Introduction
Temozolomide (TMZ) is an alkylating drug most often used in the treatment of primary brain malignancies. Herein, we report an unusual cutaneous adverse drug reaction (ADR) associated with TMZ and characterized by temporally distinct dermal and subcutaneous hypersensitivity reactions.
Report of a case
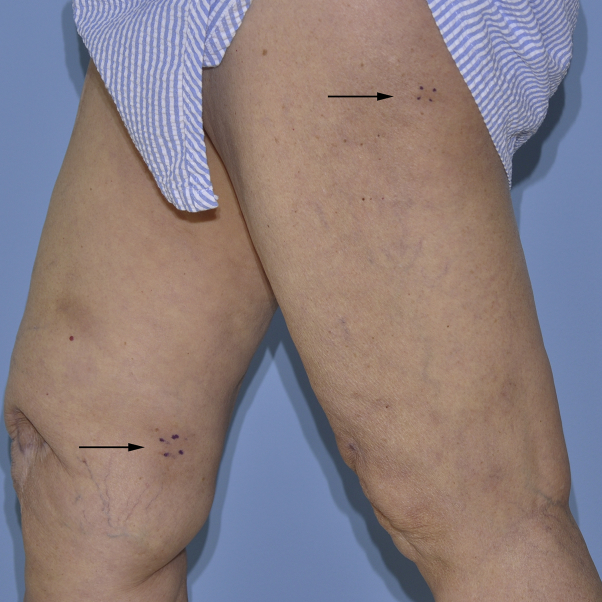
A woman in her 70s had right frontal anaplastic astrocytoma (World Health Organization grade III). The patient underwent a gross total tumor resection and a 6-week course of postoperative radiation therapy (6000 cGy) with concurrent TMZ (75 mg/m2) by mouth daily. Four weeks after completion of radiation, she started adjuvant TMZ (150 mg/m2 by mouth), following the standard schedule for high-grade gliomas (5 consecutive days per 28-day cycle). During the second cycle, she had persistent red tender subcutaneous nodules on her bilateral thighs without associated systemic symptoms (Fig 1). There was no history of any recent infections or other new medications, and there was no personal or family history of inflammatory bowel disease, sarcoidosis, lupus erythematosus, or chronic lower extremity edema. Histopathologic examination of 2 skin biopsies sections found mixed septal and lobular panniculitis with a mixed cell inflammatory infiltrate containing eosinophils (Fig 2, A). Antinuclear antibody and rheumatoid factor serologic findings were negative, and a complete blood cell count was within normal limits. The skin nodules spontaneously resolved over a few months without TMZ dose reduction or other specific intervention. TMZ was discontinued after 6 cycles.
Fig 1.
Clinical overview image of the legs shows subcutaneous red nodules on the bilateral lower extremities (black arrows).
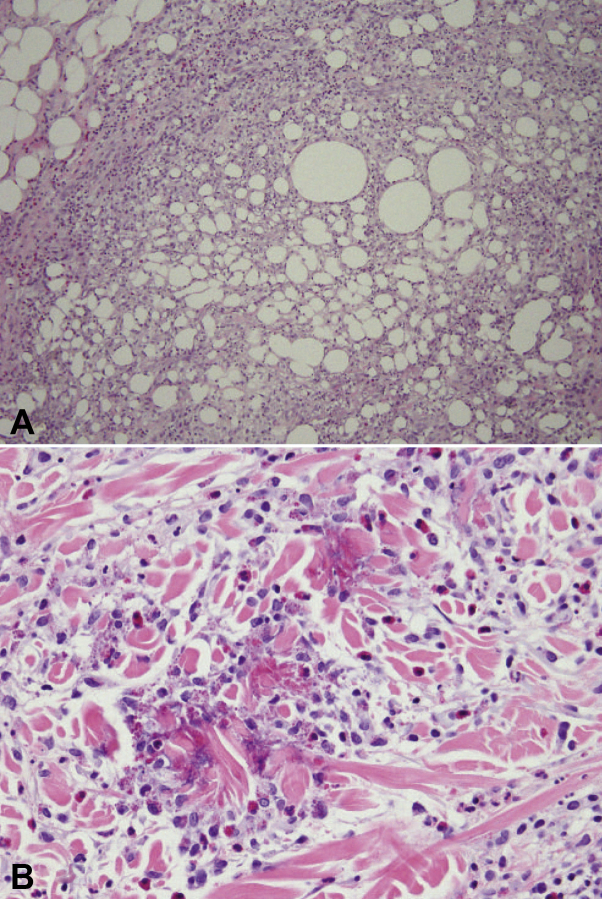
Fig 2.
Representative photomicrographs of skin biopsies. A, Septal and lobular panniculitis with a mixed cell infiltrate containing numerous eosinophils. B, Dermal mixed cell infiltrate with abundant eosinophils, early flame figures, and edema. (A and B, Hematoxylin-eosin stain; original magnifications: A, ×20; B, ×40.)
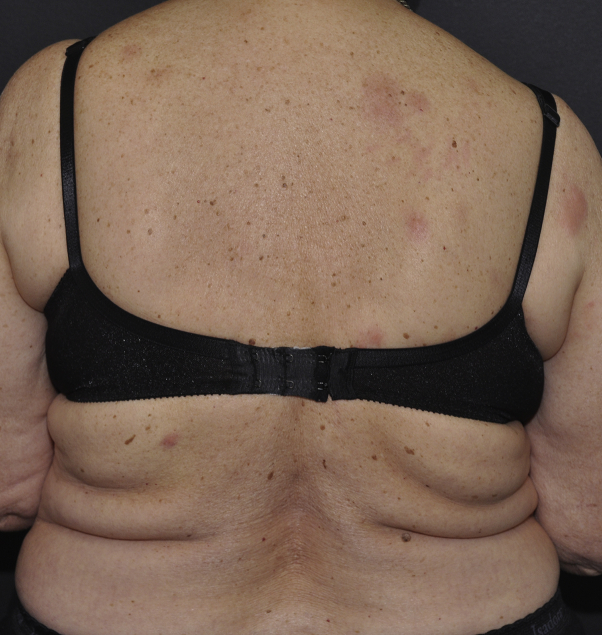
The patient remained in disease remission until 2 years later when a nodular enhancement consistent with recurrence was noted on surveillance magnetic resonance imaging. She was restarted on TMZ (150 mg/m2) by mouth daily for 5 consecutive days per 28-day cycle. Within 10 days of resuming TMZ, the patient noted an itchy rash with persistent individual lesions that did not come and go. On cutaneous examination, discrete red plaques and nodules were found on the bilateral upper extremities, thighs, and back (Fig 3). Histopathologic examination of a skin biopsy specimen found a perivascular and interstitial lymphocytic infiltrate with abundant eosinophils, early flame figures, and dermal edema, consistent with a dermal hypersensitivity reaction (Fig 2, B).1 The patient denied recent arthropod bites, travel, and new prescription or over-the-counter medications. A complete blood cell count was within normal limits. Despite use of topical clobetasol, 0.05% ointment twice daily, and hydroxyzine, 25 mg by mouth 3 times daily, the rash persisted, and she developed new persistent skin nodules. She completed a 3-week course of oral prednisone (60 mg daily for 1 week, 40 mg daily for 1 week, 20 mg daily for 1 week) with improvement but not resolution of her symptoms. Progression of disease was then noted on magnetic resonance imaging, and the patient discontinued TMZ therapy to start a clinical trial. Within 2 to 3 weeks of discontinuing TMZ, all skin lesions resolved. After 7 months of follow-up, the patient had not experienced any recurrence of her rash.
Fig 3.
Clinical overview image shows red plaques and nodules on the back.
Discussion
The reported dermatologic side effects of TMZ are limited but include urticaria, desquamative and maculopapular rash, alopecia, Stevens-Johnson syndrome/toxic epidermal necrolysis, and palmo-plantar erythrodysesthesia.2 Among 300 Korean patients with malignant glioma treated with TMZ, 16 (5.3%) had skin rash, most frequently Common Terminology Criteria for Adverse Events grade 2 or lower (n = 15, 94%).3, 4 Of note, the authors found that patients treated with TMZ and concomitant radiotherapy were more likely to have skin rash than patients treated with TMZ and no concomitant radiotherapy (7.2% vs 2.5%, P = .02).4 Pothiawala et al5 suggested that the apparently low rate of cutaneous ADRs from TMZ can be explained in part by concomitant use of corticosteroids, which are frequently used in patients with high-grade gliomas to reduce edema associated with radiation or tumor growth.
Although we were unable to identify a published report of an ADR associated with TMZ administration with similar clinicopathologic features, the likelihood that TMZ was responsible is possible (score of 3) for the first episode of rash and probable (score of 7) for the second episode of rash, according to the Naranjo ADR probability scale.6 The findings in our case emphasize the important need for clinicopathologic correlation in evaluating patients with nonspecific skin biopsies. Fung1 found that follow-up in 74 of 110 patients with a skin biopsy result consistent with dermal hypersensitivity reaction eventually revealed, most commonly, diagnoses of urticaria, drug reactions, and spongiotic (eczematous) dermatitis. In addition, the role of drugs in panniculitides is often not considered, as the clinical and histologic features of drug-induced panniculitides are often indistinguishable from those caused by other etiologies.7 Clinicians should remain cognizant that cutaneous ADRs remain great masqueraders.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Fung M.A. The clinical and histopathologic spectrum of “dermal hypersensitivity reactions,” a nonspecific histologic diagnosis that is not very useful in clinical practice, and the concept of a “dermal hypersensitivity reaction pattern”. J Am Acad Dermatol. 2002;47:898–907. doi: 10.1067/mjd.2002.120908. [DOI] [PubMed] [Google Scholar]
- 2.Alonso-Llamazares A., Vega-Castro A., Beitia-Mazuecos J.M., Mateo-Borrega B., Cardenas-Contreras R. Rapid desensitization with temozolomide in patients with delayed maculopapular rash. J Investig Allergol Clin Immunol. 2012;22:448–449. [PubMed] [Google Scholar]
- 3.National Cancer Institute. Common Terminology Criteria for Adverse Events v.4.0 (CTCAE). http://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed April 29, 2015
- 4.Bae S.H., Park M.J. Toxicity profile of temozolomide in the treatment of 300 malignant glioma patients in Korea. 2014;29:980–984. doi: 10.3346/jkms.2014.29.7.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pothiawala S., Hsu M.Y., Yang C., Kesari S., Ibrahimi O.A. Urticarial hypersensitivity reaction caused by temozolomide. J Drugs Dermatol. 2010;9:1142–1144. [PubMed] [Google Scholar]
- 6.Naranjo C.A., Busto U., Sellers E.M. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 7.Borroni G., Torti S., D'Ospina R.M., Pezzini C. Drug-induced panniculitides. G Ital Dermatol Venereol. 2014;149:263–270. [PubMed] [Google Scholar]