Abstract
This Perspective presents the fundamental principles, the elementary reactions, the initial catalytic systems, and the contemporary catalysts that have converted C−H bond functionalization from a curiosity to a reality for synthetic chemists. Many classes of elementary reactions involving transition-metal complexes cleave C−H bonds at typically unreactive positions. These reactions, coupled with a separate or simultaneous functionalization process lead to products containing new C−C, C−N, and C−O bonds. Such reactions were initially studied for the conversion of light alkanes to liquid products, but they have been used (and commercialized in some cases) most often for the synthesis of the more complex structures of natural products, medicinally active compounds, and aromatic materials. Such a change in direction of research in C−H bond functionalization is remarkable because the reactions must occur at an unactivated C−H bond over functional groups that are more reactive than the C−H bond toward classical reagents. The scope of reactions that form C−C bonds or install functionality at an unactivated C−H bond will be presented, and the potential future utility of these reactions will be discussed.
INTRODUCTION
The selective functionalization of C−H bonds with molecular catalysts has been pursued for nearly 50 years.1 One of the major synthetic targets for such reactions has been the conversion of light alkanes to alcohols or the conversion of linear alkanes to α-olefins (eq 1). The conversion of methane to methanol would
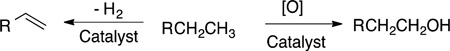 |
(1) |
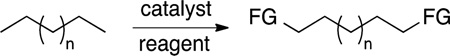
allow stranded, gaseous methane to be transported as liquid product,2,3 or the methanol to be used in reactions that form C−C bonds in higher hydrocarbons.4 The selective oxidation of alkanes at the terminal position could convert linear alkanes to detergent alcohols, while the oxidation of lighter alkanes, such as butane, to α,ω-diols (eq 2) would provide monomers for
 |
(2) |
polymers made on large scale. None of these goals have been achieved in a practical fashion. However, the knowledge gained about the mechanisms by which C−H bonds can be cleaved by transition-metal compounds resulting from studies toward these goals5,6 have begun to dramatically impact synthetic organic chemistry.
Synthetic organic chemistry has relied on the formation of carbon−carbon bonds by matching nucleophiles with electrophiles and by interconversion of functional groups attached to or contained within a skeleton of carbon atoms. Many catalytic reactions have been developed that facilitate the formation of carbon−carbon and carbon−heteroatom bonds at existing functional groups, including some of the most common reactions used in all of synthetic chemistry.7–9 Hydrogenation, cross coupling, and olefin metathesis reactions have revolutionized the way organic electronic materials, natural products, and medicinally active compounds are synthesized.
In many cases, the functional groups in these molecules are installed by the cleavage of a carbon−hydrogen bond. Of course, some C−H bonds are acidic and are often deprotonated to create a nucleophile that is used to form a carbon−carbon or carbon−heteroatom bond. In another classic set of reactions, electrophilic aromatic substitutions install functional groups or form a carbon−carbon bond at the position of a C−H bond. These reactions are some of the most valuable reactions in synthetic chemistry.
Classical organic chemistry also has shown that C−H bonds are readily cleaved by some radicals. Halogen radicals, hydroxyl radical, amidyl radicals, oxoimidyl radicals, and benzoyl radicals all cleave C−H bonds to form alkyl radicals.10 These alkyl radicals can then, according to proposed mechanisms, be quenched with oxygen, undergo oxidation to form carbocations,11 undergo pericyclic reactions12 or even react with ligands bound to transition metals to install a new functional group.13,14 However, radicals that form sufficiently strong X−H bonds (X = O, N) to cleave the C−H bond are often unselective. In particular, halogen and hydroxyl radicals cleave, with some preference, a tertiary C−H bond over a secondary C−H bond, and they cleave secondary C−H bonds over primary C−H bonds. However, the selectivity for one tertiary C−H bond over another, or one secondary C−H bond over another in a simple hydrocarbon is low.15 Moreover, none of these classes of reactions lead to the selective functionalization of primary C−H bonds without the bias of a directing group. The functionalization of the primary C−H bond in an alkane with high selectivity has been accomplished only with boron reagents (vide infra),16,17 and the selective, catalytic functionalization of methane with molecular catalysts occurs in only a few cases and without sufficient selectivity and activity for commercial application.18,19
A C−H BOND VERSUS A FUNCTIONAL GROUP AS THE SITE OF A REACTION
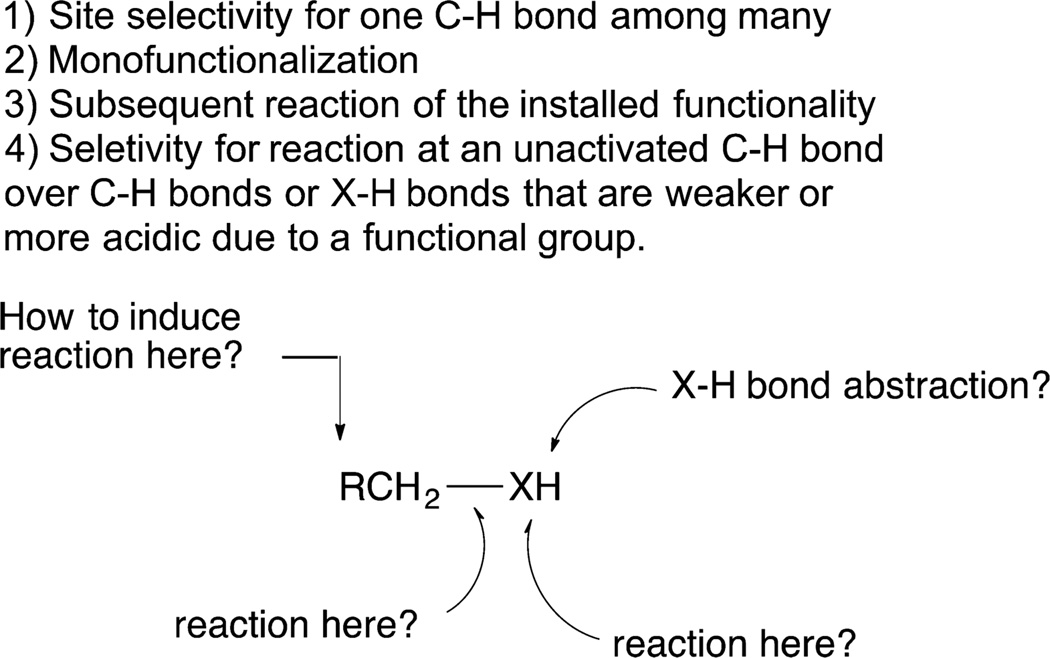
One reason the focus of C−H bond functionalization was placed originally on the transformations of alkanes, rather than the functionalization of C−H bonds in molecules containing functionality, was the expectation that a system reactive enough to cleave an unactivated C−H bond would react rapidly with existing functional groups (Figure 1). A metal center that would insert rapidly into the C−H bond of an alkane seemed likely to insert into the O−H or N−H bond of an alcohol or amine, insert into the C(O)−O bond of an ester, bind to a Lewis basic nitrile or amide, or cleave the weaker C−H bonds alpha to an oxygen or nitrogen atom.
Figure 1.
Four challenges facing selective C−H bond functionalization of a reactant containing existing functional groups.
Yet, many of these expectations have proven to be invalid or to be overcome by selective catalysts. Some radicals, whether organic or metal-based, cleave C−H bonds selectively, some transition-metal centers insert into C−H bonds in preference to reacting with the bonds of typical functional groups, and many strategies have been developed to direct the metal center to one of many C−H bonds. As a result of these strategies, the functionalization of C−H bonds is beginning to become a mainstream method for conducting organic synthesis. Many academic laboratories are conducting the synthesis of natural products and biologically active synthetic compounds with the functionalization of a C−H bond as a strategic step. Many medicinal chemists are beginning to think of creating diversity from existing compound collections by functionalization of C−H bonds on aryl and heteroaryl rings, and many materials scientists are forming polymers by C−H bond functionalization or modifying poly-aromatic structures by functionalizing the periphery at the position of a C−H bond with a catalytic process.
This Perspective will provide one view on what led to a change in mind-set about the potential of C−H bond functionalization to be used in the synthesis of complex molecules, rather than the conversion of light hydrocarbons to oxygenated or unsaturated products. As is typically the case for a trend in research, several groups were far ahead of the current research activity, and such work (often overlooked) will be noted as part of this Perspective. Today, the functionalization of C−H bonds is a mainstream topic of synthetic chemistry and one of the most investigated approaches to develop new synthetic methodology; remaining unsolved, but increasingly important due to the production of shale gas, is the original goal: the mild and selective conversion of methane and light hydrocarbons to functionalized feedstocks.
CHALLENGES FACING THE FUNCTIONALIZATION OF C−H BONDS
Most molecules that one seeks to functionalize contain many different C−H bonds. The presence of multiple C−H bonds with different properties leads to several challenges facing the selective functionalization of C−H bonds (Figure 1). One challenge is to control site selectivity. If the system is sufficiently reactive to functionalize one C−H bond, the system is likely to be sufficiently reactive to functionalize C−H bonds at several different sites, leading to a mixture of products containing a single new functional group at different sites. A second challenge is to achieve monofunctionalization. Again, if the system is sufficiently reactive to functionalize one C−H bond, it is likely to be sufficiently reactive to functionalize multiple C−H bonds in the same molecule, leading to the formation of a mixture of products containing different numbers of new functional groups. A third challenge arises from the reactivity of most products of C−H bond functionalization being greater than the reactivity of the starting material. This issue is one of the greatest challenges facing the oxidation of alkanes. Because alcohols are more reactive than alkanes, the alcohol is typically oxidized to the ketone or aldehyde, and the ketone or aldehyde can be further oxidized to the corresponding ester or acid. Thus, a mixture of products containing different states of oxidation is often formed.20 A fourth challenge facing the functionalization of “unactivated C−H bonds” results from the effect of existing functional groups on nearby C−H bonds. An existing functional group can cause adjacent C−H bonds to be weak21 or acidic22 and, therefore, more reactive than an alkyl C−H bond. For example, a carbonyl function causes the alpha C−H bonds to be acidic; a halogen, oxygen, or nitrogen atom causes the alpha C−H bonds to be weak; a C−C triple bond causes the terminal C−H bond to be acidic; and an arene or heteroarene π system creates a pathway for substitution of a C−H bond with electrophiles. Thus, developing a system that reacts preferentially at an alkyl C−H bond over a functional group or the C−H bond adjacent to a functional group is exacerbated by the need to overcome the inherent reactivity of different types of C−H bonds. Finally, the existing functional groups can coordinate to the catalyst. Such coordination can be exploited to enable site-selective reactions, but it can also make it difficult to conduct a reaction at a C−H bond distal from the functional groups.
OPPORTUNITIES OFFERED BY THE FUNCTIONALIZATION OF C−H BONDS
The justification for the functionalization of C−H bonds is often expressed as a means to conduct green or sustainable chemistry. Although several classes of C−H bond functionalization clearly eliminate the need to prepare two functionalized reagents (see the discussion of direct arylation below), few published C−H bond functionalization reactions approach the goal of green or “sustainable” synthesis because of the presence of copper or silver oxidants, additives, such as salts or acids, reagents that can be purchased, but require multiple steps to prepare, and the need for high loadings of catalysts containing complex ligands. However, C−H bond functionalization can create new classes of bond constructions that lead to new strategies for preparing complex molecules or smaller building blocks in fewer steps than would be required by classical methods. As noted in a prior review, the economy of synthetic steps typically dominates other measures of synthetic efficiency.23
When considering the value of a synthetic method, one should appreciate that the criteria for utility vary depending on the purpose of the synthesis. If one is preparing a molecule on small scale to evaluate its function, the time required to prepare the molecule and the diversity of the molecules that can be prepared by a synthetic route are the most important factors. Thus, if C−H bond functionalization can provide direct access to the desired molecule, the issues of catalyst efficiency and cost of reagents are nearly irrelevant. For example, when modifying a natural product to change the number or type of binding sites to a protein or to change its solubility, rapid access to a range of products is the primary goal, rather than minimizing waste or catalyst loading.
Likewise, when preparing a molecule for testing toxicity, access to kilogram quantities of the molecule in the shortest time is most important. In this case, the C−H bond functionalization process must be suitable to use on larger scale than is typically conducted when demonstrating a new method. Factors, such as air sensitivity, salt production, ability to purify the product from the reagent components, and reagent or catalyst accessibility are some of the important issues. However, the cost of goods is typically outweighed by the cost of time. After all, the sales of a blockbuster drug exceed millions of dollars per day—surely more than the price of a reagent or ligand on the catalyst.
In contrast, the cases when one would use a reaction on production scale should meet the criteria of green chemistry and will confront issues of catalyst lifetime. In these cases, the use of C−H bond functionalization must cut steps of a synthesis without consuming catalyst components or reagents and with a cost that competes with that of the longer synthetic route consisting of classical chemistry. Many C−H bond functionalizations have been reported, but few occur, so far, with turnover numbers and reagents that are sufficiently practical for use in commercial production of a material, an agrochemical product, or an active pharmaceutical ingredient.24–26
The remainder of this Perspective will discuss the principles of C−H bond functionalization, the catalytic processes that have been used in synthetic applications, and examples of how C−H bond functionalization has been used in organic synthesis. Because this paper is meant to highlight these issues and opportunities, rather than to provide a comprehensive review of C−H bond functionalization, the examples will be illustrative. More detailed reviews can be found in textbooks,27 edited books of review articles,28 and special or thematic issues of review journals.29–31
HISTORICAL OVERVIEW OF THE CLASSES OF CATALYTIC C−H BOND FUNCTIONALIZATION REACTIONS
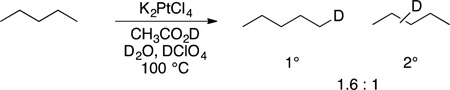
In 1969 and the early 1970s, Shulpin and Shilov published platinum-catalyzed C−H bond functionalizations.1,32–34 They showed that the platinum-catalyzed halogenations of alkanes generate distributions of products that are distinct from those generated by radical halogenation of alkanes; Hodges showed that platinum-catalyzed exchange of deuterium between acids and alkanes also occurs, and these two reactions occur with similar selectivity (eqs 3 and 4).
The similarity between the selectivity for halogenation and H/D exchange implied that the two processes occurred through an alkylplatinum intermediate. Therefore, an organometallic catalyst could affect the site selectivity for C−H bond functionalizations. Although the halogenation reactions of Shilov were catalytic in Pt(II), the reactant for halogenation was, unfortunately, limited to the Pt(IV) halide added to the system.
 |
(3) |
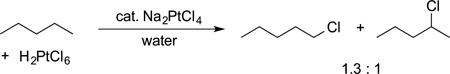 |
(4) |
Contemporaneously, Fujiwara studied the oxidative formation of carbon−carbon bonds by the cleavage of aromatic, as well as aliphatic, C−H bonds.35–37 The initial studies showed that palladium–alkene complexes containing carboxylate ligands or free alkenes and palladium acetate would react with arenes to form vinylarenes.35,36 Soon after he showed that this arene olefination could be catalyzed by palladium with copper and oxygen to reoxidize the reduced palladium (eq 5).37 This
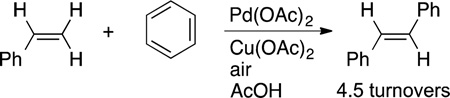 |
(5) |
oxidative coupling of an arene with an alkene, called the Fujiwara reaction, is now a classic C−H bond functionalization reaction that has been used for the synthesis of complex molecules and is the foundation of much recent research.
Fujiwara also reported the carboxylation of arenes38–40 and even the carboxylation of alkanes41 by the combination of carbon monoxide and an oxidant (eq 6). These reactions form benzoic
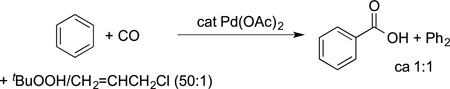 |
(6) |
acids from arenes and alkane carboxylic acids from cyclic alkanes. The regioselectivity of these reactions with arenes was similar to that of an electrophilic aromatic substitution. Thus, the mechanism for these reactions is thought to involve the addition of an electrophilic palladium to the π system of the arene.38
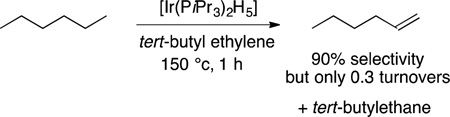
A third class of C−H bond functionalization reaction was developed about a decade later. Felkin and Crabtree reported the dehydrogenations of alkanes to form alkenes catalyzed by iridium complexes.42–45 The highest turnover numbers were obtained from the transfer dehydrogenation of cyclooctane with tert-butyl ethylene as the hydrogen acceptor (eq 7). Cyclooctane was the
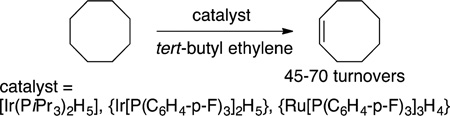 |
(7) |
most reactive alkane due to the more favorable thermodynamics for the dehydrogenation of this cycloalkane than other alkanes and the unfavorable dehydrogenation of the product to the corresponding diene. Because heterogeneous catalysts are known for alkane dehydrogenation, much effort was paid to assess whether the catalyst was homogeneous (molecular) or heterogeneous (aggregated).46 These studies showed that the catalyst reacted in the solution phase as a molecular species. Moreover, studies by Felkin showed that the kinetic selectivity for reaction of a linear alkane favored the α-olefin,42 and such selectivity is not observed with heterogeneous catalysts (eq 8).
 |
(8) |
Much effort has been spent to render the Shilov chemistry catalytic with an oxidant other than Pt(IV). Periana reported the conversion of methane to methyl bisulfate with SO3 in sulfuric acid as the oxidant and a catalyst generated from platinum chloride and bipyrimidine (eq 9).18 Although fuming sulfuric
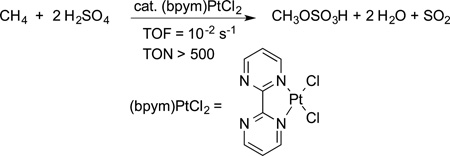 |
(9) |
acid is an inexpensive oxidant, separation of the product from the medium and hydrolysis to form the desired alcohol make this reaction unfeasible for technical conversions of methane to methanol. However, these studies did show that even the most unreactive of C−H bonds could be functionalized at modest temperatures.
Much effort has been spent to build upon the oxidative functionalizations of Fujiwara. Many examples of arene olefination are now known, and methods to render these reactions selective for a specific site on an arene have been developed.47 Applications of these reactions to the synthesis of complex molecules will be discussed in detail later in this Perspective. In addition, metal carboxylate complexes have been used as catalyst, often with an added dative ligand, for reactions that are redox neutral. For example, the reaction of an arene with an aryl halide occurs to form biaryl and heteroaryl compounds in the presence of catalysts containing carboxylate ligands (eq 10).48,49 These
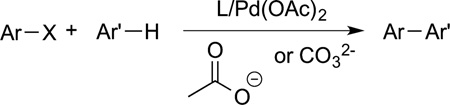 |
(10) |
reactions are one of the most utilized C−H coupling reactions on process scale because they form the biaryl units in many medicinally active compounds without the need to generate an organometallic nucleophile.
Although some of the earliest work on alkane functionalization led to systems for dehydrogenation, the selective formation of α-olefins has not been achieved in a practical way. Although an α-olefin can be the kinetic product, this initial product isomerizes to the internal olefin in the presence of the catalyst that led to alkane dehydrogenation.50 However, this reaction has been combined with olefin metathesis to create a process for the metathesis of alkanes (eq 11).51,52 Dehydrogenation of the alkane to the alkene, metathesis of the resulting alkene, and hydrogenation of the resulting alkenes leads to new alkanes that are longer and shorter than the starting alkane. This alkane metathesis is a remarkable tandem process for modifications of alkanes in homogeneous systems, but the catalysts for this reaction do not tolerate functional groups; thus, much development would be needed to make this process applicable to the synthesis of complex molecules.
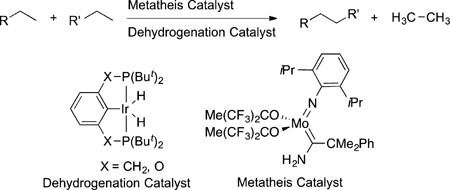 |
(11) |
In addition to these organometallic processes occurring by the formation of metal-carbon σ bonds, a large body of early work on C−H bond functionalization comprised the catalytic insertions of carbene units into C−H bonds.53 Copper and rhodium complexes catalyze both intermolecular54,55 and intramolecular56,57 insertions of carbene units into C−H bonds of alkyl groups.53,58 Intramolecular reactions of diazoesters catalyzed by dinuclear rhodium carboxylate complexes led to cyclic products from insertion of the carbene into secondary C−H bonds (eq 12), and the use of such insertions of carbenes
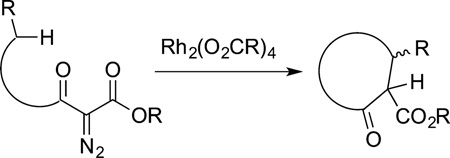 |
(12) |
into C−H bonds is particularly applicable to the synthesis of natural products and medicinally relevant compounds and will be presented later in this paper. Related rhodium dimers also catalyze the insertion of nitrenes into C−H bonds (eq 13).
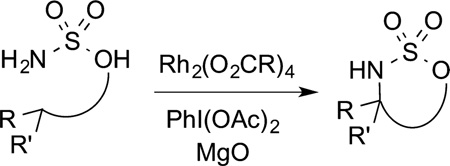 |
(13) |
Like the intramolecular insertions of carbenes, intramolecular insertion of nitrenes into alkyl C−H bonds have been developed extensively,59 and such reactions have been used in some of the most challenging syntheses of natural products. This nitrene insertion chemistry will be discussed in more detail later in this Perspective.
The final class of C−H bond functionalization that will be discussed in this Perspective occurs by abstraction of C−H bonds by metal-based radicals to form alkyl radicals or trapping of alkyl radicals by metal complexes to form the functionalized product, or both. The autoxidation of cyclohexane to cyclohexanols and cyclohexanone is conducted commercially with cobalt acetate as initiator.60 This reaction is the first step of the synthesis of adipic acid. However, this reaction has not been used for the oxidation of more complex molecules. Yet, an increasing number of studies are being conducted that exploit the selectivity of electrophilic radicals for abstraction of the more electron-rich alkyl C−H bond over abstraction of the less electron-rich C−H bond (eq 14).61
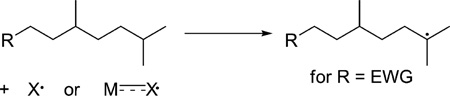 |
(14) |
Applications of this reaction manifold also will be discussed later in this Perspective.
MECHANISMS OF METAL-MEDIATED C−H BOND CLEAVAGE
Many pathways by which a transition-metal complex can cleave a C−H bond have been revealed.62 These pathways include oxidative addition, σ bond metathesis, concerted metalation–deprotonation, direct insertion of a ligand, electrophilic addition to a π system, and abstraction of a hydrogen atom. Illustrative examples of these reactions and the lessons learned that relate to the development of catalytic processes will be described in this section.
The first stoichiometric reactions of organometallic complexes with alkanes to form products containing a metal−carbon bond were reported by Crabtree,63 Bergman,64 Graham,65 Jones,66 and Watson.67 Crabtree reported the reaction of a [Ir(H)2-(acetone)2(PPh3)2]+ with cyclooctane to form the cylooctadiene complex [Ir(COD)2(PPh3)2]+ (eq 15). The mechanism of this
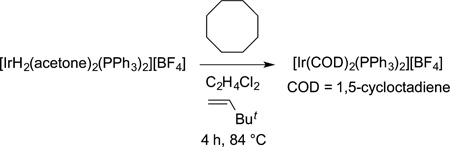 |
(15) |
reaction was unclear, but it showed that a discrete organometallic complex could cleave the C−H bond of an alkane.
Bergman and Graham reported the first oxidative additions of alkanes to form alkylmetal hydride products (eq 16, M = Ir).
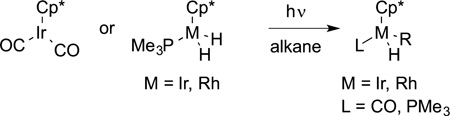 |
(16) |
This work was remarkable because it provided the first clear view of how a transition metal complex could cleave a C-H bond in solution. Jones reported the oxidative addition of linear alkanes to form primary alkylmetal hydride complexes with a second-row metal rhodium (eq 16, M = Rh). Watson reported the reaction of the lanthanide complex Cp*LuMe(OEt2) with 13CH4 to form Cp*Lu13Me(OEt2). Because this lutetium compound lacks available d electrons, this result showed that pathways other than oxidative addition can lead to the intermolecular cleavage of an alkane C−H bond (eq 17).
| (17) |
Prior to these studies on the oxidative addition of alkane C−H bonds, several examples of the oxidative addition of aryl C−H bonds had been published. For example, Tolman and Ittel at DuPont reported the exchange of arenes from (DMPE)Ru(Ar)-(H) complexes (DMPE = 1,2-bis(dimethylphosphino)ethane) (eq 18).68 These complexes did not react with alkanes. Thus, these
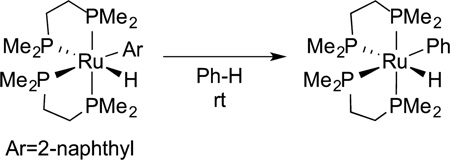 |
(18) |
studies revealed one of the general trends in reactivity of metal complexes toward C−H bonds: the oxidative addition of aryl C−H bonds occurs in preference to the oxidative addition of alkyl C−H bonds.
Many studies also showed that transition-metal centers would undergo intramolecular reactions with both aryl and alkyl C−H bonds. For example, nickelocene reacts with azobenzene to form a metallacycle containing a new nickel–aryl bond, in addition to a dative bond to the diazo unit (eq 19).69 One of the most
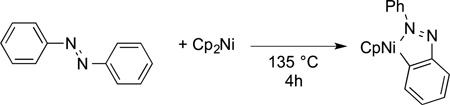 |
(19) |
illustrative examples of intramolecular oxidative addition is the reaction of neopentyl lithium with the iridium complex in eq 20 to form the dialkyl hydride product from an intramolecular oxidative addition of a methyl C−H bond.70
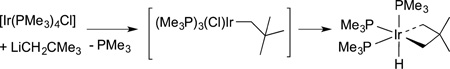 |
(20) |
The reactions of electrophilic metal carboxylate complexes with aryl and alkyl C−H bonds occurred as part of the catalytic chemistry reported by Fujiwara almost 50 years ago. However, the pathways by which metal−carboxylate complexes cleave C−H bonds have been revealed much more recently. Sakaki first computed a pathway for cleavage of the H-H bond in dihydrogen by a metal−carboxylate as part of the mechanism for the hydrogenation of CO2.71 The lowest energy pathway (eq 21)
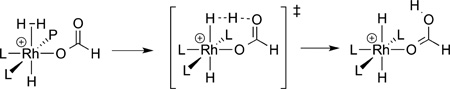 |
(21) |
consisted of the coordination of hydrogen to the metal. Such coordination acidifies the hydrogen. The carboxylate ligand then deprotonates the bound hydrogen with the carbonyl oxygen atom through a six-membered-ring transition state. The relationship between the carbonyl oxygen atom and the bound oxygen atom in a carboxylate ligand leads to a low-energy pathway.
Sakaki,72 MacGregor,73 and Gorelsky74 computed the reaction coordinate for the formation of aryl- and heteroarylpalladium complexes from a palladium carboxylate species and an aryl or heteroaryl C−H bond by a pathway that is closely related to that computed by Sakaki for reaction of hydrogen with metal carboxylates. Binding of the arene acidifies the C−H bond, and the proton in this bond is removed by the carboxylate ligand (eq 22). These reactions are particularly facile with certain
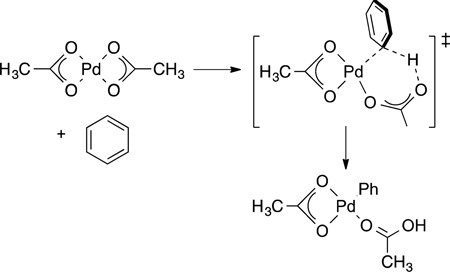 |
(22) |
heteroarenes and tend to occur at the more acidic of the C−H bonds of these substrates. The importance of the carboxylate group has been shown many times experimentally by the requirement of a carbonate or carboxylate base for the catalytic reactions to occur, but the structure of the transition state was revealed predominantly by computation.
The insertions of the carbene unit of metal−carbene complexes are a final common reaction that leads to cleavage (and direct functionalization) of an alkyl C−H bond. Typically, the formation of the metal−carbene is rate limiting, and reaction of the carbene complex with a C−H bond occurs after rate-limiting formation of the carbene. Thus, experimental mechanistic information on such complexes has been difficult to gain. Only recently has an example of the rhodium−carbene intermediate in such a reaction been generated; Davies and Berry reported the characterization of such a complex spectroscopically at low temperature.75 Thus, a majority of the mechanistic information on these reactions has been gained from computational studies.76,77
The insertions of carbene units into alkyl C−H bonds occur without formation of a metal−carbon bond from the substrate. The carbene inserts into the C−H bond by an asynchronous, concerted pathway (eq 23). The reactions occur preferentially at
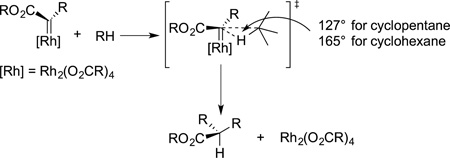 |
(23) |
the more electron-rich C−H bonds, due to the electrophilic character of the carbene unit. Thus, the reactions occur at secondary C−H bonds over primary C−H bonds. However, the reactions tend to occur with a slight preference for insertion at unhindered secondary C−H bonds over tertiary C−H bonds due to steric effects.78 The rhodium-carbene complexes are highly reactive. If they do not undergo C−H bond insertion, they undergo dimerization to form alkenes.
Finally, many studies have been conducted on the reactions of transition-metal complexes with alkanes to form alkyl radical intermediates or the trapping of alkyl radicals by metal complexes to form functionalized products. The generation of alkyl radicals by abstraction of C−H bonds with metal-oxo complexes has been studied for synthetic purposes largely to reveal the mechanism of the oxidation of alkanes by iron porphyrin complexes. Such oxo-iron complexes react with alkanes to form an iron-bound hydroxo ligand and an alkyl radical, and the alkyl radical reacts rapidly with the resulting hydroxo ligand in a “rebound” step to form the alcohol product (eq 24).79–81 Ongoing work is
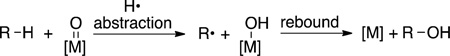 |
(24) |
occurring to decipher whether oxo- iron complexes with distinct ligand sets and oxo-metal complexes of second- and third-row metals that are less prone to undergo one-electron redox events also react with alkane C−H bonds by radical pathways or by nonradical pathways.82 Alternatively, peroxides or hypervalent iodine reagents can react with a metal complex to generate organic radicals that abstract a C−H bond. The resulting alkyl radical then can react with the resulting metal complex to form functionalize product and regenerate the complex that led to the original radical (eq 25). This sequence is the accepted mechanism for Karasch oxidation of allylic C−H bonds12,83,84
| (25) |
and has been extended recently to several aminations13,85 and new oxidations86 of alkyl C−H bonds.
FROM STOICHIOMETRIC REACTIONS AND INITIAL CATALYTIC REACTIONS TO SYNTHETIC METHODS FOR FUNCTIONALIZATION OF ARENES
For these elementary steps and initial catalytic processes to be developed into useful synthetic methodology, several conceptual advances were needed. First an approach was needed to control the site and number of C−H bonds that would undergo the functionalization process. Second, reagents were needed that would lead to the intermolecular functionalization of C−H bonds, rather than the stoichiometric oxidative addition of C−H bonds or intramolecular transformations of designed substrates. Third, systems were needed that reacted at C−H bonds faster than they reacted with typically more reactive functional groups. As discussed below, the application of directing groups to C−H bond functionalization and the identification of catalysts that react at C−H bonds in preference to conventional functional groups revolutionized the application of C−H bond functionalization. In addition, mild oxidants and main group reagents have been identified that make the functionalization processes thermodynamically favorable.
In 1993, Murai published a paper in Nature that strongly influenced the way synthetic chemists think about the potential of C−H bond functionalization.87 This paper disclosed the addition of the C−H bonds in aryl ketones ortho to the carbonyl functional group across alkenes (eq 26). The reaction did not
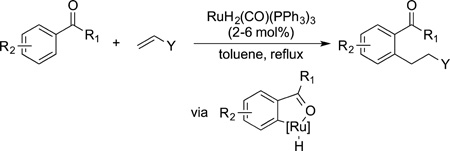 |
(26) |
occur on an arene lacking this functional group. The alkylation of an arene is a classical reaction of organic chemistry, typically conducted with an acid catalyst, giving branched products from the alkene and regioselectivity at the arene controlled by electronic effects. In contrast, this reaction occurred with an electronpoor arene, formed the linear alkylarene, and installed the alkyl group regioselectively ortho to the carbonyl group. Moreover, this reaction was the first high-yield catalytic C−H bond functionalization in which the substrate containing the reacting C−H bond was the limiting reagent. Catalysts that enable this reaction to occur under milder conditions have been developed.88 In addition, combinations of catalysts and substrates, such as aryl imines, that allow the reaction to occur without isomerization of the alkene have been developed.89 Reactions that occur intramolecularly and that occur with heteroarenes have been reported and used extensively for the synthesis of natural products and pharmaceutically active compounds.90
Particularly influential, however, has been the extension of directing groups to increase the reactivity and control the site selectivity of reactions related to Fujiwara’s palladium-catalyzed oxidation. In 1970 and 1971, Fahey at Phillips Petroleum reported the catalytic chlorination of azobenzene to form a mixture of mono- and polyhalogenation products containing chlorine in the ortho position (eq 27).91,92 In 1996, Andrienko reported the iodination of azobenzene with I2 and a combination of PdI2 and
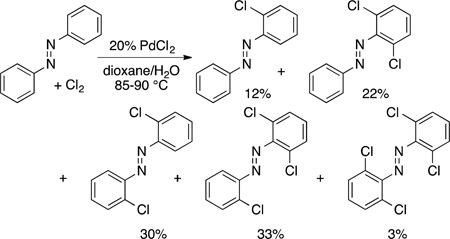 |
(27) |
CuCl2 as catalyst,93 and in 2001 Kodama filed a patent on the ortho halogenation of a precursor to an agrochemical (vide infra).25
More recently, Sanford reported oxidation, halogenation, and oxidative coupling of aryl and alkyl C−H bonds in a broader scope of reactants containing coordinating functional groups.94,95 Sanford showed that C−H bonds undergo acetoxylation proximal to a dative nitrogen donor, such as a pyridyl ring (eq 28). This chemistry was then extended to the functionalization of aromatic C−H bonds directed by a basic functional group selectively in good yield (eq 29).
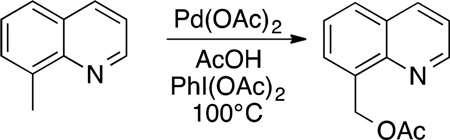 |
(28) |
 |
(29) |
Yu showed that the directed functionalization could be conducted at an unactivated alkyl C−H bond. In particular, he showed that an alkyl C−H bond near a chiral oxazoline donor undergoes halogenation diastereoselectively (eq 30).96,97 His
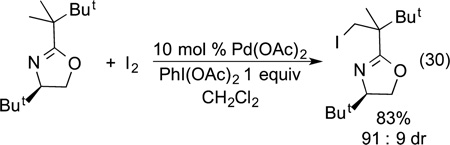 |
(30) |
group has now discovered a series of chiral, nonracemic catalysts for directed, enantioselective functionalization of achiral substrates.98–100 Of course, lithiation of aromatic C−H bonds and diastereoselective lithiation of certain aliphatic C−H bonds controlled by chiral directing groups has been a mainstay of organic chemistry for many years.101 However, these palladium-catalyzed processes allow for the directed functionalization to occur at mild temperatures and without the strongly basic conditions of ortho-lithiation.
The application of a coordinating substituent on the substrate to direct reactions ortho to a C−H bond is now used in a bewildering array of functionalizations.102,103 These functionalizations include the transformations of aryl, vinyl, and alkyl C−H bonds. These reactions include halogenation (including fluorination), amination, oxidative olefination, alkylation (with an alkyl halide), carboxylation, arylation, and alkynylation of the C−H bond located ortho to a directing group. Moreover, this approach to the functionalization of alkyl C−H bonds has led to the site-selective oxidation, amination, arylation, and alkylation of aliphatic C−H bonds. These substrates undergoing functionalization of alkyl C−H bonds in substrates ranging from alkylpyridines and imines to amino acids. Many directing groups are now known, but the 8-amidoquinoline group published by Daugulis104,105 is one of the most commonly used because it can be installed at the position of a carboxylic acid and binds to many metals, including first-row metals, that one would use for functionalization (eq 31).
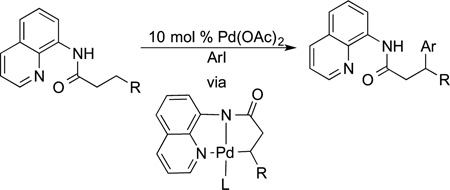 |
(31) |
These directing groups increase the rate of C−H bond cleavage due to binding of the substrate at the metal, in addition to controlling the site at which the C−H bond cleavage and functionalization occurs. Thus, researchers have been interested in developing substituents that accelerate the rate of C−H bond cleavage, but direct the functionalization to a site located more distal to the substituent.
For example, directing groups have been designed that contain a structure that positions the catalyst at a meta C−H bond. These auxiliaries are also derived from a carboxylic acid and contain a nitrile that binds to a palladium catalyst (eqs 32 and 33).106,107 These substrates undergo Fujiwara vinylation at the position meta to an acetamide group or an aryl benzyl ether linkage containing a nitrile group at a defined angle.
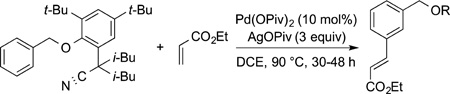 |
(32) |
 |
(33) |
ARENE FUNCTIONALIZATION WITHOUT DIRECTING GROUPS
The functionalization of arenes also has been accomplished with catalysts that react selectively at C−H bonds without the influence of directing groups. The number of catalysts that functionalize arenes without the aid of a directing group is small, but these catalysts are some of the most used for synthetic applications. Depending on the catalyst, the most reactive site can be the one that is the most available sterically, the one that is most electron rich or electron poor, or the one that is the most acidic.
Undirected Arene Functionalization with Sterically Controlled Regioselectivity
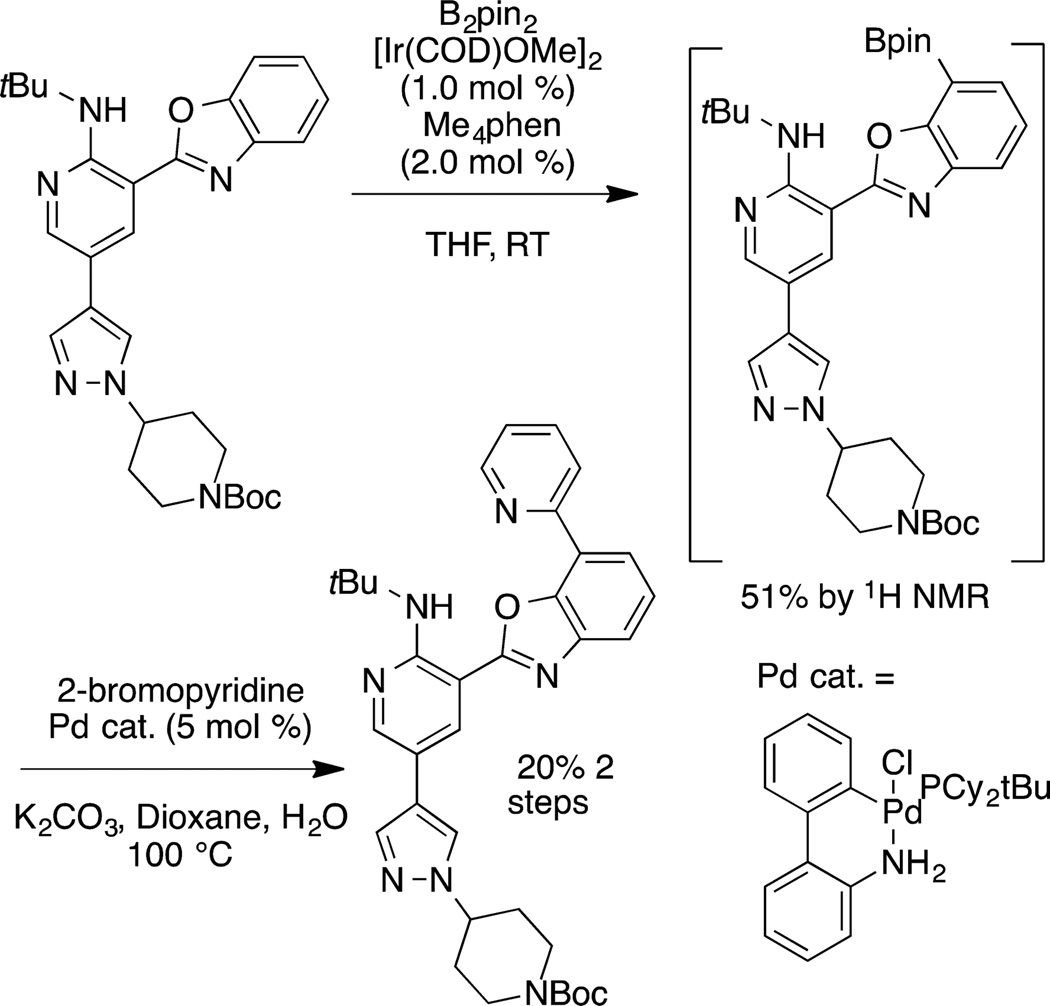
One of the most commonly used systems for the functionalization of arenes and heteroarenes is the iridium-catalyzed reaction of these substrates with boron reagents to form aryl- and heteroarylboronate esters.108 The borylation of arenes occurs, in almost all cases,109 with regioselectivity controlled by steric effects.110 The borylations of heteroarenes occur with regioselectivity controlled by a combination of steric and electronic effects.111–113 The borylation of arenes occurs by the reaction of a diboron compound, typically B2pin2 (pin = pinacolate), or a borane, typically HBpin, with an arene and a catalyst. The most active catalysts are based on the combination of an Ir(I) precursor and a chelating dative ligand bound to iridium through nitrogen (di-tert-butylbipyridine24 or tetramethylphenanthroline,114,115 for example).
Examples of the borylation of 1,3-disubstituted arenes are shown in eq 34.116 These examples illustrate the high
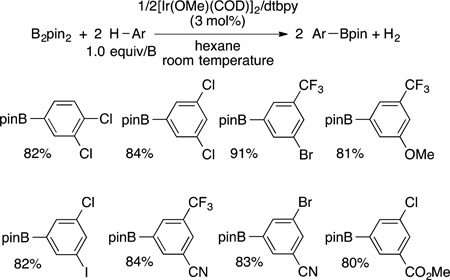 |
(34) |
regioselectivity for formation of the product from functionalization of the one C−H bond that is more sterically accessible than the other three C−H bonds. The products of these borylation reactions are common reagents for a series of coupling and oxidative transformations, such as Suzuki–Miyaura cross coupling117 and oxidative functionalizations to form phenols,118 ethers,119 amines,119 nitriles,120 and fluoroalkylarenes,121 among other products.
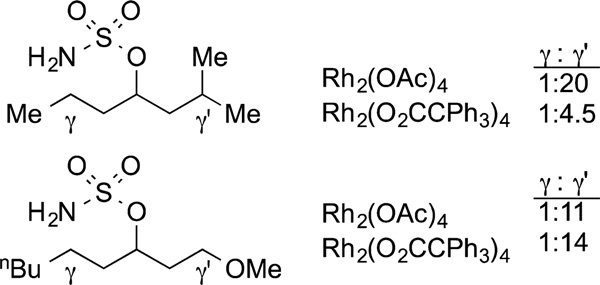
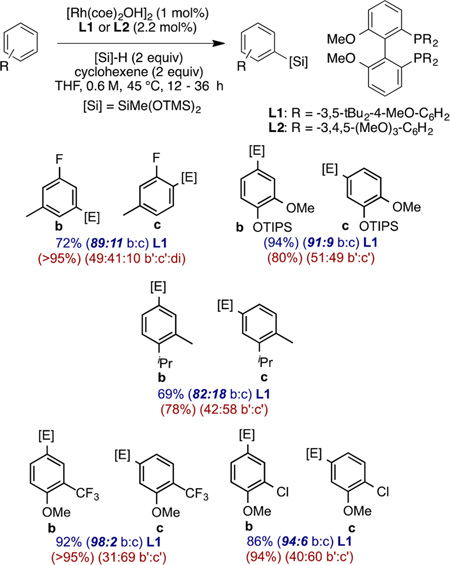
Related silylations of aryl and heteroaryl C−H bonds also have been developed.122 The most active catalysts for C−H bond silylation are rhodium complexes of hindered arylbisphosphines containing biaryl backbones123 and iridium complexes ligated by phenanthroline ligands.124–126 One remarkable feature of the silylation of C−H bonds catalyzed by Rh complexes containing large bisphosphines is the exquisite sensitivity for the size of an ortho substituent (eq 35). For example, the catalyst can distinguish between cleavage of a C−H bond ortho to one hydrogen and one fluorine and cleavage of a C−H bond ortho to two hydrogens. This catalyst and silane reagent also can distinguish between a C−H bond para to the larger of two substituents and a C−H bond meta to the larger of two substituents (eq 35).
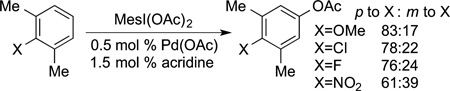
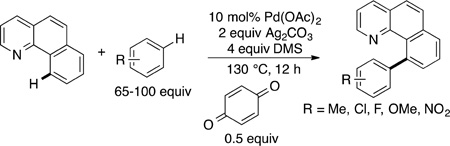
Other C−H bond functionalizations can occur with regioselectivity controlled by steric effects, but these reactions do not occur with the efficiency and scope of the borylation and silylation reactions. For example, the acetoxylation of C−H bonds in arenes catalyzed by palladium complexes ligated by nitrogen ligands occurs at the most sterically accessible C−H bond (eq 36),127 but these reactions are severely limited in scope. Likewise, palladium-catalyzed oxidative coupling of an arene containing a directing group with a substituted arene occurs at the sterically most accessible C−H bond of the substituted arene (eq 37),128 but these reactions are limited to arenes containing
 |
(35) |
 |
(36) |
 |
(37) |
strongly coordinating directing groups and a small set of relatively stable substituents.
Unidrected Arene Functionalization with Electronically Controlled Regioselectivity
The oxidative functionalization of aryl C−H bonds and many functionalizations of heteroaryl C−H bonds occur with regioselectivity controlled by the electronic properties of the C−H bond. In most cases, the reaction occurs at the most electron-rich C−H bond. In other cases, the reaction occurs at the most acidic C−H bond.
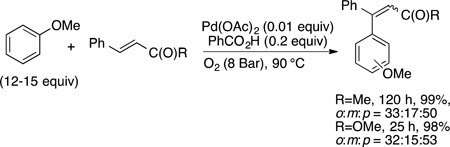
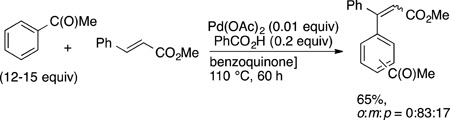
For example, the oxidative olefination of arenes first reported by Fujiwara35–37 and the subsequent versions of this reaction with arenes lacking directing groups occur at the most electron-rich C−H bond. For this reason, many researchers consider the mechanism of the C−H bond cleavage to occur by an attack of an electrophilic palladium center on the more electron-rich of the C−H bonds.47 Examples of such site-selectivity are shown in eqs 38 and 39 (refs 129 and 130, respectively). The reaction of anisole occurs preferentially at the para position over the meta position, while the reaction of acetophenone occurs preferentially at the meta position over the para position. (The extent of reactivity at the ortho position depends on the size of the substituent.)
 |
(38) |
 |
(39) |
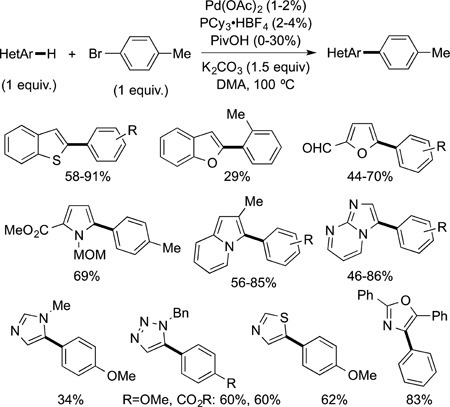
The regioselectivity of reactions of heteroarenes can be high and derived from the electronic properties of the C−H bond. The catalytic reaction of arenes with aryl halides (direct arylation) is one of the most valuable C−H bond functionalization reactions of heteroarenes.48,131 Because the electronic properties of arenes vary significantly from one position to another, this regioselectivity can be high. A thorough study of the regioselectivity of the direct arylation of heteroarenes was published by Fagnou and selected examples are shown in eq 40.132 In
 |
(40) |
most cases, the reaction occurs at the most acidic C−H bond of the heteroarene.133 The barriers computed by DFT for C−H bond cleavage in the direct arylation process by a concerted metalation–deprotonation step (in which the carboxylate deprotonates the C−H bond of a bound arene) correlate well with the regioselectivity observed experimentally.
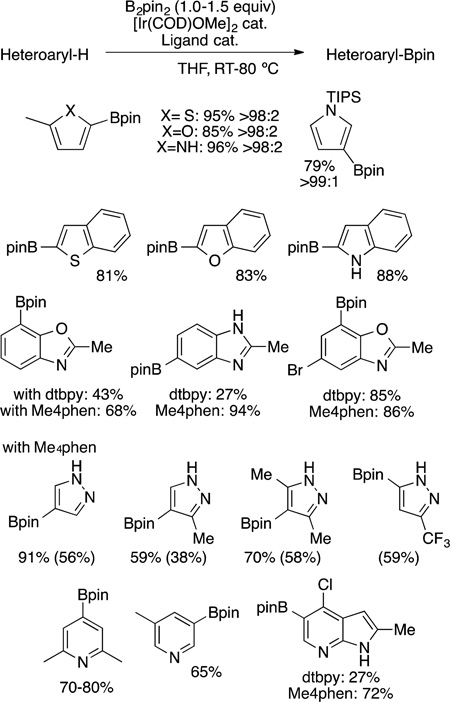
The borylation of heteroarenes, even in the absence of directing effects,134,135 also occurs with strong regioselectivity.111,136–138 Several examples illustrating this regioselectivity are shown in eq 41. The borylation of five-membered ring heteroarenes is faster than the borylation of an arene, including an arene fused to the five-membered ring. For example, the reaction of benzofuran occurs on the furan group, not the benzo-fused ring. Moreover, the reaction occurs α to the oxygen, nitrogen or sulfur of a five-membered ring containing one heteroatom. However, heteroarenes containing multiple heteroatoms give distinct, but still strong, regioselectivity.
A thorough account of such selectivities was recently reported;111 to summarize, the reaction does not occur α to
 |
(41) |
nitrogen when the ring contains a basic nitrogen atom. Thus, pyridines undergo iridium-catalyzed borylation at the 3- or 4-position, and pyrazoles undergo borylation at the 4-position. These selectivities result from multiple effects. The reaction alpha to a heteroatom has been proposed to result from the acidity of this C−H bond.139 The selectivity for reaction at the 3- and 4-positions of pyridine results form a slightly higher barrier for cleavage of the C−H bond at the 2-position and decomposition of the small amounts of 2-boryl products that would form. The reaction at the 4-position of pyrazoles results from borylation of the nitrogen, creating steric hindrance around the nonbasic nitrogen of this heterocycle, and a presumed higher barrier for C−H bond cleavage α to the basic nitrogen, as is seen in pyridine.111,140
The functionalizations of alkyl, rather than aryl, C−H bonds by oxidation, oxidative coupling, and insertions of carbenes also occur with regioselectivity controlled by the electronic properties of the C−H bond. In general, these reactions occur at a more electron-rich C−H bond than at a more electron-poor C−H bond. It has been difficult to distinguish between the secondary C−H bonds in an alkane, but oxidations by many catalysts and reagents occur at the secondary or tertiary C−H bond more distal to an electron-withdrawing functional group than at the positions more proximal to such a group.61 Because most substrates for the oxidation of C−H bonds that would form products useful for pharmaceutical or agrochemical applications contain functional groups that affect the electronic properties of C−H bonds, highly selective functionalization of C−H bonds in molecules more complex than alkanes can be accomplished.
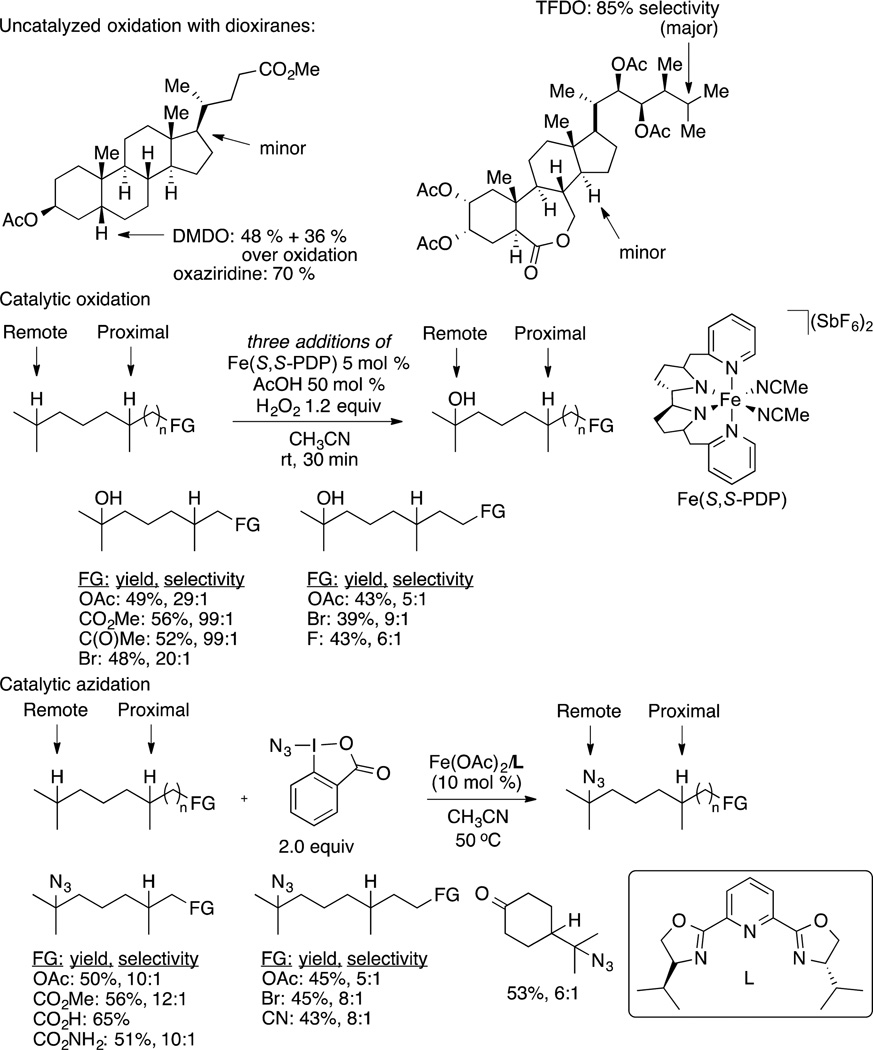
Such selectivity has been known for many years and applied to the functionalization of C−H bonds in scattered cases. In addition to the oxidation141,142 and halogenation of C−H bonds in complex structures,143,144 the introduction of nitrogen-based functionality can be accomplished by the catalytic azidation of C−H bonds.85 Figure 2 shows the site selectivity for the functionalization of C−H bonds in a series of compounds with existing functionality that influences the electron density at C−H bonds. One advantage of conducting C−H bond functionalization by the abstraction of hydrogen atoms with radical intermediates is the higher tolerance of the process toward acidic functional groups that tend to react with organometallic intermediates. However, one drawback of such pathways is the difficulty in controlling relative and absolute stereochemistry at the site of the new functional group.
Figure 2.
Examples of site-selective functionalization of alkyl C−H bonds in functionalized molecules under oxidative conditions.
This site selectivity has been observed for reactions that are proposed to occur by concerted insertion of an oxygen atom from a reagent or catalyst or by abstraction of a C−H bond by an electrophilic site to form an alkyl radical intermediate. However, similar site selectivity has been observed during radical reactions that form C−N bonds. A recently reported, iron-catalyzed azidation of C−H bonds clearly occurs by an alkyl radical intermediate, as evidenced by the stereochemical outcome of the reactions. These reactions occur with high selectivity for tertiary over secondary C−H bonds and the most electron rich of two or more C−H bonds in a molecule containing functional groups.
Although these oxidations and azidations occur via abstraction of a C−H bond by a species acting like an electrophilic radical, other catalytic reactions that clearly occur by concerted pathways also occur with site selectivity controlled by the electronic properties of a C−H bond. For example, the insertions of carbenes into aliphatic C−H bonds occurs at the C−H bond located alpha to oxygen and nitrogen in furans and pyrrolidines.145–147 Likewise, rhodium-catalyzed insertions of nitrenes occur selectively at tertiary over secondary or primary C−H bonds (Figure 3),148 even though evidence for an alkyl radical in such reactions has not been gained;149 the amination of tertiary C−H bonds occurs with retention of configuration, implying that the reactions occur without the intermediacy of an alkyl radical.150,151 Thus, the electron-rich nature of a tertiary C−H bond, rather than the stability of the tertiary alkyl radical, leads to site selective insertions of nitrenes in the absence of strong steric effects.
Figure 3.
Site selectivity for the rhodium-catalyzed amination of alkyl C−H bonds.
Although most alkyl C−H bond functionalizations occur at the most electron-rich sites, and at sites that form the most stable radical, there is evidence that some C−H bond functionalization reactions can occur with site selectivity that is distinct from that controlled by electron density and stability of an alkyl radical.
For example, some functionalizations occur at the most sterically accessible, primary alkyl C−H bond. It is well established that organometallic complexes containing a primary alkyl group are more stable, except in special cases, than those containing a secondary or a tertiary alkyl group.152–154 Thus, catalytic functionalizations occurring through organometallic alkyl intermediates can occur at primary over secondary C−H bonds. However, few catalytic reactions, particularly undirected reactions, lead to functionalization of primary C−H bonds.
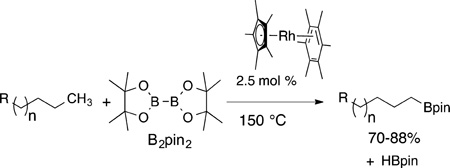
The class of reaction that, so far, leads to the functionalization of primary C−H bonds without a directing group is the formation of alkylboronate esters from alkanes (eq 42).16 This
 |
(42) |
reaction, now catalyzed by complexes of rhenium,17 rhodium,16 ruthenium,155 and iridium,156 occurs in each case with exceptionally high selectivity for primary over secondary C−H bonds. Moreover, the reaction occurs preferentially at primary C−H bonds located beta to oxygen (eq 43) or nitrogen in alkyl ethers
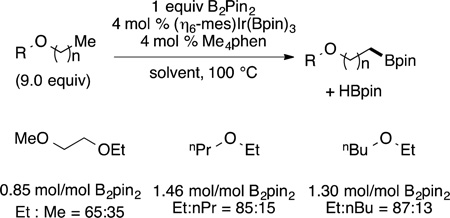 |
(43) |
and alkylamines,156 rather than the C−H bond located alpha to oxygen or nitrogen that would react if the stability of the radical controlled the site selectivity.
In some cases, directed functionalizations occur at primary C−H bonds in preference to secondary or tertiary C−H bonds. Silylations,125,157 arylations, and acetoxylations158 all have been reported to occur at primary C−H bonds. For example, the silylation of C−H bonds catalyzed by iridium and tetramethylphenanthroline occurs at the primary C−H bond located gamma to a hydroxyl group or ketone. These reactions have been shown to occur in the presence of a range of functional groups (eq 44).125 The reaction forms a silole, which is a precursor to a 1,3-diol. As shown in eq 45, the reaction occurs
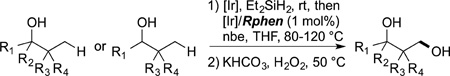 |
(44) |
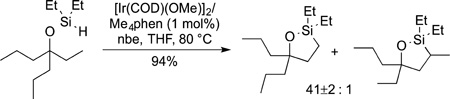 |
(45) |
approximately 50 times faster at a primary C−H bond over a secondary C−H bond, after correcting for the number of the two types of C−H bonds located gamma to oxygen in the alcohol.157
The directed functionalization of primary C−H bonds also occurs with palladium catalysts. The acetoxylation of alkyl C−H bonds located beta to an imine function by PhI(OAc)2 occurs with palladium acetate as catalyst. The reactions of substrates that probe for the relative reactivity of primary and secondary C−H bonds showed that the primary C−H bonds are more reactive. As shown in eq 46, the acetoxylation of substrates that contain
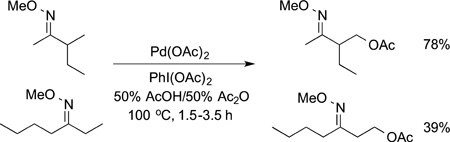 |
(46) |
primary and secondary positions beta to the oxime occur selectively at the primary position. No product from functionalization of the secondary C−H bond was observed when a primary C−H bond was accessible to the catalyst ligated to the oxime function.
APPLICATIONS OF C−H BOND FUNCTIONALIZATION TO THE SYNTHESIS OF COMPLEX MOLECULES
As noted in the introduction to this Perspective, early effort toward the functionalization of alkanes was motivated by the conversion of methane to methanol. Yet, the synthesis of complex molecules has been influenced most significantly in several ways by the developments in C−H bond functionalization. First, the synthesis of many natural products has now been completed with steps involving C−H bond functionalization. Second, although less documented in journals, C−H bond functionalization is being used for the synthesis of medicinal candidates by the functionalization of complex structures. Such complex structures include natural products and existing compound collections. Finally, some C−H bond functionalization reactions occur with yields sufficiently high for use in polymerization processes, and some reactions occur in ways that are valuable for modification of aromatic molecules of interest for materials science.
Over the past several decades, hundreds of natural products and pharmaceutical candidates have been synthesized with functionalization of a typically unreactive C−H bond as a key step.159 Many of these natural products have simple structures and were prepared to demonstrate the potential utility of a reaction; other targets are more complex and have illustrated how C−H bond functionalization can be used to prepare complex molecules, but have not led to substantially shorter synthetic routes than prior more classical approaches. However, the syntheses of several complex molecules have been completed with staggering efficiency using C−H bond functionalization as part of the strategy for assembling the core of these molecules. For example, the synthesis of tetrodotoxin was completed in 32 steps,160 versus 67 in the prior synthesis, austamide in seven steps, versus 29 in the original synthesis,161 incarvillatein in 11 steps versus 20 in the prior synthesis162, and Dramacidin in 15 steps versus 25 in a prior modern synthesis.163
Although efforts to use C−H bond functionalization in this fashion have intensified recently, one should not overlook that several classes of C−H bond functionalizations—carbene insertions and oxidative coupling of arenes and heteroarenes—have been used in this context since the 1980s. Thus, the focus has shifted from the functionalization of alkanes to alkyl and aryl groups in molecules with existing functionality, but reliable classes of C−H bond functionalization have been used in synthetic applications for many years, dating back to the time soon after they were discovered.
The following sections illustrate how C−H bond functionalization has affected organic synthesis with selected examples from the literature. Comprehensive159 and focused142,164–166 reviews have been published on this topic, and readers who seek such detailed coverage should refer to these excellent publications. Many examples of the cleavage of C−H bonds by radical initiators have been used in synthetic applications; this Perspective focuses on C−H bond functionalization by catalysts that are organometallic or react in a similar fashion to organometallic species.
Synthesis of Natural Products by the Reactions of Metal-Carbene and Nitrene Complexes with C−H bonds
Some of the earliest syntheses of natural products by C−H bond functionalization were completed by carbene insertions into C−H bonds. Thirty years ago, two syntheses of pentalenolactone E methyl ester by Taber167 and by Cane168 were completed using a rhodium-catalyzed intramolecular insertion of a carbene into a C−H bond to form the polycyclic core of this natural product. These approaches are shown in eq 47. Although an elegant and visionary application of C−H
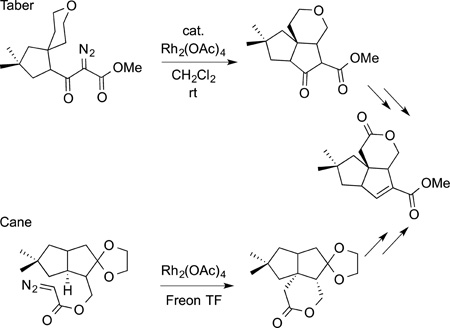 |
(47) |
bond functionalization, few additional syntheses of natural products by C−H bond functionalization were reported at that time. One exception is the synthesis of the classic target morphine completed by White in 1997 in which insertion of a carbene into a C−H bond was used to form the polycyclic core (eq 48). The advent of chiral catalysts for these carbene insertions by Doyle created the ability to use this chemistry to conduct enantioselective syntheses with the carbene insertion as the step that establishes absolute stereochemistry. An example of such a reaction in the synthesis of isodeoxypodophyllotoxin by Doyle is shown in eq 49.
More recently, catalysts and carbene precursors that react intermolecularly with C−H bonds were discovered by Davies169,170 and applied to the synthesis of natural products. The combination of enantioselective intermolecular C−H insertion
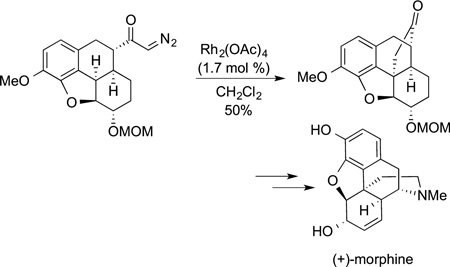 |
(48) |
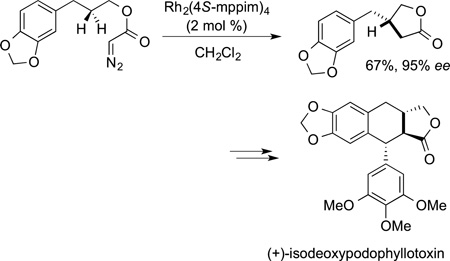 |
(49) |
of vinyl-substituted carbenes, followed by Cope rearrangements were used as steps in the synthesis of elisapterosin B and colombiasin (eq 50).171 The most complex application of carbene
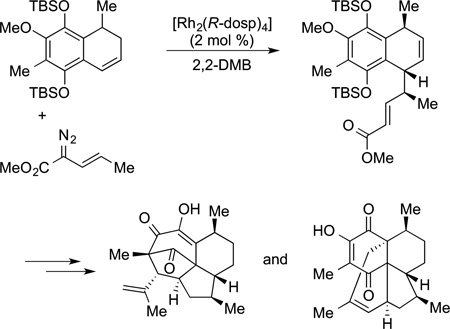 |
(50) |
insertions into C−H bonds in total synthesis is that to the synthesis of tetrodotoxin. The C−H bond insertion step and the bond of the final product formed from this reaction are shown in eq 51. This step and a nitrene insertion were elements of this synthesis by Du Bois that shortened the prior synthesis of 67 steps to 32 steps.
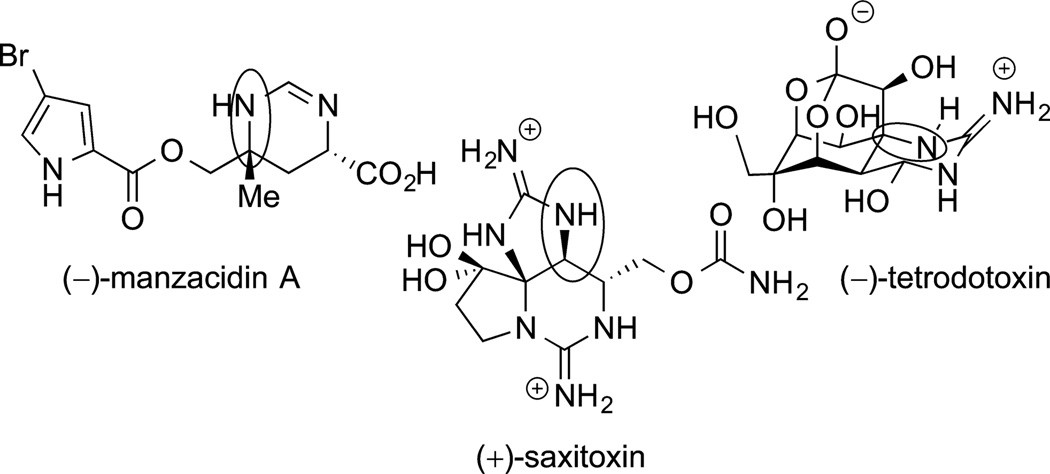
The insertions of nitrenes into C−H bonds are closely related to the insertions of carbenes. The applications of these reactions to the synthesis of complex molecules exclusively involves intramolecular variants. Similar catalysts to those used for the insertion of carbenes into C−H bonds have been used for the insertion of nitrenes into C−H bonds. Initial examples of this reaction were published over 30 years ago. However, practical systems were published recently, and applications to the synthesis of natural products are contained in this recent literature. Du Bois applied this reaction to the synthesis of the three natural products shown in Figure 4,160,172 the most complex synthesis being that of tetrodotoxin discussed above. The C−N bonds formed by nitrene
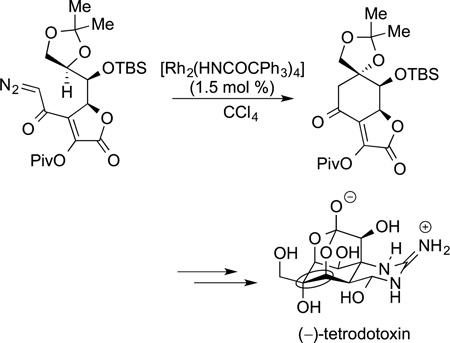 |
(51) |
insertion during the synthesis of these molecules are circled. Since that time, about a dozen natural products have been prepared with nitrene insertion as a step of the syntheses.
Figure 4.
Three natural products prepared by Du Bois and co-workers using nitrenes insertions into C−H bonds.
Synthesis of Natural Products by Generating Organometallic Intermediates from Alkyl C−H Bonds
As will be shown in the next section, the oxidative functionalization of aryl C−H bonds had been applied to the synthesis of a range of natural products and functional materials. However, one driving force indicating that C−H bond functionalization based on “C−H activation” (defined as the addition of a C−H bond to form a metal-carbon bond)173 could become a synthetic method for the synthesis of molecules of biological interest stemmed from initial studies on the functionalization of alkyl C−H bonds. The synthesis of several natural products or the cores of these natural products by mechanisms involving oxidative addition or concerted metalation-deprotonation signaled to the synthetic organic and organometallic communities that these fields could intersect at the application of the C−H activation in alkyl groups to the synthesis of complex molecules.
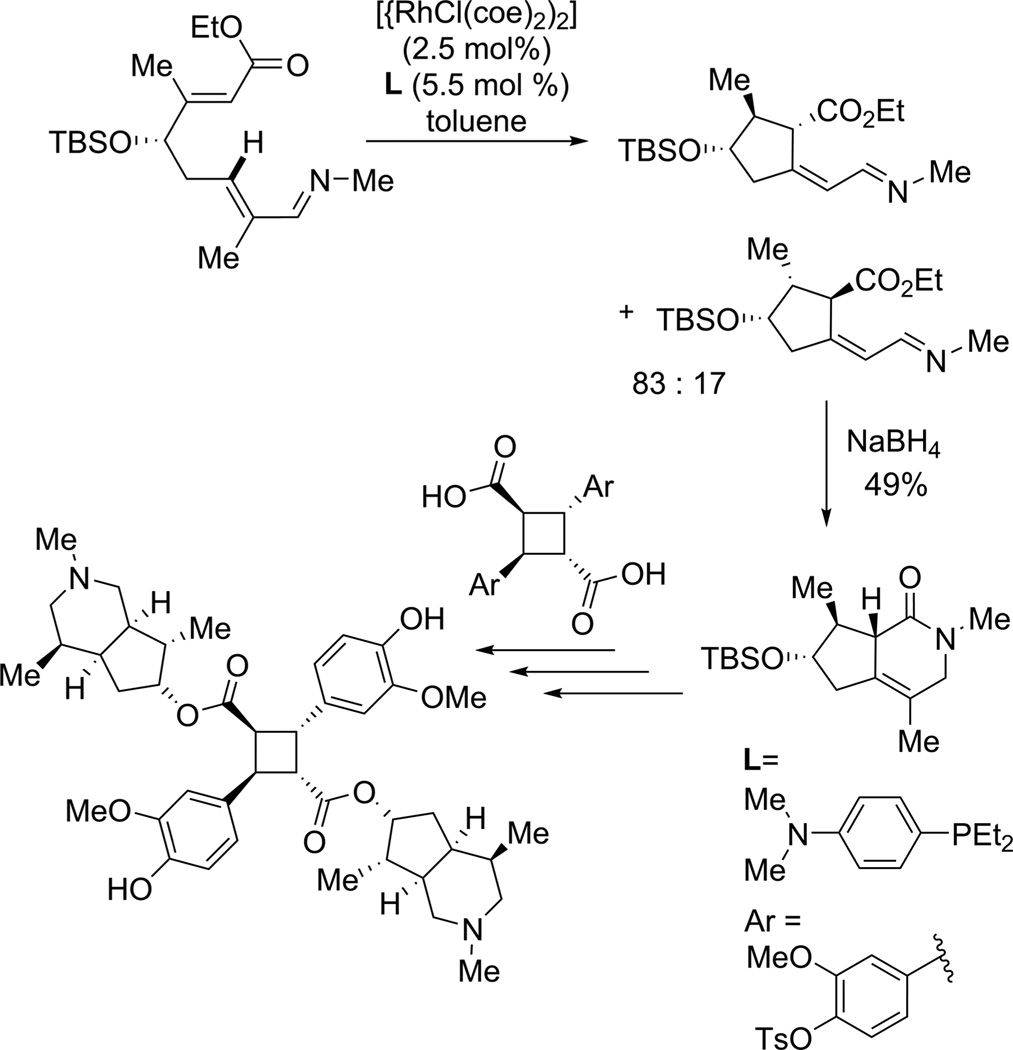
Some of the early applications of such reactions involved stoichiometric amounts of metal complexes. Given the resources required to conduct such syntheses, the use of stoichiometric metal complexes might not be a significant hurdle when preparing milligram quantities of products. One of the early examples of the application of reactions that involve metal-carbon σ bonds to the synthesis of complex molecules was the assembly of the core of rhazinalam by stoichiometric174 and later diastereoselective175 dehydrogenation of an alkyl group (eq 52). Dehydrogenation by organometallic complexes has typically been considered to have potential for the preparation of α-olefins from alkanes, but a selective process at high conversion remains unknown. In this case, the dehydrogenation of an ethyl group directed by coordination of platinum to a bisimine ligand, which was generated from an aldehyde in the synthetic intermediate,
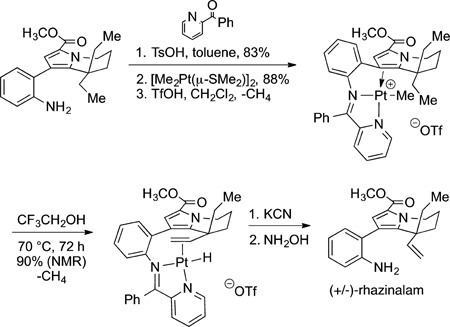 |
(52) |
led to the desired α-olefin. Carbonylation of this alkene and cyclization led to the core of this molecule.
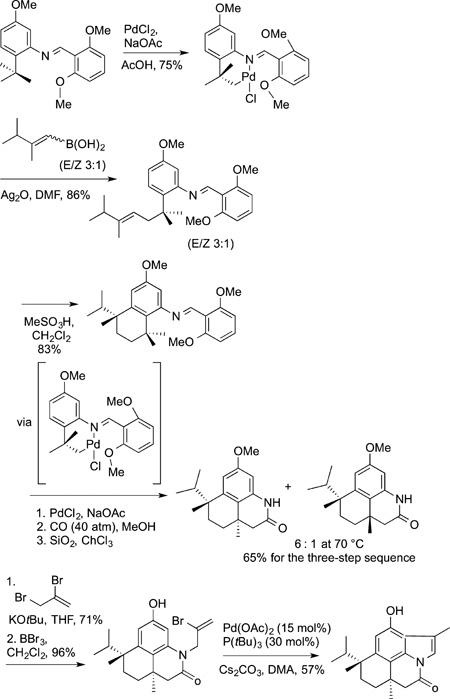
The synthesis of razinalam was followed by the synthesis of teleocidin B-4 using the functionalization of a tert-butyl group at two of the three methyl substituents as a core design element (eq 53). The first two C−H bond functionalization steps in the
 |
(53) |
synthesis of teleocidin B-4 and C−H bond functionalization steps in the synthesis of rhazinalam core are conducted with stoichiometric amounts of palladium, and directing groups are installed and removed before and after these steps, but these syntheses showed unconventional disconnections that could be part of a synthetic strategy if reactions that functionalize typically unreactive C−H bonds are used.
Directed functionalizations of alkyl C−H bonds have been developed further, and catalytic, directed functionalizations have been used as part of the synthesis of complex structures. For example, directed reactions have been used to functionalize the C−H bonds in a range of aliphatic carboxylic acids and amino acids. Chen reported the use of this example to form the noncanonical amino acid in the natural cyclic peptide celogentin C (eq 54).176
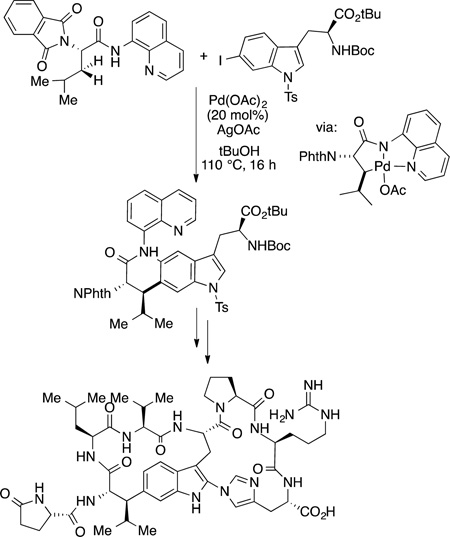 |
(54) |
Synthesis of Natural Products by Functionalization of Aryl C−H Bonds
The range of applications of aryl and heteroaryl C−H bond functionalization to the synthesis of natural products and medicinal agents is diverse. A wide range of arene functionalization reactions has been used, including the Fujiwara-Moritani oxidative couplings, the Murai hydroarylations, direct arylations, and C−H borylations. In many cases, the oxidative coupling reactions occur selectively at the 2-position of electron-rich heteroaryl rings, such as indoles, and this selectivity has enabled the efficient synthesis of many alkaloid natural products by C−H bond functionalization. The use of directing groups for the catalytic synthesis of halogenated materials in industrial laboratories was disclosed prior to the widespread use of such strategies in academic laboratories.25 The following sections provide selected applications of aryl and heteroaryl C−H bond functionalizations to the synthesis of medicinally important compounds and natural products.
Applications of the Fujiwara–Moritani Oxidative Couplings
Many examples of the use of the oxidative coupling of arenes or heteroarenes with alkenes and oxidative coupling of two arenes or an arene and a heteroarene have been reported since the reaction was disclosed in the 1970s. As noted in the introduction to this section, the functionalization of indoles by such processes has been common. In one of the most illustrative examples of how such strategies could be used to expedite the synthesis of natural products, the synthesis of racemic austamide was completed by Corey and Baran in only seven steps,177 while the prior synthesis 23 years earlier was completed in 29 steps.178 This short synthesis of austamide is summarized in eq 55. After generation of the appropriately prenylated tryptophan
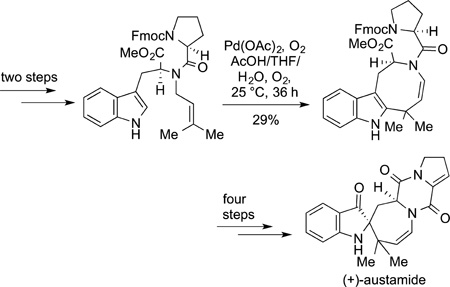 |
(55) |
derivative, the oxidative coupling of the prenyl group at the indole 2-position with 1 equiv of palladium and oxygen led to the eight-membered ring. This material was converted to the final product by the series of classical steps of the originally reported synthesis.
A related oxidative coupling was used by researchers at BMS to prepare rebeccamycin aglycone.179 In this case the intramolecular coupling of two indole units was used to prepare the carbazole core (eq 56). In a similar vein, Boger coupled two
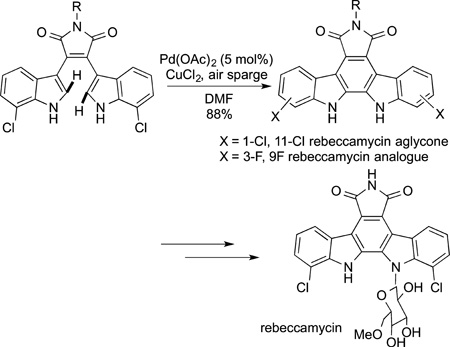 |
(56) |
pyrroles tethered through an amide to generate the C−C linkage between two of the pyrrole units of prodigiosin (eq 57).180 The
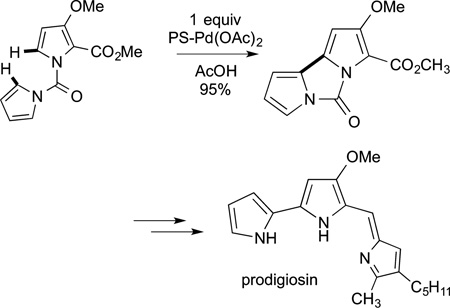 |
(57) |
carbazole core of many small natural products have been prepared by intramolecular oxidative coupling of two aryl rings.
Applications of Direct Arylation
The coupling of an arene or heteroarene with an aryl or heteroaryl halide has proven to be one of the most valuable C−H bond functionalization reactions for the synthesis of biologically important molecules. Because this reaction is the synthetic equivalent of the Suzuki-Miyaura coupling of an aryl halide with an arylboronate, many groups have sought to simplify the synthesis of the biaryl or heteroaryl products of Suzuki couplings by using the direct arylation process. Because the biaryl units generated by such chemistry are more commonly found in pharmaceuticals and pharmaceutical candidates than in natural products, the applications of direct arylation to the synthesis of complex molecules have focused on the synthesis of medicinally important compounds, rather than natural products.
Selected examples of the application of this reaction to the synthesis of important biaryl compounds are shown in eq 58 and
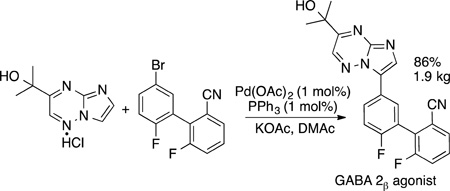 |
(58) |
 |
(59) |
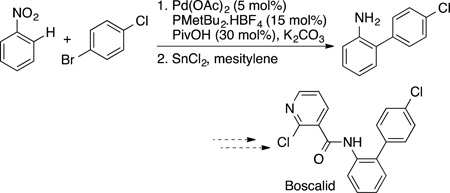
eq 59. The synthesis of the orally active GABA α2/3 agonist in eq 58 was conducted on multikilogram scale by coupling a biaryl bromide at the 3-position of an imidazo[1,2-α]pyrimidine.181 The agrochemical Boscalid in eq 59 also has been prepared by C−H bond functionalization, first in Fagnou’s laboratory by palladium-catalyzed direct arylation.182
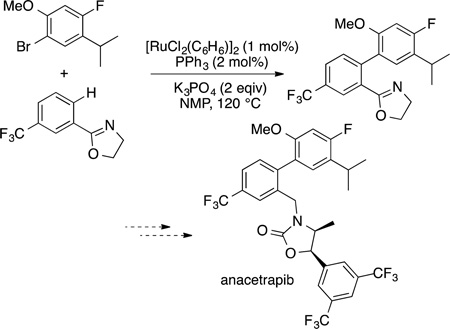
Because of the high activity of [(arene)Ru(OAc)2] complexes for direct arylation demonstrated by Ackermann183 and the lower cost of ruthenium than palladium, several commercially important biaryl compounds have been prepared by ruthenium-catalyzed direct arylation. For example, the biaryl core of anacetrapib, an inhibitor of cholesterylester transfer protein (CETP) active for hypercholesterolemia184 and members of the sartan family of antihypertensive drugs have been prepared by Rucatalyzed direct arylation. The application to the synthesis of anacetrapib is shown in eq 60.185
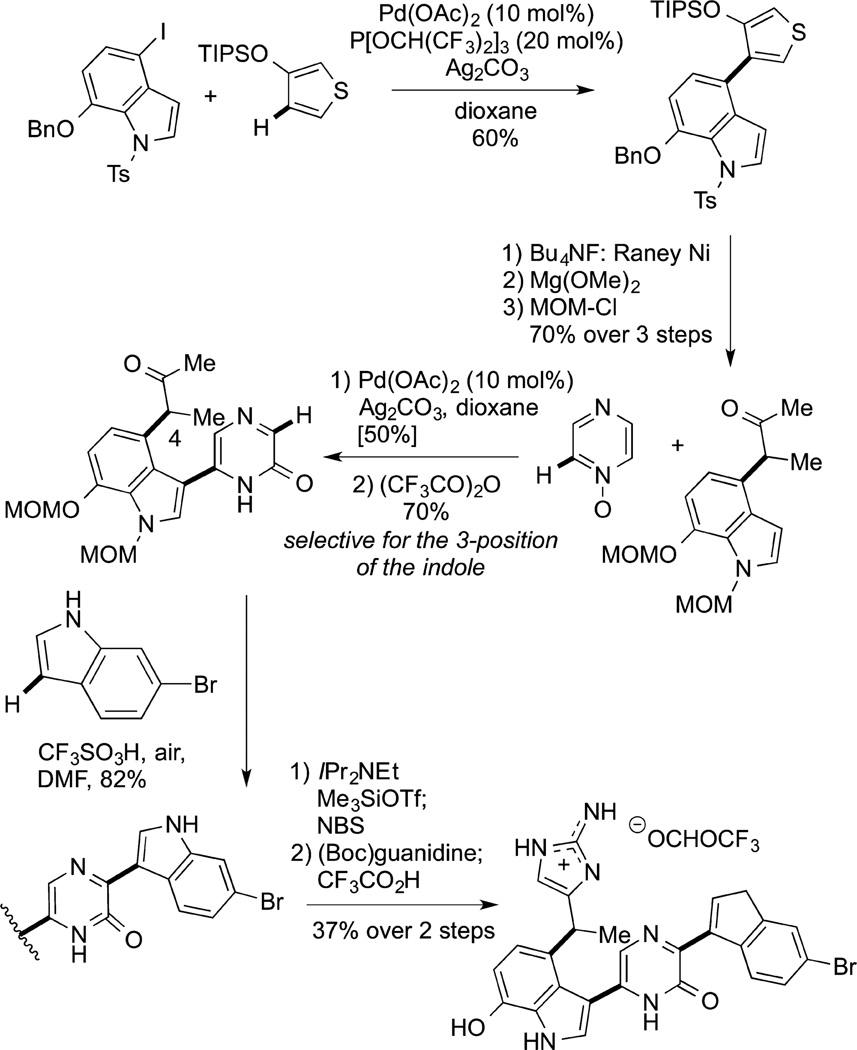
Although the majority of applications of such reactions to synthesis have focused on pharmaceutical ingredients, Itami published a dramatic example of the efficiency created by this direct arylation process, in combination with oxidative couplings, for the synthesis of the natural product dragmacidin D. His group completed the synthesis of this molecule in 15 steps, which included a series of metal-catalyzed C−H arylation reactions (Scheme 1).163 This synthesis was 10 steps shorter than a synthesis by an exceptionally talented trio using alternative catalytic methods, including Suzuki–Miyaura reactions.186
 |
(60) |
Scheme 1.
First, the direct arylation was conducted between an iodoindole and a siloxythiophene. The resulting product was converted to an α-aryl ketone by cleavage of the thiophene. Oxidative coupling of pyrazine oxide with unusual regioselectivity at the 3-position of the indole, followed by a direct arylation of a bromoindole at the pyrazidineone formed the penultimate product. This intermediate constructed by three functionalizations of aryl C−H bonds was converted to the final product by more classical construction of the guanidine unit. This synthesis illustrates the potential of C−H bond functionalization to enable a synthetic strategy that involves intuitive disconnections between two aryl or heteroaryl rings and sub-stituents on the aryl rings.
Applications of Intramolecular Hydroarylation
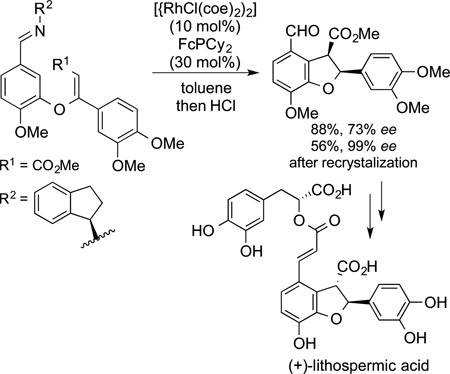
Intramolecular versions of hydrovinylation reactions that are loosely related to Murai’s directed hydroarylations, have been used in many cases by Bergman and Ellman for the synthesis of polycylic natural products. For example, the synthesis of incarvillateine was completed by the route shown in Scheme 2 in roughly one-third the steps of a previous synthesis.162 An intramolecular directed hydrovinylation reaction formed the carbocylic ring fused to the piperidine. An imine directing group facilitated the C−H bond cleavage and ensured the proper geometry of the alkene product. The piperidine was then formed by cyclization at the nitrogen of the imine directing group. After reduction steps, the resulting bicyclic structures were linked to the core by simple condensation with a diaryl-substituted squaric acid. Related imine-directed hydroarylations of alkenes were used by the same researchers to create a concise synthesis of lithospermic acid (eq 61).187
 |
(61) |
Scheme 2.
Applications of the Borylation of C−H Bonds
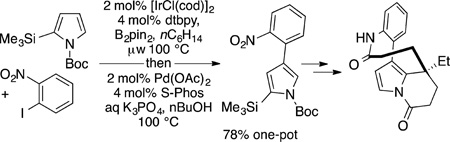
The installation of a boryl group as a synthetic intermediate has now been used several times to facilitate the synthesis of a series of natural products. In these syntheses, the resulting arylboronate group is used for cross-coupling or installation of functional groups in the final product. Gaunt used this reaction in combination with cross coupling as an early step in the synthesis of rhazinicine (eq 62).188 In this case, the borylation reaction occurs at the 3-position of a 2-silyl pyrrole derivative due to the size of the Boc group on the nitrogen. Cross coupling and subsequent steps led to the final product.
 |
(62) |
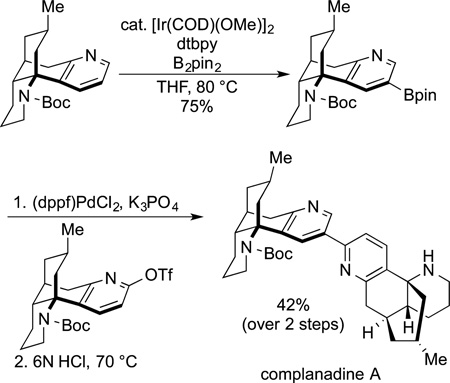
In contrast to this use of the borylation by Gaunt at the beginning of a synthetic sequence, Sarpong used the borylation of pyridine at the late stage of the synthesis of complanidine A (eq 63). In this
 |
(63) |
case, the linkage between the two pyridyl groups of the natural product was forged at the appropriate position by coupling a 2-pyridyl triflate derived from the corresponding pyridone with a 3-pyridyl boronate. The boronate group was installed at the 3-position of the pyridine by the combination of electronic and steric effects on the regioselectivity of the borylation of heteroarenes discussed earlier in this Perspective.
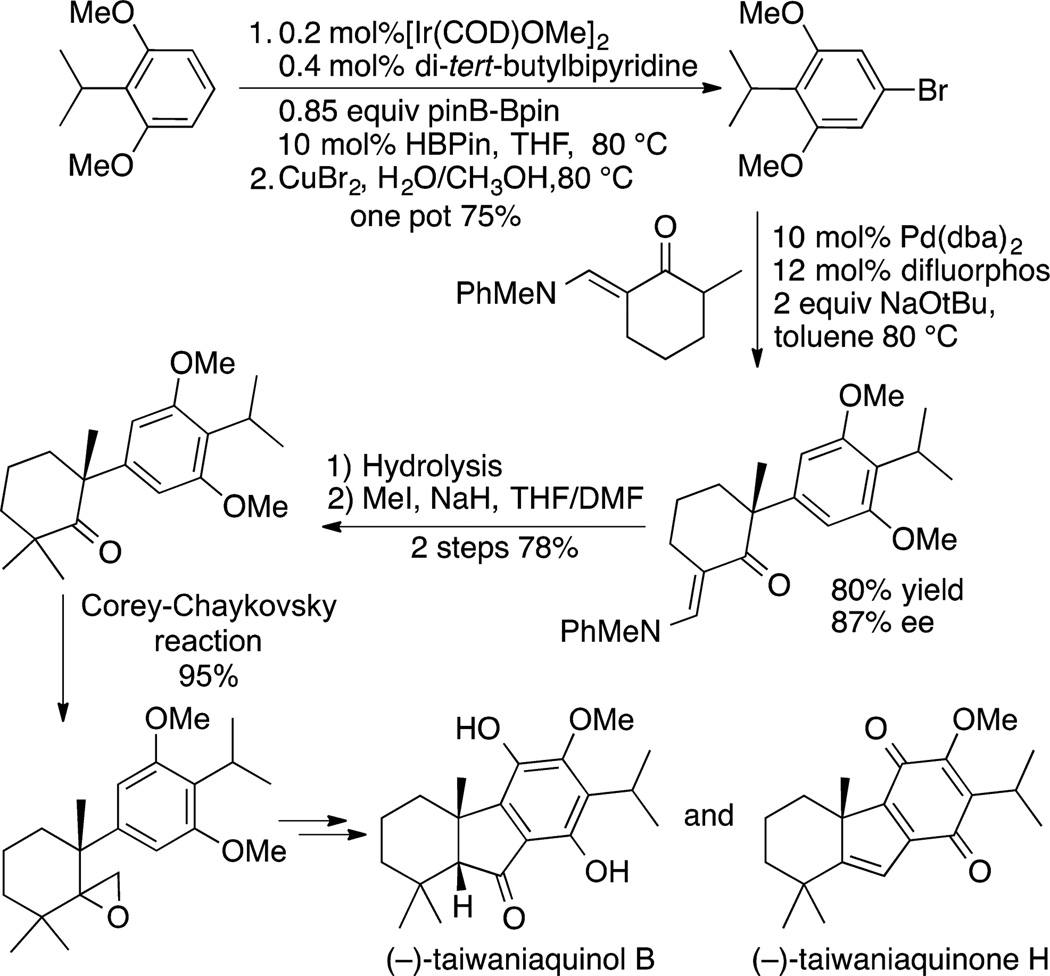
C−H borylation has also been used to form the aryl halide electrophile for an enantioselective α-arylation reaction as part of our synthesis of (−)-taiwaniaquinone H and (−)-taiwaniaquinol B (Scheme 3).189 These syntheses required the halide meta to methoxy substituents. To accomplish this goal at an early step of this synthesis, the arene was converted to the aryl bromide by a combination of borylation at the sterically most accessible C−H bond and bromination of the resulting arylboronate. This aryl bromide participated in an enantioselective α-arylation of a ketone, followed by cyclization and oxidation to form the final products. Additional complex molecules have now been prepared by related uses of arylboronate intermediates generated by C−H bond borylation.190,191
Scheme 3.
Application of Directed Halogenation to Agroscience
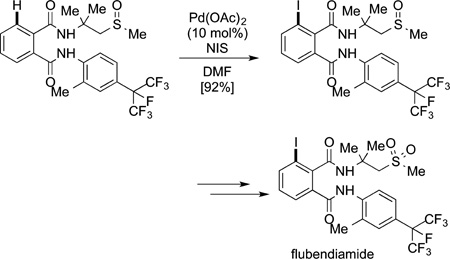
Although the synthesis just described required placement of a halogen at the position meta to a substituent, other syntheses require the installation of a halide at the position ortho to a functional group, either as a synthetic intermediate or final product. In one of the earliest applications of C−H bond functionalization to the synthesis of biologically important compounds, a palladium-catalyzed iodination of a benzamide was conducted to install the halogen ortho to the amide function. This process was patented in 2001,25 indicating that the researchers developed this process after the palladium-catalyzed directed iodination of azobenzene by Fahey and directed hydroarylation by Murai, but independent of the later academic work on palladium-catalyzed directed functionalizations of arenes. No report of this directed halogenation was published in the academic literature. Yet, the patent describes the halogenation in eq 64, which is an intermediate to the final insecticide.
 |
(64) |
Application of C−H Bond Functionalization to Materials Science
Because aromatic units are prevalent in electronic materials and are the only unit of the fragments of fullerene fragments and nanotubes, the functionalization of arene C−H bonds has been used to synthesize or modify materials. In addition, a long-standing target for materials science has been the synthesis of polyolefins containing polar functionality. Thus, C−H bond functionalization of polyolefins could be one application of aliphatic C−H bond functionalization. At this point, the applications of C−H bond functionalization to materials science are not extensive, but several papers describe synthetic strategies for materials synthesis by C−H bond functionalization. The examples shown below illustrate the potential of C−H bond functionalization to be applied to this genre of chemical synthesis.
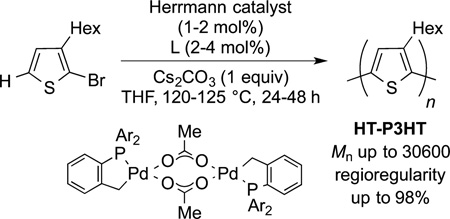
Just as direct arylation has been used to prepare medicinally important compounds, including commercial applications, direct arylation has been used to form polymers with important electronic properties.192 The important conductive polymer polythiophene has been prepared by palladium catalyzed direct arylation to form regioregular polythiophene, as shown in in eq 65. The success of this synthetic route results from the high reactivity of the C−H bonds alpha to heteroatoms in five-membered heteroarenes. In the current polymerizations, the number-average molecular weights approach 30 000 Da.
In other cases, C−H bond functionalization has been used to prepare aromatic compounds with novel electronic properties.
 |
(65) |
 |
(66) |
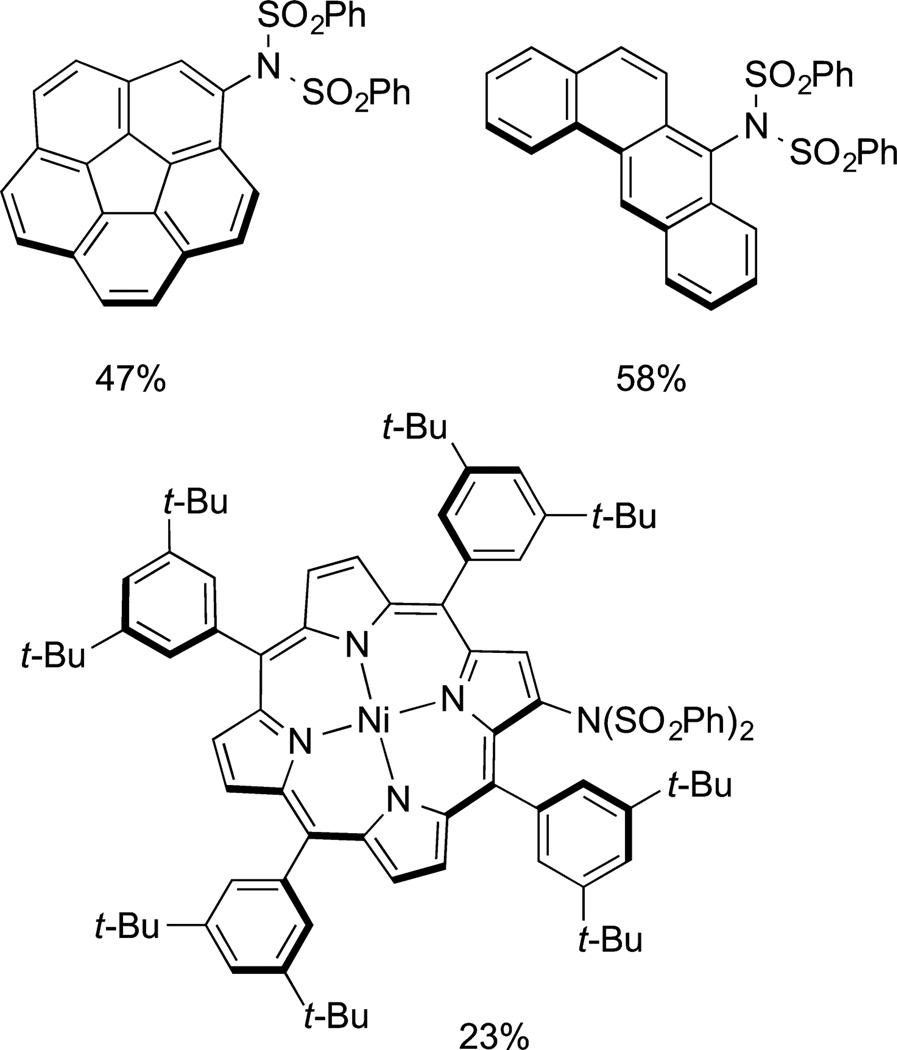
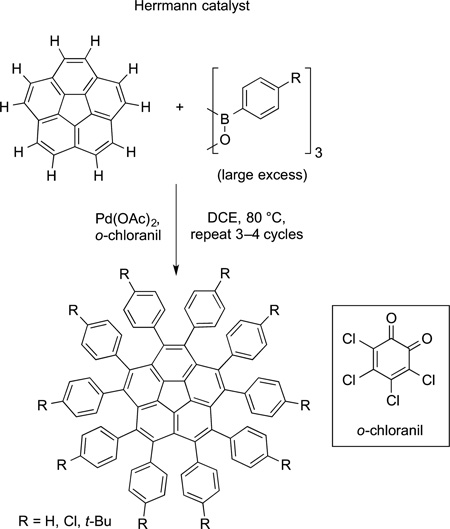
Scott and Itami used direct oxidative arylation of arenes with boroxines to prepare a series of fused polyaromatic molecules. One remarkable reaction is the decaarylation of corannulene to form a corannulene containing 10 aryl rings on the periphery. This reaction is shown in eq 66. Itami also has reported a coppercatalyzed coupling of NFSI with arenes and heteroarenes relevant to electronic materials. Several examples of the products of this imidation are shown in Figure 5.
Figure 5.
Three of many polycyclic hydrocarbons formed by selective, copper-catalyzed imidation.
Scott also has used the borylation of C−H bonds to modify corannulenes. Iridium-catalyzed borylation was used to generate a symmetric pentaborylcorannulene (eq 67). A key observation
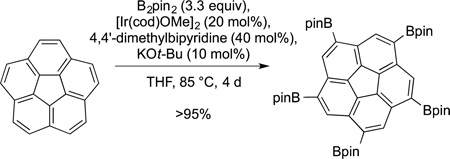 |
(67) |
that enabled this reaction to give the symmetrical product in high yield is the reversibility of the reaction in the presence of base. When run in the presence of base for 4 days, the borylation occured reversibly until the most stable, symmetrical product formed. This species was then coupled with 2-bromo-1,3-dichlorobenzene to form the precursor used to prepare a pure [5,5]nanotube. Most striking is the coupling of this material with 2-bromobiphenyl, followed by oxidative coupling to form the warped graphene segment (eq 68).
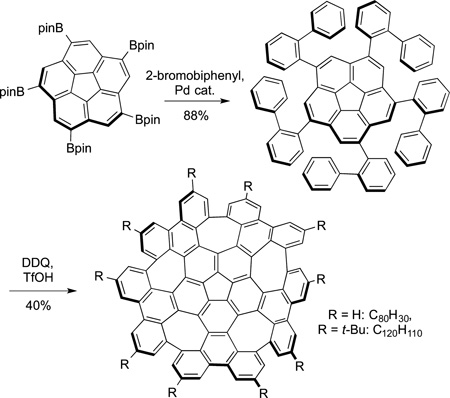 |
(68) |
Finally, as noted in the introduction to this section, the borylation of polyolefins has led to new materials. Hillmyer and Hartwig reported the borylation and subsequent functionalization of the C−B bond to form polyolefins bearing hydroxyl, formyl, and amino groups (eq 69).193–195 Although currently impractical for the preparation of large amounts of such materials, this chemistry does provide a synthetic route to materials that are difficult or impossible to prepare by alternative methods. Bae has reported the borylation of crystalline polystyrene (eq 70). The product of this reaction has been oxidized or coupled with an aryl halide to form new materials. This reaction sequence reduces the crystallinity of this material, modulating the processability without changing the molecular weight.196
Late-Stage Functionalization of Complex Molecules
A final emerging application of C−H bond functionalization is the diversification of existing compound collections and the modification of natural products to increase their polarity and modify the array of functional groups. At conferences, Merck researchers have presented studies on the combination of C−H borylation and subsequent functionalizations to form new derivatives
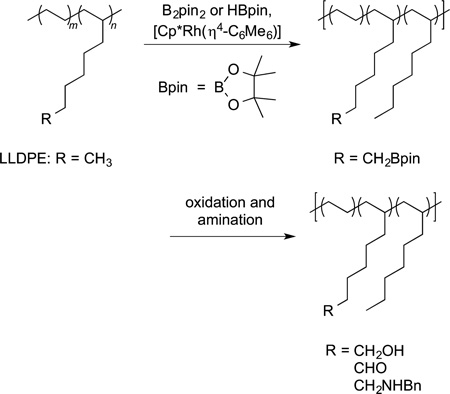 |
(69) |
 |
(70) |
of molecules being used in structure–activity relationship studies. Certainly, similar modification of pharmaceutical candidates by C−H bond functionalization is being conducted at other pharmaceutical companies.
In the published literature, Hartwig and Larsen have shown that the borylation of C−H bonds catalyzed by the combination of iridium and tetramethylphenanthroline occurs at heteroaryl C−H bonds with broad scope to add substituents to simple and more complex heteroarenes in medicinally active compounds.111 One example of the site-selective functionalization of a medicinally active compound containing basic saturated and unsaturated heterocycles is shown in Scheme 4. Hartwig and Cheng also have shown that the silylation of C−H bonds occurs with a wide range of heteroarenes, and this process can be used in combination with oxidation, cross-coupling, and halogenation to modify a range of structures of active pharmaceutical ingredients.126 Examples of these reactions are shown in eq 71.
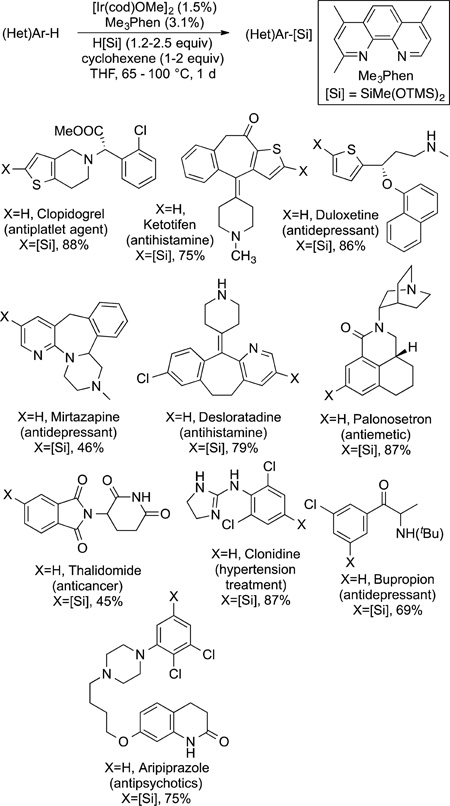 |
(71) |
Scheme 4.
The functionalization of alkyl C−H bonds also can be used to modify complex natural products or precursors to them. For example, White showed that an iron-catalyzed oxidation is suitable for the modification of artimisinin, a densely functionalized natural product used for the treatment of malaria (eq 72).141
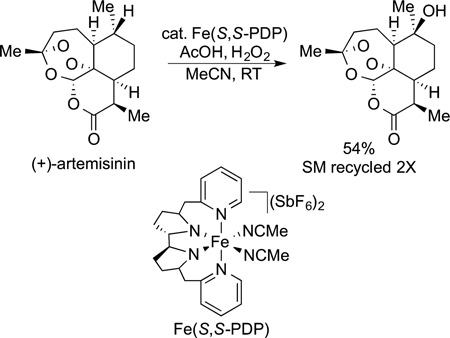 |
(72) |
Recently, Hartwig and Sharma showed that the azidation of tertiary C−H bonds in natural product derivatives can be conducted regioselectively with an iron catalyst ligated by a pybox ligand (eq 73). Most recently, Groves showed that azidation at
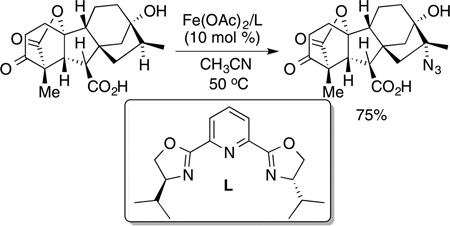 |
(73) |
secondary and tertiary C−H bonds occurs with a manganese porphyrin catalyst.197
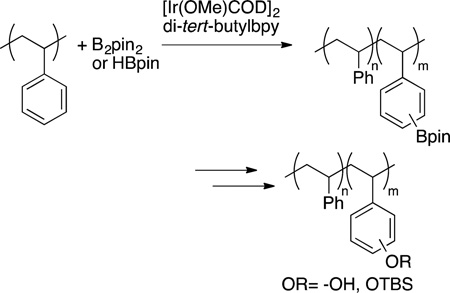
The functionalization of alkyl C−H bonds of complex molecules also can be conducted with main group reagents catalyzed by transition-metal systems. Hartwig and Simmons showed that primary C−H bonds in a series of natural products, including polycyclic, functionalized triterpenoids, can be achieved by a combination of silylation of an alcohol, cyclization by silylation at a primary C−H bond, and oxidation (eq 74).125 A closely related
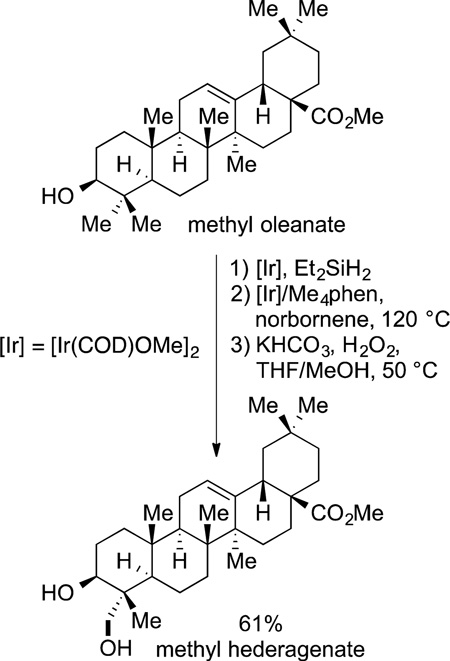 |
(74) |
sequence was conducted in prior studies in 10 steps, whereas the direct functionalization by silylation involves one set of operations to generate pure isolated product.198 Such chemistry now has been used to modify carbohydrates199 and other terpenes, in the latter case to improve their aqueous solubility.200
FUTURE PROSPECTS FOR C−H BOND FUNCTIONALIZATION
Given these accomplishments made in the field of C−H bond functionalization, what can one expect in the future to emerge from research on this topic? This Perspective closes with suggestions on the potential impact of C−H bond functionalization to many different applications.
Unresolved is whether the original goal of converting light alkanes to more valuable products will be accomplished and even should be investigated further. Two general views of the importance of the conversion of methane to higher alkanes exist in light of the newly created abundance of shale gas. In one view, the abundance of methane in shale gas makes the potential conversion of this lightest alkane to higher alkanes more important to accomplish than ever. By another view, shale gas contains more ethane than we could ever use for chemical synthesis (after dehydrogenation to ethylene), and this second view implies that the importance of methane conversion is limited to locations where methane is stranded and pipelines to transport the gas to a location where it would be a useful fuel are absent. The amount of stranded methane at these locations is on the order of trillions of cubic meters per year.201 For this reason and because of the potential that conversion of abundant methane to higher alkanes (perhaps via methanol) could be economically valuable, much investment202 has been made to commercialize new approaches to couple methane to form higher alkanes. Moreover, methods for anaerobic conversion of methane to aromatic hydrocarbons in zeolites are being investigated203 and could become economically feasible if the chemistry could be improved and routes to arenes from naphtha become limited and more costly.
Without doubt, the conversion of C2 and C3 alkanes to more valuable products could become reality. For example, the conversion of ethane to acetic acid could be feasible on large scale and would be more direct than the combination of converting coal to syngas and converting the syngas to acetic acid (although difficult to beat economically). Improvements in the dehydrogenation of propane to propene could also be made. This dehydrogenation is difficult to conduct selectively, in part because the allylic C−H bond in the product is much weaker than the C−H bonds in the alkane reactant.
The scope of potential applications of C−H bond functionalization to the synthesis of fine chemicals is even broader than has been achieved currently. Few C−H bond functionalization reactions occur with the tolerance for functional groups that would make it suitable for modification of complex structures without the installation of protective groups and directing groups. Thus, site-selective, undirected functionalization of unprotected complex molecules is one current major challenge; accomplishing these reactions with selectivity for one C−H bond over another with catalysts that override the inherent reactivity of C−H bonds due to their steric or electronic properties is an even greater challenge. The accomplishment of such goals would greatly facilitate the synthesis of analogs for studies on structure-reactivity relationships in medicinal chemistry and could create short total synthesis of important complex structures.
Few C−H bond functionalization reactions occur with the level of efficiency that allows for their use in a technical process. Of course, the requirements of catalyst loadings depend on the value of the final product. For a pharmaceutical, this value depends on dosage, patent status, and the application of the final compound. Moreover, the requirements for an application of catalysis to synthesize the active ingredient of a pharmaceutical under patent is much different from that to synthesize one that is generic, and the requirements for this application are still more forgiving than those for preparing an agrochemical. In any case, the typical catalyst loadings for C−H bond functionalization reactions, with a few exceptions,204 are much higher than those for the commonly conducted cross-coupling, hydrogenation, and carbonylation reactions. Such requirements can be alleviated by the development of catalysts based on Earth-abundant metals, rather than noble metals. However, the cost of the ligand is often greater than the cost of the metal, and the metal, not the ligand, in a process is typically recycled, thereby dampening the impact of the cost the metal used.
Finally, as noted in the introduction, a justification for C−H bond functionalization often focuses on the principles of sustainability and green chemistry. C−H bond functionalization reactions can be conducted without the need to install one or more functional groups. However, the claim of meeting the principles of green chemistry requires that the reactions lack directing groups that need to be installed and removed and that they lack additives, such as silver oxidants, cesium salts, and superstoichiometric amounts of metal additives, such as copper. Finally, the reagents used for functionalization cannot require multiple steps and cannot be prepared by halogenated intermediates or starting materials, such as iodoarenes, diazonium salts, and highly halogenated main group reagents, if arguments that the chemistry avoids halogenated reagents are to be valid.
Although academic researchers often focus on commodity chemicals and medicinally important compounds, the applications of new reaction technologies to agroscience can tremendously impact sustainability by affecting resource utilization. Many projections indicate that the food demand will increase multifold, while most of the world’s arable land is currently utilized. Expansion of arable land will have large detrimental impacts on the environment and emissions of greenhouse gases. Moreover water usage and water purity are major issues that are affected by agriculture. Thus, the yield of product per acre by improved agrochemicals discovered through synthetic chemistry can have a larger impact on the use of important resources than many of the problems receiving greater attention by synthetic chemists.
Finally, this Perspective showed that C−H bond functionalization is just beginning to be used as a synthetic tool for materials science. For C−H bond functionalization to enable step-growth polymerization, the yields of the C−H bond functionalization reactions must be higher than is typical for these reactions currently. Chain-growth polymerizations do not require the same yields as step-growth polymerizations to achieve high molecular weights, but chain-growth polymerization by C−H bond functionalization is difficult to envision. Yet, improvements in this methodology could lead to the ability to synthesize polymers by C−H bond functionalization. Perhaps more likely, C−H bond functionalization could enable the preparation of new monomers with appropriate efficiency to broaden the scope of the pool of monomers for commercial materials. In particular, it could provide practical access to heteroaromatic and complex aromatic monomers for specialty polymers, particularly aromatic and heteroaromatic monomers for electronic materials.
It is clear that the ability to develop catalytic reactions in general is nearly limitless. I hope the work described in this Perspective makes clear that many creative researchers have converted catalytic C−H bond functionalization from a curiosity to a reality. The field is progressing, but remains far from its goal of creating a wide range of reactions suitable for the practical synthesis of small and large, simple and complex molecules by C−H bond functionalization.
Acknowledgments
The NSF (1213409) and NIH (GM-58108 and GM-115812) are gratefully acknowledged for their support of our work on various projects involving C−H bond functionalization. I thank Dr. Ankit Sharma, Dr. Rashad Karimov Taegyo Lee, and Matthew Larsen for their critical comments on the manuscript.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Goldshleger NF, Tyabin MB, Shilov AE, Shteinman AA. Russ. J. Phys. Chem. USSR. 1969;43:1222. [Google Scholar]
- 2.Haynes CA, Gonzalez R. Nat. Chem. Biol. 2014;10:331. doi: 10.1038/nchembio.1509. [DOI] [PubMed] [Google Scholar]
- 3.Fei Q, Guarnieri MT, Tao L, Laurens LML, Dowe N, Pienkos PT. Biotechnol. Adv. 2014;32:596. doi: 10.1016/j.biotechadv.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Chang CD, Silvestri AJ. J. Catal. 1977;47:249. [Google Scholar]
- 5.Arndtsen BA, Bergman RG, Mobley TA, Peterson TH. Acc. Chem. Res. 1995;28:154. [Google Scholar]
- 6.Labinger JA, Bercaw JE. Nature. 2002;417:507. doi: 10.1038/417507a. [DOI] [PubMed] [Google Scholar]
- 7.Carey JS, Laffan D, Thomson C, Williams MT. Org. Biomol. Chem. 2006;4:2337. doi: 10.1039/b602413k. [DOI] [PubMed] [Google Scholar]
- 8.Dugger RW, Ragan JA, Ripin DHB. Org. Process Res. Dev. 2005;9:253. [Google Scholar]
- 9.Roughley SD, Jordan AM. J. Med. Chem. 2011;54:3451. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- 10.Renaud P, Sibi MP, editors. Radicals in Organic Synthesis. 1st. Vol. 1. Wiley-VCH: Weinheim; 2001. [Google Scholar]
- 11.Kochi JK, Mains HE. J. Org. Chem. 1965;30:1862. [Google Scholar]
- 12.Beckwith ALJ, Zavitsas AA. J. Am. Chem. Soc. 1986;108:8230. [Google Scholar]
- 13.Jang ES, McMullin CL, Kass M, Meyer K, Cundari TR, Warren TH. J. Am. Chem. Soc. 2014;136:10930. doi: 10.1021/ja5026547. [DOI] [PubMed] [Google Scholar]
- 14.Tran BL, Li B, Driess M, Hartwig JF. J. Am. Chem. Soc. 2014;136:2555. doi: 10.1021/ja411912p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clayden J, Greeves N, Warren S, Wothers P. Organic Chemistry. 1st. Oxford: Oxford University Press; 2000. [Google Scholar]
- 16.Chen H, Schlecht S, Semple TC, Hartwig JF. Science. 2000;287:1995. doi: 10.1126/science.287.5460.1995. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Hartwig JF. Angew. Chem., Int. Ed. 1999;38:3391. doi: 10.1002/(sici)1521-3773(19991115)38:22<3391::aid-anie3391>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Periana RA, Taube DJ, Gamble S, Taube H, Satoh T, Fujii H. Science. 1998;280:560. doi: 10.1126/science.280.5363.560. [DOI] [PubMed] [Google Scholar]
- 19.Ab Rahim MH, Forde MM, Jenkins RL, Hammond C, He Q, Dimitratos N, Lopez-Sanchez JA, Carley AF, Taylor SH, Willock DJ, Murphy DM, Kiely CJ, Hutchings G. J. Angew. Chem., Int. Ed. 2013;52:1280. doi: 10.1002/anie.201207717. [DOI] [PubMed] [Google Scholar]
- 20.Ellis PE, Lyons JE. Coord. Chem. Rev. 1990;105:181. [Google Scholar]
- 21.Luo YR. Handbook of Bond Dissociation Energies in Organic Compounds. Boca Raton: CRC Press; 2003. [Google Scholar]
- 22.Bordwell FG. Acc. Chem. Res. 1988;21:456. [Google Scholar]
- 23.Wender PA, Verma VA, Paxton TJ, Pillow TH. Acc. Chem. Res. 2008;41:40. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]
- 24.Ishiyama T, Takagi J, Ishida K, Miyaura N, Anastasi N, Hartwig JF. J. Am. Chem. Soc. 2002;124:390. doi: 10.1021/ja0173019. [DOI] [PubMed] [Google Scholar]
- 25.Kodama H, Katsuhira T, Nishida T, Hino T, Tsubata K. WO Patent Appl. Japan: Nihon Nohyaku Co. Ltd; 2001. PCT/JP2001/003707. [Google Scholar]
- 26.Wang X-C, Hu Y, Bonacorsi S, Hong Y, Burrell R, Yu J-Q. J. Am. Chem. Soc. 2013;135:10326. doi: 10.1021/ja4055492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartwig JF. Organotransition Metal Chemistry. Sausalito, CA: University Science Books; 2010. p. 857. [Google Scholar]
- 28.Goldberg KI, Goldman AS, editors. Activation and Functionalization of C-H Bonds. Vol. 885. Washington, DC: American Chemical Society; 2004. [Google Scholar]
- 29.Doyle MP, Goldberg KI. Acc. Chem. Res. 2012;45:777. doi: 10.1021/ar300096z. [DOI] [PubMed] [Google Scholar]
- 30.Davies HML, Du Bois J, Yu J-Q. Chem. Soc. Rev. 2011;40:1855. doi: 10.1039/c1cs90010b. [DOI] [PubMed] [Google Scholar]
- 31.Crabtree RH. Chem. Rev. 2010;110:575. doi: 10.1021/cr900388d. [DOI] [PubMed] [Google Scholar]
- 32.Shul’pin GB, Shilov AE. Activation and Catalytic Reactions of Saturated Hydrocarbons. Vol. 21. New York: Springer; 2000. [Google Scholar]
- 33.Goldshleger NF, Shteinman AA, Shilov AE, Eskova VV. Zh. Fiz. Khim. 1972;46:1353. [Google Scholar]
- 34.Goldshleger NF, Shteinman AA, Shilov AE, Eskova VV. Russ. J. Phys. Chem. USSR. 1972;46:785. [Google Scholar]
- 35.Moritanl I, Fujiwara Y. Tetrahedron Lett. 1967;8:1119. [Google Scholar]
- 36.Fujiwara Y, Moritani I, Matsuda M, Teranishi S. Tetrahedron Lett. 1968;9:633. [Google Scholar]
- 37.Fujiwara Y, Noritani I, Danno S, Asano R, Teranishi S. J. Am. Chem. Soc. 1969;91:7166. doi: 10.1021/ja01053a047. [DOI] [PubMed] [Google Scholar]
- 38.Fujiwara Y, Kawauchi T, Taniguchi H. J. Chem. Soc., Chem. Commun. 1980:220. [Google Scholar]
- 39.Jintoku T, Fujiwara Y, Kawata I, Kawauchi T, Taniguchi H. J. Organomet. Chem. 1990;385:297. [Google Scholar]
- 40.Taniguchi Y, Yamaoka Y, Nakata K, Takaki K, Fujiwara Y. Chem. Lett. 1995:345. [Google Scholar]
- 41.Fujiwara Y, Jintoku T, Uchida Y. New J. Chem. 1989;13:649. [Google Scholar]
- 42.Baudry D, Ephritikhine M, Felkin H, Zakrzewski J. J. Chem. Soc., Chem. Commun. 1982:1235. [Google Scholar]
- 43.Baudry D, Ephritikhine M, Felkin H, Holmes-Smith R. J. Chem. Soc., Chem. Commun. 1983:788. [Google Scholar]
- 44.Crabtree RH, Mellea MF, Mihelcic JM, Quirk JM. J. Am. Chem. Soc. 1982;104:107. [Google Scholar]
- 45.Burk MJ, Crabtree RH. J. Am. Chem. Soc. 1987;109:8025. [Google Scholar]
- 46.Anton DR, Crabtree RH. Organometallics. 1983;2:855. [Google Scholar]
- 47.Le Bras J, Muzart J. Chem. Rev. 2011;111:1170. doi: 10.1021/cr100209d. [DOI] [PubMed] [Google Scholar]
- 48.Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi J, Muto K, Itami K. Eur. J. Org. Chem. 2013;2013:19. [Google Scholar]
- 50.Liu F, Pak EB, Singh B, Jensen CM, Goldman AS. J. Am. Chem. Soc. 1999;121:4086. [Google Scholar]
- 51.Goldman AS, Roy AH, Huang Z, Ahuja R, Schinski W, Brookhart M. Science. 2006;312:257. doi: 10.1126/science.1123787. [DOI] [PubMed] [Google Scholar]
- 52.Vidal V, Theolier A, ThivolleCazat J, Basset JM. Science. 1997;276:99. doi: 10.1126/science.276.5309.99. [DOI] [PubMed] [Google Scholar]
- 53.Doyle MP, Duffy R, Ratnikov M, Zhou L. Chem. Rev. 2010;110:704. doi: 10.1021/cr900239n. [DOI] [PubMed] [Google Scholar]
- 54.Demonceau A, Noels AF, Hubert AJ, Teyssie P. J. Chem. Soc., Chem. Commun. 1981:688. [Google Scholar]
- 55.Demonceau A, Noels AF, Hubert AJ, Teyssie P. Bull. Soc. Chim. Belg. 1984;93:945. [Google Scholar]
- 56.Wenkert E, Davis LL, Mylari BL, Solomon MF, Da Silva RR, Shulman S, Warnet RJ, Ceccherelli P, Curini M, Pellicciari R. J. Org. Chem. 1982;47:3242. [Google Scholar]
- 57.Taber DF, Petty EH. J. Org. Chem. 1982;47:4808. [Google Scholar]
- 58.Burke SD, Grieco PA. Organic Reactions. New York: John Wiley & Sons, Inc; 2004. [Google Scholar]
- 59.Du Bois J. Org. Process Res. Dev. 2011;15:758. doi: 10.1021/op200046v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Musser MT. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH Verlag GmbH & Co; 2002. (Online) [Google Scholar]
- 61.Newhouse T, Baran PS. Angew. Chem., Int. Ed. 2011;50:3362. doi: 10.1002/anie.201006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hartwig JF. Organotransition Metal Chemistry. Sausalito, CA: University Science Books; 2010. Chapter 6: Oxidative Addition of non-Polar Reagents. [Google Scholar]
- 63.Crabtree RH, Mihelcic JM, Quirk JM. J. Am. Chem. Soc. 1979;101:7738. [Google Scholar]
- 64.Janowicz AH, Bergman RG. J. Am. Chem. Soc. 1982;104:352. [Google Scholar]
- 65.Hoyano JK, Graham WAG. J. Am. Chem. Soc. 1982;104:3723. [Google Scholar]
- 66.Jones WD, Feher F. J. Organometallics. 1983;2:562. [Google Scholar]
- 67.Watson PL. J. Am. Chem. Soc. 1983;105:6491. [Google Scholar]
- 68.Tolman CA, Ittel SD, English AD, Jesson JP. J. Am. Chem. Soc. 1979;101:1742. [Google Scholar]
- 69.Kleiman JP, Dubeck M. J. Am. Chem. Soc. 1963;85:1544. [Google Scholar]
- 70.Tulip TH, Thorn DL. J. Am. Chem. Soc. 1981;103:2448. [Google Scholar]
- 71.Musashi Y, Sakaki S. J. Am. Chem. Soc. 2002;124:7588. doi: 10.1021/ja020063c. [DOI] [PubMed] [Google Scholar]
- 72.Biswas B, Sugimoto M, Sakaki S. Organometallics. 2000;19:3895. [Google Scholar]
- 73.Davies DL, Donald SMA, Macgregor SA. J. Am. Chem. Soc. 2005;127:13754. doi: 10.1021/ja052047w. [DOI] [PubMed] [Google Scholar]
- 74.Gorelsky SI, Lapointe D, Fagnou K. J. Am. Chem. Soc. 2008;130:10848. doi: 10.1021/ja802533u. [DOI] [PubMed] [Google Scholar]
- 75.Kornecki KP, Briones JF, Boyarskikh V, Fullilove F, Autschbach J, Schrote KE, Lancaster KM, Davies HML, Berry JF. Science. 2013;342:351. doi: 10.1126/science.1243200. [DOI] [PubMed] [Google Scholar]
- 76.Nakamura E, Yoshikai N, Yamanaka M. J. Am. Chem. Soc. 2002;124:7181. doi: 10.1021/ja017823o. [DOI] [PubMed] [Google Scholar]
- 77.Hansen J, Autschbach J, Davies HML. J. Org. Chem. 2009;74:6555. doi: 10.1021/jo9009968. [DOI] [PubMed] [Google Scholar]
- 78.Davies HML, Beckwith RE. J. Chem. Rev. 2003;103:2861. doi: 10.1021/cr0200217. [DOI] [PubMed] [Google Scholar]
- 79.Groves JT, McClusky GA. J. Am. Chem. Soc. 1976;98:859. [Google Scholar]
- 80.Groves JT. J. Chem. Educ. 1985;62:928. [Google Scholar]
- 81.Groves JT. J. Inorg. Biochem. 2006;100:434. doi: 10.1016/j.jinorgbio.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 82.Shaik S, Cohen S, Wang Y, Chen H, Kumar D, Thiel W. Chem. Rev. 2010;110:949. doi: 10.1021/cr900121s. [DOI] [PubMed] [Google Scholar]
- 83.Walling C, Zavitsas AA. J. Am. Chem. Soc. 1963;85:2084. [Google Scholar]
- 84.Andrus MB, Argade AB, Chen X, Pamment MG. Tetrahedron Lett. 1995;36:2945. [Google Scholar]
- 85.Sharma A, Hartwig JF. Nature. 2015;517:600. doi: 10.1038/nature14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tran BL, Driess M, Hartwig JF. J. Am. Chem. Soc. 2014;136:17292. doi: 10.1021/ja510093x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murai S, Kakiuchi F, Sekine S, Tanaka Y, Kamatani A, Sonoda M, Chatani N. Nature. 1993;366:529. [Google Scholar]
- 88.Kakiuchi F, Murai S. Acc. Chem. Res. 2002;35:826. doi: 10.1021/ar960318p. [DOI] [PubMed] [Google Scholar]
- 89.Jun CH, Moon CW, Hong JB, Lim SG, Chung KY, Kim YH. Chem. - Eur. J. 2002;8:485. doi: 10.1002/1521-3765(20020118)8:2<485::AID-CHEM485>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 90.Colby DA, Tsai AS, Bergman RG, Ellman JA. Acc. Chem. Res. 2012;45:814. doi: 10.1021/ar200190g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fahey DR. J. Chem. Soc. D. 1970:417. [Google Scholar]
- 92.Fahey DR. J. Organomet. Chem. 1971;27:283. [Google Scholar]
- 93.Andrienko OS, Goncharov VS, Raida VS. Russ. J. Org. Chem. 1996;32:89. [Google Scholar]
- 94.Dick AR, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:2300. doi: 10.1021/ja031543m. [DOI] [PubMed] [Google Scholar]
- 95.Kalyani D, Dick AR, Anani WQ, Sanford MS. Org. Lett. 2006;8:2523. doi: 10.1021/ol060747f. [DOI] [PubMed] [Google Scholar]
- 96.Giri R, Chen X, Yu JQ. Angew. Chem., Int. Ed. 2005;44:2112. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]
- 97.Giri R, Liang J, Lei JG, Li JJ, Wang DH, Chen X, Naggar IC, Guo CY, Foxman BM, Yu JQ. Angew. Chem., Int. Ed. 2005;44:7420. doi: 10.1002/anie.200502767. [DOI] [PubMed] [Google Scholar]
- 98.Chan KSL, Fu H-Y, Yu J-Q. J. Am. Chem. Soc. 2015;137:2042. doi: 10.1021/ja512529e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao K-J, Lin DW, Miura M, Zhu R-Y, Gong W, Wasa M, Yu J-Q. J. Am. Chem. Soc. 2014;136:8138. doi: 10.1021/ja504196j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wasa M, Engle KM, Lin DW, Yoo EJ, Yu J-Q. J. Am. Chem. Soc. 2011;133:19598. doi: 10.1021/ja207607s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hartung CG, Snieckus V. The Directed Ortho Metalation Reaction. A Point of Departure for New Synthetic Aromatic Chemistry. New York: Wiley-VCH; 2002. [Google Scholar]
- 102.Lyons TW, Sanford MS. Chem. Rev. 2010;110:1147. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen X, Engle KM, Wang DH, Yu JQ. Angew. Chem., Int. Ed. 2009;48:5094. doi: 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Daugulis O, Do H-Q, Shabashov D. Acc. Chem. Res. 2009;42:1074. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shabashov D, Daugulis O. J. Am. Chem. Soc. 2010;132:3965. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leow D, Li G, Mei TS, Yu JQ. Nature. 2012;486:518. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang RY, Li G, Yu JQ. Nature. 2014;507:215. doi: 10.1038/nature12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem. Rev. 2010;110:890. doi: 10.1021/cr900206p. [DOI] [PubMed] [Google Scholar]
- 109.Chotana GA, Rak MA, Smith MI. J. Am. Chem. Soc. 2005;127:10539. doi: 10.1021/ja0428309. [DOI] [PubMed] [Google Scholar]
- 110.Hartwig JF. Chem. Soc. Rev. 2011;40:1992. doi: 10.1039/c0cs00156b. [DOI] [PubMed] [Google Scholar]
- 111.Larsen MA, Hartwig JF. J. Am. Chem. Soc. 2014;136:4287. doi: 10.1021/ja412563e. [DOI] [PubMed] [Google Scholar]
- 112.Kallepalli VA, Sanchez L, Li H, Gesmundo NJ, Turton CL, Maleczka RE, Smith MRI. Heterocycles. 2010;80:1429. [Google Scholar]
- 113.Kallepalli VA, Shi F, Paul S, Onyeozili EN, Maleczka RE, Smith MR. J. Org. Chem. 2009;74:9199. doi: 10.1021/jo901822b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liskey CW, Hartwig JF. J. Am. Chem. Soc. 2013;135:3375. doi: 10.1021/ja400103p. [DOI] [PubMed] [Google Scholar]
- 115.Liskey CW, Hartwig JF. J. Am. Chem. Soc. 2012;134:12422. doi: 10.1021/ja305596v. [DOI] [PubMed] [Google Scholar]
- 116.Ishiyama T, Takagi J, Hartwig JF, Miyaura N. Angew. Chem., Int. Ed. 2002;41:3056. doi: 10.1002/1521-3773(20020816)41:16<3056::AID-ANIE3056>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 117.Ishiyama T, Takagi J, Ishida K, Miyaura N, Anastasi NR, Hartwig JF. J. Am. Chem. Soc. 2002;124:390. doi: 10.1021/ja0173019. [DOI] [PubMed] [Google Scholar]
- 118.Maleczka RE, Shi F, Holmes D, Smith MRI. J. Am. Chem. Soc. 2003;125:7792. doi: 10.1021/ja0349857. [DOI] [PubMed] [Google Scholar]
- 119.Tzschucke CC, Murphy JM, Hartwig JF. Org. Lett. 2007;9:761. doi: 10.1021/ol062902w. [DOI] [PubMed] [Google Scholar]
- 120.Liskey CW, Liao X, Hartwig JF. J. Am. Chem. Soc. 2010;132:11389. doi: 10.1021/ja104442v. [DOI] [PubMed] [Google Scholar]
- 121.Fier PS, Luo J, Hartwig JF. J. Am. Chem. Soc. 2013;135:2552. doi: 10.1021/ja310909q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheng C, Hartwig JF. Chem. Rev. 2015;115:8946. doi: 10.1021/cr5006414. [DOI] [PubMed] [Google Scholar]
- 123.Cheng C, Hartwig JF. Science. 2014;343:853. doi: 10.1126/science.1248042. [DOI] [PubMed] [Google Scholar]
- 124.Cheng C, Simmons EM, Hartwig JF. Angew. Chem., Int. Ed. 2013;52:8984. doi: 10.1002/anie.201304084. [DOI] [PubMed] [Google Scholar]
- 125.Simmons EM, Hartwig JF. Nature. 2012;483:70. doi: 10.1038/nature10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng C, Hartwig JF. J. Am. Chem. Soc. 2015;137:592. doi: 10.1021/ja511352u. [DOI] [PubMed] [Google Scholar]
- 127.Cook AK, Emmert MH, Sanford MS. Org. Lett. 2013;15:5428. doi: 10.1021/ol4024248. [DOI] [PubMed] [Google Scholar]
- 128.Hull KL, Sanford MS. J. Am. Chem. Soc. 2007;129:11904. doi: 10.1021/ja074395z. [DOI] [PubMed] [Google Scholar]
- 129.Dams M, De Vos DE, Celen S, Jacobs PA. Angew. Chem., Int. Ed. 2003;42:3512. doi: 10.1002/anie.200351524. [DOI] [PubMed] [Google Scholar]
- 130.Zhang YH, Shi BF, Yu JQ. J. Am. Chem. Soc. 2009;131:5072. doi: 10.1021/ja900327e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miura M, Nomura M. Top. Curr. Chem. 2002;219:211. [Google Scholar]
- 132.Liégault B, Lapointe D, Caron L, Vlassova A, Fagnou K. J. Org. Chem. 2009;74:1826. doi: 10.1021/jo8026565. [DOI] [PubMed] [Google Scholar]
- 133.Lapointe D, Markiewicz T, Whipp CJ, Toderian A, Fagnou K. J. Org. Chem. 2011;76:749. doi: 10.1021/jo102081a. [DOI] [PubMed] [Google Scholar]
- 134.Kallepalli VA, Shi F, Paul S, Onyeozili EN, Maleczka RE, Smith MRI. J. Org. Chem. 2009;74:9199. doi: 10.1021/jo901822b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kawamorita S, Ohmiya H, Sawamura M. J. Org. Chem. 2010;75:3855. doi: 10.1021/jo100352b. [DOI] [PubMed] [Google Scholar]
- 136.Takagi J, Sato K, Hartwig JF, Ishiyama T, Miyaura N. Tetrahedron Lett. 2002;43:5649. [Google Scholar]
- 137.Ishiyama T, Nobuta Y, Hartwig JF, Miyaura N. Chem. Commun. 2003:2924. doi: 10.1039/b311103b. [DOI] [PubMed] [Google Scholar]
- 138.Ishiyama T, Takagi J, Yonekawa Y, Hartwig JF, Miyaura N. Adv. Synth. Catal. 2003;345:1103. [Google Scholar]
- 139.Vanchura BA, II, Preshlock SM, Roosen PC, Kallepalli VA, Staples RJ, Maleczka RE, Jr, Singleton DA, Smith MR., III Chem. Commun. 2010;46:7724. doi: 10.1039/c0cc02041a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Preshlock SM, Plattner DL, Maligres PE, Krska SW, Maleczka RE, Smith MRI. Angew. Chem., Int. Ed. 2013;52:12915. doi: 10.1002/anie.201306511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen MS, White MC. Science. 2007;318:783. doi: 10.1126/science.1148597. [DOI] [PubMed] [Google Scholar]
- 142.White MC. Science. 2012;335:807. doi: 10.1126/science.1207661. [DOI] [PubMed] [Google Scholar]
- 143.Liu W, Groves JT. Acc. Chem. Res. 2015;48:1727. doi: 10.1021/acs.accounts.5b00062. [DOI] [PubMed] [Google Scholar]
- 144.Schmidt VA, Quinn RK, Brusoe AT, Alexanian EJ. J. Am. Chem. Soc. 2014;136:14389. doi: 10.1021/ja508469u. [DOI] [PubMed] [Google Scholar]
- 145.Davies HML, Matasi JJ, Hodges LM, Huby NJS, Thornley C, Kong N, Houser JH. J. Org. Chem. 1997;62:1095. [Google Scholar]
- 146.Davies HML, Hansen T, Hopper DW, Panaro SA. J. Am. Chem. Soc. 1999;121:6509. [Google Scholar]
- 147.Davies HML, Hansen T, Churchill MR. J. Am. Chem. Soc. 2000;122:3063. [Google Scholar]
- 148.Fiori KW, Espino CG, Brodsky BH, Du Bois J. Tetrahedron. 2009;65:3042. [Google Scholar]
- 149.Harvey ME, Musaev DG, Du Bois J. J. Am. Chem. Soc. 2011;133:17207. doi: 10.1021/ja203576p. [DOI] [PubMed] [Google Scholar]
- 150.Espino CG, Du Bois J. Angew. Chem., Int. Ed. 2001;40:598. doi: 10.1002/1521-3773(20010202)40:3<598::AID-ANIE598>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 151.Fiori KW, Du Bois J. J. Am. Chem. Soc. 2007;129:562. doi: 10.1021/ja0650450. [DOI] [PubMed] [Google Scholar]
- 152.Reger DL, Culbertson EC. Inorg. Chem. 1977;16:3104. [Google Scholar]
- 153.Buchanan JM, Stryker JM, Bergman RG. J. Am. Chem. Soc. 1986;108:1537. [Google Scholar]
- 154.Harvey JN. Organometallics. 2001;20:4887. [Google Scholar]
- 155.Murphy JM, Lawrence JD, Kawamura K, Incarvito C, Hartwig JF. J. Am. Chem. Soc. 2006;128:13684. doi: 10.1021/ja064092p. [DOI] [PubMed] [Google Scholar]
- 156.Li Q, Liskey CW, Hartwig JF. J. Am. Chem. Soc. 2014;136:8755. doi: 10.1021/ja503676d. [DOI] [PubMed] [Google Scholar]
- 157.Li BJ, Driess M, Hartwig JF. J. Am. Chem. Soc. 2014;136:6586. doi: 10.1021/ja5026479. [DOI] [PubMed] [Google Scholar]
- 158.Desai LV, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:9542. doi: 10.1021/ja046831c. [DOI] [PubMed] [Google Scholar]
- 159.Yamaguchi J, Yamaguchi AD, Itami K. Angew. Chem., Int. Ed. 2012;51:8960. doi: 10.1002/anie.201201666. [DOI] [PubMed] [Google Scholar]
- 160.Hinman A, Du Bois J. J. Am. Chem. Soc. 2003;125:11510. doi: 10.1021/ja0368305. [DOI] [PubMed] [Google Scholar]
- 161.Baran PS, Corey EJ. J. Am. Chem. Soc. 2002;124:7904. doi: 10.1021/ja026663t. [DOI] [PubMed] [Google Scholar]
- 162.Tsai AS, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2008;130:6316. doi: 10.1021/ja8012159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mandal D, Yamaguchi AD, Yamaguchi J, Itami K. J. Am. Chem. Soc. 2011;133:19660. doi: 10.1021/ja209945x. [DOI] [PubMed] [Google Scholar]
- 164.Wencel-Delord J, Glorius F. Nat. Chem. 2013;5:369. doi: 10.1038/nchem.1607. [DOI] [PubMed] [Google Scholar]
- 165.Gutekunst WR, Baran PS. Chem. Soc. Rev. 2011;40:1976. doi: 10.1039/c0cs00182a. [DOI] [PubMed] [Google Scholar]
- 166.Kakiuchi F, Chatani N. Adv. Synth. Catal. 2003;345:1077. [Google Scholar]
- 167.Taber DF, Schuchardt JL. J. Am. Chem. Soc. 1985;107:5289. [Google Scholar]
- 168.Cane DE, Thomas PJ. J. Am. Chem. Soc. 1984;106:5295. [Google Scholar]
- 169.Davies HML, Manning JR. Nature. 2008;451:417. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Davies HML, Dick AR. Topics in Current Chemistry. In: Yu JQ, Shi Z, editors. C-H Activation. Vol. 292. Berlin/Heidelberg: Springer-Verlag; 2009. p. 303. [Google Scholar]
- 171.Davies HML, Dai X, Long MS. J. Am. Chem. Soc. 2006;128:2485. doi: 10.1021/ja056877l. [DOI] [PubMed] [Google Scholar]
- 172.Fleming JJ, Du Bois J. J. Am. Chem. Soc. 2006;128:3926. doi: 10.1021/ja0608545. [DOI] [PubMed] [Google Scholar]
- 173.Hartwig JF. Organotransition Metal Chemistry. Sausalito, CA: University Science Books; 2010. p. 825. [Google Scholar]
- 174.Johnson JA, Sames D. J. Am. Chem. Soc. 2000;122:6321. [Google Scholar]
- 175.Johnson JA, Li N, Sames D. J. Am. Chem. Soc. 2002;124:6900. doi: 10.1021/ja026130k. [DOI] [PubMed] [Google Scholar]
- 176.Feng Y, Chen G. Angew. Chem., Int. Ed. 2010;49:958. doi: 10.1002/anie.200905134. [DOI] [PubMed] [Google Scholar]
- 177.Baran PS, Corey EJ. J. Am. Chem. Soc. 2002;124:7904. doi: 10.1021/ja026663t. [DOI] [PubMed] [Google Scholar]
- 178.Hutchison AJ, Kishi Y. J. Am. Chem. Soc. 1979;101:6786. [Google Scholar]
- 179.Wang J, Rosingana M, Watson DJ, Dowdy ED, Discordia RP, Soundarajan N, Li W-S. Tetrahedron Lett. 2001;42:8935. [Google Scholar]
- 180.Boger DL, Patel M. J. Org. Chem. 1988;53:1405. [Google Scholar]
- 181.Gauthier DR, Limanto J, Devine PN, Desmond RA, Szumigala RH, Foster BS, Volante RP. J. Org. Chem. 2005;70:5938. doi: 10.1021/jo0507035. [DOI] [PubMed] [Google Scholar]
- 182.Caron L, Campeau L-C, Fagnou K. Org. Lett. 2008;10:4533. doi: 10.1021/ol801839w. [DOI] [PubMed] [Google Scholar]
- 183.Ackermann L, Vicente R. Top. Curr. Chem. 2009;292:211. doi: 10.1007/128_2009_9. [DOI] [PubMed] [Google Scholar]
- 184.Ouellet SG, Roy A, Molinaro C, Angelaud R, Marcoux J-F, O’Shea PD, Davies IW. J. Org. Chem. 2011;76:1436. doi: 10.1021/jo1018574. [DOI] [PubMed] [Google Scholar]
- 185.Seki M, Nagahama M. J. Org. Chem. 2011;76:10198. doi: 10.1021/jo202041e. [DOI] [PubMed] [Google Scholar]
- 186.Garg NK, Sarpong R, Stoltz BM. J. Am. Chem. Soc. 2002;124:13179. doi: 10.1021/ja027822b. [DOI] [PubMed] [Google Scholar]
- 187.O’Malley SJ, Tan KL, Watzke A, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2005;127:13496. doi: 10.1021/ja052680h. [DOI] [PubMed] [Google Scholar]
- 188.Beck EM, Hatley R, Gaunt MJ. Angew. Chem., Int. Ed. 2008;47:3004. doi: 10.1002/anie.200705005. [DOI] [PubMed] [Google Scholar]
- 189.Liao X, Stanley LM, Hartwig JF. J. Am. Chem. Soc. 2011;133:2088. doi: 10.1021/ja110215b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tomita D, Yamatsugu K, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2009;131:6946. doi: 10.1021/ja901995a. [DOI] [PubMed] [Google Scholar]
- 191.Wang C, Sperry J. J. Org. Chem. 2012;77:2584. doi: 10.1021/jo300330u. [DOI] [PubMed] [Google Scholar]
- 192.Wang Q, Takita R, Kikuzaki Y, Ozawa F. J. Am. Chem. Soc. 2010;132:11420. doi: 10.1021/ja105767z. [DOI] [PubMed] [Google Scholar]
- 193.Kondo Y, Garcia-Cuadrado D, Hartwig JF, Boaen NK, Wagner NL, Hillmyer MA. J. Am. Chem. Soc. 2002;124:1164. doi: 10.1021/ja016763j. [DOI] [PubMed] [Google Scholar]
- 194.Bae C, Hartwig JF, Boaen Harris NK, Long RO, Anderson KS, Hillmyer MA. J. Am. Chem. Soc. 2005;127:767. doi: 10.1021/ja044440s. [DOI] [PubMed] [Google Scholar]
- 195.Bae C, Hartwig JF, Chung H, Harris NK, Switek KA, Hillmyer MA. Angew. Chem., Int. Ed. 2005;44:6410. doi: 10.1002/anie.200501837. [DOI] [PubMed] [Google Scholar]
- 196.Shin J, Jensen SM, Ju J, Lee S, Xue Z, Noh SK, Bae C. Macromolecules. 2007;40:8600. [Google Scholar]
- 197.Huang X, Bergsten TM, Groves JT. J. Am. Chem. Soc. 2015;137:5300. doi: 10.1021/jacs.5b01983. [DOI] [PubMed] [Google Scholar]
- 198.García-Granados A, López PE, Melguizo E, Parra A, Simeó Y. J. Org. Chem. 2007;72:3500. doi: 10.1021/jo070116e. [DOI] [PubMed] [Google Scholar]
- 199.Frihed TG, Heuckendorff M, Pedersen CM, Bols M. Angew. Chem., Int. Ed. 2012;51:12285. doi: 10.1002/anie.201206880. [DOI] [PubMed] [Google Scholar]
- 200.Michaudel Q, Journot G, Regueiro-Ren A, Goswami A, Guo Z, Tully TP, Zou L, Ramabhadran RO, Houk KN, Baran PS. Angew. Chem. 2014;126:12287. doi: 10.1002/anie.201407016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.World LNG/GTL Review. Houston, TX: Zeus Development Inc; 2001. [Google Scholar]
- 202.Bullis K. Chasing the Dream of Half-Price Gasoline from Natural Gas. MIT Technology Review. 2014 Jan 15; http://www.technologyreview.com/news/523146/chasing-the-dream-of-half-price-gasoline-from-natural-gas/
- 203.Spivey JJ, Hutchings G. Chem. Soc. Rev. 2014;43:792. doi: 10.1039/c3cs60259a. [DOI] [PubMed] [Google Scholar]
- 204.Boller TM, Murphy JM, Hapke M, Ishiyama T, Miyaura N, Hartwig JF. J. Am. Chem. Soc. 2005;127:14263. doi: 10.1021/ja053433g. [DOI] [PubMed] [Google Scholar]