Highlight
CabZIP63, indirectly activated by CaWRKY40, positively modulates transcription of CabZIP63 and CaWRKY40, enhances the binding of CaWRKY40 to its target promoters, and, therefore, increases resistance to Ralstonia solanacearum and thermotolerance.
Key words: CabZIP63, CaWRKY40, high temperature–high humidity, pepper, Ralstonia solanacearum, transcription factors.
Abstract
CaWRKY40 is known to act as a positive regulator in the response of pepper (Capsicum annuum) to Ralstonia solanacearum inoculation (RSI) or high temperature–high humidity (HTHH), but the underlying mechanism remains elusive. Herein, we report that CabZIP63, a pepper bZIP family member, participates in this process by regulating the expression of CaWRKY40. CabZIP63 was found to localize in the nuclei, be up-regulated by RSI or HTHH, bind to promoters of both CabZIP63 (pCabZIP63) and CaWRKY40 (pCaWRKY40), and activate pCabZIP63- and pCaWRKY40-driven β-glucuronidase expression in a C- or G-box-dependent manner. Silencing of CabZIP63 by virus-induced gene silencing (VIGS) in pepper plants significantly attenuated their resistance to RSI and tolerance to HTHH, accompanied by down-regulation of immunity- or thermotolerance-associated CaPR1, CaNPR1, CaDEF1, and CaHSP24. Hypersensitive response-mediated cell death and expression of the tested immunity- and thermotolerance-associated marker genes were induced by transient overexpression (TOE) of CabZIP63, but decreased by that of CabZIP63-SRDX. Additionally, binding of CabZIP63 to pCaWRKY40 was up-regulated by RSI or HTHH, and the transcript level of CaWRKY40 and binding of CaWRKY40 to the promoters of CaPR1, CaNPR1, CaDEF1 and CaHSP24 were up-regulated by TOE of CabZIP63. On the other hand, CabZIP63 was also up-regulated transcriptionally by TOE of CaWRKY40. The data suggest collectively that CabZIP63 directly or indirectly regulates the expression of CaWRKY40 at both the transcriptional and post-transcriptional level, forming a positive feedback loop with CaWRKY40 during pepper’s response to RSI or HTHH. Altogether, our data will help to elucidate the underlying mechanism of crosstalk between pepper’s response to RSI and HTHH.
Introduction
Accumulating evidence indicates that extensive transcriptional reprogramming of multiple genes is generally activated in plants by exposure to various biotic or abiotic stresses individually or simultaneously, which leads to an appropriate downstream defense response (Atkinson and Urwin, 2012; Prasch and Sonnewald, 2013; Rasmussen et al., 2013; Zhu et al., 2013; Cao et al., 2014; Sewelam et al., 2014). Transcription factors (TFs) are extensively involved and act as regulators in the transcriptional reprogramming through recognizing and binding their cognate cis-elements in promoters of clusters of target genes, and the TFs themselves are also orchestrated by multiple upstream signaling components, constituting a complicated TF network modulating the expression of a huge number of responding genes (Atkinson and Urwin, 2012; Lindemose et al., 2013; Schweizer et al., 2013; Rabara et al., 2014). A better understanding of the mechanism underlying the network will benefit genetic engineering to improve crop tolerance/resistance to various stresses.
WRKYs are one of the largest plant-specific TF families, characterized by their conserved WRKY domain, which recognizes and bind to the cognate W-box (TTGACC) enriched in the promoters of their target genes and transcriptionally modify their expression (Eulgem et al., 2000; Ulker and Somssich, 2004; Wu et al., 2005). Besides their role in plant growth and development, the majority of WRKY family members have also been implicated in plant responses to different biotic stresses, including pathogens (Cheng et al., 2015), herbivores (Skibbe et al., 2008), and viruses (Chen et al., 2013), abiotic stresses including drought (Jiang et al., 2012; Niu et al., 2012; Luo et al., 2013), heat (Li et al., 2011), salt (Niu et al., 2012), and freezing (Niu et al., 2012), and phytohormone signaling including abscisic acid (ABA) (Rushton et al., 2012), salicylic acid (SA) (Knoth et al., 2007; Shimono et al., 2007), jasmonic acid (JA) (Gao et al., 2011), and ethylene (ET) (Chen et al., 2013). These WRKY genes are generally transcriptionally up- or down-regulated by stresses, acting as activators or repressors in the response of plants to these stresses. New findings demonstrate that the functions of WRKY TFs and the underlying mechanisms are complicated; the WRKY genes involved in plant response to different stresses generally exhibit transcriptionally inducible expression, and multiple cis-elements including the W-box are consistently present in their promoters, suggesting extensive autoregulation and cross-regulation by WRKY itself and various upstream TFs and other signaling components (Eulgem and Somssich, 2007; Rushton et al., 2010; Llorca et al., 2014). However, knowledge of the possible transcriptional regulators of WRKY TFs and how they operate is very limited so far. In addition, a single WRKY TF may be modified transcriptionally by multiple stresses, and these TFs are involved in several seemingly disparate processes (Zhang et al., 2008; Rushton et al., 2010; Chen et al., 2012; Yu et al., 2012; Zhu et al., 2013). These findings indicate that WRKY proteins might act as convergent nodes in the crosstalk between or among different biological processes, which provides great potential for plants, allowings fine-tuning of specific biological processes and co-ordination of multiple biological processes (Schweiger et al., 2014). However, the underlying mechanism remains largely unknown.
Another large TF family are the basic leucine zippers (bZIPs); the members of this family are characterized by a 40–80 amino acid conserved bZIP domain, which possesses a basic region that binds DNA and an adjacent Leu (leucine) zipper region that mediates protein dimerization (Hurst, 1995). Through preferential binding of the basic region to DNA sequences with an ACGT core cis-element, in particular the G-box (CACGTG), C-box (GACGTC), and A-box (TACGTA) (Izawa et al., 1993; Foster et al., 1994), bZIPs transcriptionally modify the expression of a vast array of target genes and play important roles in diverse physiological processes in plant growth, development, and responses to abiotic stresses, such as salt, drought (Lee et al., 2006), and nitrogen (Lopez-Berges et al., 2010), biotic stresses (Pontier et al., 2001; Kim and Delaney, 2002; Zander et al., 2010; Zhong et al., 2015), as well as signaling mediated by phytohormones such as SA, JA, ET, and ABA; some members act as negative regulators and some members act as positive regulators (Pontier et al., 2001; Zander et al., 2010, 2012). As well as being regulated at the transcriptional level, bZIPs are also modified at the post-translational level via the formation of heterodimers or homodimers (Llorca et al., 2014). In Arabidopsis, some bZIPs such as the TGA family of TFs participate in SA signaling regulation by interacting with NPR1 (Despres et al., 2000; Kim and Delaney, 2002; Shearer et al., 2012), but some clade I TGA TFs were found to act in an NPR1-independent manner (Shearer et al., 2012). As mentioned above, although bZIP and WRKY are involved in similar biological processes and some of these processes overlap, information on the functional relationship between bZIPs and WRKYs is very limited.
Pepper (Capsicum annuum) is a vegetable of great economic importance worldwide and also a typical member of the Solanaceae, with various soil-borne diseases, which generally cause heavy loss in pepper production, especially under high temperature–high humidity (HTHH) conditions. Unraveling the molecular mechanism underlying the pathogen response under HTHH will enable us to explore and manipulate crucial regulatory nodes in order to enhance disease resistance under HTHH conditions. Previously, we found that CaWRKY40, which is transcriptionally up-regulated both by high temperature under high humidity and by Ralstonia solanacearum inoculation (RSI), acts as a positive regulator in the response of pepper to R. solanacearum infection and in thermotolerance under high humidity (Dang et al., 2013). CaWRKY6, another member of the pepper WRKY family, also acts as a positive regulator in the same process by binding to the promoter of CaWRKY40 and directly activating the transcriptional expression of CaWRKY40 (Cai et al., 2015). In the present study, a positive clone was isolated by the yeast one-hybrid system from a cDNA library of pepper using the promoter of CaWRKY40 as bait. The positive clone turned out to be CabZIP63, which is up-regulated by RSI or HTHH, and acts as a positive regulator in the response of pepper to RSI or HTHH by acting directly upstream of CaWRKY40, forming a positive feedback loop with CaWRKY40 during pepper’s response to RSI or HTHH.
Materials and methods
Plant materials and growth conditions
Seeds of pepper (C. annuum) or inbred lines Zunla-1, GZ03, and XJ116, and Nicotiana benthamiana, provided by the pepper breeding group in Fujian Agriculture and Forestry University (Fuzhou, China), were sown in a soil mix [peat moss:perlite, 2:1 (v/v)] in plastic pots and placed in a growth room under at 25 °C, 60–70 µmol photons m−2 s−1, a relative humidity of 70%, and a 16h light/8h dark photoperiod.
Pathogens and inoculation procedures
Ralstonia solanacearum strain FJC100301 was isolated previously in our lab and amplified according to the method of Dang et al. (2013). The bacterial cell solution used for RSI of pepper plants for functional characterization of CabZIP63 was diluted to 108 cfu ml−1 (OD600=0.8) with 10mM MgCl2. Pepper plants were inoculated by infiltrating 10ml of the resulting R. solanacearum suspension into the third leaves of pepper plants at the eight-leaf stage from the apical meristem using a syringe without a needle, and mock inoculation was with sterile 10mM MgCl2. The leaves were harvested at the indicated time points for the preparation of RNA or for other assays such as trypan blue and 3,3′-diaminobenzidine (DAB) staining.
The virulence of R. solanacearum strain FJC100301 was assayed by irrigation of injured roots using two inbred pepper lines, GZ03 and XJ116. Each pot containing one plant at the six- to eight-leaf stage with its root injured was irrigated with 5ml of FJC100301 suspension containing 1×105 cells ml–1, and then the pots were kept in a growth room at 28 °C with soil moisture at >90%. The disease indexes of the plants were evaluated at 7 days post-inoculation (dpi) following the standards published by the Ministry of Agriculture of the People’s Republic of China (Supplementary Table S1 at JXB online).
Treatment of plants with exogenous hormones and HTHH
Pepper plants at the four-leaf stage were sprayed with 1mM SA, 100 µM methyl jasmonate (MeJA), 100 µM ABA, or 100 µM ethephon (ETH). Mock-treated plants were sprayed with the corresponding solvent or sterile ddH2O. For HTHH treatment, pepper plants at the eight-leaf stage were kept under high temperature (38 °C) and 90% humidity or normal temperature (25 °C) and 50% humidity; to ensure that cell death under HTHH did not result from photo-oxidative stress, plants were put in the dark before harvesting for further analysis.
Yeast one-hybrid screening
Screening was performed using the Matchmaker™ one-hybrid system (Clontech, Palo Alto, CA, USA). To make a target–reporter construct, a fragment in the promoter of CaWRKY40 containing a C-box and a G-box (from –1889 to –1551 where the translation start codon of CaWRKY40 was set as +1) was inserted into the KpnI and XhoI sites of plasmid pAbAi . The recombinant vector was sequenced and transformed into the yeast strain Y1HGold (Clontech) by polyethylene glycol (PEG)-mediated transformation to generate the yeast bait strain. The pepper Matchmake™ cDNA expression library (Zunla-1) constructed previously in our lab was used for screening for positive clones interacting with the C- and G-box-containing pCaWRKY40 according to the protocol provided by the Matchmaker™ one-hybrid system (Clontech). A 15ml yeast culture was transformed using 3 μg of the cDNA and plated on synthetic minimal medium containing 400ng ml–1 AbAr (Aureobasidin A), but lacking uracil. After incubation at 30 °C for 3 d, the colonies were transferred to filter paper and tested for β-galactosidase activity. Plasmids were extracted from the positive yeast colonies, amplified in Escherichia coli cells, and purified for sequencing. The sequences of the positive clones were used as a query to search the genome sequence banks (http://peppersequence.genomics.cn/page/species/index.jsp), and its corresponding promoter sequence was determined.
Vector construction
For vector construction, a Gateway cloning technique (Invitrogen, Carlsbad, CA, USA) and a series of Gateway-compatible destination vectors were employed. The full-length cDNA of CabZIP63 and CaWRKY40, and the promoter region of CabZIP63 (2000bp upstream of ATG, pCabZIP63), were initially amplified by PCR with their corresponding specific primer pair (Supplementary Table S2) flanked with attB for Gateway cloning and GXL DNA polymerase (Takara, Osaka, Japan), and confirmed by sequencing. The full-length cDNAs were cloned into the entry vector pDONR207 by BP reaction, and then into destination vectors such as pMDC83, pK7WG2, and pEarleyGate201 by LR reaction for subcellular localization, transient overexpression, and ChIP analysis, respectively. pCabZIP63 was cloned into the pMDC163 destination vector for expression assay of the pCabZIP63-driven β-glucuronidase (GUS) reporter gene in pepper plants. To construct the vectors for virus-induced gene silencing (VIGS), a fragmens of ~229bp in length in the 3′-untranslated region (UTR) of CabZIP63 or CaWRKY40 was amplified by PCR with a specific primer pair, and was cloned sequentially into entry vector pDONR207 and the destination vector, the PYL279 VIGS vector, using the Gateway cloning technique (Invitrogen) similarly to as described above. For the vector construction for the dominant repressor version of CabZIP63 or CaWRKY40, the EAR repression domain (SRDX) (Hiratsu et al., 2003) was fused to the 3′ terminus of CabZIP63 or CaWRKY40 by PCR using the primers modified according to the sequence of the SRDX domain (5′-CTCGATCTGGATCTAGAACT CCGTTTGGGTTTCGCT-3′). Subsequently the CabZIP63-SRDX or CaWRKY40-SRDX amplicon was cloned into destination vector pK7WG2 by the Gateway cloning technique (Invitrogen) similarly to as described above.
Determination of CabZIP63 subcellular localization
Agrobacterium tumefaciens strain GV3101 containing the constructs 35S::CabZIP63-GFP and 35S::GFP (used as a control) were grown overnight, and then resuspended in induction medium (10mM MES, 10mM MgCl2, pH 5.7, and 150 µM acetosyringone). Bacterial suspensions (OD600=0.8) were injected into N. benthamiana leaves using a syringe without a needle. At 48h post-infiltration (hpi), green fluorescent protein (GFP) fluorescence was imaged using a laser scanning confocal microscope (TCS SP8, Leica, Solms, Germany) with an excitation wavelength of 488nm and a 505–530nm band-pass emission filter.
Transient overexpression of CabZIP63 (–SRDX) or CaWKRY40 (–SRDX) in pepper leaves
For transient overexpression analysis, A. tumefaciens strain GV3101 harboring the 35S::CabZIP63 (–SRDX) or 35S::CaWRKKY40 (–SRDX) vector was grown overnight, and then resuspended in induction medium. The bacterial suspension (OD600=0.8) was injected into leaves of pepper plants at the eight-leaf stage, and the injected leaves were harvested at 24 hpi for further use.
VIGS of CabZIP63 in pepper plants
For CabZIP63 silencing analysis, the 3′-UTR of CabZIP63 was used for VIGS vector construction; its sequence specificity was confirmed by genome-wide homology sequence searching by BLAST against sequences in the CM334 and Zunla-1 databases (http://peppergenome.snu.ac.kr/ and http://peppersequence.genomics.cn/page/species/blast.jsp). We did not find any homologous sequence in other pepper genes. The resulting Tobacco rattle virus (TRV)-based vectors TRV2-CabZIP63 and TRV1 were transformed into A. tumefaciens strain GV3101. GV3101 cells harboring TRV1 and TRV2-CabZIP63 or TRV2 as a negative control (resuspended in the induction medium at a 1:1 ratio, OD600=0.6) were co-infiltrated into cotyledons of 2-week-old pepper plants. The details of the process were as described in our previous studies (Dang et al., 2014; Cai et al., 2015; Zhang et al., 2015b).
Histochemical staining
Staining with trypan blue and DAB was carried out according to the previously published method of Choi et al. (2012), following the process as detailed in our previous studies (Dang et al., 2014; Cai et al., 2015; Liu et al., 2015).
Quantitative real-time RT–PCR
To determine the relative transcription levels of selected genes, real-time reverse transcription–PCR (RT–PCR) was performed with specific primers (Supplementary Table S3) according to the manufacturer’s instructions for the BIO-RAD Real-time RT-PCR system (Foster City, CA, USA) and the SYBR Premix Ex Taq II system (TaKaRa). Total RNA preparation and real-time RT–PCR were carried out following procedures used in our previous studies (Dang et al., 2014; Cai et al., 2015; Zhang et al., 2015b). At least three replications of each experiment were performed. Data were analyzed by the Livak method (Livak and Schmittgen, 2001) and expressed as a normalized relative expression level (2-ΔΔCT) of the respective genes. The relative transcript levels of the analyzed pepper were normalized to the transcript levels of CaACTIN (GQ339766) and 18S rRNA (EF564281). In each case, three technical replications were performed for each of at least three independent biological replicates.
Chromatin immunoprecipitation analysis
ChIP assays were performed as described by Cai et al. (2015). The GV3101 strain containing 35S::CabZIP63-HA or 35S::CaWRKY40-HA was infiltrated into the leaves of pepper plants at the eight-leaf stage; the plants were harvested and ~2g of pepper leaves were treated with either 10mM β-mercaptoethanol or DMSO (solvent control) for 16h and subsequently fixed with 1.0% formaldehyde for 5min. The chromatin was sheared to an average length of 500bp by sonication, and immunoprecipitated with antibody against hemagglutinin (HA; Santa Cruz Biotechnology). A 10mg aliquot of antibodies was used for each ChIP analysis, and the immunoprecipitated DNA was analyzed for enrichment of CabZIP63 or CaWRKY40 at the promoter region of target genes by quantitative real-time RT–PCR. Fold increases of immunoprecipitated DNA were calculated relative to the input DNA and the internal control CaACTIN or 18S rRNA. Each sample was quantified at least in triplicate. The primers used for real-time RT–PCR analysis in ChIP assays are listed in Supplementary Table S4.
Fluorometric GUS enzymatic assay
A fluorometric GUS enzymatic assay for measuring GUS activity in pepper plant extracts was performed as described previously (Jefferson et al., 1987). Leaves were lysed in extraction buffer (50mM phosphate buffer, pH 7.0, 10mM EDTA, 0.1% Triton X-100, 0.1% sodium lauryl sarcosine, and 10mM β-mercaptoethanol) by freezing with liquid nitrogen, and were ground using a pestle and mortar. Aliquots of the extracts (100 μl) were added to 1ml of assay buffer (extraction buffer containing 1mM MU), pre-warmed, and incubated at 37 °C. After 0, 5, and 20min of incubation, 100 μl samples were removed and placed in 1.9ml of stop buffer (200 μM sodium carbonate). Fluorescence was measured using a Multi Detection Microplate Reader (Bio-TEK Synergy™ HT, Bad Friedrichshall, Germany). The total protein content in plant extracts was estimated by the Bradford method using BSA as a standard (Bradford, 1976).
Results
Cloning and sequence analysis of CabZIP63
To isolate the possible TFs that transcriptionally modify the expression of CaWRKY40, a yeast one-hybrid system screening of a pepper cDNA library constructed from leaves of Zunla-1 using an ~400bp promoter region of CaWRKY40 containing a G-box and a C-box as bait was performed. Among the 12 positive clones acquired, one clone turned out to be full-length cDNA of a gene encoding a bZIP protein. It is 1389bp in length, contains a 1272bp ORF, and a conserved bZIP domain was found in its deduced amino acid sequence and a nuclear localization signal (NLS) in its C-terminus. It shared 62.56, 56.74, 53.86, 52.74, 52.74, 51.88, and 52.93% deduced amino acid sequence identities with the products of NtbZIP1, CsbZIP6, PtbZIP, GmSBZ1, GsCPRF2, GaCPRF2, and MtbZIP88, respectively. Since it exhibited the highest sequence identity (43%) with that of the product of AtbZIP63 among all of the bZIPs in Arabidopsis, it was designated CabZIP63 (Capsicum annuum bZIP63) (see Supplementary Fig. S1). Although AtbZIP63 was found to act as a sensitive integrator of transient ABA and glucose signals (Matiolli et al., 2011), the role of CabZIP63 in pepper has not been characterized so far.
CabZIP63 is localized to nuclei
An NLS (397LEHLQKRIRGD407, Supplementary Fig. S1) present in CabZIP63 indicates its nuclear localization; to confirm this probability, we assayed its subcellular localization by expression of the constructs p35S::CabZIP63-GFP and p35S::GFP (control) individually in N. benthamiana leaves. Typical results showed the exclusive localization of CabZIP63–GFP in nuclei, whereas the GFP control was observed in multiple subcellular compartments including the cytoplasm and nuclei (Supplementary Fig. S2).
The expression of CabZIP63 was enhanced by HTHH and RSI as well as exogenous applied SA, MeJA, ETH, and ABA
Since CaWRKY40 acts as positive regulator in pepper’s response to HTHH and RSI, and as CabZIP63 can bind to the CaWRKY40 promoter, CabZIP63 is likely to play a role in the above defense responses. To test if this is the case, the activity of the CabZIP63 promoter in response to HTHH and RSI was examined using Agrobacterium-mediated transient overexpression in pepper leaves. The construct pCabZIP63::GUS was transformed into Agrobacterium GV3101, and cells containing pCabZIP63::GUS were infiltrated into pepper leaves, at 24 hpi. The infiltrated pepper leaves were further inoculated with R. solanacearum (OD600=0.6) or treated with heat stress (38 °C) under 90% humidity; after RSI or HTHH treatment, the leaves were harvested at appropriate time points and the GUS activities were measured in the pepper leaves. The results showed that both RSI and HTHH significantly up-regulated the expression of CabZIP63 compared with the mock treatment (Supplementary Fig. S3A). The relative transcription levels of CabZIP63 were also measured at appropriate time points after RSI or HTHH by real-time RT–PCR, and the result showed that the transcript levels of CabZIP63 were enhanced more intensively than those of pCabZIP63-driven GUS by RSI or HTHH; this might be due to post-transcriptional regulation of GUS expression and the difference in feedback regulation between expression of GUS and CabZIP63 in planta (Supplementary Fig. S3B). As phytohormones such as SA, JA, ET, and ABA have typically been found to be involved in plant defense signaling against different biotic and abiotic stresses, to test if CabZIP63 is involved in the pathways mediated by these hormones, the expression of pCabZIP63-driven GUS after exogenous applications of SA, MeJA, ETH, and ABA was measured. The results showed that GUS expression was induced by all of the four test hormones (Supplementary Fig. S3C).
CabZIP63 is transcriptionally regulated by CabZIP63 itself
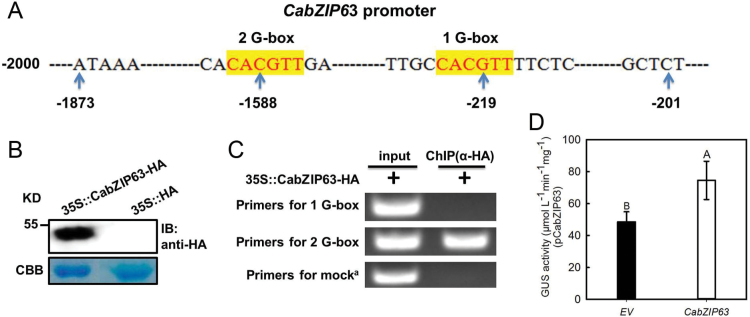
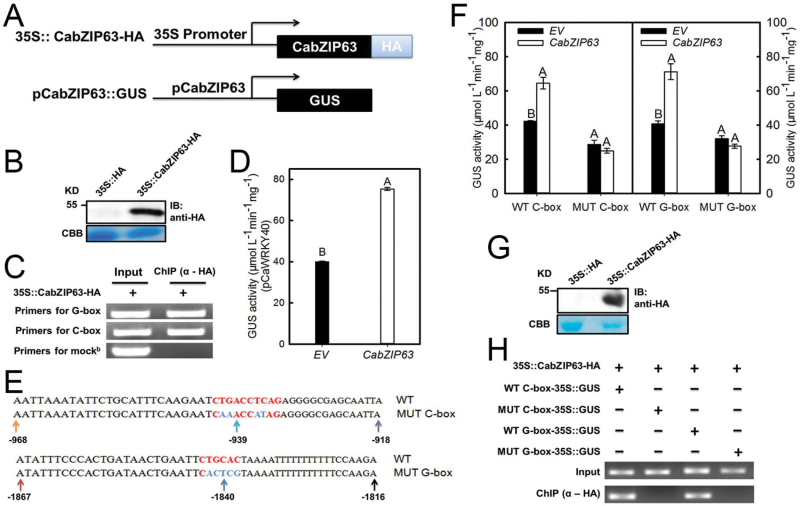
The presence of a G-box and C-box, which were previously found to be bound by bZIP TFs (Izawa et al., 1993), in the promoter region of CabZIP63 implies that the transcriptional expression of CabZIP63 is probably self-regulated. To test this possibility, we performed a ChIP assay to test if CabZIP63 can also bind its own promoter. GV3101 cells containing 35S::CabZIP63-HA were infiltrated into the leaves of pepper plants; 48h later, the leaves were harvested for chromatin preparation and ChIP analysis. The stable expression of fused protein CabZIP63-HA was confirmed by western blot analysis with anti-HA antibody. The result showed that a specific primer pair flanking one of the two G-boxes amplified the product of the DNA fragments immunoprecipitated by anti-HA antibody as templates, indicating that CabZIP63 can bind to it own promoter (Fig. 1A–C). To test the possible self-regulation of CabZIP63 further, pCabZIP63-driven GUS expression was analyzed after transient overexpression of CabZIP63 in pepper plants, and the result showed that a significantly enhanced expression of GUS was triggered by transient overexpression of CabZIP63 (Fig. 1D).
Fig. 1.
CabZIP63 is transcriptionally regulated by CabZIP63 itself. (A) Schematic representation and sequence of elements within the –200 to –1873bp region of pCabZIP63. (B) Transient overexpression of CabZIP63-HA in pepper leaves as detected by immunoblotting (IB). CBB, Coomassie Brilliant Blue. (C) ChIP assay indicated that CabZIP63 binds to its own promoter. Pepper leaves were infiltrated with GV3101 cells carrying 35S::CabZIP63-HA. The infiltrated leaves were harvested and cross-linked with 1% formaldehyde for chromatin preparation. The sheared chromatin was immunoprecipitated with an anti-HA antibody. The acquired DNA samples were adjusted to the same concentration and PCRs were performed using specific primer pairs according to flanking sequences of the two G-boxes. Lanes 1, input (total DNA–protein complex); lanes 2, DNA–protein complex immunoprecipitated with an anti-HA antibody. Mocka (Supplementary Table S4) is a DNA fragment that was distant from the cis-element of the two G-boxes in pCabZIP63, and was used as a control for ChIP assay. (D) The expression of pCabZIP63-driven GUS was induced by transient overexpression of CabZIP63 in pepper leaves. Data represent the means ±SD from four independent biological replicates. Different upper case letters indicate significantly different means, as analyzed by Fisher’s protected LSD test (P<0.01). (This figure is available in colour at JXB online.)
Effect of CabZIP63 silencing on resistance of pepper to R. solanacearum and thermotolerance, and the expression of marker genes
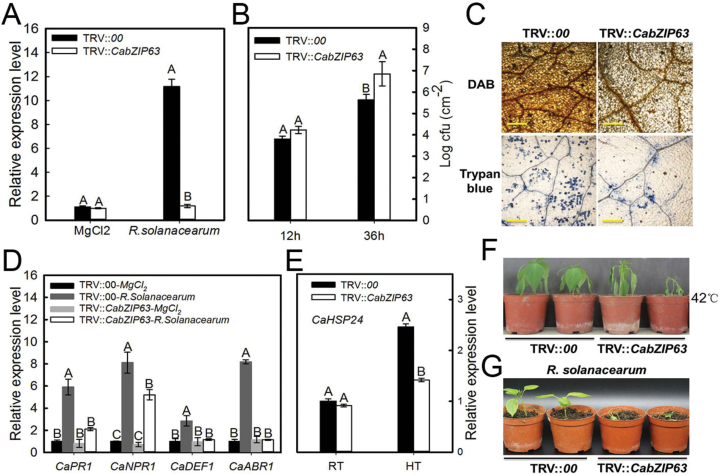
To test the role of CabZIP63 in immunity and thermotolerance under high humidity, we performed loss-of-function experiments in pepper seedlings in which CabZIP63 was silenced by VIGS. We used TRV::CaPDS, which silences the phytoene desaturase (PDS) gene and induces a photobleaching phenotype, as an additional control to determine the success of gene silencing. The two vectors TRV1 (PYL192) and TRV2 (PYL279) were separately transformed into A. tumefaciens GV3101. The two resulting GV3101 strains were mixed and co-injected into leaves of pepper seedlings, and seedlings were incubated at 16 °C for 56h; after this they were kept at 25 °C. Four independent experiments were performed, and we obtained ~100 plants of TRV::00 and 100 plants of TRV::CabZIP63, respectively. Six plants were randomly selected to check the efficiency of gene silencing by inoculation with cells of the virulent R. solanacearum strain FJC100301, which was detected by root irrigation to be virulent to pepper plants using two pepper inbred lines, GZ03, a line moderately resistant to R. solanacearum, and XJ116, a line susceptible to R. solanacearum (Supplementary Fig. S4). The result showed that in FJC100301-challenged TRV::CabZIP63 pepper plants, CabZIP63 transcript levels were reduced to ~30% of those in TRV::00 plants, suggesting the success of CabZIP63 silencing (Fig. 2A). With these CabZIP63-silenced pepper plants, the effects of CabZIP63 silencing on pepper immunity and thermotolerance were assayed; 60 TRV2::CabZIP63 and 60 TRV2::00 plants were randomly selected and inoculated with FJC100301. Definite wilting symptoms were observed in TRV2::CabZIP63 plants at 14 dpi, with an average disease index of 3.0, while TRV2::00 plants exhibited only faint wilting symptoms, with an average disease index of 1.2 (Supplementary Table S5). Consistently, our data also showed that the growth of R. solanacearum was significantly increased in CabZIP63-silenced pepper plants, manifested by higher cfu values compared with those in the control plants at 36 hpi (Fig. 2B), and dark-brown DAB (indicator of H2O2 accumulation) and trypan blue (indicator of cell death or necrosis) staining was detected in the leaves of TRV2:00 plants at 48 hpi, whereas the intensities of DAB and trypan blue staining were distinctly reduced in CabZIP63-silenced leaves (Fig. 2C). Additionally, the expression of defense-related CaPR1, CaNPR1, CaDEF1, and CaABR1 was significantly lower in leaves of R. solanacearum-inoculated CabZIP63-silenced pepper plants at 24 hpi compared with that in control plants (Fig. 2D). On the other hand, when challenged with high temperature (42 °C) under 90% humidity, the (i) TRV2::CabZIP63 plants exhibited significantly increased thermosensitivity compared with the wild-type control plants; (ii) the thermotolerance-associated CaHSP24 was much lower in TRV2::CabZIP63 plants compared with the TRV2::00 plants (Fig. 2E). The results strongly suggest that silencing of CabZIP63 significantly impairs resistance/tolerance of pepper plants to RSI or HTHH (Fig. 2F, G).
Fig. 2.
Distinct responses of CabZIP63-silenced pepper plants to RSI and HTHH. (A) Real-time RT–PCR analysis of CabZIP63 expression in R. solanacearum-inoculated or mock-treated (inoculated with solution of MgCl2) CabZIP63-silenced pepper (TRV::CabZIP63) and control (TRV::00). (B) Detection of growth of R. solanacearum in CabZIP63-silenced or control pepper plants inoculated with R. solanacearum at 12h and 36h. (C) Trypan blue staining and DAB staining in R. solanacearum-inoculated CabZIP63-silenced (TRV::CabZIP63) and empty vector (TRV::00) pepper leaves at 2 days post-inoculation (dpi). Scale bars=50 μm. (D) Real-time RT–PCR analyses of transcription levels of the tested defense-related genes in CabZIP63-silenced pepper (TRV::CabZIP63) and control (TRV::00) after inoculation with or without R. solanacearum. (E) Real-time RT–PCR analyses of transcription levels of the thermotolerance-related CaHSP24 in CabZIP63-silenced pepper (TRV::CabZIP63) and control (TRV::00) with or without high temperature (HT) treatment. (F) The pepper plants were treated at 42 °C for 24h, and then kept under normal temperature conditions (25 °C) for 24h before checking the phenotype. (G) Phenotypic effect of R. solanacearum attack on CabZIP63-silenced (TRV::CabZIP63) and control (TRV::00) plants at 14 dpi. Data represent the means ±SD from four independent experiments. Different letters indicate significant differences, as determined by Fisher’s protected LSD test (P<0.01). (This figure is available in colour at JXB online.)
Transient overexpression of CabZIP63 or CabZIP63–SRDX modifies the cell death, immunity and thermotolerance-associated marker gene expression in pepper plants
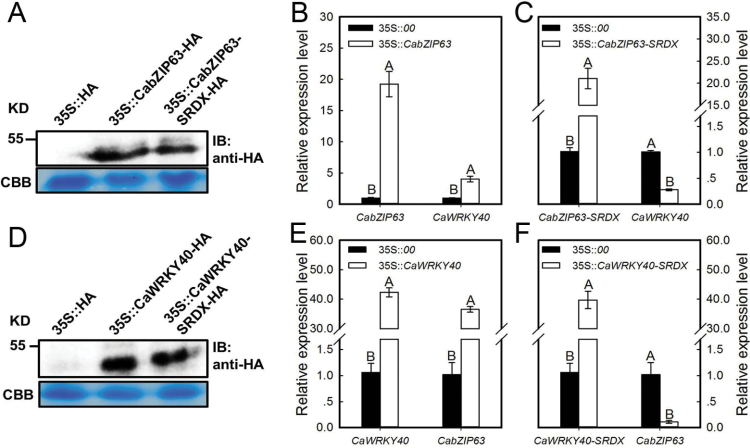
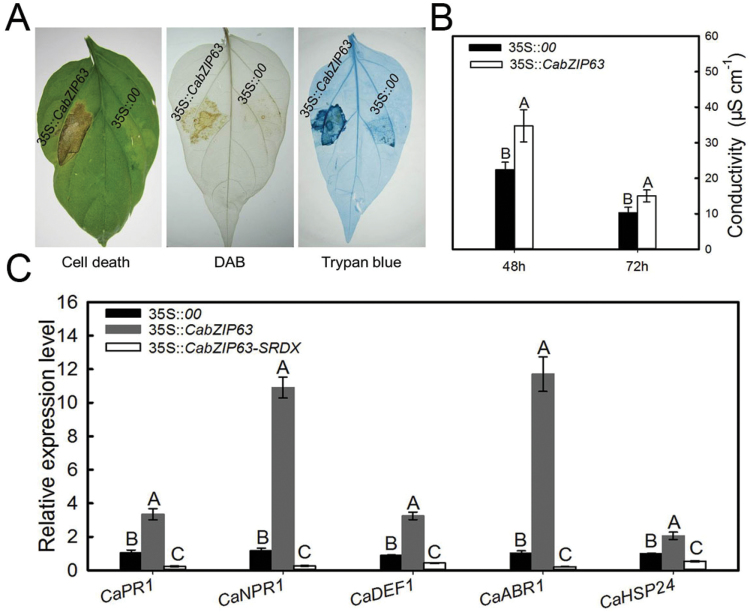
To confirm further the finding that CabZIP63 acts as a positive regulator in pepper’s defense response to both HTHH and RSI, CabZIP63 was transiently expressed in pepper leaves by infiltration with GV3101 cells carrying 35S::00 (empty vector) or 35S::CabZIP63. Real-time RT–PCR and immunoblot analysis showed that the HA-tagged CabZIP63 mRNA and protein were stably expressed in pepper plants (Fig. 5C). HR-mediated cell death and H2O2 accumulation were assessed by staining with trypan blue and DAB, respectively; the result showed that the transient overexpression of CabZIP63 induced both extensive HR-mediated cell death and accumulation of H2O2 in pepper plants (Fig. 3A). We also detected ion leakage to measure the severity of cell necrosis caused by transient overexpression of CabZIP63, and the result showed that pepper leaves transiently overexpressing CabZIP63 exhibited more ion leakage at 48 and 72 hpi than leaves expressing the empty vector control (Fig. 3B). We also examined changes in the expression of defense-related genes including CaNPR1, CaPR1, CaDEF1, and CaHSP24, and the results showed that the relative transcription levels of CaPR1, CaNPR1, CaDEF1, and CaHSP24 increased continuously during transient overexpression of CabZIP63. In contrast, the transient overexpression of CabZIP63-SRDX, a repressor version of CabZIP63, was also performed in pepper leaves, and the success of fused CabZIP63-SRDX mRNA was confirmed by real-time RT–PCR. The result showed that the overexpression of CabZIP63-SRDX markedly decreased the expression of the tested marker genes (Fig. 3C). These data indicated that CabZIP63 might act as a positive regulator in the response of pepper to pathogen and heat stress.
Fig. 5.
Inter-relationship between the expression of CabZIP63 and CaWRKY40 at the transcriptional level. (A) The effect of transient overexpression of 35S::CabZIP63 on the transcript level of CaWRKY40 in pepper leaves. (B) The effect of transient overexpression of 35S::CabZIP63-SRDX on the transcript level of CaWRKY40 in pepper leaves. (C) Transient overexpression of CabZIP63-HA and CabZIP63-SRDX-HA in pepper leaves as detected by immunoblotting (IB). CBB, Coomassie Brilliant Blue. (D) The effect of transient overexpression of 35S::CaWRKY40 on the transcript level of CabZIP63 in pepper leaves. (E) The effect of transient overexpression of 35S::CaWRKY40-SRDX on the transcript level of CabZIP63 in pepper leaves. (F) Transient overexpression of CaWRKY40-HA and CaWRKY40-SRDX-HA in pepper leaves as detected by immunoblotting. The pepper leaves were infiltrated with GV3101 cells (OD600=0.8) containing different constructs, which were harvested at 24 hpi for total RNA extraction; the transcript levels of CaWRKY40 or CabZIP63 were determined by real-time RT–PCR with specific primer pairs. Data represent the means ±SD from four independent biological replicates. Different upper case letters indicate significantly different means, as analyzed by Fisher’s protected LSD test (P<0.01). (This figure is available in colour at JXB online.)
Fig. 3.
Cell death and expression of immunity- or thermotolerance-related marker genes were triggered by transient overexpression of 35S::CabZIP63. (A) Cell death triggered by transient overexpression of 35S::CabZIP63, displayed with phenotype, DAB staining, and trypan blue staining at 4 dpi, respectively. (B) Quantification of electrolyte leakage as ion conductivity to assess the cell death response in leaf discs. (C) Quantitative real-time RT–PCR analysis of the expression of immunity- or thermotolerance associated marker genes in 35S::CabZIP63 and 35S::CabZIP63-SRDX expressed in pepper leaves at 24 hpi, respectively. Data represent the means ±SD from four independent biological replicates. Different letters above the bars indicate significantly different means (P<0.01), as analyzed by Fisher’s protected LSD test. (This figure is available in colour at JXB online.)
CabZIP63 binds to both the G-box and the C-box in the promoter of CaWRKY40
As CabZIP63 responded transcriptionally to HTHH and RSI similarly to CaWRKY40 (Dang et al., 2013), and the G-box and C-box, which were previously found to be preferentially bound by bZIPs (Izawa et al., 1993), are present in the promoter region of CaWRKY40, we speculated that CaWRKY40 might be transcriptionally regulated by CabZIP63 in pepper’s response to HTHH and RSI. To test this possibility, we performed ChIP analysis to test if CabZIP63 can also bind the promoter of CaWRKY40 via the G-box or C-box. The result showed that the fused CabZIP63-HA was successfully expressed by transient overexpression in pepper plants, and both the specific primers pairs flanking the G-box or the C-box amplified products with the DNA fragments immunoprecipitated by anti-HA antibody as templates, indicating that CabZIP63 can bind to the CaWRKY40 promoter (Fig. 4A–D). To confirm this result further and to determine whether the C-box or the G-box or both the boxes are responsible for the specific binding, we constructed pW40C, pW40G, pW40CM, and pW40GM, and analyzed the possible contribution of binding of CabZIP63 to the C-box or G-box in the transcriptional regulation of CaWRKY40 by CabZIP63. The results showed that the overexpression of CabZIP63 activated the expression of GUS driven by pW40C and pW40G, but failed to activate the expression of GUS driven by either pW40CM or pW40GM (Fig. 4E, F). With these four vectors, we also tested the possible binding of CabZIP63 to the C- or G-box by ChIP through transient overexpression in N. benthamiana leaves; the results showed that CabZIP63 bound to both the C-box and the G-box (Fig. 4G, H).
Fig. 4.
The binding of CabZIP63 to pCaWRKY40 and GUS expression in a C- or G-box-dependent manner. (A) Schematic diagram of transient overexpression and GUS reporter constructs used for co-transfection in pepper leaves. (B) Transient overexpression of CabZIP63-HA in pepper leaves for ChIP assay as detected by immunoblotting (IB). CBB, Coomassie Brillaint Blue. (C) ChIP assay indicated that CabZIP63 bound to pCaWRKY40. Chromatin was isolated from infiltrated pepper leaves cross-linked with 1% formaldehyde, and was sheared, and immunoprecipitated with an anti-HA antibody. The acquired DNA samples adjusted to the same concentration were used as templates for PCRs with specific primer pairs based on C-box- or G-box-flanking sequences. Lanes 1, input (total DNA–protein complex); lanes 2, DNA–protein complex immunoprecipitated with an anti-HA antibody. Mockb (Supplementary Table S4) is a DNA fragment distant from the C-box and G-box in pCaWRKY40, and was used as a control for ChIP assay. (D) pCaWRKY40-driven GUS expression was triggered by transient overexpression of CabZIP63 by Agrobacterium infiltration in pepper leaves. The pepper leaves were co-infiltrated with GV3101 cells carrying pCaWRKY40::GUS or 35S::CabZIP63. (E) Schematic representation and sequence of elements that were mutagenized within the –918 to –968bp and –1816 to –1867bp regions in pCaWRKY40; the fragment harboring each C- or G-box and their corresponding mutants were amplified by PCR and were cloned into vector pMDC163, in which the original CaMV35S promoter was replaced by the core promoter of CaMV35S (–46bp to +8bp). The acquired vectors were named pW40C, pW40CM, pW40G, and pW40GM, respectively. (F) The constructs of pW40C, pW40CM, pW40G, and pW40GM were transformed into GV3101 individually, and the resulting cells containing the individual constructs were co-infiltrated with GV3101 cells containing 35S::CabZIP63-HA into pepper leaves, which were harvested for GUS expression assay at the appropriate time points. (G) Transient overexpression of CabZIP63-HA in N. benthamiana leaves for ChIP assay as detected by immunoblotting (IB). (H) The result of ChIP indicated that CabZIP63 failed to bind to the mutant C- or G-box. GV3101 cells containing 35S::CabZIP63-HA were co-infiltrated into N. benthamiana leaves with GV3101 cells carrying pW40C, pW40CM, pW40G, and pW40GM for ChIP assay. Data represent the means ±SD from four independent biological replicates. Different upper case letters indicate significantly different means, as analyzed by Fisher’s protected LSD test (P<0.01). (This figure is available in colour at JXB online.)
The effect of RSI or HTHH on the binding of CabZIP63 to pCaWRKY40
As CabZIP63 is enhanced by both HTHH and RSI, and CabZIP63 binds to the promoter of CaWRKY40, the binding of CabZIP63 to the promoter of CaWRKY40 might contribute to the transcriptional activation of CaWRKY40 against RSI or HTHH. To test this possibility, the binding of CabZIP63 to pCaWRKY40 under RSI or HTHH was assayed by ChIP, during which 35S::CabZIP63-HA was transiently overexpressed in pepper leaves by Agrobacterium infiltration. The infiltrated leaves were harvested at 48 hpi for chromatin preparation, excision, and immunoprecipitation, and the resulting DNA fragments were used as template for real-time RT–PCR with specific primer pairs for pCaWRKY40 (Supplementary Fig. S5). The results showed that both RSI and HTHH enhanced the binding of CabZIP63 to pCaWRKY40.
Inter-relationship between the expression of CabZIP63 and CaWRKY40 at the transcriptional level
As both CabZIP63 and CaWRKY40 are up-regulated transcriptionally by RSI and HTHH, and CabZIP63 binds to pCaWRKY40, it is presumed that CabZIP63 might be transcriptionally up-regulated by both RSI and HTHH and could then activate the transcription of CaWRKY40. To test this possibility, the effect of transient overexpression of CabZIP63 in pepper leaves on the transcript level of CaWRKY40 was measured by real-time RT–PCR, and the results showed that the transient overexpression of CabZIP63 enhanced the transcript level of CaWRKY40 in pepper leaves (Fig. 5A, B). In contrast, transient overexpression of CabZIP63-SRDX was performed in pepper plants, and the transcript and protein of CabZIP63-SRDX were confirmed by real-time RT–PCR and western blot analysis, respectively. The result showed that the overexpression of CabZIP63-SRDX significantly decreased the transcription level of CaWRKY40 by real-time RT–PCR using their corresponding specific primers designed according to the sequence in its 3′-UTR (Fig. 5A, C). Consistently, pCaWRKY40-driven GUS expression assay showed that the GUS expression was significantly promoted by transient overexpression of CabZIP63 (Fig. 4D). Interestingly, our data also showed that the transient overexpression of CaWRKY40 enhanced the transcription level of CabZIP63 in pepper leaves (Fig. 5D, E). In contrast, the transient overexpression of CaWRKY40-SRDX, which was confirmed by both real-time RT–PCR and western blot analysis, significantly decreased the transcription level of endogenous CaWRKY40 and CabZIP63 by real-time RT–PCR using the specific primers designed according to of the sequence in their 3′-UTRs (Fig. 5D, F; Supplementary Fig. S6A, B).
The effect of transient overexpression of CabZIP63 on the binding of CaWRKY40 to the promoters of its target genes
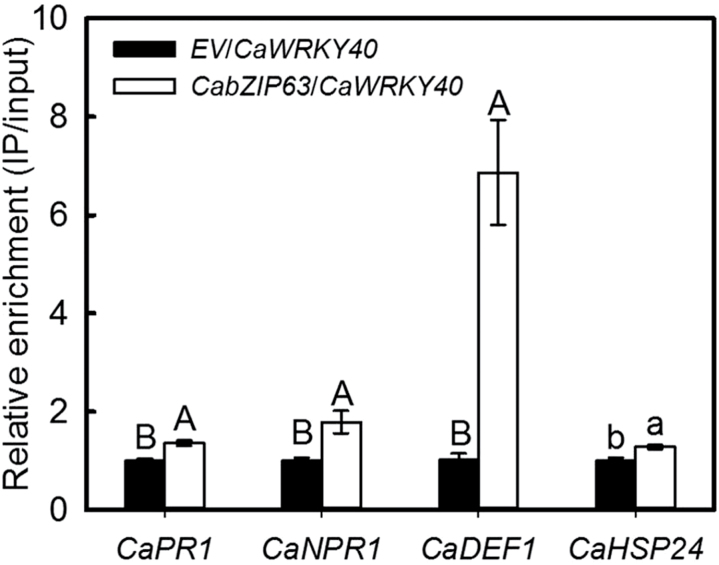
Our previous data showed that CaWRKY40 achieves its function in pepper’s response to RSI and HTHH by transcriptional modification of its targets genes including CaPR1, CaNPR1, CaDEF1, and CaHSP24, and data in the present study showed that CabZIP63 manipulates the expression of CaWRKY40, implying that the up-regulation of CabZIP63 might ultimately enhance the binding of CaWRKY40 to the promoters of its target genes. To confirm this possibility, we assayed the effect of CabZIP63 overexpression on the direct binding of CaWRKY40 to the promoters of its target genes by transient overexpression in pepper leaves. The results showed that the enrichment of CaWRKY40 at the promoters of CaNPR1, CaPR1, CaDEF1, and CaHSP24 was significantly enhanced by transient overexpression of CabZIP63 compared with the control pepper leaves (Fig. 6).
Fig. 6.
The DNA binding of CaWRKY40 to the promoters of its target genes was potentiated by transient overexpression of 35S::CabZIP63 in pepper plants. GV3101 cells containing 35S::CaWRKY40-HA and 35S::CabZIP63-83 were mixed at a ratio of 1:1 and were co-infiltrated into pepper leaves, with GV3101 cells containing 35S::00 as mock treatment. The leaves were harvested at 48 hpi for chromatin preparation (relative enrichment levels of samples of CaWRKY40 were set to 1 after normalization by input). Data represent the means ±SD from three independent biological replicates. Different upper case letters indicate significant differences from three independent biological replicates based on the LSD test (P<0.01). Different lower case letters indicate significant differences from three independent experiments based on the LSD test (P<0.05).
Discussion
In our previous study, CaWRKY40 was found to be up-regulated transcriptionally by HTHH or RSI, and to act as a positive regulator in pepper’s response to these stresses (Dang et al., 2013); however, their underlying mechanism remained to be elucidated. In the present study, we provide evidence that CabZIP63, a member of the bZIP family in pepper, acts as a positive TF modulating the expression of CaWRKY40, forming a positive feedback loop with CaWRKY40 during pepper’s defense response to HTHH or RSI.
The evidence that CabZIP63 is a bZIP family member comes from the following. First, CabZIP63 contains a conserved domain, which is generally present in bZIP proteins, in its deduced amino acid sequence, and also exhibits sequence identity with bZIP orthologs in other plant species such as, for example, bZIP63 in Arabidopsis, bZIP1 in Nicotiana tabacum, bZIP6 in Camellia sinensis, SBZ1 in Glycine max, and bZIP88 in Medicago truncatula. Secondly, CabZIP63 contains an NLS in its C-terminus and was consistently localized in the nuclei in cells of N. benthamiana leaves during subcellular localization using transient overexpression by Agrobacterium infiltration, which is a general characteristic of the majority of TFs (Boulikas, 1994) including bZIPs (Liu et al., 2012). Thirdly, CabZIP63 was found to bind to the G-box- and C-box-containing CaWRKY40 promoter by ChIP in the present study, and can activate the expression of a GUS reporter gene in a G-box- or C-box-dependent manner. In a previous study, bZIPs were found to bind preferentially via their basic region to DNA sequences with an ACGT core cis-element, in particular like the G-box, C-box, and A-box (Izawa et al., 1993; Foster et al., 1994; Heinekamp et al., 2002). All these results indicate that CabZIP63 is a member of the pepper bZIP family.
Accumulated evidence indicates that plant growth, development, and response to environmental stress are largely regulated at the transcriptional level (Baena-Gonzalez et al., 2007; Baena-Gonzalez and Sheen, 2008; Rymen and Sugimoto, 2012; Buscaill and Rivas, 2014), and genes up-regulated during plant response to stresses can have important roles in plant resistance/tolerance to stresses (Bartsch et al., 2006; Dang et al., 2013, 2014; Cai et al., 2015; Sun et al., 2015). Our data showed that the expression of the GUS reporter gene driven by the CabZIP63 promoter was induced by RSI or HTHH. Consistently, the transcript level of CabZIP63 was also found to be enhanced by both RSI and HTHH, implying that CabZIP63 might act as a positive regulator in pepper’s defense response to these stresses. This possibility was corroborated by the data from the loss- and gain-of function study of CabZIP63. Pepper plants with CabZIP63 silenced by VIGS exhibited a significantly decreased resistance and tolerance to RSI and HTHH compared with control plants, accompanied by down-regulation of thermotolerance-associated CaHSP24 (Pivovarova et al., 2005; Baek et al., 2014) under HTHH, and down-regulation of immunity-associated CaNPR1 (Ustun et al., 2013), CaPR1 (Kim and Hwang, 2012), CaDEF1 (Choi et al., 2008), and CaABR1 (Choi and Hwang, 2011) under RSI. In contrast, the transient overexpression of CabZIP63 activated HR-mediated cell death compared with the control, revealed by a high level of ion leakage and darker trypan blue and DAB staining (Choi et al., 2012; Dang et al., 2013), coupled with up-regulation of CaABR1, CaPR1, CaNPR1, CaDEF1, and CaHSP24 in pepper leaves transiently overexpressing CabZIP63. These results strongly suggest that CabZIP63 acts as positive regulator in pepper’s response to both HTHH and RSI. Although bZIPs have been extensively studied in plant immunity (Pontier et al., 2001; Kim and Delaney, 2002; Zander et al., 2010; Du et al., 2014), and in abiotic stresses such as salinity (Orellana et al., 2010; Lakra et al., 2015; Zhang et al., 2015a), drought (Orellana et al., 2010; Lakra et al., 2015), cold (Hwang et al., 2005; Zhang et al., 2015a), and heat (Li et al., 2012; Srivastava et al., 2014), and AtbZIP63 was previously found to be a sensitive integrator of transient ABA and glucose signals, as well as low energy response (Matiolli et al., 2011; Mair et al., 2015), no information is available about the involvement of bZIPs in responses of plants to both pathogen infection and heat stress.
Our data also indicate a close relationship between CabZIP63 and CaWRKY40, as they showed a synergistic response not only to RSI and HTHH, but also to exogenously applied SA, MeJA, ETH, and ABA (Dang et al., 2013). Importantly, CabZIP63 was found to bind pCaWRKY40 and activated GUS expression in a C- and G-box-dependent manner, and this binding was found to be potentiated by both RSI and HTHH, suggesting that CabZIP63 acts as a TF of CaWRKY40. Since a multitude of cis-elements including a W-, G-, and C-box are present in pCaWRKY40, and CaWRKY6 was previously found to act as a positive regulator by directly activating the transcriptional expression of CaWRKY40 during pepper response to RSI and HTHH (Cai et al., 2015), indicating that the actions of CaWRKY40 are orchestrated by multiple TFs, how these TFs co-ordinate to fine-tune the expression of CaWRKY40 remains to be determined. In the present study, positive feedback loops were found in the transcriptional regulation of CabZIP63 and between CabZIP63 and CaWRKY40. The expression of CabZIP63 was found to be induced by the transient overexpression of CabZIP63 itself and by that of CaWRKY40, while it was decreased by the transient overexpression of CabZIP63-SRDX or CaWRKY40-SRDX, in which the SRDX domain was used to transform CabZIP63 and CaWRKY40 into dominant-negative repressor versions and has been widely used to assess the roles of TFs (Grunewald et al., 2013; Takada, 2013; Figueroa and Browse, 2015). This suggests an indirect regulation of CabZIP63 by CaWRKY40 possibly via unknown upstream components, since CaWRKY40 failed to bind to pCabZIP63 (data not shown). Similar positive feedback loops have also been found during pepper’s response to RSI and HTHH between CaWRKY6 and CaWRKY40 (Cai et al., 2015), as well as in plant response to a wide array of stresses (Miersch and Wasternack, 2000; Arimura et al., 2002; Yan et al., 2015; Zhang et al., 2015b). For example, positive feedback loops have been found in signaling against stresses mediated by brassinosteroid (BR) (Yan et al., 2015) and ABA (Xiong et al., 2002), as well as JA and ET (Arimura et al., 2002). These positive feedback loops might allow plants to respond to stresses more efficiently. Interestingly, compared with up-regulation of CabZIP63 by the transient overexpression of CaWRKY40, the transient overexpression of CabZIP63 only slightly activated the transcriptional expression of CaWRKY40, as the function of bZIPs has been found to be modified by other proteins such as NPR1, WRKY TFs, and other bZIPs in a protein–protein interaction manner (Spoel et al., 2003; Alves et al., 2013; Caarls et al., 2015). We speculate that the lower level of CaWRKY40 activation by transient overexpression of CabZIP63 might be due to the absence of its interacting partners under this condition. In addition to the transcription level, CaWRKY40 appears to be modulated by CabZIP63 at the post-transcriptional level, since the binding of CaWRKY40 to its target genes was found to be up-regulated by transient overexpression of CabZIP63 in pepper leaves in the present study; however, the underlying mechanism remains to be elucidated. The above results also collectively suggest a close link between RSI resistance and HTHH tolerance in pepper plants, which might occur in multiple nodes including CaWRKY6, CaWRKY40, and CabZIP63, as well as other unidentified components, and these components may be functionally connected, forming a transcriptional network composed of positive and negative feedback loops and feed-forward modules. This arrangement may provide great regulatory potential for plants to trigger appropriate disease resistance and thermotolerance, and co-ordinate different biological processes including growth, development, and response to other stresses. The synergistic response of pepper to HTHH and pathogen infection might be a result of its evolution under simultaneously or alternately occurring HTHH and pathogen infection in their natural habitats.
Our data also showed that CaNPR1 is a target of CaWRKY40, the expression of CabZIP63 and the binding of CabZIP63 to pCaWRKY40 was up-regulated by HTHH and RSI, and expression of CaWRKY40 was also enhanced by transient overexpression of CabZIP63, suggesting that the up-regulation of CabZIP63 by HTHH and RSI might result in accumulation of CaNPR1. As CaNPR1 acts as an important regulator by interacting with TGA, a member of the bZIP family, in plant immunity mediated by SA signaling (Spoel et al., 2003; Caarls et al., 2015), we speculate that CaNPR1 might interact with CabZIP63, which may play a role in modulating expression of CaWRKY40; further investigation is required to confirm this hypothesis and to elucidate the possible underlying mechanism.
Collectively, our data indicate that CabZIP63 acts as an activator of CaWRKY40 in the response of pepper to RSI or HTHH. Upon exposure of RSI or HTHH, the expression of CabZIP63 and its binding to pCaWRKY40 are up-regulated; therefore, the expression of CaWRKY40 and the binding of CaWRKY40 to its target genes are also activated, and eventually lead to the transcriptional modulation of target genes of CaWRKY40 and the defense response of pepper to RSI and HTHH. Our results will facilitate the dissection of the crosstalk between pepper’s response to HTHH and R. solanacearum.
Supplementary data
Supplementary data are available at JXB online
Figure S1. Comparison of amino acid sequences deduced from pepper CabZIP63 with the representative closely related proteins from other plant species.
Figure S2. The expression of CabZIP63 was induced by RSI and HTHH as well as exogenously applied SA, MeJA, ETH, or ABA.
Figure S3. CabZIP63 is transcriptionally regulated by CabZIP63 itself.
Figure S4. The detection of virulence of R. solanacearum strain FJC100301 by root irrigation.
Figure S5. The binding of CabZIP63 to pCaWRKY40 and GUS expression in a C- or G-box-dependent manner.
Figure S6. Relative expression level of endogenous CabZIP63 or CaWRKY40 in pepper leaves transiently overexpressing CabZIP63-SRDX or CaWRKY40-SRDX by real-time RT–PCR.
Table S1. Grading standards for evaluation of disease resistance of pepper plants to R. solanacearum by root irrigation.
Table S2. Pepper primers used for vector construction in this study.
Table S3. Pepper primers used for real-time RT–PCR in this study.
Table S4. Pepper primers used for ChIP PCR or real-time RT–PCR in this study.
Table S5. The plant numbers with different levels of disease resistance in the VIGS assay of CabZIP63 against R. solanacearum strain FJC100301 inoculation.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31372061, 31401890, 31401312, 31301254, 31060263, and 31260482) and major science and technology project in Fujian Province (2013NZ0002-3). We thank Mark D. Curtis for kindly providing the Gateway destination vectors, Bernd Weisshaar for pBT10-GUS, and Dr S.P. Dinesh-Kumar (Yale University) for the pTRV1 and pTRV2 vectors. We also would like to thank Dr Zonghua Wang (Key Laboratory of Bio-pesticide and Chemistry Biology, Ministry of Education, Fujian Agricultural and Forestry University, China) for providing the Co-IP plasmids (pEarleyGate201).
Glossary
Abbreviations:
- dpi
days post-inoculation;
- hpi
hours post-inoculation
- HTHH
high temperature–high humidity
- RSI
Ralstonia solanacearum inoculation
- TRV
Tobacco rattle virus
- VIGS
virus-induced gene silencing.
References
- Alves MS, Dadalto SP, Goncalves AB, De Souza GB, Barros VA, Fietto LG. 2013. Plant bZIP transcription factors responsive to pathogens: a review. International Journal of Molecular Sciences 14, 7815–7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura G, Ozawa R, Nishioka T, Boland W, Koch T, Kuhnemann F, Takabayashi J. 2002. Herbivore-induced volatiles induce the emission of ethylene in neighboring lima bean plants. The Plant Journal 29, 87–98. [DOI] [PubMed] [Google Scholar]
- Atkinson NJ, Urwin PE. 2012. The interaction of plant biotic and abiotic stresses: from genes to the field. Journal of Experimental Botany 63, 3523–3543. [DOI] [PubMed] [Google Scholar]
- Baek JH, Park JA, Kim JM, Oh JM, Park SM, Kim DH. 2014. Functional analysis of a tannic-acid-inducible and hypoviral-regulated small heat-shock protein Hsp24 from the chestnut blight fungus Cryphonectria parasitica. Molecular Plant-Microbe Interactions 27, 56–65. [DOI] [PubMed] [Google Scholar]
- Baena-Gonzalez E, Rolland F, Thevelein JM, Sheen J. 2007. A central integrator of transcription networks in plant stress and energy signalling. Nature 448, 938–942. [DOI] [PubMed] [Google Scholar]
- Baena-Gonzalez E, Sheen J. 2008. Convergent energy and stress signaling. Trends in Plant Science 13, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch M, Gobbato E, Bednarek P, Debey S, Schultze JL, Bautor J, Parker JE. 2006. Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. The Plant Cell 18, 1038–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulikas T. 1994. Putative nuclear localization signals (NLS) in protein transcription factors. Journal of Cellular Biochemistry 55, 32–58. [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Buscaill P, Rivas S. 2014. Transcriptional control of plant defence responses. Current Opinion in Plant Biology 20, 35–46. [DOI] [PubMed] [Google Scholar]
- Caarls L, Pieterse CM, Van Wees SC. 2015. How salicylic acid takes transcriptional control over jasmonic acid signaling. Frontiers in Plant Science 6, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Yang S, Yan Y, et al. 2015. CaWRKY6 transcriptionally activates CaWRKY40, regulates Ralstonia solanacearum resistance, and confers high-temperature and high-humidity tolerance in pepper. Journal of Experimental Botany 66, 3163–3174. [DOI] [PubMed] [Google Scholar]
- Cao F, Chen F, Sun H, Zhang G, Chen ZH, Wu F. 2014. Genome-wide transcriptome and functional analysis of two contrasting genotypes reveals key genes for cadmium tolerance in barley. BMC Genomics 15, 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Song Y, Li S, Zhang L, Zou C, Yu D. 2012. The role of WRKY transcription factors in plant abiotic stresses. Biochimica et Biophysica Acta 1819, 120–128. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang L, Li D, Wang F, Yu D. 2013. WRKY8 transcription factor functions in the TMV-cg defense response by mediating both abscisic acid and ethylene signaling in Arabidopsis. Proceedings of the National Academy of Sciences, USA 110, E1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Li H, Deng Y, Xiao J, Li X, Wang S. 2015. The WRKY45-2–WRKY13–WRKY42 transcriptional regulatory cascade is required for rice resistance to fungal pathogen. Plant Physiology 167, 1087–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Hwang BK. 2011. Proteomics and functional analyses of pepper abscisic acid-responsive 1 (ABR1), which is involved in cell death and defense signaling. The Plant Cell 23, 823–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Hwang IS, Hwang BK. 2012. Requirement of the cytosolic interaction between PATHOGENESIS-RELATED PROTEIN10 and LEUCINE-RICH REPEAT PROTEIN1 for cell death and defense signaling in pepper. The Plant Cell 24, 1675–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HW, Lee BG, Kim NH, Park Y, Lim CW, Song HK, Hwang BK. 2008. A role for a menthone reductase in resistance against microbial pathogens in plants. Plant Physiology 148, 383–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang F, Wang Y, She J, et al. 2014. Overexpression of CaWRKY27, a subgroup IIe WRKY transcription factor of Capsicum annuum, positively regulates tobacco resistance to Ralstonia solanacearum infection. Physiologia Plantarum 150, 397–411. [DOI] [PubMed] [Google Scholar]
- Dang FF, Wang YN, Yu L, et al. 2013. CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection. Plant, Cell and Environment 36, 757–774. [DOI] [PubMed] [Google Scholar]
- Despres C, DeLong C, Glaze S, Liu E, Fobert PR. 2000. The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. The Plant Cell 12, 279–290. [PMC free article] [PubMed] [Google Scholar]
- Du Z, Chen A, Chen W, Liao Q, Zhang H, Bao Y, Roossinck MJ, Carr JP. 2014. Nuclear–cytoplasmic partitioning of cucumber mosaic virus protein 2b determines the balance between its roles as a virulence determinant and an RNA-silencing suppressor. Journal of Virology 88, 5228–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulgem T, Rushton PJ, Robatzek S, Somssich IE. 2000. The WRKY superfamily of plant transcription factors. Trends in Plant Science 5, 199–206. [DOI] [PubMed] [Google Scholar]
- Eulgem T, Somssich IE. 2007. Networks of WRKY transcription factors in defense signaling. Current Opinion in Plant Biology 10, 366–371. [DOI] [PubMed] [Google Scholar]
- Figueroa P, Browse J. 2015. Male sterility in Arabidopsis induced by overexpression of a MYC5–SRDX chimeric repressor. The Plant Journal 81, 849–860. [DOI] [PubMed] [Google Scholar]
- Foster R, Izawa T, Chua NH. 1994. Plant bZIP proteins gather at ACGT elements. FASEB Journal 8, 192–200. [DOI] [PubMed] [Google Scholar]
- Gao QM, Venugopal S, Navarre D, Kachroo A. 2011. Low oleic acid-derived repression of jasmonic acid-inducible defense responses requires the WRKY50 and WRKY51 proteins. Plant Physiology 155, 464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald W, De Smet I, De Rybel B, Robert HS, van de Cotte B, Willemsen V, Gheysen G, Weijers D, Friml J, Beeckman T. 2013. Tightly controlled WRKY23 expression mediates Arabidopsis embryo development. EMBO Reports 14, 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinekamp T, Kuhlmann M, Lenk A, Strathmann A, Droge-Laser W. 2002. The tobacco bZIP transcription factor BZI-1 binds to G-box elements in the promoters of phenylpropanoid pathway genes in vitro, but it is not involved in their regulation in vivo. Molecular Genetics and Genomics 267, 16–26. [DOI] [PubMed] [Google Scholar]
- Hiratsu K, Matsui K, Koyama T, Ohme-Takagi M. 2003. Dominant repression of target genes by chimeric repressors that include the EAR motif, a repression domain, in Arabidopsis. The Plant Journal 34, 733–739. [DOI] [PubMed] [Google Scholar]
- Hurst HC. 1995. Transcription factors 1: bZIP proteins. Protein Profile 2, 101–168. [PubMed] [Google Scholar]
- Hwang EW, Kim KA, Park SC, Jeong MJ, Byun MO, Kwon HB. 2005. Expression profiles of hot pepper (Capsicum annum) genes under cold stress conditions. Journal of Biosciences 30, 657–667. [DOI] [PubMed] [Google Scholar]
- Izawa T, Foster R, Chua NH. 1993. Plant bZIP protein DNA binding specificity. Journal of Molecular Biology 230, 1131–1144. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liang G, Yu D. 2012. Activated expression of WRKY57 confers drought tolerance in Arabidopsis. Molecular Plant 5, 1375–1388. [DOI] [PubMed] [Google Scholar]
- Kim DS, Hwang BK. 2012. The pepper MLO gene, CaMLO2, is involved in the susceptibility cell-death response and bacterial and oomycete proliferation. The Plant Journal 72, 843–855. [DOI] [PubMed] [Google Scholar]
- Kim HS, Delaney TP. 2002. Over-expression of TGA5, which encodes a bZIP transcription factor that interacts with NIM1/NPR1, confers SAR-independent resistance in Arabidopsis thaliana to Peronospora parasitica. The Plant Journal 32, 151–163. [DOI] [PubMed] [Google Scholar]
- Knoth C, Ringler J, Dangl JL, Eulgem T. 2007. Arabidopsis WRKY70 is required for full RPP4-mediated disease resistance and basal defense against Hyaloperonospora parasitica. Molecular Plant-Microbe Interactions 20, 120–128. [DOI] [PubMed] [Google Scholar]
- Lakra N, Nutan KK, Das P, Anwar K, Singla-Pareek SL, Pareek A. 2015. A nuclear-localized histone-gene binding protein from rice (OsHBP1b) functions in salinity and drought stress tolerance by maintaining chlorophyll content and improving the antioxidant machinery. Journal of Plant Physiology 176, 36–46. [DOI] [PubMed] [Google Scholar]
- Lee SC, Choi HW, Hwang IS, Choi DS, Hwang BK. 2006. Functional roles of the pepper pathogen-induced bZIP transcription factor, CAbZIP1, in enhanced resistance to pathogen infection and environmental stresses. Planta 224, 1209–1225. [DOI] [PubMed] [Google Scholar]
- Li S, Fu Q, Chen L, Huang W, Yu D. 2011. Arabidopsis thaliana WRKY25, WRKY26, and WRKY33 coordinate induction of plant thermotolerance. Planta 233, 1237–1252. [DOI] [PubMed] [Google Scholar]
- Li Y, Humbert S, Howell SH. 2012. ZmbZIP60 mRNA is spliced in maize in response to ER stress. BMC Research Notes 5, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemose S, O’Shea C, Jensen MK, Skriver K. 2013. Structure, function and networks of transcription factors involved in abiotic stress responses. International Journal of Molecular Sciences 14, 5842–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Wu Y, Wang X. 2012. bZIP transcription factor OsbZIP52/RISBZ5: a potential negative regulator of cold and drought stress response in rice. Planta 235, 1157–1169. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, Qiu AL, Shi LP, et al. 2015. SRC2-1 is required in PcINF1-induced pepper immunity by acting as an interacting partner of PcINF1. Journal of Experimental Botany 66, 3683–3698. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Llorca CM, Potschin M, Zentgraf U. 2014. bZIPs and WRKYs: two large transcription factor families executing two different functional strategies. Frontiers in Plant Science 5, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Berges MS, Rispail N, Prados-Rosales RC, Di Pietro A. 2010. A nitrogen response pathway regulates virulence functions in Fusarium oxysporum via the protein kinase TOR and the bZIP protein MeaB. The Plant Cell 22, 2459–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Bai X, Sun X, et al. 2013. Expression of wild soybean WRKY20 in Arabidopsis enhances drought tolerance and regulates ABA signalling. Journal of Experimental Botany 64, 2155–2169. [DOI] [PubMed] [Google Scholar]
- Mair A, Pedrotti L, Wurzinger B, et al. 2015. SnRK1-triggered switch of bZIP63 dimerization mediates the low-energy response in plants. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matiolli CC, Tomaz JP, Duarte GT, et al. 2011. The Arabidopsis bZIP gene AtbZIP63 is a sensitive integrator of transient abscisic acid and glucose signals. Plant Physiology 157, 692–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miersch O, Wasternack C. 2000. Octadecanoid and jasmonate signaling in tomato (Lycopersicon esculentum Mill.) leaves: endogenous jasmonates do not induce jasmonate biosynthesis. Biological Chemistry 381, 715–722. [DOI] [PubMed] [Google Scholar]
- Niu CF, Wei W, Zhou QY, et al. 2012. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant, Cell and Environment 35, 1156–1170. [DOI] [PubMed] [Google Scholar]
- Orellana S, Yanez M, Espinoza A, Verdugo I, Gonzalez E, Ruiz-Lara S, Casaretto JA. 2010. The transcription factor SlAREB1 confers drought, salt stress tolerance and regulates biotic and abiotic stress-related genes in tomato. Plant, Cell and Environment 33, 2191–2208. [DOI] [PubMed] [Google Scholar]
- Pivovarova AV, Mikhailova VV, Chernik IS, Chebotareva NA, Levitsky DI, Gusev NB. 2005. Effects of small heat shock proteins on the thermal denaturation and aggregation of F-actin. Biochemical and Biophysical Research Communications 331, 1548–1553. [DOI] [PubMed] [Google Scholar]
- Pontier D, Miao ZH, Lam E. 2001. Trans-dominant suppression of plant TGA factors reveals their negative and positive roles in plant defense responses. The Plant Journal 27, 529–538. [DOI] [PubMed] [Google Scholar]
- Prasch CM, Sonnewald U. 2013. Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiology 162, 1849–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabara RC, Tripathi P, Rushton PJ. 2014. The potential of transcription factor-based genetic engineering in improving crop tolerance to drought. Omics 18, 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S, Barah P, Suarez-Rodriguez MC, Bressendorff S, Friis P, Costantino P, Bones AM, Nielsen HB, Mundy J. 2013. Transcriptome responses to combinations of stresses in Arabidopsis. Plant Physiology 161, 1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton DL, Tripathi P, Rabara RC, et al. 2012. WRKY transcription factors: key components in abscisic acid signalling. Plant Biotechnology Journal 10, 2–11. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Somssich IE, Ringler P, Shen QJ. 2010. WRKY transcription factors. Trends in Plant Science 15, 247–258. [DOI] [PubMed] [Google Scholar]
- Rymen B, Sugimoto K. 2012. Tuning growth to the environmental demands. Current Opinion in Plant Biology 15, 683–690. [DOI] [PubMed] [Google Scholar]
- Schweiger R, Heise AM, Persicke M, Muller C. 2014. Interactions between the jasmonic and salicylic acid pathway modulate the plant metabolome and affect herbivores of different feeding types. Plant, Cell and Environment 37, 1574–1585. [DOI] [PubMed] [Google Scholar]
- Schweizer F, Bodenhausen N, Lassueur S, Masclaux FG, Reymond P. 2013. Differential contribution of transcription factors to Arabidopsis thaliana defense against Spodoptera littoralis. Frontiers in Plant Science 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewelam N, Oshima Y, Mitsuda N, Ohme-Takagi M. 2014. A step towards understanding plant responses to multiple environmental stresses: a genome-wide study. Plant, Cell and Environment 37, 2024–2035. [DOI] [PubMed] [Google Scholar]
- Shearer HL, Cheng YT, Wang L, Liu J, Boyle P, Despres C, Zhang Y, Li X, Fobert PR. 2012. Arabidopsis clade I TGA transcription factors regulate plant defenses in an NPR1-independent fashion. Molecular Plant-Microbe Interactions 25, 1459–1468. [DOI] [PubMed] [Google Scholar]
- Shimono M, Sugano S, Nakayama A, Jiang CJ, Ono K, Toki S, Takatsuji H. 2007. Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. The Plant Cell 19, 2064–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibbe M, Qu N, Galis I, Baldwin IT. 2008. Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. The Plant Cell 20, 1984–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Koornneef A, Claessens SM, et al. 2003. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. The Plant Cell 15, 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R, Deng Y, Howell SH. 2014. Stress sensing in plants by an ER stress sensor/transducer, bZIP28. Frontiers in Plant Science 5, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Liu M, Zhang W, Yang W, Bei X, Ma H, Qiao F, Qi X. 2015. Bean metal-responsive element-binding transcription factor confers cadmium resistance in tobacco. Plant Physiology 167, 1136–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S. 2013. Post-embryonic induction of ATML1-SRDX alters the morphology of seedlings. PLoS One 8, e79312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulker B, Somssich IE. 2004. WRKY transcription factors: from DNA binding towards biological function. Current Opinion in Plant Biology 7, 491–498. [DOI] [PubMed] [Google Scholar]
- Ustun S, Bartetzko V, Bornke F. 2013. The Xanthomonas campestris type III effector XopJ targets the host cell proteasome to suppress salicylic-acid mediated plant defence. PLoS Pathogens 9, e1003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KL, Guo ZJ, Wang HH, Li J. 2005. The WRKY family of transcription factors in rice and Arabidopsis and their origins. DNA Research 12, 9–26. [DOI] [PubMed] [Google Scholar]
- Xiong L, Lee H, Ishitani M, Zhu JK. 2002. Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. Journal of Biological Chemistry 277, 8588–8596. [DOI] [PubMed] [Google Scholar]
- Yan J, Guan L, Sun Y, Zhu Y, Liu L, Lu R, Jiang M, Tan M, Zhang A. 2015. Calcium and ZmCCaMK are involved in brassinosteroid-induced antioxidant defense in maize leaves. Plant and Cell Physiology 56, 883–896. [DOI] [PubMed] [Google Scholar]
- Yu F, Huaxia Y, Lu W, Wu C, Cao X, Guo X. 2012. GhWRKY15, a member of the WRKY transcription factor family identified from cotton (Gossypium hirsutum L.), is involved in disease resistance and plant development. BMC Plant Biology 12, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander M, Chen S, Imkampe J, Thurow C, Gatz C. 2012. Repression of the Arabidopsis thaliana jasmonic acid/ethylene-induced defense pathway by TGA-interacting glutaredoxins depends on their C-terminal ALWL motif. Molecular Plant 5, 831–840. [DOI] [PubMed] [Google Scholar]
- Zander M, La Camera S, Lamotte O, Metraux JP, Gatz C. 2010. Arabidopsis thaliana class-II TGA transcription factors are essential activators of jasmonic acid/ethylene-induced defense responses. The Plant Journal 61, 200–210. [DOI] [PubMed] [Google Scholar]
- Zhang J, Peng Y, Guo Z. 2008. Constitutive expression of pathogen-inducible OsWRKY31 enhances disease resistance and affects root growth and auxin response in transgenic rice plants. Cell Research 18, 508–521. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang L, Xia C, Zhao G, Liu J, Jia J, Kong X. 2015. A novel wheat bZIP transcription factor, TabZIP60, confers multiple abiotic stress tolerances in transgenic Arabidopsis. Physiologia Plantarum 153, 538–554. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Kuang H, Chen C, Yan J, Do-Umehara HC, Liu XY, Dada L, Ridge KM, Chandel NS, Liu J. 2015. Corrigendum: The kinase Jnk2 promotes stress-induced mitophagy by targeting the small mitochondrial form of the tumor suppressor ARF for degradation. Nature Immunology 16, 785. [DOI] [PubMed] [Google Scholar]
- Zhong X, Xi L, Lian Q, Luo X, Wu Z, Seng S, Yuan X, Yi M. 2015. The NPR1 homolog GhNPR1 plays an important role in the defense response of Gladiolus hybridus. Plant Cell Reports 34, 1063–1074. [DOI] [PubMed] [Google Scholar]
- Zhu YN, Shi DQ, Ruan MB, Zhang LL, Meng ZH, Liu J, Yang WC. 2013. Transcriptome analysis reveals crosstalk of responsive genes to multiple abiotic stresses in cotton (Gossypium hirsutum L.). PLoS One 8, e80218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.