Abstract
Learning abnormalities have long been centrally implicated in posttraumatic psychopathology. Indeed of all anxiety disorders, PTSD may be most clearly attributable to discrete, aversive learning events. In PTSD, such learning is acquired during the traumatic encounter and is expressed as both conditioned fear to stimuli associated with the event and more general over-reactivity—or failure to adapt—to intense, novel, or fear-related stimuli. The relatively straightforward link between PTSD and these basic, evolutionarily old, learning processes of conditioning, sensitization, and habituation affords models of PTSD comprised of fundamental, experimentally tractable mechanisms of learning that have been well characterized across a variety of mammalian species including humans. Though such learning mechanisms have featured prominently in explanatory models of psychological maladjustment to trauma for at least 90 years, much of the empirical testing of these models has occurred only in the past two decades. The current review delineates the variety of theories forming this longstanding tradition of learning-based models of PTSD, details empirical evidence for such models, attempts an integrative account of results from this literature, and specifies limitations of, and future directions for, studies testing learning models of PTSD.
Keywords: PTSD, fear-conditioning, extinction, overgeneralization, sensitization, habituation
1. Introduction
From the learning perspective, symptoms of PTSD stem largely from maladaptive learning occurring during and after a traumatic encounter. Such learning manifests in both associative and non-associative forms. Through associative fear-conditioning (Pavlov, 1927), neutral stimuli (people, places, and things) associated with the aversive trauma acquire the capacity to trigger and maintain anxiety well after the occurrence of the traumatic episode. This conditioning process is thought to contribute centrally to the re-experiencing (e.g., distressing recollections) and avoidance symptom-clusters that are often triggered by exposure to stimuli that resemble aspects of the trauma, but are in fact indicative of no genuine danger. Maladaptive forms of non-associative learning in PTSD are evidenced by broad anxious reactivity—to novel, intense, or fear-relevant stimuli in the environment—that is both resistant to degradation via habituation, and susceptible to intensification through sensitization. These non-associative learning correlates of PTSD promote sustained sympathetic activation thought to result in such hyperarousal symptoms of PTSD as hypervigilance, exaggerated startle, difficulty concentrating and irritability. In this review, we will explore, in depth, the variety of learning mechanisms implicated in the onset and maintenance of PTSD through outlining associative- and non-associative-learning models of PTSD; detailing extant empirical support for such models; and delineating the research limitations and future directions for the field. A summary of learning-based theories of PTSD is provided in Table 1.
Table 1.
Delineation of learning based theories of PTSD, clinical illustrations, and empirical evidence.
| Theory | Thesis | Clinical Vignette | Lab-Based Evidence |
|---|---|---|---|
| A USA combat veteran with PTSD acquired Pavlovian fear to a roadside object (CS+) used to encase an improvised explosive device (US) by which he was injured while on street patrol in Iraq. | |||
| I. Associative | |||
| 1. Resistance to Extinction | The persistence of conditioned fear to CS+ that are no longer indicative of environmental danger (i.e., CS+ no longer paired with the US), contributes to PTSD symptomatology. | Though, upon return to civilian life in the USA, this veteran’s initial display of conditioned fear to roadside objects (CS+) would be considered normative, if this veteran fails to extinguish fear through repeated exposures to benign roadside objects (trash, debris, fire hydrants), conditioned fear would pathogenically contribute to his post-deployment functioning. | • Inconsistent evidence for impaired acquisition of extinction learning in PTSD. • Accumulating support for impaired recall of extinction learning over time in PTSD. |
| 1a. Increased fear excitation (hyper-conditionability) | Individuals with, or at risk for PTSD have a heightened tendency to form aversive associations reflected by abnormally strong excitatory fear-conditioning to the CS+, resulting in resistance to extinguish fear to the CS+. | If this soldier forms overly strong associations between roadside objects (CS+) and the explosion (US), upon return to the USA the roadside objects will be particularly salient trauma cues that will resist extinction via exposures to roadside objects in the absence of the US, and thereby maintain traumatic fear over time. | • Little support from empirical literature as most lab-based studies do not show heightened fear conditioning to the CS+ in PTSD. |
| 1b. Incubation | Heightened excitatory fear-conditioning in those with, or at risk for PTSD results in a CR (fear) that is aversive enough to serve as a US-substitute. In turn, the CR continues to reinforce the CS+ in the absence of US pairings, blocking extinction, and perhaps even ‘incubating’, or enhancing, the conditioned fear response with repeated, unpaired CS+ exposures. | Upon return to the USA, the veteran’s conditioned fear response to roadside objects is sufficiently acute and aversive to serve as a ‘US substitute’ that continues to aversively reinforce the CS+ (roadside objects) in the absence of the US (explosions). This prevents the veteran from extinguishing fear to the CS+ and may result in enhanced conditioned fear responses over time. | • No evidence. |
| 1c. Two-stage learning | Heightened Pavlovian fear in those with, or at risk for PTSD (Stage 1) is proposed to act as a drive that motivates and reinforces avoidance of the CS+ (Stage 2). Such avoidance prevents extinction by denying the individual future opportunities to experience the CS+ in the absence of the US. | Strong Pavlovian fear to roadside objects motivates the returning veteran to avoid driving or walking on streets to avert conditioned fear elicited by roadside objects. By so doing, the veteran is denied the opportunity to extinguish this conditioned response, in their now safe post-deployment environment, through exposure to roadside objects in the absence of dangerous outcomes. | • No evidence. |
| 1d. Reduced fear inhibition | Individuals with, or at risk for PTSD have an impaired ability to inhibit fear to CSs previously, but no longer, paired with an aversive US, blocking or retarding extinction of conditioned fear. | The veteran’s memories of post-deployment exposures to roadside objects, in the absence of any aversive outcomes, are insufficiently strong to inhibit the excitatory fear memory of the CS-US association acquired in Iraq, resulting in a failure to extinguish fear. | • Theory supported by fMRI data linking poor retention of extinction in PTSD to under-functioning mPFC: a brain region contributing to fear reduction through inhibition of amygdaloid neurons. |
| 2. Associative learning deficits & Sustained contextual anxiety | The impaired ability to learn environmental cues (CS+) of danger (US) denies those with, or at risk for PTSD the awareness of safety periods, resulting in chronic anxiety. Further, the unawareness of danger cues leads the individual to more generally associate the unpleasant US with the environment in which the US is experienced, resulting in heightened contextual anxiety. | While still in Iraq, the veteran fails to distinguish between danger and safety due to insufficient learning of danger cues. This leads to chronic anxiety during deployment and heightened contextual anxiety to the deployment environment, which are both risk factors for PTSD. | • Mixed evidence for associative learning deficits in PTSD. • Substantial lab-based evidence of heightened contextual anxiety in PTSD. |
| 3. Over-generalization | A heightened tendency among those with, or at risk for PTSD, to transfer conditioned fear from the CS+ to stimuli resembling the CS+. Such overgeneralization results in the undue spreading of conditioned fear to stimuli that resemble features of the traumatic encounter. | Though conditioned-fear was acquired to a particular encasement of a roadside explosive device, upon return to the USA the veteran’s fears may generalize to such ‘benign’ roadside objects as trash cans, fire hydrants, or other roadside debris. This may promote frequent trauma-related anxiety in his benign posttraumatic environment because these roadside objects are ubiquitous in his neighborhood and town. | • Preliminary support derives from differential conditioning studies in PTSD evidencing heightened fear reactivity to CS-sharing multiple stimulus features (e.g., shape, size, duration, spatial location) with the conditioned danger-cue. • Recent support from ‘generalization gradient’ studies identifying less steep gradients of generalization in PTSD. |
| 4. Failure to inhibit fear in the presence of safety cues | A compromised capacity for fear inhibition results in the failure to suppress fear in the presence of safety cues, and maintains fear responding during periods of safety among those with, or at risk for PTSD. | Upon return to the USA, the veteran encounters roadside objects (CS+) coincident with many potential environmental cues of safety including neighborhood people, shops, parks, and houses. These safety cues are however unable to exert inhibitory control over the conditioned response to roadside objects leading to a continuation of the traumatic response in the benign, civilian context. | • Support derives from ‘conditional discrimination’ findings of impaired inhibition of fear to a conditioned danger-cue when presented in tandem with a conditioned safety-cue among PTSD patients. • Additional support is provided by neuroimaging studies documenting reduced activation in brain areas associated with fear inhibition (mPFC) among those with, versus without, PTSD during exposure to traumatic pictures and sounds, script driven traumatic imagery, and fearful faces. |
| II. Non-Associative | |||
| 1. Failure-to-habituate | Trauma induces an impaired ability to autonomically adapt, or habituate, to intense, novel, or fear-relevant environmental stimuli (whether or not they resemble aspects of the trauma) among those with, or at risk for PTSD. The failure of this type of habituation is proposed as a central contributor to the hyper-arousal cluster of PTSD symptoms. | Upon return to the USA, this veteran may display persistent autonomic responding to reoccurring, and more or less irrelevant, sensory stimuli (sounds, sights, touches), as displayed by exaggerated startle responses and hypervigilance. Additionally, the inability to filter out these reoccurring sensory stimuli compromises concentration by exhausting attentional resources otherwise available for processing more consequential stimulus events. | • Supported by the well replicated finding of less steep habituation slopes, measured via SCR (but not HR or startle EMG) to intense acoustic tones. |
| 2. Stress sensitization | Trauma induces autonomic hyper-excitability (i.e., stress sensitization) to intense, novel, or fear related stimuli (whether or not they resemble aspects of the trauma) among those with, or at risk for PTSD. This hyper-excitability is proposed as an underlying mechanism for the hyper-arousal cluster of PTSD symptoms. | Upon return to the USA, this veteran may display increasing autonomic responding to reoccurring, and more or less irrelevant, sensory stimuli (sounds, sights, touches), resulting in the same hyper-arousal symptoms described above for habituation failure (exaggerated startle, hypervigilance, poor concentration). | • Supported by replicated findings of increased HR (and SCR and startle EMG to some degree) responses to repeatedly presented, high intensity noises in those with vs. without PTSD. |
| 2a. Amygdala kindling | A variant of stress sensitization theory in which the traumatic experience is proposed to stimulate, or kindle, the amygdala-based fear circuit rendering the fear system hyper-excitable to either future trauma or fear-relevant stimuli. | Because of trauma-related kindling of this veteran’s fear circuit, upon return to the US, he displays heightened anxiety to fear-relevant stimuli contributing to the maintenance of PTSD symptomatology. | • Supported by replicated neuroimaging findings of stronger amygdala activation to fearful and neutral faces in those with vs. without PTSD. • Epileptic discharges in the human temporal lobe (the cerebral structure encasing the amygdala) are associated with marked increases in reported fear and anxiety. |
CS+ = conditioned danger-cue; CS− = conditioned safety-cue; US=unconditioned stimulus; CR=conditioned response; EMG=electromyography; HR=heart-rate; SCR=skin conductance response; fMRI=functional magnetic resonance imaging; mPFC=medial prefrontal cortex.
From the outset, it should be noted that this review does not comprise an exhaustive examination of all relevant empirical findings. Rather, this review is theoretically driven, in that it endeavors to describe the weight of the evidence for and against all prevalent learning-based theories of PTSD. Search terms used for retrieval of articles thus included ‘traumatic stress’ plus theory-specific terms such as: ‘resistance to extinguish’, ‘extinction’, or ‘extinction recall’; ‘conditionability’, ‘incubation’, or ‘acquisition of conditioned fear’; ‘associative learning deficits’, ‘contextual anxiety’, or ‘unpredictable threat’; ‘two-stage learning’ or ‘avoidance’; ‘generalization’; ‘failure to inhibit’ or ‘conditioned inhibition’; ‘habituation’; and ‘sensitization’, or ‘kindling’. This approach was designed to ensure retrieval of articles covering all prevailing learning-based theories of PTSD.
2. Associative fear-learning
Fear-conditioning—the associative learning process whereby a naturally benign conditioned stimulus (CS) acquires anxiogenic properties by virtue of its pairing with a naturally aversive unconditioned stimulus (US)—has figured prominently in accounts of maladaptive psychological reactions to trauma for at least 90 years (Pavlov, 1927; Watson & Rayner, 1920). During this period, a variety of mechanisms through which fear-conditioning exerts pathological influence have been theorized, including: 1) resistance to extinguish conditioned fear, 2) Mowrer’s two-stage learning, 3) stimulus generalization, 4) hyper-conditionability, and 5) associative-learning deficits leading to contextual anxiety.
2.1. Resistance to extinction
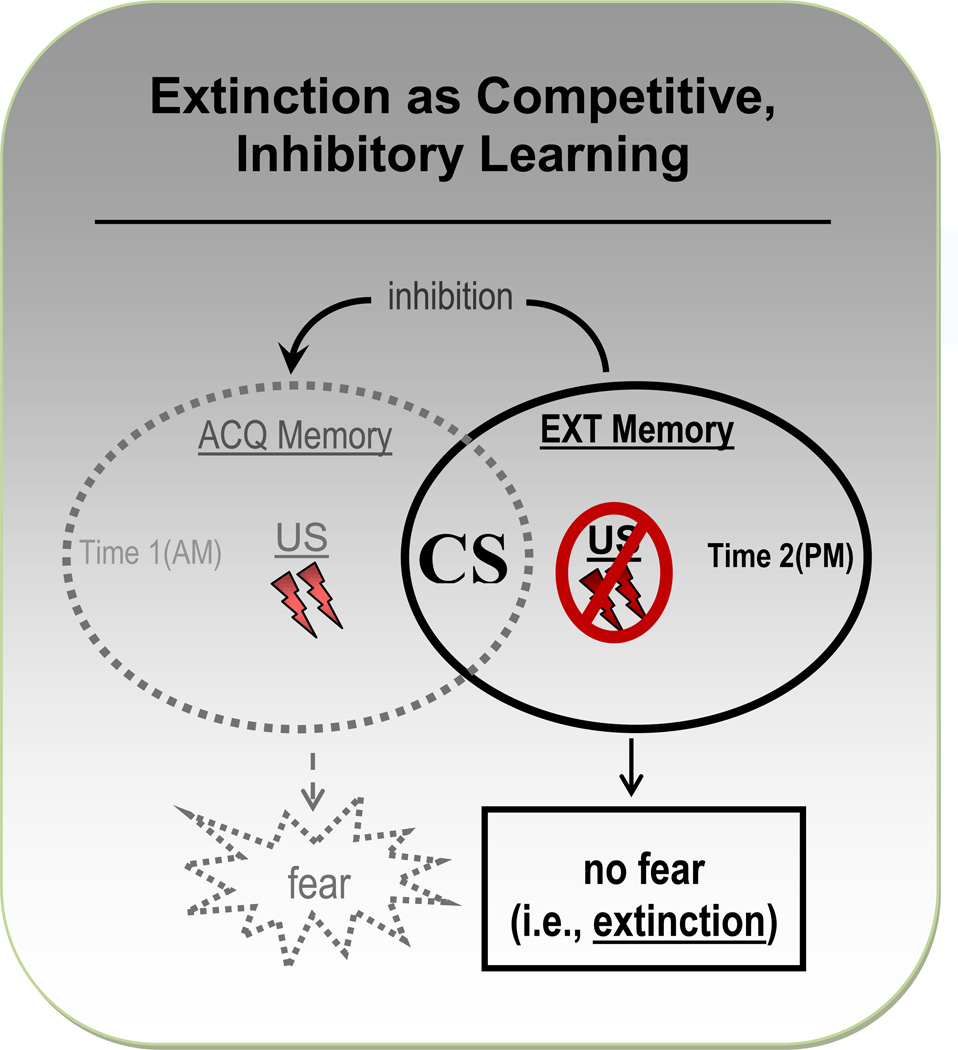
Many conditioning models of PTSD posit a resistance to extinguish conditioned fear as the central pathogen. In the context of fear-conditioning, extinction refers to a decline in fear responding to a conditioned danger-cue (i.e., CS) presented one or more times in the absence of the aversive US. Failure to extinguish entails the persistence of fear to stimuli that are no longer indicative of environmental danger and thus constitutes a maladaptive expression of anxiety. Importantly, extinction is a new learning that inhibits, rather than erases, the acquired CS/US association (for a review, see Bouton, 2004). Specifically, extinction involves the encoding of a second learning experience with the CS (i.e., the CS as benign) that competes for activation with the original acquisition learning experience (i.e., the CS as a signal of danger: Bouton, 1991). Extinction of conditioned responding occurs only when the extinction learning is strong enough to outcompete the fear memory encoded at acquisition for activation (see Figure 1).
Figure 1.
Schematic display of the competing nature of acquisition (ACQ) and extinction (EXT) memories of the conditioned stimulus (CS). In this example, ACQ of conditioned fear follows from presentations of the CS together with an electric-shock US during a morning (AM) testing session. The EXT memory is encoded later that afternoon (PM) during a testing session in which the CS is repeatedly presented in the absence of the US. These ACQ and EXT learning experiences generate two competing memories of the CS, with the former representing the CS as an anxiogenic predictor of aversive shock and the latter characterizing the CS as a benign stimulus associated with no aversive outcome. Activation of the ACQ memory of the CS elicits fear, while activation of the EXT memory of the CS elicits no fear. Successful EXT learning depends on an EXT memory of the benign CS that is strong enough to outcompete the ACQ memory for excitation, thereby inhibiting both activation of the ACQ memory and the associated fear reactivity to the CS.
From this competition-theory perspective, extinction accounts of PTSD derive from two mechanisms through which the inhibitory influences of extinction are outstripped by excitatory influences of fear acquisition. The first results from elevated levels of acquisition that overpower the inhibitory effects of extinction. The second involves a deficit in extinction (inhibitory) learning that confers a competitive edge to the fear acquisition memory. Drawing from the first of these two mechanisms, the conditionability theory by Pitman and colleagues (e.g., Orr et al., 2000) imputes PTSD to hyper-conditionability: a disposition toward forming aversive associations instantiated by abnormally strong acquisition and a resulting resistance to extinguish. The second mechanism is reflected in theories by Davis and colleagues (Davis, Falls, & Gewirtz, 2000) and Jovanovic and Ressler (2010) linking PTSD to deficits in extinction, and other forms of inhibitory fear learning, resulting in the failure to suppress what are otherwise normative levels of fear acquisition.
An additional formulation of the extinction model comes from Eysenck (1979) who argues that the conditioned response (i.e., an internal state of fear) is sufficiently “nocive” or uncomfortable in those disposed toward clinical anxiety to serve as an aversive US-substitute in the absence of the genuine US. That is, extinction of fear to a CS previously paired with an aversive outcome may be slowed or prevented in those for whom anxiety reactions to the CS are sufficiently strong to function as aversive reinforcement of the CS during extinction learning. Eysenck not only predicts slowed extinction but goes further by expecting a kind of “incubation”, or enhancement of the conditioned fear response, in the absence of the genuine US, in those disposed to anxiety. Such incubation is proposed to operate through a positive feedback loop whereby repeated reinforcement of the CS by the fear response strengthens the aversive valence of the CS, which subsequently increases levels of fearful CS-reactivity and provides further aversive reinforcement of the CS.
To illustrate extinction-failure in the context of PTSD, consider the example of a combat soldier who acquires conditioned fear to a roadside, box-shaped object (CS) used to encase an improvised explosive device (US) by which he is injured while on street patrol in Iraq. Though, upon return to civilian life, the initial display of conditioned fear to roadside objects is normative, failure to extinguish fear through repeated exposure to benign roadside objects (i.e., the CS in the absence of the US) is thought to be the maladaptive consequence of either: 1) an overly strong acquisition memory (i.e., conditionability hypothesis, 2) an insufficiently strong extinction memory (i.e., failure-to-inhibit hypothesis, or 3) an especially nocive conditioned response that continues to aversively reinforce roadside objects (i.e., incubation hypothesis).
2.1.1. Empirical evidence
Much support for the role of extinction in PTSD derives from the clinical effectiveness of “extinction-like” exposure therapy for the treatment of PTSD (e.g., Rothbaum & Davis, 2003; Rothbaum & Foa, 2002). Specifically, the use of this treatment is predicated on the notion that patients manifest a resistance to extinguish trauma-related fear that is surmountable through clinical facilitation of the extinction process. Unfortunately, the lab-based evidence is less convincing, with some demonstrating weaker extinction learning in PTSD (e.g., Blechert, Michael, Vriends, Margraf, & Wilhelm, 2007; Orr et al., 2000; Wessa & Flor, 2007), but not others (e.g., Grillon & Morgan, 1999; Peri, Ben Shakhar, Orr, & Shalev; 2000). This heterogeneity in extinction results is difficult to disentangle given the absence of methodological or sample characteristics that clearly differentiate studies producing positive versus null findings. For example, findings are inconsistent for: 1) studies assessing extinction psychophysiologically (compare Orr et al., 2000 vs. Peri, Ben Shakhar, Orr, & Shalev, 2000) or through subjective ratings (compare Wessa & Flor, 2007 vs. Blechert et al., 2007); 2) studies employing electric shock (Orr et al., 2000 vs. Grillon & Morgan, 1999) or more mild aversive events (Wessa & Flor, 2007 vs. Peri et al., 2000) as unconditioned stimuli; and 3) studies testing patients with combat-related PTSD (Milad et al., 2008 vs. Grillon & Morgan, 1999) or non-combat related PTSD (Wessa & Flor, 2007 vs. Bremner et al., 2005). One possibility is that extinction deficits relate specifically to the re-experiencing cluster of PTSD symptoms (Norrholm et al, 2011), and that relating extinction deficits to overall levels of PTSD, without considering the severity of individual symptom clusters, frustrates attempts to link extinction abnormalities to PTSD.
More recently, interest has accrued in studying PTSD-control differences in retention of extinction learning over time (Milad et al., 2008; Milad et al., 2009; Orr et al., 2006; Shvil et al., 2014). Two of three such studies document impaired recall of extinction learning in PTSD patients during a second day of testing, in the absence of abnormalities in the original extinction learning (Milad et al., 2008; Milad et al., 2009). Recently, a fourth such study demonstrated deficits in extinction recall in male but not female patients with PTSD (Shvil et al., 2014). Twin data from one study (Milad et al., 2008) characterizes deficient extinction recall in PTSD as a consequence of traumatic exposure rather than a preexisting risk factor. Such findings link PTSD to an intact ability to extinguish conditioned fear during the course of extinction (exposure) training, but a trauma-induced impairment in maintaining benefits of this training over time. Whether impaired extinction-recall emerges as a more replicable marker of PTSD awaits further testing. For now these results seem to highlight the importance of repeated follow-up assessments of PTSD, and repeated treatment sessions if needed, for patients undergoing exposure-based treatments.
As portrayed in Figure 1, the competition theory of extinction implies two mechanisms by which extinction failure might occur: 1) an overly strong acquisition memory of the CS as threatening that is refractory to the inhibitory effects of the extinction memory, as implied by the hyper-conditionability theory (e.g., Orr et al., 2000); or 2) an insufficiently strong, inhibitory extinction memory of the CS as benign that is unable to outcompete the acquisition memory for activation, as posited by the failure-to inhibit theory (e.g., Davis et al., 2000). A degree of doubt is cast on the former mechanism by a strong majority of lab-based conditioning studies evidencing normative levels of fear acquisition to the CS in PTSD (e.g., Blechert et al., 2007; Grillon & Morgan, 1999; Milad et al., 2008; Milad et al., 2009; Peri et al., 2000). Such findings suggest that extinction failure in PTSD, via dominance of the acquisition memory over the extinction memory, is unlikely attributable to unduly strong, initial levels of acquisition in patients. That retention of extinction may constitute a more robust marker of PTSD leaves open the possibility for abnormally strong retention of acquisition in patients that, over time, results in acquisition memories too strong for inhibition by extinction memories. Importantly, the absence of abnormally strong conditioned responding immediately following acquisition among those with PTSD, in the presence of heightened retention of conditioned fear over time, seems consistent with the observation that retention of PTSD symptoms, rather than the initial acquisition of such symptoms, distinguishes trauma survivors with, versus without, PTSD (e.g., Rothbaum & Foa, 1993). Indeed, the majority of trauma survivors (65%–94%) display posttraumatic symptoms in the early aftermath of the trauma and only a minority of survivors meet diagnostic criteria for PTSD (11%–42%) by retaining such symptoms beyond the first few months post-trauma (estimated percentages from Rothbaum & Foa, 1993). Though the weight of available data suggest no abnormalities in acquisition of conditioned fear to the conditioned danger cue in PTSD, such data, by and large, reflect levels of conditioning during and immediately following acquisition. Future studies assessing retention of fear to conditioned danger-cues in PTSD are needed before ruling out heightened responding to learned danger-cues as a conditioning marker of PTSD.
The second mechanism, imputing extinction failure in PTSD to inadequate inhibition of the acquisition memory, has begun to receive support from results suggesting impaired processes of fear inhibition in PTSD (for a review, see Jovanovic & Ressler, 2010). Because these data are directly relevant to both failure-to-extinguish and failure-to-inhibit theories of PTSD, such data will be discussed in the context of both models in the failure-to-inhibit section of this review. Of note, though Eysenck’s incubation version of extinction theory is appealing on its face, no empirical support for this model in PTSD has been garnered to date.
2.2. Associative-learning deficits and sustained contextual anxiety
In dramatic contrast to the conditionability theory of Pitman and colleagues (Orr et al., 2000), the associative-learning-deficits model attributes PTSD to an impaired ability to form aversive associations through classical conditioning (Grillon, 2002). During a traumatic experience, this deficit is said to prevent the individual from learning environmental cues predicting danger. To not know cues for danger is to be unaware of safety in their absence, leaving the individual in a chronic state of anxiety (Seligman & Binik, 1977). Furthermore, the absence of threat cues leads the individual to more generally associate the unpleasant US with the environment in which the US is experienced, resulting in heightened contextual anxiety—a form of learning further contributing to chronic anxiety by maintaining diffuse anxiety for the duration of exposure to the traumatic milieu. Thus chronic anxiety—fueled by both the absence of safety periods and contextual conditioning—is viewed as the pathologic consequence of associative-learning deficits in those with, or disposed toward, PTSD (Grillon, 2002).
2.2.1. Empirical evidence
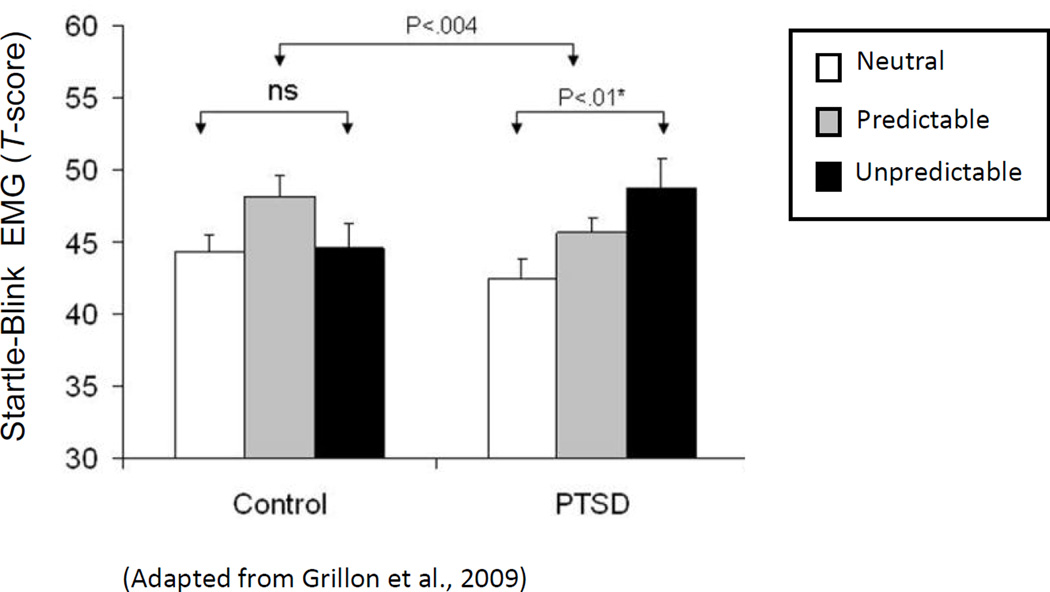
Whereas evidence for associative-learning deficits in PTSD is mixed, with support coming from some studies (Burriss, Ayers, Ginsberg, & Powell, 2008) but not others (Burriss, Ayers & Powell, 2007; Geuze, Vermetten, Ruf, de Kloet, Westenberg, 2008; Werner, et al. 2009), the psychopathological endpoint of this model (sustained contextual anxiety) is substantiated by startle-EMG studies evidencing heightened contextual anxiety in PTSD. For example, Grillon and Baas (2003) identify a pattern of results across multiple studies in which heightened startle magnitudes in PTSD are found in aversive, but not benign, experimental contexts (for a review see Grillon & Baas, 2003), suggesting enhanced sensitivity to contextual anxiety among those with PTSD. Moreover, a study by Grillon and colleagues (Grillon, Pine, Lissek, Rabin, Bonne, & Vythilingam, 2009) further examined this link using an instructed threat paradigm designed for within-subject manipulations of contextual danger (Grillon, Baas, Lissek, Smith, & Milstein, 2004). Specifically, startle magnitudes were assessed during three sustained contexts (duration = 2 minutes) of increasing aversiveness: 1) a neutral (N) context where no unpleasant events were possible; 2) a mildly aversive context where unpleasant stimuli were presented predictably (P); 3) and a more highly aversive context in which unpleasant stimuli were presented unpredictably (U). The outcome variable was fear-potentiated startle: the reliable enhancement of the startle reflex when an organism is in a state of fear (Davis & Astrachan, 1978; Grillon, Ameli, Woods, Merikangas, Davis, 1991). As can be seen in Figure 2, fear-potentiated startle to the most aversive context (U), relative to the least (N), is significantly stronger in PTSD patients. Because this enhanced contextual-potentiation in patients was derived from startle responses collected across the 2-minute duration of N, P, and U conditions; such data link PTSD with sustained contextual anxiety. Importantly, this result was accompanied by normative levels of startle potentiation to discrete (non-contextual) cues reliably predicting imminent delivery of unpleasant stimulation, specifically linking PTSD to abnormal processes of sustained contextual anxiety rather than more phasic, discretely-cued, fear responses. Consistently, a prospective study of police cadets exposed to police-related trauma, found pre-trauma levels of subjective anxiety to contextual threat, but not discretely cued threat, to be predictive of post-trauma symptoms of PTSD (Pole et al., 2009). Such results also implicate increased contextual anxiety as a pre-morbid risk factor for PTSD.
Figure 2.
(Adapted from Grillon et al., 2009) Average startle magnitudes in PTSD patients and healthy controls across three levels of contextual aversiveness: 1) neutral context (low threat), during which no unpleasant stimulation was delivered, 2) predictable context (moderate threat) during which unpleasant stimulation was given predictably, and (3) unpredictable context during which unpleasant stimulation could be given at any time.
2.3. Two-stage learning
A variant of extinction theory, the two-stage learning model views avoidance of the CS as the primary force behind extinction failure. Specifically, classically conditioned fear is proposed to act as a drive that motivates and reinforces avoidance of the CS, thereby denying an individual the opportunity to extinguish via exposure to the CS in the absence of the US (Eysenck, 1976, 1979; Eysenck & Rachman, 1965; Miller, 1948; Mowrer, 1947, 1960). The explanatory power of this model derives largely from its clinical and intuitive appeal. Specifically, avoidance of learned trauma-cues is central to the clinical presentation of PTSD, which, by definition, denies patients the exposure necessary to extinguish conditioned fear.
In terms of the competitive-learning model of extinction detailed in Figure 1, such avoidance results in an acquisition memory of the CS that receives no competition from an inhibitory extinction memory, leaving only the memory of the CS as a danger cue available for activation. In our example, the returning veteran may avoid driving or walking on streets to avert conditioned fear elicited by roadside objects resembling improvised explosive device (IED) encasements encountered during combat. By so doing, the veteran is denied the opportunity to extinguish this conditioned response, in their now safe post-deployment environment, through exposure to roadside objects in the absence of dangerous outcomes.
2.3.1 Empirical evidence
Although the first classical fear-conditioning stage of this model has received substantial experimentation in PTSD (see above), the extent to which this initial conditioning motivates the second instrumental avoidance stage has been the target of little lab-based testing. Indeed, most assessments of avoidance in PTSD have explored correlations between PTSD symptom severity and experiential avoidance: the intrapsychic attempt to evade thoughts, emotions, and sensations related to the trauma (e.g., Marx & Sloan, 2005; Simpson, Jakupcak, & Luterek, 2006). Though this work has helped elucidate the contribution of mental avoidance to the onset and course of PTSD, it has not allowed for systematic assays of learning mechanisms subserving, the more objectively measurable, behavioral constituents of avoidance in PTSD. Future studies are needed to assess the way acquisition of lab-based classical fear-conditioning antecedes behavioral avoidance of conditioned stimuli. Our lab is currently undertaking such work, and recently validated paradigm demonstrating robust relations between Pavlovian fear-potentiated startle to a CS+ and subsequent behavioral avoidance in the presence of the CS+ (van Meurs, Wiggert, Wicker, and Lissek, 2014). This paradigm will next be applied to test for heightened levels behavioral avoidance, induced by Pavlovian fear, in those suffering from posttraumatic psychopathology.
2.4. Over-generalization
Conditioned responses have long been known to transfer, or generalize, to stimuli resembling the original CS (Pavlov, 1927). Evidence linking the conditioned generalization process to pathologic anxiety dates back to Watson and Rayner (1920) who famously demonstrated generalization of conditioned fear to all things fury in a toddler (‘Little Albert’) following acquisition of fear-conditioning to a white rat. This kind of conditioned generalization has since been adopted as a core feature of PTSD, through which fear during a traumatic event extends to safe conditions ‘resembling’ the distressing event (American Psychiatric Association, 2013). The pathogenic contribution of conditioned generalization follows from the undue proliferation of trauma cues in the individual’s environment. That is, generalization results in the spreading of traumatic reactions to stimuli that resemble the CS, but are themselves benign. Precipitating this proliferation of trauma cues may be an underlying disposition toward reduced thresholds for threat reactivity, resulting in less danger information (i.e., less resemblance to the CS) required for activation of the fear circuit among those with, or prone toward, clinical anxiety (Lissek et al., 2010).
The maladaptive effects of this generalization process can be illustrated by an IED exposed combat veteran with PTSD. Though conditioned-fear was acquired to a box-shaped encasement of the IED, the veteran’s fears may generalize to such other roadside objects in his post-deployment environment as trash cans, fire hydrants, or other roadside debris. This may promote frequent trauma-related anxiety in his benign posttraumatic environment because these roadside objects are ubiquitous in his neighborhood and town.
2.4.1 Empirical evidence
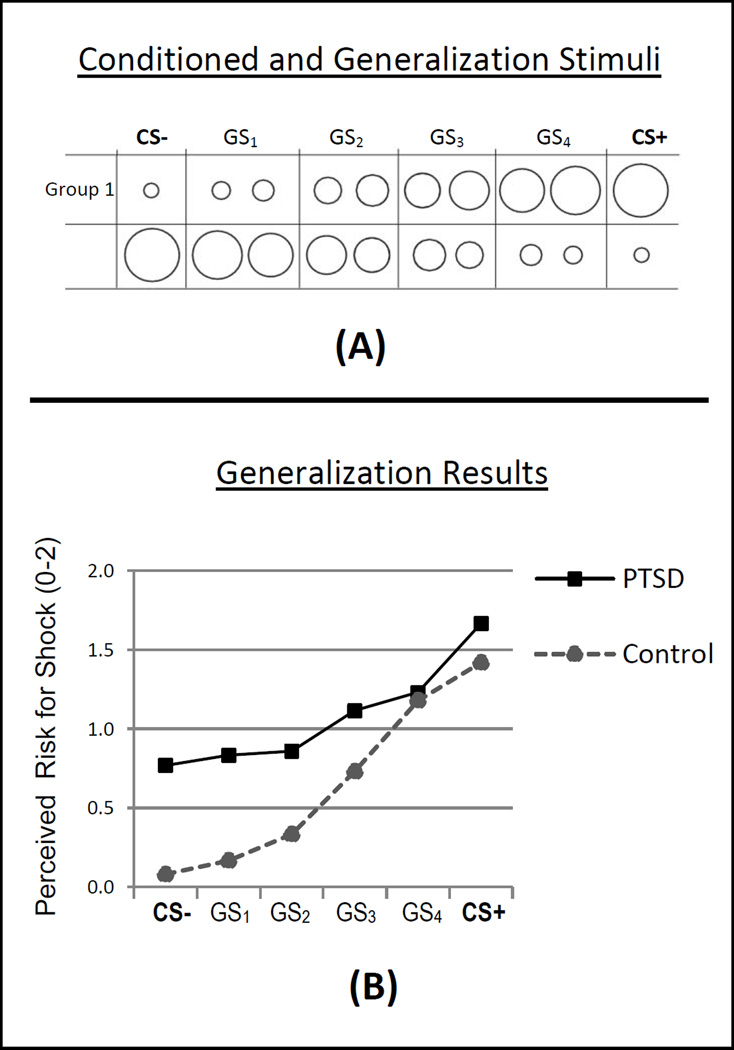
Though generalization of conditioned fear has featured prominently in etiologic accounts of maladaptive anxiety at least since Watson and Raynor’s “Little Albert” experiment (1920), lab-based testing of this idea has been sparse (for a review see Lissek et al., 2008). More recently, this idea has been the target of increased empirical attention, prompted—in part—by meta-analytic results implicating over-generalization of conditioned fear as one of the more robust conditioning correlates of clinical anxiety generally, and PTSD in particular (Lissek et al., 2005). This result was driven by studies evidencing over-reactivity, among PTSD patients, to safety cues sharing stimulus features (e.g., shape, size, duration, spatial location) with the conditioned danger-cue (e.g., Grillon & Morgan, 1999; Orr et al., 2000; Peri et al., 2000), an effect implying heightened transfer, or generalization, of conditioned fear from the learned danger-cue to resembling stimuli in PTSD patients. In further pursuit of this finding, our group developed a conditioned fear-generalization paradigm incorporating systematic methods developed and tested in animals (e.g., Armony, Servan-Schreiber, Romanski, Cohen, & LeDoux, 1997) in which fear responses are assessed to both the conditioned danger-cue and generalization stimuli (GS) parametrically varying in similarity to the danger cue. As in work with animals, such methods applied to humans yielded generalization gradients, or slopes, with the highest level of fear responding to the conditioned danger-cue and gradually decreasing levels of fear generalization to GSs of decreasing similarity to the danger cue (Lissek et al., 2008). The steepness of this gradient indexes generalization, with less steep downward gradients indicating more generalization. A more detailed description of this method as well as preliminary results, suggesting less steep gradients of generalization in PTSD versus healthy comparisons, can be found in Figure 3.
Figure 3.
(A) Conditioned and generalization stimuli included 10 rings of gradually increasing size with extremes serving as the conditioned danger-cue paired with electric shock (CS+) and the conditioned safety-cue unpaired with shock (CS−). For half of participants (Group 1), the largest ring was the CS+ and the smallest was the CS−, and for the other half (Group 2) this was reversed. The eight rings of intermediary size served as generalization stimuli (GSs) and created a continuum-of-similarity from CS+ to CS−. Before analysis, responses to every two intermediaries were collapsed into a single class of stimulus, leaving four classes of generalization stimuli (GS1, GS2, GS3, GS4). Collapsing was implemented to avoid an unduly large number of trials while maintaining a gradual continuum-of-size across rings. Immediately following presentation of each ring, participants were asked to rate their perceived risk for receiving a shock on a scale of 0–2 (where 0 = no risk, 1= some risk, and 2= a lot of risk) to assess the degree to which the conditioned threat value of the CS+ generalized to resembling GSs. (B) Generalization results for 13 adult PTSD patients with mixed traumatic histories (sexual abuse [n=2], physical assault [n=4], car accident [n=3], other [n=4]) and 13 age- and sex-matched healthy comparisons indicate less steep gradients of perceived risk in the PTSD group (Group × Stimulus-type interaction, p=.003). Though this suggests overgeneralization in patients, higher overall risk ratings in PTSD patients (p=.02) imply the additional influence of non-associative sensitization on the shape of response gradients in patients.
As illustrated in Figure 3, findings of less steep gradients of responding in PTSD patients are unlikely due purely to over-generalization of conditioned fear, but rather seem to reflect the combined influence of (associative) generalization and (non-associative) sensitization processes. Specifically, generalization is evidenced by the downward slope in PTSD responses as the presented, ring-shaped stimulus increasingly differentiates in size from the conditioned danger-cue, and sensitization is evidenced by the degree to which PTSD responding to most classes of ringed stimuli (i.e., GS3, GS2, GS1, & CS−) is elevated relative to healthy comparisons. The possibility, however, exists that elevated responding to most ring sizes in PTSD, seemingly reflecting a sensitization process, actually stems from conditioned responding that has generalized from the ring-shaped danger cue to all ringed stimuli.
This possibility was recently tested in individuals with and without PTSD using a modified version of this paradigm that include assessment of responses to a non-ringed (triangular), control stimulus (Kaczkurkin, Burton, Chazin, & Lissek, 2013). The triangular control stimulus allows for the separation of sensitization and generalization processes. Specifically, if PTSD patients over-respond to rings, but not the triangular control stimulus, the group difference can be attributed to overgeneralization in PTSD. If, however, PTSD patients over-respond to both rings and the triangular control, group differences would be attributable to over-sensitization in PTSD. Results of this recent study demonstrate less steep downward gradients of generalization to ringed stimuli among PTSD patients (indicative of over-generalization), in the absence of over-reactivity to the non-ringed control stimulus. Such results suggest overgeneralization rather than over-sensitization as the learning mechanism underlying group differences. To date, much of the lab-based evidence for over-generalization in PTSD has come from our group and the field would benefit from confirmation or disconfirmation of these findings from other research groups.
Clinically, the link between PTSD and overgeneralization prescribes a therapeutic focus on stimulus events resembling features of the traumatic encounter in addition to the actual features of the encounter. Specifically, exposure treatments might focus on reducing fear-reactivity to both stimuli associated with the trauma and stimuli approximating those associated with the trauma.
2.5. Failure to inhibit fear
This final associative learning framework links PTSD to impaired mechanisms of fear inhibition, resulting in the expression of fear in the presence of safety cues (Davis et al., 2000; Jovanovic & Ressler, 2010). This model comes as a logical corollary of the above described theory of extinction from Davis and colleagues (2000) and Jovanovic and Ressler (2010), according to which impaired mechanisms of inhibitory fear-conditioning in PTSD are responsible for extinction-failure. From this perspective, all processes dependent on fear-inhibition, including but not limited to extinction, should be compromised in PTSD. Safety learning in the traumatic context is one such process because cues signaling periods of safety are effective only if inhibition of ongoing anxiety to the ambient threat of the environment is achieved. This model thus links PTSD to an inability to suppress fear in the presence of safety cues.
According to this perspective, our IED exposed veteran who continues to display conditioned fear to roadside objects, upon return to their benign pre-deployment environment, is suffering the effects of failed inhibition of fear in the presence of safety signals. Specifically, such roadside objects are experienced coincident with many potential environmental cues of safety including neighborhood people, shops, parks, and houses associated with, what may have been, the relative safety of his childhood years. Such safety cues are however unable to exert inhibitory control over the conditioned response to roadside objects leading to a continuation of the traumatic response in the benign, civilian context.
2.5.1 Empirical evidence
Support for the role of deficient fear-inhibition in PTSD-related conditioning abnormalities draws from neuroimaging data linking poor retention of extinction in PTSD to an under-functioning medial-prefrontal cortex (mPFC: Milad et al., 2009): a brain region thought to subserve fear reduction through its demonstrated inhibition of amygdaloid neurons (e.g., Quirk et al., 2003; Rosenkrantz et al., 2003). Further support for impaired mPFC engagement by fear-inducing stimuli in PTSD, that may instantiate compromised fear inhibition, is found by neuroimaging studies documenting reduced mPFC activation in those with, versus without, PTSD during exposure to traumatic pictures and sounds (Bremner et al., 1999), script driven traumatic imagery (Shin et al., 1999), and fearful faces (Shin et al., 2005; Williams et al., 2006). Additionally, two such studies found inverse relations between the severity of PTSD symptomatology and mPFC responses in PTSD patients (Shin et al., 2005; Williams et al., 2006), supporting the clinical relevance of decreased mPFC engagement during fear evocations.
A final line of work supporting impaired inhibition of fear in PTSD comes from studies applying a conditional discrimination paradigm (AX+/BX−) designed to assess the degree to which fear to a conditioned danger-cue is reduced when presented in tandum with a conditioned safety-cue. Results provide evidence for both decreased inhibition of fear-potentiated startle in the presence of a conditioned safety-cue (Jovanovic et al., 2010) and less transfer of that inhibition during the combined (configural) presentation of the conditioned danger- and safety-cue, among PTSD patients (Jovanovic et al., 2010, 2013). This reduced transfer of inhibition has recently been found to predict levels of PTSD in trauma exposed, deployed soldiers 7 months after testing (Sijbrandij, Engelhard, Lommen, Leer, and Baas, 2013), suggesting that impairments in fear inhibition may serve as a premorbid risk factor for later development of PTSD.
3. Non-associative fear-learning
In contrast to fear-conditioning, non-associative fear learning involves changes in reactivity to environmental stimuli that are not associatively linked to aversive outcomes. In the context of PTSD, non-associative mechanisms are thought to generate increases, or resistance to decreases, in fear reactivity to novel, intense, or fear-relevant stimuli. Contrasting the multiplicity of associative models of PTSD, non-associative accounts largely center on two mechanism of learning: habituation and sensitization.
3.1. Habituation
One of the most fundamental and ubiquitous forms of learning is habituation, whereby responding progressively declines with repeated stimulation (Groves & Thompson, 1970). In the context of traumatic responding, habituation refers to decreases in autonomic, behavioral, or neural responses to repeatedly presented novel, intense, or fear-relevant (unconditioned) stimuli. The failure of this type of habituation is proposed as a central contributor to the hyper-arousal cluster of PTSD symptoms (e.g., hyper-vigilance, exaggerated startle, and difficulty concentrating). For example, hyper-vigilance and exaggerated startle may be maintained in the benign post-traumatic context through persistent autonomic responding to reoccurring, and more or less irrelevant, stimuli. Additionally, the inability to filter out these reoccurring sensory stimuli likely compromises concentration by exhausting attentional resources otherwise available for processing more consequential stimulus events.
3.1.1. Empirical evidence
The failure-to-habituate theory of PTSD receives support from the well replicated finding of less steep habituation slopes to repeatedly presented intense acoustic tones in trauma survivors with, versus without, PTSD when assessing habituation with the skin conductance response (SCR: e.g., Orr et al., 1995; Orr, Solomon, et al., 1997; Shalev et al., 1992): an index of sympathetic arousal. Indeed, a fairly recent meta-analysis identified this SCR habituation effect as the most robust psychophysiological correlate of PTSD (Pole, 2007). These findings link PTSD to an impaired ability to autonomically adapt, or habituate, to intense environmental stimuli, and serve as experimental analogues of the sustained hyper-arousal and hyper-vigilance seen clinically. Although such data do not provide clarification on whether decreased SCR habituation in PTSD precedes or follows the traumatic exposure, a recent prospective study provides support for reduced SCR habituation as a pre-trauma risk factor for PTSD (Pole et al., 2009). These data are further supported by findings in monozygotic twins documenting strong genetic contributions to rates of SCR habituation, thus supporting SCR habituation as a premorbid, dispositional trait (Lykken et al., 1988). Unlike SCR measures of habituation, the weight of habituation effects assessed by heart-rate (HR) and eyeblink-startle responses to intense tones do not evidence attenuated habituation in PTSD (Pole, 2007). The reason for these differences across measures is unclear, though the presence of habituation effects in PTSD when measured with SCR: an index of sympathetic activation, but not HR: a measure that is sensitive to both sympathetic and parasympathetic influences, suggests that reduced habituation in PTSD is attributable to sympathetic abnormalities.
3.2. Sensitization
The direct inverse of habituation is sensitization: a non-associative learning mechanism whereby responses are amplified through repeated stimulation (Groves & Thompson, 1970). In the context of fear and anxiety, sensitization generally refers to the increase in fear-related responses to novel, intense, or fear-relevant unconditioned stimuli following activation of the fear system (Marks & Tobena, 1990). Essentially, fear sensitization is thought to arise from a fear-system rendered hyperexcitable by previous activation (Rosen & Schulkin, 1998).
A large body of work in animals has documented stress-related sensitization with some elucidation of its underlying mechanisms. The most common approach involves assessing changes in fear-relevant behaviors in rats following exposure to highly stressful conditions. In one such line of work, defensive responding—operationalized by startle reactivity to intense bursts of white noise—is reliably enhanced in rats after administration of strong electric footshocks (e.g., Davis, 1989). This startle potentiation, referred to as shock sensitization, may model the non-associative hyper-reactivity to intense, novel stimuli seen in PTSD, though it is worth noting that the non-associative nature of this effect has been brought into question by evidence imputing shock sensitization to associative fear of the context in which shock was delivered (for a review, see Richardson & Elsayed, 1998).
A second line of work exposing rats to aversive electric shocks in the service of assessing sensitization demonstrates the imperative of stressor uncontrollability for displays of stress-related sensitization (for a review, see Maier & Watkins, 2005). Specifically, rats exposed to inescapable (uncontrollable) versus escapable (controllable) shocks exhibit time-limited enhancement (sensitization) of such stress-related processes as avoidance of cat odors (William & Groux, 1993), avoidance of novel situations and flavors (Job & Barnes, 1995; Minor, 1990), fear-conditioning (Desiderato & Newman, 1971), suppression of appetitive behaviors (Maier, unpublished data), and avoidance of social interactions (Short & Maier, 1993). Importantly, these effects of uncontrollable stress are likely due to non-associative sensitization as they occur in contexts with no apparent resemblance to the shock-delivery environment and, as such, are unlikely the result of contextual conditioning. The possibility thus exists that oversensitivity to novel, intense, or fear-relevant stimuli of the kind seen in PTSD stems from trauma-induced stress-sensitization that is dependent on the uncontrollable nature of the trauma. The plausibility of this theory is supported by the view that the uncontrollable nature of trauma is central to the etiology of PTSD (e.g., Foa, Zinbarg, & Rothbaum, 1992; Volpicelli, Balaraman, Hahn, Wallace, & Bux, 1999).
One final related animal model induces sensitization through electrically stimulating, or partially kindling, the amygdala-based fear circuit with the aim of increasing the excitability of this circuit (for a review, see Rosen & Schulkin, 1998)1. Indeed, a number of studies document increases in fear-related behaviors following amygdala kindling in animals (e.g., Adamec, 1994. For example, Rosen and colleagues (1996) found partial kindling (only two stimulations) of the amygdala to enhance fear-potentiated startle (Rosen et al., 1996).
Additionally, affective consequences of epileptic discharges in the human temporal lobe (the cerebral structure encasing the amygdala) are marked by increases in reported fear and anxiety (e.g., Gloor, 1978). These findings support the theory that trauma-evoked anxiety sensitizes the fear system through a partial kindling of the amygdala, leaving it hyper-reactive to future traumatic encounters, and conferring risk for PTSD from subsequent trauma. Consistent with this idea are findings demonstrating a heightened incidence of PTSD among those with previous histories of trauma-exposure (e.g., Bremner et al., 1993; Breslau et al., 1999; Davidson, Hughes, & Blazer, 1991).
3.2.1. Empirical evidence
Perhaps the strongest evidence for greater sensitization of fear-related autonomic responses in PTSD, is psychophysiology studies assessing HR responses to repeatedly presented, high intensity acoustic stimuli, the majority of which document larger average HR responses in PTSD patients (e.g., Carson et al., 2007; Jovanovic et al., 2009; Metzger et al., 1999; Orr et al., 1995, 1997, 2003; Shalev et al., 1992, 2000). Several studies have also found stronger SCR to these same acoustic stimuli in PTSD (e.g., Shalev et al., 1992, 1997), though somewhat less robustly than HR elevations (for a review, see Pole, 2007). Because these psychophysiological results were largely found in trauma exposed individuals with versus without PTSD, such findings represent viable support for stronger, trauma-related sensitization of the autonomic nervous system as a non-associative learning correlate of PTSD. Although it could be argued that this type of increased HR responding is due to risk factors predating the trauma (e.g., genetic predisposition) rather than de novo learning, both behavioral-genetic data (Orr et al., 2003) and prospective assessments (Shalev et al., 2000) characterize this effect as an acquired (e.g., learned) marker of PTSD rather than a pre-existing risk factor conferred by heredity or the pre-traumatic environment.
In contrast to HR findings, enhanced psychophysiological activation to high intensity acoustic stimuli is not consistently found in PTSD when operationalized by baseline measures of the startle-blink reflex (Grillon & Baas, 2003). The mixed nature of these results is surprising given that exaggerated startle is a diagnostic feature of PTSD (American Psychiatric Association, 2013). Indeed, findings in rodents and humans reliably demonstrate the potentiation of startle magnitudes when an organism is in a state of anxious arousal (Davis & Astrachan, 1978; Grillon, Ameli, Woods, Merikangas, & Davis, 1991), an emotional state clearly axiomatic to the PTSD diagnosis. Importantly, a review of this literature reveals an interesting pattern of results whereby elevated baseline startle magnitudes in PTSD are found in experimental contexts in which stressful procedures ensue (e.g., threat of electric shock), but are not found in contexts relatively free of experimental stress (Grillon & Baas, 2003). This dissociation may best be understood from a kindling perspective on PTSD (e.g., Pitman, Orr, & Shalev, 1993; Post & Weiss, 1998; Rosen & Schulkin, 1998), according to which psychological trauma sensitizes the fear system among those prone to PTSD, and renders the amygdala-based fear circuit hyper-excitable to such future fear-evoking situations as stressful experimental contexts. On its face, this conceptualization seems at odds with findings of null differences in startle potentiation between survivors with and without PTSD to instructed, discrete cues signaling imminent delivery of aversive stimulation (e.g., Grillon, Morgan, Davis, & Southwick, 1998; Grillon et al., 2009). That is, the kindling model would predict greater trauma-related sensitization of the fear circuit in PTSD leaving the circuit hyper-excitable to threat, and culminating in stronger fear-potentiated startle to both contextual and discretely-cued signals of danger. This apparent inconsistency may be reconciled within the strong situation framework (Lissek, Pine, & Grillon, 2006), whereby strong threats constitute cues of potent, imminent, and certain danger that evoke the adaptive fear response among anxiety patients and healthy controls alike; and weaker threats constitute cues of less potent, imminent, and certain danger to which those with an anxiety disorder are hyper-reactive.
In our instance, the situational strength of discrete threat cues is stronger than that of contextual threat by virtue of the increased imminence and certainty of the discretely cued danger. Thus, discrete threat cues should elicit a fear response regardless of whether or not the individual’s fear system has been made excitable through stress-sensitization, and regardless of whether or not the individual has PTSD. By contrast, such weak situations as contextual threat may elicit fear only in individuals for whom hyper-excitability of the fear system was achieved through sensitization (e.g., PTSD patients), with all others experiencing only sub-threshold levels of fear activation. Increased baseline startle in stressful contexts found in PTSD, therefore, supports a role for sensitization via kindling in the disorder, despite null patient-control differences in startle potentiation to discrete threat cues.
Further findings consistent with the kindling model of PTSD derive from neuroimaging studies tracking the neural correlates of fearful-face processing across those with and without PTSD. Presentations of fearful facial expressions reliably activate the human amygdala (e.g., Hariri et al., 2003; Morris et al., 1996; Phillips et al., 1997), and have become a noninvasive means of probing amygdala function in the intact human brain. If, as held by the kindling model, trauma-evoked fear renders the amygdala-based fear circuit hyper-excitable in survivors who develop PTSD, post-traumatic presentations of fearful faces should produce stronger amygdala reactions in those with PTSD. Several studies confirm this prediction with fearful faces (vs. happy faces) evoking stronger amygdala reactions in trauma survivors with PTSD compared to those without PTSD (e.g., Rauch et al., 2000; Shin et al., 2005; Felmingham et al., 2010). Additional findings of amygdala hyper-excitability to neutral faces in those with versus without PTSD, indicate that the effect may not be restricted to ‘fearful’ faces (Brunetti et al., 2010). It should be noted that the increased amygdala reactivity in PTSD may reflect a premorbid risk factor rather than abnormal stress sensitization of this circuit; prospective research on amygdala responses to faces in PTSD is needed to clarify this issue.
The above described experimental support for the sensitization-by-kindling hypothesis of PTSD is well complimented by phenomenological data revealing greater risk for PTSD among survivors with a history of previous traumatic exposures (e.g., Breslau, Chilcoat, Kessler, & Davis, 1999; Brewin, Andrews, Valentine, 2000; Ozer, Best, Lipsey, & Weiss, 2003). These findings suggest that psychological trauma kindles the fear-system, and renders it maladaptively hyper-reactive to future trauma.
The final sensitization theory reviewed herein imputes non-associative learning correlates of PTSD to the uncontrollable nature of the stressor (trauma). This theory of PTSD has received little experimental testing, which is unfortunate given the general view that uncontrollability and unpredictability are critical features of PTSD-inducing events (e.g., Foa et al., 1992; Mineka & Zinbarg, 2006; Volpicelli et al., 1999). This general view stems largely from animal data documenting behavioral consequences of uncontrollable and unpredictable threat that are analogous to many symptoms of PTSD (for a review, see Foa et al., 1992). Non-experimental evidence for this perspective derives from studies documenting inverse relations between perceptions of control and PTSD symptoms among survivors of sexual victimization (Bolstad & Zinbarg, 1997; Regehr, Cadell, & Jansen, 1999) and criminal assault (Kushner, Riggs, Foa, & Miller, 1993). Extant experimental support comes from initiatives testing the relation between clinical anxiety and threat predictability (e.g., Grillon et al., 2008), which is a characteristic of threat distinct from, but highly related to, controllability. In a recent study conducted by Grillon et al. (2009), PTSD patients displayed normative levels of fear-potentiated startle to threat cues signaling imminent aversive noises (e.g., predictable threat), but elevated fear-potentiated startle during a sustained period in which aversive noises were delivered at random (i.e., unpredictable threat). These findings demonstrate a heightened reactivity to threat-unpredictability in PTSD, and provide a degree of support for the hypothesis that uncontrollable/unpredictable threat differentially sensitizes the fear system of those with, versus without, PTSD.
4. Toward an integrated account of learning findings in PTSD
Studies reviewed thus far report a variety of results across a multiplicity of stress-related learning mechanisms with proposed relevance to PTSD. Although no unified conceptualization of these results is readily obvious, the social psychological concept of the strong situation may provide an interpretive framework with which to deduce the beginnings of a unified account. As described previously, the strong threat situation represents an experimental condition that unambiguously signals aversive events of high certainty and imminence, and evokes the adaptive fear response among anxiety patients and healthy controls alike. Weakening the experimental situation by reducing the temporal proximity of the stressor, decreasing the predictive value of the danger cue, and/or increasing the ambiguity of the threat information is predicted to facilitate the emergence of patient-control differences in anxious reactivity.
In our context, the discrete conditioned danger cue is something of a strong situation by virtue of its relatively unambiguous signaling of certain and imminent danger. Contextual threat, unpredictable threat, and safety cues resembling threat cues (to which fear generalizes) may be thought to constitute weaker threats. That is, compared to discrete conditioned danger cues, contextual threat communicates danger of less imminence; unpredictable threat signals danger of less certainty; and cues approximating the likeness of the danger cue constitute threats of greater ambiguity. Consistent with predictions of the strong situation framework, PTSD-control differences in anxious reactivity have not been reliably found to the stronger discrete conditioned danger cue and seem to emerge under weaker situations of contextual threat (e.g., Grillon & Baas, 2003), unpredictable threat (Grillon et al., 2009), and perceptually degraded danger cues (Kaczkurkin, Burton, Chazin, & Lissek, 2013). Although this framework does not thoroughly account for the rich variety of findings in this literature, it may help set the stage for future, more comprehensive, integrative explanations.
5. Research limitations and future directions
The past two decades have seen substantial increases in lab-based testing of associative and non-associative learning abnormalities in PTSD. Although tests of extinction failure in PTSD are well represented in this recent literature, the variety of other promising learning theories of PTSD detailed herein have, to date, received relatively little empirical attention. Indeed, PTSD abnormalities in contextual anxiety, conditioned generalization, and failure to inhibit fear to safety cues have each, more or less, been completed by single laboratories, and the field awaits replication of these findings by other laboratories. Additionally, the vast majority of conditioning studies in PTSD have targeted processes of Pavlovian fear-conditioning (extinction, over-generalization, etc.) and have not tested abnormalities in instrumental fear-conditioning (i.e., learned avoidance). This is a significant omission, given the centrality of conditioned avoidance of trauma reminders to posttraumatic psychopathology (American Psychiatric Association, 2013). In fact, some evidence from clinical research implicates avoidance as the class of symptoms most detrimental to psychosocial functioning in trauma survivors (Hendrix, Erdmann, Briggs, 1998; Samper, Taft, King, King, 2004; Solomon & Mikulincer, 2007), and may predict posttraumatic psychopathology better than other trauma-related symptom clusters (Bryant, Marosszeky, Crooks, Baguley, & Gurka, 2000; North et al., 1999; North, Oliver, & Pandya, 2012).
In addition to this problem of investigative scope, existing studies often identify PTSD–related learning abnormalities that are not unambiguously attributable to a single learning mechanism. For example, over-responding to the conditioned safety cue (CS−) repeatedly found in PTSD patients, could reflect over-responding to all novel cues (i.e., sensitization), heightened transfer of responding to cues resembling the CS+ (overgeneralization), an impaired ability to accurately learn the link between the CS+ and US (associative learning deficits) or failure to inhibit fear to safety cues. Additionally, resistance to extinguish fear could reflect overly strong excitation learning to the CS+ at acquisition, or too little learned fear inhibition to the CS+ at extinction. Some of these interpretive problems can be addressed by way of improved experimental design. For example, further tests of over-reactivity to the CS− in PTSD would benefit from use of generalization methods in which fear responding is assessed to CS− that are perceptually similar and dissimilar to the CS+. That is, over-responding in PTSD patients to perceptually similar but not dissimilar CS− reflects an over-generalization process rather than a general over-responding to either all CS− (as predicted by the failure-to-inhibit hypothesis) or all novel stimuli (as predicted by the sensitization hypothesis).
Other efforts to link specific learning abnormalities to PTSD may benefit from brain imaging techniques. For example, the contributions of fear excitation versus fear inhibition processes toward extinction abnormalities in PTSD may be dissociated by the specific brain correlates of these abnormalities, with perturbed activation in areas attributed to fear excitation (e.g., amygdala, anterior insula) implicating excitatory abnormalities and perturbations in brain areas associated with fear inhibition (e.g., ventromedial prefrontal cortex) suggestive of inhibitory abnormalities. This approach has begun to yield fruit, with brain imaging results attributing deficiencies in extinction recall to perturbations in circuitry associated with fear inhibition (e.g., Milad et al., 2009).
Once methodologies for isolating specific learning abnormalities are identified or developed, the field would benefit from studies designed to assess multiple learning mechanisms in samples of traumatic stress patients large enough to distinguish different types of learning abnormalities in different clusters of patients. Because it is likely different learning mechanisms (e.g., sensitization, extinction failure, generalization, contextual anxiety) contribute differently to PTSD across different individuals, it would be useful to identify subclasses of PTSD patients affected by different mechanism alone or in combination. Targeting a single learning mechanism putatively associated with PTSD, as done by the majority of extant studies, may underestimate learning effects in PTSD by capturing learning aberrancies in only the subset of PTSD patients displaying abnormalities in the particularly targeted learning mechanism, and missing individuals with abnormalities in other learning processes.
An additional limitation of this literature that besets much of experimental psychopathology is whether abnormalities in patients generated by lab-based testing reflect pre-morbid risk factors or ongoing disease processes. The dissociation of the former from the latter is facilitated by prospective data relating pre-morbid to post-morbid findings, as well as behavioral genetic methods designed to disentangle the influence of genetic risk from that of environment, on onset and maintenance of the disorder. The available data of this type in the PTSD literature on learning abnormalities, characterizes effects of poor recall of extinction and over-sensitization as acquired markers of PTSD (Milad et al., 2008; Orr et al., 2003; Shalev et al., 2000), and deficits in original extinction learning (Guthrie and Bryant, 2006; Lommen, Engelhard, Sijbrandij, van den Hout, and Herman, 2013), enhanced contextual anxiety (Pole et al., 2009) and deficient habituation (Pole et al., 2009) as pre-trauma risk factors for PTSD. Nevertheless, more data is needed before conclusions can be drawn, with confidence, regarding the value of a given learning abnormality as a vulnerability factor for, versus a diagnostic marker of, PTSD. One fortuitous consequence of the cross-species relevance of associative and non-associative learning processes implicated in the etiology of PTSD, is the availability of rich neuroscience findings in animals from which to generate and test psychobiological accounts of PTSD. For example, data in animals suggests that sensitization-like PTSD symptoms in the hyper-arousal cluster are, in part, instantiated by the activation of a subset of serotonergic neurons in the dorsal raphe nucleus (DRN) projecting to the basolateral amygdala during uncontrollable stress (for a review, see Maier & Watkins, 2005). Additionally, fear-conditioning research in animals employing lesion and single-cell-recording methods have linked extinction and its retention to the vmPFC (e.g., Milad & Quirk, 2002; Quirk, Russo, Barron, & Lebron, 2000), which is a brain region strongly implicated in fear reduction through inhibitory influences on the amygdala (Quirk et al., 2003; Rosenkranz et al., 2003). These animal findings have inspired a fruitful line of human work testing the medial prefrontal cortex as a neural locus of extinction abnormalities in PTSD (e.g., Milad et al., 2009). Future studies testing learning models of PTSD should continue to test neural hypotheses informed by the animal literature. For example, animal findings suggest the importance of testing the role of the bed-nucleus-of-the-stria-terminalis, hippocampus, and vmPFC in putative, PTSD abnormalities in contextual anxiety, overgeneralization of conditioned fear, and failure to inhibit fear, respectively. Additionally, animal data have important implications for up- and down-regulation of these learning processes and should be brought to bear by efforts to develop novel treatments for PTSD.
In addition to the importance of future efforts to neurally characterize learning correlates of PTSD, genetic work in this area is needed. Although historically, learning theories posit stimulus-response accounts of behavior that draw exclusively on environmental causes of behavior (e.g., Skinner, 1953), the emergence of PTSD among some survivors of comparable traumas is evidence of gene by environment interactions. As such, modern learning theories of PTSD must account for the genetic liabilities that dispose some trauma survivors to maladaptive learning following traumatic encounters. Though this effort is underway (for a review see Amstadter, Nugent, & Koenen, 2009), more work of this kind is needed.
A final limitation of this literature lies in the inability of learning models to account for the full richness of PTSD phenomenology. Though associative and non-associative learning abnormalities are well positioned to account for a subset of PTSD symptoms (re-experiencing, avoidance, hyper-arousal), they do not seem particularly well suited to explain the full clinical picture of PTSD, including symptoms related to guilt, shame, dissociation, and anger. Future learning research should assess the relatedness of learning abnormalities to these other types of symptoms. Though learning mechanisms will probably not provide a full explanation of such symptoms, important interactions may emerge.
6. Summary and Concluding thoughts
A rich array of learning-based theories of PTSD have emerged during the century following Watson and Rayner’s seminal experiment on “Little Albert” (1920). Such learning models have offered mechanized accounts of PTSD based on basic learning processes that are readily probed and objectively quantified in the laboratory environment. It is thus surprising that empirical testing of these models has occurred primarily in the most recent two decades. During this time, evidence for a variety of PTSD-related abnormalities in basic learning processes have been found, the most promising of which include slowed habituation of skin conductance responses (SCRs) to intense environmental stimuli, heightened sensitization of heart-rate responses to loud acoustic noises, failure to retain extinction learning as indicated by increased SCRs and reduced activation in brain areas associated with fear inhibition during exposure to the previously extinguished CS+, overgeneralization of conditioned fear as indicated by elevated perceptions of shock risk to stimuli resembling the CS+, heightened contextual anxiety as indicated by larger fear-potentiated startle during experimental conditions of unpredictable threat, and deficits inhibiting fear to safety cues as indicated by reduced suppression of fear-potentiated startle in the presence of safety cues. Importantly, the cross-species relevance of such learning processes brings to bear a wealth of animal data from which to infer the neural basis of these PTSD-related learning abnormalities. This translational bridge between animal and PTSD findings promises to contribute essentially to the field’s dedicated efforts toward future brain-based diagnostics and neutrally-targeted interventions for PTSD.
Highlights.
Associative and non-associative learning accounts of PTSD are described.
Empirical evidence for and against these accounts are reviewed.
Learning abnormalities in PTSD most supported by the literature are identified, and include slowed habituation, heightened sensitization, failure to retain extinction learning, overgeneralization of conditioned fear, heightened contextual anxiety, and deficits inhibiting fear to safety cues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Full kindling produces seizures in the activated tissue while partial kindling is usually limited to only two stimulations and produces no behavioral signs of seizure.
References
- Adamec RE, Morgan HD. The effect of kindling of different nuclei in the left and right amygdala on anxiety in the rat. Physiology and Behavior. 1994;55:1–12. doi: 10.1016/0031-9384(94)90002-7. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Amstadter AB, Nugent NR, Koenen KC. Genetics of PTSD: Fear Conditioning as a Model for Future Research. Psychiatr Ann. 2009;39:358–367. doi: 10.3928/00485713-20090526-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: Effects of auditory cortex lesions in a computational model and in rats. Cerebral Cortex. 1997;7:157–165. doi: 10.1093/cercor/7.2.157. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: Evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behaviour Research and Therapy. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bolstad B, Zinbarg R. Sexual victimization, generalized perception of control, and posttraumatic stress disorder symptom severity. Journal of Anxiety Disorders. 1997;11:523–540. doi: 10.1016/s0887-6185(97)00028-5. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and Behavioral Processes in Extinction. Learning and Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Swartzentruber D. Sources of relapse after extinction in Pavlovian and instrumental learning. Clinical Psychology Review. 1991;11(2):123–140. [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. American Journal of Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. American Journal of Psychiatry. 1993;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biological Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, et al. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychological Medicine. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Chilcoat HD, Kessler RC, Davis GC. Previous Exposure to Trauma and PTSD Effects of Subsequent Trauma: Results From the Detroit Area Survey of Trauma. American Journal of Psychiatry. 1999;156:902–907. doi: 10.1176/ajp.156.6.902. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Marosszeky JE, Crooks J, Baguley I, Gurka J. Coping style and post-traumatic stress disorder following severe traumatic brain injury. Brain injury. 2000;14:175–180. doi: 10.1080/026990500120826. [DOI] [PubMed] [Google Scholar]
- Brunetti M, Sepede G, Mingoia G, Catani C, Ferretti A, Merla A, et al. Elevated response of human amygdala to neutral stimuli in mild post traumatic stress disorder: neural correlates of generalized emotional response. Neuroscience. 2010;168:670–679. doi: 10.1016/j.neuroscience.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Burriss L, Ayers E, Ginsberg J, Powell DA. Learning and memory impairment in PTSD: Relationship to depression. Depression and Anxiety. 2008;25:149–157. doi: 10.1002/da.20291. [DOI] [PubMed] [Google Scholar]
- Burriss L, Ayers E, Powell DA. Combat veterans show normal discrimination during differential trace eyeblink conditioning, but increased responsivity to the conditioned and unconditioned stimulus. Journal of Psychiatr Research. 2007;41:785–794. doi: 10.1016/j.jpsychires.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Carson MA, Metzger LJ, Lasko NB, Paulus LA, Morse AE, Pitman RK, Orr SP. Physiologic reactivity to startling tones in female Vietnam nurse veterans with PTSD. J Trauma Stress. 2007;20:657–666. doi: 10.1002/jts.20218. [DOI] [PubMed] [Google Scholar]
- Davidson JRT, Hughes D, Blazer DG. Post-traumatic stress disorder in the community: an epidemiological study. Psychological Medicine. 1991;21:713–721. doi: 10.1017/s0033291700022352. [DOI] [PubMed] [Google Scholar]
- Davis M. Sensitization of the acoustic startle reflex by footshock. Behavioral Neuroscience. 1989;103:495–503. [PubMed] [Google Scholar]
- Davis M, Astrachan DI. Conditioned fear and startle magnitude: effects of different footshock or backshock intensities used in training. Journal of Experimental Psychology Animal Behavior Process. 1978;4:95–103. doi: 10.1037//0097-7403.4.2.95. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Gewirtz J. Neural systems involved in fear inhibition: extinction and conditioned inhibition. In: Myslobodsky M, Weiner I, editors. Contemporary issues in modeling psychopathology. 2000. pp. 113–142. [Google Scholar]
- Desiderato O, Newman A. Conditioned suppression produced in rats by tones paired with escapable or inescapable shock. Journal of Comparative Physiology and Psychology. 1971;77:427–443. doi: 10.1037/h0031880. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. The learning theory model of neurosis: a new approach. Behaviour Research and Therapy. 1976;14:251–267. doi: 10.1016/0005-7967(76)90001-2. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. The conditioning model of neurosis. Behavioral and Brain Sciences. 1979;2:155–199. [Google Scholar]
- Eysenck HJ, Rachman SJ. Causes and cures of neurosis. London: Routledge & Kegan Paul; 1965. [Google Scholar]
- Felmingham K, et al. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. Journal of Abnormal Psychology. 2010;119:241–247. doi: 10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: An animal model. Psychological Bulletin. 1992;112:218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- Geuze E, Vermetten E, Ruf M, de Kloet CS, Westenberg HG. Neural correlates of associative learning and memory in veterans with posttraumatic stress disorder. Journal of Psychiatric Research. 2008;42:659–669. doi: 10.1016/j.jpsychires.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Gloor P. Inputs and outputs of the amygdala: What the amygdala is trying to tell the rest of the brain. New York: Plenum Press; 1978. [Google Scholar]
- Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biological Psychiatry. 2002;51:851–858. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clinical Neurophysiology. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behavioral Neuroscience. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C, Lissek S, Rabin S, McDowell D, Dvir S, Pine DS. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. American Journal of Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CA., III Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with post-traumatic stress disorder. Biological Psychiatry. 1998;44:1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biological Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosomatic medicine. 2006;68(2):307–311. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Hendrix CC, Erdmann MA, Briggs K. Impact of Vietnam veterans’ arousal and avoidance on spouses’ perceptions of family life. American journal of Family Therapy. 1998;26:115–128. [Google Scholar]
- Job RF, Barnes BW. Stress and consumption: Inescapable shock, neophobia, and quinine finickiness in rats. Behavioral Neuroscience. 1995;109:106–116. doi: 10.1037//0735-7044.109.1.106. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. American Journal of Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, Kozarić-Kovacić D. Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. International Journal of Psychophysiology. 2009;71:264–268. doi: 10.1016/j.ijpsycho.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Sakoman AJ, Kozarić-Kovačić D, Meštrović AH, Duncan EJ, Davis M, Norrholm SD. Acute stress disorder versus chronic posttraumatic stress disorder: inhibition of fear as a function of time since trauma. Depression and Anxiety. 2013;30(3):217–224. doi: 10.1002/da.21991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin AN, Burton PC, Chazin SM, Lissek SM. Generalization of conditioned fear in individuals with PTSD. In: Kaczkurkin A, Lissek S, editors. Psychophysiological and neurobiological indices of internalizing psychopathology; Symposium presented at the annual conference of the Society for Psychophysiological Research; Florence, Italy. 2013. (Chairs) [Google Scholar]
- Kushner MG, Riggs DS, Foa EB, Miller SM. Perceived controllability and the development of posttraumatic stress disorder (PTSD) in crime victims. Behaviour Research and Therapy. 1993;31:1105–1110. doi: 10.1016/0005-7967(93)90048-y. [DOI] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin S, Cornwell BR, Alvarez RP, Pine DS, Grillon C. Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behaviour Research and Therapy. 2008;46:678–687. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Pine DS, Grillon C. The strong situation: A potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology. 2006;72:265–270. doi: 10.1016/j.biopsycho.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear-conditioning in the anxiety disorders: A meta-analysis. Behaviour Research and Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, Heller RE, Luckenbaugh D, Geraci M, Pine DS, Grillon C. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. American Journal of Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommen MJ, Engelhard IM, Sijbrandij M, van den Hout MA, Hermans D. Pre-trauma individual differences in extinction learning predict posttraumatic stress. Behaviour research and therapy. 2013;51:63–67. doi: 10.1016/j.brat.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Iacono WG, Haroian K, McGue M, Bouchard TJ. Habituation of the skin conductance responses to strong stimuli: A twin study. Psychophysiology. 1988;25:4–15. doi: 10.1111/j.1469-8986.1988.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: The roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience and Biobehavioral Reviews. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Marks I, Tobena A. Learning and unlearning fear: A clinical and evolutionary perspective. Neuroscience and Biobehavioral Reviews. 1990;14:365–384. doi: 10.1016/s0149-7634(05)80059-4. [DOI] [PubMed] [Google Scholar]
- Marx BP, Sloan DM. Peritraumatic dissociation and experiential avoidance as predictors of posttraumatic stress symptomatology. Behaviour Research and Therapy. 2005;43:569–583. doi: 10.1016/j.brat.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Metzger LJ, Orr SP, Berry NJ, et al. Physiologic reactivity to startling tones in women with PTSD. Journal of Abnormal Psychology. 1999;108:347–352. doi: 10.1037//0021-843x.108.2.347. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. Journal of Psychiatric Research. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Miller NE. Studies of fear as a learnable drive: I. Fear as motivation and fear reduction as reinforcement in the learning of new responses. Journal of Experimental Psychology. 1948;38:89–101. doi: 10.1037/h0058455. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: it's not what you thought it was. American Psychologist. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Minor TR. Conditioned fear and neophobia following inescapable shock. Animal Learning and Behavior. 1990;18:222–226. [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Mowrer OH. On the dual nature of learning: a reinterpretation of “conditioning” and “problem solving”. Harvard Educational Review. 1947;17:102–148. [Google Scholar]
- Mowrer OH. Learning theory and behavior. New York: Wiley; 1960. [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biological Pychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North CS, et al. Psychiatric disorders among survivors of the Oklahoma City bombing. JAMA. 1999;282:755–762. doi: 10.1001/jama.282.8.755. [DOI] [PubMed] [Google Scholar]
- North CS, Oliver J, Pandya A. Examining a comprehensive model of disaster-related posttraumatic stress disorder in systematically studied survivors of 10 disasters. Am J Public Health. 2012;102:e40–e48. doi: 10.2105/AJPH.2012.300689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Metzger LJ, et al. Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1995;104:75–82. doi: 10.1037//0021-843x.104.1.75. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Hu FB, Shalev AY, et al. Physiologic responses to sudden loud tones in monozygotic twins discordant for combat exposure: Association with posttraumatic stress disorder. Archives of General Psychiatry. 2003;60:283–288. doi: 10.1001/archpsyc.60.3.283. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109:290–298. [PubMed] [Google Scholar]
- Orr SP, Milad MR, Metzger LJ, Lasko NB, Gilbertson MW, Pitman RK. Effects of beta blockade, PTSD diagnosis, and explicit threat on the extinction and retention of an aversively conditioned response. Biological Psychology. 2006;73:262–271. doi: 10.1016/j.biopsycho.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Orr SP, Solomon Z, Peri T, et al. Physiologic responses to loud tones in Israeli veterans of the 1973 Yom Kippur War. Biological Psychiatry. 1997;41:319–326. doi: 10.1016/s0006-3223(95)00671-0. [DOI] [PubMed] [Google Scholar]
- Ozer E, Best S, Lipsey T, Weiss D. Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychological Bulletin. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Conditioned reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Peri T, Ben Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in postraumatic stress disorder. Biological Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Pittman RK, Orr SP, Shalev AY. Once bitten, twice shy: Beyond the conditioning model of PTSD. Biol Psychiatry. 1993;44:193–206. doi: 10.1016/0006-3223(93)90132-w. [DOI] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR. Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biological Psychiatry. 2009;65:235–240. doi: 10.1016/j.biopsych.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, Weiss SRB. Sensitization and kindling phenomena in mood, anxiety, and obsessive–compulsive disorders: the role of serotonergic mechanisms in illness progression. Biological Psychiatry. 1998;44:193–206. doi: 10.1016/s0006-3223(98)00144-9. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. Journal of Neuroscience. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. Journal of Neuroscience. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: A functional MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Regehr C, Cadell S, Jansen K. Perceptions of control and long-term recovery from rape. American Journal of Orthopsychiatry. 1999;69:110–115. doi: 10.1037/h0080386. [DOI] [PubMed] [Google Scholar]
- Richardson R, Elsayed H. Shock sensitization of startle in rats: The role of contextual conditioning. Behavioral Neuroscience. 1998;112:1136–1141. doi: 10.1037//0735-7044.112.5.1136. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Hamerman E, Sitcoske M, Glowa JR, Schulkin J. Hyperexcitability: Exaggerated fear-potentiated startle produced by partial amygdala kindling. Behavioral Neuroscience. 1996;110:43–50. doi: 10.1037//0735-7044.110.1.43. [DOI] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychological Review. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. Journal of Neuroscience. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BA, Foa EB. Subtypes of posttraumatic stress disorder and duration of symptoms. In: Davidson JR, Foa EB, editors. Posttraumatic Stress Disorder: DSM-IV and Beyond. 23–35. Washington, DC: American Psychiatric Press; 1993. [Google Scholar]
- Rothbaum BO, Foa EB. Post-traumatic stress disorder and sleep. New England Journal of Medicine. 2002;346:1334–1335. [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Annals of the New York Academy of Science. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Seligman MEP, Binik YM. The safety signal hypothesis. In: Davis H, Hurwitz HMB, editors. Operant-Pavlovian Interactions. New York: Hillsdale; 1977. pp. 165–187. [Google Scholar]
- Shalev AY, Orr SP, Peri T, Schreiber S, Pitman RK. Physiologic responses to loud tones in Israeli patients with posttraumatic stress disorder. Archives of General Psychiatry. 1992;49:870–875. doi: 10.1001/archpsyc.1992.01820110034005. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Peri T, Brandia D, et al. Auditory startle response in trauma survivors with posttraumatic stress disorder: a prospective study. American Journal of Psychiatry. 2000;157:255–261. doi: 10.1176/appi.ajp.157.2.255. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Peri T, Orr SP, et al. Auditory startle responses in help-seeking trauma survivors. Psychiatry Research. 1997;69:1–7. doi: 10.1016/s0165-1781(96)03001-6. [DOI] [PubMed] [Google Scholar]
- Shin LM, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, et al. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. American Journal of Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Short KR, Maier SF. Stressor controllability, social interaction, and benzodiazepine systems. Pharmacology, Biochemistry and Behavior. 1993;45:1–9. doi: 10.1016/0091-3057(93)90128-g. [DOI] [PubMed] [Google Scholar]
- Shvil E, Sullivan GM, Schafer S, Markowitz JC, Campeas M, Wager TD, Neria Y. Sex differences in extinction recall in posttraumatic stress disorder: A pilot fMRI study. Neurobiology of learning and memory. 2014 doi: 10.1016/j.nlm.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijbrandij M, Engelhard IM, Lommen MJ, Leer A, Baas JM. Impaired fear inhibition learning predicts the persistence of symptoms of posttraumatic stress disorder (PTSD) Journal of Psychiatric Research. 2013;47(12):1991–1997. doi: 10.1016/j.jpsychires.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Simpson T, Jakupcak M, Luterek JA. Fear and avoidance of internal experiences among patients with substance use disorders and PTSD: The centrality of anxiety sensitivity. Journal of Traumatic Stress. 2006;19:481–491. doi: 10.1002/jts.20128. [DOI] [PubMed] [Google Scholar]
- Samper RE, Taft CT, King DW, King LA. Posttraumatic stress disorder symptoms and parenting satisfaction among a national sample of male vietnam veterans. J Trauma Stress. 2004;17:311–315. doi: 10.1023/B:JOTS.0000038479.30903.ed. [DOI] [PubMed] [Google Scholar]
- Skinner BF. Science and Human Behavior. New York: Macmillan; 1953. [Google Scholar]
- Solomon Z, Mikulincer M. Posttraumatic intrusion, avoidance, and social functioning: a 20-year longitudinal study. J Consult Clin Psychol. 2007;75:316–324. doi: 10.1037/0022-006X.75.2.316. [DOI] [PubMed] [Google Scholar]
- van Meurs B, Wiggert N, Wicker I, Lissek S. Maladaptive behavioral consequences of conditioned fear-generalization: A pronounced, yet sparsely studied, feature of anxiety pathology. Behaviour Research and Therapy. 2014;57:29–37. doi: 10.1016/j.brat.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli J, Balaraman G, Hahn J, Wallace H, Bux D. The Role of uncontrollable trauma in the development of PTSD and alcohol addiction. Alcohol Research & Health. 1999;23:256–262. [PMC free article] [PubMed] [Google Scholar]
- Watson JB, Rayner R. Conditioned emotional reactions. Journal of Experimental Psychology. 1920;3:1–14. [Google Scholar]
- Werner NS, Meindl T, Engel RR, Rosner R, Riedel M, Reiser M, Fast K. Hippocampal function during associative learning in patients with posttraumatic stress disorder. Journal of Psychiatric Research. 2009;43:309–318. doi: 10.1016/j.jpsychires.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: Evidence from second-order conditioning. American Journal of Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Williams JL, Groux ML. Exposure to various stressors alters preferences for natural odors in rats (Rattus norvegicus) Journal of Comparative Psychology. 1993;107:39–47. doi: 10.1037/0735-7036.107.1.39. [DOI] [PubMed] [Google Scholar]
- Williams LM, et al. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. NeuroImage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]