Abstract
Background
The development of more effective anti-tuberculosis vaccines would contribute to the control of the global problem of infection with Mycobacterium tuberculosis (MTB). Recently, increasing evidences showed that HIV-Tat protein transduction domain is implicated in promotion of vaccines by inducing cellular immuno-response. However, it is rare known about the role of TAT in vaccines against MTB.
Methods
In this study, we expressed recombinant protein-fused Ag85B with TAT (TAT-Ag85B) which was used as a vaccine to inoculate mice infected with MTB.
Results
As s result, both IgG2a in serum and IFN-γ or TNFα produced by spleen cells were all increased significantly in the mice inoculated by TAT-Ag85B. Furthermore, consistently, TAT-Ag85B inoculation significantly reduced MTB loads both in lung and spleen.
Conclusions
These findings demonstrate that a novel protein vaccine of TAT-Ag85B enhances immune response both in humoral and cellular immunity, and contributes to protective efficacy against MTB.
Keywords: TAT, Protein transduction domain, Mycobacterium tuberculosis, Ag85B, Protective immunity
Introduction
Mycobacterium tuberculosis (MTB) infection remains a major cause of morbidity and mortality throughout the world, with 9 million new cases of tuberculosis (TB) in 2011 resulting in 1.4 million deaths.1 The current vaccine, Mycobacterium bovis bacille Calmette–Guérin (BCG), has failed to prevent the adult pulmonary TB epidemic with a wide range of efficacy.2 Hence, more effective vaccines are urgently needed for disease prevention.
HIV-TAT peptide is a protein transduction domain (PTD) that can efficiently deliver macromolecules including 20–200-kDa proteins and peptides into cells.3–5 The TAT peptide is composed of an 11-amino acid peptide (TAT47-57: YGRKKRRQRRR) which can traverse most biomembranes either alone or fused with other proteins or peptides.6 A large body of evidence provides the fact that Tat can enhance immune responses by promoting macrophage-derived dendritic cell maturation and their antigen-presenting properties.7,8 However, there is no research using the TAT peptide to enhance immune responses against M. tuberculosis infection.
Ag85B, a 30-kDa fibronectin-binding protein, is a major protein secreted by all Mycobacterium species and was shown to induce protective responses to M. tuberculosis challenge.9 A number of studies have demonstrated a significant protective effect in the lungs of mice immunized with Ag85B.10 A recombinant M. bovis BCG overexpressing Ag85B also showed more potent immune responses and more enduring protection against M. tuberculosis infection than BCG as a vaccine.11 However, little is known about the ability of TAT-fused Ag85B to induce protective immunity against TB.
Our previous study has indicated that T-bet is an efficacious Th1-inducing adjuvant in the context of the Ag85B DNA-based vaccination against tuberculosis.12 T-bet is a member of the T-box family of transcription factors, which is a key controller of Th1 differentiation by activating the hallmark Th1 cytokine, IFN-γ.13 As the infection with MTB results in a decreased T helper (Th)1-type immune response, accompanied by an increased Th2 response,14 T-bet can polarize Th1 immune response and inhibit MTB replication.
In this study, we investigated whether a TAT-Ag85B fusion protein as a vaccine alone was able to enhance Ag85B-specific immune responses and anti-tuberculosis protection in mice. The result showed that the TAT-Ag85B exhibited a dramatic increase in Ag85B-specific Th1 responses and an impressive anti-tuberculosis effect.
Materials and Methods
Plasmids and animals
As previously described, the virulent MTB strain H37Rv, the pcDNA3.1-FLAG-T-bet were prepared.12 To construct pET28a-Ag85B, the Ag85B gene was amplified from the genomic DNA of H37Rv by PCR with specific primers (sense – cgggaattcatgacagacgtgagccgaaag; antisense – aatgtcgacgccggcgcctaacgaac), then the PCR product was subsequently cloned into the pET28a vectors. Similarly, to construct pET28a-TAT-Ag85B, two synthesized TAT47-57 oligonucleotides (sense – ctagcggctacggccgcaagaaacgccgccagcgccgccgcggtg antisense – gatccaccgcggcggcgctggcggcgtttcttgcggccgtagccg) were obtained and annealed to generate a double-stranded oligonucleotide encoding 11 amino acids (YGRKKRRQRRR) of TAT47-57. The products were subcloned into the pET28a-Ag85B plasmid. These primers were synthesized by Sangon Biotech Company. Female Balb/c mice (6–8 weeks old) were purchased from the animal centre of Anhui University of Science and Technology and raised carefully in accordance with the National Institutes of Health guidelines on animal care. All experimental procedures were approved by the Animal Care and Use Committee of Anhui University of Science and Technology (Permit numbers: AUST 2012-0032).
Expression and purification of fusion proteins
The plasmids of pET28a-Ag85B, pET28a-TAT-Ag85B were transformed into E. coli BL21 (DE3) cells to express fusion proteins. The BL21 were grown in Luria–Bertani broth containing 100 μg/mL kanamycin. The culture was then shake-incubated (37 °C, 250 rpm) in a 1-L flask. When the OD600 of the culture reached 0.6–0.7, the fusion protein was induced at a temperature of 28 °C for 8 h with 0.5 mmol/L isopropyl β-D-thiogalactopyranoside (IPTG). Cells were harvested and disrupted by sonication. The lysate was centrifuged at 12000 × g at 4 °C for 30 min and inclusion bodies were collected for further purification. After centrifugation, the inclusion bodies were washed in a 10-mL wash buffer A (50 mmol/L Tris, 1 mmol/L EDTA, 100 mmol/L Nacl, 2 mol/L urea, 0.5%(V/V) TritonX-100) for two times, and then washed in a wash buffer B (50 mmol/L Tris, 1 mmol/L EDTA, 100 mmol/L NaCl, 4 mol/L urea). Subsequently, the inclusion bodies were lysed in a 5-mL lysis buffer (58 mmol/L Na2HPO4, 17 mmol/L NaH2PO4, 68 mmol/L NaCl) containing 8-mol urea and the fusion proteins were purified on a Ni-NTA superflow chromatography column (Qiagen). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to detect expression of the target proteins. Western blot was used to confirm expression of the recombinant TAT-Ag85B proteins by mouse anti-His tag antibody (Sigma). Protein concentrations were estimated via Bradford’s method using bovine serum albumin as a standard. Ag85B protein as control protein was purified from pET28a-Ag85B expression vector with same protocol for TAT-Ag85B.
SDS-PAGE and immunoblotting
Samples were boiled for 5 min in the presence of 4 × SDS-PAGE-loading buffer (250 mmol/L Tris, pH 6.8, 40% glycerol, 8% SDS, 0.57 mol/L β-mercaptoethanol, 0.12% bromophenol blue). Equal amounts of protein were run on 12% SDS-PAGE gels and transferred onto a PVDF membrane. The membrane was blocked 3 h at room temperature in 5% milk in PBS/Tween 20 (0.1%) and then probed with anti-His tag antibodies (Protein Technology Group) overnight at 4 °C. After washing with PBST, the membrane was probed with appropriate HRP-conjugated goat anti-rabbit IgG (Protein Technology Group) for 1 h at 37 °C. Finally, primary antibodies were visualized with the enhanced chemiluminescence.
Animal immunization
Female Balb/c mice 6–8 week of age were used for vaccination and further infection. At first, they were randomly divided into three groups: PBS, Ag85B and TAT-Ag85B. Five mice per group were injected intramuscularly three times with aluminum hydroxide-adjuvant TAT-Ag85B (50 μg) or Ag85B (50 μg) and equal column(50 μL) PBS, at 2 weeks apart.
Challenge with M. tuberculosis
Three months after the final immunization, different groups of mice were challenged intravenously at the tail vein with 1 × 105 CFU of MTB H37Rv strain. The mice spleens and lungs were dissected out after two months postinfection. The spleen and lung tissues were homogenized and subjected to serial dilutions. An aliquot was plated on solid Middlebrook 7H10 agar plates and incubated at 37 °C for 4 weeks. The colonies were counted and the results were expressed as log10CFU.
Anti-Ag85B IgG assay
The titers of anti-Ag85B IgG and IgG2a antibodies in the sera from immunized mice were detected by ELISA using recombinant Ag85B, as previously described.12
Cytokine release assay
Five months after the last immunization, the mice were sacrificed and their splenic lymphocytes were prepared by a routine method. Splenocytes were stimulated in duplicate with 10 μg/mL rAg85B in 24-well plates for each mouse. After 72 h, culture supernatants were harvested and detected for IFN-γ, TNFα, IL-10 and IL-4 levels using ELISA kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions, as previously described.15
Acid-fast staining of lung slices
The histological sections were primarily stained with Carbol-fuchsin solution by heating on fire for 5 min. After natural cooling, destaining with 5% hydrochloric acid and restaining with 0.3% alkaline methylene blue solution. Finally, they were dehydrated and mounted as usually.9
Statistics
Data are presented as the means ± SD. The unpaired two-tailed Student’s t-test was used to determine significant differences between two groups. Differences were considered statistically significant if p < 0.05.
Results
Identification of recombinant TAT-Ag85B proteins
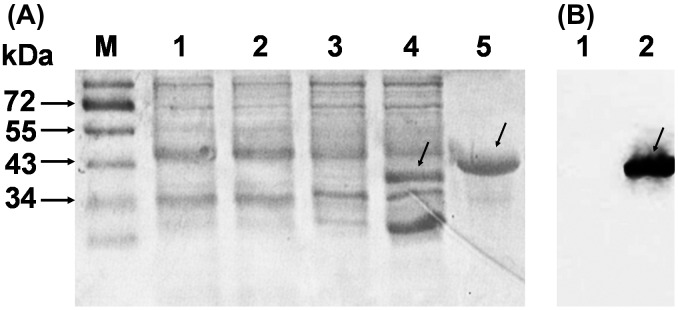
DNA sequencing and enzyme digestion confirmed the successful constructions. E. coli BL21 (DE3) strains habouring plasmid pET28a-Ag85B or pET28a-TAT-Ag85B were induced with IPTG and purified on a Ni-NTA column, respectively. The final purified products of recombinant TAT-Ag85B (40 kDa) protein were identified by 12% SDS-PAGE as expected molecular weight (Figure 1A). Furthermore, E. coli-expressed histidine-tagged TAT-Ag 85B protein was identified using immunoblot analysis with anti-His antibody (Figure 1B).
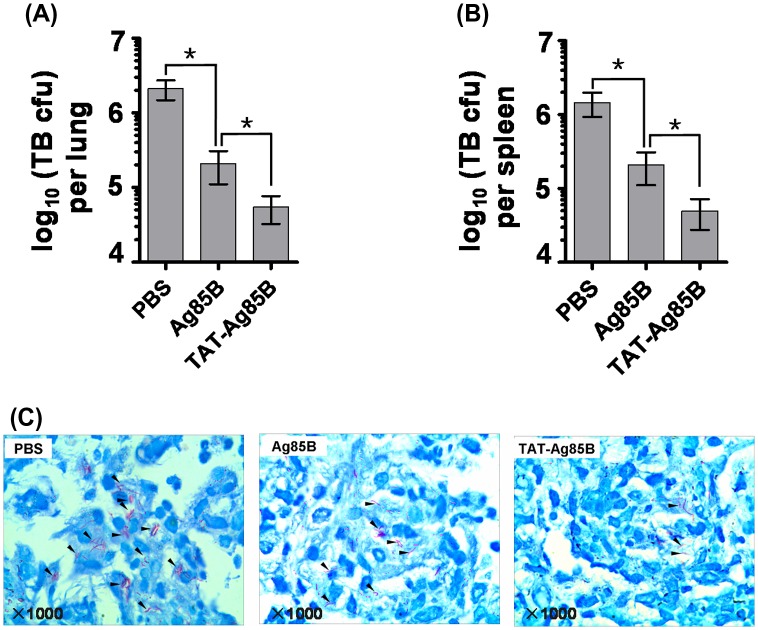
Figure 3.
The protection in the lung and spleen by vaccination following M. tuberculosis H37Rv infection.
Mice were immunized with PBS, Ag85B and TAT-Ag85B three times 2 weeks apart. Three months later, the immunized mice were challenged intravenously with 1 × 105 CFU M. tuberculosis H37Rv bacilli. At week 24, the bacterial loads (CFU ± SEM) were determined in the lung A or spleen B. Furthermore, M. tuberculosis in lung slice was detected by acid-fast staining as indicated by black arrowheads, 20 fields per slice were examined C. The differences of the bacterial growth between two groups of immunized mice were analysed by non-parametric Mann–Whitney test. Data, means ± SD (*p < 0.05).
TAT-Ag85B enhances Ag85B-specific antibody response and stimulates the secretion of cytokines
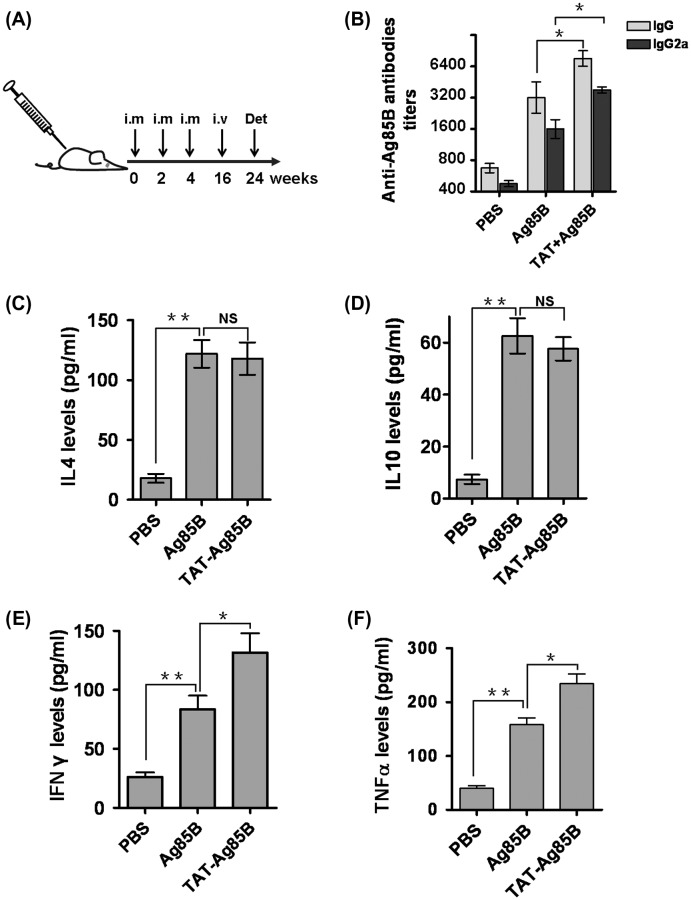
After 8 weeks postinfection, mice were sacrificed as described in Figure 2A. Five months after the final vaccination, TAT-Ag85B group mice still expressed high levels of specific IgG- or IgG2a-antibodies, indicating an activation of immuno-memory induced by TAT-Ag85B. Compared to Ag85B vaccination, higher Ig2a was found in TAT-Ag85B group, suggesting a Th1-dominant immunity. To clarify it, we detected the Th1-type cytokine (IFN-γ and TNFα) and Th2-type cytokine (IL-4 and IL-10) produced by spleen cells. As shown in Figure 2C–F, after Ag85B stimulation, significant increase in IFN-γ and TNFα was found in TAT-Ag85-vaccinated mice. Whereas, the levels of IL-4 or IL-10 in TAT-Ag85B group did not differ significantly from Ag85B-vaccinated mice. The higher levels of IFN-γ and TNFα demonstrate that TAT-Ag85B induces Th1-dominant responses, confirming the elevated IgG2a in Figure 2B.
Figure 2.
Immunization, antibody titer and cytokine production.
Female Balb/c mice 6–8 week of age were randomly assigned into three groups for different immunization regimens: PBS, Ag85B and TAT-Ag85B. A Five mice per group were immunized intramuscularly (i.m) three times at 2-week intervals, then injected through tail vein (i.v) with MTB at week 16, eventually sacrificed for many detections (Det) at week 24. B The level of anti-Ag85B IgG and IgG2a in sera was determined by ELISA. Sera from pre-immunized mice were used as a negative control. C–F For detecting cytokines responded to vaccination, 106 splenocytes from vaccinated mice were stimulated in vitro with Ag85B for 72 h and the supernatants were harvested for IFN-γ, TNFα, IL-4 and IL-10 determination using ELISA. All independent experiments were performed in triplicate, data are presented as the means ± SD (*p < 0.05, **p < 0.01).
The protection of TAT-Ag85B on bacterial load
To determine the protection of mycobacterial vaccines in lung and spleen against MTB H37Rv infection, we examined the replication of infected MTB in vivo. After vaccination, mice were challenged with MTB H37Rv in week 16, and the numbers of MTB loads in the spleens and lungs were determined by in vitro colony-formation assays. As shown in Figure 3, two months after challenge, the MTB loads were detected. Both in lungs and spleens, a significant reduction of bacterial loads was found in TAT-Ag85B-vaccinated mice compared to Ag85B vaccination. The repression of MTB loads indicated the superior potency of TAT-Ag85B in initiating protective immunity against MTB infection. Subsequently, we also compared the levels of MTB distributed in lung and found that Acid-fast Mycobacterium significantly decreased in TAT-Ag85B group compared to Ag85B group (Figure 3B).
Figure 1.
Identification of TAT-Ag85B.
Purification of the protein A. 1: E. coli BL21 containing pet-28a; 2: IPTG-induced BL21 containing pet-28a; 3: E. coli BL21 containing pet-TAT-Ag85B; 4: IPTG-induced E. coli BL21 containing pet-TAT-Ag85B; 5: purified TAT-Ag85B using Nickel affinity column; M: protein markers. Immunoblot analysis of TAT-Ag85B using anti-histidine antibody B. 1: IPTG-induced E. coli BL21 containing pet-28a; 2: IPTG-induced E. coli BL21 containing pet-TAT-Ag85B.
Discussion
In spite of the distinct advantage in safety, protein vaccines remain inferior due to the attenuate ability that fails to induce Th1-type cellular immunity enough to eliminate MTB. Therefore, how to induce a strong anti-tuberculosis immunity and protective efficacy is still a fundamental problem. Previous results indicated that one of the effective mechanisms of enhancing specific immune response depends on the delivery of proteins into cells.16 Discovery of the HIV-Tat PTD has opened avenues for directing in vitro and in vivo delivery of proteins into cells. Therefore, we linked up Ag85B to the TAT peptide to promote Ag85B‑based vaccination against tuberculosis, exhibiting a dramatic increase in Ag85B-specific Th1 responses and an impressive anti-tuberculosis efficacy.
Firstly, our study showed that vaccination with TAT-Ag85B resulted in much higher anti-Ag85B-specific antibody and inflammatory cytokines production than vaccination with Ag85B. This strengthened response may relate to enhancement of Ag85B presentation aided by Tat PTD transduction. Similar results can be demonstrated by TAT-transferred antigens that induce Th1-type response.17 TAT-mediated antigen presentation was also supported by Chen X, who found that recombinant PTD-HBcAg could penetrate into DC cytoplasm to promote DC maturation, and enhance T cells response to generate HBcAg-specific CTLs.18
Subsequently, both in humoral and in cellular immunity, TAT-Ag85B was characterized by the superior potency to induce Th1-dominant immune response. We found the TAT-Ag85B elicited higher levels of IgG2a compared to the Ag85B. Furthermore, the classic Th1-type cytokines, IFN-γ and TNFα were dramatically upregulated in mice vaccinated by TAT-Ag85B. Contrarily, TAT-Ag85B seems not to change the levels of IL-4 and IL-10, compared to Ag85B. These results demonstrated that vaccination with the TAT-Ag85B induced vigorous Th1 responses in mice, and even skewed the Th2-biased immune response established by a protein boost back to a Th1-type response. This shift of immune types may relate to involvement of T-bet in Th1 functional polarization.19,20
Eventually, the efficacy of a vaccine mainly relies on its protection from bacterial infection. TAT-Ag85B-induced protection can be demonstrated by reduced MTB loads both in lungs and in spleens. Additionally, a long-term efficacy induced by TAT-Ag85B can be evaluated because the protection period is at least for five months since the last vaccination. This protection is probably a consequence of the dominant Th1-type immune response, as CD4+ T cells and IFN-γ responses are important in protection against tuberculosis.21–23 Unfortunately, we failed to analyse the MTB infection-related lung inflammation in mice. Thus, the therapeutic effectiveness of TAT-Ag85B vaccine remains uncertain. Further studies are required for the safety of the vaccine. However, these data did confirme the efficacy of an anti-tuberculosis vaccine, TAT-Ag85B, in a murine model of tuberculosis.
In summary, our results demonstrate that the TAT-Ag85B exhibits a strong immunity and protection in a murine model of tuberculosis. Therefore, the findings provide a new potent strategy for the development of improved vaccine.
Author Contributions
HD, WJ and ZR contributed to study design, data analysis and drafted the manuscript. HD, WJ and WW-Y performed laboratory assays. XY, DZ, DJ, WW and HJ assisted with laboratory assays. XY, NS and XC assisted with data analysis.
Acknowledgements
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China, [grant number 81571528], [grant number 81202294], [grant number 81172778], [grant number 81302524], [grant number 61170172]; Anhui Provincial Natural Science Foundation of China [grant number KJ2013A105].
Conflict of Interest
There is no conflict of interest in this study.
References
- 1.World Health Organization Global tuberculosis control, chapter 2;2012. p. 8–28. [Google Scholar]
- 2.Ritz N, Strach M, Yau C, Dutta B, Tebruegge M, Connell TG, et al. A comparative analysis of polyfunctional T cells and secreted cytokines induced by Bacille Calmette-Guérin immunisation in children and adults. PLoS ONE 2012;7:e37535. 10.1371/journal.pone.0037535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JS, Kim HS, Park HM, Kim CH, Kim TG. Efficient induction of anti-tumor immunity by a TAT-CEA fusion protein vaccine with poly(I:C) in a murine colorectal tumor model. Vaccine 2011;29(47):8642–8. 10.1016/j.vaccine.2011.09.052 [DOI] [PubMed] [Google Scholar]
- 4.Fittipaldi A, Giacca M. Transcellular protein transduction using the Tat protein of HIV-1. Adv Drug Deliv Rev. 2005;57(4):597–608. 10.1016/j.addr.2004.10.011 [DOI] [PubMed] [Google Scholar]
- 5.Wadia JS, Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv Drug Deliv Rev. 2005;57(4):579–96. 10.1016/j.addr.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 6.Wu Y, Ren C, Gao Y, Hou B, Chen T, Zhang C. A novel method for promoting heterologous protein expression in Escherichia coli by fusion with the HIV-1 TAT core domain. Amino Acids 2010;39(3):811–20. 10.1007/s00726-010-0534-2 [DOI] [PubMed] [Google Scholar]
- 7.Park JS, Kim HS, Park HM, Kim CH, Kim TG. Efficient induction of anti-tumor immunity by a TAT-CEA fusion protein vaccine with poly(I:C) in a murine colorectal tumor model. Vaccine 2011;29(47):8642–8. 10.1016/j.vaccine.2011.09.052 [DOI] [PubMed] [Google Scholar]
- 8.Fanales-Belasio E, Moretti S, Fiorelli V, Tripiciano A, Pavone Cossut MR, Scoglio A, et al. HIV-1 tat addresses dendritic cells to induce a predominant Th1-type adaptive immune response that appears prevalent in the asymptomatic stage of infection. J Immunol. 2009;182(5):2888–97. 10.4049/jimmunol.0711406 [DOI] [PubMed] [Google Scholar]
- 9.Dong H, Jing W, Yabo Y, Xiaokang Y, Wan W, Min M, et al. Establishment of rat model of silicotuberculosis and its pathological characteristic. Pathog Glob Health 2014;108(7):312–6. 10.1179/2047773214Y.0000000157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty TM, Olsen AW, Weischenfeldt J, Huygen K, D’Souza S, Kondratieva TK, et al. Comparative analysis of different vaccine constructs expressing defined antigens from Mycobacterium tuberculosis. J Infect Dis. 2004;190(12):2146–53. 10.1086/jid.2004.190.issue-12 [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Fu R, Chen Z, Tan K, Chen L, Teng X, et al. Immunogenicity and protective efficacy of a novel recombinant BCG strain overexpressing antigens Ag85A and Ag85B. Clin Dev Immunol. 2012;2012:563838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu D, Wu J, Zhang R, Chen L. T-bet acts as a powerful adjuvant in Ag85B DNA-based vaccination against tuberculosis. Mol Med Rep. 2012;6(1):139–44. [DOI] [PubMed] [Google Scholar]
- 13.Kanhere A, Hertweck A, Bhatia U, Gökmen MR, Perucha E, Jackson I, et al. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun. 2012;3:1268. 10.1038/ncomms2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Józefowski S, Sobota A, Kwiatkowska K. How Mycobacterium tuberculosis subverts host immune responses. BioEssays 2008;30(10):943–54. 10.1002/bies.v30:10 [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Hu D, Du JW, Tao XR, Qi XL, Zhang RB. Th1 immunity is not required for the effect of lipopolysaccharide exposure on modifying asthmatic responses of mice before sensitization. Chin Med J (Engl). 2010;123:1047–51. [PubMed] [Google Scholar]
- 16.Chen X, Lai J, Pan Q, Tang Z, Yu Y, Zang G. The delivery of HBcAg via Tat-PTD enhances specific immune response and inhibits Hepatitis B virus replication in transgenic mice. Vaccine 2010;28(23):3913–9. 10.1016/j.vaccine.2010.03.070 [DOI] [PubMed] [Google Scholar]
- 17.Yang M, Wang S, Wang S, Ma J, Xu X, Mao C, et al. Tat-mediated intracellular delivery of T-bet protein into THP-1 cells can induce Th1-Type response. Immunol Invest. 2008;37(2):97–111. 10.1080/08820130701690725 [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Yu Y, Pan Q, Tang Z, Han J, Zang G. Enhancement of cytotoxic T lymphocyte activity by dendritic cells loaded with Tat-protein transduction domain-fused hepatitis B virus core antigen. Acta Biochim Biophys Sin (Shanghai). 2008;40(12):996–1004. 10.1111/abbs.2008.40.issue-12 [DOI] [PubMed] [Google Scholar]
- 19.Kanhere A, Hertweck A, Bhatia U, Gökmen MR, Perucha E, Jackson I, et al. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun. 2012;3:1268. 10.1038/ncomms2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipscomb MW, Chen L, Taylor JL, Goldbach C, Watkins SC, Kalinski P, et al. Ectopic T-bet expression licenses dendritic cells for IL-12-independent priming of type 1 T cells in vitro. J Immunol. 2009;183(11):7250–8. 10.4049/jimmunol.0901477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green AM, DiFazio R, Flynn JL. IFN-γ from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J Immunol. 2013;190(1):270–7. 10.4049/jimmunol.1200061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker LF, Brennan MJ, Rosenstein PK, Sadoff JC. Tuberculosis vaccine research: the impact of immunology. Curr Opin Immunol. 2009;21(3):331–8. 10.1016/j.coi.2009.05.017 [DOI] [PubMed] [Google Scholar]
- 23.Dheda K, Schwander SK, Zhu B, van Zyl-Smit RN, Zhang Y. The immunology of tuberculosis: from bench to bedside. Respirology 2010;15(3):433–50. 10.1111/res.2010.15.issue-3 [DOI] [PMC free article] [PubMed] [Google Scholar]