Highlight
An ethylene-insensitive mutant of pea with enhanced nodulation and arbuscular mycorrhizal development showed that gibberellin and brassinosteroid deficiency affect nodule number via ethylene levels.
Key words: Arbuscular mycorrhizae, brassinosteroids, ein2, ethylene insensitivity, gibberellins, hormone mutants, nodulation, peas, root growth.
Abstract
The regulation of arbuscular mycorrhizal development and nodulation involves complex interactions between the plant and its microbial symbionts. In this study, we use the recently identified ethylene-insensitive ein2 mutant in pea (Pisum sativum L.) to explore the role of ethylene in the development of these symbioses. We show that ethylene acts as a strong negative regulator of nodulation, confirming reports in other legumes. Minor changes in gibberellin1 and indole-3-acetic acid levels in ein2 roots appear insufficient to explain the differences in nodulation. Double mutants produced by crosses between ein2 and the severely gibberellin-deficient na and brassinosteroid-deficient lk mutants showed increased nodule numbers and reduced nodule spacing compared with the na and lk single mutants, but nodule numbers and spacing were typical of ein2 plants, suggesting that the reduced number of nodules in na and lk plants is largely due to the elevated ethylene levels previously reported in these mutants. We show that ethylene can also negatively regulate mycorrhizae development when ethylene levels are elevated above basal levels, consistent with a role for ethylene in reducing symbiotic development under stressful conditions. In contrast to the hormone interactions in nodulation, ein2 does not override the effect of lk or na on the development of arbuscular mycorrhizae, suggesting that brassinosteroids and gibberellins influence this process largely independently of ethylene.
Introduction
Symbioses between plants and microorganisms are common and are important for the acquisition of at least two key macronutrients, nitrogen and phosphorus (Ferguson et al., 2010; Foo et al., 2013b; Gu et al., 2011). Phosphorus acquisition is enhanced by an arbuscular mycorrhizal symbiosis with fungi of the phylum Glomeromycota, a symbiosis estimated to be formed by more than 80% of land plants (Smith and Read, 2008). The symbiosis between plants and rhizobial bacteria is less common but is important for nitrogen acquisition, especially in legumes (Ferguson et al., 2010). Abiotic stresses such as drought, salinity, and waterlogging influence these important symbioses and subsequent nutrient uptake, but our understanding of the specific mechanisms through which this occurs are still emerging (Belimov et al., 2009; Capoen et al., 2010; Larrainzar et al., 2014; Singleton and Bohlool, 1984). One possibility is that these stresses may modify pathways for hormone synthesis or signalling, including stress hormones such as ethylene (Alonso et al., 1999; Jones et al., 2015) and growth-promoting hormones such as gibberellins and brassinosteroids (Ferguson et al., 2005, 2011; Foo et al., 2013a). Indeed, one recent report suggests the involvement of ethylene in the regulation of nitrogen fixation by drought (Larrainzar et al., 2014). Ethylene is known to be involved in the regulation of many other aspects of plant development (Davies, 2010). Most detailed work on ethylene has been conducted in Arabidopsis, a species that does not form arbuscules when infected with mycorrhizal fungi (Veiga et al., 2013) or rhizobial symbioses. The other prominent model species in ethylene research, especially in relation to fruit ripening, is tomato; while tomato forms arbuscular mycorrhizal symbioses, it does not form nodules, and is therefore not suitable for studying the effects of plant hormones on the two symbioses in the same system.
Recently, an ethylene signalling mutant, Psein2, was identified in pea (Pisum sativum L.) (Weller et al., 2015), opening up the prospect of exploring the role of ethylene signalling in these symbioses in an important agricultural crop. EIN2 encodes an N-RAMP metal-transporter-like protein and is a single-copy gene in temperate legumes such as pea and Medicago truncatula, unlike Lotus japonicus and the tropical legumes Glycine max and Phaseolus vulgaris, in which two copies occur (Miyata et al., 2013; Weller et al., 2015). Based on evidence from ein2 in Arabidopsis, and the single-copy nature of PsEIN2 (Lin et al., 2009; Merchante et al., 2013; Weller et al., 2015), it is likely that all ethylene signalling in pea occurs through EIN2. Ethylene binding results in inactivation of the ethylene receptor CTR1, which in turn dephosphorylates EIN2 and enables its proteolytic cleavage. Release of the EIN2 C-terminal fragment allows it to enter the nucleus, where it inhibits degradation of the EIN3/EIL1 transcription factors and promotes the expression of ethylene-responsive genes (Qiao et al., 2012). In the shoots of pea the product of this gene has been shown to regulate petal senescence and the response of plants to low-intensity red and blue light (Weller et al., 2015), but its effects in pea on root development and symbioses with microorganisms have not been examined. Previous application studies by Lee and LaRue (1992) suggested that ethylene inhibits nodulation in peas. In M. truncatula, a closely related temperate legume that also forms indeterminate nodules with a persistent meristem, the EIN2 homologue negatively regulates nodulation (Penmetsa et al., 2008). The relationship between ethylene and nodulation appears more complex in legumes that form determinate nodules (Chan et al. 2013; Hunter, 1993: Miyata et al., 2013; Schmidt et al., 1999). While findings support a general negative role for ethylene, the control has recently been shown to be complex, with positive effects of ethylene early in rhizobial symbiosis (Larrainzar et al., 2015). Consequently, the effect of ethylene sensitivity on nodulation still merits clarification in a wider range of legume species.
Two growth-promoting hormones, gibberellins and brassinosteroids, have been shown to increase nodule number in legumes at physiological levels (Ferguson et al., 2005; Lievens et al., 2005), although for gibberellin an optimum level is reached, above which a decrease in nodulation occurs (Ferguson et al., 2005). These results come mainly from studies in pea, which used hormone-deficient mutants that display reduced nodulation (Ferguson et al., 2005, 2011). The mutants used were na, which is defective in ent-kaurenoic acid oxidase, causing reduced gibberellin levels (Davidson et al., 2003), and lk, which expresses a truncated steroid 5α-reductase enzyme that results in reduced brassinosteroid levels (Nomura et al., 2004). These severely hormone-deficient mutants have also been shown to produce elevated levels of ethylene (Ferguson et al., 2011; Ross and Reid, 1986). The elevated ethylene levels raise the possibility that at least some of the developmental changes in the mutants resulting from the reduced gibberellin and brassinosteroid levels may occur via effects on ethylene. Indeed, the shoot phenotype of the brassinosteroid-deficient lk mutant has some ethylene-related characteristics that can be partially reversed by treatment with the ethylene synthesis inhibitor amino-ethoxyvinyl glycine (Ferguson et al., 2011; Ross and Reid, 1986). In addition, the decrease in nodulation seen in gibberellin-deficient na mutants can be reversed by treatment with amino-ethoxyvinyl glycine (Ferguson et al., 2011), and recently it has been shown that the gibberellin biosynthetic pathway is regulated by ethylene during the development of the rhizobial symbiosis (Larrainzar et al., 2015). Further studies are required to clarify the role of such interactions between ethylene, brassinosteroids, and gibberellins in the control of nodulation.
The results from studies using ethylene mutants to examine arbuscular mycorrhizae formation are inconclusive. Ethylene mutants have been examined in both M. truncatula and tomato. However, the reports are difficult to interpret, with the ethylene-insensitive mutant in M. truncatula reported to have increased mycorrhizae, and both ethylene-insensitive and ethylene-overproducing tomato plants reported to exhibit a small elevation, reduction, or no change in mycorrhizal colonization (Chan et al., 2013; Riedel et al., 2008; Torres de Los Santos et al., 2011; Zsögön et al., 2008). An inhibitory role for ethylene in mycorrhizal colonization was also suggested by enhanced colonization of mutants disrupted in the RIN gene, which encodes a MADS-box transcription factor that blocks ripening, including climacteric ethylene production (Torres de Los Santos et al., 2011; Vrebalov et al., 2002). Application studies in pea suggest that ethylene inhibits arbuscular mycorrhizal formation (Geil et al., 2001) and the consensus view based on both mutant and application studies appears to be that ethylene is inhibitory for mycorrhizal formation (Geil et al., 2001; Penmetsa et al., 2008; Vela et al., 2007). However, the role of ethylene in arbuscular mycorrhizae formation requires clarification and deserves further exploration, as ethylene signalling may be an important mechanism for plants to limit fungal colonization under stress.
To date, there has been little examination of how other hormones implicated in mycorrhizal development may interact with ethylene in the control of mycorrhizal development. Recent reports suggest a positive role for brassinosteroids in mycorrhizal development in both tomato and rice (Bitterlich et al., 2014a, b). Given, as outlined above, that severe brassinosteroid deficiency causes elevated ethylene production in pea, it is important to know whether this brassinosteroid/ethylene interaction may influence mycorrhizal development.
In this paper we use the recently identified ein2 mutant of pea (Weller et al., 2015) to explore the role of the hormone ethylene on nodule and arbuscular mycorrhizal development. The ein2 mutation eliminates the C-terminal domain of the encoded protein (Weller et al., 2015), which is essential for its function in Arabidopsis (Wen et al., 2012). We show that the resultant ethylene insensitivity leads to a greater number of nodules, and also to enhanced mycorrhizal colonization under conditions where ethylene levels are elevated. The ein2 mutant does not appear to affect these symbioses by markedly altering the level of other hormones such as gibberellin1 (GA1), indole-3-acetic acid (IAA), and abscisic acid (ABA). We also generated double mutants to test the interactions between gibberellin deficiency and brassinosteroid deficiency and ethylene insensitivity on nodule number and mycorrhizal development. We show that the reduction in nodule number in gibberellin- and brassinosteroid-deficient plants is probably due to the elevated production of ethylene by these mutants. In contrast, ethylene is likely to act independently of gibberellin and brassinosteroids to influence interactions with arbuscular mycorrhizal fungi.
Materials and methods
Plant material and growth conditions
The AF145 mutant line carrying the ein2 mutation was generated by ethyl methanesulfonate mutagenesis of cv. Torsdag (Weller et al., 2015) and selected after back-crossing to Torsdag three times. Crosses to produce the double mutants ein2 na and ein2 lk involved AF145 crossed to lines carrying either the na-1 or lk mutant alleles that had been previously back-crossed to Torsdag six and three times, respectively. All plants were grown in 140mm pots in a 1:1 mixture of vermiculite and dolerite chips topped with 4cm of pure vermiculite in a temperature-limited glasshouse under a natural photoperiod extended to 18h. The exceptions were for plants in Fig. 1, which were grown in tubes as described by Weston et al. (2009) on 1.3% agar containing the appropriate level of either IAA (Sigma-Aldrich; St Louis, MO, USA) or 1-N-naphthylphthalamic acid (NPA) (Sigma-Aldrich), and for those grown for ethylene analysis as described below. For root architecture measurements, plants were grown for 14 d and root parameters were recorded, including the total number of secondary roots, the average length of the top 10 secondary roots, and the number of tertiary roots borne on these roots. Plants in the experiment receiving ethephon had either 10 μg of ethephon in 10 μl of ethanol or just 10 μl of ethanol applied every 2 weeks after planting to the uppermost expanded leaflet. This application method was used in order to achieve a minimal dose that did not markedly affect root or shoot development and to avoid any direct effect of ethephon on mycorrhizal fungi in the soil.
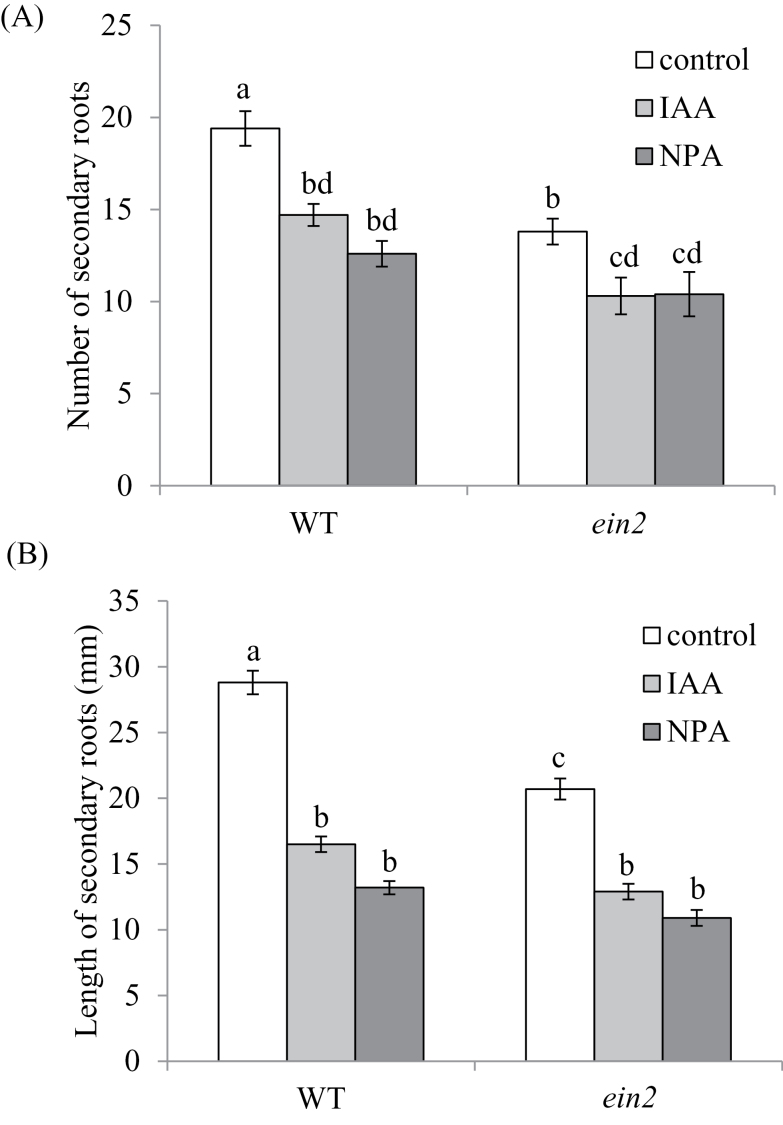
Fig. 1.
Response of roots to IAA and NPA. (A) Number and (B) average length of secondary roots of 7-day-old wild-type pea (cv. Torsdag) and ein2 plants treated with either 1mM IAA or 50mM NPA, or left untreated. Mean±SE (n=9). Within each panel, values with different letters above the bars are significantly different at P<0.05.
Symbiosis experiments
Nodulation experiments were carried out as described in Foo and Davies (2011). Briefly, plants were grown two per pot, inoculated at day 7 with a 3-day-old culture of Rhizobium leguminosarum bv viciae (RLV248) grown in yeast-mannitol broth, and received modified Long Ashton solution containing 5mM NaH2PO4 and no nitrogen weekly. Plants were scored for phenotypic characters at the ages indicated. At the time of scoring, the total number of nodules on a root system was counted. Nodule spacing data were obtained by placing secondary roots on a grid and selecting nodules that intersected the grid, as described by Foo et al. (2014). Root, shoot, and nodule dry weights were obtained and nodule number expressed as the number of nodules per g root dry weight to account for small differences in root size (Foo et al., 2014).
Mycorrhizae experiments were conducted as outlined in Foo et al. (2013a) with plants grown two per pot in vermiculite and dolerite chips containing a mixture of Rhizophagus irregularis spores (approximately 8000 spores per pot; Premier Tech Pty Ltd, Quebec, Canada) and propagules obtained from leek or spring onion R. irregularis pot cultures (1:4 by volume of inoculum to vermiculite and dolerite chips). Plants received modified Long Ashton solution containing 10mM KNO3 and 0.05mM NaH2PO4 weekly. Plants were removed from the soil and the whole root system was cut into 1cm segments and mixed. A subset of roots was cleared using boiling 5% KOH and stained using the ink and vinegar method (Vierheilig et al., 1998). Mycorrhizal colonization of roots was scored according to McGonigle et al. (1990), where 150 intersects were observed from 25 root segments per plant and scored for the presence of arbuscules, vesicles, and/or intraradical hyphae. Root colonization was calculated from the percentage of intersects that contained any internal fungal structure (arbuscule, hyphae, or vesicle), and arbuscule frequency was calculated from the percentage of intersects that contained arbuscules. Some variation occurred between experiments due to seasonal affects. For experiments with na single mutants and na ein2 double mutants, where some mutant lines contained an increased number of cortical cell layers compared with wild-type lines, the colonization rate for all plants was expressed relative to the number of cell layers, as described in Foo et al., (2013a).
Hormone analyses
Levels of IAA and GA1 were determined from mature whole roots as described by Jager et al. (2005, 2008). Briefly, samples were homogenized and extracted into 80% (v/v) methanol and deuterated internal standards were added. Samples were passed through C18 Sep-Pak cartridges (Waters, Australia) and fractionated using a reverse-phase C18 HPLC system (Waters Associates, Milford, USA). The individual fractions containing gibberellins, IAA, or ABA were pooled separately. Gibberellin-containing samples were methylated. Gibberellin and IAA samples were then analysed by gas chromatography-mass spectrometry-selected ion monitoring using a Hewlett Packard 5890 gas chromatograph coupled to a Kratos Concept ISQ mass spectrometer controlled by a Mach 3 data system (Jager et al., 2005). Pooled fractions containing ABA were partitioned against diethyl ether, resuspended in chloroform, and GC-MS-MS analysis was performed with a Varian 8400 Autosampler and a Varian 3800 GC coupled to a Varian 1200 triple quadrupole MS, as described by Jager et al. (2008). Endogenous hormone levels were calculated from the ratio of endogenous to standard peak areas after mass spectroscopy and normalized to per g root fresh weight.
For ethylene analysis, wild-type and ein2 mutant plants were grown three per jar in unsealed 600ml glass jars, filled to one-third of the volume with damp vermiculite. Plants were grown in a growth cabinet under an 18h photoperiod (100 µmol quanta m−2 s−1 at the top of the jar) at 20 °C/15 °C day/night temperatures. After 11 d, when plants had two expanded leaves, each jar was sealed with a gas-tight lid fitted with a septum. After a further 30h growth in the cabinet, 400 µl samples of headspace gas were withdrawn with syringes from four replicate jars of each genotype. GC-MS was performed as described by Foo et al. (2006). A known concentration of acetylene gas (4 ppm in 100 µl) was mixed with each sample and a standard of mixed ethylene and acetylene (both 4 ppm in 500 µl) was analysed between each replicate so that the concentration of ethylene in the samples could be calculated. After analysis, the plants were removed from the jars and whole-plant fresh weight was determined.
Data analysis
Statistical analyses were performed using Excel Stat Plus. Percentage data were arc-sin transformed before analysis. For pairwise comparisons t-tests were performed, and for all other analyses ANOVAs were performed followed by Tukey’s post-hoc test (P<0.05).
Results
Root phenotype and the response to auxin of a pea ein2 mutant
Although the ein2 mutant does not display a classical triple response to applied ethylene (Goeschl et al., 1967; Weller et al., 2015), the roots of Psein2 mutants have only a subtle phenotype. The secondary roots are slightly shorter than those of the wild-type progenitor cv. Torsdag (P<0.05, Table 1) and the number of tertiary roots is reduced (P<0.01) when grown in potting medium, but there is no effect on the ratio of shoot to root fresh weights. There is also a reduction in secondary root numbers and lengths in some circumstances, such as when seedlings are grown in tubes on agar medium (P<0.01, Fig. 1). Auxin plays a central role in the regulation of root growth in many species, and several authors have suggested that it may do so in part by modulating ethylene levels or response (Clark et al., 1999; Stepanova et al., 2007). Application of the auxin IAA and an inhibitor of auxin transport, NPA, significantly inhibited secondary root number and length in wild-type peas (Fig. 1). A similar response was observed in the ein2 mutant. There was no significant interaction between genotype and treatment in a two-way ANOVA. This suggests that these compounds do not act primarily in the roots by affecting EIN2-dependent ethylene signalling, in agreement with the work of Eliasson et al. (1989). However, Prayitno et al. (2006) have shown that in Medicago Mtein2 can influence auxin transport and subsequent nodulation.
Table 1.
The effect of ein2 on root growth
| Genotype | Number of secondary roots | Average length of top 10 secondary roots (mm) | Number of tertiary roots | Shoot FW: root FW |
|---|---|---|---|---|
| WT | 71.5±8.3 | 136.0±4.5 | 5.7±0.6 | 1.3±0.2 |
| ein2 | 82.5±6.03 | 123.0±2.8* | 2.8±0.2** | 1.1±0.1 |
Comparison of the parental wild-type (WT) cv. Torsdag with the ein2 mutant grown under glasshouse conditions for 14 d for the number of secondary and tertiary roots, the average length of the uppermost 10 secondary roots per plant, and the shoot fresh weight:root fresh weight ratio. Mean±SE (n=4–8). * P<0.05, ** P<0.01.
Hormone levels in the ein2 mutant
To examine whether ethylene insensitivity may influence root development by modifying the levels of other hormones known to regulate root growth, we examined bioactive GA1, IAA, and ABA levels in ein2 roots. No substantial differences in the levels of these hormones (see Ross et al., 2011) were observed between the wild-type plants and the Psein2 mutant roots, even though in the cases of IAA and GA1 they were significant at the 5% level (Table 2). We also determined the level of ethylene produced by ein2 plants and found that the level was significantly increased, by approximately twofold, compared with wild-type plants (Supplementary Fig. S1 at JXB online). This is similar to the increase seen in Atein2 plants (Guzmán and Ecker, 1990) and is typical of the feedback mechanisms seen in hormone-insensitive mutants (e.g. Nomura et al., 1999).
Table 2.
Hormone levels in whole mature roots
| Genotype | GA1 (ng g–1 FW root) | IAA (ng g–1 FW root) | ABA (ng g–1 FW root) |
|---|---|---|---|
| WT | 0.11±0.004 | 10.78±0.2 | 8.1±2.8 |
| ein2 | 0.078±0.004* | 12.0±0.3* | 5.8±1.2 |
Levels of GA1, IAA, and ABA in whole root systems of glasshouse-grown 18-day-old wild-type (WT) cv. Torsdag plants and ein2 mutants. Mean±SE (n=3). * P<0.05.
Symbiotic development in Psein2 mutants
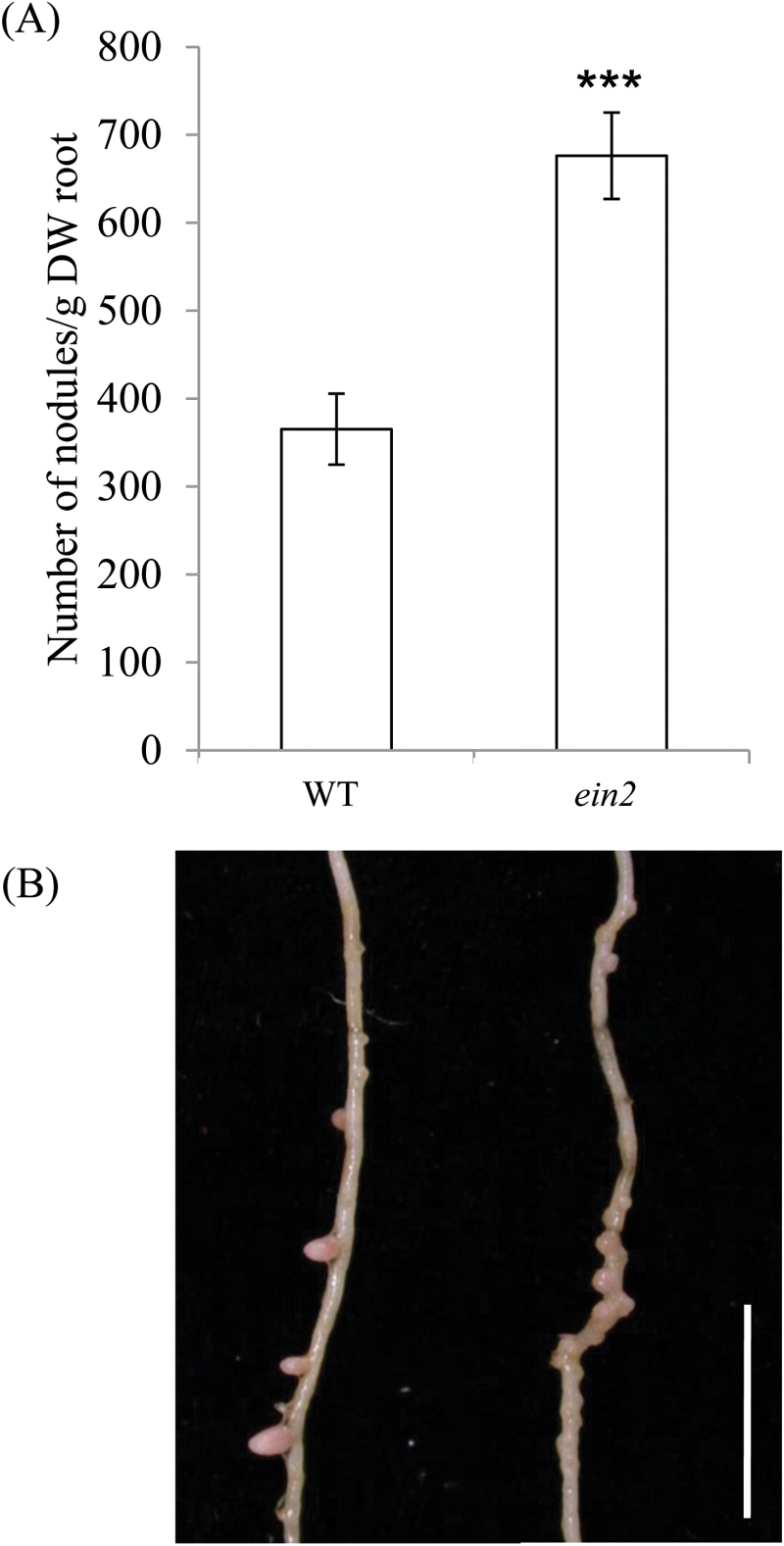
In pea, Psein2 mutants exhibit a significant increase in the number of nodules formed for a given root mass compared with wild-type plants (P<0.001; Fig. 2). These nodules are smaller than those on wild-type plants and are more closely spaced (Fig. 2B), a characteristic of nodulation in ethylene-insensitive lines (Miyata et al., 2013; Penmetsa and Cook, 1997). Our results from pea, a temperate species with indeterminate nodules, therefore support an inhibitory role of ethylene in nodule formation (e.g. Lee and LaRue, 1992, Penmetsa and Cook, 1997).
Fig. 2.
Nodulation in ein2 and wild-type plants. (A) Number of nodules per g dry root weight. Values are mean±SE (n=5). ***P<0.001. (B) Photograph of nodules on a secondary root of (left) wild-type and (right) ein2 plants (tertiary roots have been removed) grown under nitrate-free conditions for 28 days. Scale bar=1cm. (This figure is available in colour at JXB online.)
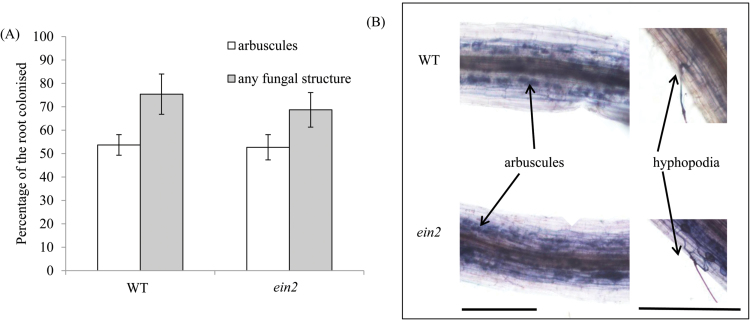
As outlined previously (Foo et al., 2013a), a generally negative role for ethylene in mycorrhizal symbioses has been reported. We found no significant effect of the ein2 mutation on mycorrhizal colonization in pea when grown in relatively unstressed conditions in pots. When grown with R. irregularis fungi, Psein2 roots were colonized to a similar extent to wild-type roots (Fig. 3A), including a similar percentage of the root containing arbuscules (the nutrient exchange unit of the symbiosis) (Fig. 3A), and formed normal internal fungal structures (hyphae and arbuscules; Fig. 3B). Hyphopodia, when observed, also appeared normal (Fig. 3B).
Fig. 3.
Mycorrhizal colonization of roots of wild-type and ein2 mutants. The plants were grown for 6 weeks in pot culture with Rhizophagus irregularis under low phosphate (0.05mM NaH2PO4). (A) The roots were scored for the percentage of roots possessing arbuscules or any internal fungal structures (hyphae, arbuscules, and/or vesicles). Values are mean±SE (n=6). (B) Photomicrograph of colonized roots showing arbuscules and hyphopodia. Scale bars=100 µm. (This figure is available in colour at JXB online.)
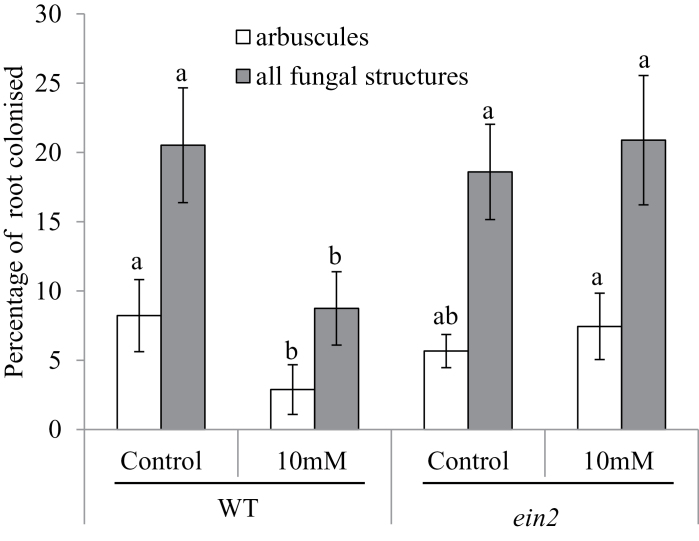
In order to examine whether ein2 mutants had altered mycorrhizal colonization under elevated ethylene, as may be experienced by plants under stress, we treated wild-type and ein2 mutants with ethephon (Fig. 4), an ethylene-releasing compound (Yang, 1969). Ethephon significantly suppresses overall shoot and root growth in wild-type plants, but ein2 plants show no significant response (Weller et al., 2015; data not shown). Treatment with ethephon resulted in a significant reduction in the percentage of the root colonized with fungal structures and arbuscules in wild-type plants; this response was absent in ein2 mutants (Fig. 4). A two-way ANOVA confirmed this interaction between genotype and ethephon treatment for the percentage of the root colonized by fungal structures (P<0.05), indicating that ethylene may be a negative regulator of mycorrhizal colonization in pea when ethylene levels are elevated above the baseline level.
Fig. 4.
Mycorrhizal colonization of roots treated with ethephon. Wild-type and ein2 mutants were treated with 0 (control) or 10 μg of ethephon applied to the leaf fortnightly. Plants were grown for 6 weeks in pot culture with Rhizophagus irregularis under low phosphate (0.05mM NaH2PO4) and scored for the percentage of roots possessing arbuscules or any internal fungal structures (hyphae, arbuscules, and/or vesicles). Values are mean±SE (n=6). Within a parameter, values with different letters above the bars indicate differences at P<0.05.
Gibberellin and brassinosteroid deficiency affect nodule number via ethylene
Bioactive gibberellins and brassinosteroids can act as positive regulators of nodulation in pea (Ferguson et al., 2005). The gibberellin-deficient extreme dwarf na mutant forms a small number of incompletely developed nodules, while the brassinosteroid-deficient lk mutant has a reduced number of nodules, which may be larger than those on wild-type plants and appear fully functional (Ferguson et al., 2005). However, both na and lk mutant plants have been shown to produce more ethylene than comparable wild-type lines, and some aspects of their phenotypes appear to at least be partly due to these elevated ethylene levels (Ferguson et al., 2011; Ross and Reid, 1986). In order to examine the interactions between these hormones, the double mutants ein2 lk and ein2 na were produced and their nodulation phenotypes examined.
The ein2 lk double mutant plants had a significantly different shoot phenotype from those of the single mutant lk plants. The internodes of the ein2 lk plants were more than 50% longer than those of the single-mutant lk plants (P<0.001, Fig. 5A). However, the plants were still much shorter than wild-type or single-mutant ein2 plants (34% and 37% of the length respectively; data not shown). This clearly shows that while the elevated level of ethylene produced by lk plants (Ross and Reid, 1986) has a minor effect on shoot elongation, the major effect on elongation caused by brassinosteroid deficiency is not due to elevated ethylene levels. This is consistent with the stimulatory effect of ethylene synthesis inhibitors on the shoot growth of a brassinosteroid-deficient mutant (Ross and Reid, 1986).
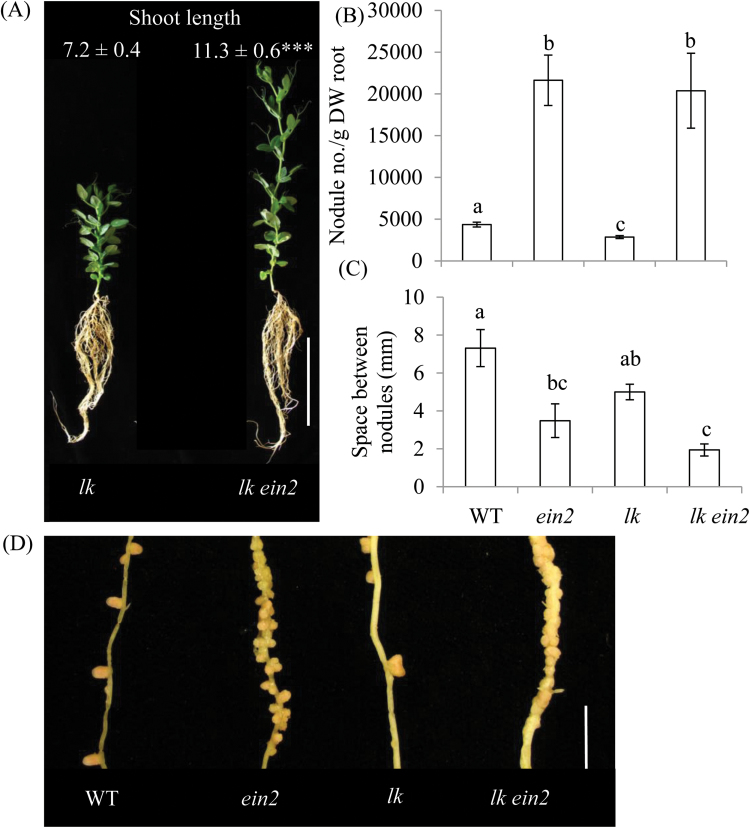
Fig. 5.
Nodulation in wild-type, ein2, lk and lk ein2 double mutant plants. Plants were grown under nitrate-free conditions for 35 days. (A) Photograph of whole plants, including shoot height (length between nodes 1 and 9 in cm; scale bar=5cm). (B) Number of nodules per g dry weight of roots. (C) Average space between nodules. (D) Photograph of nodules on a secondary root of wild-type, ein2, lk, and lk ein2 plants (tertiary roots have been removed; scale bar=1cm). For A *** P<0.001; for B and C, values with different letters above the bars indicate differences at P<0.05. Values are mean±SE (n=6). (This figure is available in colour at JXB online.)
As previously reported, nodule number was significantly reduced in the single lk mutant plants and significantly elevated in single ein2 mutant plants compared with wild-type plants (Ferguson et al., 2005; Figs. 2 and 5B, D). For ein2, this was also reflected in a decrease in nodule spacing (Fig. 5C). The nodules on lk plants were similar in weight to those on wild-type plants and pink in colour, suggesting that they were functional (Fig. 5D; data not shown). The ein2 mutation appears to be fully epistatic to lk in terms of nodule number, despite the fact that lk ein2 double-mutant shoots are very much reduced in size compared with those of wild-type and ein2 plants. This suggests a direct effect of the ethylene insensitivity on nodule number. However, nodules on lk ein2 double mutants were pink and appeared to be functional (Fig. 5D). This suggests that brassinosteroids may be stimulatory for nodule initiation via an effect on ethylene levels but do not affect subsequent nodule development.
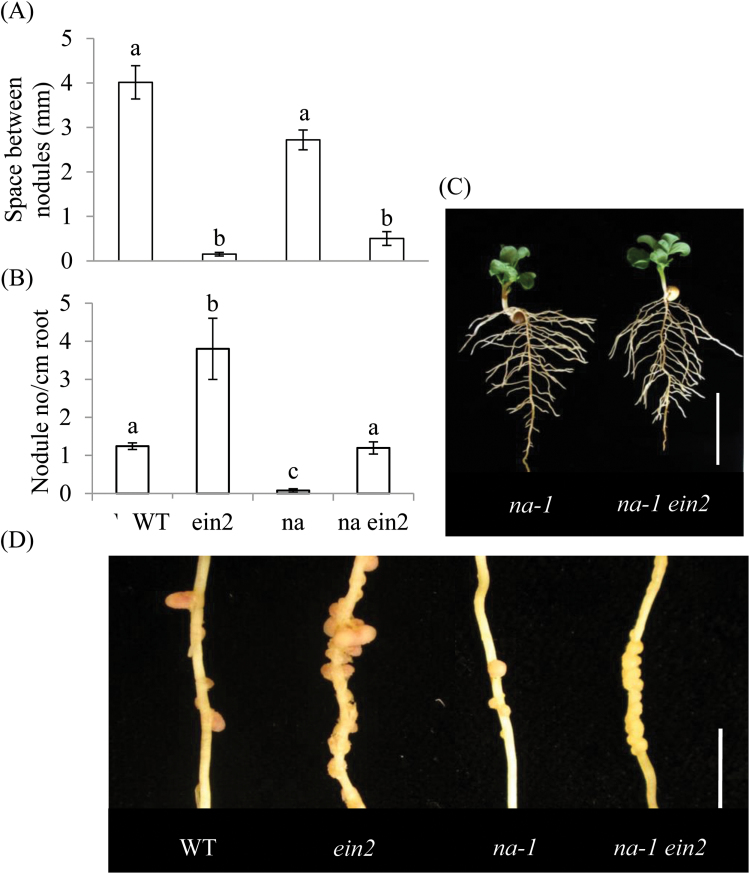
The ein2 na double-mutant plants had an extremely dwarfed shoot, similar to those of the na plants, showing that ethylene is not involved with the manifestation of this gibberellin-deficient phenotype (Fig. 6). The roots of double-mutant plants were also still thickened and showed reduced branching compared with wild-type plants (data not shown), phenotypically similar to the root phenotype of the single-mutant na plants (Fig. 6C; Ferguson et al., 2011). However, the ein2 na roots had a distinctly different nodulation phenotype from those of na single-mutant plants (Fig. 6A–D). The spacing between nodules was dramatically reduced in the double mutant compared with wild-type plants, almost to the same extent as in the ein2 plants (Fig. 6A). Double-mutant plants also displayed an increased number of nodules per cm of root compared with na single-mutant plants (Fig 6B). The effect of na on nodule number is well understood (Ferguson et al., 2005, 2011) and was obvious in these plants (Fig. 6). Overall, these results suggest that the elevated level of ethylene in na plants could be at least partly responsible for the substantial reduction in nodule number. However, the nodules produced on the ein2 na plants were still small and white in colour (Fig. 6D), suggesting that they were non-functional, unlike the nodules on ein2 lk plants. This suggests that while gibberellin levels influence nodule number at least partially through ethylene levels and subsequent EIN2-dependent ethylene signalling (similar to brassinosteroid-deficient lk plants), gibberellins also appear to be involved with subsequent nodule development independent of ethylene signalling.
Fig. 6.
Nodulation in wild-type, ein2, na, and na ein2 double-mutant plants. Plants were grown under nitrate-free conditions for 32 days. (A) Average space between nodules. (B) Number of nodules per cm of root. (C) Photograph of whole plants of na and na ein2 (scale bar=5cm). (D) Photograph of nodules on a secondary root (tertiary roots have been removed; scale bar=1cm). Values with different letters above the bars indicate differences at P<0.05. Values are mean±SE (n=6). (This figure is available in colour at JXB online.)
Lack of interaction of brassinosteroids and gibberellins with ethylene during mycorrhizal development
Recent reports suggest that brassinosteroids promote mycorrhizal development in tomato and rice (Bitterlich et al., 2014a, b), while gibberellin signalling suppresses arbuscule development, including in pea (Floss et al., 2013; Foo et al., 2013a; Yu et al., 2014). In pea, the brassinosteroid mutant lkb causes a partial block in the conversion of 24-methylenecholesterol to campesterol during brassinosteroid biosynthesis (Nomura et al., 1999) due to a mutation in the pea homologue of DIM in Arabidopsis (Schultz et al., 2001). This mutation does not influence arbuscular mycorrhizal development, but it is not clear whether this is due to the leaky nature of the mutation or that brassinosteroids do not influence mycorrhizal development in pea (Foo et al., 2013a).
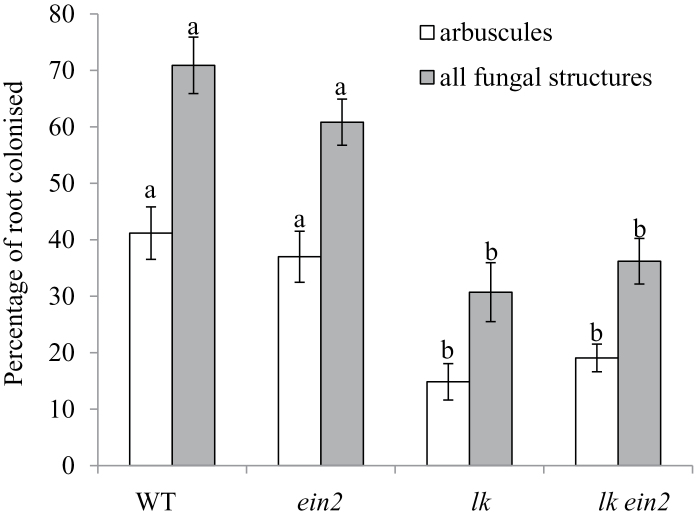
To differentiate between these two alternatives, we used the more severe lk mutant of pea (Reid, 1986; Ross and Reid, 1989), which carries a putative null mutation in the ortholog of the steroid 5α-reductase DET2 and shows a dramatic reduction in brassinosteroid levels (Nomura et al., 2004). The results clearly show that the lk mutation reduces total root colonization by the fungus, including a significant decrease in the percentage of roots containing arbuscules compared with wild-type roots (Fig. 7; P<0.01). This is consistent with the findings in tomato and rice (Bitterlich et al., 2014a, b), although the previous report in the leaky lkb mutant of pea (Foo et al., 2013a) suggests that the reduction in brassinosteroid level must be large before an effect is observed. This supports the hypothesis that brassinosteroids do influence mycorrhizal development in pea.
Fig. 7.
Mycorrhizal colonization of wild-type, ein2, lk, and lk ein2 plants. Plants were grown for 6 weeks in pot culture with Rhizophagus irregularis under low phosphate (0.05mM NaH2PO4) and the roots were scored for the percentage of roots possessing arbuscules or any internal fungal structures (hyphae, arbuscules, and/or vesicles). Within a parameter values, with different letters above the bars indicate differences at P<0.01. Values are mean±SE (n=7–11).
As outlined in the previous section, both na and lk mutant plants have been shown to produce more ethylene than comparable wild-type lines, and this may account for at least part of their reduced nodulation phenotypes (Figs 5 and 6). We also examined whether elevated ethylene may influence the mycorrhizal phenotype of these gibberellin- and brassinosteroid-deficient lines by examining mycorrhizal development in the double mutants containing the ein2 ethylene-insensitive mutation.
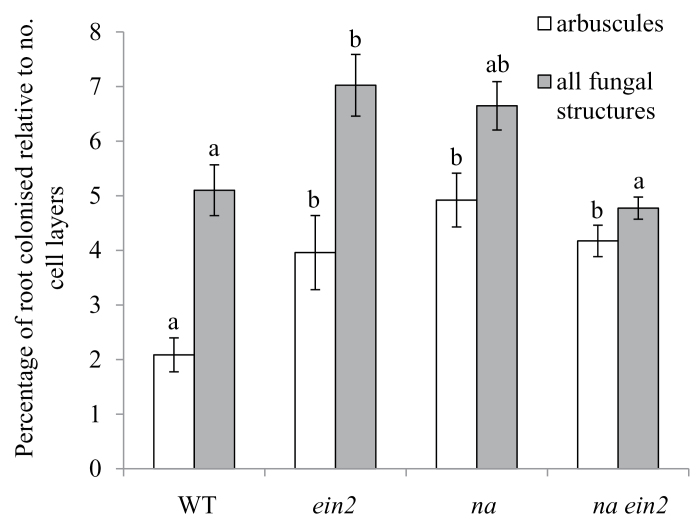
Interestingly, in one of these experiments we observed a small but significant increase in the percentage of the root colonized by fungi and arbuscules in ein2 single-mutant plants compared with wild-type plants (Fig. 8; P<0.05). This is in contrast to previous experiments, where no effect of ein2 was observed (Figs 3, 4, and 7). It is possible that this was due to somewhat higher ethylene production in this particular experiment, which might have caused a small suppression of mycorrhizal development in ethylene-sensitive wild-type plants, as seen for ethephon treatment in Fig. 4. However, elevated ethylene could not explain the decrease in mycorrhizal colonization observed in lk mutant plants, as colonization of the lk ein2 double mutant did not differ from that of single-mutant lk plants (Fig. 7). Indeed, like lk single mutants, lk ein2 double mutants formed significantly fewer arbuscules than wild-type plants. Likewise, the increase in arbuscule development in single-mutant na plants (compared with wild-type plants, P<0.05; Fig. 8) is similar to that observed in na ein2 double mutants (Fig. 8). These data suggest that brassinosteroids and gibberellins each have a primary effect on mycorrhizal colonization rather than acting indirectly through altered ethylene production.
Fig. 8.
Mycorrhizal colonization of wild-type, ein2, na, and na ein2 plants. Plants were grown for 6 weeks in pot culture with Rhizophagus irregularis under low phosphate (0.05mM NaH2PO4). The roots were scored for the percentage of roots possessing arbuscules or any internal fungal structures (hyphae, arbuscules, and/or vesicles), and this is expressed relative to the number of cortical cell layers to account for the increase in cortical cell layers in roots of na and na ein2 mutants compared with wild-type plants and the ein2 mutant. Within a parameter, values with different letters above the bars indicate differences at P<0.05. Values are mean±SE (n=7–11).
Discussion
While there has been debate in the literature about the role of ethylene in both rhizobial and arbuscular mycorrhizal symbioses, the results with the Psein2 mutant are clear and generally in agreement with the results seen in the Medicago Mtein2 mutant (Penmetsa and Cook, 1997; Penmetsa et al., 2008). Nodulation is dramatically increased in the Psein2 mutant, and this closely aligns with the conclusion from mutant studies in M. truncatula (Larrainzar et al., 2015; Penmetsa and Cook, 1997) and L. japonicus (Miyata et al., 2013) that ethylene is a negative regulator of nodulation. Indeed, localized production of ethylene has been shown to regulate nodule positioning (Chan et al., 2013). The contrary results in relation to the role of ethylene in nodulation from other legumes (e.g. soybeans; Hunter, 1993) or other reports in L. japonicus possibly reflect redundancy in the EIN2 pathway (Chan et al., 2013; Desbrosses and Stougaard, 2011). A promotive effect of Psein2 on arbuscular mycorrhizal colonization is seen under stressful conditions or when ethylene levels have been raised by the addition of ethephon (Fig. 4). Overall, it appears that ethylene can act as a negative regulator of arbuscular mycorrhizal colonization, as was shown by Geil et al. (2001). However, in contrast to its effects on nodulation, in pea ethylene levels may need to reach a threshold above the basal level to influence arbuscular mycorrhizal colonization, as might occur under stressful environmental conditions; this may explain why, in contrast to Mtein2 mutants (Penmetsa et al., 2008), Psein2 mutants do not always display elevated mycorrhizal development.
Ethylene insensitivity, conferred by the Psein2 mutation, has only a minor effect on root growth in pea under glasshouse conditions (Table 1), indicating that altered root development is not the cause of the altered symbiosis phenotypes of this mutant. Basal ethylene levels in wild-type peas do not appear to substantially limit root growth, and the response seen in Psein2 plants is consistent with the effects of ethylene insensitivity in other species such as Arabidopsis (Stepanova et al., 2007) and M. truncatula (Penmetsa and Cook, 1997). We also found no evidence that ethylene signalling through EIN2 is necessary for IAA-induced inhibition of root growth in pea plants grown in vitro (Fig. 1), consistent with previous reports in Arabidopsis (Stepanova et al. 2007).
The gibberellin-deficient na plants and the brassinosteroid-deficient lk plants both have substantially dwarfed shoot phenotypes compared with wild-type and ein2 plants. However, dwarfism was not correlated with the symbiosis phenotypes observed in the roots, because in an ein2 background dwarf na (Fig. 6) and lk (Fig. 5) plants had substantially increased nodule numbers (and reduced nodule spacing) compared with single-mutant na or lk parents. Furthermore, lk was epistatic to ein2 in terms of the extent of mycorrhizal colonization of the roots (Fig. 7). This suggests that the effect of gibberellins and brassinosteroids on symbioses is not an indirect effect of their influence on general plant growth, but may represent a more direct developmental regulation.
While there is strong evidence that gibberellin and brassinosteroid levels influence ethylene levels in na and lk plants (Ferguson et al., 2011; Ross and Reid, 1986), there is little evidence that the loss of ethylene sensitivity in ein2 plants meaningfully alters the level of GA1, IAA, or ABA by a physiologically relevant amount (Table 2; see Ross et al., 2011). This suggests that the observed effects of ein2 on nodulation do not result from changes in the level of these hormones, at least at the gross whole-root level. However, the increased nodulation of double-mutant ein2 lk and ein2 na plants suggests that gibberellins and brassinosteroids influence ethylene levels, which in turn influence nodule numbers.
In contrast, there is no clear evidence that gibberellins or brassinosteroids interact with ethylene to regulate the symbiosis with arbuscular mycorrhizal fungi. Studies in several species, including pea, indicate that gibberellin signalling through DELLA proteins inhibits arbuscule development (Floss et al., 2013; Foo et al., 2013a; Yu et al., 2014). Consistent with this, severely gibberellin-deficient na mutants of pea have increased arbuscule formation (Foo et al., 2013a; Fig. 8). We found that double-mutant na ein2 plants also displayed elevated arbuscule formation, indicating that elevated ethylene cannot explain the na mycorrhizal phenotype and that gibberellin is likely to act directly on mycorrhizal development. In contrast, the low arbuscular mycorrhizal colonization rates observed in lk mutants (Fig. 7) are consistent with their elevated ethylene production (Ross and Reid, 1986). However, like lk single mutants, lk ein2 double mutants still displayed reduced arbuscular mycorrhizal colonization compared with wild-type plants (Fig. 7), indicating that brassinosteroid deficiency has a more direct effect on arbuscule formation, rather than via an indirect effect on ethylene signalling.
In conclusion, genetic studies using an ein2 mutant of pea show that ethylene influences nodule number and arbuscular mycorrhizal colonization. The characterization of double mutants combining gibberellin or brassinosteroid deficiency with ein2 suggests that a major part of the effect of these hormones on nodule number may be due to changes in ethylene levels, but this is not the case for their effects on colonization by mycorrhizal fungi. The fact that we have been able to delineate different interactions and roles for gibberellin, brassinosteroids, and ethylene in the regulation of nodule number and arbuscular mycorrhizal colonization is intriguing, and these interactions and the proposed sequence of their actions and the genes involved are outlined in Fig. 9. Although ethylene generally acts to inhibit these carbon-intensive symbioses, how elevated ethylene, such as that generated when plants are under stress, may impinge upon these processes may also be influenced by the status of other hormones. Given that both gibberellin and brassinosteroids have been implicated in protection against stress, including drought, oxidative stress, and salinity (e.g., Bajguz and Hayat, 2009; Colebrook et al., 2014; Jager et al., 2008; Kagale et al., 2007; Kohli et al., 2013), such an insight may be important for future studies examining how plants balance symbioses with plant growth under stressful conditions.
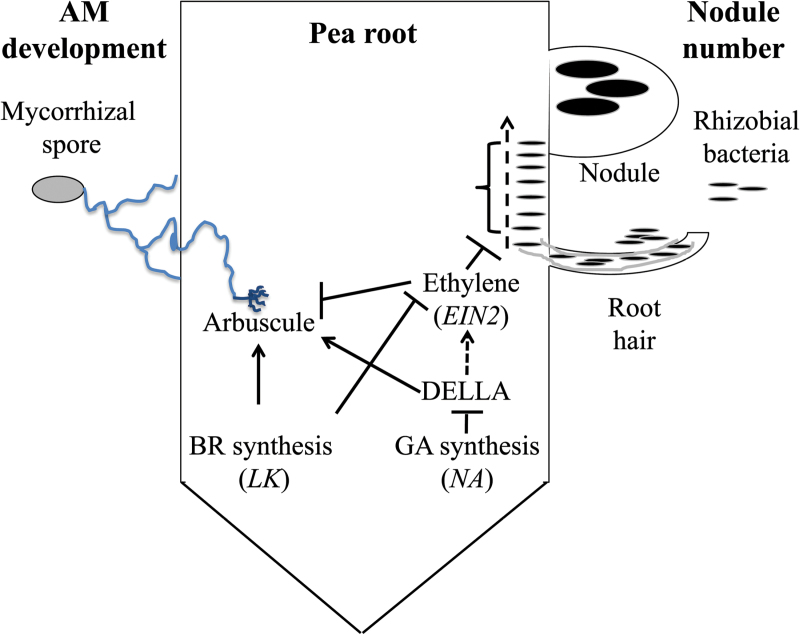
Fig. 9.
Model for the roles of gibberellin (GA), brassinosteroid (BR), and ethylene in nodule number and arbuscular mycorrhizal (AM) development in pea. Flat-ended lines indicate a negative influence, while arrows indicate a positive influence. (This figure is available in colour at JXB online.)
Supplementary data
Figure S1. Endogenous ethylene level emitted by 12-day-old wild-type and ein2 mutant pea (Pisum sativum) plants.
Acknowledgements
We thank Jennifer Smith, Shelley Urquhart, and Cassandra Hugill for assistance with scoring the plants, Tracey Winterbottom, and Michelle Lang for care of the plants and set-up and maintenance of growth conditions. We also thank Noel Davies for assistance with hormone analyses. This work was supported by the Australian Research Council through Discovery grants to EF, JLW, and JBR.
References
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. 1999. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284, 2148–2152. [DOI] [PubMed] [Google Scholar]
- Bajguz A, Hayat S. 2009. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiology and Biochemistry 47, 1–8. [DOI] [PubMed] [Google Scholar]
- Belimov AA, Davies WJ, Dodd IC, Hontzeas N, Theobald JC, Safronova VI. 2009. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytology 181, 413–442. [DOI] [PubMed] [Google Scholar]
- Bitterlich M, Boldt-Burisch K, Franken P, Krügel U, Kühn C. 2014. a. The sucrose transporter SISUT2 from tomato interacts with brassinosteroid functioning and affects arbuscular mycorrhiza formation. The Plant Journal 78, 877–889. [DOI] [PubMed] [Google Scholar]
- Bitterlich M, Krugel U, Boldt-Burisch, Franken P, Kuhn C. 2014. b. Interaction of brassinosteroid functions and sucrose transporter SISUT2 regulate the formation of arbuscular mycorrhiza. Plant Signalling & Behavior 9, e970426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capoen W, Goormachtig S, Holsters M. 2010. Water-tolerant legume nodulation. Journal of Experimental Botany 61, 1251–1255. [DOI] [PubMed] [Google Scholar]
- Chan PK, Biswas B, Gresshoff PM. 2013. Classical ethylene insensitive mutants of the Arabidopsis EIN2 orthologue lack the expected ‘hypernodulation’ response in Lotus japonicus. Journal of Integrative Biology 55, 395–408. [DOI] [PubMed] [Google Scholar]
- Clark DG, Gubrium EK, Barrett JE, Nell TA, Klee HJ. 1999. Root formation in ethylene-insensitive plants. Plant Physiology 121, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebrook EH, Thomas SG, Phillips AL, Hedden P. 2014. The role of gibberellin signalling in plant responses to abiotic stress. Journal of Experimental Biology 217, 67–75. [DOI] [PubMed] [Google Scholar]
- Davidson SE, Elliott RC, Helliwell CA, Poole AT, Reid JB. 2003. The pea gene NA encodes ent-kaurenoic acid oxidase. Plant Physiology 131, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ. 2010. The plant hormones: their nature, occurrence and functions. In: Davies PJ. ed. Plant hormones: biosynthesis, signal transduction, action! Dordrecht: Springer, 1–15. [Google Scholar]
- Desbrosses GJ, Stougaard J. 2011. Root nodulation: a paradigm for how plant-microbe symbiosis influences host developmental pathways. Cell Host & Microbe 10, 348–358. [DOI] [PubMed] [Google Scholar]
- Eliasson L, Bertell G, Bolander E. 1989. Inhibitory action of auxin on root elongation not mediated by ethylene. Plant Physiology 91, 310–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BJ, Foo E, Ross JJ, Reid JB. 2011. Relationship between gibberellin, ethylene and nodulation in Pisum sativum. New Phytologist 189, 829–842. [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Indrasumunar A, Hayashi S, Lin M, Lin Y, Reid DE, Gresshoff PM. 2010. Molecular analysis of legume nodule development and autoregulation. Journal of Integrative Plant Biology 52, 61–76. [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Ross JJ, Reid JB. 2005. Nodulation phenotypes of gibberellin and brassinosteroid mutants of pea. Plant Physiology 138, 2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floss DS, Levy JG, Lévesque-Tremblay V, Pumplin N, Harrison MJ. 2013. DELLA proteins regulate arbuscule formation in arbuscular mycorrhizal symbiosis. Proceedings of the National Academy of Sciences of the United States of America 110, E5025–E5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Davies NW. 2011. Strigolactones promote nodulation in pea. Planta 234, 1073–1081. [DOI] [PubMed] [Google Scholar]
- Foo E, Ferguson BJ, Reid JB. 2014. The potential roles of strigolactones and brassinosteroids in the autoregulation of nodulation pathway. Annals of Botany 113, 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Ross JJ, Davies NW, Reid JB, Weller JL. 2006. A role for ethylene in the phytochrome‐mediated control of vegetative development. The Plant Journal . 46, 911–921. [DOI] [PubMed] [Google Scholar]
- Foo E, Ross JJ, Jones WT, Reid JB. 2013. a. Plant hormones in arbuscular mycorrhizal symbioses: an emerging role for gibberellins. Annals of Botany 111, 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo E, Yoneyama K, Hugill C, Quittenden L, Reid JB. 2013. b. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Annals of Botany 113, 1037–1045 [DOI] [PubMed] [Google Scholar]
- Geil RD, Peterson LR, Guinel F. 2001. Morphological alterations of pea (Pisum sativum cv. Sparkle) arbuscular mycorrhizas as a result of exogenous ethylene treatment. Mycorrhiza 11, 137–143. [DOI] [PubMed] [Google Scholar]
- Goeschl JD, Pratt HK, Bonner BA. 1967. An effect of light on the production of ethylene and the growth of the plumular portion of etiolated pea seedlings. Plant Physiology 42, 1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Chen A, Dai X, Liu W, Xu G. 2011. How does phosphate status influence the development of the arbuscular mycorrhizal symbiosis? Plant Signaling & Behavior 6, 1300–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. 1990. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. The Plant Cell 2, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter WJ. 1993. Ethylene production by root nodules and effect of ethylene on nodulation in Glycine max . Applied Environmental Microbiology 59, 1947–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager CE, Symons GM, Ross JJ, Reid JB. 2008. Do brassinosteroids mediate the water stress response? Physiologia Plantarum 133, 417–425. [DOI] [PubMed] [Google Scholar]
- Jager CE, Symons GM, Ross JJ, Smith JJ, Reid JB. 2005. The brassinosteroid growth response in pea is not mediated by changes in gibberellin content. Planta 221, 141–148. [DOI] [PubMed] [Google Scholar]
- Jones JMC, Clairmont L, MacDonald ES, Weiner CA, Emery RJN, Guinel FC. 2015. E151 (sym15), a pleiotropic mutant of pea (Pisum sativum L.), displays low nodule number, enhanced mycorrhizae, delayed lateral root emergence, and high root cytokinin levels. Journal of Experimental Botany , 66, 4047–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagale S, Divi UK, Krochko JE, Keller WA, Krishna P. 2007. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 225, 353–364. [DOI] [PubMed] [Google Scholar]
- Kohli A, Sreenivasulu N, Lakshmanan P, Kumar PP. 2013. The phytohormone crosstalk paradigm takes center stage in understanding how plants respond to abiotic stresses. Plant Cell Reports 32, 945–957. [DOI] [PubMed] [Google Scholar]
- Larrainzar E, Alibert B, Arrese-Igor C, Gil-Quintana E, Gonzalez EM, Limami AM, Molenaar JA, Wienkoop S. 2014. Drought stress provokes the down- regulation of methionine and ethylene biosynthesis pathways in Medicago truncatula roots and nodules. Plant, Cell and Environment 37, 2051–2063. [DOI] [PubMed] [Google Scholar]
- Larrainzar E, Riely BK, Kim SC, et al. 2015. Deep sequencing of the Medicago truncatula root transcriptome reveals a massive and early interaction between Nod factor and ethylene signals. Plant Physiology 169, 233–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, LaRue TA. 1992. Exogenous ethylene inhibits nodulation of Pisum sativum L.cv Sparkle. Plant Physiology , 100, 1759–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievens S, Goormachtig S, Den Herder J, Capoen W, Mathis R, Hedden P, Holster M. 2005. Gibberellins are involved in nodulation of Sesbania rostrata . Plant Physiology 139, 1366–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Zhong S, Grierson D. 2009. Recent advances in ethylene research. Journal of Experimental Botany 60, 3311–3336. [DOI] [PubMed] [Google Scholar]
- McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. 1990. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytologist 115, 495–501. [DOI] [PubMed] [Google Scholar]
- Merchante C, Alonso JM, Stepanova AN. 2013. Ethylene signaling: simple ligand, complex regulation. Current Opinion in Plant Biology 16, 554–560. [DOI] [PubMed] [Google Scholar]
- Miyata K, Kawaguchi M, Nakagawa T. 2013. Two distinct EIN2 genes cooperatively regulate ethylene signaling in Lotus japonicus . Plant and Cell Physiology 54, 1469–1477. [DOI] [PubMed] [Google Scholar]
- Nomura T, Jager CE, Kitasaka Y, et al. 2004. Brassinosteroid deficiency due to truncated steroid 5α-reductase causes dwarfism in the lk mutant of pea. Plant Physiology 135, 2220–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Kitasaka Y, Takasuto S, Reid JB, Fukami M, Yokota T. 1999. Brassinosteroid/sterol synthesis and plant growth as affected by lka and lkb mutations of pea. Plant Physiology 119, 1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penmetsa RV, Cook DR. 1997. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275, 527–530 [DOI] [PubMed] [Google Scholar]
- Penmetsa RV, Uribe P, Anderson J, et al. 2008. The Medicago truncatula ortholog of Arabidopsis EIN2, sickle, is a negative regulator of symbiotic and pathogenic microbial associations. The Plant Journal 55, 580–595. [DOI] [PubMed] [Google Scholar]
- Prayitno J, Rolfe BG, Mathesius U. 2006. The ethylene insensitive sickle mutant of Medicago truncatula shows altered auxin transport regulation during nodulation. Plant Physiology 142, 168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H, Shen Z, Huang SS, Schmitz RJ, Urich MA, Briggs SP, Ecker JR. 2012. Processing and subcellular trafficking of ER-tethered EIN2 control response to ethylene gas. Science 338, 390–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid JB. 1986. Internode length in Pisum. Three further loci, lh, ls and lk . Annals of Botany 57, 577–592. [Google Scholar]
- Riedel T, Groten K, Baldwin IT. 2008. Symbiosis between Nicotiana attenuata and Glomus intraradices: ethylene plays a role, jasmonic acid does not. Plant, Cell and Environment 31, 1203–1213. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Reid JB. 1986. Internode length in Pisum. The involvement of ethylene with the gibberellin-insensitive erectoides phenotype. Physiologia Plantarum 67, 673–679. [Google Scholar]
- Ross JJ, Reid JB. 1989. Internode length in Pisum. Two further genes, lka and lkb . Physiologia Plantarum 75, 81–88. [Google Scholar]
- Ross JJ, Weston DE, Davidson SE, Reid JB. 2011. Plant hormone interactions: how complex are they? Physiologia Plantarum 141, 299–309. [DOI] [PubMed] [Google Scholar]
- Schmidt JS, Harper JE, Hoffman TK, Bent AF. 1999. Regulation of soybean nodulation independent of ethylene signalling. Plant Physiology 119, 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz L, Kerckhoffs LHJ, Klahre U, Yokota T, Reid JB. 2001. Molecular characterisation of the brassinosteroid-deficient lkb mutant in the pea. Plant Molecular Biology 47, 491–498. [DOI] [PubMed] [Google Scholar]
- Singleton PW, Bohlool BB. 1984. Effect of salinity on nodule formation by soybean. Plant Physiology 74, 72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Read DJ. 2008. Mycorrhizal symbiosis , 3rd edn. San Diego: Academic Press. [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. 2007. Multilevel interactions between ethylene and auxin in Arabidopsis roots. The Plant Cell 19, 2169–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres de Los Santos R, Vierheilig H, Ocampo JA, García-Garrido JM. 2011. Altered pattern of arbuscular mycorrhizal formation in tomato ethylene mutants. Plant Signaling & Behavior 6, 755–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga RSL, Faccio A, Genre A, Pieterse CMJ, Bonfante P, van der Heijden MGA. 2013. Arbuscular mycorrhizal fungi reduce growth and infect roots of the non-host plant Arabidopsis thaliana . Plant, Cell & Environment 36, 1926–1937. [DOI] [PubMed] [Google Scholar]
- Vela GM, Molinero-Rosales N, Ocampo JA, Garcia Garrido JM. 2007. Endocellulase activity is associated with arbuscular mycorrhizal spread in pea symbiotic mutants but not with its ethylene content in root. Soil Biology and Chemistry 39, 786–792. [Google Scholar]
- Vierheilig H, Cougland AP, Wyss U, Piche Y. 1998. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Applied and Environmental Microbiology 64, 5004–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrebalov J, Medrano D, Drake R, Giovanonni J, Padmanabhan V, Ruezinsky D, Schuch W, White R. 2002. A MADS-box gene necessary for fruit ripening at the tomato Ripening-inhibitor (Rin) locus. Science 296, 343–346. [DOI] [PubMed] [Google Scholar]
- Weller JL, Foo E, Hecht V, Ridge S, Vander Schoor JK, Reid JB. 2015. Ethylene signalling influences light-regulated development in pea. Plant Physiology 169, 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Zhang C, Ji Y, Zhao Q, He W, An F, Jiang L, Guo H. 2012. Activation of ethylene signaling is mediated by nuclear translocation of the cleaved EIN2 carboxyl terminus. Cell Research 22, 1613–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston DE, Reid JB, Ross JJ. 2009. Auxin regulation of gibberellin biosynthesis in the roots of pea (Pisum sativum L.). Functional Plant Biology 36, 362–369. [DOI] [PubMed] [Google Scholar]
- Yang SF. 1969. Ethylene evolution from 2-chloroethylphosphonic acid. Plant Physiology 44, 1203–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N, Luo D, Zhang X, et al. 2014. A DELLA protein complex controls the arbuscular mycorrhizal symbiosis in plants. Cell Research 24, 130–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsögön A, Lambais MR, Benedito VA, Figueira AVO, Peres LEP. 2008. Reduced arbuscular mycorrhizal colonization in tomato ethylene mutants. Scientia Agricola 65, 259–267. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.