Highlight
We report evidence of the diversification and interaction of anthocyanin-related MYB activators and basic helix-loop-helix cofactors, which regulate anthocyanin biosynthesis in the potato tuber.
Key words: Anthocyanin, cofactors, diversification, interaction, MYB transcription factors, potato.
Abstract
In potato (Solanum tuberosum L.), R2R3 MYBs are involved in the regulation of anthocyanin biosynthesis. We examined sequences of these MYBs in cultivated potatoes, which are more complex than diploid potato due to ploidy and heterozygosity. We found amino acid variants in the C-terminus of the MYB StAN1, termed R0, R1, and R3, due to the presence of a repeated 10-amino acid motif. These variant MYBs showed some expression in both white and pigmented tubers. We found several new alleles or gene family members of R2R3 MYBs, StMYBA1 and StMYB113, which were also expressed in white potato tubers. From functional analysis in tobacco, we showed that the presence of a C-terminal 10-amino acid motif is optimal for activating anthocyanin accumulation. Engineering a motif back into a MYB lacking this sequence enhanced its activating ability. Versions of StMYBA1 and StMYB113 can also activate anthocyanin accumulation in tobacco leaves, with the exception of StMYB113-3, which has a partial R2R3 domain. We isolated five family members of potato StbHLH1, and one StJAF13, to test their ability to interact with MYB variants. The results showed that two alleles of StbHLH1 from white skin and red skin are non-functional, while three other StbHLH1s have different co-regulating abilities, and need to be activated by StJAF13. Combined with expression analysis in potato tuber, results suggest that StbHLH1 and StJAF13 are key co-regulators of anthocyanin biosynthesis, while the transcripts of MYB variants StAN1, StMYBA1, and StMYB113 are well expressed, even in the absence of pigmentation.
Introduction
Anthocyanins, the largest group of plant flavonoids, are the main pigments responsible for the red-blue colour of many plant species (Grotewold, 2006; Holton and Cornish, 1995; Petroni and Tonelli, 2011). Besides attracting pollinators and aiding in seed dispersal in flowers and fruits, anthocyanins have key roles in protection against UV radiation and cold temperatures (Christie et al., 1994; Sarma and Sharma, 1999), and response to drought stress (André et al., 2009; Castellarin et al., 2007). Moreover, anthocyanins have potential health benefits in humans, such as protection against some cancers and neuronal and cardiovascular diseases (Crowe et al., 2011; He and Giusti, 2010; Mink et al., 2007).
The cytosol-located anthocyanin biosynthetic pathway enzymes are encoded by a series of well-described genes (Grotewold, 2006; Holton and Cornish, 1995; Payyavula et al., 2013). After biosynthesis, anthocyanins are transported to vacuoles or cell walls (Koes et al., 2005). Expression of the pathway genes is controlled by a complex of MYB transcription factors (TFs), basic helix-loop-helix (bHLH) TFs, and WD-repeat proteins, the MYB-bHLH-WD40 ‘MBW’ complex (Baudry et al., 2004; Patra et al., 2013). The MYB superfamily constitutes one of the most abundant groups of TFs described in plants. MYB proteins have two distinct regions, an N-terminal conserved MYB DNA-binding domain, which is approximately 52 amino acid residues in length, and a diverse C-terminal modulator region that is responsible for the regulatory activity of the protein. Based on the number of highly conserved imperfect repeats in the MYB domain, the MYB family can be divided into four classes, 1R-, R2R3-, 3R-, and 4R-MYB proteins (Dubos et al., 2010; Rosinski and Atchley, 1998). Among these MYB TFs, R2R3 MYBs constitute the largest TF gene family in plants, with 126 R2R3 MYB genes identified in Arabidopsis and divided into 22 subgroups on the basis of conserved motifs (Stracke et al., 2001). The R2R3 MYB family plays an important role in regulating the expression of catalytic enzymes, including the anthocyanin pathway (Allan et al., 2008). Many R2R3 MYB regulators have been demonstrated to be transcriptional activators of the anthocyanin biosynthetic pathway from many species, such as Arabidopsis (Borevitz et al., 2000; Gonzalez et al., 2008), petunia (Quattrocchio et al., 1999), tomato (Mathews et al., 2003), grapevine (Deluc et al., 2008; Kobayashi et al., 2005; Walker et al., 2007), maize (Paz-Ares et al., 1987), pepper (Borovsky et al., 2004), potato StAN1 (Jung et al., 2005, 2009; Zhang et al., 2009b), sweet potato (Chu et al., 2013), and apple (Ban et al., 2007; Espley et al., 2007; Takos et al., 2006). In addition to the transcriptional activators, several MYB TFs have been identified as repressors of the anthocyanin biosynthetic pathway from several species, for example, strawberry (Aharoni et al., 2001), snapdragon (Tamagnone et al., 1998), apple (Lin-Wang et al., 2011), grapevine (Cavallini et al., 2015), Arabidopsis (Jin et al., 2000), petunia (Albert et al., 2011), and ginkgo (Xu et al., 2014), as well as a single MYB-repeat AtMYBL2 and At CPC (Dubos et al., 2008; Matsui et al., 2008; Zhu et al., 2009).
Another crucial TF involved in anthocyanin biosynthesis is the bHLH protein. The first studied bHLH TF regulating the anthocyanin pathway was Lc from maize, which was shown to cooperate with the MYB factor C1 (Ludwig et al., 1989); bHLH TFs were subsequently studied in other plants, such as AtbHLH42 (AtTT8) in Arabidopsis, AN1/JAF13 bHLHs in petunia, MdbHLH3 in apple, and VvMYC1 and VvMYCA1 in grapevine (Chandler et al., 1989; Espley et al., 2007; Goodrich et al., 1992; Hichri et al., 2010; Matus et al., 2010). The bHLH TF is essential for the activity of the R2R3 MYB partner, as they interact with each other to form transcriptional complexes with the promoters of biosynthetic genes. For example, the R2R3 MYB C1 protein in maize (Zea mays) interacts with a bHLH TF (either of the genes termed B or R) to activate the promoter of dihydroflavonol reductase (DFR) (Sainz et al., 1997). In contrast, maize P1 MYB activates some flavonoid genes in the absence of a bHLH (Grotewold et al., 2000).
The variation of colour intensity, or the location of pigmentation, has been attributed to mutations in the genes or promoters of TFs. A retrotransposon-induced mutation in grapevine (Vitis vinifera) in the promoter region of MYBA1 leads to a loss of anthocyanin accumulation in berry skin (Kobayashi et al., 2004). Multiple repeats of a promoter segment causes TF autoregulation in red-fleshed apples (Espley et al., 2009), while a motif of two 228bp fragments forming tandem repeats in the promoter region of the MYB TF RLC1 causes red leaf coloration in cotton under light (Gao et al., 2013). The Rc mutation in rice (Oryza sativa) accounts for 97% of white pericarp varieties (Sweeney et al., 2007) and was shown to be a 14bp deletion in the bHLH Rc gene.
Potato (Solanum tuberosum) is a major staple food and the fourth largest crop grown worldwide. It has been found that pigmented potato cultivars are a rich source of anthocyanins, in particular acylated derivatives (Fossen and Andersen, 2000; Rodriguez-Saona et al., 1999). The concentration of anthocyanins in purple-fleshed tubers is similar to that of the highest anthocyanin-producing crops, such as blueberries, blackberries, cranberries, or grapes (Puértolas et al., 2013). Potato peel shows a higher anthocyanin content and antioxidant activity than the corresponding flesh (Lewis et al., 1998). Anthocyanin synthesis in the tuber periderm of tetraploid potato is controlled by three loci, D, P, and R, which have been localized to chromosomes 11, 2, and 10, respectively. P and R were found to be genes encoding the biosynthetic enzymes F3′5′H and DFR, whereas D encodes an R2R3 MYB named StAN1, which is similar to petunia AN2 (Jung et al., 2005, 2009; Zhang et al., 2009b). StAN1 is not only a crucial regulator of anthocyanin biosynthesis in skin coloration of potato, but also a key regulator of other tuber phenylpropanoids and is regulated by sucrose (Payyavula et al., 2013). An additional MYB with high homology to AN1 protein, StMYBA1, corresponds to the translated sequence of the published StAN3 and is a possible AN1 pseudogene (D’Amelia et al., 2014; Jung et al., 2009). StMYB113 is homologous to AtMYB113, which positively regulates phenylpropanoid metabolism in Arabidopsis thaliana (Borevitz et al., 2000). StbHLH1 shows a strong association with phenylpropanoid expression in the potato tuber and may contribute to the anthocyanin accumulation in potato leaves, while StJAF13 acts as a putative AN1 co-regulator for anthocyanin gene expression in potato leaves (D’Amelia et al., 2014; Payyavula et al., 2013).
In this study, functional characterization of alleles or gene family members of the R2R3 MYBs StAN1, StMYBA1, and StMYB113 from different cultivated potatoes was performed. The function of the different versions of StAN1, showing variable C-terminal repeats, was verified, while StMYBA1 and StMYB113 also showed activation activity. Furthermore, five alleles or gene family members of StbHLH1, and one StJAF13, were also isolated and their function in anthocyanin biosynthesis was studied. The results showed that two alleles of StbHLH1 from white and red cultivars were non-functional, and other alleles from purple and red cultivars had different levels of co-regulating ability. In potato, real-time quantitative PCR (qPCR) analysis of skin and flesh of tubers suggested that a lack of expression of StbHLH1 and StJAF13 limits anthocyanin biosynthesis, while StAN1, StMYBA1, and StMYB113 are expressed in both white and pigmented tissues. However, the majority of transcripts of StAN1 are truncated in unpigmented tissues. In white potato tubers, failure to activate expression of StbHLH1 and StJAF13 may be due to differential processing of the StAN1 transcripts and non-functional alleles of StbHLH1.
Materials and methods
Plant materials
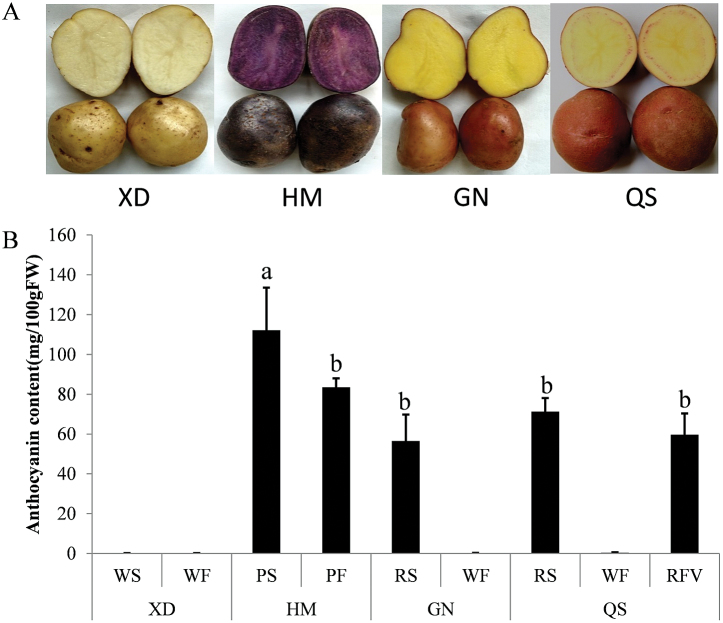
The white potato (Solanum tuberosum L.) cultivar ‘Xin Daping’ (XD; white skin and white flesh), purple potato cultivar ‘Hei Meiren’ (HM; purple skin and purple flesh), and red potato cultivars ‘Gannongshu No.5’ (GN; red skin and white flesh) and ‘Qinshu No.9’ (QS; red skin, white flesh and red vascular ring) (Fig. 1A) were grown in a greenhouse at Gansu Agricultural University, China. GN was cultivated by Gansu Agricultural University, HM and XD are local cultivars in Gansu province, and QS was cultivated by Qinghai Academy of Agriculture and Forestry Sciences of Qinghai Province. Five fresh tubers (diameter 4–5cm) were harvested and skin tissue was carefully separated from cortex tissue using a scalpel to minimize flesh contamination. The flesh tissue was isolated with at least 5mm distance from the skin to eliminate skin contamination. The red vascular ring was separated from surrounding white flesh tissue using a scalpel. The skin and flesh of these potatoes were frozen in liquid nitrogen and stored at –80 °C.
Fig. 1.
Characterization of the four genotypes used in this study. (A) Skin and flesh colour of Xin Daping (XD), Hei Meiren (HM), Gannongshu No.5 (GN) and Qingshu No.9 (QS). (B) Total anthocyanin content of skin, flesh, and red vascular ring of the four genotypes. The data represent the means±SE of three biological replicates. Statistical significance was determined by one-way ANOVA; significant differences between means [Least Significant Difference (LSD), P<0.05] are indicated where letters above the bars differ. WS, white skin; WF, white flesh; PS, purple skin; PF, purple flesh; RS, red skin; RFV, red vascular ring.
Nicotiana tabacum and Nicotiana benthamiana were grown under glasshouse conditions in full potting mix, using natural light with daylight extension to 16h.
Determination of anthocyanin content of potato skin and flesh
Anthocyanin content was determined by the pH differential spectrophotometry method described by Zhang et al (2009a). Anthocyanins were extracted from 1g samples in methanol/0.05 % HCl and absorbance of the extracts was measured by a spectrophotometer (UV-2550, Shimadzu, Japan) at 510 and 700nm. Absorbance (Abs) was calculated as Abs=(A510 nm–A700 nm)pH1.0–(A510 nm–A700 nm)pH4.5 with a molar extinction coefficient for cyanidin 3-glucoside of 26900 (Yang et al., 2012; Zhang et al., 2009a). Total anthocyanin content (TAC) was calculated using the following equation and expressed as milligrams of cyanidin 3-glucoside equivalents per 100g dry material.
Where e is cyanidin 3-glucoside molar absorbance [26900mL (mmol·cm)–1], L is the cell path length (1cm), MW is the molecular weight of anthocyanin (449.2g mol–1), D is a dilution factor, V is the final volume (ml), and G is the mass of dry material (mg).
DNA and RNA extraction
Total RNA of skin and flesh from the four potato cultivars, and from young leaves and roots of tobacco, were extracted using the PureLink Plant RNA Reagent Kit (Invitrogen, USA) according to the manufacturer’s instructions. The RNA was quantified by using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, USA) and quality was assayed on a 1% agarose gel. Removal of genomic DNA and first-strand cDNA synthesis were carried out using oligo(dT) (QuantiTect Reverse Transcription Kit, Qiagen). Genomic DNA was extracted from tuber tissue using the cetyl trimethyl ammonium bromide method of Murray and Thompson (1980).
Gene cloning and sequence analysis
Full-length coding sequences of the alleles of the potato MYBs StAN1, StMYBA1, and StMYB113, and the potato bHLHs StbHLH1 and StJAF13, were amplified from cDNA of skin and flesh of four cultivars using Platinum Taq DNA Polymerase High Fidelity (Invitrogen, USA). A truncated version of StAN1, StAN1-R0T, was amplified from cDNA of white skin. Full-length fragments were ligated into the binary vectors pSAK277 or pHEX2. Promoter sequences were isolated from the four potato cultivars and inserted into the pGreenII 0800-LUC vector (Hellens et al., 2005). Details of all the cloning procedures are shown in the Supplementary Methods at JXB online and primers used are described in Supplementary Table S3.
The sequences of non-functional StMYB113-3, StbHLH1-1, and StbHLH1-4 are described in Supplementary Table S4. Conserved cis-element motifs located in promoters were scanned by using the online software PLACE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ and http://www.dna.affrc.go.jp/PLACE/). All constructs were individually electroporated into Agrobacterium tumefaciens GV3101 (MP90). The NtAN1 RNAi and NtJAF13 RNAi binary vector pTKO2 and the promoter of Arabidopsis DFR (TT3, AT5g42800) were from the New Zealand Institute for Plant & Food Research Ltd (Hellens et al., 2005; Lin-Wang et al., 2010).
Phylogenetic analysis
Phylogenetic trees of MYB TFs and bHLH TFs were developed using MEGA6.0 (Tamura et al., 2007). The evolutionary history was inferred using the minimum evolution phylogeny test and 1000 bootstrap replicates. The evolutionary distances were computed using the Poisson correction method with units of the number of amino acid substitutions per site.
Transient assays of gene function
Transient assays, or dual luciferase assays, were performed in tobacco (N. benthamiana or N. tabacum) as previously reported (Espley et al., 2009; Lin-Wang et al., 2010). Details of all the transient assay procedures are shown in the Supplementary Methods.
In a separate colour assay, NtAN1 RNAi and NtJAF13 RNAi stably transformed tobacco plants were used (Montefiori et al., 2015). Digital photographs of anthocyanin development in these patches were taken at 4 days post-infiltration.
Transformation of tobacco
StAN1-R0, StAN1-R1, and StAN1-R3 were transformed in tobacco leaves (N. tabacum) using the leaf disc method (Horsch et al., 1985). After inoculation of leaf discs on medium with kanamycin and Timentin (ticarcillin disodium and clavulanate potassium), resistant shoots were regenerated from the cut surface of the explants. These shoots were separated from the explants and roots were induced. For each construct, 12 transgenic lines were obtained. Transgenic plants were identified by kanamycin selection and by qPCR analysis.
HPLC measurement of tobacco leaves and roots
For the stably transformed tobacco plants, three tobacco leaves were taken and pooled together from each of five transgenic lines, and roots were taken from each of three transgenic lines for each construct, and empty vector pSAK277 was used as a negative control. For transient assays, three patches of tobacco leaf were pooled together for each treatment, with three biological replicates, and GUS was used as a negative control. Freeze-dried tissue was pulverized and resuspended in methanol (with 0.1% HCl) at a ratio of 5ml solvent to 1g of original fresh weight (FW). The mixture of powdered sample and solvent was extracted at room temperature for 3h in the dark, and then centrifuged at 3500rpm for 10min. The supernatant was diluted 20-fold with 20% methanol. A 400 μl aliquot of the diluted supernatant was used for anthocyanin HPLC measurement of tobacco leaves (McGhie et al., 2005).
qPCR
Real-time qPCR DNA amplification and analysis was carried out using the LightCycler 480 Real-Time PCR System (Roche), with LightCycler 480 software version 1.5. The LightCycler 480 SYBR Green I Master Mix (Roche) was used. qPCR conditions were 5min at 95 °C, followed by 40 cycles of 5s at 95 °C, 5s at 60 °C, and 10s at 72 °C, followed by 65–95 °C melting curve detection. The qPCR efficiency of each gene was obtained by analysing the standard curve of a cDNA serial dilution of that gene. Relative abundance was calculated with the ΔCT method using actin (X55752) and elongation factor-1 (AB061263) of potato, and actin (EU938079) and elongation factor-1 (D63396) of tobacco for template normalization. The primers are listed in Supplementary Table S3.
R repeat function verification
A synthesized cDNA version of StAN1-R0 (with a R motif inserted), termed StAN1-R0M, in pUC57 cloning vector (GenScript), was used for R repeat function verification. The R repeat was inserted in the same position in StR0M as it is found in StR1 (amino acids 132–143), then cloned into the pSAK277 expression vector. This construct was infiltrated into N. benthamiana and N. tabacum leaves, as described previously, in the presence of the prom-3-StDFR promoter to test the R repeat function.
Statistical analysis
For qPCR analysis, data are presented as means (±SE) of four biological replicates. For transient transformation promoter activation assays, data are presented as means (±SE) of four biological replicates. For the analysis of anthocyanins, data are presented as means (±SE) of three biological replicates. Statistical significance was determined by one-way ANOVA.
Results
Tuber anthocyanin content in four potato genotypes
Four genotypes, HM (purple skin and purple flesh), GN (red skin and white flesh), QS (red skin, white flesh and red vascular ring), and XD (white skin and white flesh), were analysed (Fig. 1A). Anthocyanins were detectable only in red or purple skin or flesh, with the highest concentration in the purple skin (112.2±21.29mg 100g–1 FW). The concentrations of anthocyanins in the purple flesh (83.5±4.49mg 100g–1 FW), red skin and flesh (71.3±6.70mg 100g–1 FW and 56.54±13.25mg 100g–1 FW, respectively), and red vascular ring (59.68±10.62mg 100g–1 FW) were not significantly different (Fig. 1B).
Analysis of StAN1, StMYBA1, and StMYB113 gene sequences in differentially pigmented cultivars
PCR amplification and genotyping were used to determine the sequences of StAN1 variants in differentially pigmented tissues of potato cultivars. Full-length coding sequences of three variants, termed StAN1-R0, StAN1-R1, and StAN1-R3, were amplified from cDNA of tuber skin and flesh of the four cultivars (Fig. 1, Table 1). Distinguishing these variants were three perfect duplications of 30 nucleotides (CTATTGCTCCTCAACCACAAGAAGGAATTA) termed R (coding for 10 amino acids: TIAPQPQEGI) in the third exon of StAN1-R3 (Fig. 2). A truncated version of StAN1-R0, termed StAN1-R0T, was amplified from StAN1-R0 at positions 1–302bp according to our previous RNA-seq result (Liu et al., 2015) (Table 1).
Table 1.
Presence of different transcription factor genes and alleles in tetraploid potatoes used in this study.
| Cultivar and tissue | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| XD | HM | GN | QS | ||||||
| Transcript | WS | WF | PS | PF | RS | WF | RS | WF | RFV |
| StAN1-R0 | √ | ||||||||
| StAN1-R1 | √ | √ | √ | √√ | |||||
| StAN1-R3 | √√ | √ | √ | √√ | √√ | √ | |||
| StAN1-R0T | √ | ||||||||
| StMYBA1-1 | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| StMYBA1-2 | √ | ||||||||
| StMYB113-1 | √ | √ | |||||||
| StMYB113-2 | √ | √ | |||||||
| StMYB113-3* | √ | ||||||||
| StbHLH1-1* | √ | ||||||||
| StbHLH1-2 | √ | √ | √ | ||||||
| StbHLH1-3 | √ | √ | |||||||
| StbHLH1-4* | √ | ||||||||
| StbHLH1-5 | √ | ||||||||
| StJAF13 | √ | √ | |||||||
WS, white skin; WF, white flesh; PS, purple skin; PF, purple flesh; RS, red skin; RFV, red vascular ring. * Premature stop codon in this transcript. √ Full-length transcript identified by PCR and sequencing. √√ Identified by qPCR melting curve analysis followed by sequencing of the qPCR fragment.
Fig. 2.
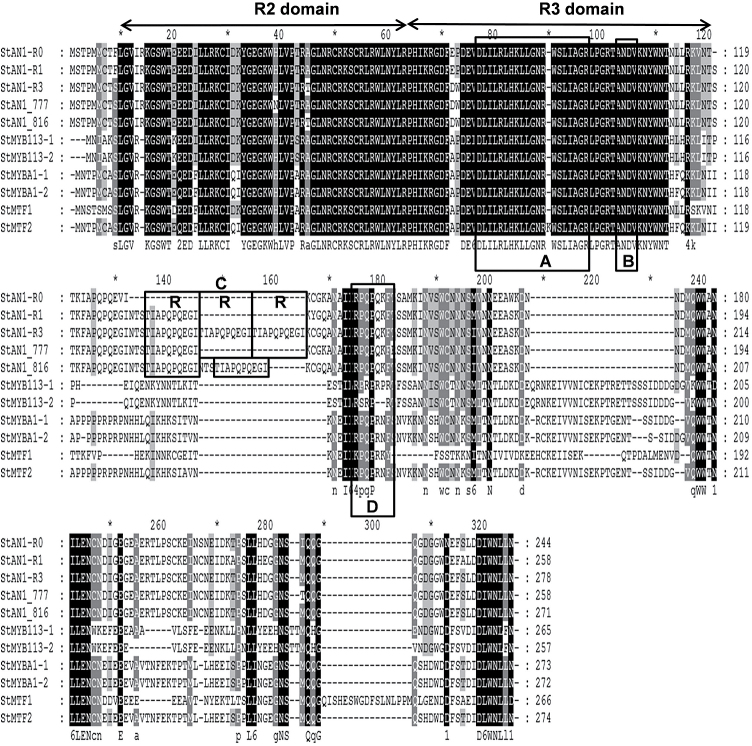
Protein sequence alignment of anthocyanin MYB regulators from potato. The R2 and R3 repeat domains are indicated by arrows. Box (A) indicates the conserved region of the bHLH interacting motif ([DE]Lx2[RK]x3Lx6Lx3R). Box (B) indicates a conserved motif [A/S/G]NDV in the R2R3 domain for dicot anthocyanin-promoting MYBs. Box (C) indicates the perfect repeat TIAPQPQEGI. Box (D) indicates a C-terminal-conserved motif [R/K]Px[P/A/R]xx[F/Y] for anthocyanin-regulating MYBs (Hichri et al., 2010). NCBI protein accession numbers: StAN1-R0, AKA95391; StAN1-R1, AKA95392; StAN-R3, AKA95392; StAN1_777, AAX53089; StAN1_816, AAX53087; StMYB113-1, ALA13583; StMYB113-2, ALA13584; StMYBA1-1, ALA13581; StMYBA1-2, ALA13582; StMTF1, ABY40370; StMTF2, ABY40371.
Melting curve analysis and sequencing of the qPCR fragments confirmed expression of two variants, StAN1-R0 and StAN1-R3, in white skin, but just one variant, StAN1-R1, was expressed in white flesh of the white cultivar XD (Table 1). Only StAN1-R1 was expressed in purple skin and purple flesh. In red cultivars, StAN1-R3 was expressed in red skin, white flesh, and the red vascular ring, while StAN1-R1 was also present in the white flesh of the red cultivar GN (Supplementary Fig. S1).
Two variants of StMYBA1 were also cloned, termed StMYBA1-1 (expressed in all tissues) and StMYBA1-2 (isolated only from HM purple skin). Distinguishing the two variants were three nucleotides (CCT) at positions 370–372 in the third exon of StMYBA1-1. Three differentially expressed variants of StMYB113 were isolated, termed StMYBA113-1, StMYBA113-2, and StMYBA113-3 (Fig. 2, Table 1). A 130bp deletion in StMYBA113-3 at nucleotide positions 125–254 resulted in a premature stop codon at amino acid position 9. Compared with StMYBA113-1, there are several deletions and amino acid changes in StMYBA113-2, as shown in Fig. 2.
StAN1-R0, StAN1-R1, StAN1-R3, StMYBA1-1, StMYBA1-2, StMYB113-1, and StMYB113-2 encoded R2R3 MYB TFs that contain the highly conserved R2 and R3 MYB domains in the N-terminal region. In addition, they all contained other conserved motifs in the C-terminal region, including the [D/E]Lx2[R/K]x3Lx6Lx3R motif (Box A in Fig. 2) critical for interaction with R/B-like bHLH proteins (Zimmermann et al., 2004) and the conserved ANDV motif (Box B in Fig. 2) identified from MYB regulators of the anthocyanin pathway in Rosaceae (Lin-Wang et al., 2010). StAN1-R0, StAN1-R1, StAN1-R3, StMYBA1-1, and StMYBA1-2 contained the motif [R/K]Px[P/A/R]xx[F/Y] (Box D in Fig. 2), which is highly conserved in the anthocyanin-activating MYBs of some plant species (Lin-Wang et al., 2010), while in StMYB113-1 and StMYB113-2 this motif is [R/K] [P/S]x[P/A/R]xx[F/Y/R].
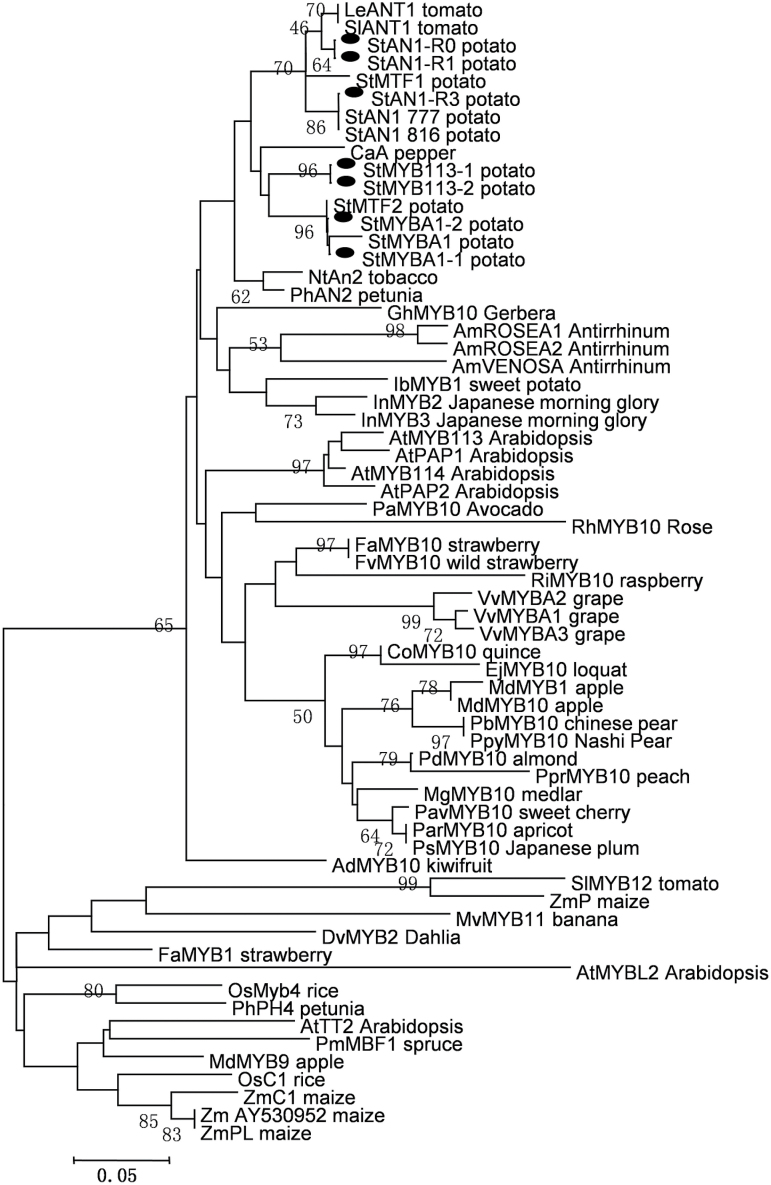
Newly identified StAN1, StMYBA1, and StMYB113 sequences were highly homologous to previously identified MYBs in diploid (Jung et al., 2009) and tetraploid potato: StAN1-777, StAN1-816 (AY841129 and AY841127), MTF1 and StMTF1 (EU310399), and StMTF2 (EU310400). Phylogenetic analysis (Supplementary Fig. S2) showed that StAN1-R0, StAN1-R1, StAN1-R3, StMYBA1-1, StMYBA1-2, StMYB113-1, and StMYB113-2 clustered with published regulators of anthocyanin biosynthesis such as PAP1, as well as other anthocyanin-promoting MYBs from dicot species. Monocot sequences, such as C1 of rice and P of maize, as well as the gymnosperm Picea mariana MBF1, clustered outside this group. Furthermore, the MYB proteins clustered according to their taxonomic relationships in Solanaceae and other anthocyanin-promoting MYBs from other species (Fig. 3). StAN1-R0, StAN1-R1, and StAN1-R3 were closely associated with StAN1-777, StAN1-816, and StMFT1. StMYBA1s were closely clustered with the StMFT2 and StMYB113s, as well as MYBs of other solanaceous species, SlANT1, LeANT1, PhAN2, NtAN2, and CaA, known to regulate anthocyanins.
Fig. 3.
Phylogenetic relationship analysis of potato MYBs and known anthocyanin MYB regulators from other species. Sequences were aligned using Geneious v.6.1.6 (Drummond et al., 2011) with a cost matrix of 65%, a gap open penalty of 12, and a gap extension penalty of 3. Phylogenetic and molecular evolutionary analysis was conducted using MEGA version 6.0. The evolutionary history was inferred using the neighbour-joining method and 1000 bootstrap replicates; bootstrap values less than 50 are not shown. The predicated proteins of StAN1-R0, StAN1-R1, StAN1-R3, StMYBA1-1, StMYBA1-2, StMYB113-1, and StMYB113-2 are indicated by black oval dots.
qPCR analysis of StAN1, StMYBA1, and StMYB113 in four potato genotypes
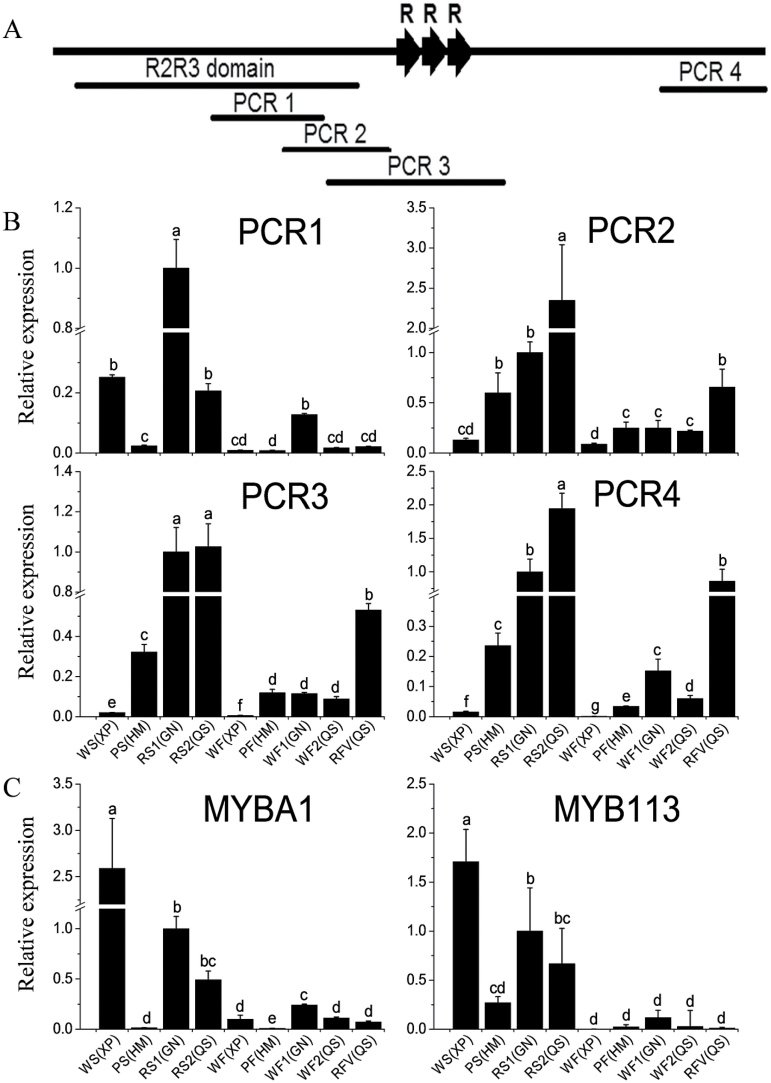
To investigate the expression profiles of the different variants of StAN1 in potato tubers, four pairs of qPCR primers were designed to different regions of StAN1 (Fig. 4A). These were designated as PCR 1–4. PCR 1 was in the R2R3 domain, PCR 2 was before the repeat region including part of R2R3 domain, PCR 3 flanked the repeat region, and PCR 4 was in the C-terminus (Fig. 4A). These were used to test the different variants present in skin and flesh according to Tm value and sequence results.
Fig. 4.
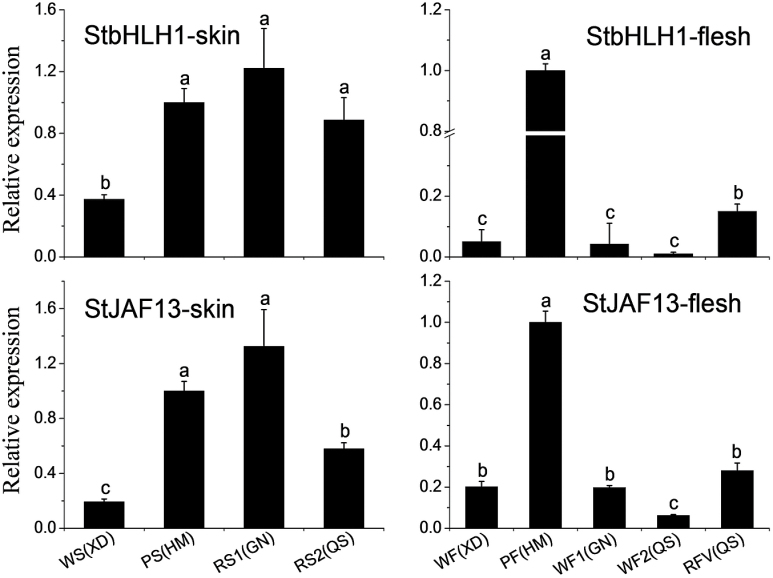
Expression analysis of StAN1s, StMYBA1s, and StMYB113s in four potato cultivars. (A) Schematic of the StAN1 gene. PCR 1–4 represent the different regions. (B) qPCR expression of the different regions of StAN1 in skin, flesh, and red vascular ring of the four genotypes, XD, HM, GN, and QS. Red skin of GN was set as a calibrator. (C) qPCR expression of StMYBA1s and StMYB113s in skin, flesh and red vascular ring of the four genotypes. Red skin of GN was set as a calibrator. The data represent the means±SE of three biological replicates. Statistical significance was determined by one-way ANOVA; significant differences between means (LSD, P<0.05) are indicated where letters above the bars differ. WS, white skin; PS, purple skin; RS, red skin; WF, white flesh; PF, purple flesh; RVF, red vascular ring.
Surprisingly, in the white skin of the white cultivar XD, StAN1 expression in the region encoding the R2R3 domain (PCR 1) was much higher than in the purple skin of the purple cultivar (Fig. 4B). However, reduced expression was detected using primers for PCR 2 and PCR 3 regions, and there was very low expression in the white cultivar if the primers were targeted to the 3′-end of the cDNA (PCR 4). In contrast, only a small drop in amplification between PCR 1 and PCR 2, and consistent expression between PCR 2 and 3, was observed for the pigmented tissue and white flesh of the pigmented cultivars HM, GN, and QS. Amplification with primers targeted to the 3′-end of the cDNA was consistently detected in pigmented tissues, particularly in red cultivars. This is in agreement with our previous RNA-seq analysis (Liu et al., 2015) where, in a white skin library, 1187 reads mapped to the R2R3 domain and only 13 reads mapped to the C-terminus. In a purple skin library, 579 total reads mapped evenly to both the R2R3 domain (297 reads) and the C-terminus (282 reads). These results suggest that most of the StAN1 transcripts in the cultivar with white skin and white flesh lack the 3′-end, which encodes the C-terminus. Potentially, these truncated StAN1 transcripts are unable to regulate anthocyanin biosynthesis.
In purple flesh, StAN1 expression is moderate, but a full-length coding sequence is transcribed. However, there was little difference in expression between white flesh of red cultivars and the purple flesh, suggesting that, in addition to the expression level, the function of the three StAN1 variants may differ. The expression profiles of StMYBA1 and StMYB113 were similar (Fig. 4C). Both had higher expression in the white skin of the white cultivar than in the purple skin of the purple cultivar. Their expression levels in the flesh of the four genotypes were lower than that in skin, and there was almost no expression of StMYBA1 in purple flesh.
Analysis of StbHLH TFs in differentially pigmented cultivars
Full-length coding sequences of five alleles or variants of the potato StbHLH1 and one version of StJAF13 were amplified from cDNA of tuber skin and flesh of the four cultivars (Table 1). These were termed StbHLH1-1 (from skin of the white cultivar XD), StbHLH1-2 (from skin of the white cultivar XD, and purple skin and purple flesh of HM), StbHLH1-3 (from purple skin and purple flesh of cultivar HM), StbHLH1-4, and StbHLH1-5 (from red skin of GN). StJAF13 was isolated from purple skin of cultivar HM and red skin of GN. Supplementary Table S1 summarizes potential mutations found in the five StbHLH1 variants with respect to published StbHLH1 sequence. We found that the coding sequences of StbHLH1-1 and StbHLH1-2 were similar, except that StbHLH1-1 showed a G nucleotide deletion at nucleotide position 1589, which resulted in a premature stop codon. The coding sequence of StbHLH1-4 had an insertion of 50 nucleotides at nucleotide position 478–527, which also resulted in a premature stop codon. StbHLH1-2 was the same as the published StbHLH1. StbHLH1-3 had two deletions of 6 and 15 nucleotides, and six single nucleotide polymorphism (SNP) differences. StbHLH1-5 showed a deletion of 24 nucleotides and 34 SNP differences (Supplementary Fig. S3, Table S1). There was 80.6, 100, 96.8, 35.2, and 96.8% identity between the five StbHLH1 variants and published StbHLH1, respectively. StJAF13 shared 99.8% identity with published StJAF13. Phylogenetic analysis showed that the five variants of StbHLH grouped with tobacco An1a and An1b, and petunia An1 (Supplementary Fig. S4). StJAF13 is grouped with tomato GLABRA3-like, tobacco JAF13a and JAF13b, and petunia JAF13.
qPCR analysis of StbHLHs in four potato genotypes
It has been previously shown in Solanaceous species that there is a hierarchy of bHLHs partnering with anthocyanin-activating MYBs (Montefiori et al., 2015). We examined the expression of StbHLH1 (Payyavula et al., 2013) and StJAF13 (D’Amelia et al., 2014) in potato tubers. qPCR primers were designed based on the conserved region of the five alleles of bHLH1, allowing all versions to be detected. Both bHLHs showed similar expression profiles, with higher levels detected in genotypes with pigmented skin and flesh (Fig. 5, Table 1). The expression level was less in white skin than in pigmented skin. There was little expression of StbHLH1 and StJAF13 in white flesh of white and red-skinned cultivars, whereas they were both highly expressed in purple flesh. Expression was also elevated in the red vascular ring of the flesh in the QS cultivar (Fig. 5). Therefore, both StbHLH1 and StJAF13 correlate with anthocyanin biosynthesis in potato tuber skin and flesh.
Fig. 5.
Quantitative analysis of transcript levels of StbHLH1 and StJAF13 in skin, flesh and red vascular ring of four genotypes. Purple skin and purple flesh of HM were set as calibrators. The data represent the means±SE of three biological replicates. Statistical significance was determined by one-way ANOVA; significant differences between means (LSD, P<0.05) are indicated where letters above the bars differ. WS, white skin; PS, purple skin; RS, red skin; WF, white flesh; PF, purple flesh; RVF, red vascular ring.
Functional assays of StAN1, StMYBA1 and StMYB113 in tobacco
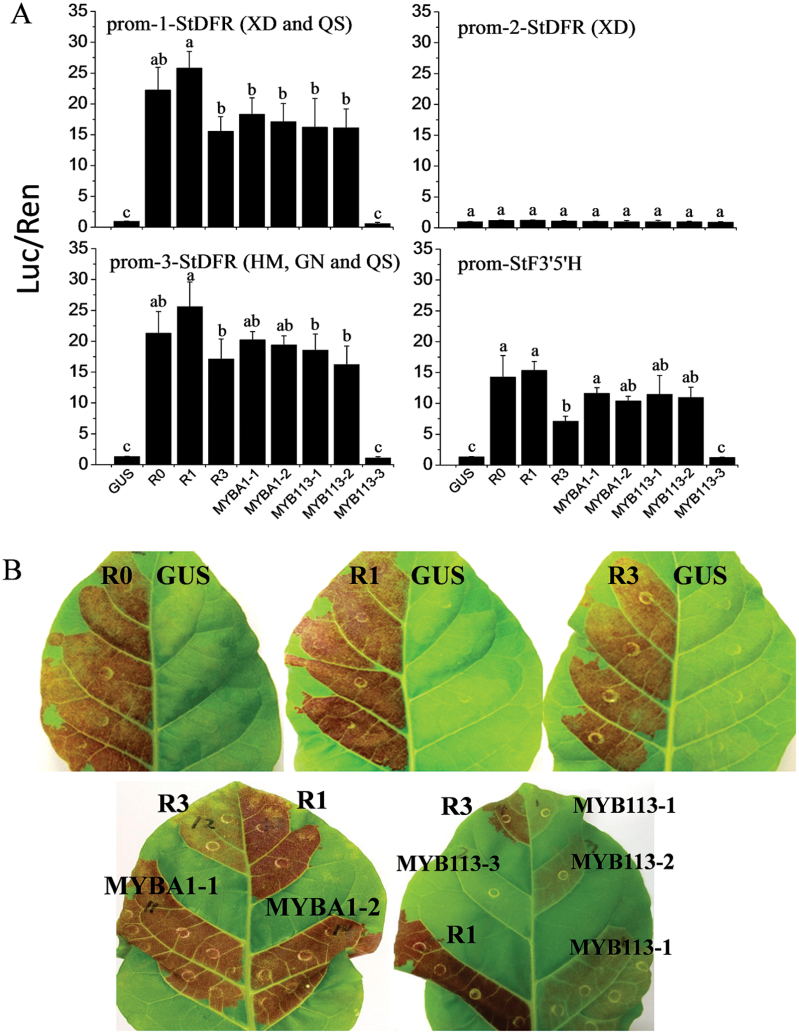
In order to functionally test the different MYB genes and their variants, transient luciferase assays in N. benthamiana were used to measure transactivation of potato DFR and F3′5′H promoters. Sequencing of a ~2000bp region upstream of the ATG translation start codon of StDFR (chr02:40292119..40294109 PGSC, http://solanaceae.plantbiology.msu.edu/pgsc_download.shtml) (Potato Genome Sequencing Consortium, 2011) revealed substantial variation in the promoter. There were at least three alleles of StDFR in four genotypes, of which prom-1-StDFR and prom-2-StDFR were found in the white genotype XD, prom-3-StDFR was found in the purple and red cultivars HM and GN, and prom-1-StDFR and prom-3-StDFR were found in the other red cultivar QS. The StF3′5′H promoters of four genotypes were all identical. Numerous cis-acting regulatory elements were identified and the most abundant motifs were light-responsive elements such as G-Box, defence and stress-responsive elements (TC-rich repeats), and MYB binding sites (MYBCORE). A circadian element, methyljasmonate responsive element (CGTCA motif), Plant MYB binding site (MYBPLANT), and MYC recognition sequence (MYCATERD1) were present in all DFR promoters, but not in the StF3′5′H promoter (Supplementary Table S2).
Full-length cDNAs of StAN1-R0, StAN1-R1, StAN1-R3, StMYBA1-1, StMYBA1-2, StMYB113-1, StMYB113-2, and StMYB113-3 under control of the 35S promoter were co-infiltrated into N. benthamiana leaves with prom-StDFRs-LUC and prom-StF3′5′H-LUC in a second Agrobacterium strain (Hellens et al., 2005). There was significant activation of prom-1-StDFR and prom-3-StDFR by seven of the eight potato MYBs, compared with the negative control (Fig. 6A). In contrast, prom-2-StDFR, cloned from the white cultivar, could not be activated by any of the eight MYBs. For the prom-1-StDFR and prom-3-StDFR promoters, activation by StAN1-R1 was higher than by StAN1-R3, StMYB113-1, and StMYB113-2. Activation of the StF3′5′H promoter by StAN1-R3 was significantly lower than by the other MYB TFs. StMYB113-3 appears to be non-functional as it lacks part of the R2R3 region.
Fig. 6.
Transient assays of StAN1-R0, StAN1-R1, StAN1-R3, StMYBA1-1, StMYBA1-2, StMYB113-1, StMYB113-2, and StMYB113-3. (A) Activation of three alleles of potato DFR promoters and F3′5′H promoter. Error bars are the SE of three independent experiments with four replicate reactions. Statistical significance was determined by one-way ANOVA; significant differences between means (LSD, P<0.05) are indicated where letters above the bars differ. (B) Patches of anthocyanin production in tobacco leaves induced by infiltration with StAN1-R0, StAN1-R1, StAN1-R3, StMYBA1-1, StMYBA1-2, StMYB113-1, StMYB113-2, StMYB113-3, and GUS.
The transient activation of an anthocyanic patch in tobacco was examined after infiltration with individual MYBs. No anthocyanin was observed with GUS or StMYB113-3. Only weak activation was detected upon infiltration of StMYB113-1 and StMYB113-2, and relatively strong activation was observed with the remaining MYBs (Fig. 6B). Significantly higher anthocyanin accumulation (as quantified by HPLC; Supplementary Fig. S5C) was observed upon infiltration of StAN1-R1 compared with StAN1-R3 (Fig. 6B), suggesting that the presence of a single R repeat provides optimal activation. This was confirmed using a mutant version of StAN1-R0, designated StAN1-R0M, in which an R motif is inserted into the same position as that found in StAN1-R1. Subsequent prom-1-StDFR transactivation assays combined with quantification of anthocyanin accumulation demonstrated a 1.22-fold increased activation capacity over StAN1-R0 (Supplementary Fig. S5). The truncated version of StAN1-R0, StAN1-R0T, did not induce any anthocyanin or significantly inhibit the activity of full-length StAN1-R0, R1, or R3 (Supplementary Fig. S6). These results suggest that the R repeat is an important functional domain in StAN1, with the single R motif enhancing the ability of StAN1 to activate anthocyanin synthesis.
StAN1-R0, StAN1-R1, and StAN1-R3 activate all the anthocyanin biosynthetic genes in leaves and roots of transformed tobacco lines
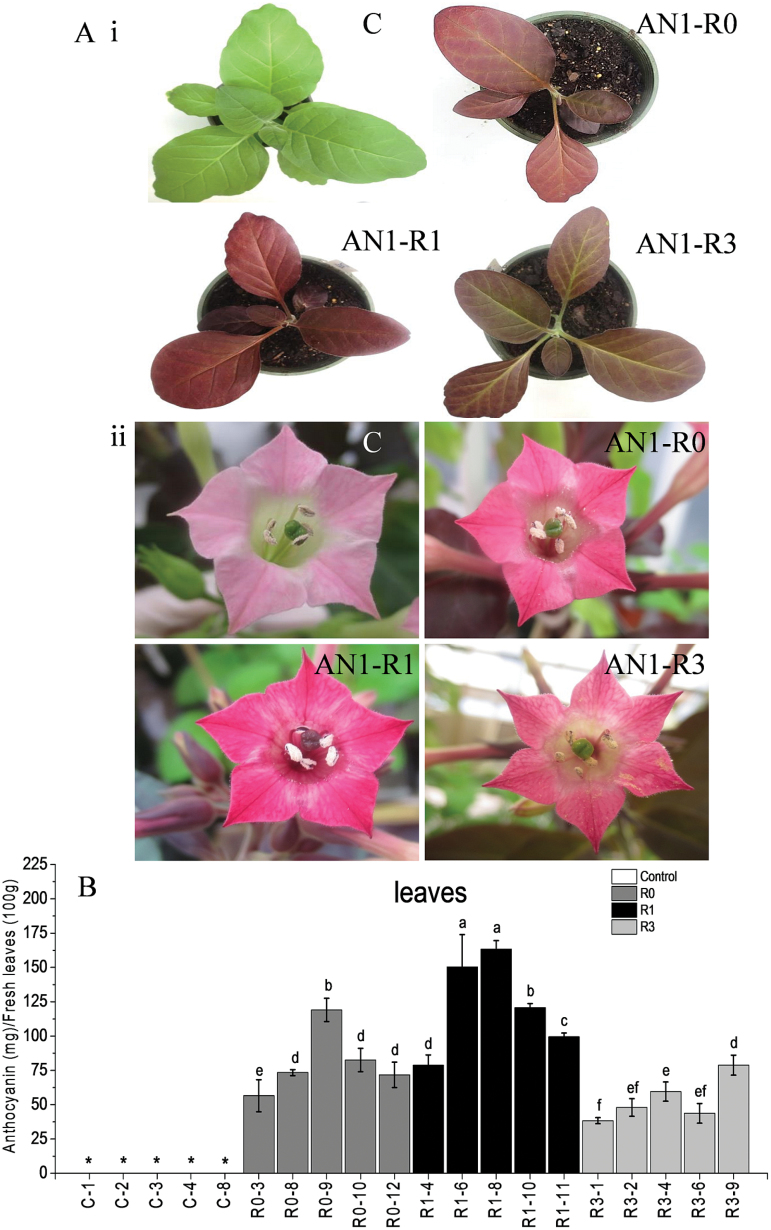
In order to further examine the efficiencies of StAN1-R0, StAN1-R1, and StAN1-R3 in inducing anthocyanin biosynthesis, stably transformed lines of tobacco were generated. Transformed plants were tested by genomic PCR to confirm the presence of each transgene, and by qPCR to determine the individual expression levels of StAN1-R0, StAN1-R1, and StAN1-R3. For each construct, five independent lines were selected for phenotypic analysis.
The lines overexpressing StAN1-R1 accumulated significantly higher levels of anthocyanin in the leaves and flowers compared with those overexpressing StAN1-R0 and StAN1-R3 (Fig. 7A, B). The average foliar anthocyanin content in StAN1-R1 transgenic lines was higher than in the StAN1-R0 and StAN1-R3 transgenic lines, with the highest anthocyanin content in the leaves of line 8 StAN1-R1, reaching 163.3±6.24mg 100g–1 FW (Fig. 7B).
Fig. 7.
Phenotypes and anthocyanin content of transgenic tobacco plants transformed with empty vector, StAN1-R0, StAN1-R1, and StAN1-R3. (A) Visible reddening was seen in leaves (i) and flowers (ii) of plants transformed with StAN1-R0, StAN1-R1, and StAN1-R3. (B) Anthocyanin content from five independent transgenic lines of each construct showed the highest concentration in two out of the five StAN1-R1 lines and the lowest concentration in three out of five StAN1-R3 lines. C represents empty vector controls. Error bars are the SE for three replicate extracts per line. Statistical significance was determined by one-way ANOVA; significant differences between means (LSD, P<0.05) are indicated where letters above the bars differ. * indicates no detectable levels of anthocyanin content.
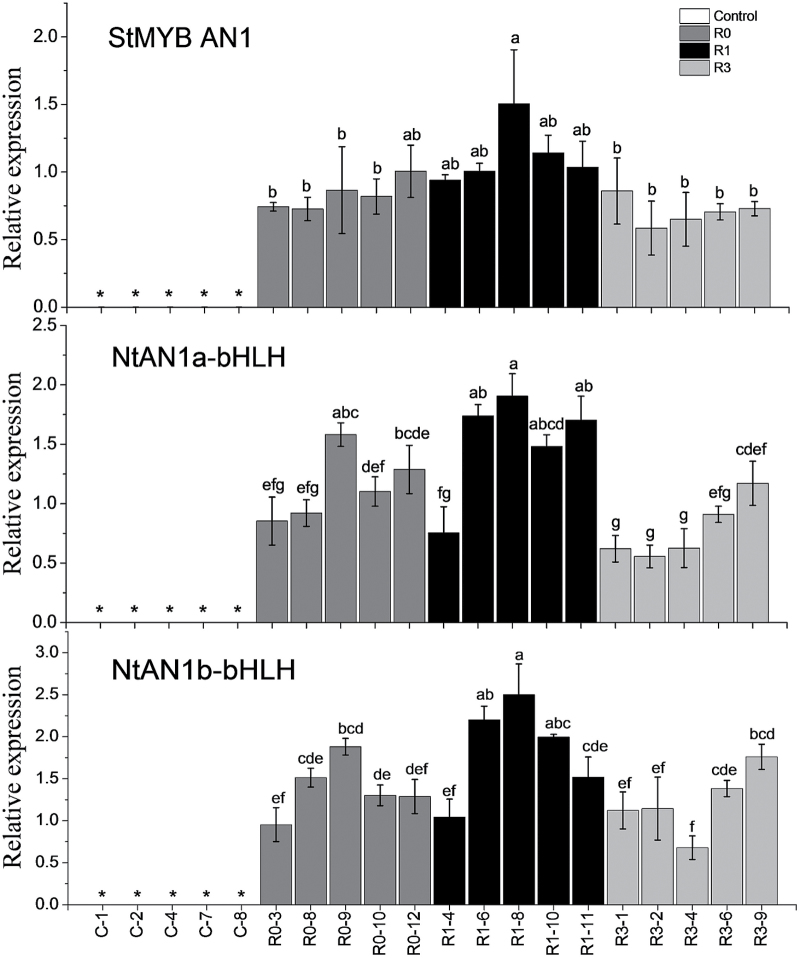
The expression of StAN1 and the endogenous tobacco bHLH TFs NtAN1a and NtAN1b were analysed in transgenic lines. The results showed that StAN1-R0, StAN1-R1, and StAN1-R3 overexpression resulted in an induction of the endogenous bHLH TFs NtAN1a and NtAN1b. The induction of NtAN1a and NtAN1b was greatest in the leaves of StAN1-R1 transgenic lines (Fig. 8). The tobacco MYB NtAN2, controlling tobacco anthocyanin production (Pattanaik et al., 2010), was not induced by overexpression of the potato MYBs (not detectable by qPCR; not shown).
Fig. 8.
Quantitative analysis of transcript levels of StMYB AN1, NtAN1a-bHLH, and NtAN1b-bHLH in tobacco transgenic lines. The data represent the means±SE of three biological replicates. Statistical significance was determined by one-way ANOVA; significant differences between means (LSD, P<0.05) are indicated where letters above the bars differ. Genotypes denoted by * showed no detectable levels of expression.
The transcript levels of several anthocyanin biosynthetic genes were also analysed, such as chalcone synthase (NtCHS), chalcone isomerase (NtCHI), flavonoid 3′-monooxygenase (NtF3′H), flavanone-3-beta-hydroxylase (NtF3H), and leucoanthocyanidin dioxygenase/anthocyanidin synthase (NtLDOX/NtANS). These were expressed at higher levels in StAN1-R1 transgenic lines compared with StAN1-R0 and StAN1-R3 transgenic lines. The expression profile correlated with both the anthocyanin levels and endogenous bHLH TFs NtAN1a and NtAN1b (Supplementary Fig. S7).
StAN1, StMYBA1, and StMYB113 partner with StbHLH1 and StJAF13 to regulate anthocyanin biosynthesis
As previously shown, StAN1-R0, StAN1-R1, StAN1-R3, StMYBA1-1, StMYBA1-2, StMYB113-1, and StMYB113-2 can all regulate anthocyanin biosynthesis in tobacco. In potato, StAN1-R0, StAN1-R1, and StAN1-R3 are highly expressed in pigmented tissues, but they are also expressed in white skin and white flesh. qPCR and read mapping of RNA-seq suggests that the StAN1 transcript is short in white tubers. However, StMYBA1 and StMYB113 are highly expressed in white skin of the white cultivar XD (Fig. 4C). This suggests that the presence or absence of StbHLH TFs may be crucial in regulating anthocyanin biosynthesis in potato. To further examine the interaction between StbHLH1 and StJAF13 with StAN1, StMYBA1, and StMYB113, transient assays were carried out in N. tabacum stably transformed with RNAi NtAn1 (including NtAn1a and NtAn1b) and RNAi StJAF13. The aim of using these tobacco lines was to minimize the activity of the endogenous bHLHs.
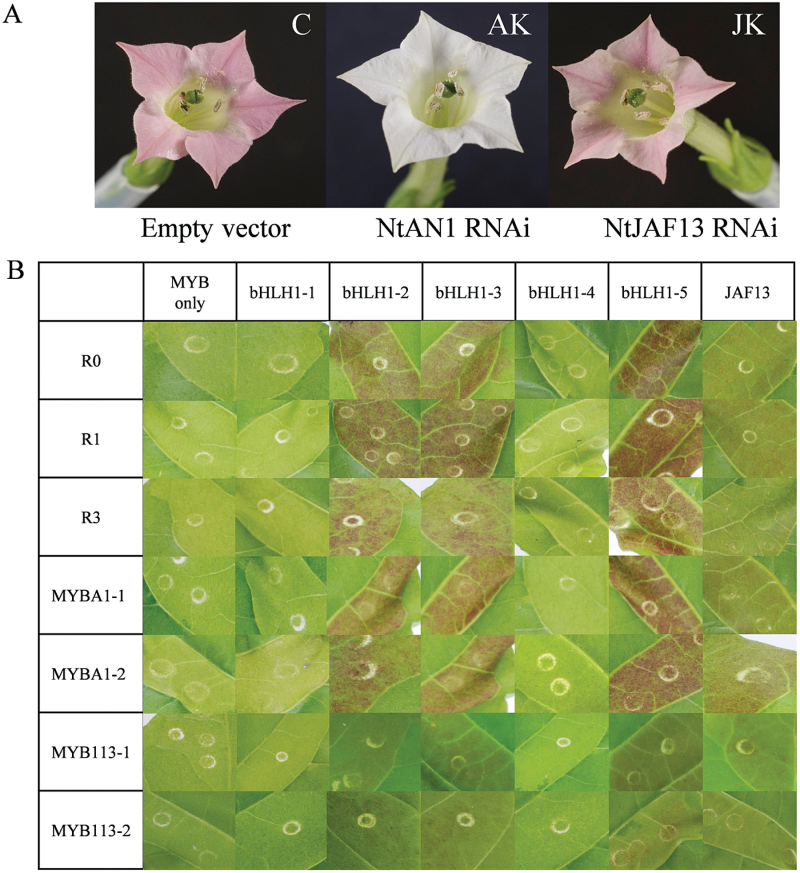
As previously reported (Montefiori et al., 2015), tobacco lines of RNAi NtAn1 had white flowers and RNAi NtJAF13 had pale pink flowers (Fig. 9A). The leaves of RNAi NtAn1 plants were infiltrated with variants of StAN1 (StAN1-R0, StAN1-R1, and StAN1-R3), StMYBA1 (StMYBA1-1 and StMYBA1-2), or StMYB113 (StMYBA113-1 and StMYB113-2) alone, or combined with one of five variants of StbHLH1 (StbHLH1-1–StbHLH1-5) and one StJAF13. The RNAi NtAn1 construct may have a negative effect on the potato bHLH transgenes. However, induction of anthocyanic patches indicates that this interference is minimal. When TFs were transiently transformed into RNAi NtAn1 tobacco leaves, anthocyanin accumulation was observed in the treatments with StAN1, StMYBA1, and StMYBA113 in the presence of StbHLH1-2, StbHLH1-3, StbHLH1-5, or StJAF13, but not with StbHLH1-1 and StbHLH1-4 or MYB TFs alone. The strongest anthocyanin accumulation was observed in leaves infiltrated with a MYB TF and StbHLH1-5. The weakest interaction appeared to be with the MYBs and StJAF13 (Fig. 9B).
Fig. 9.
Transient activation of anthocyanic responses by StAN1, StMYBA1, and StMYB113 combined with StbHLH1 and with StJAF13. (A) Flowers of transgenic tobacco plants transformed with empty vector, NtAn1 RNAi, or NtJAF13 RNAi. (B) Patches of anthocyanin production in NtAn1 RNAi tobacco leaves infiltrated by MYBs alone or combined with five variants of StbHLH1 and StJAF13.
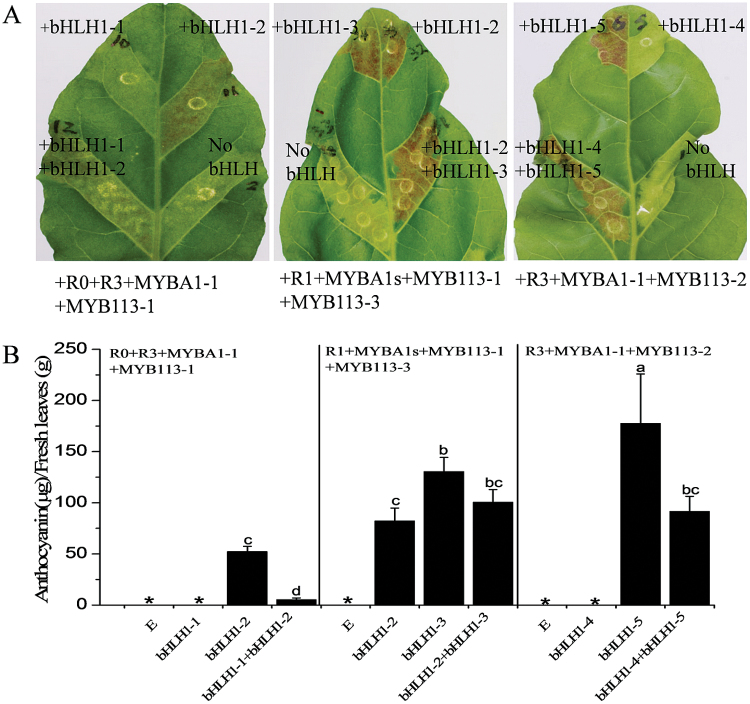
To simulate the TFs present in the white potato skin, the MYBs StAN1-R0, StAN1-R3, StMYBA1-1, and StMYB113-1 were mixed with StbHLH1-1 and StbHLH1-2 in a tobacco background lacking NtAn1 (Fig. 10A). Anthocyanin production induced by the MYBs combined with StbHLH1-1 and StbHLH1-2 was significantly reduced compared with production induced by MYB TFs with StbHLH1-2 alone (Fig. 10A, B). This raises the possibility that the non-functional StbHLH1-1 and functional StbHLH1-2 compete with each other, which limits anthocyanin accumulation.
Fig. 10.
Transient activation of anthocyanic responses in tobacco by simulating transcription factors present in potato skin. (A) Patches of anthocyanin production in NtAn1 RNAi tobacco leaves induced by the combinations as shown. (B) Anthocyanin was extracted from patches of each combination. Error bars are the SE of three biological replicates. Statistical significance was determined by one-way ANOVA; significant differences between means (LSD, P<0.05) are indicated where letters above the bars differ. E represents empty vector control. Genotypes denoted by * showed no detectable levels of anthocyanin content.
In the purple skin of purple tubers, expression of the MYBs StAN1-R1, StMYBA1-1, StMYBA1-2, StMYB113-1, and StMYB113-3, and the bHLHs StbHLH1-2 and StbHLH1-3, was evident. This combination induced high levels of anthocyanin accumulation in tobacco leaves. In the red skin of the red cultivar GN, StAN1-R3, StMYBA1-1, and StMYB113-2, and StbHLH1-4 and StbHLH1-5 transcripts were present, and this combination also induced anthocyanin accumulation in the NtAn1 RNAi tobacco leaves. The truncated non-functional StbHLH1-4 did not prevent the accumulation of anthocyanin induced by the MYBs, yet had some inhibitory effect on the functional StbHLH1-5 (Fig. 10A, B).
When the same combinations described above were transformed into NtJAF13 RNAi tobacco leaves, anthocyanin accumulation was prevented unless the MYBs were co-infiltrated with a functional bHLH (Supplementary Fig. S8). This supports observations that in the Solanaceae MYB TFs need to co-partner with NtJAF13 to stimulate NtAn1, which then up-regulates the anthocyanin biosynthetic genes (Montefiori et al., 2015). In the potato tuber, there was significantly lower expression of StJAF13 in both white skin and white flesh (Fig. 5), suggesting that both StJAF13 and StbHLH1s are important factors in controlling tuber anthocyanin biosynthesis.
Discussion
For potato tuber skin and flesh, genetic analysis has revealed three major loci controlling pigmentation, D, R, and P (De Jong et al., 2004). StAN1, coding for a R2R3 MYB, was shown to control potato skin colour, and StMYBA1 was suggested as a possible AN1 pseudogene (Jung et al., 2009). In the present study, we characterized three variants of StAN1 from four differentially pigmented genotypes and tested the importance of a repeated domain in the third exon of StAN1. We examined the function of StMYBA1 and a novel StMYB113, both of which are highly expressed in white skin of white tubers. Results suggest that the StbHLH TFs are the limiting regulators in anthocyanin biosynthesis in the tuber, as MYBs (StAN1-R0, StAN1-R1, StAN1-R3, StMYBA1, and StMYB113) can be well expressed even in the absence of pigmentation.
Three major variants of StAN1 present in four different pigmented cultivars
In petunia, Schwinn et al. (2006) reported that striking effects on floral phenotype could be caused by small changes in MYB sequence. Recently, D’Amelia et al. (2014) reported that potato AN1 displays high intraspecific sequence variability in both coding and non-coding sequences of AN1 and that its expression in leaves is associated with high anthocyanin content. In studying the potato tuber, we confirmed this variability in StAN1 and explored the function of many of the variants of the gene. Apart from several SNPs, the main difference between the variants was three perfect duplications of 30 nucleotides (termed R) in the third exon of StAN1. These indels would be consistent with the results of Jung et al. (2009), who found bands of different sizes of StAN1 from different tetraploid potatoes. Their precise role is difficult to determine in this highly heterozygous crop. We therefore investigated the function of StAN1-R0, StAN1-R1, StAN1-R3, and the R repeat in more detail.
The involvement of StAN1-R0, StAN1-R1, and StAN1-R3 in regulating anthocyanin biosynthesis
Since there are few other sequence differences between StAN1-R0, StAN1-R1, and StAN1-R3, it appears likely that this set of mutations occurred recently in the evolution or cultivation of potato. Our transient analysis revealed that StAN1-R1, harbouring one R-motif, was optimal for the regulation of anthocyanin levels. We further confirmed that the R-motif can enhance the ability of StAN1 to regulate anthocyanin biosynthesis by inserting an R-motif into StAN1-R0.
The C-terminal variants of StAN1 were further investigated using stable transformation of tobacco. Our results showed that heterologous expression of StAN1-R0, StAN1-R1, and StAN1-R3 activated the anthocyanin biosynthetic pathway and induced anthocyanin pigmentation. Activated biosynthetic genes included the early biosynthetic genes NtCHS, NtCHI, NtF3H, and NtF3′H, and the late biosynthetic genes NtDFR, NtANS, and NtUFGT. In Arabidopsis, the expression of early and late biosynthetic genes appears to be controlled separately, by different TFs (Dubos et al., 2010). In addition, the endogenous tobacco bHLH TFs NtAN1a and NtAN1b were highly induced. However, the endogenous MYB NtAN2 was not induced by StAN1-R0, StAN1-R1, or StAN1-R3. This is in contrast to results found when bayberry MrMYB1 was overexpressed in tobacco petals (Huang et al., 2013).
StbHLH1 and StJAF13 are limiting regulators of anthocyanin biosynthesis in potato tubers
The results of stable transformation showed that overexpression of StAN1 leads to high expression of the bHLH genes NtAN1a and NtAN1b in tobacco. The expression profiles of the bHLH genes and biosynthetic genes are largely overlapping (Fig. 8, Supplementary Fig. S7). Conversely, in potato, StAN1, StMYBA1, and StMYB113 can be highly expressed in white skin, but the expression levels of the bHLH partners appeared limiting. This suggests that bHLHs may be crucial in regulating anthocyanin biosynthesis in potato (De Jong et al., 2004; Payyavula et al., 2013). It was hypothesized by Jung et al. (2009) that for tuber flesh, the allelic configuration of different loci, like the bHLHs, may influence the phenotype when AN1 is constitutively expressed. In Arabidopsis seeds, TT8/AtBHLH042 is involved in the control of the expression of the DFR and BAN genes (Nesi et al., 2000). Appelhagen et al. (2011) identified EGL3 and TT8 as necessary regulators of anthocyanin accumulation in developing Arabidopsis seedlings. Butelli et al. (2012) observed that the RUBY MYB from orange (Citrus sinensis) promoted a stronger pigmentation of transformed tobacco plants when co-expressed with snapdragon bHLHs.
Based on qPCR in potato and transformation of tobacco, it appears that the production of anthocyanin in tubers is associated with two overlapping mechanisms. One requires a high level of expression of a full-length transcript of StAN1, and expression of StbHLH1 and StJAF13, to activate anthocyanin biosynthesis. There is expression of a shortened StAN1 transcript in the white skin of white tubers, while in white flesh of a red cultivar there is low expression of StbHLH1 and StJAF13. Thus, there is no anthocyanin accumulation in these tissues despite the presence of high expression of StMYBA1 and StMYB113. The other potential mechanism is linked to certain alleles of StAN1 and StbHLH1, which influence anthocyanin biosynthesis. Different variants of StAN1 have different anthocyanin-regulating abilities due to the presence of the R motif. Furthermore, amino acid substitutions in StbHLH1-2, StbHLH1-3, and StbHLH1-5 cause alterations in the interaction with StAN1, and premature stop codons in StbHLH1-1 and StbHLH1-4 result in alleles which could be inhibitory in white-skinned tubers.
We also found that MYB TFs (StAN1, StMYBA1, and StMYB113) co-partner with NtJAF13 to first activate the bHLH NtAN1, as previously shown (Montefiori et al., 2015). The potato MYB TFs and the endogenous tobacco bHLHs then function together to up-regulate the anthocyanin biosynthetic genes. This is consistent with qPCR results of StbHLH1 and StJAF13 in potato, especially in flesh, where StJAF13 expression patterns overlap with those of StbHLH1. D’Amelia et al. (2014) reported AN1/StJAF13 and AN1/StbHLH1 interactions in potato leaf; these are consistent with our results in tubers. It appears that StJAF13 also has an important role in regulating anthocyanin biosynthesis in tubers.
In conclusion, these data show at least two important mechanisms controlling potato tuber pigmentation—an allelic diversity of variants of R2R3 MYBs and their interacting bHLH partners, as well as differential expression of key genes. Three AN1 alleles (StAN1-R0, StAN1-R1, and StAN1-R3) have functionally important variations in the C-terminal domain, while StMYBA1 and StMYB113 are also potentially functional. We demonstrated that StbHLH1 and StJAF13 are also limiting factors in anthocyanin biosynthesis. Future work could focus on the alleles of StAN1 and StbHLH1 that potentially act as repressors of tuber pigmentation.
Supplementary data
Supplementary Methods. Gene cloning and sequence analysis; transient assays of gene function.
Supplementary Table S1. Summary of missense mutations found with respect to StbHLH1 (JX848660) sequence.
Supplementary Table S2. List of putative regulatory elements present in promoters of StDFR and StF3′5′H.
Supplementary Table S3. Primers for real-time quantitative PCR.
Supplementary Table S4. The sequences of non-functional StMYB113-3, StbHLH1-1, and StbHLH1-4.
Supplementary Fig. S1. Different variants of StAN1 presented in skin, flesh, and red vascular ring of four genotypes by qPCR melting curve analysis.
Supplementary Fig. S2. Phylogenetic relationship between Arabidopsis MYB transcription factors and anthocyanin-related MYBs of potato and other species.
Supplementary Fig. S3. Protein sequence alignment of five alleles of StbHLH1 and one allele of StJAF13 in differentially pigmented potatoes.
Supplementary Fig. S4. Phylogenetic relationship of anthocyanin-related bHLH genes of potato and other species.
Supplementary Fig. S5. Transient expression assays to probe the function of StAN1-R0M.
Supplementary Fig. S6. Transient expression assays to probe the function of StAN1-R0T.
Supplementary Fig. S7. Quantitative analysis of transcript levels of anthocyanin biosynthetic genes in transgenic tobacco leaves.
Supplementary Fig. S8. Patches of anthocyanin production in NtJAF13 RNAi tobacco leaves.
Acknowledgements
This research program is financially supported by the Fostering Foundation for the Excellent PhD Dissertation of Gansu Agricultural University (2013), Agricultural Biotechnology Research and Application Development Project of Gansu Province (grant no. GNSW-2015-15), Basic Research of Innovative Group of Gansu Province (grant no. 1308RJIA005), and is the part of a collaborative programme with the New Zealand Institute of Plant and Food Research. We would like to thank Tim Holmes for photography and Monica Dragulescu for looking after the plants in the glasshouse.
References
- Aharoni A, De Vos C, Wein M, Sun Z, Greco R, Kroon A, Mol JN, O’Connell AP. 2001. The strawberry FaMYB1 transcription factor suppresses anthocyanin and flavonol accumulation in transgenic tobacco. The Plant Journal 28, 319–332. [DOI] [PubMed] [Google Scholar]
- Albert NW, Lewis DH, Zhang H, Schwinn KE, Jameson PE, Davies KM. 2011. Members of an R2R3-MYB transcription factor family in Petunia are developmentally and environmentally regulated to control complex floral and vegetative pigmentation patterning. The Plant Journal 65, 771–784. [DOI] [PubMed] [Google Scholar]
- Allan AC, Hellens RP, Laing WA. 2008. MYB transcription factors that colour our fruit. Trends in Plant Science 13, 99–102. [DOI] [PubMed] [Google Scholar]
- André CM, Schafleitner R, Legay S, Lefèvre I, Aliaga CAA, Nomberto G, Hoffmann L, Hausman J-F, Larondelle Y, Evers D. 2009. Gene expression changes related to the production of phenolic compounds in potato tubers grown under drought stress. Phytochemistry 70, 1107–1116. [DOI] [PubMed] [Google Scholar]
- Appelhagen I, Jahns O, Bartelniewoehner L, Sagasser M, Weisshaar B, Stracke R. 2011. Leucoanthocyanidin dioxygenase in Arabidopsis thaliana: characterization of mutant alleles and regulation by MYB-BHLH-TTG1 transcription factor complexes. Gene 484, 61–68. [DOI] [PubMed] [Google Scholar]
- Ban Y, Honda C, Hatsuyama Y, Igarashi M, Bessho H, Moriguchi T. 2007. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant and Cell Physiology 48, 958–970. [DOI] [PubMed] [Google Scholar]
- Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshaar B, Lepiniec L. 2004. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana . The Plant Journal 39, 366–380. [DOI] [PubMed] [Google Scholar]
- Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C. 2000. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. The Plant Cell 12, 2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovsky Y, Oren-Shamir M, Ovadia R, De Jong W, Paran I. 2004. The A locus that controls anthocyanin accumulation in pepper encodes a MYB transcription factor homologous to Anthocyanin2 of Petunia . Theoretical and Applied Genetics 109, 23–29. [DOI] [PubMed] [Google Scholar]
- Butelli E, Licciardello C, Zhang Y, Liu J, Mackay S, Bailey P, Reforgiato-Recupero G, Martin C. 2012. Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in blood oranges. The Plant Cell 24, 1242–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellarin SD, Pfeiffer A, Sivilotti P, Degan M, Peterlunger E, Di Gaspero G. 2007. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant, Cell & Environment 30, 1381–1399. [DOI] [PubMed] [Google Scholar]
- Cavallini E, Matus JT, Finezzo L, Zenoni S, Loyola R, Guzzo F, Schlechter R, Ageorges A, Arce-Johnson P, Tornielli GB. 2015. The phenylpropanoid pathway is controlled at different branches by a set of R2R3-MYB C2 repressors in grapevine. Plant Physiology 167, 1448–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler VL, Radicella JP, Robbins TP, Chen J, Turks D. 1989. Two regulatory genes of the maize anthocyanin pathway are homologous: isolation of B utilizing R genomic sequences. The Plant Cell 1, 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie PJ, Alfenito MR, Walbot V. 1994. Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 194, 541–549. [Google Scholar]
- Chu H, Jeong JC, Kim WJ, Chung DM, Jeon HK, Ahn YO, Kim SH, Lee HS, Kwak SS, Kim CY. 2013. Expression of the sweetpotato R2R3-type IbMYB1a gene induces anthocyanin accumulation in Arabidopsis . Physiologia Plantarum 148, 189–199. [DOI] [PubMed] [Google Scholar]
- Crowe FL, Roddam AW, Key TJ, Appleby PN, Overvad K, Jakobsen MU, Tjønneland A, Hansen L, Boeing H, Weikert C. 2011. Fruit and vegetable intake and mortality from ischaemic heart disease: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heart study. European Heart Journal 32, 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amelia V, Aversano R, Batelli G, Caruso I, Castellano Moreno M, Castro-Sanz AB, Chiaiese P, Fasano C, Palomba F, Carputo D. 2014. High AN1 variability and interaction with basic helix-loop-helix co-factors related to anthocyanin biosynthesis in potato leaves. The Plant Journal 80, 527–540. [DOI] [PubMed] [Google Scholar]
- De Jong W, Eannetta N, De Jong D, Bodis M. 2004. Candidate gene analysis of anthocyanin pigmentation loci in the Solanaceae . Theoretical and Applied Genetics 108, 423–432. [DOI] [PubMed] [Google Scholar]
- Deluc L, Bogs J, Walker AR, Ferrier T, Decendit A, Merillon J-M, Robinson SP, Barrieu F. 2008. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiology 147, 2041–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A, Ashton B, Buxton S, Cheung M, Cooper A, Duran C, Field M, Heled J, Kearse M, Markowitz S. 2011. Geneious v5.4. [Google Scholar]
- Dubos C, Le Gourrierec J, Baudry A, Huep G, Lanet E, Debeaujon I, Routaboul JM, Alboresi A, Weisshaar B, Lepiniec L. 2008. MYBL2 is a new regulator of flavonoid biosynthesis in Arabidopsis thaliana . The Plant Journal 55, 940–953. [DOI] [PubMed] [Google Scholar]
- Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. 2010. MYB transcription factors in Arabidopsis . Trends in Plant Science 15, 573–581. [DOI] [PubMed] [Google Scholar]
- Espley RV, Brendolise C, Chagné D, Kutty-Amma S, Green S, Volz R, Putterill J, Schouten HJ, Gardiner SE, Hellens RP. 2009. Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples. The Plant Cell 21, 168–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC. 2007. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. The Plant Journal 49, 414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossen T, Andersen Ø. 2000. Anthocyanins from tubers and shoots of the purple potato, Solanum tuberosum . Journal of Horticultural Science and Biotechnology 75, 360–363. [Google Scholar]
- Gao Z, Liu C, Zhang Y, Li Y, Yi K, Zhao X, Cui M-L. 2013. The promoter structure differentiation of a MYB transcription factor RLC1 causes red leaf coloration in empire red leaf cotton under light. PLOS ONE 8, e77891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Zhao M, Leavitt JM, Lloyd AM. 2008. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. The Plant Journal 53, 814–827. [DOI] [PubMed] [Google Scholar]
- Goodrich J, Carpenter R, Coen ES. 1992. A common gene regulates pigmentation pattern in diverse plant species. Cell 68, 955–964. [DOI] [PubMed] [Google Scholar]
- Grotewold E. 2006. The genetics and biochemistry of floral pigments. Annual Review of Plant Biology 57, 761–780. [DOI] [PubMed] [Google Scholar]
- Grotewold E, Sainz MB, Tagliani L, Hernandez JM, Bowen B, Chandler VL. 2000. Identification of the residues in the Myb domain of maize C1 that specify the interaction with the bHLH cofactor R. Proceedings of the National Academy of Sciences of the United States of America 97, 13579–13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Giusti MM. 2010. Anthocyanins: natural colorants with health-promoting properties. Annual Review of Food Science and Technology 1, 163–187. [DOI] [PubMed] [Google Scholar]
- Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA. 2005. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hichri I, Heppel SC, Pillet J, Léon C, Czemmel S, Delrot S, Lauvergeat V, Bogs J. 2010. The basic helix-loop-helix transcription factor MYC1 is involved in the regulation of the flavonoid biosynthesis pathway in grapevine. Molecular Plant 3, 509–523. [DOI] [PubMed] [Google Scholar]
- Holton TA, Cornish EC. 1995. Genetics and biochemistry of anthocyanin biosynthesis. The Plant Cell 7, 1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch R, Fry J, Hoffmann N, Eichholtz D, Rogers S, Fraley R. 1985. A simple and general method for transferring genes into plants. Science 227, 1229–1231. [DOI] [PubMed] [Google Scholar]
- Huang Y-J, Song S, Allan AC, Liu X-F, Yin X-R, Xu C-J, Chen K-S. 2013. Differential activation of anthocyanin biosynthesis in Arabidopsis and tobacco over-expressing an R2R3 MYB from Chinese bayberry. Plant Cell, Tissue and Organ Culture 113, 491–499. [Google Scholar]
- Jin H, Cominelli E, Bailey P, Parr A, Mehrtens F, Jones J, Tonelli C, Weisshaar B, Martin C. 2000. Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis . The EMBO Journal 19, 6150–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CS, Griffiths HM, De Jong DM, Cheng S, Bodis M, De Jong WS. 2005. The potato P locus codes for flavonoid 3′,5′-hydroxylase. Theoretical and Applied Genetics 110, 269–275. [DOI] [PubMed] [Google Scholar]
- Jung CS, Griffiths HM, De Jong DM, Cheng S, Bodis M, Kim TS, De Jong WS. 2009. The potato developer (D) locus encodes an R2R3 MYB transcription factor that regulates expression of multiple anthocyanin structural genes in tuber skin. Theoretical and Applied Genetics 120, 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Goto-Yamamoto N, Hirochika H. 2004. Retrotransposon-induced mutations in grape skin color. Science 304, 982. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Goto-Yamamoto N, Hirochika H. 2005. Association of VvmybA1 gene expression with anthocyanin production in grape (Vitis vinifera) skin-color mutants. Journal of the Japanese Society for Horticultural Science 74, 196–203. [Google Scholar]
- Koes R, Verweij W, Quattrocchio F. 2005. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends in Plant Science 10, 236–242. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Walker JR, Lancaster JE, Sutton KH. 1998. Determination of anthocyanins, flavonoids and phenolic acids in potatoes. I: Coloured cultivars of Solanum tuberosum L. Journal of the Science of Food and Agriculture 77, 45–57. [Google Scholar]
- Lin-Wang K, Bolitho K, Grafton K, Kortstee A, Karunairetnam S, McGhie TK, Espley RV, Hellens RP, Allan AC. 2010. An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biology 10, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Wang K, Micheletti D, Palmer J, et al. 2011. High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex. Plant, Cell & Environment 34, 1176–1190. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lin-Wang K, Deng C, et al. 2015. Comparative transcriptome analysis of white and purple potato to identify genes involved in anthocyanin biosynthesis. PLOS ONE 10, e0129148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig SR, Habera LF, Dellaporta SL, Wessler SR. 1989. Lc, a member of the maize R gene family responsible for tissue-specific anthocyanin production, encodes a protein similar to transcriptional activators and contains the myc-homology region. Proceedings of the National Academy of Sciences of the United States of America 86, 7092–7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews H, Clendennen SK, Caldwell CG, Liu XL, Connors K, Matheis N, Schuster DK, Menasco D, Wagoner W, Lightner J. 2003. Activation tagging in tomato identifies a transcriptional regulator of anthocyanin biosynthesis, modification, and transport. The Plant Cell 15, 1689–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Umemura Y, Ohme-Takagi M. 2008. AtMYBL2, a protein with a single MYB domain, acts as a negative regulator of anthocyanin biosynthesis in Arabidopsis. The Plant Journal 55, 954–967. [DOI] [PubMed] [Google Scholar]
- Matus J, Poupin M, Cañón P, Bordeu E, Alcalde J, Arce-Johnson P. 2010. Isolation of WDR and bHLH genes related to flavonoid synthesis in grapevine (Vitis vinifera L.). Plant Molecular Biology 72, 607–620. [DOI] [PubMed] [Google Scholar]
- McGhie TK, Hunt M, Barnett LE. 2005. Cultivar and growing region determine the antioxidant polyphenolic concentration and composition of apples grown in New Zealand. Journal of Agricultural and Food Chemistry 53, 3065–3070. [DOI] [PubMed] [Google Scholar]
- Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong C-P, Nettleton JA, Jacobs DR. 2007. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. The American journal of clinical nutrition 85, 895–909. [DOI] [PubMed] [Google Scholar]
- Montefiori M, Brendolise C, Dare AP, Lin-Wang K, Davies KM, Hellens RP, Allan AC. 2015. In the Solanaceae, a hierarchy of bHLHs confer distinct target specificity to the anthocyanin regulatory complex. Journal of Experimental Botany 66, 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M, Thompson WF. 1980. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research 8, 4321–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesi N, Debeaujon I, Jond C, Pelletier G, Caboche M, Lepiniec L. 2000. The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. The Plant Cell 12, 1863–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra B, Schluttenhofer C, Wu Y, Pattanaik S, Yuan L. 2013. Transcriptional regulation of secondary metabolite biosynthesis in plants. Biochimica et Biophysica Acta 1829, 1236–1247. [DOI] [PubMed] [Google Scholar]
- Pattanaik S, Kong Q, Zaitlin D, Werkman JR, Xie CH, Patra B, Yuan L. 2010. Isolation and functional characterization of a floral tissue-specific R2R3 MYB regulator from tobacco. Planta 231, 1061–1076. [DOI] [PubMed] [Google Scholar]
- Payyavula RS, Singh RK, Navarre DA. 2013. Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism. Journal of Experimental Botany 64, 5115–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares J, Ghosal D, Wienand U, Peterson P, Saedler H. 1987. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. The EMBO Journal 6, 3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroni K, Tonelli C. 2011. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Science 181, 219–229. [DOI] [PubMed] [Google Scholar]
- Puértolas E, Cregenzán O, Luengo E, Álvarez I, Raso J. 2013. Pulsed-electric-field-assisted extraction of anthocyanins from purple-fleshed potato. Food Chemistry 136, 1330–1336. [DOI] [PubMed] [Google Scholar]
- Potato Genome Sequencing Consortium 2011. Genome sequence and analysis of the tuber crop potato. Nature 475, 189–195. [DOI] [PubMed] [Google Scholar]
- Quattrocchio F, Wing J, van der Woude K, Souer E, de Vetten N, Mol J, Koes R. 1999. Molecular analysis of the anthocyanin2 gene of petunia and its role in the evolution of flower color. The Plant Cell 11, 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Saona L, Wrolstad R, Pereira C. 1999. Glycoalkaloid content and anthocyanin stability to alkaline treatment of red-fleshed potato extracts. Journal of Food Science 64, 445–450. [Google Scholar]
- Rosinski JA, Atchley WR. 1998. Molecular evolution of the Myb family of transcription factors: evidence for polyphyletic origin. Journal of Molecular Evolution 46, 74–83. [DOI] [PubMed] [Google Scholar]
- Sainz MB, Grotewold E, Chandler VL. 1997. Evidence for direct activation of an anthocyanin promoter by the maize C1 protein and comparison of DNA binding by related Myb domain proteins. The Plant Cell 9, 611–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma AD, Sharma R. 1999. Anthocyanin-DNA copigmentation complex: mutual protection against oxidative damage. Phytochemistry 52, 1313–1318. [Google Scholar]
- Schwinn K, Venail J, Shang Y, Mackay S, Alm V, Butelli E, Oyama R, Bailey P, Davies K, Martin C. 2006. A small family of MYB-regulatory genes controls floral pigmentation intensity and patterning in the genus Antirrhinum . The Plant Cell 18, 831–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Werber M, Weisshaar B. 2001. The R2R3-MYB gene family in Arabidopsis thaliana . Current Opinion in Plant Biology 4, 447–456. [DOI] [PubMed] [Google Scholar]
- Sweeney MT, Thomson MJ, Cho YG, Park YJ, Williamson SH, Bustamante CD, McCouch SR. 2007. Global dissemination of a single mutation conferring white pericarp in rice. PLOS Genetics 3, e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takos AM, Jaffé FW, Jacob SR, Bogs J, Robinson SP, Walker AR. 2006. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiology 142, 1216–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L, Merida A, Parr A, Mackay S, Culianez-Macia FA, Roberts K, Martin C. 1998. The AmMYB308 and AmMYB330 transcription factors from Antirrhinum regulate phenylpropanoid and lignin biosynthesis in transgenic tobacco. The Plant Cell 10, 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24, 1596–1599. [DOI] [PubMed] [Google Scholar]
- Walker AR, Lee E, Bogs J, McDavid DA, Thomas MR, Robinson SP. 2007. White grapes arose through the mutation of two similar and adjacent regulatory genes. The Plant Journal 49, 772–785. [DOI] [PubMed] [Google Scholar]
- Xu F, Ning Y, Zhang W, Liao Y, Li L, Cheng H, Cheng S. 2014. An R2R3-MYB transcription factor as a negative regulator of the flavonoid biosynthesis pathway in Ginkgo biloba . Functional & Integrative Genomics , 14, 177–189. [DOI] [PubMed] [Google Scholar]
- Yang J-H, Park H-Y, Kim Y-S, Choi I-W, Kim S-S, Choi H-D. 2012. Quality characteristics of vacuum-fried snacks prepared from various sweet potato cultivars. Food Science and Biotechnology 21, 525–530. [Google Scholar]
- Zhang C, Ma Y, Zhao X, Mu J. 2009a. Influence of copigmentation on stability of anthocyanins from purple potato peel in both liquid state and solid state. Journal of Agricultural and Food Chemistry 57, 9503–9508. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cheng S, De Jong D, Griffiths H, Halitschke R, De Jong W. 2009b. The potato R locus codes for dihydroflavonol 4-reductase. Theoretical and Applied Genetics 119, 931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H-F, Fitzsimmons K, Khandelwal A, Kranz RG. 2009. CPC, a single-repeat R3 MYB, is a negative regulator of anthocyanin biosynthesis in Arabidopsis . Molecular Plant 2, 790–802. [DOI] [PubMed] [Google Scholar]
- Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF. 2004. Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. The Plant Journal 40, 22–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.