Abstract
Neuroactive steroids are efficacious modulators of γ-aminobutyric acid type A receptor (GABAA) receptor function. The effects of steroids on the GABAA receptor are typically determined by comparing steady-state single-channel open probability or macroscopic peak responses elicited by GABA in the absence and presence of a steroid. Due to differences in activation conditions (exposure duration, concentration of agonist), it is not obvious whether modulation measured using typical experimental protocols can be used to accurately predict the effect of a modulator on native receptors under physiologic conditions. In the present study, we examined the effects of 14 neuroactive steroids and analogs on the properties of spontaneous inhibitory postsynaptic currents (sIPSCs) in cultured rat hippocampal neurons. The goal was to determine whether the magnitude of modulation of the decay time course of sIPSCs correlates with the extent of modulation and kinetic properties of potentiation as determined in previous single-channel studies. The steroids were selected to cover a wide range of efficacy on heterologously expressed rat α1β2γ2L GABAA receptors, ranging from essentially inert to highly efficacious (strong potentiators of single-channel and macroscopic peak responses). The data indicate a strong correlation between prolongation of the decay time course of sIPSCs and potentiation of single-channel open probability. Furthermore, changes in intracluster closed time distributions were the single best predictor of prolongation of sIPSCs. We infer that the information obtained in steady-state single-channel recordings can be used to forecast modulation of synaptic currents.
Introduction
The γ-aminobutyric acid type A receptor (GABAA) receptor is an inhibitory ionotropic transmitter-gated ion channel whose activation in mature neurons leads to hyperpolarization of the cell or dampening of the effects of excitatory channels. Drugs capable of enhancing GABAA receptor function have possible applications as anxiolytics, anticonvulsants, and sedatives (Rudolph and Mohler, 2006; Franks, 2008).
Many neuroactive steroids and analogs are potentiators of the mammalian GABAA receptor. In electrophysiologic experiments, potentiation is observed as augmentation of the whole-cell peak response when a steroid is coapplied with a low concentration of transmitter (Callachan et al., 1987; Harrison et al., 1987a). Studies employing a single-channel patch clamp have revealed that the increase in macroscopic current response is mediated by up to three specific changes in the open and closed time distributions (Akk et al., 2004). Strong potentiators, such as the endogenous steroid (3α,5α)-3-hydroxypregnan-20-one (3α5αP) and the synthetic anesthetic steroid (3α,5α)-3-hydroxypregnane-11,20-dione (3α5αP11O), act by decreasing the prevalence of the long-lived closed state and increasing both the prevalence and mean duration of the long-lived open state (Akk et al., 2005). Weak potentiating steroids, such as (3α,5β)-3-hydroxyandrostan-17-one (3α5β17O), act through changes in a single kinetic component, such as an increase in the prevalence of dwells in the long-lived open state (Li et al., 2007a). There is a good correlation between the magnitude of potentiation of whole-cell peak response and the increase in single-channel open probability (Akk et al., 2010).
Experimental conditions in macroscopic and single-channel studies do not, however, reflect the physiologic conditions in brain. Native GABAA receptors are continuously bathed in a mixture of steroids of endogenous origin while clearance of exogenously-applied anesthetic steroids occurs slowly, with a timescale of minutes or hours (Ram et al., 2001; Visser et al., 2002). In contrast, drug applications in macroscopic measurements are of finite length, typically lasting a few seconds followed by rapid washout. We have previously found that there is a correlation between the reciprocal of application length and EC50 of potentiation (Li et al., 2007b), likely due to initial redistribution of the steroid among the lipid fractions in the cell, that conceals the true extent of drug effect in short applications. Single-channel recordings in the cell-attached configuration are long-lasting, but redistribution of steroid from the patch to the rest of cell, which is acting as a sink, reduces steroid concentration in the patch thereby affecting potency estimates (Li et al., 2007b).
Another shortcoming is the fact that single-channel measurements are obtained under steady-state conditions, but synaptic activity occurs far from the steady state. Native synaptic-type GABAA receptors are activated by brief (likely ≤1 millisecond) pulses of a saturating concentration of transmitter. The distribution of kinetic states occupied during such brief activation may differ from that during prolonged exposure to submaximal agonist concentration in a single-channel recording. Spatial spread and variable rebinding of transmitter are additional potential complications of physiologic, synaptic activation that are not evident in steady-state channel recordings.
The differences in exposure conditions cast some doubt on our ability to accurately predict effects of modulators on native GABAA receptors under physiologic conditions from the data generated using typical electrophysiologic experimental protocols. In the present study, we have examined the effects of several neuroactive steroids and analogs on the properties of spontaneous inhibitory postsynaptic currents (sIPSCs) in cultured rat hippocampal neurons. The goal was to determine whether the extent of modulation observed in synaptic responses correlates with the kinetic properties of potentiation determined in single-channel studies. On the whole, we infer that the information obtained from steady-state single-channel recordings can be used to forecast modulation of synaptic currents.
Materials and Methods
Cultured rat hippocampal neurons were prepared as described previously elsewhere (Emnett et al., 2015). Rat pups (postnatal day 1–3) were anesthetized with isoflurane, and the hippocampus was dissected and cut into slices (500 µm thickness). The slices were digested with 1 mg/ml papain in oxygenated Leibovitz L-15 medium (Invitrogen, Gaithersburg, MD) followed by mechanical trituration in modified Eagle’s medium (Invitrogen) containing 5% horse serum, 5% fetal calf serum, 17 mM d-glucose, 400 μM glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. Cells were seeded in modified Eagle’s medium at a density of ∼650 cells mm−2 onto 25-mm cover glasses coated with 5 mg/ml of collagen or 0.1 mg/ml of poly-d-lysine with 1 mg/ml laminin. Cultures were incubated at 37°C in a humidified chamber with 5% CO2/95% air. Cytosine arabinoside (6.7 μM) was added 3 to 4 days after plating to inhibit glial proliferation, followed by replacement of half of the culture medium with Neurobasal medium (Life Technologies, Carlsbad, CA) plus B27 supplement (Life Technologies) the following day. The animal procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The protocol was approved by the Animal Studies Committee of Washington University in St. Louis.
We recorded sIPSCs from neurons cultured for 10 to 14 days. For recordings, coverslips with cells were transferred to a new dish with extracellular solution containing (in mM): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 d-glucose, and 10 HEPES (pH 7.4 with NaOH). To block glutamate receptors, 5 µM 6-cyano-7-nitroquinoxalone-2,3-dione (CNQX) and 25 µM dl-2-amino-5-phosphono-valeric acid (DL-APV) were added to bath. Steroids and analogs were added to the bath at the indicated concentration at least 10 minutes before recording to reach full equilibration with the drug (Zimmerman et al., 1994). Each coverslip with neurons was exposed to only one kind of drug due to difficulties associated with complete washout of these lipophilic compounds from the cells. The pipette solution contained (in mM): 140 CsCl, 4 NaCl, 4 MgCl2, 0.5 CaCl2, 5 EGTA, 10 HEPES (pH 7.4 with CsOH). Neurons were identified visually and clamped at −70 mV. All experiments were done at room temperature.
Currents were amplified with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA), low-pass filtered at 1 kHz, and digitized with a Digidata 1322A interface (Molecular Devices) at 5 kHz. The detection and analysis of synaptic currents were conducted using pClamp 10 software (Molecular Devices). First, a template was created by averaging one to three random events under a drug condition. This template was used to identify all the events under that drug condition. The template search parameters were set to detect negative-going peaks of variable amplitude with a template match threshold set at the default value 4, which provides balance between missed events and false positives. The program automatically detected the spontaneous firing events that were then visually inspected and manually accepted or rejected. The overlay plot of all the events thus selected was then saved in a separate file. The events from each recording were averaged, and subsequent analysis was conducted on the averaged traces. Because decay times of the events varied with drug conditions, different templates representing each drug condition had to be created. Decay time courses were fitted to sums of two exponentials. The data are presented in weighted time constants, calculated as τw = A1τ1 + A2τ2, where τ1 and τ2 are the time constants of the two components and A1 and A2 are the proportions of the two components.
Concentration–response curves were fitted for pooled data with the following equation:
 |
(1) |
where EC50 is the concentration of steroid producing a half-maximal effect, nH describes the slope of relationship, and Ymin and Ymax are the low and high concentration asymptotes, respectively. Fitting was conducted using the NFIT software (University of Texas, Medical Branch at Galveston, Galveston, TX).
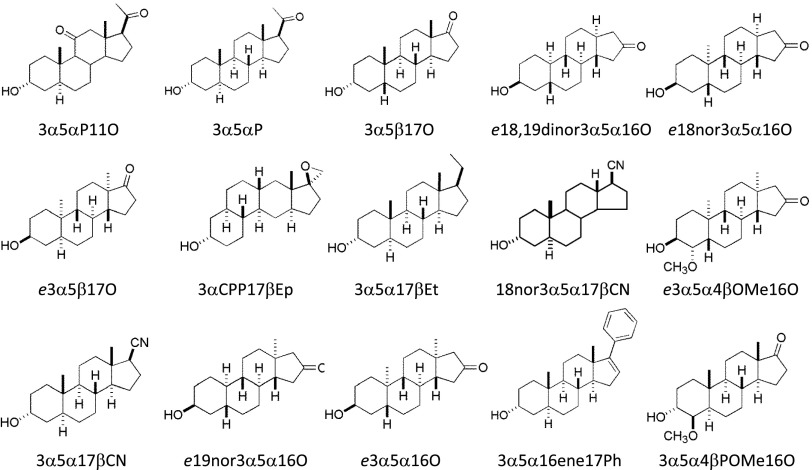
Inorganic salts used in the buffers CNQX, and DL-APV were bought from Sigma-Aldrich (St. Louis, MO). Steroids were bought from Sigma-Aldrich or Steraloids (Newport, RI), or synthesized locally as described previously elsewhere (Hu et al., 1993; Scaglione et al., 2008; Li et al., 2009; Qian et al., 2014). Structures of steroids used are given in Fig. 1. Stock solutions of steroids and analogs were made in dimethylsulfoxide (DMSO) at a 10–20 mM concentration. Stock solutions were kept at room temperature and further diluted as needed on the day of the experiment. The highest final concentration of DMSO was 0.1% (v/v). This concentration of DMSO is without effect on currents from recombinant α1β2γ2L GABAA receptors and GABAA receptor-mediated synaptic currents (Li et al., 2007a; Mitchell et al., 2007).
Fig. 1.
Structures of steroids and analogs tested in the study. Enantiomeric steroids (e18,19dinor3α5α16O, e18nor3α5α16O, e3α5β17O, e3α5α4βOMe16O, e19nor3α5α16O, and e3α5α16) are inverted relative to natural steroids when bound to GABAA receptors. For models of the modes of binding natural steroids and their enantiomers, see (Krishnan et al., 2012; Qian et al., 2014).
Open probability (Po) of single-channel activity elicited by 50 µM GABA in the absence or presence of steroids was calculated from the previously published individual intracluster open and closed time distributions using the following equation:
| (2) |
where OTi and frOTi are the mean duration and fraction of the individual open time components and CTi and frCTi are the mean duration and fraction of the individual closed time components.
Analysis of single-channel currents and simulation of synaptic events was conducted using the QuB Suite (www.qub.buffalo.edu). Previous single-channel data (Li et al., 2009; Qian et al., 2014) were first reanalyzed using Model 1 (Lema and Auerbach, 2006):
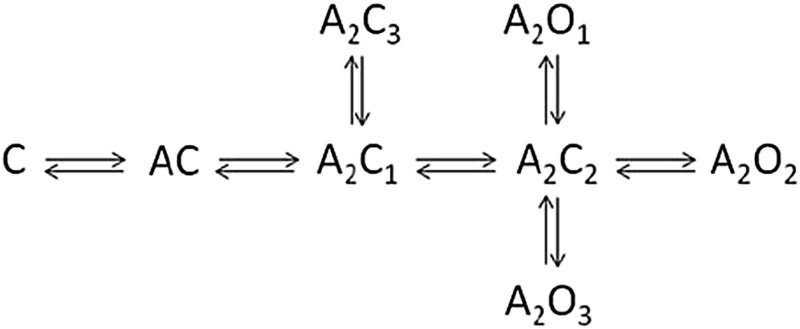
This model predicts three open states, differing in their mean duration, associated with fully liganded receptors. The model and the fitted rate constants were then used to simulate synaptic events. Exposure to a vesicle of GABA was mimicked by an agonist profile with a duration of 1 millisecond and a concentration of 10 mM. The starting state was the unliganded, closed state (C). The decay time courses of resulting macroscopic currents were fitted to a single-exponential, using Origin (OriginLab, Northampton, MA).
Results
Effects of Steroids on the Decay Time Course of sIPSCs.
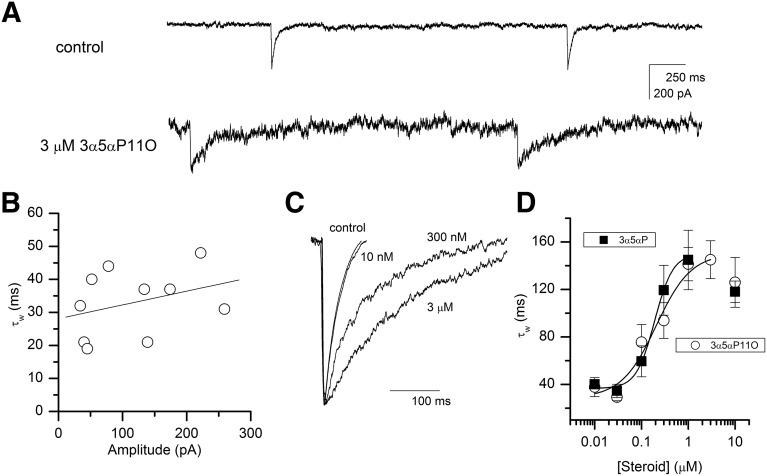
Cells cultured for 10 to 14 days exhibited spontaneous IPSCs in the presence of glutamate receptor blockers CNQX and DL-APV (Fig. 2A). In 12 cells, the mean frequency of events was 0.72 ± 0.69 Hz. The sIPSCs were sensitive to GABAA receptor blockers and eliminated during bath application of 10 µM gabazine (not shown). The decay time course of averaged sIPSCs was fitted to a sum of two exponentials, yielding the mean weighted time constant (τw) of 34 ± 3 milliseconds (mean ± S.E.M.; 12 cells; Fig. 2B). This is similar to several previous estimates for decay times of miniature and spontaneous IPSCs from hippocampal neurons (e.g., Poisbeau et al., 1997; Zorumski et al., 1998; Banks and Pearce, 1999; Park et al., 2011). The amplitudes of sIPSCs varied considerably from cell to cell. There was, however, no correlation between mean amplitude and decay time of sIPSC (Fig. 2B).
Fig. 2.
Properties of sIPSCs. (A) Sample traces showing spontaneous activity under control conditions and in the presence of 3 µM 3α5αP11O. Exposure to the steroid results in prolongation of decay time course and an increase in noise. (B) A relationship between the mean weighted decay time constant and the mean amplitude of sIPSCs under control conditions. An increase in the mean amplitude is not associated with an increase in decay time constant (R2 = 0.33, P = 0.35). (C) Decay time course of sIPSCs under control conditions and in the presence of 10 nM, 300 nM, or 3 µM 3α5αP11O. The traces are averaged from 35 to 297 events per condition and have their amplitudes normalized for better illustration of the effect of steroid on decay time course. (D) Dose-response relationship for steroid-induced prolongation of the weighted decay time constant. The curves were fitted to eq. 1 (Materials and Methods). For 3α5αP, Ymin = 36 ± 4 milliseconds, Ymax = 150 ± 7 milliseconds, EC50 = 0.19 ± 0.02 µM, nH = 2.2 ± 0.4. For 3α5αP11O, Ymin = 29 ± 5 milliseconds, Ymax = 150 ± 22 milliseconds, EC50 = 0.21 ± 0.11 µM, nH = 1.2 ± 0.8. Exposure to 10 µM 3α5αP or 3α5αP11O resulted in a small reduction of the effect. These data points were not included in the fit.
Addition of potentiating steroid to the extracellular medium led to an increase in the decay time constant of sIPSCs (Fig. 2A lower trace and Fig. 2C). In the presence of 3 µM 3α5αP11O, the τw was 145 ± 16 milliseconds (four cells). The increase in decay time was not accompanied by changes in mean amplitude (149 ± 34 pA versus 175 ± 62 pA under control conditions). Concentration–response measurements conducted in the presence of 10 nM to 3 µM 3α5αP11O yielded an EC50 of 0.21 ± 0.11 µM and a Hill coefficient of 1.2 ± 0.8 (data combined from three to five cells at each concentration; Fig. 2D).
The fitted low concentration asymptote (29 milliseconds) was similar to the decay time constant under control conditions. Addition of the endogenous steroid 3α5αP to the extracellular medium also resulted in prolonged sIPSCs. In the presence of 1 µM 3α5αP the τw was 145 ± 25 milliseconds (3 cells). The EC50 for prolongation of decay time constant was 0.19 ± 0.02 µM. The Hill coefficent was 2.2 ± 0.4, and the low concentration asymptote was at 36 ± 4 milliseconds (Fig. 2D). The maximal fitted values for τw in the presence of 3α5αP11O or 3α5αP were indistinguishable (150 ± 22 and 150 ± 7 milliseconds, respectively).
Single-channel experiments have shown that potentiating steroids act on the synaptic-type α1β2γ2L GABAA receptor via changes in gating properties that manifest as one or more of the following: an increase in the mean duration and prevalence of long openings (duration and % OT3) and a decrease in the prevalence of the closed state associated with channel closing (% CT3). The largest effect on open probability or macroscopic peak response is observed with steroids possessing all three effects (Akk et al., 2010). The steroids 3α5αP and 3α5αP11O modify all three parameters (Akk et al., 2005; unpublished data).
To probe the relationship between the single-channel mechanism of potentiation and prolongation of τw of sIPSCs, we measured spontaneous synaptic activity in the presence of several previously characterized steroids and analogs. Each compound was added to the extracellular solution at a concentration (3 to 10 µM) that was known to produce a saturating response in single-channel or whole-cell peak response measurements.
As expected, steroids that only affect open time distributions in single-channel recordings and have a relatively small effect on macroscopic peak response, had a tendency toward smaller effect on τw. In the presence of 10 µM 3α5β17O or (3β,5β,8α,9β,10α,13α,14β)-3-hydroxygonan-16-one [e18,19dinor3α5α16O], whose sole effect in single-channel recordings is to increase the relative frequency of long openings (Li et al., 2007a; Qian et al., 2014), the τw was 94 ± 2 milliseconds (5 cells) or 53 ± 10 milliseconds (four cells), respectively.
We examined the effects of five steroid analogs, (3β,5β,8α,9β,10α,13α,14β)-18-nor-3-hydroxyandrostan-16-one [e18nor3α5α16O], (3β,5α,8α,9β,10α,13α,14β)-3-hydroxyandrostan-17-one [e3α5β17O], (2ʹS,3S,4aR,6aR,7aS,10aS,11aR,11bR)-hexadecahydro-7a-methyl-spiro[8H-cyclopenta[b]phenanthrene-8,2ʹ-oxiran]-3-ol [3αCPP17βEp], (3α,5α)-pregnan-3-ol [3α5α17βEt], and (3α,5α,17β)-18-nor-3-hydroxyandrostane-17-carbonitrile [18nor3α5α17βCN], that were known to increase both the duration and prevalence of long openings, but not affect intracluster closed times (Li et al., 2007a, 2009; Scaglione et al., 2008; Qian et al., 2014). Exposure to these compounds ranged from no effect on the decay time constant in the presence of e18nor3α5α16O (τw = 36 ± 2 milliseconds, four cells) to a more than 4-fold prolongation in the presence of e3α5β17O (τw = 143 ± 15 milliseconds, five cells).
In addition to 3α5αP11O and 3α5αP discussed previously, we measured the effects of two additional steroids ((3β,4α,5β,8α,9β,10α,13α,14β)-3-hydroxy-4-methoxy-androstan-16-one [e3α5α4βOMe16O] and (3α,5α,17β)-18-nor-3-hydroxyandrostane-17-carbonitrile [3α5α17βCN]) that modify both open and closed times in single-channel recordings producing a strong effect on the peak response (Akk et al., 2004; Qian et al., 2014).
Both compounds also strongly increased the decay time constant of sIPSCs. The τw was 243 ± 24 milliseconds (4 cells) in the presence of e3α5α4βOMe16O and 132 ± 12 milliseconds (4 cells) in the presence of 3α5α17βCN. The steroid (3β,5β,8α,9β,10α,13α,14β)-3-hydroxyestran-16-one [e19nor3α5α16O] that only affects closed time properties in single-channel recordings (Qian et al., 2014) was also an efficacious potentiator of the decay time course. In six cells, the decay time constant was 206 ± 36 milliseconds. We tested the effect of (3β,5β,8α,9β,10α,13α,14β)-3-hydroxyandrostan-16-one [e3α5α16O], that in single-channel recordings increases the fraction of long openings and decreases the fraction of long closed times (Qian et al., 2014). The τw was 241 ± 21 milliseconds (6 cells) in the presence of 1 µM e3α5α16O.
The steroid (3α5α)-17-phenylandrost-16-en-3-ol (3α5α16ene17Ph) increases the prevalence of OT3 without affecting its mean duration or the closed time distributions. In whole-cell recording, this steroid is essentially without effect on the peak response (Mennerick et al., 2004). Application of 3α5α16ene17Ph weakly increased the decay time of sIPSCs (61 ± 10 milliseconds; five cells). The data are summarized in Table 1.
TABLE 1.
Summary of the effects of steroids on decay time course
The table shows weighted time constants (mean ± S.E.M.) under control conditions (no steroid) and in the presence of saturating concentrations (1–10 μM) of fourteen steroids. The single-channel open probability (Po) of receptors activated by 50 μM GABA in the absence (control) and presence of steroids is provided for comparison. Po was calculated from pooled intracluster open and closed time data published previously (Akk et al., 2004, 2005; Li et al., 2007a, 2009; Scaglione et al., 2008; Qian et al., 2014).
| Steroid |
τw |
Po |
|---|---|---|
| ms | ||
| None (control) | 34 ± 3 | 0.41 |
| 3α5αP11O | 145 ± 16 | 0.93 |
| 3α5αP | 145 ± 25 | 0.84 |
| 3α5β17O | 94 ± 2 | 0.44 |
| e18,19dinor3α5α16O | 53 ± 10 | 0.46 |
| e18nor3α5α16O | 36 ± 2 | 0.49 |
| e3α5β17O | 143 ± 15 | 0.72 |
| 3αCPP17βEp | 86 ± 9 | 0.83 |
| 3α5α17βEt | 86 ± 11 | 0.77 |
| 18nor3α5α17βCN | 77 ± 16 | 0.82 |
| e3α5α4βOMe16O | 243 ± 24 | 0.86 |
| 3α5α17βCN | 132 ± 12 | 0.71 |
| e19nor3α5α16O | 206 ± 36 | 0.58 |
| e3α5α16O | 241 ± 21 | 0.84 |
| 3α5α16ene17Ph | 61 ± 10 | 0.50 |
As negative control, we measured the effect of (3α,4β,5α)-3-hydroxyandrostan-17-one (3α5α4βOMe16O) on the time course of sIPSCs. Prior macroscopic measurements on heterologously expressed α1β2γ2L receptors had shown that this steroid does not modulate the peak response (Qian et al., 2014). In four cells bathed in 1 µM 3α5α4βOMe16O the τw was 33 ± 4 milliseconds, not different from the value under control conditions (P > 0.8; t test).
Correlation of Effects on Decay of sIPSCs with Effects on Single-Channel Currents.
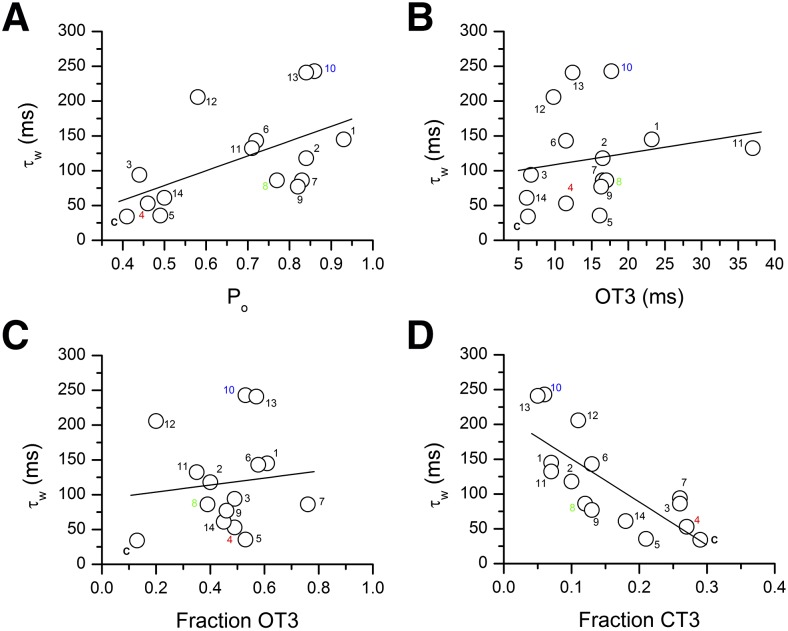
We next determined whether the magnitude of effects observed on decay time course of sIPSCs correlates with changes in single-channel open and closed time properties in the presence of steroid. For that, we calculated the open probability of the receptor, using eq. 2 (Materials and Methods) and previously determined open and closed time parameters (Akk et al., 2004, 2005; Li et al., 2007a; Scaglione et al., 2008; Qian et al., 2014). Results of the calculations and the linear regression fit are shown in Fig. 3A. The analysis indicates correlation between the increases in τw and open probability (R2 = 0.56, P = 0.029).
Fig. 3.
Correlation between steroid-induced prolongation of sIPSCs and its effect on α1β2γ2L GABAA receptor single-channel properties. (A) Steroid-induced increase in τw correlates with steroid-induced increase in open probability of single-channel clusters elicited by 50 µM GABA (R2 = 0.56, P = 0.029). Each symbol represents data for one condition (control or in the presence of one steroid or analog). C, control (no steroid); 1, 3α5αP11O; 2, 3α5αP; 3, 3α5β17O; 4, e18,19dinor3α5α16O; 5, e18nor3α5α16O; 6, e3α5β17O; 7, 3αCPP17βEp; 8, 3α5α17βEt; 9, 18nor3α5α17βCN; 10, e3α5α4βOMe16O; 11, 3α5α17βCN; 12, e19nor3α5α16O; 13, e3α5α16O; 14, 3α5α16ene17Ph. The structures of steroids are shown in Fig. 1. The data for steroid-effects on single-channel properties are from Akk et al. (2004, 2005); Li et al., (2007a, 2009); Scaglione et al., (2008); and Qian et al., (2014). The data for 3α5α4βOMe16O are not shown. This compound does not enhance the peak macroscopic response; however, its effects on single-channel properties have not been studied. Steroids e18,19dinor3α5α16O, 3α5α17βEt, and e3α5α4βOMe16O (numbering shown in red, green, and blue, respectively) were used for additional kinetic analysis and modeling summarized in Table 2 and Fig. 4. (B) Steroid-induced increase in τw does not correlate with steroid-induced increase in the mean duration of the longest-lived open time component, OT3 (R2 = 0.19, P = 0.50). Symbols are coded as in A. (C) Steroid-induced increase in τw does not correlate with steroid-induced increase in the fraction (relative frequency) of OT3 (R2 = 0.12, P = 0.68). Symbols are coded as in A. (D) Steroid-induced increase in τw correlates with steroid-induced decrease in the fraction of the longest-lived intracluster closed time component, CT3 (R2 = −0.76, P = 0.0001). Symbols are coded as in A.
The increase in open probability mainly results from increases in the mean duration and prevalence of the longest-lived open time component, OT3, and a decrease in the prevalence of the longest-lived intracluster closed time component, CT3 (Akk et al., 2004, 2010). To determine whether any single kinetic component correlates with modulation of sIPSCs, we plotted τw as a function of changes in each of the three kinetic properties (Fig. 3B–D). The data show no significant correlation with the mean duration of OT3 (R2 = 0.19, P = 0.50) or the fraction of OT3 (R2 = 0.12, P = 0.68). However, the increase in τw showed significant correlation (R2 = −0.76, P = 0.0001) with the decrease in the fraction of CT3. We previously (Steinbach and Akk, 2001) assigned this closed state to dwells in the mono- and unliganded closed states. The relative frequency of CT3 indicates how often does the channel return from the numerous diliganded open and closed states to the monoliganded closed state. Thus, steroid modulation of this transition is the best predictor of changes in the decay time constant of sIPSCs.
Modeling of sIPSCs Based on Single-Channel Activation Parameters.
We employed Model 1 (Materials and Methods; Lema and Auerbach, 2006) to determine whether steroid-induced changes in individual transition rates in single-channel recordings can be used to simulate changes observed in sIPSCs. Modeling was conducted for four conditions: GABA alone, GABA + e18,19dinor3α5α16O, GABA + 3α5α17βEt, and GABA + e3α5α4βOMe16O. These steroids were selected because they differed in how strongly they modified single-channel responses (Fig. 3). Exposure to e18,19dinor3α5α16O results in an increase in the fraction of OT3 (Qian et al., 2014). Both 3α5α17βEt (Li et al., 2009) and e3α5α4βOMe16O (Qian et al., 2014) increase both the duration and fraction of OT3, and reduce the fraction of CT3. However, the two steroids differ in the extent of modulation of the fraction of CT3 (Fig. 3D); as a result, the compounds have unequal effects on Po. The overall rank order of potentiation is: e3α5α4βOMe16O > 3α5α17βEt > e18,19dinor3α5α16O.
For each condition, single-channel data (Li et al., 2009; Qian et al., 2014) from four to six patches were combined and analyzed by fitting to Model 1. Some of the transition rates were fixed to previously determined values. We constrained the GABA association and dissociation rate constants to 3 µM−1s−1 and 300 second−1, respectively (Lema and Auerbach, 2006). Our earlier single-channel data indicate that potentiating steroids do not modify receptor affinity to GABA (Akk et al., 2004). Accordingly, the same values were used in characterization of steroid data. We also constrained the rate constant governing transition from A2C1 to A2C3 at 300 second−1 to improve convergence of fits. The fitted rate constants for all experimental conditions are provided in Table 2.
TABLE 2.
Results of kinetic modeling
Results of kinetic modeling of single-channel currents elicited by 50 µM GABA alone or in the presence of e18,19dinor3α5α16O, 3α5α17βEt, or e3α5α4βOMe16O. Data from four to six patches at each condition were combined and analyzed using Model 1. The rate constants (and standard deviations estimated from the Hessian matrix) are in s−1. The association (3 µM−1s−1) and dissociation rate constants (300 s−1) for GABA, and the rate for A2C1→A2C3 transition (300 s−1) were constrained to values determined in a previous analysis (Lema and Auerbach, 2006). Single-channel data were filtered at 2 kHz. The dead time was 90 µs. The rate constants in this table were used to simulate synaptic events (Fig. 4).
| Transition | GABA | +e18,19dinor3α5α16O | +3α5α17βEt | + e3α5α4βOMe16O |
|---|---|---|---|---|
| A2C1→A2C2 | 1590 ± 86 | 1902 ± 103 | 1972 ± 69 | 3029 ± 290 |
| A2C2→A2C1 | 4032 ± 238 | 3689 ± 287 | 1846 ± 61 | 1645 ± 191 |
| A2C3→A2C1 | 332 ± 28 | 474 ± 54 | 586 ± 55 | 615 ± 67 |
| A2C2→A2O1 | 1491 ± 116 | 1185 ± 118 | 1895 ± 75 | 1348 ± 79 |
| A2O1→A2C2 | 4591 ± 317 | 4648 ± 347 | 2660 ± 95 | 2259 ± 120 |
| A2C2→A2O2 | 3059 ± 254 | 836 ± 81 | 920 ± 47 | 742 ± 80 |
| A2O2→A2C2 | 404 ± 28 | 822 ± 107 | 548 ± 47 | 206 ± 23 |
| A2C2→A2O3 | 443 ± 237 | 1534 ± 106 | 1401 ± 44 | 895 ± 86 |
| A2O3→A2C2 | 138 ± 22 | 88 ± 3 | 69 ± 2 | 47 ± 3 |
This analysis provides kinetic correlates to the effects observed in single-channel recordings. All three steroids increase the prevalence of long-lived openings, offset by a decrease in the prevalence of the intermediate-duration open state. This effect is mediated by an increase in the rate of transition from A2C2 to A2O3, accompanied by a decrease in the rate of transition from A2C2 to A2O2. Increase in the mean duration of OT3 is mediated by reduction in the A2O3 → A2C2 transition rate. The effect on the prevalence of CT3 is mediated by the rates governing forward and reverse transitions between A2C1 and A2C2. Interestingly, 3α5α17βEt and e3α5α4βOMe16O, that differ in their maximal effects on the prevalence of CT3, had nonidentical effects on these transitions. Both compounds reduced the rate for A2C2 → A2C1, but only e3α5α4βOMe16O increased the rate for A2C1 → A2C2 step.
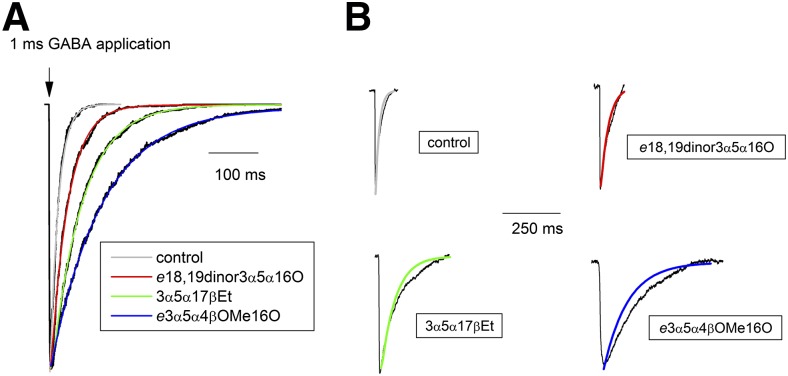
We then simulated synaptic responses using Model 1 and the rate constants in Table 2. The response was driven by a 1-millisecond square-pulse application of 10 mM agonist, with the unliganded, closed state (C) as the starting state. The simulated responses are shown in Fig. 4A along with fits to a single-exponential decay. The predicted decay time constants under the four conditions show the same rank order as measured weighted time constants (GABA + e3α5α4βOMe16O > GABA + 3α5α17βEt > GABA + e18,19dinor3α5α16O > control). The actual values for measured and predicted decay times were within a factor of 3. For receptors activated by GABA alone, we predict that the decay time constant is 18 milliseconds while the average measured τw in the absence of steroid was 34 milliseconds. Coapplication of e18,19dinor3α5α16O, 3α5α17βEt, or e3α5α4βOMe16O with GABA prolonged the predicted decay time constant to 37 milliseconds, 66 milliseconds, or 112 milliseconds, respectively. The measured τw in the presence of these steroids was 52 milliseconds, 86 milliseconds, and 271 milliseconds. Comparison of predicted and measured responses is shown in Fig. 4B.
Fig. 4.
Modeling of synaptic events. (A) Synaptic events were simulated according to Model 1 and the rate constants given in Table 2. Receptor activation was driven by a 1 millisecond-long square-pulse of 10 mM GABA (arrow) in the absence (control) and presence of saturating concentrations of e18,19dinor3α5α16O, 3α5α17βEt, or e3α5α4βOMe16O. Overlaid on the data traces are single-exponential fits that yielded 18 milliseconds, 37 milliseconds, 66 milliseconds, and 112 milliseconds for control, e18,19dinor3α5α16O, 3α5α17βEt, or e3α5α4βOMe16O, respectively. (B) The fitted lines from (A) are overlaid on the averaged sIPSCs obtained under control conditions (no steroid), and in the presence of e18,19dinor3α5α16O, 3α5α17βEt, or e3α5α4βOMe16O.
Discussion
There are several notable differences between commonly used electrophysiologic recording protocols and the drug exposure conditions in brain. First, exposure times to the modulator are different. In typical experimental protocols, where modulation is determined by comparing responses to an agonist in the absence and presence of a modulator, drug applications normally last from a few seconds in small cells like human embryonic kidney cells or fibroblasts to a few tens of seconds in the case of large cells such as Xenopus oocytes. In contrast, the buildup and clearance of many drugs, including lipophilic steroids and analogs, in the brain has a timescale of minutes or hours (Ram et al., 2001; Visser et al., 2002). Our previous work has shown that steroid redistribution to internal lipid compartments affects modulation of cell membrane localized receptors, and that prolonged drug applications result in lower estimated EC50 (Li et al., 2007b).
A second issue relates to agonist profile. Both the agonist concentration and application duration are different in the two settings. Experimental modulation is usually measured in the presence of some arbitrary low concentration of agonist, such as EC5 or EC20 GABA for α1β2γ2 receptors, whereas native synaptic GABAA receptors are alternately bathed in millimolar (saturating) concentrations of GABA after the release of transmitter from presynaptic nerve terminals followed by longer periods where the surrounding medium contains submicromolar (<EC1) concentrations of the agonist. We recently showed that anesthetic drugs readily potentiate the small steady-state currents elicited by submicromolar GABA, intended to mimic ambient GABA between synaptic events (Li and Akk, 2015). It is, however, less clear whether synaptic events can be potentiated, given the high, near-maximal open probability of α1β2γ2 receptors in the presence of saturating GABA. In any case, given the widely different exposure times to the agonist, the occupancy of the various states, and the effects of state-dependent modulators, are likely to be qualitatively different.
We have previously characterized mechanisms of steady-state modulation for several potentiating steroids (e.g., Akk et al., 2004). Steroids act by modulating up to three specific parameters of open and closed time distributions. Kinetic components of potentiation detected in single-channel recordings and the resulting changes in receptor open probability are generally a good predictor of magnitude of modulation of peak responses in whole-cell measurements (Li et al., 2007b, 2009; Scaglione et al., 2008; Qian et al., 2014). However, no direct comparison with modulation of synaptic responses is available.
It is known from previous work that addition of neuroactive steroids, such as 3α5αP and 3α5αP11O, to the extracellular medium leads to prolongation of inhibitory postsynaptic currents (Harrison et al., 1987b; Zorumski et al., 1998; Spigelman et al., 2003; Haage et al., 2005). In the present study, we set out to determine whether the magnitude of this effect correlates with the degree of potentiation observed in steady-state single-channel patch clamp recordings, and whether an effect on synaptic currents can be predicted from the kinetic profile of a steroid as determined in single-channel studies.
Based on measuring the effects of fourteen neuroactive steroids and analogs, we conclude that there is a strong positive correlation between steroid-induced changes in single-channel open probability and prolongation of decay time of sIPSCs in the presence of steroids. Steroids that most efficaciously potentiate Po typically had the strongest effect on τdec. When we separated the increase in Po into major components that produce potentiation, we found that the decrease in the prevalence of the longest-lived intracluster closed time component was the sole predictor of prolongation of sIPSCs. This finding is not necessarily surprising because the decrease in the prevalence of CT3 is most strongly associated with enhancement of single-channel Po and the macroscopic peak response (Akk et al., 2010). In the framework of Model 1, this kinetic effect is jointly produced by the increase in the rate of the A2C1 → A2C2 transition and a decrease in the rate of A2C2 → A2C1. We interpret the lack of correlation between open time properties and τdec as an independence of steroid’s ability to prolong the mean open duration and its effects on closed times.
Overall, our data indicate that studies of steroid-induced changes in steady-state single-channel currents can be employed to predict steroid effects on transient, synaptic responses. We also infer that the α1β2γ2L receptor is an acceptable model system to mimic and study synaptic-type GABAA receptors.
Several prior studies have observed prolongation of the decay time course of sIPSCs or deactivation time constant of heterologously expressed α1β2γ2L receptors in the presence of potentiating steroids (Harrison et al., 1987b; Wohlfarth et al., 2002; Spigelman et al., 2003; Haage et al., 2005) or volatile anesthetics such as halothane and isoflurance (Banks and Pearce, 1999). Based on kinetic modeling simulations, Haage et al. (2005) proposed that 3α5αP increases the decay time by reducing the GABA unbinding rate. A similar conclusion was reached for halothane-induced prolongation of decay time course (Li and Pearce, 2000). However, mechanistic conclusions can be dependent on the activation model selected for analysis. Changes in the occupancies of any of the fully liganded states, including various short-lived nonconducting states, would modify the macroscopic deactivation time course (Bianchi and Macdonald, 2001; Bianchi et al., 2007). We previously showed that receptor affinity to GABA, i.e., the binding and unbinding rates, or the maximal effective opening rate in the presence of GABA are not affected by potentiating steroids (Akk et al., 2004). Our current modeling results are in agreement with this, showing that steroid effects can be fully accounted for by changes in transitions between fully liganded states.
Strictly speaking, an increase in the decay time course does not necessarily lead to an increase in charge transfer. Sojourns in intraburst nonconducting states have been associated with prolonged decay after brief applications of agonist (Jones and Westbrook, 1995). However, a compound whose sole effect is an introduction of such nonconducting states will also reduce the open probability within the burst. In fact, the prolongation of the burst duration will be exactly offset by the reduction in open probability within the burst. It is noteworthy that the effects observed in the presence of e18,19dinor3α5α16O, 3α5α17βEt, or e3α5α4βOMe16O increase both the mean duration and the open probability of a burst.
Acknowledgments
The authors thank Ann Benz for technical help preparing cultures, Lily Cao for assistance with electrophysiologic recordings, and Joe Henry Steinbach for helpful comments on the manuscript.
Abbreviations
- 3α5α16ene17Ph
(3α5α)-17-phenylandrost-16-en-3-ol
- 3α5α17βCN
(3α,5α,17β)-3-hydroxyandrostane-17-carbonitrile
- 3α5α17βEt
(3α,5α)-pregnan-3-ol
- 3α5αP
(3α,5α)-3-hydroxypregnan-20-one
- 3α5αP11O
(3α,5α)-3-hydroxypregnane-11,20-dione
- 3α5α4βOMe16O
(3α,4β,5α)-3-hydroxyandrostan-17-one
- 3α5β17O
(3α,5β)-3-hydroxyandrostan-17-one
- 3αCPP17βEp
(2ʹS,3S,4aR,6aR,7aS,10aS,11aR,11bR)-hexadecahydro-7a-methyl-spiro[8H-cyclopenta[b]phenanthrene-8,2ʹ-oxiran]-3-ol
- 18nor3α5α17βCN
(3α,5α,17β)-18-nor-3-hydroxyandrostane-17-carbonitrile
- e3α5β17O
(3β,5α,8α,9β,10α,13α,14β)-3-hydroxyandrostan-17-one
- e18,19dinor3α5α16O
(3β,5β,8α,9β,10α,13α,14β)-3-hydroxygonan-16-one
- e19nor3α5α16O
(3β,5β,8α,9β,10α,13α,14β)-3-hydroxyestran-16-one
- e18nor3α5α16O
(3β,5β,8α,9β,10α,13α,14β)-18-nor-3-hydroxyandrostan-16-one
- e3α5α4βOMe16O
(3β,4α,5β,8α,9β,10α,13α,14β)-3-hydroxy-4-methoxy-androstan-16-one
- e3α5α16O
(3β,5β,8α,9β,10α,13α,14β)-3-hydroxyandrostan-16-one
- CNQX
6-cyano-7-nitroquinoxalone-2,3-dione
- CT
closed time
- DL-APV
dl-2-amino-5-phosphono-valeric acid
- DMSO
dimethylsulfoxide
- GABAA receptor
γ-aminobutyric acid type A receptor
- OT
open time
- Po
open probability
- sIPSC
spontaneous inhibitory postsynaptic current
Authorship Contributions
Participated in research design: Chakrabarti, Covey, Mennerick, Akk.
Conducted experiments: Chakrabarti.
Contributed new reagents or analytic tools: Qian, Krishnan.
Performed data analysis: Chakrabarti, Akk.
Wrote or contributed to the writing of the manuscript: Chakrabarti, Covey, Mennerick, Akk.
Footnotes
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grants R01GM108580, R21MH104506, R01MH101874], and funds from the Taylor Family Institute for Innovative Psychiatric Research.
References
- Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, Steinbach JH. (2004) Neuroactive steroids have multiple actions to potentiate GABAA receptors. J Physiol 558:59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Covey DF, Evers AS, Mennerick S, Zorumski CF, Steinbach JH. (2010) Kinetic and structural determinants for GABA-A receptor potentiation by neuroactive steroids. Curr Neuropharmacol 8:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. (2005) Neurosteroid access to the GABAA receptor. J Neurosci 25:11605–11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Pearce RA. (1999) Dual actions of volatile anesthetics on GABA(A) IPSCs: dissociation of blocking and prolonging effects. Anesthesiology 90:120–134. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Botzolakis EJ, Haas KF, Fisher JL, Macdonald RL. (2007) Microscopic kinetic determinants of macroscopic currents: insights from coupling and uncoupling of GABAA receptor desensitization and deactivation. J Physiol 584:769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. (2001) Agonist trapping by GABAA receptor channels. J Neurosci 21:9083–9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. (1987) Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci 231:359–369. [DOI] [PubMed] [Google Scholar]
- Emnett CM, Eisenman LN, Mohan J, Taylor AA, Doherty JJ, Paul SM, Zorumski CF, Mennerick S. (2015) Interaction between positive allosteric modulators and trapping blockers of the NMDA receptor channel. Br J Pharmacol 172:1333–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP. (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9:370–386. [DOI] [PubMed] [Google Scholar]
- Haage D, Bäckström T, Johansson S. (2005) Interaction between allopregnanolone and pregnenolone sulfate in modulating GABA-mediated synaptic currents in neurons from the rat medial preoptic nucleus. Brain Res 1033:58–67. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. (1987a) Structure-activity relationships for steroid interaction with the γ-aminobutyric acidA receptor complex. J Pharmacol Exp Ther 241:346–353. [PubMed] [Google Scholar]
- Harrison NL, Vicini S, Barker JL. (1987b) A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci 7:604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Zorumski CF, Covey DF. (1993) Neurosteroid analogues: structure-activity studies of benz[e]indene modulators of GABAA receptor function. 1. The effect of 6-methyl substitution on the electrophysiological activity of 7-substituted benz[e]indene-3-carbonitriles. J Med Chem 36:3956–3967. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. (1995) Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron 15:181–191. [DOI] [PubMed] [Google Scholar]
- Krishnan K, Manion BD, Taylor A, Bracamontes J, Steinbach JH, Reichert DE, Evers AS, Zorumski CF, Mennerick S, Covey DF. (2012) Neurosteroid analogues. 17. Inverted binding orientations of androsterone enantiomers at the steroid potentiation site on γ-aminobutyric acid type A receptors. J Med Chem 55:1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lema GM, Auerbach A. (2006) Modes and models of GABA(A) receptor gating. J Physiol 572:183–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Akk G. (2015) Synaptic-type α1β2γ2L GABAA receptors produce large persistent currents in the presence of ambient GABA and anesthetic drugs. Mol Pharmacol 87:776–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Bandyopadhyaya AK, Covey DF, Steinbach JH, Akk G. (2009) Hydrogen bonding between the 17β-substituent of a neurosteroid and the GABA(A) receptor is not obligatory for channel potentiation. Br J Pharmacol 158:1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Bracamontes J, Katona BW, Covey DF, Steinbach JH, Akk G. (2007a) Natural and enantiomeric etiocholanolone interact with distinct sites on the rat α1β2γ2L GABAA receptor. Mol Pharmacol 71:1582–1590. [DOI] [PubMed] [Google Scholar]
- Li P, Shu HJ, Wang C, Mennerick S, Zorumski CF, Covey DF, Steinbach JH, Akk G. (2007b) Neurosteroid migration to intracellular compartments reduces steroid concentration in the membrane and diminishes GABA-A receptor potentiation. J Physiol 584:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Pearce RA. (2000) Effects of halothane on GABA(A) receptor kinetics: evidence for slowed agonist unbinding. J Neurosci 20:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, He Y, Jiang X, Manion BD, Wang M, Shute A, Benz A, Evers AS, Covey DF, Zorumski CF. (2004) Selective antagonism of 5α-reduced neurosteroid effects at GABA(A) receptors. Mol Pharmacol 65:1191–1197. [DOI] [PubMed] [Google Scholar]
- Mitchell EA, Gentet LJ, Dempster J, Belelli D. (2007) GABAA and glycine receptor-mediated transmission in rat lamina II neurones: relevance to the analgesic actions of neuroactive steroids. J Physiol 583:1021–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HM, Choi IS, Nakamura M, Cho JH, Lee MG, Jang IS. (2011) Multiple effects of allopregnanolone on GABAergic responses in single hippocampal CA3 pyramidal neurons. Eur J Pharmacol 652:46–54. [DOI] [PubMed] [Google Scholar]
- Poisbeau P, Feltz P, Schlichter R. (1997) Modulation of GABAA receptor-mediated IPSCs by neuroactive steroids in a rat hypothalamo-hypophyseal coculture model. J Physiol 500:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian M, Krishnan K, Kudova E, Li P, Manion BD, Taylor A, Elias G, Akk G, Evers AS, Zorumski CF, et al. (2014) Neurosteroid analogues. 18. Structure-activity studies of ent-steroid potentiators of γ-aminobutyric acid type A receptors and comparison of their activities with those of alphaxalone and allopregnanolone. J Med Chem 57:171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram K, Lam GN, Chien B. (2001) A high-performance liquid chromatography-tandem mass spectrometric method for the determination of pharmacokinetics of ganaxolone in rat, monkey, dog and human plasma. J Chromatogr B Biomed Sci Appl 751:49–59. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. (2006) GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol 6:18–23. [DOI] [PubMed] [Google Scholar]
- Scaglione JB, Jastrzebska I, Krishnan K, Li P, Akk G, Manion BD, Benz A, Taylor A, Rath NP, Evers AS, et al. (2008) Neurosteroid analogues. 14. Alternative ring system scaffolds: GABA modulatory and anesthetic actions of cyclopenta[b]phenanthrenes and cyclopenta[b]anthracenes. J Med Chem 51:1309–1318. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Liang J, Cagetti E, Samzadeh S, Mihalek RM, Homanics GE, Olsen RW. (2003) Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABA(A) receptor δ subunit. J Neurophysiol 90:903–910. [DOI] [PubMed] [Google Scholar]
- Steinbach JH, Akk G. (2001) Modulation of GABA(A) receptor channel gating by pentobarbital. J Physiol 537:715–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser SA, Smulders CJ, Reijers BP, Van der Graaf PH, Peletier LA, Danhof M. (2002) Mechanism-based pharmacokinetic-pharmacodynamic modeling of concentration-dependent hysteresis and biphasic electroencephalogram effects of alphaxalone in rats. J Pharmacol Exp Ther 302:1158–1167. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. (2002) Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the δ subunit. J Neurosci 22:1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SA, Jones MV, Harrison NL. (1994) Potentiation of γ-aminobutyric acidA receptor Cl- current correlates with in vivo anesthetic potency. J Pharmacol Exp Ther 270:987–991. [PubMed] [Google Scholar]
- Zorumski CF, Mennerick SJ, Covey DF. (1998) Enantioselective modulation of GABAergic synaptic transmission by steroids and benz[e]indenes in hippocampal microcultures. Synapse 29:162–171. [DOI] [PubMed] [Google Scholar]