99]. RICT-1/mTORC2 is required for activation of the SGK-1 kinase [99,103,104], which phosphorylates and inhibits SKN-1 [20,121]. The difference in how these two bacterial strains affect SKN-1 activity in mTORC2- or SGK-1-inhibited animals appears to derive from metabolic or other signals produced by the bacteria, not nutrient intake per se [99]. This suggests the interesting possibility that mammalian Nrf/CNC proteins might be influenced by signals from the intestinal microbiota. The drug rapamycin increases C. elegans lifespan in an skn-1-dependent manner, apparently by reducing activity of both mTORC1 and mTORC2 (Fig. 6B) [29]. This is consistent with evidence that rapamycin disrupts activity of both mTORC complexes in mammals [122,123].
Dietary restriction (DR), or the reduction of food intake to levels just above starvation, increases lifespan in essentially all eukaryotes [94,108,124]. In C. elegans, different DR regimens show different genetic requirements for lifespan extension [125]. SKN-1 is required in the eat-2 genetic DR model [126] and two liquid-culture DR protocols (Fig. 6B) [19,127]. Nutrient availability in the environment is sensed by neurons, and lifespan extension from DR is prevented by ablation of the single pair of ASI sensory neurons, in which SKN-1b is prominently expressed (Fig. 2B) [19]. Reductions in food intake increase SKN-1 levels in these neurons [19,128,129], an effect that seems to be mediated by certain miRNAs [83,129]. In one study, ASI neuronal expression of SKN-1b restored the requirement for skn-1 in DR lifespan extension [19]. This requirement appeared to reflect an skn-1-dependent increase in oxygen consumption by the animal, raising the interesting question of how this nonautonomous metabolic regulation is mediated.
C. elegans lifespan can also be extended by surgical or genetic ablation of germline stem cells (GSCs) [86,114,130]. This effect seems to be evolutionarily conserved, at least in some species, and may reflect strategies for preserving reproductive capacity and metabolic homeostasis under adverse conditions. In C. elegans, a striking effect of GSC loss is that the animals store excess fat, but also show multiple alterations in lipid metabolism, including increases in lipolytic activities [30,86,114,130]. Many lipid metabolism enzymes are critical for the lifespan extension associated with GSC loss, raising the intriguing question of whether this lifespan extension might involve production and storage of beneficial lipids.
Recent evidence suggests that the excess fat seen in animals that lack GSCs may derive largely from accumulation of yolk-associated lipids that had been produced for reproduction, rather than specifically induced synthesis and storage of excess lipids [30]. Along with other transcription factors that influence metabolism [86,114,130], SKN-1 is required for GSC loss to extend lifespan [30]. Interestingly, SKN-1 is induced to accumulate in nuclei by the high levels of lipids that are present in these animals. Genetic analysis suggests that this SKN-1 activation does not derive simply from stress associated with elevated fat levels, but instead from specific lipid-based signals. SKN-1 responds to these signals by up-regulating many genes, including a large number involved in lipid metabolism, thereby ameliorating the fat overload and presumably altering the balance of lipids present, and enhancing stress resistance and longevity [30]. Analysis of GSC-ablated C. elegans may provide a powerful system for unraveling how Nrf/CNC proteins are regulated by lipids and associated signals, how they might influence synthesis of lipids that have signaling functions, and how they cooperate with other transcription factors to affect lipid metabolism.
The list of other signals or pharmacological agents that increase C. elegans lifespan in an skn-1-dependent manner is too long to cite in its entirety, but some of these are illustrative of the range of SKN-1 functions and responses. Amyloid-binding compounds that include the dye Thioflavin T (ThT) and the spice curcumin enhance proteostasis and extend lifespan dependent upon skn-1 [131,132]. SKN-1 is also required for lifespan extensions associated with knockdown of a set of genes that are down-regulated during spaceflight, a condition that also reduces protein aggregation during aging [133]. A recent body of work indicates that lifespan is increased when mitochondrial ROS production is increased to levels that trigger protective stress responses, but are not acutely toxic [128,134,135]. In some studies SKN-1 is the central mediator of this “mitohormesis” effect on lifespan (Fig. 6B) [128,136,137]. It is an intriguing question how mitohormesis relates to the recently described response of SKN-1 to impaired mitophagy and mitochondrial function, which appears to depend upon Ca++ signaling [68]. Under some conditions interference with mitochondrial function increases lifespan independent of SKN-1 [20,126,135], illustrating how much remains to be learned about the relationship between mitochondrial signaling, SKN-1, and aging. Various other interventions do not need SKN-1 to increase lifespan, including certain DR protocols [125,138], activation of dauer-related mechanisms in adults [43] and hypoxia [139]. It is not understood whether such mechanisms promote longevity through different downstream processes from those regulated by SKN-1 or generally mobilize the same or overlapping mechanisms by acting through other regulators such as DAF-16/FOXO.
5. SKN-1-regulated mechanisms that increase lifespan
As mechanisms that increase C. elegans lifespan have been described, genetic and gene expression studies have identified downstream cellular processes that are enhanced by these interventions and are critical for longevity. These mechanisms include detoxification of xenobiotics and ROS, proteasome and chaperone activity, autophagy, innate immunity, mitochondrial biogenesis, and metabolic changes [68,87,90,92,114,140]. As described in previous sections, many of these processes are regulated by SKN-1 (Fig. 3). Supporting the idea that SKN-1 controls cellular processes that are intimately linked to longevity, RNAi knockdown of many SKN-1-up- or –down-regulated genes respectively reduces or increases lifespan [41,43], and overexpression of the individual SKN-1 target genes gst-10 (glutathione-S-transferase) [141], gsr-1 (glutathione reductase) [142], or pbs-5 (proteasome beta subunit) [143] is sufficient to prolong lifespan. In the last case, lifespan extension was shown to be skn-1-dependent [143], as would be predicted from the importance of SKN-1 for expression of most proteasome subunit genes [41,50].
An unexpected mechanism through which SKN-1 promotes longevity was identified through analysis of C. elegans in which IIS was reduced under conditions where dauer processes were inactive [43]. Expression profiling of these animals indicated that, as expected, detoxification mechanisms are prominent among processes activated by SKN-1. Surprisingly, however, collagens and other extracellular matrix (ECM) genes were by far the most overrepresented categories among SKN-1-up-regulated genes, and subsequent analyses showed that ECM remodeling is critical for longevity [43]. Expression of particular ECM genes declines with age [144], but is increased and maintained by essentially every longevity intervention [43]. Certain collagens are essential for lifespan extension by these interventions, and overexpression of these collagens individually is sufficient to increase lifespan [43]. The C. elegans cuticle is remodeled during aging, and longevity interventions increase adulthood ECM deposition. Most remarkably, when adulthood expression of these collagens is disrupted, otherwise long-lived animals do not simply become sick, but show accelerated onset of aging markers, including changes in gene expression. This suggests that the ECM plays a regulatory role during aging and is critical for signaling mechanisms that promote longevity systemically [43]. This is an area of great importance in the aging field, because it has become apparent that interactions between tissues are important for lifespan extension, and coordinating responses to stress [90,140]. Interestingly, SKN-1 appears to regulate most ECM genes indirectly during aging, suggesting that its effects might be exerted through downstream regulators, or might involve effects on redox or proteostasis processes that coordinate ECM deposition and regulation [43].
The precedents identified in C. elegans predict that Nrf/CNC proteins are likely to be important for longevity assurance in higher organisms. This is clearly true in Drosophila, in which elevated Nrf2/CNC expression increases lifespan [2,145]. It is more difficult to perform epistasis and other genetic analyses of Nrf/CNC proteins in mammals, in part because in mice Nrf2 activity that is unrestrained by Keap1 is deleterious [3,146], and because Nrf1 is essential for embryonic development [35]. Nevertheless, various findings suggest that Nrf/CNC proteins may be important for longevity. It is possible that Nrf proteins are themselves directly involved in pathways and mechanisms that promote lifespan, as in the nematode and fruit fly. In the mouse, lifespan can be increased by reductions in either insulin/IGF-1 or mTOR signaling [108,147]. Treatment with an IGF-1 inhibitor activates an Nrf2-regulated reporter in a human cell line [148], rapamycin treatment activates Nrf2 in human fibroblasts [149] and up-regulates Nrf2 targets in the liver in mice [29,150], and Nrf2 is required for the anti-tumor effect of DR [151]. On the other hand, in proliferating cells, PI3 kinase activity increases Nrf2 activity [3], possibly by inhibiting GSK-3, and rapamycin has been observed to reduce Nrf2 activity in a cell culture model [152]. Clearly much remains to be learned about how prolongevity interventions influence the activity of mammalian Nrf/CNC proteins.
Perhaps the most compelling evidence for involvement of Nrf/CNC proteins in mammalian longevity comes from examination of downstream processes that these proteins regulate. Nrf2 targets and other detoxification genes are up-regulated in vivo in numerous mouse models in which aging has been slowed by genetic, dietary, or pharmacological interventions [150,153–156]. ECM genes also appear to be responsive to Nrf2 and up-regulated in long-lived mice [43]. Finally, cells obtained from an extremely long-lived rodent, the naked mole rat, are notable for dramatically elevated proteasome, Nrf2, and Nrf2 target gene activity and oxidative stress resistance [156–158]. While we may ultimately find that Nrf2 and other Nrf/CNC proteins help drive lifespan extension in mammals, it is also possible that in higher organisms the activity of Nrf/CNC-regulated processes is increased by longevity interventions through other mechanisms. In either case, it appears likely that we will continue to uncover additional connections between these fascinating transcription regulators and aging.
6. Perspectives.
It is clear that the functions and regulation of SKN-1 are remarkably complex. This may reflect its being involved in some of the most fundamental mechanisms by which cells and organisms protect themselves from exogenous insults and endogenous perturbations (Fig. 3). Given how little we understand still about how SKN-1 controls these various processes and responds to so many different stresses and situations, it seems likely that we are a long way from grasping the full scope of this complexity. By unraveling how SKN-1 interacts with other regulators and signals within cells and across tissues, we will gain valuable knowledge about how organisms protect themselves and maintain regulatory and metabolic homeostasis. While it is safe to assume that the situation in mammals will be considerably more complex, the insights gained from the worm should continue to suggest valuable ideas and directions for further investigation with respect to regulation and functions of mammalian Nrf/CNC proteins. Similarly, our increasing appreciation of the importance of SKN-1 in mechanisms that determine C. elegans lifespan suggests that further exploration of these areas with regard to mammalian aging should be a priority. Cross-pollination between studies in mammalian models and in simpler systems such as C. elegans and Drosophila is likely to accelerate the process by which we devise strategies for developing therapeutic or preventive interventions that involve Nrf/CNC proteins in humans.
Acknowledgments
The work was supported by funding from the NIH to TKB (R01GM062891, R01GM094398, and R21AG043949) and MJS (T32DK007260), Swiss National Science funding to CYE (P300P3_154633), an AFAR fellowship to MI, and a Diabetes Research Center award to the Joslin Diabetes Center (P30DK036836).
References
- 1.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 2.Sykiotis GP, Bohmann D. Stress-activated cap'n'collar transcription factors in aging and human disease. Sci Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011;16:123–140. doi: 10.1111/j.1365-2443.2010.01473.x. [DOI] [PubMed] [Google Scholar]
- 4.Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Uruno A, Yagishita Y, Yamamoto M. The Keap1-Nrf2 system and diabetes mellitus. Arch Biochem Biophys. 2015;566:76–84. doi: 10.1016/j.abb.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Walker AK, See R, Batchelder C, Kophengnavong T, Gronniger JT, Shi Y, Blackwell TK. A conserved transcription motif suggesting functional parallels between Caenorhabditis elegans SKN-1 and Cap'n'Collar-related basic leucine zipper proteins. J Biol Chem. 2000;275:22166–22167. doi: 10.1074/jbc.M001746200. [DOI] [PubMed] [Google Scholar]
- 7.An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–1893. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riddle DL. C. elegans II. Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- 9.Itoh K, Igarashi K, Hayashi N, Nishizawa M, Yamamoto M. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol Cell Biol. 1995;15:4184–4193. doi: 10.1128/mcb.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowerman B, Eaton BA, Priess JR. skn-1, a maternally expressed gene required to specify the fate of ventral blastomeres in the early C. elegans embryo. Cell. 1992;68:1061–1075. doi: 10.1016/0092-8674(92)90078-q. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell TK, Bowerman B, Priess JR, Weintraub H. Formation of a monomeric DNA binding domain by Skn-1 bZIP and homeodomain elements. Science. 1994;266:621–628. doi: 10.1126/science.7939715. [DOI] [PubMed] [Google Scholar]
- 12.Carroll AS, Gilbert DE, Liu X, Cheung JW, Michnowicz JE, Wagner G, Ellenberger TE, Blackwell TK. SKN-1 domain folding and basic region monomer stabilization upon DNA binding. Genes Dev. 1997;11:2227–2238. doi: 10.1101/gad.11.17.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo MC, Ha S, Pelczer I, Pal S, Walker S. The solution structure of the DNA-binding domain of Skn-1. Proc Natl Acad Sci U S A. 1998;95:8455–8460. doi: 10.1073/pnas.95.15.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rupert PB, Daughdrill GW, Bowerman B, Matthews BW. A new DNA-binding motif in the Skn-1 binding domain-DNA complex. Nat Struct Biol. 1998;5:484–491. doi: 10.1038/nsb0698-484. [DOI] [PubMed] [Google Scholar]
- 15.Kophengnavong T, Carroll AS, Blackwell TK. The SKN-1 amino-terminal arm is a DNA specificity segment. Mol Cell Biol. 1999;19:3039–3050. doi: 10.1128/mcb.19.4.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung CK, Wang Y, Deonarine A, Tang L, Prasse S, Choe KP. A negative-feedback loop between the detoxification/antioxidant response factor SKN-1 and its repressor WDR-23 matches organism needs with environmental conditions. Mol Cell Biol. 2013;33:3524–3537. doi: 10.1128/MCB.00245-13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Leung CK, Hasegawa K, Wang Y, Deonarine A, Tang L, Miwa J, Choe KP. Direct interaction between the WD40 repeat protein WDR-23 and SKN-1/Nrf inhibits binding to target DNA. Mol Cell Biol. 2014;34:3156–3167. doi: 10.1128/MCB.00114-14. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Sun Z, Chin YE, Zhang DD. Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol. 2009;29:2658–2672. doi: 10.1128/MCB.01639-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop NA, Guarente L. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature. 2007;447:545–549. doi: 10.1038/nature05904. [DOI] [PubMed] [Google Scholar]
- 20.Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 22.Kell A, Ventura N, Kahn N, Johnson TE. Activation of SKN-1 by novel kinases in Caenorhabditis elegans. Free Radic Biol Med. 2007;43:1560–1566. doi: 10.1016/j.freeradbiomed.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paek J, Lo JY, Narasimhan SD, Nguyen TN, Glover-Cutter K, Robida-Stubbs S, Suzuki T, Yamamoto M, Blackwell TK, Curran SP. Mitochondrial SKN-1/Nrf mediates a conserved starvation response. Cell Metab. 2012;16:526–537. doi: 10.1016/j.cmet.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glover-Cutter KM, Lin S, Blackwell TK. Integration of the unfolded protein and oxidative stress responses through SKN-1/Nrf. PLoS Genet. 2013;9:e1003701. doi: 10.1371/journal.pgen.1003701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Crouch DH, Yamamoto M, Hayes JD. Negative regulation of the Nrf1 transcription factor by its N-terminal domain is independent of Keap1: Nrf1, but not Nrf2, is targeted to the endoplasmic reticulum. Biochem J. 2006;399:373–385. doi: 10.1042/BJ20060725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radhakrishnan SK, den Besten W, Deshaies RJ. p97-dependent retrotranslocation and proteolytic processing govern formation of active Nrf1 upon proteasome inhibition. Elife. 2014;3:e01856. doi: 10.7554/eLife.01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 28.Choe KP, Przybysz AJ, Strange K. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol. 2009;29:2704–2715. doi: 10.1128/MCB.01811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, Blackwell TK. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15:713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinbaugh MJ, Narasimhan SD, Robida-Stubbs S, Moronetti Mazzeo LE, Dreyfuss JM, Hourihan JM, Raghavan P, Operaña TN, Esmaillie R, Blackwell TK. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. Elife. 2015 doi: 10.7554/eLife.07836. http://dx.doi.org/10.7554/eLife.07836. [DOI] [PMC free article] [PubMed]
- 31.Staab TA, Griffen TC, Corcoran C, Evgrafov O, Knowles JA, Sieburth D. The conserved SKN-1/Nrf2 stress response pathway regulates synaptic function in Caenorhabditis elegans. PLoS Genet. 2013;9:e1003354. doi: 10.1371/journal.pgen.1003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staab TA, Evgrafov O, Knowles JA, Sieburth D. Regulation of synaptic nlg- 1/neuroligin abundance by the skn-1/Nrf stress response pathway protects against oxidative stress. PLoS Genet. 2014;10:e1004100. doi: 10.1371/journal.pgen.1004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowerman B, Draper BW, Mello CC, Priess JR. The maternal gene skn-1 encodes a protein that is distributed unequally in early C. elegans embryos. Cell. 1993;74:443–452. doi: 10.1016/0092-8674(93)80046-h. [DOI] [PubMed] [Google Scholar]
- 34.Farmer SC, Sun CW, Winnier GE, Hogan BL, Townes TM. The bZIP transcription factor LCR-F1 is essential for mesoderm formation in mouse development. Genes Dev. 1997;11:786–798. doi: 10.1101/gad.11.6.786. [DOI] [PubMed] [Google Scholar]
- 35.Chan JY, Kwong M, Lu R, Chang J, Wang B, Yen TS, Kan YW. Targeted disruption of the ubiquitous CNC-bZIP transcription factor, Nrf-1, results in anemia and embryonic lethality in mice. EMBO J. 1998;17:1779–1787. doi: 10.1093/emboj/17.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J Biol Chem. 2003;278:48021–48029. doi: 10.1074/jbc.M308439200. [DOI] [PubMed] [Google Scholar]
- 37.Gehring WJ, Ikeo K. Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15:371–377. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- 38.Gaudet J, Mango SE. Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science. 2002;295:821–825. doi: 10.1126/science.1065175. [DOI] [PubMed] [Google Scholar]
- 39.Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/ transport by xenobiotics. Arch Pharm Res. 2005;28:249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- 40.Lindblom TH, Dodd AK. Xenobiotic detoxification in the nematode Caenorhabditis elegans. J Exp Zool A Comp Exp Biol. 2006;305:720–730. doi: 10.1002/jez.a.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveira RP, Porter Abate J, Dilks K, Landis J, Ashraf J, Murphy CT, Blackwell TK. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell. 2009;8:524–541. doi: 10.1111/j.1474-9726.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SK, Tedesco PM, Johnson TE. Oxidative stress and longevity in Caenorhabditis elegans as mediated by SKN-1. Aging Cell. 2009;8:258–269. doi: 10.1111/j.1474-9726.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ewald CY, Landis JN, Porter Abate J, Murphy CT, Blackwell TK. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature. 2015;519:97–101. doi: 10.1038/nature14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niu W, Lu ZJ, Zhong M, Sarov M, Murray JI, Brdlik CM, Janette J, Chen C, Alves P, Preston E, et al. Diverse transcription factor binding features revealed by genome-wide ChIP-seq in C. elegans. Genome Res. 2011;21:245–254. doi: 10.1101/gr.114587.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, Matsumoto K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005;19:2278–2283. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicholas HR, Hodgkin J. Responses to infection and possible recognition strategies in the innate immune system of Caenorhabditis elegans. Mol Immunol. 2004;41:479–493. doi: 10.1016/j.molimm.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 47.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–516. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoeven R, McCallum KC, Cruz MR, Garsin DA. Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PLoS Pathog. 2011;7:e1002453. doi: 10.1371/journal.ppat.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papp D, Csermely P, Soti C. A role for SKN-1/Nrf in pathogen resistance and immunosenescence in Caenorhabditis elegans. PLoS Pathog. 2012;8:e1002673. doi: 10.1371/journal.ppat.1002673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li X, Matilainen O, Jin C, Glover-Cutter KM, Holmberg CI, Blackwell TK. Specific SKN-1/Nrf stress responses to perturbations in translation elongation and proteasome activity. PLoS Genet. 2011;7:e1002119. doi: 10.1371/journal.pgen.1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 52.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 53.Pickering AM, Staab TA, Tower J, Sieburth D, Davies KJ. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. J Exp Biol. 2013;216:543–553. doi: 10.1242/jeb.074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radhakrishnan SK, Lee CS, Young P, Beskow A, Chan JY, Deshaies RJ. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol Cell. 2010;38:17–28. doi: 10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sha Z, Goldberg AL. Proteasome-mediated processing of Nrf1 is essential for coordinate induction of all proteasome subunits and p97. Curr Biol. 2014;24:1573–1583. doi: 10.1016/j.cub.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steffen J, Seeger M, Koch A, Kruger E. Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol Cell. 2010;40:147–158. doi: 10.1016/j.molcel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 57.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. J Biol Chem. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 59.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 60.Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 62.Pang S, Lynn DA, Lo JY, Paek J, Curran SP. SKN-1 and Nrf2 couples proline catabolism with lipid metabolism during nutrient deprivation. Nat Commun. 2014;5:5048. doi: 10.1038/ncomms6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsujita T, Peirce V, Baird L, Matsuyama Y, Takaku M, Walsh SV, Griffin JL, Uruno A, Yamamoto M, Hayes JD. Transcription factor Nrf1 negatively regulates the cystine/glutamate transporter and lipid-metabolizing enzymes. Mol Cell Biol. 2014;34:3800–3816. doi: 10.1128/MCB.00110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu Z, Chen L, Leung L, Yen TS, Lee C, Chan JY. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc Natl Acad Sci U S A. 2005;102:4120–4125. doi: 10.1073/pnas.0500660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee LY, Kohler UA, Zhang L, Roenneburg D, Werner S, Johnson JA, Foley DP. Activation of the Nrf2-ARE pathway in hepatocytes protects against steatosis in nutritionally induced non-alcoholic steatohepatitis in mice. Toxicol Sci. 2014 doi: 10.1093/toxsci/kfu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okada K, Warabi E, Sugimoto H, Horie M, Gotoh N, Tokushige K, Hashimoto E, Utsunomiya H, Takahashi H, Ishii T, et al. Deletion of Nrf2 leads to rapid progression of steatohepatitis in mice fed atherogenic plus high-fat diet. J Gastroenterol. 2013;48:620–632. doi: 10.1007/s00535-012-0659-z. [DOI] [PubMed] [Google Scholar]
- 67.Lee CS, Ho DV, Chan JY. Nuclear factor-erythroid 2-related factor 1 regulates expression of proteasome genes in hepatocytes and protects against endoplasmic reticulum stress and steatosis in mice. FEBS J. 2013;280:3609–3620. doi: 10.1111/febs.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palikaras K, Lionaki E, Tavernarakis N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature. 2015;521:525–528. doi: 10.1038/nature14300. [DOI] [PubMed] [Google Scholar]
- 69.Mitsuishi Y, Motohashi H, Yamamoto M. The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol. 2012;2:200. doi: 10.3389/fonc.2012.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 71.Lo SC, Hannink M. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp Cell Res. 2008;314:1789–1803. doi: 10.1016/j.yexcr.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.An JH, Vranas K, Lucke M, Inoue H, Hisamoto N, Matsumoto K, Blackwell TK. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc Natl Acad Sci U S A. 2005;102:16275–16280. doi: 10.1073/pnas.0508105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, Tobon-Velasco JC, Devijver H, Garcia-Mayoral MF, Van Leuven F, et al. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol Cell Biol. 2012;32:3486–3499. doi: 10.1128/MCB.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hebeisen M, Roy R. CDC-25.1 stability is regulated by distinct domains to restrict cell division during embryogenesis in C. elegans. Development. 2008;135:1259–1269. doi: 10.1242/dev.014969. [DOI] [PubMed] [Google Scholar]
- 76.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 77.Cai B, Xia Z. p38 MAP kinase mediates arsenite-induced apoptosis through FOXO3a activation and induction of Bim transcription. Apoptosis. 2008;13:803–810. doi: 10.1007/s10495-008-0218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J, Robida-Stubbs S, Tullet JM, Rual JF, Vidal M, Blackwell TK. RNAi screening implicates a SKN-1-dependent transcriptional response in stress resistance and longevity deriving from translation inhibition. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buckley BJ, Marshall ZM, Whorton AR. Nitric oxide stimulates Nrf2 nuclear translocation in vascular endothelium. Biochem Biophys Res Commun. 2003;307:973–979. doi: 10.1016/s0006-291x(03)01308-1. [DOI] [PubMed] [Google Scholar]
- 80.Ishikado A, Sono Y, Matsumoto M, Robida-Stubbs S, Okuno A, Goto M, King GL, Blackwell TK, Makino T. Willow bark extract increases antioxidant enzymes and reduces oxidative stress through activation of Nrf2 in vascular endothelial cells and Caenorhabditis elegans. Free Radic Biol Med. 2013;65:1506–1515. doi: 10.1016/j.freeradbiomed.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun Z, Huang Z, Zhang DD. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS One. 2009;4:e6588. doi: 10.1371/journal.pone.0006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Okuyama T, Inoue H, Ookuma S, Satoh T, Kano K, Honjoh S, Hisamoto N, Matsumoto K, Nishida E. The ERK-MAPK pathway regulates longevity through SKN-1 and insulin-like signaling in Caenorhabditis elegans. J Biol Chem. 2010;285:30274–30281. doi: 10.1074/jbc.M110.146274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu F, He CX, Luo LJ, Zou QL, Zhao YX, Saini R, Han SF, Knolker HJ, Wang LS, Ge BX. Nuclear hormone receptor regulation of microRNAs controls innate immune responses in C. elegans. PLoS Pathog. 2013;9:e1003545. doi: 10.1371/journal.ppat.1003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith-Vikos T, de Lencastre A, Inukai S, Shlomchik M, Holtrup B, Slack FJ. MicroRNAs mediate dietary-restriction-induced longevity through PHA-4/ FOXA and SKN-1/Nrf transcription factors. Curr Biol. 2014;24:2238–2246. doi: 10.1016/j.cub.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rizki G, Picard CL, Pereyra C, Lee SS. Host cell factor 1 inhibits SKN-1 to modulate oxidative stress responses in Caenorhabditis elegans. Aging Cell. 2012;11:717–721. doi: 10.1111/j.1474-9726.2012.00831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 87.Shore DE, Ruvkun G. A cytoprotective perspective on longevity regulation. Trends Cell Biol. 2013;23:409–420. doi: 10.1016/j.tcb.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klass MR. A method for the isolation of longevity mutants in the nematode Caenorhabditis elegans and initial results. Mech Ageing Dev. 1983;22:279–286. doi: 10.1016/0047-6374(83)90082-9. [DOI] [PubMed] [Google Scholar]
- 89.Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 91.Hansen M, Flatt T, Aguilaniu H. Reproduction, fat metabolism, and life span: what is the connection? Cell Metab. 2013;17:10–19. doi: 10.1016/j.cmet.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bansal A, Zhu LJ, Yen K, Tissenbaum HA. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc Natl Acad Sci U S A. 2015;112:E277–E286. doi: 10.1073/pnas.1412192112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Przybysz AJ, Choe KP, Roberts LJ, Strange K. Increased age reduces DAF-16 and SKN-1 signaling and the hormetic response of Caenorhabditis elegans to the xenobiotic juglone. Mech Ageing Dev. 2009;130:357–369. doi: 10.1016/j.mad.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rahman MM, Sykiotis GP, Nishimura M, Bodmer R, Bohmann D. Declining signal dependence of Nrf2-MafS-regulated gene expression correlates with aging phenotypes. Aging Cell. 2013;12:554–562. doi: 10.1111/acel.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Milman S, Atzmon G, Huffman DM, Wan J, Crandall JP, Cohen P, Barzilai N. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. 2014;13:769–771. doi: 10.1111/acel.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Kuhlow D, Guthke R, Platzer M, Kahn CR, Ristow M. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial l-proline catabolism to induce a transient ROS signal. Cell Metab. 2012;15:451–465. doi: 10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mizunuma M, Neumann-Haefelin E, Moroz N, Li Y, Blackwell TK. mTORC2-SGK-1 acts in two environmentally responsive pathways with opposing effects on longevity. Aging Cell. 2014;13:869–878. doi: 10.1111/acel.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 101.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 102.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jones KT, Greer ER, Pearce D, Ashrafi K. Rictor/TORC2 regulates Caenorhabditis elegans fat storage, body size, and development through sgk-1. PLoS Biol. 2009;7:e60. doi: 10.1371/journal.pbio.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Soukas AA, Kane EA, Carr CE, Melo JA, Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23:496–511. doi: 10.1101/gad.1775409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell. 2011;144:757–768. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 106.Wang T, Blumhagen R, Lao U, Kuo Y, Edgar BA. LST8 regulates cell growth via target-of-rapamycin complex 2 (TORC2) Mol Cell Biol. 2012;32:2203–2213. doi: 10.1128/MCB.06474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- 111.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892. [DOI] [PubMed] [Google Scholar]
- 113.Henderson ST, Bonafe M, Johnson TE. daf-16 protects the nematode Caenorhabditis elegans during food deprivation. J Gerontol A Biol Sci Med Sci. 2006;61:444–460. doi: 10.1093/gerona/61.5.444. [DOI] [PubMed] [Google Scholar]
- 114.Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 115.Tohyama D, Yamaguchi A, Yamashita T. Inhibition of a eukaryotic initiation factor (eIF2Bδ/F11A3.2) during adulthood extends lifespan in Caenorhabditis elegans. FASEB J. 2008;22:4327–4337. doi: 10.1096/fj.08-112953. [DOI] [PubMed] [Google Scholar]
- 116.Rogers AN, Chen D, McColl G, Czerwieniec G, Felkey K, Gibson BW, Hubbard A, Melov S, Lithgow GJ, Kapahi P. Life span extension via eIF4G inhibition is mediated by posttranscriptional remodeling of stress response gene expression in C. elegans. Cell Metab. 2011;14:55–66. doi: 10.1016/j.cmet.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xiao R, Chun L, Ronan EA, Friedman DI, Liu J, Xu XZ. RNAi Interrogation of dietary modulation of development, metabolism, behavior, and aging in C. elegans. Cell Rep. 2015;11:1123–1133. doi: 10.1016/j.celrep.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xiao R, Zhang B, Dong Y, Gong J, Xu T, Liu J, Xu XZ. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell. 2013;152:806–817. doi: 10.1016/j.cell.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ruf V, Holzem C, Peyman T, Walz G, Blackwell TK, Neumann-Haefelin E. TORC2 signaling antagonizes SKN-1 to induce C. elegans mesendodermal embryonic development. Dev Biol. 2013;384:214–227. doi: 10.1016/j.ydbio.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 125.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Park SK, Link CD, Johnson TE. Life-span extension by dietary restriction is mediated by NLP-7 signaling and coelomocyte endocytosis in C. elegans. FASEB J. 2010;24:383–392. doi: 10.1096/fj.09-142984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, Blackwell TK. Dietary restriction involves NAD+-dependent mechanisms and a shift toward oxidative metabolism. Aging Cell. 2014 doi: 10.1111/acel.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schmeisser S, Priebe S, Groth M, Monajembashi S, Hemmerich P, Guthke R, Platzer M, Ristow M. Neuronal ROS signaling rather than AMPK/ sirtuin-mediated energy sensing links dietary restriction to lifespan extension. Mol Metab. 2013;2:92–102. doi: 10.1016/j.molmet.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vora M, Shah M, Ostafi S, Onken B, Xue J, Ni JZ, Gu S, Driscoll M. Deletion of microRNA-80 activates dietary restriction to extend C. elegans healthspan and lifespan. PLoS Genet. 2013;9:e1003737. doi: 10.1371/journal.pgen.1003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Antebi A. Regulation of longevity by the reproductive system. Exp Gerontol. 2013;48:596–602. doi: 10.1016/j.exger.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alavez S, Vantipalli MC, Zucker DJ, Klang IM, Lithgow GJ. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature. 2011;472:226–229. doi: 10.1038/nature09873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liao VH, Yu CW, Chu YJ, Li WH, Hsieh YC, Wang TT. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech Ageing Dev. 2011;132:480–487. doi: 10.1016/j.mad.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 133.Honda Y, Higashibata A, Matsunaga Y, Yonezawa Y, Kawano T, Higashitani A, Kuriyama K, Shimazu T, Tanaka M, Szewczyk NJ, et al. Genes down-regulated in spaceflight are involved in the control of longevity in Caenorhabditis elegans. Sci Rep. 2012;2:487. doi: 10.1038/srep00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010;20:2131–2136. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hunt PR, Son TG, Wilson MA, Yu QS, Wood WH, Zhang Y, Becker KG, Greig NH, Mattson MP, Camandola S, et al. Extension of lifespan in C. elegans by naphthoquinones that act through stress hormesis mechanisms. PLoS One. 2011;6:e21922. doi: 10.1371/journal.pone.0021922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schmeisser S, Schmeisser K, Weimer S, Groth M, Priebe S, Fazius E, Kuhlow D, Pick D, Einax JW, Guthke R, et al. Mitochondrial hormesis links low-dose arsenite exposure to lifespan extension. Aging Cell. 2013;12:508–517. doi: 10.1111/acel.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Honjoh S, Yamamoto T, Uno M, Nishida E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature. 2009;457:726–730. doi: 10.1038/nature07583. [DOI] [PubMed] [Google Scholar]
- 139.Leiser SF, Fletcher M, Begun A, Kaeberlein M. Life-span extension from hypoxia in Caenorhabditis elegans requires both HIF-1 and DAF-16 and is antagonized by SKN-1. J Gerontol A Biol Sci Med Sci. 2013;68:1135–1144. doi: 10.1093/gerona/glt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Taylor RC, Berendzen KM, Dillin A. Systemic stress signalling: understanding the cell non-autonomous control of proteostasis. Nat Rev Mol Cell Biol. 2014;15:211–217. doi: 10.1038/nrm3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ayyadevara S, Dandapat A, Singh SP, Benes H, Zimniak L, Shmookler Reis RJ, Zimniak P. Lifespan extension in hypomorphic daf-2 mutants of Caenorhabditis elegans is partially mediated by glutathione transferase CeGSTP2-2. Aging Cell. 2005;4:299–307. doi: 10.1111/j.1474-9726.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- 142.Luersen K, Stegehake D, Daniel J, Drescher M, Ajonina I, Ajonina C, Hertel P, Woltersdorf C, Liebau E. The glutathione reductase GSR-1 determines stress tolerance and longevity in Caenorhabditis elegans. PLoS One. 2013;8:e60731. doi: 10.1371/journal.pone.0060731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chondrogianni N, Georgila K, Kourtis N, Tavernarakis N, Gonos ES. 20S proteasome activation promotes life span extension and resistance to proteotoxicity in Caenorhabditis elegans. FASEB J. 2015;29:611–622. doi: 10.1096/fj.14-252189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Budovskaya YV, Wu K, Southworth LK, Jiang M, Tedesco P, Johnson TE, Kim SK. An elt-3/elt-5/elt-6 GATA transcription circuit guides aging in C. elegans. Cell. 2008;134:291–303. doi: 10.1016/j.cell.2008.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis. 2010;31:90–99. doi: 10.1093/carcin/bgp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bartke A, Sun LY, Longo V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev. 2013;93:571–598. doi: 10.1152/physrev.00006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McMahon M, Campbell KH, MacLeod AK, McLaughlin LA, Henderson CJ, Wolf CR. HDAC inhibitors increase Nrf2-signaling in tumour cells and blunt the efficacy of co-adminstered cytotoxic agents. PLoS One. 2014;9:e114055. doi: 10.1371/journal.pone.0114055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lerner C, Bitto A, Pulliam D, Nacarelli T, Konigsberg M, Van Remmen H, Torres C, Sell C. Reduced mammalian target of rapamycin activity facilitates mitochondrial retrograde signaling and increases life span in normal human fibroblasts. Aging Cell. 2013;12:966–977. doi: 10.1111/acel.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Steinbaugh MJ, Sun LY, Bartke A, Miller RA. Activation of genes involved in xenobiotic metabolism is a shared signature of mouse models with extended lifespan. Am J Physiol Endocrinol Metab. 2012;303:E488–E495. doi: 10.1152/ajpendo.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, et al. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51:618–631. doi: 10.1016/j.molcel.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 153.Swindell WR. Gene expression profiling of long-lived dwarf mice: longevity- associated genes and relationships with diet, gender and aging. BMC Genomics. 2007;8:353. doi: 10.1186/1471-2164-8-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leiser SF, Miller RA. Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol. 2010;30:871–884. doi: 10.1128/MCB.01145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun LY, Spong A, Swindell WR, Fang Y, Hill C, Huber JA, Boehm JD, Westbrook R, Salvatori R, Bartke A. Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. Elife. 2013;2:e01098. doi: 10.7554/eLife.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lewis KN, Wason E, Edrey YH, Kristan DM, Nevo E, Buffenstein R. Regulation of Nrf2 signaling and longevity in naturally long-lived rodents. Proc Natl Acad Sci U S A. 2015;112:3722–3727. doi: 10.1073/pnas.1417566112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Perez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, Mele J, Andziak B, Yang T, Edrey Y, Friguet B, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci U S A. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rodriguez KA, Edrey YH, Osmulski P, Gaczynska M, Buffenstein R. Altered composition of liver proteasome assemblies contributes to enhanced proteasome activity in the exceptionally long-lived naked mole-rat. PLoS One. 2012;7:e35890. doi: 10.1371/journal.pone.0035890. [DOI] [PMC free article] [PubMed] [Google Scholar]