Highlight
GORK channels play an important role in the disruption of auxin homeostasis in Arabidopsis root tip and the subsequent inhibition of root growth caused by Trichokonin VI from Trichoderma.
Key words: Arabidopsis thaliana, auxin, GORK, peptaibols, Trichoderma, Trichokonin VI.
Abstract
Trichoderma spp. are well known biocontrol agents that produce a variety of antibiotics. Peptaibols are a class of linear peptide antibiotics mainly produced by Trichoderma. Alamethicin, the most studied peptaibol, is reported as toxic to plants at certain concentrations, while the mechanisms involved are unclear. We illustrated the toxic mechanisms of peptaibols by studying the growth-inhibitory effect of Trichokonin VI (TK VI), a peptaibol from Trichoderma longibrachiatum SMF2, on Arabidopsis primary roots. TK VI inhibited root growth by suppressing cell division and cell elongation, and disrupting root stem cell niche maintenance. TK VI increased auxin content and disrupted auxin response gradients in root tips. Further, we screened the Arabidopsis TK VI-resistant mutant tkr1. tkr1 harbors a point mutation in GORK, which encodes gated outwardly rectifying K+ channel proteins. This mutation alleviated TK VI-induced suppression of K+ efflux in roots, thereby stabilizing the auxin gradient. The tkr1 mutant also resisted the phytotoxicity of alamethicin. Our results indicate that GORK channels play a key role in peptaibol–plant interaction and that there is an inter-relationship between GORK channels and maintenance of auxin homeostasis. The cellular and molecular insight into the peptaibol-induced inhibition of plant root growth advances our understanding of Trichoderma–plant interactions.
Introduction
Trichoderma spp. have long been recognized as agents that can be utilized for the control of plant disease (Benítez et al., 2004). Some Trichoderma spp. can produce auxins or volatiles to promote plant growth or protect plants from biotic and abiotic stresses (Contreras-Cornejo et al., 2009, 2014a, b; Hung et al., 2013). The presence of diverse antibiotics correlates well with the biocontrol abilities of Trichoderma (Lin et al., 1994; Wilhite et al., 1994) and provides a rich resource for screening new pesticides. In a recent review, the authors emphasized the potential of applications of specific secondary metabolites of Trichoderma for the control of phytopathogens as substitutes for whole-organism formulations (Keswani et al., 2014). However, many Trichoderma antibiotics are reported to be phytotoxic (Haraguchi et al., 1996; Marfori et al., 2003; Keswani et al., 2014). It was also found that some Trichoderma antibiotics, such as harzianic acid and harzianolide, promote plant growth and/or induce plant resistance at low concentrations, but inhibit plant growth at relatively high concentrations (Vinale et al., 2009; Cai et al., 2013). To promote the use of Trichoderma and Trichoderma antibiotics, a detailed understanding of the phytotoxicity of these antibiotics is necessary.
Peptaibols, a class of linear peptide antibiotics, are mainly produced by Trichoderma. Peptaibols are made up of 5–20 amino acid residues, which are synthesized by non-ribosomal peptide synthetases (NRPSs) (Szekeres et al., 2005). Peptaibols can be divided into three groups, which are distinguished by the chain lengths of the amino acid sequences. These groups are as follows: long-sequence peptaibols with 18–20 amino acid residues; short-sequence peptaibols with 11–16 residues; and lipopeptaibols with 6 or 10 residues (Daniel and Filho, 2007). The amphipathic nature of peptaibols allows many of them to form voltage-dependent ion channels in lipid membranes, which is the likely basis of their biological activities (Béven et al., 1999; Chugh and Wallace, 2001), such as antimicrobial activity, antitumor activity, and the ability to elicit plant defense (Szekeres et al., 2005). Alamethicin, the most extensively studied long-sequence peptaibol, is well known for its antimicrobial activity and the ability to induce plant resistance (Leitgeb et al., 2007; Kredics et al., 2013). However, alamethicin can also be toxic to plants. Alamethicin causes lesions on Arabidopsis leaves at 5–30 μM (Rippa et al., 2010) and induces rRNA cleavage-associated rapid death in Arabidopsis at 50 μM (Rippa et al., 2007). Alamethicin also permeabilizes the plasma membrane and the mitochondria in tobacco suspension cells and induces cell death at ~22 μM (Matic et al., 2005). The mechanisms involved are not yet fully understood.
Post-embryonic root growth in higher plants is maintained by the root meristem. Within the root meristem, the mitotically inactive quiescent center (QC) cells and the surrounding stem cells constitute a specialized microenvironment known as the root stem cell niche, which serves as the source of all differentiated cell types during root development (Dello Ioio et al., 2008; Dinneny and Benfey, 2008). The phytohormone auxin plays a crucial role in root patterning. It is generally believed that local auxin biosynthesis and polar auxin transport, which depends on the PIN-FORMED (PIN) family of auxin efflux facilitators, function together to establish a state of auxin homeostasis that is required for root meristem patterning and maintenance (Jiang and Feldman, 2003; Blilou et al., 2005; Zhao, 2010).
Trichoderma longibrachiatum SMF2 (hereafter SMF2) is a biocontrol fungus that produces several long-sequence peptaibols known as Trichokonins (TKs), which have broad-spectrum antimicrobial activity (Song et al., 2006; Shi et al., 2012). TK VI is the main component of the TKs produced by SMF2 (Song et al., 2007). In addition to being an antibiotic, TK VI has other biological functions, including the elicitation of systemic resistance in tobacco and Chinese cabbage (Luo et al., 2010; Li et al., 2014) and the induction of programmed cell death in tumor cells (Shi et al., 2010). However, the effect of TK VI on plant growth has not yet been studied.
In this study, we investigated the cellular and molecular mechanisms of TK VI-induced inhibition of primary root growth in Arabidopsis thaliana. TK VI inhibited both cell division and cell elongation in the primary root, and disturbed the maintenance of the root stem cell niche. TK VI also increased the auxin content and disrupted the auxin response gradients in the root tip by modulating local auxin biosynthesis and polar auxin transport. We screened the dominant mutant tkr1, which resisted TK VI toxicity, to further elucidate the molecular mechanisms involved. Map-based cloning revealed a mutation in the GORK gene, which encodes a gated outwardly rectifying K+ channel protein. Using the non-invasive micro-test technique (NMT) and patch-clamp whole-cell recordings, we found that K+ efflux was suppressed by TK VI in the wild type and this suppression was much alleviated in tkr1, enabling tkr1 to maintain K+ and auxin homeostasis better than the wild type when TK VI was supplied. Moreover, we found that tkr1 was also resistant to root growth inhibition caused by alamethicin. Our results revealed the cellular and molecular mechanisms involved in the peptaibol-induced inhibition of plant root growth, which advances our understanding of the interactions between Trichoderma and plants.
Materials and methods
Plant materials and growth conditions
The A. thaliana ecotypes Columbia-0 (Col-0) and Landsberg erecta (Ler) were used. Some of the plant materials used in this study were previously described CYCB1;1 pro :GFP (Zheng et al., 2011); QC25 (Sabatini et al., 1999); DR5 pro :GUS (Ulmasov et al., 1997); IAA2 pro :GUS (Swarup et al., 2007); PIN1 pro :GUS, PIN2 pro :GUS, PIN3 pro :GUS, and PIN7 pro :GUS (Vieten et al., 2005); ASA1 pro :GUS and ASB1 pro :GUS (Stepanova et al., 2005); axr1-1 (Lincoln et al., 1990); pin1-5 (Sohlberg et al., 2006); pin2 pin3 pin4, pin3 pin4 pin7, and pin2 pin3 pin7 (Blilou et al., 2005); asa1-1 (Sun et al., 2009); PLT1 pro :CFP, PLT1 pro :PLT1:YFP, PLT2 pro :CFP, and PLT2 pro :PLT2:YFP (Kornet and Scheres, 2009); SHR pro :SHR:GFP (Nakajima et al., 2001); and SCR pro :GFP (Helariutta et al., 2000). Salk_082258C seeds were obtained from the A. thaliana Biological Resource Center.
Seeds were surface-sterilized for 15min in 10% (v/v) commercial bleach, washed five times with sterile water, and plated on half-strength Murashige and Skoog (MS) medium with 1% (w/v) sucrose and 0.8% (w/v) agar. The seeds were stratified at 4 °C for 2 d in the dark and then transferred to a phytotron set at 22 °C with a 16h light/8h dark photoperiod (with a light intensity of 120 μmol photons m–2 s–1) in vertically or horizontally oriented Petri dishes.
Purification and preparation of TK VI
TK VI was prepared from solid-state fermented SMF2 using a previously described method (Song et al., 2007). Purified TK VI powder (purity >95%) was dissolved in methanol to obtain a 5mM stock solution for further use.
Phenotypic analysis, statistics, and microscopy
The representative seedlings were transplanted to a new Petri dish to be photographed, and root lengths were measured using Image J. The size of the root meristem was assessed as the number of cells between the QC and the first elongating cell in the cortex cell file (Dello Ioio et al., 2007). Whole seedlings or different tissues were cleared in HCG solution (chloroacetaldehyde:water:glycerol=8:3:1) for several minutes before microscopic analysis. Microscopy was performed using a Leica Microsystems DM5000B microscope and DFC490 charge-coupled device (CCD) camera. Statistical significance was evaluated using Student’s t-test. The presented data are mean values of at least three biological repeats with SD.
For Lugol staining of the roots, tissues were incubated in Lugol solution (Sigma-Aldrich) for 3–5min, washed in water once, and mounted in HCG solution for microscopic analysis (Chen et al., 2011). Histochemical staining for β-glucuronidase (GUS) activity in transgenic plants was performed as previously described (Jefferson et al., 1987). Confocal imaging was obtained using a Leica TCS SP5 confocal laser-scanning microscope. Seedlings were stained with 10mg ml–1 propidium iodide for 5min and washed once in water. Fluorescence was quantified using the LAS AF Lite program. Approximately 10 seedlings per image were examined. At least three independent experiments were performed to obtain consistent and statistically significant results.
Gene expression analysis
For quantitative reverse transcription–PCR (qRT–PCR) analysis, whole seedlings or root tip tissues were harvested and frozen in liquid nitrogen for RNA extraction. RNA was extracted using the RNeasy kit (Qiagen). First-strand cDNA was synthesized from 2 μg of total RNA using Superscript III reverse transcriptase (Invitrogen) and oligo(dT) primers. qRT–PCRs were performed using a cycler apparatus (Bio-Rad) and the RealMasterMix kit (SYBR Green, Tiangen) according to the manufacturer’s instructions. The expression levels of the target genes were normalized to those of ACTIN2. Statistical significance was evaluated by Student’s t-test. The primers used to quantify gene expression levels are listed in Supplementary Table S1 at JXB online.
Free IAA measurement
Five-day-old Col-0 seedlings were transplanted to half-strength MS medium without or with 5 μM TK VI for 3h. Then, 2mm root tips were collected for indole acetic acid (IAA) measurements. Approximately 150mg (fresh weight) of root tips was used for IAA extraction and measurement, which followed a published method (Kojima et al., 2009) with minor modifications (Zhou et al., 2010).
Polar auxin transport assay
Five-day-old seedlings grown vertically on plates were transferred to medium without or with 5 μM TK VI for 24h. Seedlings were then transferred to half-strength MS medium, and 1cm of the apical ends of the roots was removed for polar auxin transport assays as previously described (Lewis and Muday, 2009) with minor modifications (Qi et al., 2012).
Mutant screening and map-based cloning of the GORK gene
The seeds of an ethylmethane sulfonate (EMS)-mutagenized population of Arabidopsis ecotype Col-0 were grown vertically on medium supplied with 5 μM TK VI and screened for seedlings with longer roots compared with those of the wild type. Seedlings with longer primary roots were transplanted to soil so that seeds could be harvested for further verification. One mutant, named tkr1, displayed consistent TK VI resistance. The original tkr1 mutant was backcrossed to Col-0 for three generations, and the resulting homozygous progeny were used in this study.
For mapping analysis, the tkr1 mutant in the Col-0 background was crossed with the polymorphic ecotype Ler. The resulting F1 plants were self-pollinated to yield an F2 population segregating for tkr1 mutant phenotypes. A total of 1200 individual F2 plants were selected for GORK chromosomal mapping.
To generate the DR5 pro :GUS reporter line in the tkr1 mutant background, homozygous tkr1 plants were crossed with a transgenic line harboring the DR5 pro :GUS construct to produce an F2 population. Plants that were homozygous for the tkr1 mutation and the DR5 pro :GUS construct were identified from the F2 population and analyzed in the next generation. In each analysis, parental transgenic lines were used for comparisons with corresponding mutants.
Plasmid construction and plant transformation
The GORK promoter region was PCR amplified using the primers 5'-CGCGGATCCATTGTTATAGATCACTTTAAG-3' (BamHI) and 5'-CCGGAATTCGTTTTCAAGAATCGGTTAAATGAAA TC-3' (EcoRI). The PCR product was cloned into the BamHI/EcoRI sites of the binary vector pCAMBIA1391-Z (CAMBIA), resulting in a transcriptional fusion of the GORK promoter and the GUS-coding region.
Full-length GORK-coding sequences were amplified from wild-type and tkr1 genomes using Gateway-compatible primers. The PCR products were cloned using pENTR Directional TOPO cloning kits (Invitrogen) and then recombined with the binary vector pGWB2 (Nakagawa et al., 2007) to generate the 35S pro :GORK and 35S pro :tkr1 constructs. The primers used were 5'-CACCATGGGACGTCTCCGGAGA-3' and 5'-TTATGTTTGATCAGTAGTATCACTG-3'.
The GORK promoter region was amplified using the primers 5'-AACTGCAGATTGTTATAGATCACTTTAAG-3' (PstI) and 5'-CGGGGTACCGTTTTCAAGAATCGGTTAAATGAAATC-3' (KpnI) for cloning into the PstI/KpnI sites of vector pCAMBIA1300 (CAMBIA). The full-length GORK-coding sequence in tkr1 was amplified using the primers 5'-CGGGGTACCATG GGACGTCTCCGGAGACGGCAAG-3' (KpnI) and 5'-CGG GGTACCTGTTTGATCAGTAGTATCACTG-3' (KpnI) for cloning into the KpnI site of the former construct to generate the GORK pro :tkr1 construct. The orientation of the insertion was confirmed by sequencing.
The above constructs were then transformed into the Agrobacterium tumefaciens strain GV3101 (pMP90), which was used for the transformation of Arabidopsis plants by vacuum infiltration (Bechtold and Pelletier, 1998). Transformants were selected based on their resistance to hygromycin (Roche).
Measurement of net K+ flux using NMT
Transient K+ flux was measured using a BIO-IM Series NMT system (YoungerUSA) at the Xuyue Beijing NMT Research Service Center as previously described (Vincent et al., 2005). Five-day-old seedlings were incubated in measuring solution (0.1mM CaCl2, 0.1mM KCl, and 0.3mM MES, pH 5.8) for 15min to equilibrate before the K+ ion flux measurements were recorded for 10–15min. Then, 12 µM TK VI was added to a final concentration of 3 µM. The K+ ion flux recordings were restarted and continued for 14min. The data measured during the first 2min after the TK VI shock were discarded because of diffusion effects from the addition of the stock solution. Net fluxes were calculated using JCal V3.2.2 (http://youngerusa.com or http://ifluxes.com/jcal).
Patch-clamp whole-cell recordings of protoplasts isolated from the root elongation zone epidermis
The standard and enzyme solutions for protoplasts isolation were the same as those previously described (Xu et al., 2006). Root tips (2–3mm) were isolated and incubated in enzyme solution at 23 ºC for 30min. It was confirmed by direct observation that protoplasts were released solely from the elongation zone epidermis (Demidchik et al., 2003). Protoplasts were filtered through a 50 µm nylon mesh and washed twice with standard solution. The bath and pipette solutions used for whole-cell recordings were the same as those previously described (Ivashikina et al., 2001).
Whole-cell currents were recorded 10min after whole-cell configuration was achieved using an Axopatch-200B amplifier (Axon Instruments, USA). For the recordings, the holding potential was set as –60 mV; the test voltage steps were from –120 mV to 100 mV, with +20 mV increments and a 5s duration for each test voltage. For TK VI-treated samples, 3 μM TK VI was added to the bath solution immediately after the formation of the whole-cell configuration. Currents were recorded after 10min. The software used for data recording and analysis was pCLAMP 10.2. The Student’s t-test was used for the comparison of current magnitudes. Results with P≤0.001 were considered to be significantly different.
Results
TKs produced by Trichoderma longibranchiatum SMF2 inhibit the growth of Arabidopsis seedlings
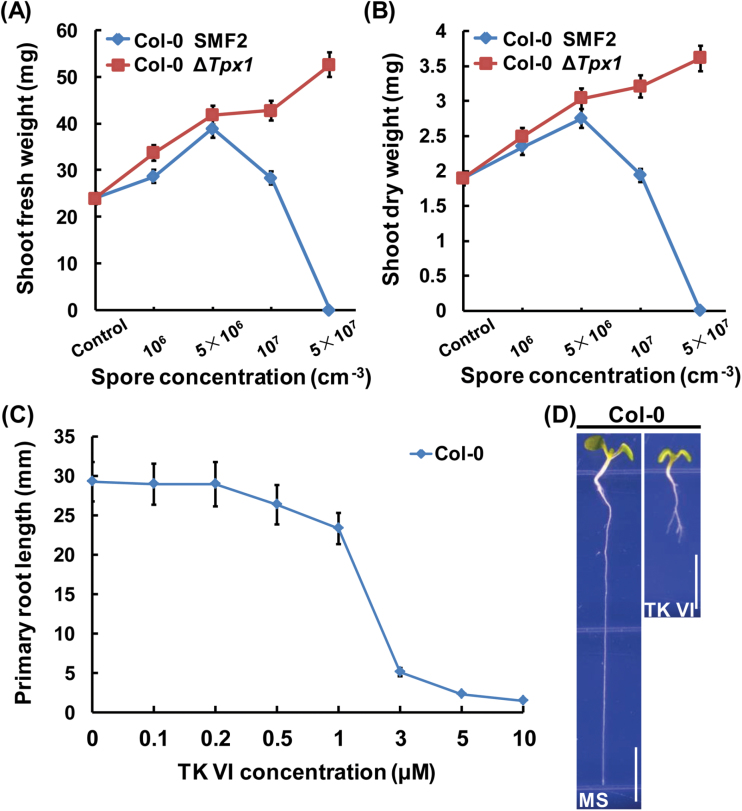
To study the effect of TKs on plant growth in SMF2–plant interactions, we grew Arabidopsis seedlings in soil supplied with different concentrations of spores from the wild-type SMF2 strain and the ΔTpx1 strain. ΔTpx1 is a mutant of SMF2 that does not produce TKs (Supplementary Fig. S1). Low concentrations (≤5×106 cm–3) of wild-type SMF2 spores supplied in the soil promoted the growth of Arabidopsis seedlings. However, high concentrations (≥1×107 cm–3) of wild-type SMF2 spores counteracted this growth promotion effect or inhibited the growth of the Arabidopsis seedlings. Treatment with spores of the ΔTpx1 strain did not show any negative effect, but produced a dose-dependent promotion effect on the growth of the Arabidopsis seedlings (Fig. 1A, B; Supplementary Fig. S2). These results suggest that SMF2 TKs can counteract the plant growth promotion effect of SMF2 or even inhibit plant growth.
Fig. 1.
Growth inhibition of Col-0 seedlings by TKs produced by SMF2. (A, B) Statistical analysis of shoot fresh weight (A) and shoot dry weight (B) of Col-0 seedlings grown in soil without (Control) or with different concentrations of spores from wild-type SMF2 and ΔTpx1. ΔTpx1 is a mutant of SMF2 that does not produce TKs. Eight-day-old Col-0 seedlings were transplanted to soil without or with the indicated concentrations of spores from wild-type SMF2 and ΔTpx1. Plants were grown for an additional 2 weeks before their shoots were removed and weighed immediately (A) or weighed after drying at 65 °C for 3 d (B). Data shown are representative of at least three independent experiments. Each point represents the mean of 36 seedlings, and the error bars represent the SD of triplicate measurements. (C) Primary root length of 6 DAG (days after germination) Col-0 seedlings grown on medium with 0–10 μM TK VI. Data shown are averages with the SD (n>20). (D) Col-0 seedlings grown on medium without (MS) or with 5 μM TK VI at 6 DAG. Scale bars=5mm. (This figure is available in colour at JXB online.)
TK VI is the main component of the TKs produced by SMF2. To confirm further that TKs affect plant growth, we studied the effect of TK VI on the growth of Arabidopsis seedlings. We measured the primary root length of Arabidopsis seedlings by germinating seeds in medium containing different concentrations of TK VI. TK VI had no obvious effect on primary root growth at concentrations <0.5 µM, but inhibited primary root growth in a dose-dependent manner at concentrations within a range of 0.5–10 µM, with a reduction of 92% of root growth by 5 μM TK VI (Fig. 1C, D) The biomass of Arabidopsis shoots and roots was consistently and significantly reduced by 5 µM TK VI (Supplementary Fig. S3).
TK VI-induced inhibition of primary root growth involves reductions in both root meristem activity and root elongation
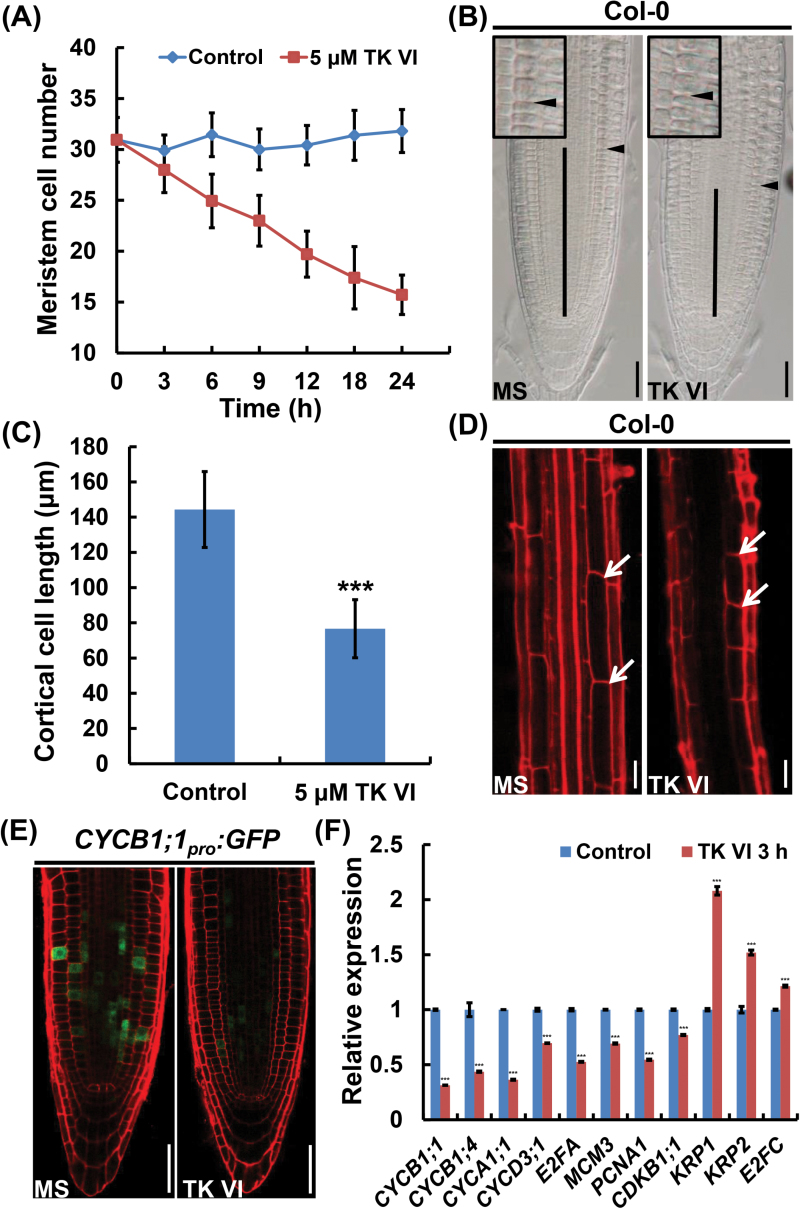
Because root growth depends on both cell division in the meristem zone and cell extension in the elongation region, we investigated TK VI-induced cellular changes in these two zones. As expected, the application of TK VI decreased the meristem size due to a progressive reduction in the number of meristematic cells (Fig. 2A, B). Additionally, shortened cortex cells in the root differentiation zone indicated that cell extension was also inhibited by TK VI in the elongation zone (Fig. 2C, D).
Fig. 2.
TK VI-induced inhibition of cell proliferation and cell elongation in the Col-0 root tip. (A) Time course of TK VI-induced reductions in root meristem size in Col-0 seedlings at 5 DAG. Data shown are averages with the SD (n=20). (B) Root meristems of 5 DAG Col-0 seedlings transplanted to medium without (MS) or with 5 μM TK VI for 12h. The meristem is marked with a vertical black line. The boundary between the meristem zone and the elongation zone is marked with a black arrowhead. The black box is an enlarged image of the boundary between the meristem zone and the elongation zone. (C) Cortical cell length in the differentiation zone of 5 DAG Col-0 seedlings grown on medium without (Control) or with 5 μM TK VI. Data shown are averages with the SD (n=20). The asterisks denote Student’s t-test significance compared with untreated plants: ***P<0.001.
(D) Differentiation zones of seedlings mentioned in (C). The white arrowheads indicate the length of a single cortical cell. (E) TK VI-induced reduction in CYCB1;1 pro :GFP expression in Col-0. Six-day-old seedlings were transferred to medium without (MS) or with 5 μM TK VI for 3h before GFP fluorescence was monitored. (F) The effect of TK VI on the expression of cell cycle-related genes in the Col-0 root tip. Six-day-old Col-0 seedlings were transplanted to medium without (Control) or with 5 μM TK VI for 3h, and the 2mm root tips were harvested for RNA extraction and qRT–PCR analysis. The transcript levels of the indicated genes in Col-0 without TK VI treatment were arbitrarily set to 1. The error bars represent the SD of triplicate reactions. The asterisks denote Student’s t-test significance compared with untreated plants: ***P<0.001. Scale bars=50 μm (B, D, E).
To determine whether the TK VI-induced decrease in the root meristem cell number was a result of a negative effect on cell division, we monitored the expression of the transcriptional fusion reporter CYCB1;1 pro :GFP, a marker that indicates the G2/M phase of the cell cycle. CYCB1;1 pro :GFP was expressed in actively dividing cells of the root meristem, and the application of TK VI markedly reduced the expression range of CYCB1;1 pro :GFP (Fig. 2E). In a qRT–PCR assay, TK VI markedly reduced the expression levels of multiple cell cycle-related genes that positively regulate cell division, including CYCB1;1, CYCB1;4, CYCA1;1, CYCD3;1, E2FA, MCM3, PCNA1, and CDKB1;1, while the expression of three negative regulators of cell division in Arabidopsis, KRP1, KRP2, and E2FC, was up-regulated accordingly (Fig. 2F) (Gutierrez, 2009).
TK VI disrupts the maintenance of the root stem cell niche
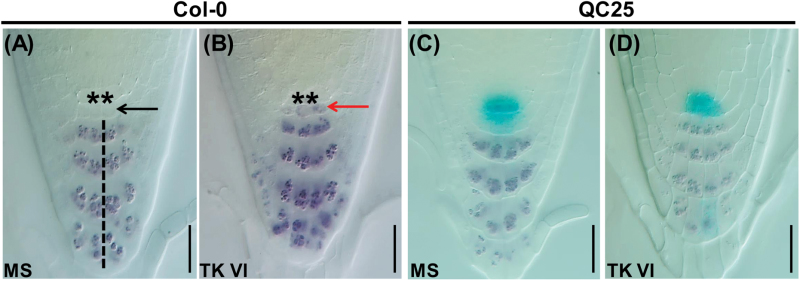
Our finding that TK VI alters root meristem activity prompted us to investigate its possible effect on the cellular organization of the QC and its surrounding stem cells. We analyzed the columella stem cells (CSCs), the cell layer immediately below the QC, to assess the possible effect of TK VI on stem cell maintenance. In the Lugol staining assay, untreated CSCs did not show starch granule accumulation (Fig. 3A, black arrow), which were thus distinct from the underlying well-organized columella cells that contained accumulated starch granules (Fig. 3A, black dashed line). After 12h of TK VI treatment, irregular CSCs containing starch granules were clearly observed immediately below QC cells (Fig. 3B, red arrow), indicating that the CSCs were in a state of differentiation. In the GUS and Lugol double-stained QC marker line QC25, aberrant cellular organization in the QC was visible after prolonged treatment with TK VI for 48h, and the accumulation of starch granules in CSCs was much more severe (Fig. 3C, D).
Fig. 3.
TK VI-induced disruption of the maintenance of the root stem cell niche in Col-0.
(A, B) TK VI-induced CSC differentiation in Col-0 as shown by Lugol staining. Six-day-old seedlings were transferred to medium without (MS) (A) or with (B) 5 μM TK VI for 12h before Lugol staining (dark brown). The asterisks denote QC cells. The black dashed line indicates the columella cell layers. The black arrowhead indicates a lack of starch accumulation in non-differentiated CSCs. The red arrowhead shows starch accumulation in TK VI-treated CSCs. (C, D) Disorganized QC cells and differentiated CSCs as shown by Lugol staining of the QC25 marker line. Six-day-old seedlings were transplanted to medium without (MS) (C) or with (D) 5 μM TK VI for 48h before double staining with GUS and Lugol was performed. Scale bars=20 µm (A–D).
TK VI increases auxin content and alters auxin response gradients in the root tip
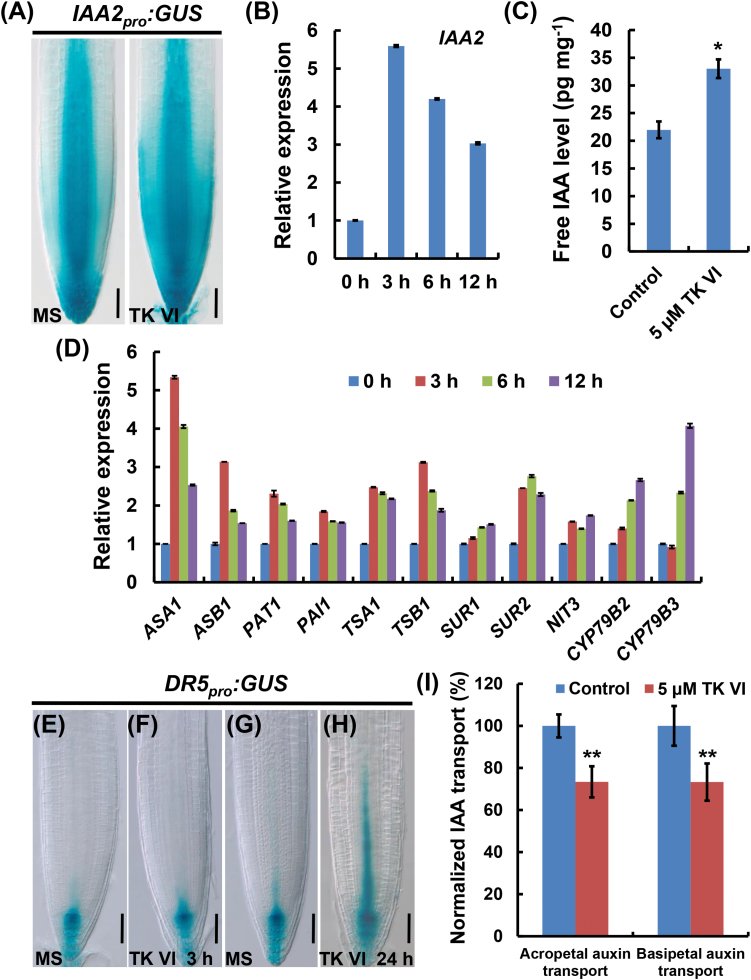
Considering the critical role of the phytohormone auxin in root growth and development, the auxin response in Arabidopsis root tips upon TK VI treatment was monitored using the auxin-inducible reporter IAA2 pro :GUS. Upon TK VI treatment, IAA2 pro :GUS was ectopically induced in the root tip (Fig. 4A). This finding, together with the qRT–PCR analysis of IAA2 expression (Fig. 4B), indicated that auxin was probably accumulated in the root tip. We then measured free IAA in the root tip. As expected, the free IAA level in TK VI-treated root tips was 50% greater than that in untreated root tips (Fig. 4C). Using qRT–PCR, we found that TK VI treatment increased the transcription levels of several auxin biosynthesis-related genes in the root tip (Fig. 4D). Our promoter–GUS assays also indicated that TK VI significantly enhanced the expression of ASA1 and ASB1 (Supplementary Fig. S4). Collectively, these data suggest that TK VI induces IAA biosynthesis in the Arabidopsis root tip through the transcriptional activation of auxin biosynthetic genes.
Fig. 4.
TK VI-induced increase of auxin content and alteration of auxin response gradients in the Col-0 root tip. (A) TK VI-induced enhancement of IAA2 pro :GUS expression in the Col-0 root tip. Six-day-old seedlings were transferred to medium without (MS) or with 5 μM TK VI for 12h before GUS staining. (B) Time course of expression of IAA2 in response to TK VI treatment. (C) Free IAA measurement in Col-0 root tips in response to TK VI treatment. Six-day-old Col-0 seedlings were transplanted to medium without (Control) or with 5 μM TK VI for 3h before the 2mm root tips were harvested. Free IAA levels were then measured. The error bars represent the SD of triplicate measurements. The asterisks denote Student’s t-test significance compared with untreated plants: *P<0.05.
(D) Time course of expression of auxin biosynthesis-related genes in response to TK VI treatment. For (B) and (D), 6-day-old Col-0 seedlings were treated with 5 μM TK VI for the indicated time periods, and the 2mm root tips were harvested for RNA extraction and qRT–PCR analysis. Transcript levels of the indicated genes in Col-0 without TK VI treatment were arbitrarily set to 1. The error bars represent the SD of triplicate reactions.
(E, F, G, H) TK VI-induced disturbance of DR5 pro :GUS expression in the Col-0 root tip. Six-day-old seedlings were transferred to medium without (MS) or with 5 μM TK VI for the indicated times before GUS staining. (I) TK VI-induced repression of acropetal and basipetal auxin transport. Six-day-old Col-0 seedlings were transplanted to medium without (Control) or with 5 μM TK VI for 24h before acropetal and basipetal auxin transport assays were performed. The acropetal and basipetal auxin transport levels without TK VI treatment were arbitrarily set to 100%. Data shown are mean values of five biological repeats with the SD. The asterisks denote Student’s t-test significance compared with untreated plants: **P<0.01. Scale bars=50 µm (A, E, F, G, H).
The accumulation of IAA in the root tip prompted us to examine the possible effect of TK VI on auxin gradients, which are mainly generated by PIN gene-mediated polar auxin transport (Blilou et al., 2005). As shown using the auxin-responsive DR5 pro :GUS reporter, auxin is asymmetrically distributed in the root tip, with an apparent high concentration (auxin maximum) in the QC/columella initial region (Fig. 4E, G), and auxin response gradients in the root tip were not altered after TK VI treatment for 3h (Fig. 4F). Accordingly, the expression levels of the auxin efflux transporter genes, PIN1, PIN2, PIN3, and PIN7, as indicated by PIN1 pro :GUS, PIN2 pro :GUS, PIN3 pro :GUS, and PIN7 pro :GUS, were not visibly affected by 3h TK VI treatment (Supplementary Fig. S5). Instead, DR5 pro :GUS expression was induced above the QC/columella initial region in the stele after prolonged treatment with TK VI for 24h (Fig. 4H), indicating the disruption of auxin response gradients in the root tip. Moreover, with TK VI treatment for 24h, the acropetal and basipetal auxin transport rates in the root tip both reduced by ~27% compared with that of the controls (Fig. 4I).
Isolation and phenotyping of the Arabidopsis TK VI-resistant mutant tkr1
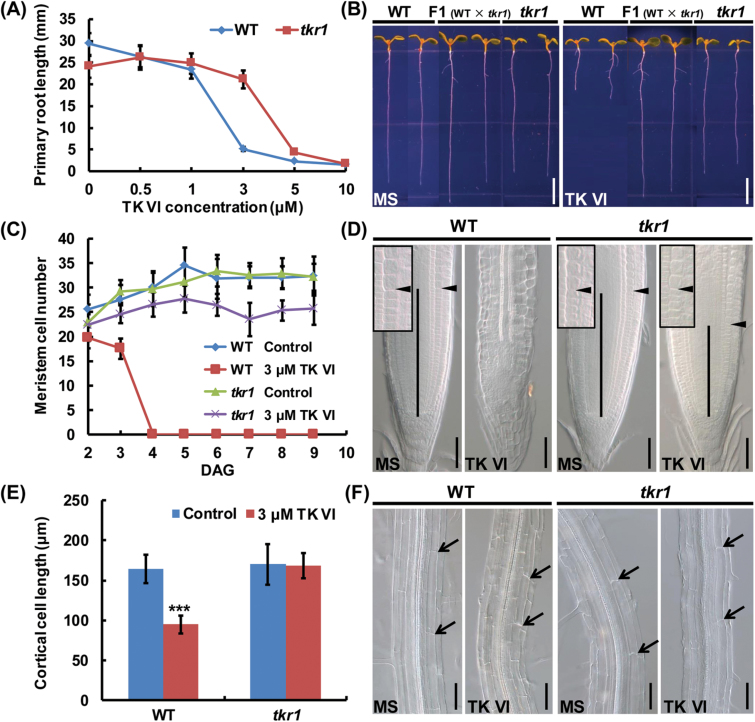
To investigate further the mechanisms involved in the TK VI-induced inhibition effect on Arabidopsis roots, Arabidopsis TK VI-resistant (tkr) mutants were screened from EMS-mutagenized M2 seedlings. Among a number of putative M3 mutants, tkr1 showed significant TK VI endurance capacity compared with the wild type when treated with 1–5 μM TK VI (especially 3 μM), which could be seen in the primary root length (Fig. 5A, B). The meristem size of the tkr1 mutant was approximately the same as that of the wild type at 6 DAG (days after germination) when cultivated on half-strength MS medium. However, the meristem zone of the wild type vanished at 4 DAG when 3 μM TK VI was applied, while the meristem cell number of TK VI-treated tkr1 seedlings only decreased by ~20% compared with untreated tkr1 seedlings even at 9 DAG (Fig. 5C, D). In tkr1 seedlings, the length of cortical cells in the differentiation zone was not affected by 3 μM TK VI (Fig. 5E, F). Taken together, we conclude that tkr1 is a dose-dependent TK VI-resistant mutant.
Fig. 5.
Isolation and phenotyping of the TK VI-resistant mutant tkr1. (A) Primary root length of 6 DAG wild-type (WT) and tkr1 seedlings grown on medium with 0–10 μM TK VI. Data shown are averages with SD (n>20). (B) Phenotyping of 6 DAG seedlings of the WT, tkr1 mutant, and F1 progeny from a cross between the wild type and tkr1 grown on medium without (MS) or with 3 μM TK VI. (C) Root meristem size of WT and tkr1 seedlings grown on medium without (Control) or with 3 μM TK VI at 2–9 DAG. Data shown are averages with SD (n=20). (D) Root meristems of 9 DAG WT and tkr1 seedlings grown on medium without (MS) or with 3 μM TK VI. The meristem zone is marked with a vertical black line. The black arrowhead indicates the boundary between the meristem and the elongation zone. The black box is an enlarged image of the boundary between the meristem zone and the elongation zone. (E) Cortical cell length in the differentiation zone of 9 DAG WT and tkr1 seedlings grown on medium without (Control) or with 3 μM TK VI. Data shown are averages with SD (n=20). Asterisks denote Student’s t-test significance compared with untreated plants: ***P<0.001. (F) Images of the differentiation zone of the seedlings described in (E). Black arrowheads indicate the length of a single cortical cell. Scale bars=5mm (B), 50 µm (D, F).
A genetic analysis revealed that the phenotype of the F1 generation from a cross between tkr1 and the wild type mimics that of tkr1 (Fig. 5B). F2 plants showed a 3:1 segregation ratio of tkr1 over the wild type (Supplementary Table S2). These results indicate that tkr1 harbors a monogenic dominant mutation in a nuclear gene.
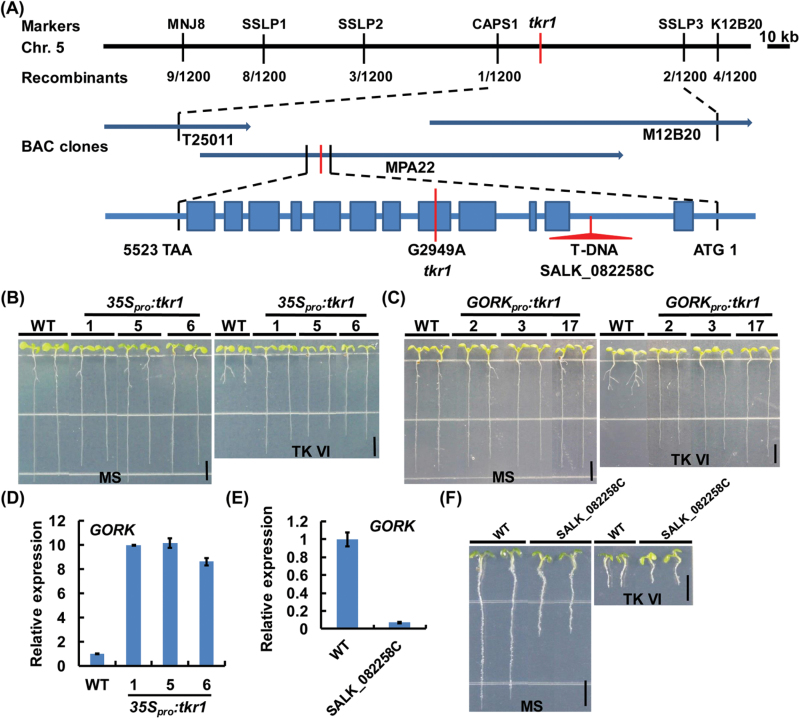
Map-based cloning of GORK, which encodes a gated outwardly rectifying K+ channel protein
Map-based cloning was carried out using an F2 population. The mutated gene was determined to be located within bacterial artificial chromosome (BAC) clone MPA22 of chromosome 5 and was ultimately identified as encoding a gated outwardly rectifying K+ channel (GORK) protein (Fig. 6A). GORKs can form tetrameric gated outwardly rectifying K+ channels, which belong to the shaker family of K+ channels. Such channels are activated upon membrane depolarization (Ache et al., 2000; Mäser et al., 2001).
Fig. 6.
Map-based cloning of the GORK gene. (A) Fine genetic and physical mapping of GORK. The target gene was initially mapped to a genetic interval between the markers MNJ8 and K12B20 on Arabidopsis chromosome 5. The analysis of a mapping population consisting of 1200 plants identified the target gene encoding GORK (At5g37500). In the GORK structure illustration, the filled boxes represent exons and the lines represent introns. Mutation and T-DNA insertion sites are shown in red. (B) Wild-type (WT) and 35S pro :tkr1 seedlings grown on medium without (MS) or with 3 μM TK VI at 6 DAG.
(C) WT and GORK pro :tkr1 seedlings grown on medium without (MS) or with 3 μM TK VI at 6 DAG. (D) qRT–PCR analysis of GORK expression in 35S pro :tkr1 seedlings. (E) qRT–PCR analysis of GORK expression in SALK_082258C. For (D) and (E), 10-day-old seedlings were harvested for RNA extraction and qRT–PCR analysis. The transcript level of GORK in the WT was arbitrarily set to 1. The error bars represent the SD of triplicate reactions. (F) WT and SALK_082258C seedlings grown on medium without (MS) or with 3 μM TK VI at 6 DAG. Scale bars=5mm (B, C, F).
Sequence analysis showed that a single nucleotide substitution in the tkr1 allele, A2949 for G2949 (Fig. 6A), results in a change of Gly313 to Ser313 in the amino acid sequence of GORK. The K+ channels of the shaker family possess six transmembrane segments (S1–S6) and a pore-forming domain (P) between S5 and S6 (Jan and Jan, 1992; Gaymard et al., 1998). SKOR is another member of the shaker family of K+ channels, and has the closest phylogenetic relationship to GORK based on phylogenetic analysis (Mäser et al., 2001). A protein sequence alignment between GORK and SKOR (Gaymard et al., 1998) indicated that Gly313 is next to the last amino acid residue of S6 and links S6 with the C-terminal cytoplasmic region of the subunit (Supplementary Fig. S6). We then constructed 35S pro :tkr1 and GORK pro :tkr1 transgenic lines in the wild-type background and analyzed their response upon TK VI treatment. These lines all showed significantly increased TK VI resistance compared with that of the wild type (Fig. 6B, C), confirming our mapping result. The overexpression of the mutated GORK gene in the 35S pro :tkr1 transgenic lines was verified by qRT–PCR (Fig. 6D).
We tested the TK VI resistance of the T-DNA insertion line SALK_082258C, which contains a T-DNA insertion in the first intron of GORK (Fig. 6A). The T-DNA insertion in SALK_082258C disrupts the transcription of GORK (Fig. 6E). Unlike tkr1, the GORK knockout T-DNA insertion line displayed a wild-type response upon TK VI treatment (Fig. 6F). Similarly, the exogenous application of the K+ channel blockers TEA+ or Ba2+ (Demidchik et al., 2010) did not alleviate the inhibition effect of TK VI on primary root growth (Supplementary Fig. S7). We then constructed 35S pro :GORK transgenic lines and examined their response to TK VI treatment. All of the GORK overexpression lines showed a wild-type response when treated with TK VI (Supplementary Fig. S8). Together, these observations indicate that the TK VI-enduring ability of tkr1 is not the result of either the loss of function of GORK or its overexpression, indicating that the single amino acid substitution of Ser313 for Gly313 most probably changes the function of GORK channels.
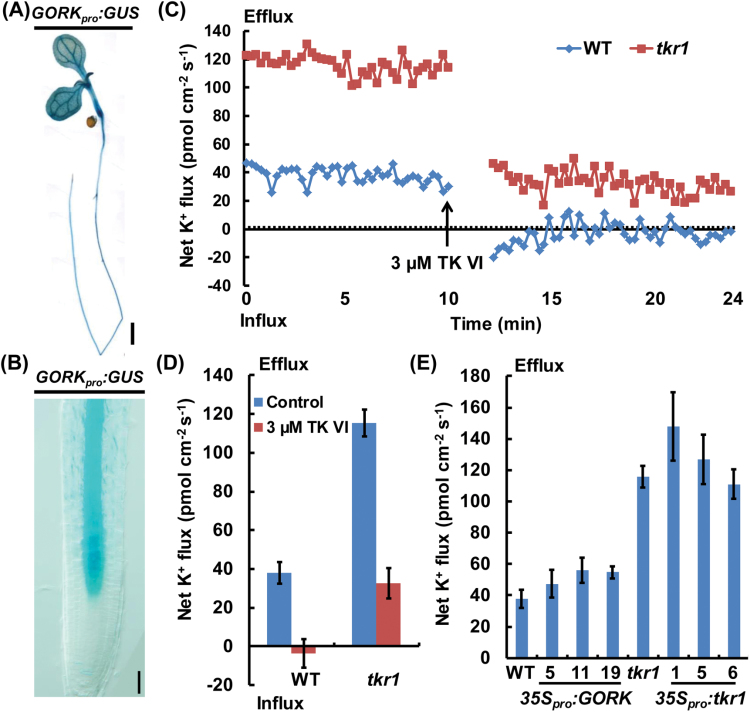
TK VI represses the outward K+ currents mediated by GORK channels, resulting in the disruption of auxin gradients
The function of K+ efflux through GORK channels in stomatal closure has been studied in detail (Hosy et al., 2003). Recent data have demonstrated that GORK-mediated K+ efflux is tightly linked to the production of stress-induced reactive oxygen species and may also be involved in programmed cell death (Demidchik et al., 2010). We first investigated the effect of the mutation in tkr1 on K+ flux before and after TK VI treatment using NMT. As indicated by our GORK pro :GUS reporter, GORK was expressed throughout the entire root except the root tip (Fig. 7A, B), in agreement with previous reports (Ache et al., 2000; Ivashikina et al., 2001). Because GORK-mediated K+ efflux is more sensitive to outer stimuli in the elongation zone than in the mature area (Demidchik et al; 2010), all of the measurements were carried out in the elongation zone of the primary root. Without TK VI treatment, an average K+ efflux of ~38 pmol cm−2 s−1 was recorded in the wild type, while the K+ efflux in tkr1 was three times higher than this value under the same conditions. Upon TK VI shock, the K+ efflux in the wild type was almost completely turned off, and an average K+ efflux of ~33 pmol cm−2 s−1 still remained in tkr1 despite a severe reduction (Fig. 7C, D). K+ flux was also measured in several independent 35S pro :GORK and 35S pro :tkr1 transgenic lines under control conditions. The K+ efflux rate in 35S pro :GORK lines was similar to that of the wild-type seedlings, and the K+ efflux rate in 35S pro :tkr1 lines resembled that of the tkr1 seedlings (Fig. 7E). These results indicate that the overexpression of GORK in 35S pro :GORK transgenic lines has no obvious effect on K+ efflux modulation, which is in agreement with their wild-type response upon TK VI treatment.
Fig. 7.
K+ flux analysis using NMT. (A, B) GORK pro :GUS expression pattern in seedlings at 5 DAG. (C) Transient K+ flux measurements upon TK VI (3 μM) shock in the elongation zone of the wild type (WT) and tkr1. Before the TK VI shock, steady K+ flux was examined for 10min. The data in the following 2min after TK VI shock are not shown for TK VI diffusion. Data shown are representative of at least three independent experiments. Each point represents the mean of six seedlings. (D) Mean efflux of K+ before and after TK VI shock in the WT and tkr1 seedlings described in (C). Data shown are averages with the SD (n=6) and are representative of at least three independent experiments. (E) Mean efflux of K+ in WT, tkr1, 35S pro :GORK, and 35S pro :tkr1 seedlings under control conditions. Steady K+ efflux in the indicated seedlings was examined for 15min. Data shown are averages with the SD (n=6) and are representative of at least three independent experiments. Scale bar=5mm (A), 50 µm (B).
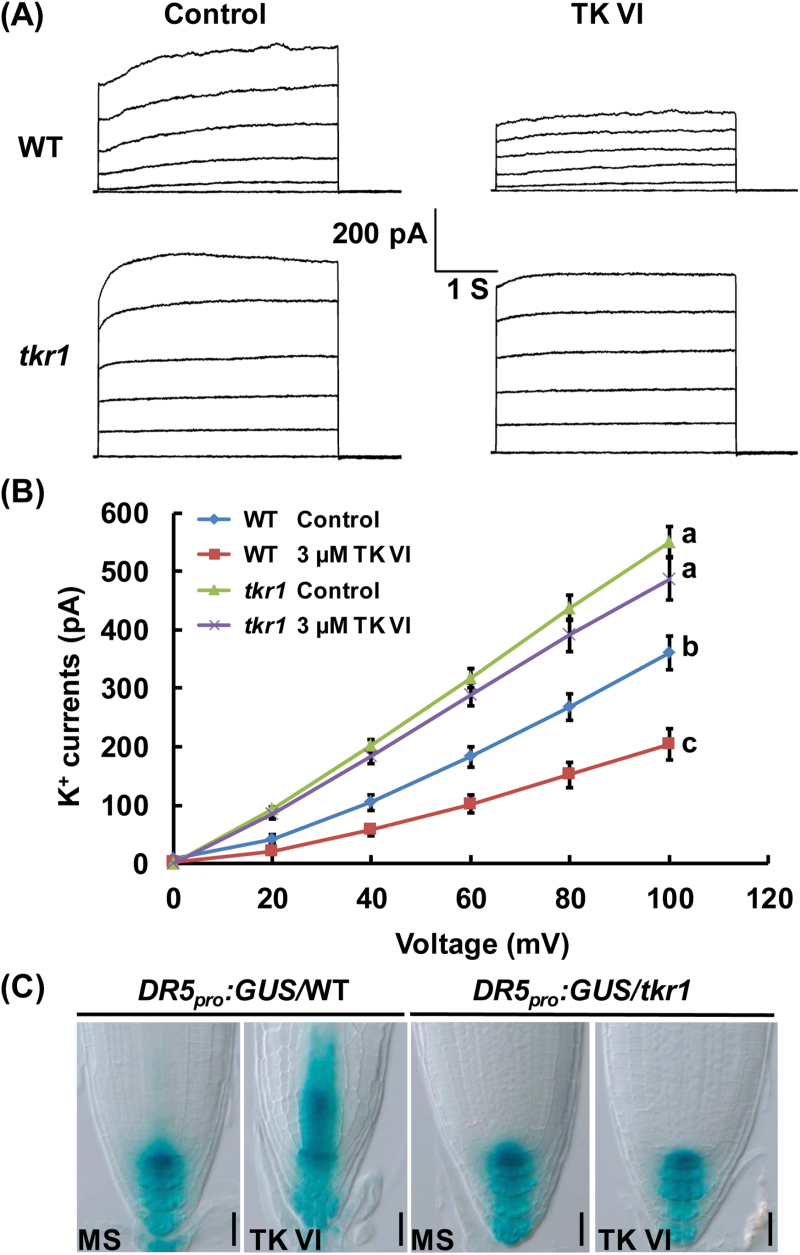
To confirm further the TK VI-induced suppression of K+ efflux, we conducted patch-clamp whole-cell recordings using root cell protoplasts isolated from the primary root elongation zone epidermis. In the absence of TK VI, outward K+ currents in tkr1 were much larger than those in the wild type; in the presence of 3 µM TK VI, outward K+ currents were significantly reduced in the wild type and only slightly suppressed in tkr1 (Fig. 8A, B).
Fig. 8.
The effect of TK VI on outward K+ currents as analyzed by patch-clamp whole-cell recordings. (A) Patch-clamp whole-cell recordings of outward K+ current traces in wild-type (WT) and tkr1 root cell protoplasts without (Control) or with 3 μM TK VI. Time and current scales shown in the middle apply to all traces. (B) The current/voltage relationship of the whole-cell outward K+ currents as illustrated in (A). The number of protoplasts measured for the curves were 16 (for WT), 14 (for WT treated with 3 μM TK VI), 11 (for tkr1), and 11 (for tkr1 treated with 3 μM TK VI). Data shown are averages with the SD. Samples with different letters are significantly different; P<0.001. (C) Effect of TK VI on auxin distribution gradients in the WT and tkr1. DR5 pro :GUS/WT and DR5 pro :GUS/tkr1 seeds were germinated on medium without (MS) or with 3 μM TK VI for 5 DAG before GUS staining assays were performed. Scale bars=50 µm.
Taken together, these results indicate that TK VI negatively regulates GORK-mediated K+ efflux. The substitution of Ser313 for Gly313 in the GORK amino acid sequence results in an enhanced K+ efflux magnitude and alleviates TK VI-induced suppression of K+ efflux in roots.
To test whether the TK VI-induced disruption of auxin response gradients in the Arabidopsis root tip is related to the suppression of GORK-mediated K+ efflux, we introduced the DR5 pro :GUS reporter into the tkr1 background and analyzed the effect of TK VI on auxin distribution in the root tip. As shown by the DR5 pro :GUS reporter, without TK VI treatment, the DR5 pro :GUS expression pattern in the root tip of the tkr1 seedlings was the same as that in the root tip of the wild-type seedlings. After growing on medium supplied with 3 µM TK VI for 5 DAG, the auxin response gradients in the wild-type seedlings were badly disrupted, with the auxin maximum lying above the QC/columella initial region, while auxin distribution in the root tip of the TK VI-treated tkr1 seedlings remained the same as that in the untreated tkr1 seedlings (Fig. 8C). These results indicate that the TK VI-induced disruption of auxin gradients in the Arabidopsis root tip results from TK VI-induced suppression of GORK-mediated K+ efflux.
The tkr1 mutant is also resistant to alamethicin, the most studied long-sequence peptaibol
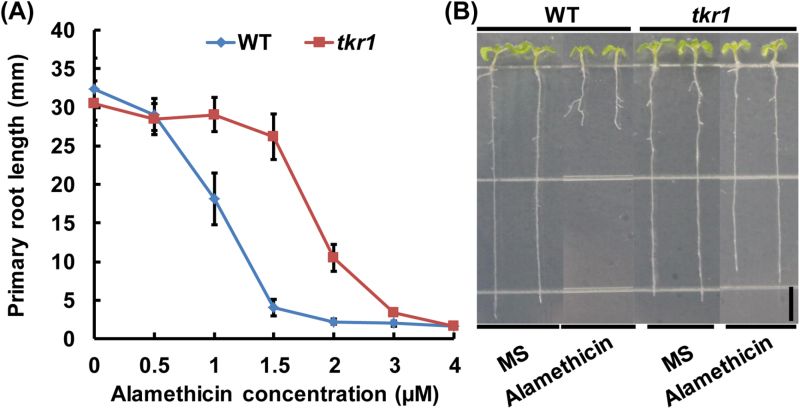
Long-sequence peptaibols are thought to function through the formation of ion channels in lipid membranes according to the ‘barrel-stave model’ (Chugh and Wallace, 2001). To determine whether long-sequence peptaibols inhibit plant growth through the same mechanism as TK VI, we investigated the effect of alamethicin on the primary root growth of the wild type and the tkr1 mutant. We found that alamethicin inhibited the primary root growth of Arabidopsis in a dose-dependent manner as previously described (Matic et al., 2005; Rippa et al., 2007, 2010), and the tkr1 mutant exhibited significantly increased resistance to alamethicin doses of 1–3 µM (especially 1.5 µM) (Fig. 9A, B). This result indicates that alamethicin probably inhibits plant growth through the same mechanism as TK VI.
Fig. 9.
Alamethicin effects on primary root growth in the wild type (WT) and tkr1 mutant.
(A) Primary root length of 6 DAG WT and tkr1 seedlings grown on medium with 0–4 μM alamethicin (Sigma, purity ≥98%). Data shown are averages with the SD (n>20). (B) WT and tkr1 seedlings grown on medium without (MS) or with 1.5 μM alamethicin at 6 DAG. Scale bars=5mm.
Discussion
TK VI-induced inhibition of primary root growth is a complex process involving multiple cellular and molecular processes
Our results indicated that TK VI inhibited both cell division and cell elongation in Arabidopsis roots; the maintenance of the root stem cell niche was also disrupted by TK VI. According to our data, TK VI-induced auxin accumulation (at 3h) occurs much earlier than the TK VI-induced disruption of auxin response gradients (at 24h) in Arabidopsis root tips, which means that the early accumulation of auxin in the root tip does not affect auxin distribution gradients. The disruption of auxin response gradients is probably caused by the inhibition of polar auxin transport in the root tip, which results in the reduction of auxin being transported to the QC/columella initial region to maintain the auxin maximum. TK VI severely affected the auxin content and auxin response gradients in wild-type Arabidopsis root tips. However, unlike axr1-1, which is an auxin-resistant mutant (Lincoln et al., 1990), tkr1 is not resistant to IAA (Supplementary Fig. S9A). In addition, in the tested auxin-related mutants, including the auxin signaling pathway mutant axr1-1, the auxin transport mutants pin1-5, pin2 pin3 pin4, pin2 pin3 pin7, and pin3 pin4 pin7, and the auxin biosynthesis mutant asa1-1, no TK VI resistance was observed (Supplementary Fig. S9B). These results indicate that auxin-related pathways are not the direct or specific targets of TK VI in the Arabidopsis–TK VI interaction process, and other key regulators of root patterning are also involved. PLETHORA (PLT) proteins are auxin-induced transcription factors that are essential for root stem cell niche patterning and root meristem activity (Aida et al., 2004; Mähönen et al., 2014). The expression of PLT1 and PLT2 was not notably affected during the first 12h of TK VI treatment, but was dramatically decreased after 24h of treatment (Supplementary Fig. S10A–E). The expression of SHORT-ROOT (SHR) and SCARECROW (SCR), which act in parallel with the PLT pathway to provide positional information for the stem cell niche (Helariutta et al., 2000; Sabatini et al., 2003), was also remarkably suppressed (Supplementary Fig. S10F, G). These data suggest that TK VI-induced inhibition of root growth is a complex process that involves multiple cellular and molecular processes.
K+ homeostasis is important for the maintenance of auxin gradients
As one of the most abundant and irreplaceable macronutrient in plants, K+ plays crucial roles in many fundamental processes in living cells, such as electrical neutralization, osmoregulation, membrane potential regulation, and the activation of crucial enzymatic reactions (Clarkson and Hanson, 1980). TRH1, a member of the AtKT/AtKUP/HAK K+ transporter family, is required for polar auxin transport in Arabidopsis roots (Rigas et al., 2001; Vicente-Agullo et al., 2004). Although the physiological mechanism by which TRH1 interacts with root auxin transport remains to be clarified, a connection exists between K+ transport and polar auxin transport. As demonstrated using the DR5 pro :GUS reporter, TK VI-induced disruption of auxin gradients was recovered to normal levels in the tkr1 mutant, in which the function of GORK channels is altered due to a substitution of Ser313 for Gly313. Our data hint at an inter-relationship between GORK channels and the maintenance of auxin homeostasis, though the mechanism involved is unclear. The trh1 mutant phenotype was not restored by high external K+ concentrations (Rigas et al., 2001); similarly, TK VI-induced inhibition of root growth was not rescued by 50mM external K+ (Supplementary Fig. S11). Additionally, trilongins, peptaibols also from T. longibrachiatum, are reported to form voltage-dependent Na+/K+-permeable channels to cause mammalian cell toxicity (Mikkola et al., 2012). These data suggest that TK VI-induced disruption of auxin gradients is not due to K+ starvation; instead, this disruption is most probably caused by alterations in K+ homeostasis.
We applied both NMT and patch-clamp whole-cell recordings to analyze the effect of the substitution of Ser313 for Gly313 on the function of GORK channels. According to these data, K+ efflux is suppressed by TK VI in the wild type, and this TK VI-induced suppression is much alleviated in tkr1, although how the mutation changes the function of GORK channels is unclear. It is well known that some K+ channels in animal cells are regulated by specific kinases (Jan and Jan, 1997). The function of AKT1, another member of the shaker family of K+ channels (Mäser et al., 2001), has been reported to be directly regulated by protein phosphorylation (Xu et al., 2006). It is unknown whether GORK is regulated by phosphorylation and whether the change of Gly313 to Ser313, an amino acid that can be phosphorylated by protein kinase, affects the phosphorylation of GORK, which merits further study.
Mechanisms of peptaibol-induced inhibition of primary root growth in Arabidopsis
Based on the results of this study and those of other studies, the mechanisms of TK VI-induced inhibition of primary root growth in Arabidopsis can be proposed. When Arabidopsis seedlings are treated with TK VI, ion channels are formed in the plasma membrane of the root cells and the ion balance of root cells is disrupted. GORK channels somehow sense this disruption in ion balance and act immediately to decrease K+ efflux. This alteration in K+ homeostasis reduces meristem size by suppressing cell division, enhances auxin content by activating local auxin biosynthesis, and disrupts auxin gradients by suppressing polar auxin transport in the root tip. The TK VI-induced disruption of auxin homeostasis and other key regulators involved in root patterning, such as PLT1/PLT2 and SHR/SCR, results in the disruption of maintenance of the root stem cell niche. Post-embryonic root growth is ultimately terminated by TK VI due to a lack of a root stem cell niche, which serves as the source of all differentiated cell types during root development. In addition, the TK VI-resistant mutant tkr1 is also resistant to alamethicin, which suggests that long-sequence peptaibols probably inhibit primary root growth in Arabidopsis via the same mechanism as TK VI.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Construction of the ΔTpx1 mutant by knocking out the Tpx1 gene that encodes the NRPS responsible for TKs biosynthesis in SMF2.
Figure S2. Arabidopsis (Col-0) seedlings grown in soil supplied with different concentrations of spores from the wild-type SMF2 or the ΔTpx1 mutant.
Figure S3. Biomass loss in TK VI-treated Arabidopsis (Col-0) shoots and roots.
Figure S4. TK VI-induced expression of ASA1 and ASB1 analyzed by promoter–GUS reporters.
Figure S5. TK VI effect on the expression of the auxin efflux transporter genes.
Figure S6. Location of GORK Gly313 by amino acid sequence alignment with SKOR.
Figure S7. Effect of K+ channel blockers on TK VI-induced inhibition of primary root growth in wild-type Arabidopsis (Col-0) and tkr1 seedlings.
Figure S8. Phenotyping of independent 35S pro :GORK transgenic lines upon TK VI treatment.
Figure S9. The auxin resistance of tkr1 and phenotyping of auxin-related Arabidopsis mutants upon TK VI treatment.
Figure S10. TK VI-induced repression of the expression of PLT1/PLT2 and SHR/SCR.
Figure S11. The effect of 50mM external K+ on TK VI-induced inhibition of root growth.
Table S1. DNA primers used for qRT–PCR assays.
Table S2. Genetic analysis of the tkr1 mutant.
Acknowledgements
This work was supported by the National Science Foundation of China (31270064), the National Basic Research Program of China (2015CB942900), the Hi-Tech Research and Development program of China (2011AA090704), and the Program of Shandong for Taishan Scholars (2009TS079).
References
- Ache P, Becker D, Ivashikina N, Dietrich P, Roelfsema MRG, Hedrich R. 2000. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Letters 486, 93–98. [DOI] [PubMed] [Google Scholar]
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B. 2004. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119, 109–120. [DOI] [PubMed] [Google Scholar]
- Bechtold N, Pelletier G. 1998. In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods in Molecular Biology 82, 259–266. [DOI] [PubMed] [Google Scholar]
- Benítez T, Rincón AM, Limón MC, Codón AC. 2004. Biocontrol mechanisms of Trichoderma strains. International Microbiology 7, 249–260. [PubMed] [Google Scholar]
- Béven L, Helluin O, Molle G, Duclohier H, Wróblewski H. 1999. Correlation between anti-bacterial activity and pore-sizes of two classes of voltage-dependent channel-forming peptides. Biochimica et Biophysica Acta 1420, 53–63. [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. 2005. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433, 39–44. [DOI] [PubMed] [Google Scholar]
- Cai F, Yu G, Wang P, Wei Z, Fu L, Shen Q, Chen W. 2013. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum . Plant Physiology and Biochemistry 73, 106–113. [DOI] [PubMed] [Google Scholar]
- Chen Q, Sun J, Zhai Q, et al. 2011. The basic helix–loop–helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis . The Plant Cell 23, 3335–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh JK, Wallace BA. 2001. Peptaibols: models for ion channels. Biochemical Society Transactions 29, 565–570. [DOI] [PubMed] [Google Scholar]
- Clarkson DT, Hanson JB. 1980. The mineral nutrition of higher plants. Annual Review of Plant Physiology 31, 239–298. [Google Scholar]
- Contreras-Cornejo HA, Macías-Rodríguez L, Alfaro-Cuevas R, López-Bucio J. 2014. a Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Molecular Plant-Microbe Interactions 27, 503–514. [DOI] [PubMed] [Google Scholar]
- Contreras-Cornejo HA, Macías-Rodríguez L, Cortés-Penagos C, López-Bucio J. 2009. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis . Plant Physiology 149, 1579–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras-Cornejo HA, Macías-Rodríguez L, Herrera-Estrella A, López-Bucio J. 2014. b The 4-phosphopantetheinyl transferase of Trichoderma virens plays a role in plant protection against Botrytis cinerea through volatile organic compound emission. Plant and Soil 379, 261–274. [Google Scholar]
- Daniel JF, Filho ER. 2007. Peptaibols of Trichoderma . Natural Product Reports 24, 1128–1141. [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, Costantino P, Sabatini S. 2007. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Current Biology 17, 678–682. [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S. 2008. A genetic framework for the control of cell division and differentiation in the root meristem. Science 322, 1380–1384. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Cuin TA, Svistunenko D, Smith SJ, Miller AJ, Shabala S, Sokolik A, Yurin V. 2010. Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. Journal of Cell Science 123, 1468–1479. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM. 2003. Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. Journal of Cell Science 116, 81–88. [DOI] [PubMed] [Google Scholar]
- Dinneny JR, Benfey PN. 2008. Plant stem cell niches: standing the test of time. Cell 132, 553–557. [DOI] [PubMed] [Google Scholar]
- Gaymard F, Pilot G, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferrière N, Thibaud JB, Sentenac H. 1998. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 94, 647–655. [DOI] [PubMed] [Google Scholar]
- Gutierrez C. 2009. The Arabidopsis cell division cycle. Arabidopsis Book 7, e0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi H, Hamatani Y, Hamada M, Fujii-Tachino A. 1996. Effect of gliotoxin on growth and branched-chain amino acid biosynthesis in plants. Phytochemistry 42, 645–648. [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser MT, Benfey PN. 2000. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101, 555–567. [DOI] [PubMed] [Google Scholar]
- Hosy E, Vavasseur A, Mouline K, Dreyer I, Gaymard F, Poree F. 2003. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proceedings of the National Academy of Sciences, USA 100, 5549–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung R, Lee S, Bennett JW. 2013. Arabidopsis thaliana as a model system for testing the effect of Trichoderma volatile organic compounds. Fungal Ecology 6, 19–26. [Google Scholar]
- Ivashikina N, Becker D, Ache P, Meyerhoff O, Felle HH, Hedrich R. 2001. K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Letters 508, 463–469. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. 1992. Tracing the roots of ion channels. Cell 69, 715–718. [DOI] [PubMed] [Google Scholar]
- Jan LY, Jan YN. 1997. Cloned potassium channels from eukaryotes and prokaryotes. Annual Review of Neuroscience 20, 91–123. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Feldman LJ. 2003. Root meristem establishment and maintenance: the role of auxin. Journal of Plant Growth and Regulation 21, 432–440. [Google Scholar]
- Keswani C, Mishra S, Sarma BK, Singh SP, Singh HB. 2014. Unraveling the efficient applications of secondary metabolites of various Trichoderma spp. Applied Microbiology and Biotechnology 98, 533–544. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kamada-Nobusada T, Komatsu H, et al. 2009. Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography-tandem mass spectrometry: an application for hormone profiling in Oryza sativa . Plant and Cell Physiology 50, 1201–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornet N, Scheres B. 2009. Members of the GCN5 histone acetyltransferase complex regulate PLETHORA-mediated root stem cell niche maintenance and transit amplifying cell proliferation in Arabidopsis . The Plant Cell 21, 1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredics L, Szekeres A, Czifra D, Vágvölgyi C, Leitgeb B. 2013. Recent results in alamethicin research. Chemistry and Biodiversity 10, 744–771. [DOI] [PubMed] [Google Scholar]
- Leitgeb B, Szekeres A, Manczinger L, Vágvölgyi C, Kredics L. 2007. The history of alamethicin: a review of the most extensively studied peptaibol. Chemistry and Biodiversity 4, 1027–1051. [DOI] [PubMed] [Google Scholar]
- Lewis DR, Muday GK. 2009. Measurement of auxin transport in Arabidopsis thaliana . Nature Protocols 4, 437–451. [DOI] [PubMed] [Google Scholar]
- Li HY, Luo Y, Zhang XS, Shi WL, Gong ZT, Shi M, Chen LL, Chen XL, Zhang YZ, Song XY. 2014. Trichokonins from Trichoderma pseudokoningii SMF2 induce resistance against Gram-negative Pectobacterium carotovorum subsp. carotovorum in Chinese cabbage. FEMS Microbiology Letters 354, 75–82. [DOI] [PubMed] [Google Scholar]
- Lin A, Lee TM, Rern JC. 1994. Tricholin, a new antifungal agent from Trichoderma viride, and its action in biological control of Rhizoctonia solan . Journal of Antibiotics 47, 799–805. [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M. 1990. Growth and development of the axr1 mutants of Arabidopsis . The Plant Cell 2, 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhang DD, Dong XW, Zhao PB, Chen LL, Song XY, Wang XJ, Chen XL, Shi M, Zhang YZ. 2010. Antimicrobial peptaibols induce defense responses and systemic resistance in tobacco against tobacco mosaic virus. FEMS Microbiology Letters 313, 120–126. [DOI] [PubMed] [Google Scholar]
- Mähönen AP, ten Tusscher K, Siligato R, Smetana O, Díaz-Triviño S, Salojärvi J, Wachsman G, Prasad K, Heidstra R, Scheres B. 2014. PLETHORA gradient formation mechanism separates auxin responses. Nature 515, 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfori EC, Kajiyama S, Fukusaki E, Kobayashi A. 2003. Phytotoxicity of the tetramic acid metabolite trichosetin. Phytochemistry 62, 715–721. [DOI] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, et al. 2001. Phylogenetic relationships within cation transporter families of Arabidopsis . Plant Physiology 126, 1646–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matic S, Geisler DA, Møller IM, Widell S, Rasmusson AG. 2005. Alamethicin permeabilizes the plasma membrane and mitochondria but not the tonoplast in tobacco (Nicotiana tabacum L. cv Bright Yellow) suspension cells. Biochemical Journal 389, 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola R, Andersson MA, Kredics L, Grigoriev PA, Sundell N, Salkinoja-Salonen MS. 2012. 20-Residue and 11-residue peptaibols from the fungus Trichoderma longibrachiatum are synergistic in forming Na+/K+-permeable channels and adverse action towards mammalian cells. FEBS Journal 279, 4172–4190. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. 2007. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. Journal of Bioscience and Bioengineering 104, 34–41. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Sena G, Nawy T, Benfey PN. 2001. Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413, 307–311. [DOI] [PubMed] [Google Scholar]
- Qi L, Yan J, Li Y, Jiang H, Sun J, Chen Q, Li H, Chu J, Yan C, Sun X. 2012. Arabidopsis thaliana plants differentially modulate auxin biosynthesis and transport during defense responses to the necrotrophic pathogen Alternaria brassicicola . New Phytologist 195, 872–882. [DOI] [PubMed] [Google Scholar]
- Rigas S, Debrosses G, Haralampidis K, Vicente-Agullo F, Feldmann KA, Grabov A, Dolan L, Hatzopoulos P. 2001. TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. The Plant Cell 13, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippa S, Adenier H, Derbaly M, Béven L. 2007. The peptaibol alamethicin induces an rRNA-cleavage-associated death in Arabidopsis thaliana . Chemistry and Biodiversity 4, 1360–1373. [DOI] [PubMed] [Google Scholar]
- Rippa S, Eid M, Formaggio F, Toniolo C, Béven L. 2010. Hypersensitive-like response to the pore-former peptaibol alamethicin in Arabidopsis thaliana . Chembiochem 11, 2042–2049. [DOI] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, et al. 1999. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99, 463–472. [DOI] [PubMed] [Google Scholar]
- Sabatini S, Heidstra R, Wildwater M, Scheres B. 2003. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes and Development 17, 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Chen L, Wang XW, Zhang T, Zhao PB, Song XY, Sun CY, Chen XL, Zhou BC, Zhang YZ. 2012. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology 158, 166–175. [DOI] [PubMed] [Google Scholar]
- Shi M, Wang HN, Xie ST, Luo Y, Sun CY, Chen XL, Zhang YZ. 2010. Antimicrobial peptaibols, novel suppressors of tumor cells, targeted calcium-mediated apoptosis and autophagy in human hepatocellular carcinoma cells. Molecular Cancer 9, 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlberg JJ, Myrenås M, Kuusk S, Lagercrantz U, Kowalczyk M, Sandberg G, Sundberg E. 2006. STY1 regulates auxin homeostasis and affects apical–basal patterning of the Arabidopsis gynoecium. The Plant Journal 47, 112–123. [DOI] [PubMed] [Google Scholar]
- Song XY, Shen QT, Xie ST, Chen XL, Sun CY, Zhang YZ. 2006. Broad-spectrum antimicrobial activity and high stability of Trichokonins from Trichoderma koningii SMF2 against plant pathogens. FEMS Microbiology Letters 260, 119–125. [DOI] [PubMed] [Google Scholar]
- Song XY, Xie ST, Chen XL, Sun CY, Zhang YZ. 2007. Solid-state fermentation for Trichokonins production from Trichoderma koningii SMF2 and preparative purification of Trichokonin VI by a simple protocol. Journal of Biotechnology 131, 209–215. [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. 2005. A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis . The Plant Cell 17, 2230–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Xu Y, Ye S, et al. 2009. Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. The Plant Cell 21, 1495–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ. 2007. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. The Plant Cell 19, 2186–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres A, Leitgeb B, Kredics L, Antal Z, Hatvani L, Manczinger L, Vágvölgyi C. 2005. Peptaibols and related peptaibiotics of Trichoderma . Acta Microbiologica et Immunologica Hungarica 52, 137–168. [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. 1997. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. The Plant Cell 9, 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Agullo F, Rigas S, Desbrosses G, Dolan L, Hatzopoulos P, Grabov A. 2004. Potassium carrier TRH1 is required for auxin transport in Arabidopsis roots. The Plant Journal 40, 523–535. [DOI] [PubMed] [Google Scholar]
- Vieten A, Vanneste S, Wisniewska J, Benková E, Benjamins R, Beeckman T, Luschnig C, Friml J. 2005. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132, 4521–4531. [DOI] [PubMed] [Google Scholar]
- Vinale F, Flematti G, Sivasithamparam K, Lorito M, Marra R, Skelton BW, Ghisalberti EL. 2009. Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum . Journal of Natural Products 72, 2032–2035. [DOI] [PubMed] [Google Scholar]
- Vincent P, Chua M, Nogue F, Fairbrother A, Mekeel H, Xu Y, Allen N, Bibikova TN, Gilroy S, Bankaitis VA. 2005. A Sec14p-nodulin domain phosphatidylinositol transfer protein polarizes membrane growth of Arabidopsis thaliana root hairs. Journal of Cell Biology 168, 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhite SE, Lumsden RD, Straney DC. 1994. Mutational analysis of gliotoxin production by the biocontrol fungus Gliocladium virens in relation to suppression of Pythium damping-off. Phytopathology 84, 816–821. [Google Scholar]
- Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH. 2006. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis . Cell 125, 1347–1360. [DOI] [PubMed] [Google Scholar]
- Zhao Y. 2010. Auxin biosynthesis and its role in plant development. Annual Review of Plant Biology 61, 49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Chen X, McCormick S. 2011. The anaphase-promoting complex is a dual integrator that regulates both MicroRNA-mediated transcriptional regulation of cyclin B1 and degradation of Cyclin B1 during Arabidopsis male gametophyte development. The Plant Cell 23, 1033–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wei L, Xu J, Zhai Q, Jiang H, Chen R, Chen Q, Sun J, Chu J, Zhu L. 2010. Arabidopsis tyrosylprotein sulfotransferase acts in the auxin/PLETHORA pathway in regulating postembryonic maintenance of the root stem cell niche. The Plant Cell 22, 3692–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.