Abstract
Mitochondrial reactive oxygen species production has emerged as an important pathological mechanism in myocardial ischemia/reperfusion injury. Attempts at targeting reactive oxygen species by scavenging using antioxidants have, however, been clinically disappointing. This review will provide an overview of the current understanding of mitochondrial reactive oxygen species in ischemia/reperfusion injury. We will outline novel therapeutic approaches designed to directly target the mitochondrial respiratory chain and prevent excessive reactive oxygen species production and its associated pathology. This approach could lead to more effective interventions in an area where there is an urgent need for new treatments.
Keywords: antioxidants, mitochondria, oxygen, reactive oxygen species, reperfusion injury
Mitochondria are an important source of reactive oxygen species (ROS) in mammalian cells and play a critical role in cardiac function. Under physiological conditions, low levels of ROS are produced as a by-product of mitochondrial respiration and act as essential cellular mediators in a variety of biological processes, including regulation of the immune response and autophagy.1–3 Stress or injury can, however, cause ROS to increase significantly, overwhelming endogenous antioxidant mechanisms, and resulting in severe oxidative damage to cellular components, such as lipids, proteins, and DNA.4 Mitochondrial ROS are now known to be key mediators of mitochondrial dysfunction and disease pathology in a range of cardiovascular conditions, including atherosclerosis, cardiac hypertrophy, chronic heart failure, ventricular remodeling, and ischemia/reperfusion (IR) injury.5–7 On reperfusion of ischemic myocardium, the rapid reintroduction of oxygen into the cell leads to a burst of ROS generation that triggers opening of the mitochondrial permeability transition (mPTP) pore and myocardial cell death. Significant progress has been made in the field of inhibiting or scavenging ROS in an attempt to preserve mitochondrial and cardiomyocyte function. However, despite the large body of evidence supporting the inhibition of oxidative stress as a valuable therapeutic strategy, treatment with antioxidants has failed to deliver clinically significant benefits.8 In the present review, we will discuss the role of mitochondrial ROS in cardiac IR injury, describing the current mechanisms that are thought to drive its production. Furthermore we will highlight current methods at targeting mitochondria ROS production with a particular focus on interventions that inhibit complexes I and II.
IR Injury
IR injury remains a leading cause of death worldwide and the primary cause of chronic heart failure. Although the past few decades have seen a marked improvement in outcomes in patients treated with early reperfusion therapy, currently 1 in 4 patients will die or present with heart failure within 1 year postinjury.9 Reperfusion of the ischemic myocardium is essential to salvage viable tissue but paradoxically the rapid restoration of blood flow can induce injury beyond that of the initial ischemic insult. Known as reperfusion injury, studies have shown that it can account for ≤50% of the total tissue damage7 for which there is currently no effective therapy available in the clinic. The mechanisms underlying IR injury are multifactorial and have been extensively reviewed elsewhere.7,10 However, it is generally accepted that mitochondrial dysfunction is central to the pathology of both IR injury and chronic heart failure, with the mitochondrion not only being the main producer of ROS but also a primary target of ROS damage.
Cardiac metabolism is predominantly aerobic. As such, the maintenance of normal cardiac function and viability is highly dependent on the constant delivery of oxygen. During periods of severe myocardial ischemia, profound disturbances in metabolism occur resulting in a shift toward anaerobic glycolysis. ATP depletion and lactic acidosis drive cytosolic sodium accumulation via the sodium/hydrogen exchanger and as a consequence excess Na+ is extruded through the reverse action of the plasma membrane sodium/calcium exchanger.11 Typical calcium (Ca2+) management by the sarcoplasmic reticulum Ca2+-ATPase is prevented because of depletion of mitochondrially derived ATP, resulting in cytosolic Ca2+ overload. Furthermore, there is an accumulation of metabolic end-products, including hypoxanthine, xanthine, and succinate,12–14 and the formation of proinflammatory mediators that promote the infiltration and activation of neutrophils. All these events are thought to prime the heart for the large burst of ROS generation on reperfusion. Reoxygenation of the cell at reperfusion and rapid restoration of the mitochondrial membrane potential (ΔΨm) result in a large Ca2+ influx into mitochondria. Together with a burst of ROS production15 and normalization of pH,16 opening of the mPTP is induced.17 The prolonged opening of the mPTP is now generally agreed to be decisive in committing cells to death on reperfusion. The mPTP is a highly conducting channel in the mitochondrial inner membrane. Although the exact nature of the pore is under debate, recent evidence suggests that the FoF1-ATP synthase is a major component.18,19 Although the low pH present during ischemia prevents formation of the mPTP, the normalization of pH at reperfusion results in mPTP formation and subsequent collapse of ΔΨm, cytochrome c release, ATP depletion, and cellular death. Opening of the mPTP is therefore a critical component in reperfusion injury pathology.10,19–21
The mitochondrial electron transport chain is an important source of ROS during IR injury but several other sources can also contribute. They include monoamine oxidase on the surface of the mitochondrial outer membrane, xanthine oxidases, NAD(P)H oxidases, and uncoupled nitric oxide synthases.22–24 The contribution of these enzymes to total IR-induced ROS production is, however, thought to be lower than that of mitochondria and to occur later in the IR injury process, so will not be discussed further in this review.
Mitochondrial Respiratory Chain
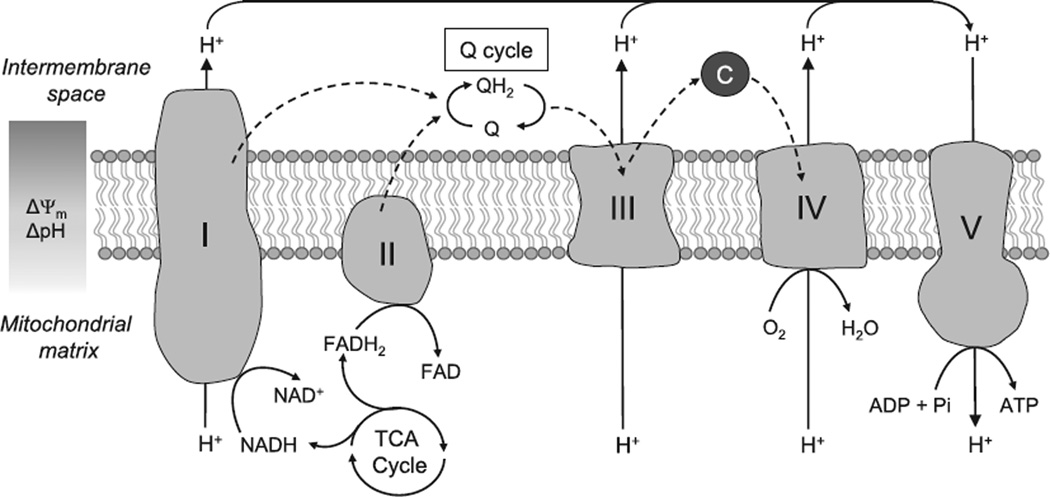
Because this review is aimed at a general audience, a brief primer on mitochondrial respiratory activity is provided here, and is illustrated in Figure 1. Substrates (pyruvate from glycolysis or acetyl-CoA from β-oxidation) are decarboxylated in the tricarboxylic acid cycle to yield reducing equivalents NADH and FADH2. Electrons are then passed onto complexes I or II, respectively, and then to the mobile electron carrier coenzyme Q10 (ubiquinone), reducing it to ubiquinol. Ubiquinol is reoxidized by complex III, passing electrons to cytochrome c, then cytochrome oxidase, and finally oxygen, generating H2O. The respiratory complexes are electron-driven proton pumps, such that this passage of electrons is coupled to the generation of a transmembrane proton electrochemical potential gradient (positive outside). The electrochemical energy in this gradient is then used by the FoF1-ATP synthase to generate ATP. It is important to note that the sharing of Co-Q as a common electron carrier by complexes I, II, and III is what permits these complexes to exist in multiple configurations, such that electrons can flow from I to III, II to III, I to II, and II to I, as shown in Figure 2.
Figure 1. The mitochondrial electron transport chain.
Electrons derived from the oxidation of NADH and FADH2 enter the electron transport chain at complexes I (NADH ubiquinone oxidoreductase) and II (Succinate dehydrogenase). They are then funneled through the electron carriers, Coenzyme Q, and complex III (Ubiquinol cytochrome c oxidoreductase), until they reach complex IV (cytochrome c oxidase) where they are used to reduce molecular oxygen to water. This transfer of electrons is coupled to the extrusion of protons at complexes I, III, and IV generating an electrochemical gradient across the mitochondrial membrane. Protons in the intermembrane space are then used to drive the synthesis of ATP at complex V (ATP synthase). C indicates cytochrome c; and TCA, tricarboxylic acid. Dashed arrows indicate path of electrons.
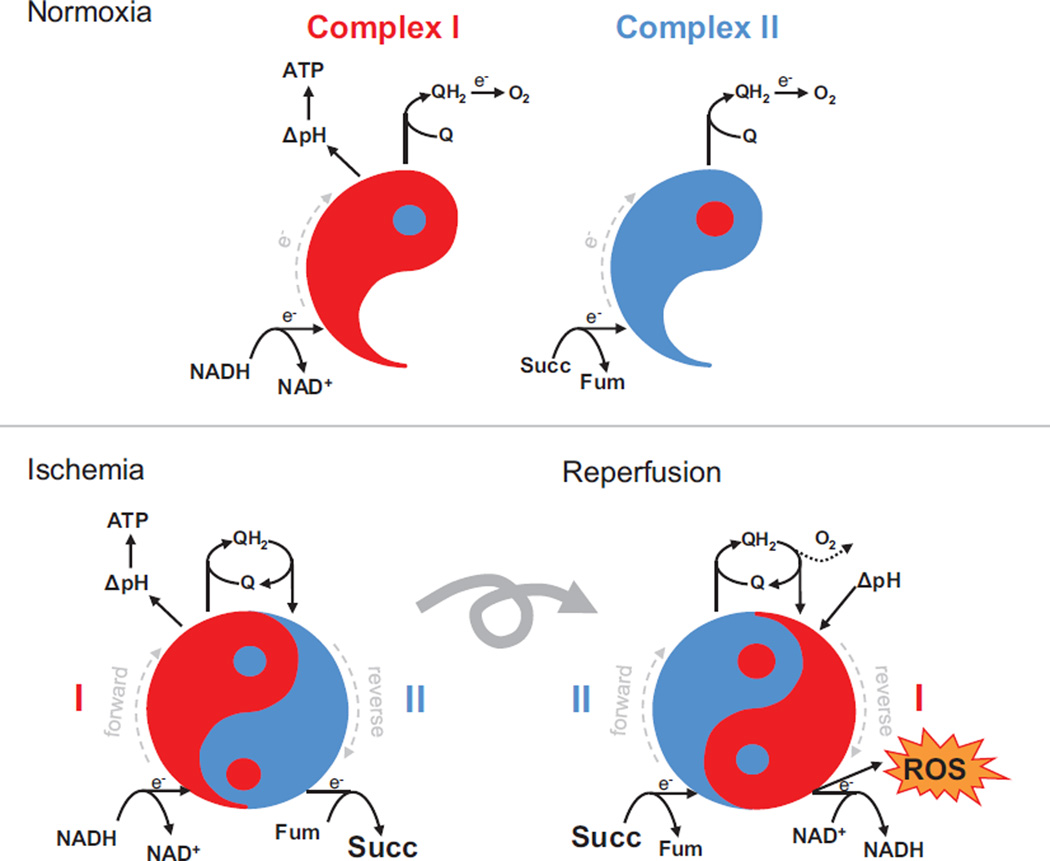
Figure 2. Respiratory Complex I and II Yin-Yang during ischemia and reperfusion.
Under normoxic conditions, both complex I (red) and complex II (blue) work in the forward direction (dashed gray line indicates direction of electron flow), taking electrons from NADH and succinate, respectively, and reducing ubiquinone (Q) to ubiquinol (QH2). Electrons are eventually passed down the respiratory chain to O2, and complex I pumps protons to generate a transmembrane ΔpH. During ischemia, QH2 generated by complex I working forward, is oxidized by complex II working in reverse. In this Yin-Yang formation, fumarate acts as an electron acceptor, resulting in accumulation of succinate. This process allows complex I to continue pumping protons during ischemia. At reperfusion, the rapid consumption of accumulated succinate generates too much QH2 for the reoxygenated terminal respiratory chain to handle (dotted line). Coupled with residual acidic pH from ischemia, this drives reverse electron transfer in complex I, resulting in the generation of significant amounts of reactive oxygen species (ROS).
Central Role of Mitochondria
Mitochondria are essential organelles for normal cellular function. Occupying ≤30% of total cardiomyocyte volume, they are the main source of ATP for the contracting cell through oxidative phosphorylation.25 Mitochondria are also a key source of cellular ROS production with oxygen being converted by mitochondria to superoxide at complexes I and III.26 However, although the amounts of superoxide produced by isolated mitochondria can be readily estimated, the amount produced in vivo and the factors that regulate this production remain obscure. The superoxide produced in the mitochondrial matrix is then largely dismutated to hydrogen peroxide (H2O2) by manganese superoxide dismutase (Mn-SOD).27 Although several other sources of ROS within mitochondria have been documented (eg, α-ketoglutarate dehydrogenase, monoamine oxidase, ETF-QOR of β-oxidation, α-glycoerophosphate dehydrogenase),28,29 their relative importance under in vivo conditions is poorly understood. Thus, the rest of this review will focus primarily on complex I because this seems to be quantitatively the most important source of ROS in the setting of IR injury.4
In the context of IR pathology, elevated mitochondrial ROS levels drive oxidative damage to mitochondria, which results in disruption of the respiratory machinery and ATP generation. In addition, in conjunction with dysregulated calcium levels, mitochondrial ROS lead to induction of the mPTP, contributing to both apoptotic and necrotic cell death caused by IR. Because of the central role of mitochondrial ROS in IR pathology, many investigations have focused on characterizing the pathways that underlie their generation. In the past decade, such studies have increasingly highlighted a central role for mitochondrial complex I as the most significant superoxide source during IR.30,31 More recently, it has been shown that generation of superoxide from complex I during IR is dependent on electron supply from the mitochondrial citric acid cycle intermediate succinate.14 Succinate, which accumulates significantly during ischemia through the reverse action of complex II, is rapidly oxidized in the first minutes of reperfusion. This rapid oxidation drives reverse electron transport (RET) at complex I, in which electrons are forced from reduced Coenzyme Q (CoQ) back to complex I generating large amounts of superoxide. This process can be described as a yin-yang formation in which during ischemia, QH2 generated by complex I working forward is oxidized by complex II working in reverse. At reperfusion, complex II acting in forward mode consumes the accumulated succinate driving RET at complex I (Figure 2). Most interestingly, from a therapeutic perspective, it has been shown that this generation of damaging ROS on reperfusion can be inhibited, either by preventing the accumulation of succinate during ischemia or by inhibiting the succinate-dependent superoxide production by transient inactivation of complex I.14,32 Both approaches will be discussed further below.
Good Versus Bad ROS
An intriguing aspect to ROS production in the heart is that depending on the circumstances and context, it can be considered either good or bad. That is, not all amounts of ROS are damaging and only when levels reach beyond the capacity of endogenous antioxidant mechanisms will ROS become detrimental to cell function and contribute to IR pathology. Conversely, ROS production has also been found to be a trigger for protection against IR injury particularly through the activation of survival programs during ischemic preconditioning (IPC) and ischemic postconditioning (IPost).33,34 IPC, first demonstrated by Murray in 1986,35 is a phenomenon in which brief cycles of IR protect the heart from reperfusion injury after a prolonged ischemic insult. ROS generated from these IR cycles are recognized as triggers for a cascade of signaling events that result in reduced tissue damage with the mitochondrion considered as a primary source.36 Pre-treating isolated rabbit hearts with oxygen radicals can reproduce the beneficial effect of IPC on infarct size,37 whereas giving ROS scavengers before ischemia abolishes IPC-induced protection.34 The most straightforward interpretation of this intriguing observation is that although low levels of ROS can be beneficial by upregulating protective mechanisms, a larger amount of ROS has detrimental effects. This counterbalance between good and bad levels, known as mitohormesis,2 is supported by an increasing body of work in which low levels of ROS are thought to act as signaling molecules to promote health and extend lifespan.2 In the context of IR injury, a small increase in ROS, sufficient to lead to transient mPTP opening, has been shown to be protective against subsequent IR injury.38 On the contrary, prolonged ROS exposure leading to sustained mPTP opening inevitably leads to irreversible mitochondrial damage and ultimately cell death. The threshold at which ROS production transitions from being protective to becoming harmful may be modulated by a variety of factors, such as diabetes mellitus, sex, and age; and risk factors which are already established to affect the efficacy of cardioprotective strategies.39 For example, one way in which sex may determine the mPTP response to ROS is in the levels of nitric oxide. It is known that endothelial nitric oxide synthase is regulated by estrogen,40 and this may directly impact ROS levels, in addition to nitric oxide being a direct inhibitor of the pore.41 Similarly for aging, the sensitivity of the Keap1/Nrf2 signaling axis, a key genetic response to oxidative stress, is known to decline with age.42
Another hypothesis explaining the protean roles of ROS could be ascribed to the spatial distribution of its sites of production. It is well established that for many signaling pathways, the intracellular location of the signal plays a crucial role; for example, the compartmentalization of the cGMP—guanylate cyclase pathway.43 Unfortunately, the details of the localization of ROS signaling are difficult to assess in vivo. However, given that different classes of mitochondria exist in the heart (subsarcolemmal versus intrafibrillar populations) behave differently during IPC and IPost,44,45 it seems likely that the spatial distribution of mitochondrial ROS generation may also be a key variable. Finally, the timing of ROS generation could be important during IR with ROS being beneficial as a trigger of preconditioning-like signaling before a prolonged period of ischemia, whereas the large ROS burst at reperfusion induces many detrimental downstream effects.
Therapeutic Implications: Preventing Excessive ROS Generation
The compelling body of evidence linking reperfusion-induced ROS production to cardiac pathology has not surprisingly led to the testing of a wide range of antioxidant approaches to mitigate the detrimental effects of oxidative stress on reperfusion. Although many antioxidant strategies have shown benefit when applied to in vitro and in vivo model systems, only a tiny fraction has translated to improvements in major clinical end-points in human trials.46 For example, antioxidants including Vitamin C, Vitamin E, Edavarone, and Coenzyme Q10 have shown disappointing or conflicting outcomes in patients.8 Many possible reasons for these poor results have been considered; the dosage of drug may not be optimal to achieve sufficient myocardial levels at reperfusion; the timing of the intervention in relation to the onset of ischemia or point of reperfusion may be incorrect and preclinical models used may not be appropriate for screening new compounds for human use.47,48 The development of mitochondria-targeted antioxidants in which compounds are localized to the mitochondrion by conjugation to a triphenylphosphonium (TPP+) cation may address some difficulties inherent in using untargeted antioxidants that do not accumulate in mitochondria.49 MitoQ is a TPP+ modified ubiquinol which on delivery to mitochondria decreases oxidative damage, and has been shown to be protective against both cardiac50 and liver IR injury51 in vivo, as well as protecting against oxidative damage in a murine model of heart transplantation.52 The potential benefit of these targeted compounds against IR injury in humans has yet to be determined. A further consideration is that a more effective strategy may be to block the excessive ROS production that occurs on reperfusion at its source, rather than scavenge it after it has been produced. Moreover, this approach could in principle allow the blockade of ROS production only when it becomes pathological, avoiding the potential disruption to cellular homeostasis by altering physiologically important cellular signals by good ROS through chronic antioxidant treatment. Given that the mitochondrial respiratory chain is a critical source of ROS on reperfusion, it has become a major target for novel compounds aimed at ameliorating IR injury, and this strategy will be discussed in the next section.
Pharmacological Inhibitors of the Respiratory Chain as Therapeutics for IR Injury
Despite the lack of oxygen during ischemia or hypoxia leading to inhibition of the respiratory chain, a wide variety of respiratory inhibitors have been demonstrated to afford protection against IR injury. The Table lists several such inhibitors and their sites of action within the respiratory chain. Until recently, it was thought the mechanism of action for these respiratory inhibitors centered on the gradual wake up hypothesis of reperfusion therapy.84 In this paradigm, a rapid reestablishment of respiratory activity at reperfusion leads to a surge of mitochondrial Ca2+ uptake and ROS generation which contribute to mPTP opening. It was hypothesized that the washout of a respiratory inhibitor present at reperfusion would permit a more gradual wake up of metabolism, thus avoiding these pathogenic effects. However, the recent identification of the source of ROS at reperfusion, namely the reverse electron transfer at complex I, forces a further focusing of this paradigm.14 Namely, it cannot go unnoticed that ≈85% of the protective respiratory inhibitors listed in the Table act at the level of complex I or II. Although the prevalence of agents hitting a given pharmacological target cannot be taken as evidence of the central biological importance of the target, it is notable that there are well-known inhibitors of other parts of the respiratory chain (eg, cyanide for complex IV, myxothiazol for complex III) that have not been found useful in a therapeutic setting. Furthermore, although many of the molecules in the Table act at a pleiotropic level, there are some exquisitely specific drugs targeted at complexes I and II (eg, rotenone and atpenin A5), which are most likely mediating their effects via these complexes and not through off-target mechanisms. We will now discuss these complexes in turn and the current evidence for their modulation in protecting the myocardium during IR injury.
Table.
Mitochondrial Respiratory Inhibitors That Have Been Shown to Protect the Heart or Brain From Ischemia/Reperfusion Injury, and Their Sites of Action
| Site of Action | Inhibitor | References |
|---|---|---|
| Complex I | Rotenone | Lesnefsky et al31 |
| Amobarbital | Stewart et al53 | |
| S-nitrosothiols | Nadtochiy et al,54 Prime et al,55 Burwell et al,56 and Nadtochiy et al57 | |
| Nitrite | Shiva et al58 and Dezfulian et al59 | |
| Metformin | Owen et al,60 Legtenberg et al,61 Bhamra et al,62 and Matsuzaki et al63 | |
| Capsaicin | D’Alonzo et al64 and Satoh et al65 | |
| Isoflurane | Hanley et al66 and Cope et al67 | |
| Ranolazine | Wyatt et al68 | |
| Complex II | Atpenin A5 | Wojtovich et al69 |
| Diazoxide | Anastacio et al70 and Wang et al71 | |
| Malonate | Chouchani et al,14 Boylston et al,72 and Wojtovich et al73 | |
| Nitroxyl | Pagliaro et al74 and Shiva et al75 | |
| 3-Nitropropionate | Riepe et al76 | |
| Nitro-alkenes | Nadtochiy et al77 | |
| Complex III | Antimycin A | Kabir et al78 |
| Complex IV | Nitric oxide | Zhao et al79 |
| Carbon monoxide | Clark et al80 | |
| Hydrogen sulfide | Khan et al,81 Pan et al,82 and Elrod et al83 |
Note: Some references are paired such that the phenomenon of a molecule inhibiting a respiratory complex and the phenomenon of it being protective in ischemia/reperfusion injury are not necessarily co-observed in the same experimental system. Inclusion of a molecule in this table should not be misconstrued as claiming that the mechanism of its protection is dependent on its effects on a given respiratory complex.
Complex I
Complex I (NADH ubiquinone oxidoreductase) is the primary point of electron entry within mitochondria responsible for the oxidation of NADH, derived from glycolysis, the citric acid cycle, and the β-oxidation of fatty acids. Complex I transfers electrons to CoQ, and protons are transported across the inner membrane contributing to the mitochondrial proton motive force. In addition, it is an important site for ROS generation with complex I producing large amounts of superoxide in the presence of a high NADH/NAD+ ratio, where oxygen reacts with a fully reduced flavin mononucleotide site.4 Complex I can also produce a large amount of ROS during RET, where in the presence of highly reduced CoQ pool and close to maximal proton motive force, electrons are pushed backward from CoQH2 through complex I reducing NAD+ to NADH and also producing superoxide.85,86 Although the physiological relevance of RET in vivo is only now being elucidated, it produces the largest rate of mitochondrial ROS production known to occur within mitochondria. Furthermore, this process of superoxide production by RET at complex I seems to be the major source of ROS early during IR injury.14
During prolonged ischemia, when complex I is not oxidizing NADH because of the lack of oxygen, the protein converts to a deactive state.87,88 Reperfusion of the tissue results in the rapid reactivation of complex I and the generation of large amounts of cytotoxic ROS by RET.14 Inhibitors of complex I including rotenone31 and amobarbital53 have found to be protective when given during cardiac IR injury, indicating that preventing the reactivation of complex I on reperfusion is a promising potential therapeutic strategy. Of course, the use of irreversible complex I inhibitors is not viable as a therapy, but interestingly when complex I undergoes the deactive transition a critical cysteine, cysteine 39, on the ND3 subunit becomes exposed to modification.87,88 This residue can be reversibly inhibited by its S-nitrosation by S-nitrosothiols such as SNO-MPG54 or MitoSNO.32 Further supporting a role for this cysteine residue in cardioprotection, recent work has shown that damage protection during IR, IPC, and IPost correlates highly with the persistent S-nitrosation of mitochondrial protein thiols, with complex I as a chief target.32,89–91 One example of this protective mechanism is MitoSNO, a mitochondria-targeted drug that prevents ROS production from complex I during early reperfusion after IR injury.32 MitoSNO is a mitochondria-targeted S-nitrosothiol based on the NO donor S-nitroso-N-acetylpenicillamine coupled to the TPP+ cation which leads to its rapid, several hundred-fold accumulation, driven by both the plasma and mitochondrial membrane potentials, into the mitochondrial matrix where it accumulates within minutes of intravenous injection.55,92 On uptake into mitochondria, MitoSNO reacts rapidly with intramitochondrial thiols and S-nitrosates cysteine 39 on subunit ND3 of complex I locking the enzyme in its deactive form at reperfusion, and thereby preventing the excessive burst of ROS on reperfusion.32 The modification is reversed with a half-life of ≈5 minutes by the endogenous mitochondrial glutathione and thioredoxin systems, allowing complex I to return to full levels of activity a few minutes after reperfusion.14 Our studies have shown that MitoSNO not only protected against IR injury in vivo32 but also greatly enhanced long-term cardiac function post-IR injury.93
Complex II
Complex II (succinate dehydrogenase) catalyzes the oxidation of succinate to fumarate, resulting in the donation of electrons to the respiratory chain via the reduction of FAD to FADH2. Unlike the other respiratory complexes, it does not pump protons across the inner membrane but instead acts to maintain a reduced CoQ pool which has been largely considered to be its primary function.94 This sequence also creates a direct link between 2 major mitochondrial pathways, the citric acid cycle and the respiratory chain. Several roles for complex II have, however, also been recently proposed that expand beyond this with evidence now for direct complex II–mediated ROS generation,95 as well as a mechanistic link with the putative mitochondrial ATP-sensitive potassium channel (mtKATP).96 Complex II is also now recognized as a key modulator of mitochondrial ROS production by other respiratory complexes, particularly complex I.
The accumulation of excessive ischemic succinate, via the reverse action of complex II, is considered a critical driver of ROS formation at reperfusion. Preventing either its build-up during ischemia or its rapid oxidation at reperfusion are therefore potential valuable therapeutic strategies to reduce detrimental ROS generation and protect against IR injury. In agreement with this, an extensive body of work exists demonstrating the inhibition of respiration at complex II can decrease ROS production.97 Inhibitors such as dimethyl malonate, diazoxide, and atpenin A5 all protect against IR injury when given before ischemia.14,69,70,72 Moreover, protection afforded by dimethyl malonate in vivo was attributed directly to the attenuation of ischemic levels of succinate and inhibition of mitochondrial ROS generation at reperfusion.14 There is also some evidence that malonate itself may act as an endogenous protector against IR with the compound being generated endogenously in mitochondria under conditions mimicking IPC.73 However, whether these compounds exert cardioprotective effects solely via complex II inhibition and ROS generation or whether the mtKATP channel is involved remains a controversially and actively discussed issue. Moreover, although these strategies may be highly useful in situations of predictable ischemia, including elective surgery and organ transplantation, they are not clinically appropriate, given patients undergoing myocardial infarction (MI) arrive at hospital with an already occluded artery. Succinate accumulation during ischemia becomes pathological only on its rapid oxidation at reperfusion in which it drives RET-mediated ROS production through complex I. By suppressing succinate oxidation at the point of reperfusion through complex II inhibition, compounds such as dimethyl malonate could be potentially cardioprotective. It is therefore essential to determine whether complex II inhibitors are equally effective at ameliorating cardiac injury when used later in IR, such as just before reperfusion. Indeed, recent work in the isolated mouse heart has demonstrated that the administration of malonate during the first 15 minutes of reperfusion was cardioprotective only through the inhibition of succinate reoxidation and the reduction in ROS production and mPTP opening.98 Whether this important result can be translated to in vivo models, however, remains to be determined.
Future Perspectives and Translational Significance
An important consideration for the potential future use of respiratory inhibition as a therapy for IR injury is the timing of delivery. Clearly, although the inhibition of complex I or II at early reperfusion would be anticipated to minimize ROS generation from RET, it is not immediately clear that inhibition of these complexes during ischemia itself would be beneficial. This is because of the yin-yang nature of complexes I and II during ischemia, in which complex I continues to operate as a proton pump allowing to some extent the ΔΨm to be maintained. As such, inhibition of complex I during ischemia may have unforeseen detrimental effects by removing this important function. A further consideration in moving such molecules into a clinical setting is their ease of washout, that is, their tightness of binding to their targets. In the case of rotenone and other tight-binding lipophilic molecules, inhibition would be expected to reverse rather slowly, if at all, whereas the complex II inhibitor, 3-nitropropionate, is a suicide inhibitor that covalently modifies complex II potentially resulting in long-term toxic effects on organ function.99 Furthermore, currently available inhibitors of mitochondrial respiratory complexes are not tissue-specific and are therefore present in other important tissues such as the brain. Consequently, the chronic delivery of a respiratory inhibitor would be expected to elicit toxic side-effects such as neurodegenerative disease. Specifically, long-term inhibition of complex I is associated with Parkinson disease, complex II Huntington disease, and complex IV Alzheimer disease.100,101 With this regard, another advantage to nitric oxide donors and other short-lived species, such as MitoSNO as mitochondrial respiratory inhibitors, is their short time of action and rapid metabolism, which would permit reestablishment of normal mitochondrial function once the initial early-reperfusion danger-period has passed.
Recently, it has been shown that treatment with a P2Y12 inhibitor, such as clopidogrel or ticagrelor, was highly protective in animal models of acute MI as well as in small human studies, and that many conditioning strategies, such as IPost do not offer additional benefits in reducing infarct size.102,103 This evidence could well be the reason for the failures of many recent clinical trials of either IPost or interventions mimicking conditioning. To translate any of the above-mentioned compounds targeting complex I or III is therefore crucial to test whether they have additive effects on top of an effective treatment with P2Y12 inhibitors.104 Further aspects on how to translate preclinical findings in patient care and the challenges especially in acute MI have been extensively reviewed elsewhere.105
Summary
There is a pressing need for therapeutic approaches to be applied in conjunction with reperfusion therapy to reduce infarction injury and long-term outcome in patients with MI. Modulation of the respiratory chain through inhibiting complexes I and II is an important emerging strategy. These interventions can now be considered as potential rational therapies, arising from the view that the initial burst of ROS from complex I on reperfusion is because of the accumulation of succinate by the reversal of complex II during ischemia, which then drives the initial burst of ROS at reperfusion by RET at complex I. The reversible inhibition of complexes I and II would therefore prevent this burst of ROS and protect against infarction. Currently, approaches that prevent the accumulation of succinate during ischemia, such as dimethyl malonate, or stabilize the deactive form of complex I by S-nitrosation, such as MitoSNO, have been shown to be effective in animal models. Whether these results will translate into the clinic remains to be seen. Certainly, the next stages are to see whether it is possible to extend and optimize these targets with new and better drugs. However, the model of succinate-driven ROS production mediated by complexes I and II should facilitate the future development of novel-targeted therapies against the generation of excessive mitochondrial ROS in a range of pathologies, such as MI and stroke.
Acknowledgments
Work in our laboratories is supported by the Medical Research Council (United Kingdom) and the British Heart Foundation.
Drs Chouchani, Murphy, Krieg have filed patents in the area of therapies designed to prevent mitochondrial reactive oxygen species production during cardiac ischemia/reperfusion injury.
Nonstandard Abbreviations and Acronyms
- I/R
ischemia/reperfusion
- IPC
ischemic preconditioning
- IPost
ischemic postconditioning
- MI
myocardial infarction
- mPTP
mitochondrial permeability transition
- RET
reverse electron transport
- ROS
reactive oxygen species
Footnotes
Disclosures
The other authors report no conflicts.
References
- 1.Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yun J, Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawyer DB, Colucci WS. Mitochondrial oxidative stress in heart failure: “oxygen wastage” revisited. Circ Res. 2000;86:119–120. doi: 10.1161/01.res.86.2.119. [DOI] [PubMed] [Google Scholar]
- 6.Morrell CN. Reactive oxygen species: finding the right balance. Circ Res. 2008;103:571–572. doi: 10.1161/CIRCRESAHA.108.184325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugamura K, Keaney JF., Jr Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cung TT, Morel O, Cayla G, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 10.Di Lisa F, Bernardi P. Mitochondria and ischemia-reperfusion injury of the heart: fixing a hole. Cardiovasc Res. 2006;70:191–199. doi: 10.1016/j.cardiores.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Karmazyn M. The myocardial sodium-hydrogen exchanger (NHE) and its role in mediating ischemic and reperfusion injury. Keio J Med. 1998;47:65–72. doi: 10.2302/kjm.47.65. [DOI] [PubMed] [Google Scholar]
- 12.Taegtmeyer H. Metabolic responses to cardiac hypoxia. Increased production of succinate by rabbit papillary muscles. Circ Res. 1978;43:808–815. doi: 10.1161/01.res.43.5.808. [DOI] [PubMed] [Google Scholar]
- 13.Pisarenko O, Studneva I, Khlopkov V, Solomatina E, Ruuge E. An assessment of anaerobic metabolism during ischemia and reperfusion in isolated guinea pig heart. Biochim Biophys Acta. 1988;934:55–63. doi: 10.1016/0005-2728(88)90119-3. [DOI] [PubMed] [Google Scholar]
- 14.Chouchani ET, Pell VR, Gaude E, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemasters JJ, Bond JM, Chacon E, Harper IS, Kaplan SH, Ohata H, Trollinger DR, Herman B, Cascio WE. The pH paradox in ischemia-reperfusion injury to cardiac myocytes. EXS. 1996;76:99–114. doi: 10.1007/978-3-0348-8988-9_7. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307(pt 1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabó I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA, Jr, Jonas EA. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- 21.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion–a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 22.Kaludercic N, Carpi A, Menabò R, Di Lisa F, Paolocci N. Monoamine oxidases (MAO) in the pathogenesis of heart failure and ischemia/reperfusion injury. Biochim Biophys Acta. 2011;1813:1323–1332. doi: 10.1016/j.bbamcr.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lassègue B, San Martín A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granger DN, Kvietys PR. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tait SW, Green DR. Mitochondria and cell signalling. J Cell Sci. 2012;125:807–815. doi: 10.1242/jcs.099234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res. 2008;77:334–343. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- 28.Adam-Vizi V, Tretter L. The role of mitochondrial dehydrogenases in the generation of oxidative stress. Neurochem Int. 2013;62:757–763. doi: 10.1016/j.neuint.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013;1:304–312. doi: 10.1016/j.redox.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–C147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- 31.Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- 32.Chouchani ET, Methner C, Nadtochiy SM, et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ambrosio G, Tritto I, Chiariello M. The role of oxygen free radicals in preconditioning. J Mol Cell Cardiol. 1995;27:1035–1039. doi: 10.1016/0022-2828(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 34.Baines CP, Goto M, Downey JM. Oxygen radicals released during ischemic preconditioning contribute to cardioprotection in the rabbit myocardium. J Mol Cell Cardiol. 1997;29:207–216. doi: 10.1006/jmcc.1996.0265. [DOI] [PubMed] [Google Scholar]
- 35.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 36.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 37.Tritto I, D’Andrea D, Eramo N, Scognamiglio A, De Simone C, Violante A, Esposito A, Chiariello M, Ambrosio G. Oxygen radicals can induce preconditioning in rabbit hearts. Circ Res. 1997;80:743–748. doi: 10.1161/01.res.80.5.743. [DOI] [PubMed] [Google Scholar]
- 38.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109:1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 39.Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142–1174. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 40.Kypreos KE, Zafirovic S, Petropoulou PI, Bjelogrlic P, Resanovic I, Traish A, Isenovic ER. Regulation of endothelial nitric oxide synthase and high-density lipoprotein quality by estradiol in cardiovascular pathology. J Cardiovasc Pharmacol Ther. 2014;19:256–268. doi: 10.1177/1074248413513499. [DOI] [PubMed] [Google Scholar]
- 41.Brookes PS, Salinas EP, Darley-Usmar K, Eiserich JP, Freeman BA, Darley-Usmar VM, Anderson PG. Concentration-dependent effects of nitric oxide on mitochondrial permeability transition and cytochrome c release. J Biol Chem. 2000;275:20474–20479. doi: 10.1074/jbc.M001077200. [DOI] [PubMed] [Google Scholar]
- 42.Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99:816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- 44.Boengler K, Stahlhofen S, van de Sand A, Gres P, Ruiz-Meana M, Garcia-Dorado D, Heusch G, Schulz R. Presence of connexin 43 in subsarcolemmal, but not in interfibrillar cardiomyocyte mitochondria. Basic Res Cardiol. 2009;104:141–147. doi: 10.1007/s00395-009-0007-5. [DOI] [PubMed] [Google Scholar]
- 45.Sun J, Nguyen T, Aponte AM, Menazza S, Kohr MJ, Roth DM, Patel HH, Murphy E, Steenbergen C. Ischaemic preconditioning preferentially increases protein S-nitrosylation in subsarcolemmal mitochondria. Cardiovasc Res. 2015;106:227–236. doi: 10.1093/cvr/cvv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ekeløf S, Jensen SE, Rosenberg J, Gögenur I. Reduced oxidative stress in STEMI patients treated by primary percutaneous coronary intervention and with antioxidant therapy: a systematic review. Cardiovasc Drugs Ther. 2014;28:173–181. doi: 10.1007/s10557-014-6511-3. [DOI] [PubMed] [Google Scholar]
- 47.Miura T, Miki T. Limitation of myocardial infarct size in the clinical setting: current status and challenges in translating animal experiments into clinical therapy. Basic Res Cardiol. 2008;103:501–513. doi: 10.1007/s00395-008-0743-y. [DOI] [PubMed] [Google Scholar]
- 48.Downey JM, Cohen MV. Why do we still not have cardioprotective drugs? Circ J. 2009;73:1171–1177. doi: 10.1253/circj.cj-09-0338. [DOI] [PubMed] [Google Scholar]
- 49.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 50.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 51.Dare AJ, Bolton EA, Pettigrew GJ, Bradley JA, Saeb-Parsy K, Murphy MP. Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol. 2015;5:163–168. doi: 10.1016/j.redox.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dare AJ, Logan A, Prime TA, Rogatti S, Goddard M, Bolton EM, Bradley JA, Pettigrew GJ, Murphy MP, Saeb-Parsy K. The mitochondria-targeted anti-oxidant MitoQ decreases ischemia-reperfusion injury in a murine syngeneic heart transplant model. J Heart Lung Transplant. 2015;34:1471–1480. doi: 10.1016/j.healun.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart S, Lesnefsky EJ, Chen Q. Reversible blockade of electron transport with amobarbital at the onset of reperfusion attenuates cardiac injury. Transl Res. 2009;153:224–231. doi: 10.1016/j.trsl.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Nadtochiy SM, Burwell LS, Ingraham CA, Spencer CM, Friedman AE, Pinkert CA, Brookes PS. In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. J Mol Cell Cardiol. 2009;46:960–968. doi: 10.1016/j.yjmcc.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prime TA, Blaikie FH, Evans C, et al. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2009;106:10764–10769. doi: 10.1073/pnas.0903250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nadtochiy SM, Burwell LS, Brookes PS. Cardioprotection and mitochondrial S-nitrosation: effects of S-nitroso-2-mercaptopropionyl glycine (SNO-MPG) in cardiac ischemia-reperfusion injury. J Mol Cell Cardiol. 2007;42:812–825. doi: 10.1016/j.yjmcc.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- 61.Legtenberg RJ, Houston RJ, Oeseburg B, Smits P. Metformin improves cardiac functional recovery after ischemia in rats. Horm Metab Res. 2002;34:182–185. doi: 10.1055/s-2002-26705. [DOI] [PubMed] [Google Scholar]
- 62.Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103:274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 63.Matsuzaki S, Humphries KM. Selective inhibition of deactivated mitochondrial complex I by biguanides. Biochemistry. 2015;54:2011–2021. doi: 10.1021/bi501473h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.D’Alonzo AJ, Grover GJ, Darbenzio RB, Hess TA, Sleph PG, Dzwonczyk S, Zhu JL, Sewter JC. In vitro effects of capsaicin: antiarrhythmic and antiischemic activity. Eur J Pharmacol. 1995;272:269–278. doi: 10.1016/0014-2999(94)00653-o. [DOI] [PubMed] [Google Scholar]
- 65.Satoh T, Miyoshi H, Sakamoto K, Iwamura H. Comparison of the inhibitory action of synthetic capsaicin analogues with various NADH-ubiquinone oxidoreductases. Biochim Biophys Acta. 1996;1273:21–30. doi: 10.1016/0005-2728(95)00131-x. [DOI] [PubMed] [Google Scholar]
- 66.Hanley PJ, Ray J, Brandt U, Daut J. Halothane, isoflurane and sevoflurane inhibit NADH: ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J Physiol. 2002;544:687–693. doi: 10.1113/jphysiol.2002.025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cope DK, Impastato WK, Cohen MV, Downey JM. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology. 1997;86:699–709. doi: 10.1097/00000542-199703000-00023. [DOI] [PubMed] [Google Scholar]
- 68.Wyatt KM, Skene C, Veitch K, Hue L, McCormack JG. The antianginal agent ranolazine is a weak inhibitor of the respiratory complex I, but with greater potency in broken or uncoupled than in coupled mitochondria. Biochem Pharmacol. 1995;50:1599–1606. doi: 10.1016/0006-2952(95)02042-x. [DOI] [PubMed] [Google Scholar]
- 69.Wojtovich AP, Brookes PS. The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels. Basic Res Cardiol. 2009;104:121–129. doi: 10.1007/s00395-009-0001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anastacio MM, Kanter EM, Makepeace C, Keith AD, Zhang H, Schuessler RB, Nichols CG, Lawton JS. Cardioprotective mechanism of diazoxide involves the inhibition of succinate dehydrogenase. Ann Thorac Surg. 2013;95:2042–2050. doi: 10.1016/j.athoracsur.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X, Wei M, Kuukasjärvi P, Laurikka J, Järvinen O, Rinne T, Honkonen EL, Tarkka M. Novel pharmacological preconditioning with diazoxide attenuates myocardial stunning in coronary artery bypass grafting. Eur J Cardiothorac Surg. 2003;24:967–973. doi: 10.1016/s1010-7940(03)00438-x. [DOI] [PubMed] [Google Scholar]
- 72.Boylston JA, Sun J, Chen Y, Gucek M, Sack MN, Murphy E. Characterization of the cardiac succinylome and its role in ischemia-reperfusion injury. J Mol Cell Cardiol. 2015;88:73–81. doi: 10.1016/j.yjmcc.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wojtovich AP, Brookes PS. The endogenous mitochondrial complex II inhibitor malonate regulates mitochondrial ATP-sensitive potassium channels: implications for ischemic preconditioning. Biochim Biophys Acta. 2008;1777:882–889. doi: 10.1016/j.bbabio.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pagliaro P, Mancardi D, Rastaldo R, Penna C, Gattullo D, Miranda KM, Feelisch M, Wink DA, Kass DA, Paolocci N. Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radic Biol Med. 2003;34:33–43. doi: 10.1016/s0891-5849(02)01179-6. [DOI] [PubMed] [Google Scholar]
- 75.Shiva S, Crawford JH, Ramachandran A, Ceaser EK, Hillson T, Brookes PS, Patel RP, Darley-Usmar VM. Mechanisms of the interaction of nitroxyl with mitochondria. Biochem J. 2004;379:359–366. doi: 10.1042/BJ20031758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Riepe MW, Esclaire F, Kasischke K, Schreiber S, Nakase H, Kempski O, Ludolph AC, Dirnagl U, Hugon J. Increased hypoxic tolerance by chemical inhibition of oxidative phosphorylation: “chemical preconditioning”. J Cereb Blood Flow Metab. 1997;17:257–264. doi: 10.1097/00004647-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 77.Nadtochiy SM, Baker PR, Freeman BA, Brookes PS. Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc Res. 2009;82:333–340. doi: 10.1093/cvr/cvn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kabir AM, Cao X, Gorog DA, Tanno M, Bassi R, Bellahcene M, Quinlan RA, Davis RJ, Flavell RA, Shattock MJ, Marber MS. Antimycin A induced cardioprotection is dependent on pre-ischemic p38-MAPK activation but independent of MKK3. J Mol Cell Cardiol. 2005;39:709–717. doi: 10.1016/j.yjmcc.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 79.Zhao X, He G, Chen YR, Pandian RP, Kuppusamy P, Zweier JL. Endothelium-derived nitric oxide regulates postischemic myocardial oxygenation and oxygen consumption by modulation of mitochondrial electron transport. Circulation. 2005;111:2966–2972. doi: 10.1161/CIRCULATIONAHA.104.527226. [DOI] [PubMed] [Google Scholar]
- 80.Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, Foresti R, Motterlini R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003;93:e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 81.Khan AA, Schuler MM, Prior MG, Yong S, Coppock RW, Florence LZ, Lillie LE. Effects of hydrogen sulfide exposure on lung mitochondrial respiratory chain enzymes in rats. Toxicol Appl Pharmacol. 1990;103:482–490. doi: 10.1016/0041-008x(90)90321-k. [DOI] [PubMed] [Google Scholar]
- 82.Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol. 2006;40:119–130. doi: 10.1016/j.yjmcc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burwell LS, Nadtochiy SM, Brookes PS. Cardioprotection by metabolic shut-down and gradual wake-up. J Mol Cell Cardiol. 2009;46:804–810. doi: 10.1016/j.yjmcc.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chance B. The interaction of energy and electron transfer reactions in mitochondria. V. The energy transfer pathway. J Biol Chem. 1961;236:1569–1576. [PubMed] [Google Scholar]
- 86.Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27:639–645. doi: 10.1016/j.tips.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 87.Kotlyar AB, Vinogradov AD. Slow active/inactive transition of the mitochondrial NADH-ubiquinone reductase. Biochim Biophys Acta. 1990;1019:151–158. doi: 10.1016/0005-2728(90)90137-s. [DOI] [PubMed] [Google Scholar]
- 88.Galkin A, Moncada S. S-nitrosation of mitochondrial complex I depends on its structural conformation. J Biol Chem. 2007;282:37448–37453. doi: 10.1074/jbc.M707543200. [DOI] [PubMed] [Google Scholar]
- 89.Kohr MJ, Sun J, Aponte A, Wang G, Gucek M, Murphy E, Steenbergen C. Simultaneous measurement of protein oxidation and S-nitrosylation during preconditioning and ischemia/reperfusion injury with resin- assisted capture. Circ Res. 2011;108:418–426. doi: 10.1161/CIRCRESAHA.110.232173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murphy E, Kohr M, Sun J, Nguyen T, Steenbergen C. S-nitrosylation: a radical way to protect the heart. J Mol Cell Cardiol. 2012;52:568–577. doi: 10.1016/j.yjmcc.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tong G, Aponte AM, Kohr MJ, Steenbergen C, Murphy E, Sun J. Postconditioning leads to an increase in protein S-nitrosylation. Am J Physiol Heart Circ Physiol. 2014;306:H825–H832. doi: 10.1152/ajpheart.00660.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Porteous CM, Logan A, Evans C, Ledgerwood EC, Menon DK, Aigbirhio F, Smith RA, Murphy MP. Rapid uptake of lipophilic triphenylphosphonium cations by mitochondria in vivo following intravenous injection: implications for mitochondria-specific therapies and probes. Biochim Biophys Acta. 2010;1800:1009–1017. doi: 10.1016/j.bbagen.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 93.Methner C, Chouchani ET, Buonincontri G, Pell VR, Sawiak SJ, Murphy MP, Krieg T. Mitochondria selective S-nitrosation by mitochondria-targeted S-nitrosothiol protects against post-infarct heart failure in mouse hearts. Eur J Heart Fail. 2014;16:712–717. doi: 10.1002/ejhf.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wojtovich AP, Smith CO, Haynes CM, Nehrke KW, Brookes PS. Physiological consequences of complex II inhibition for aging, disease, and the mKATP channel. Biochim Biophys Acta. 2013;1827:598–611. doi: 10.1016/j.bbabio.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Siebels I, Dröse S. Q-site inhibitor induced ROS production of mitochondrial complex II is attenuated by TCA cycle dicarboxylates. Biochim Biophys Acta. 2013;1827:1156–1164. doi: 10.1016/j.bbabio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 96.Ardehali H, Chen Z, Ko Y, Mejía-Alvarez R, Marbán E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci U S A. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Quarrie R, Cramer BM, Lee DS, Steinbaugh GE, Erdahl W, Pfeiffer DR, Zweier JL, Crestanello JA. Ischemic preconditioning decreases mitochondrial proton leak and reactive oxygen species production in the postischemic heart. J Surg Res. 2011;165:5–14. doi: 10.1016/j.jss.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Valls-Lacalle L, Barba I, Miró-Casas E, Alburquerque-Béjar JJ, Ruiz-Meana M, Fuertes-Agudo M, Rodríguez-Sinovas A, García-Dorado D. Succinate dehydrogenase inhibition with malonate during reperfuion reduces infarct size by preventing mitochondrial permeability transition [published online ahead of print December 23, 2015] Cardiovasc Res. 2015 doi: 10.1093/cvr/cvv279. [DOI] [PubMed] [Google Scholar]
- 99.Coles CJ, Edmondson DE, Singer TP. Inactivation of succinate dehydrogenase by 3-nitropropionate. J Biol Chem. 1979;254:5161–5167. [PubMed] [Google Scholar]
- 100.Chaturvedi RK, Flint Beal M. Mitochondrial diseases of the brain. Free Radic Biol Med. 2013;63:1–29. doi: 10.1016/j.freeradbiomed.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 101.Hoekstra AS, Bayley JP. The role of complex II in disease. Biochim Biophys Acta. 2013;1827:543–551. doi: 10.1016/j.bbabio.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 102.Roubille F, Lairez O, Mewton N, Rioufol G, Ranc S, Sanchez I, Cung TT, Elbaz M, Piot C, Ovize M. Cardioprotection by clopidogrel in acute ST-elevated myocardial infarction patients: a retrospective analysis. Basic Res Cardiol. 2012;107:275. doi: 10.1007/s00395-012-0275-3. [DOI] [PubMed] [Google Scholar]
- 103.Yang XM, Liu Y, Cui L, Yang X, Liu Y, Tandon N, Kambayashi J, Downey JM, Cohen MV. Platelet P2Y12 blockers confer direct postconditioning-like protection in reperfused rabbit hearts. J Cardiovasc Pharmacol Ther. 2013;18:251–262. doi: 10.1177/1074248412467692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cohen MV, Downey JM. Combined cardioprotectant and antithrombotic actions of platelet P2Y12 receptor antagonists in acute coronary syndrome: just what the doctor ordered. J Cardiovasc Pharmacol Ther. 2014;19:179–190. doi: 10.1177/1074248413508465. [DOI] [PubMed] [Google Scholar]
- 105.Bulluck H, Yellon DM, Hausenloy DJ. Reducing myocardial infarct size: challenges and future opportunities [published online ahead of print December 16, 2015] Heart. 2015 doi: 10.1136/heartjnl-2015-307855. [DOI] [PMC free article] [PubMed] [Google Scholar]