Abstract
The Gαi/o-coupled dopamine D2-like receptor family comprises three subtypes: the D2 receptor (D2R), with short and long isoform variants (D2SR and D2LR), D3 receptor (D3R), and D4 receptor (D4R), with several polymorphic variants. The common overlap of norepinephrine innervation and D2-like receptor expression patterns prompts the question of a possible noncanonical action by norepinephrine. In fact, previous studies have suggested that norepinephrine can functionally interact with D4R. To our knowledge, significant interactions between norepinephrine and D2R or D3R receptors have not been demonstrated. By using radioligand binding and bioluminescent resonance energy transfer (BRET) assays in transfected cells, the present study attempted a careful comparison between dopamine and norepinephrine in their possible activation of all D2-like receptors, including the two D2R isoforms and the most common D4R polymorphic variants. Functional BRET assays included activation of G proteins with all Gαi/o subunits, adenylyl cyclase inhibition, and β arrestin recruitment. Norepinephrine acted as a potent agonist for all D2-like receptor subtypes, with the general rank order of potency of D3R > D4R ≥ D2SR ≥ D2L. However, for both dopamine and norepinephrine, differences depended on the Gαi/o protein subunit involved. The most striking differences were observed with Gαi2, where the rank order of potencies for both dopamine and norepinephrine were D4R > D2SR = D2LR >> D3R. Furthermore the results do not support the existence of differences in the ability of dopamine and norepinephrine to activate different human D4R variants. The potency of norepinephrine for adrenergic α2A receptor was only about 20-fold higher compared with D3R and D4R across the three functional assays.
Introduction
As with any other G protein–coupled receptors (GPCR), by classic radioligand binding studies, monoaminergic neurotransmitter receptors have been defined into subclasses of receptors with the name of the neurotransmitter with the highest affinity. Dopamine receptors are classified in two families, the Gs/olf-coupled D1-like receptors, comprising D1 and D5 receptors (D1R and D5R) and the Gi/o-coupled D2-like receptors, comprising D2, D3, and D4 receptors (D2R, D3R, and D4R) (Beaulieu and Gainetdinov, 2011). D2-like receptors are expressed in brain areas involved in neuropsychiatric disorders and constitute targets for neurologic and antipsychotic medications. Structurally similar, the catecholamine norepinephrine is a metabolite of dopamine that requires dopamine β monooxygenase for the conversion. Therefore, the presence of the enzyme by the cell type distinguishes norepinephrine from dopamine release sites.
In general, norepinephrine and dopamine innervation coincides with the distribution of adrenergic and dopaminergic receptors, respectively, but this afferent-receptor coincidence is not always the case in the brain. Particularly in rodents, the cerebral cortex receives dense and widespread noradrenergic innervation, whereas dopaminergic terminals are more concentrated in the prefrontal cortex (Descarries et al., 1987; Séguéla et al., 1990). However, dopamine receptors are expressed throughout the cortex, and their localization exceeds that of the dopaminergic terminals (Lidow et al., 1989; Richfield et al., 1989; Goldsmith and Joyce, 1994; Wędzony et al., 2000). It has therefore been suggested that dopamine is a coneurotransmitter in noradrenergic neurons (Devoto and Flore, 2006) or that there is a long-range volume transmission of catecholamines (Rivera et al., 2008; Agnati et al., 2010). In fact, noradrenergic asynaptic varicosities constitute a majority of releasing sites in the cortex (Séguéla et al., 1990). Another mechanism, but specifically invoked for D4R (Lanau et al., 1997; Newman-Tancredi et al., 1997; Rivera et al., 2008; Cummings et al., 2010; Root et al., 2015), is the possibility that dopamine receptors can also be activated by endogenous norepinephrine.
Our current study investigates how norepinephrine compares with dopamine in ligand binding to effector-specific functional outcomes using bioluminescence resonance energy transfer (BRET) assays for all the D2-like receptors. A canonical Gi/o noradrenergic receptor, the α2A adrenergic receptor (α2AR), was also analyzed for comparison. This study included the short and long isoforms of D2R (D2SR and D2LR, respectively) and the three most common human polymorphic variants of the D4R gene, with 2-, 4-, or 7-nucleotide tandem repeats in the sequence encoding the third intracellular loop (D4.2R, D4.4R, and D4.7R). Thus, another unresolved issue about D2-like receptors is the possible functional distinction among different isoform and polymorphic variants. For D2SR and D2LR, distinct presynaptic versus postsynaptic localization patterns respectively have been confirmed by various studies (Khan et al., 1998; Centonze et al., 2003; Lindgren et al., 2003), but there is no consensus about their main functional differences at the cellular level. Nevertheless, some studies indicate a differential dependence of D2SR and D2LR on Gαi/o protein subunits for agonist-induced signaling (Gardner et al., 1996; Xu et al., 2002). Although initial studies seemed to indicate that the D4.7R signals with less efficiency than the most common variant D4.4R (Asghari et al., 1995; Jovanovic et al., 1999), more recent studies suggest that their differences might arise from specific protein interactions, including dopamine receptor heteromerization (González et al., 2012b; Ferré et al., 2014).
Materials and Methods
Radioligand Binding Experiments.
Human embryonic kidney (HEK) 293 cells stably expressing human D2R, D3R, or D4.4R were grown in a 50:50 mix of Dulbecco’s modified Eagle’s medium and Ham’s F-12 culture medium, supplemented with 20 mM HEPES, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1X antibiotic/antimycotic, 10% heat-inactivated fetal bovine serum, and 200 µg/ml hygromycin (Life Technologies, Grand Island, NY) and kept in an incubator at 37°C and 5% CO2. Upon reaching 80% to 90% confluence, cells were harvested using premixed Earle’s Balanced Salt Solution with 5 µM EDTA (Life Technologies) and centrifuged at 3000 rpm for 10 minutes at 21°C. The supernatant was removed, and the pellet was resuspended in 10 ml hypotonic lysis buffer (5 mM MgCl2, 5 mM Tris, pH 7.4 at 4°C) and centrifuged at 20,000 rpm for 30 minutes at 4°C. The pellet was then resuspended in fresh binding buffer (50 mM Tris, 10 mM MgCl2, 1 mM EDTA, pH 7.4). A Bradford protein assay (Bio-Rad Laboratories, Hercules, CA) was used to determine the protein concentration.
All test compounds were freshly dissolved in 30% dimethylsulfoxide (DMSO) and 70% H2O to a stock concentration of 1 mM or 100 µM. Each test compound was then diluted into 11 half-log serial dilutions using 30% DMSO vehicle; final test concentrations ranged from 10 µM to 10 pM in duplicates. Competitive-inhibition experiments were conducted in 96-well plates containing 300 µl fresh binding buffer, 50 µl of diluted test compound, 100 µl of membranes suspension (80 µg/well for D2R, 40 µg/well for D3R and 60 µg/well for D4.4R), and 50 µl of radioligand diluted in binding buffer (7-hydroxy-N,N-di-n-propyl-2-aminotetralin: [3H]7-OH-DPAT; 1.5 nM final concentration for D2R, 0.5 nM final concentration for D3R, 3 nM final concentration for D4.4R; ARC, Saint Louis, MO). Nonspecific binding was determined using 10 µM (+)-butaclamol (Sigma-Aldrich, St. Louis, MO), and total binding was determined with 30% DMSO vehicle.
The reaction was incubated for 90 minutes at room temperature and terminated by filtration through Uni-Filter-96 GF/B (PerkinElmer, Waltham, MA), presoaked in 0.5% polyethylenimine, using a Brandel 96-Well Plates Harvester Manifold (Brandel Instruments, Gaithersburg, MD). Filters were washed 3 times with 3 ml of ice-cold binding buffer; 65 µl PerkinElmer MicroScint 20 Scintillation Cocktail was added, and the filters were counted using a PerkinElmer MicroBeta Microplate Counter. The IC50 values for each compound were determined from dose–response curves, and the Ki values were calculated using the Cheng-Prusoff equation in GraphPad Prism 5 (GraphPad Software, San Diego, CA). Reported Ki values were determined from at least three independent experiments.
DNA Constructs and Transfection.
For all the receptor constructs, a signal peptide followed by a Flag epitope tag were fused to the N terminus for enhanced cell surface expression (Guan et al., 1992) and their detection (Guo et al., 2008). Human receptor constructs were used for D2SR, D2LR, D3R, D4.2R, D4.4R, and D4.7R (Guitart et al., 2014; Frederick et al., 2015). For fusion receptor constructs with Renilla luciferase 8 (RLuc8; provided by Dr. S. Gambhir, Stanford University, Stanford, CA), the cDNA encoding full-length RLuc8 was fused in frame to the C terminus of receptors as reported elsewhere (Urizar et al., 2011). The following human G protein constructs were used: Gαi1-mVenus with mVenus inserted at position 91, Gαi1-, Gαi2-, Gαi3-, Gαo1-, or Gαo2-RLuc8 with RLuc8 inserted at position 91, Gαs-RLuc8 with RLuc8 inserted at position 67, Gαq-RLuc8 with RLuc8 inserted at position 97, untagged Gβ1, untagged Gγ2, and Gγ2 fused to full-length mVenus at its N terminus. The Gα-Rluc8 constructs were kindly provided by Dr. Céline Galés (INSERM, Toulouse, France). The cAMP sensor with yellow fluorescence protein (YFP)-Epac-RLuc (CAMYEL) was obtained from the American Type Culture Collection (no. MBA-277; ATCC, Manassas, VA) (Jiang et al., 2007). G protein-coupled receptor kinase 2 (GRK2) and mVenus-fused β arrestin-2 (Vishnivetskiy et al., 2011) constructs were obtained from Dr. J. Javitch (Columbia University, New York, NY). All the constructs were confirmed by sequencing analysis.
A constant amount of plasmid cDNA (15 µg) was transfected into HEK 293T cells using polyethylenimine (Sigma-Aldrich) in a 1 to 2 ratio in 10-cm dishes. Cells were maintained in culture with Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and kept in an incubator at 37°C and 5% CO2. The transfected amount and ratio among the receptor and heterotrimeric G proteins were tested for optimized dynamic range in drug-induced BRET. The experiments were performed approximately 48-hours after transfection.
BRET Assay.
Variations of BRET were performed to detect receptor ligand-induced events for: 1) Gi/o protein activation, 2) adenylyl cyclase inhibition, and 3) β arrestin recruitment.
Gi/o protein activation assay uses RLuc-fused Gαi/o protein subunit and mVenus-fused Gγ2 protein for resonance energy transfer (RET) pair. Flag-tagged receptor and untagged Gβ1 constructs were cotransfected.
The adenylyl cyclase inhibition assay uses CAMYEL biosensor construct that contains RLuc and YFP allowing detection of intracellular cAMP change (Jiang et al., 2007). To study Gαi dependent inhibition activity, cells were prestimulated with 10 µM forskolin (Sigma-Aldrich) in the presence of 10 µM propranolol 10 minutes before sample reading to ensure isolation of response from D2-like receptors or α2AR.
The β arrestin recruitment uses RLuc-fused receptor and mVenus-fused β arrestin-2 for the RET pair. GRK2 was cotransfected to assist an enhanced phosphorylation required for the β arrestin recruitment.
As reported previously (Urizar et al., 2011), cells were harvested, washed, and resuspended in phosphate-buffered saline). Approximately 200,000 cells/well were distributed in 96-well plates, and 5 µM coelenterazine H (substrate for luciferase) was added to each well. One minute after addition of coelenterazine H, dopamine (Sigma-Aldrich) or l-(−)-norepinephrine (Sigma-Aldrich) was added to each well. Antagonists were added 15 minutes before the addition of agonist. The fluorescence of the acceptor was quantified (excitation at 500 nm and emission at 540 nm for 1-second recordings) in Mithras LB940 (Berthold Technologies, Bad Wildbad, Germany) to confirm the constant expression levels across experiments.
In parallel, the BRET signal from the same batch of cells was determined as the ratio of the light emitted by mVenus (510–540 nm) over that emitted by RLuc (485 nm). Results are calculated for the BRET change (BRET ratio for the corresponding drug minus BRET ratio in the absence of the drug). Emax values are expressed as the subtracted BRET change times 1000 and in the dose–response graphs are normalized as percentage of maximal value. Data and statistical analysis were performed with Prism 5 (GraphPad Software).
FACS Assay.
Fluorescence-activated cell sorting (FACS) was performed to determine the surface expression of each receptor construct, as described elsewhere (Costagliola et al., 1998), with FACS Canto II (BD Biosciences, San Jose, CA). Briefly, a fraction of the same cells used for BRET (∼500,000 cells) were harvested and incubated with anti-Flag monoclonal antibody (Sigma-Aldrich) in FACS buffer (phosphate-buffered saline with 1% bovine serum albumin and 0.1% sodium azide), washed, and incubated with the secondary anti-mouse antibody conjugated with Alexa Fluor 647 (Invitrogen, Carlsbad, CA).
Statistics.
Differences in Ki or EC50 values of norepinephrine and dopamine between different receptors were statistically analyzed by one-way analysis of variance followed by Newman-Keuls post hoc test (P values are as indicated in figure legends).
Results
Norepinephrine Binds to All Dopamine D2-Like Receptor Subtypes with High Affinity in Stably Transfected HEK-293 Cells.
Radioligand competition experiments with the D2-like receptor agonist [3H]7-OH-DPAT (Lévesque et al., 1992) were performed in HEK-293 cells stably transfected with D2SR, D2LR, D3R, or D4.4R, to compare the affinities of dopamine and norepinephrine for the D2-like receptors. Competition experiments revealed the high affinity (low Ki values) of dopamine for all the D2-like receptors, with a rank order of D3R > D4.4R ≥ D2SR = D2LR (Table 1). Consistent with previous reports (Missale et al., 1998), dopamine showed a Ki value for D3R significantly lower (higher affinity) than for D2LR. Norepinephrine also showed high and similar affinity (around 50 nM) for the four D2-like receptor subtypes, with only 4-fold (D2LR) to 15-fold (D3R) less affinity compared with dopamine (Table 1).
TABLE 1.
Binding affinities of dopamine and norepinephrine for D2LR, D2SR, D3R, and D4.4R
Results are from competitive inhibition experiments of [3H]7-OH-DPAT versus dopamine or norepinephrine in HEK-293 cells stably expressing D2LR, D2SR, D3R, or D4.4R. The equilibrium inhibition constant (Ki; nM) is shown for dopamine or norepinephrine. Values are the mean ± S.E.M. of three to six independent experiments.
| Receptor |
Ki (nM) |
NE/DA (Ki) | |
|---|---|---|---|
| Dopamine | Norepinephrine | ||
| D2LR | 16 ± 4 | 62 ± 17 | 3.9 |
| D2SR | 12.5 ± 3.1 | 75 ± 10 | 6 |
| D3R | 3 ± 1a | 43 ± 15 | 14.3 |
| D4.4R | 6.4 ± 2.0 | 68 ± 25 | 10.6 |
NE/DA, ratio between Ki values of norepinephrine and dopamine for each receptor.
P < 0.05, statistically significantly different compared with D2LR (one-way analysis of variance, followed by post hoc Newman-Keuls test).
Norepinephrine Signals with Similar Potency through All Dopamine D2-Like Receptor Subtypes in Transiently Transfected HEK-293T Cells.
D2-like receptors, by activating Gi/o proteins, inhibit adenylyl cyclase, therefore decreasing intracellular cAMP levels; consequently, the activation of cAMP targets, such as cyclic nucleotide gated channels, exchange protein directly activated by cAMP (Epac) and cAMP-dependent protein kinase or protein kinase A (Antoni, 2012). To find a functional correlate of the results obtained with radioligand binding experiments, we measured D2-like receptor-mediated inhibition of adenylyl-cyclase by measuring cAMP levels in intact cells transiently transfected with D2LR, D2SR, D3R or the D4R variants D4.2R, D4.4R, or D4.7R, using cAMP sensor with CAMYEL, a kinase-dead Epac I–based cAMP BRET biosensor. The model takes advantage of the conformational changes in Epac that are induced upon its binding to cAMP.
The conformational change triggered by cAMP results in a decrease in BRET due to luciferase and YFP changing their relative orientation from each other. A decrease in cAMP levels is therefore observed as an increase in BRET (Fig. 1) (Jiang et al., 2007). HEK-293T cells are reported to endogenously express β adrenergic receptors (Atwood et al., 2011). Accordingly, norepinephrine in nontransfected cells stimulated Gs-mediated cAMP increase, which could be completely inhibited by the selective β adrenergic blocker propranolol (10 μM; (Supplemental Fig. 1). Therefore, propranolol was added throughout the cAMP detection experiments to determine transfected D2-like receptor-mediated responses.
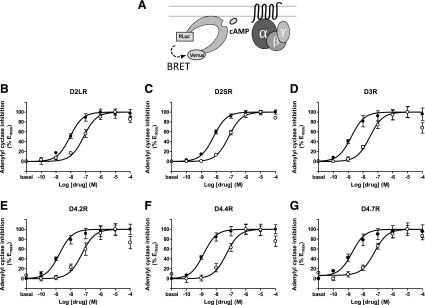
Fig. 1.
(A) Scheme of the CAMYEL sensor configuration where the intramolecular conformational change triggered by cAMP results in a decrease in BRET due to RLuc8 and YFP changing their relative orientation from each other. (B–G) Dose–response experiments of adenylyl cyclase inhibition by dopamine (●) or norepinephrine (○) mediated by D2LR, D2SR, D3R, D4.2R, D4.4R, and D4.7R in HEK-293T cells transiently expressing the CAMYEL sensor and the indicated D2-like receptor: (B) D2LR, (C) D2SR, (D) D3R, (E) D4.2R, (F) D4.4R, or (G) D4.7R. Cells were treated with forskolin (10 µM) for 10 minutes followed by dopamine or norepinephrine and BRET values obtained by forskolin alone were subtracted from BRET values for each agonist concentration. Data represent the mean ± S.E.M. of 6 to 11 experiments performed in triplicate and are shown as a percentage of the maximal effect (see Table 2 for EC50 and Emax values and statistical comparisons).
The assay showed a high sensitivity and provided EC50 values for dopamine and norepinephrine that were similar, albeit slightly lower, to the Ki values observed in radioligand binding experiments (Fig. 1, Table 2). The slightly higher potency, particularly of dopamine, in the adenylyl cyclase inhibition assay compared with the radioligand displacement assay may be due to an artifactual ceiling effect set by potentially low cellular expression of the biosensor in a certain population of transfected cells. Depending on the proportion of low-expressing cells, the response of the decrease in cAMP production, read as an increase in BRET, may be seen prematurely as fully efficacious, giving a low estimate of EC50.
TABLE 2.
Adenylyl cyclase inhibition by dopamine and norepinephrine mediated by D2LR, D2SR, D3R, D4.2R, D4.4R, and D4.7R
Results are from adenylyl cyclase inhibition-BRET experiments in HEK-293T cells transiently transfected with the CAMYEL sensor and one of the indicated receptors. Data were fit by nonlinear regression to a sigmoidal dose–response relationship against the agonist concentration. The EC50 and Emax values are mean ± S.E.M. of 5 to 11 experiments performed in triplicate. Emax values are expressed as 1000 × [BRETmax − BRETbasal].
| Receptor | Dopamine |
Norepinephrine |
NE/DA (EC50) | ||
|---|---|---|---|---|---|
| EC50 (nM) | Emax | EC50 (nM) | Emax | ||
| D2LR | 9.4 ± 1.1 | 94 ± 8 | 88 ± 17 | 94 ± 6 | 9.4 |
| D2SR | 6.7 ± 0.5a | 140 ± 7a | 62 ± 6 | 149 ± 8a | 9.3 |
| D3R | 1.6 ± 0.2a | 48 ± 7a | 19 ± 3a | 50 ± 5a | 12 |
| D4.2R | 1.8 ± 0.2a | 74 ± 8 | 54 ± 8 | 69 ± 8 | 30 |
| D4.4R | 1.4 ± 0.2a | 98 ± 9 | 53 ± 8 | 79 ± 6 | 38 |
| D4.7R | 1.6 ± 0.3a | 83 ± 11 | 53 ± 13 | 84 ± 6 | 33 |
NE/DA, ratio between EC50 values of norepinephrine and dopamine for each receptor.
P < 0.001, significantly statistically different compared with D2LR (one-way analysis of variance, followed by post hoc Newman-Keuls test).
Same as the binding experiments, both dopamine and norepinephrine showed low EC50 values with D3R, which were significantly different compared with D2LR (Table 2). In comparison with the binding experiments, both for dopamine and norepinephrine the EC50 values were moderately higher for D2LR than D2SR, although not statistically different for norepinephrine (Table 2). For dopamine, the EC50 values with the three D4R isoforms were as low as with D3R, and no statistically significant differences were obtained between the EC50 values for dopamine and norepinephrine in cells transfected with D4.2R, D4.4R, or D4.7R. In terms of efficacy, the Emax values for both dopamine and norepinephrine were statistically significantly higher for D2SR and lower for D3R compared with D2L, and no differences were observed among the D4R variants (Table 2).
Norepinephrine Activates Most G Proteins with Different Gαi/o Subunits via D2-Like Receptors at Submicromolar Concentrations in Transiently Transfected HEK-293T Cells.
Gαi/o protein subunits are associated with the canonical inhibition of adenylyl cyclase and can be grouped in Gαi, comprising the Gαi1, Gαi2, and Gαi3 subunits, and Gαo, comprising the Gαo1 and Gαo2 splice variants. Studies have suggested the existence of differences in the ability of GPCR to activate G proteins with different Gαi/o subunits, including different D2-like receptor subtypes (see Discussion and Montmayeur et al., 1993; Liu et al., 1994; Lane et al., 2008), which coincides with different proportions of G protein subunits expressed in different cell types and brain regions (Orford et al., 1991; Jiang et al., 2001).
Therefore, using BRET methodology, we compared the ability of dopamine and norepinephrine to activate Gi/o proteins with different Gαi/o subunits via all different D2-like receptor subtypes, including the two D2R isoform variants and the three main D4R polymorphic variants. For this purpose, we used luciferase-fused Gαi1, Gαi2, Gαi3, Gαo1, or Gαo2 and Venus-fused γ2 subunit constructs. β1 and γ2 were chosen because of their wide expression in the brain.
As characterized thoroughly in previous reports (Galés et al., 2005, 2006), the ligand-induced G protein conformational change results in negative BRET change, consistent with the opening and distancing between the Gαi and γ2 proteins, which is interpreted as the activation of the heterotrimeric G protein. Taking advantage of a high sequence homology among the five Gαi/o protein subunits, constructs with a luciferase insertion in the same position (i.e., amino acid 91) were used. Luminescence and fluorescence were always measured to control for similar expression levels of transiently transfected G proteins (luciferase-Gαi, β1, and Venus-γ2). Receptor constructs were fused to a Flag epitope at the N terminus, which allowed measurements of cell-surface expression by anti-Flag immunostaining by FACS.
To avoid a significant influence by spare receptors, an amount for the receptor cDNA that proportionately increased the surface receptor expression as well as Emax was chosen for the study (data not shown). FACS analysis also showed no statistically significant differences in the cell surface expression among the D2R and D4R variants transfected (Supplemental Fig. 2).
The EC50 values for both dopamine and norepinephrine were in the submicromolar range for most D2-like receptors and Gαi/o protein subunits, and they were relatively higher than those obtained in radioligand displacement and adenylyl cyclase inhibition experiments (Fig. 2, Table 3). Gi/o protein coupling specificity was demonstrated by the absence of Gs or Gq protein activation by D2-like receptors; the absence of dopamine- or norepinephrine-induced Gαi1 protein activation via endogenously expressed receptors was confirmed in cells without transfected D2-like receptors (Supplemental Fig. 3). In addition, selective involvement of transfected D2-like receptors in dopamine and norepinephrine-induced G protein activation (with the Gαi1 subunit) was demonstrated by dose-dependent blockade with the D4R antagonist L745,870 (3-{[4-(4-chlorophenyl)piperazin-1-yl]methyl}-1H-pyrrolo[2,3-b]pyridine) and the D2R-D3R antagonist raclopride (Supplemental Fig. 4).
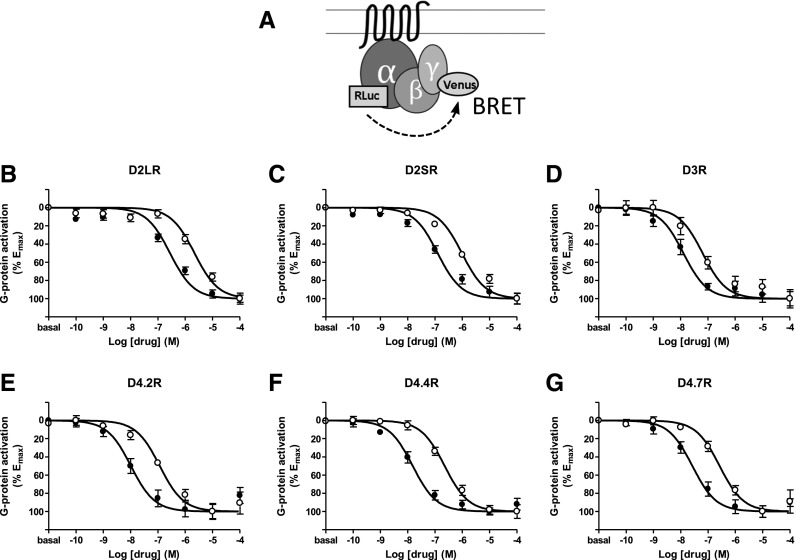
Fig. 2.
(A) Scheme of the constructs used for G-protein activation BRET experiments where RLuc8 is fused to the Gαi1 subunit and mVenus is fused to the γ2 subunit. (B–G) Dose–response experiments of G protein activation by dopamine (●) or norepinephrine (○) mediated by D2LR, D2SR, D3R, D4.2R, D4.4R, and D4.7R in HEK-293T cells transiently expressing the G protein subunits Gαi1-RLuc8, unfused β1 and γ2-mVenus and the indicated D2-like receptor: (B) D2LR, (C) D2SR, (D) D3R, (E) D4.2R, (F) D4.4R, or (G) D4.7R. Cells were treated with coelenterazine H followed by increasing concentrations of dopamine or norepinephrine. After 10 minutes, BRET between G-protein subunits was measured as described in Materials and Methods. BRET values in the absence of ligands were subtracted from the BRET values for each agonist concentration. Data were fit by nonlinear regression to a sigmoidal dose–response relationship against the agonist concentration and are shown as a percentage of the maximal effect. Data represent the mean ± S.E.M. of 11 to 18 experiments performed in triplicate (see Table 3 for EC50 and Emax values and statistical comparisons).
TABLE 3.
G-protein activation by dopamine and norepinephrine mediated by D2LR, D2SR, D3R, D4.2R, D4.4R, and D4.7R involving different Giα/o subtypes
Results were obtained from G protein activation-BRET experiments in HEK-293T cells transiently transfected with different Gαi-RLuc8 subtypes (Gαi1, Gαi2, Gαi3, Gαo1, Gαo2) and γ2-mVenus in cells expressing different D2-like receptors. Data were fit by nonlinear regression to a sigmoidal dose–response relationship against the agonist concentration. The EC50 and Emax values are the mean ± S.E.M. of 5 to 18 experiments performed in triplicate. Emax values are expressed as 1000 × [BRETmax − BRETbasal].
| Gα Subunit | Receptor | Dopamine |
Norepinephrine |
NE/DA (EC50) | ||
|---|---|---|---|---|---|---|
| EC50 (nM) | Emax | EC50 (nM) | Emax | |||
| Gαi1 | D2LR | 393 ± 43 | 19 ± 1 | 3609 ± 549 | 17 ± 1 | 9.2 |
| D2SR | 161 ± 24a | 30 ± 1a | 987 ± 171a | 26 ± 1a | 6.1 | |
| D3R | 12 ± 3a | 15 ± 1 | 83 ± 33a | 15 ± 1 | 6.9 | |
| D4.2R | 18 ± 5a | 17 ± 1 | 116 ± 28a | 16 ± 2 | 6.4 | |
| D4.4R | 21 ± 6a | 22 ± 1 | 215 ± 31a | 20 ± 1 | 10.2 | |
| D4.7R | 26 ± 5a | 25 ± 2b | 229 ± 44a | 19 ± 1 | 8.8 | |
| Gαi2 | D2LR | 177 ± 46 | 21 ± 1 | 1765 ± 391 | 24 ± 4 | 10 |
| D2SR | 92 ± 10b | 41 ± 2a | 1280 ± 309 | 39 ± 2c | 14 | |
| D3R | ND | ND | ND | ND | — | |
| D4.2R | 24 ± 6a | 37 ± 3a | 340 ± 69c | 31 ± 5 | 14 | |
| D4.4R | 19 ± 2a | 37 ± 4a | 134 ± 30c | 35 ± 2b | 7 | |
| D4.7R | 28 ± 8c | 45 ± 3a | 285 ± 46c | 47 ± 3a | 10.2 | |
| Gαi3 | D2LR | 132 ± 34 | 26 ± 2 | 1198 ± 160 | 26 ± 3 | 9.1 |
| D2SR | 52 ± 8a | 40 ± 2a | 379 ± 103a | 40 ± 2c | 7.3 | |
| D3R | 3.6 ± 0.6a | 16 ± 1a | 49.4 ± 13a | 17 ± 1b | 13.7 | |
| D4.2R | 21 ± 7a | 29 ± 2 | 316 ± 70a | 30 ± 6 | 15 | |
| D4.4R | 12 ± 3a | 42 ± 5a | 476 ± 116a | 34 ± 3 | 39.7 | |
| D4.7R | 15 ± 2c | 38 ± 3b | 398 ± 102a | 39 ± 4b | 26.5 | |
| Gαo1 | D2LR | 182 ± 42 | 35 ± 2 | 534 ± 67 | 31 ± 2 | 2.9 |
| D2SR | 24 ± 4b | 36 ± 1 | 159 ± 26b | 36 ± 2 | 6.6 | |
| D3R | 2 ± 0.3b | 22 ± 1b | 33 ± 11b | 21 ± 2 | 16.5 | |
| D4.2R | 197 ± 45 | 53 ± 3c | 408 ± 118 | 54 ± 4a | 2.1 | |
| D4.4R | 225 ± 44 | 57 ± 3a | 403 ± 78 | 54 ± 3a | 1.8 | |
| D4.7R | 228 ± 38 | 60 ± 4a | 557 ± 114 | 58 ± 4a | 2.4 | |
| Gαo2 | D2LR | 227 ± 41 | 64 ± 1 | 727 ± 53 | 55 ± 2 | 3.2 |
| D2SR | 49 ± 14a | 64 ± 2 | 298 ± 68a | 60 ± 3 | 6.1 | |
| D3R | 3 ± 0.4a | 33 ± 2a | 49 ± 19a | 30 ± 3a | 16.3 | |
| D4.2R | 36 ± 16a | 72 ± 6 | 81 ± 26a | 61 ± 5 | 2.3 | |
| D4.4R | 50 ± 14a | 69 ± 4 | 275 ± 55a | 63 ± 3 | 5.5 | |
| D4.7R | 128 ± 51b | 63 ± 5 | 122 ± 36a | 57 ± 5 | 0.95 | |
ND, not determined; NE/DA, ratio between EC50 values of norepinephrine and dopamine.
P < 0.001 compared with D2LR (one-way analysis of variance, followed by post hoc Newman-Keuls test).
P < 0.05 compared with D2LR (one-way analysis of variance, followed by post hoc Newman-Keuls test).
P < 0.01 compared with D2LR (one-way analysis of variance, followed by post hoc Newman-Keuls test).
Dopamine was more potent than norepinephrine for most D2-like receptors and Gαi/o protein subunits, but significant Gαi/o protein subunit-dependent differences were observed (Table 3). For the two D2R and the three D4R variants, dopamine was around 10 times more potent than norepinephrine when coupling to Gαi1, Gαi2, or Gαi3, but only around 2 times more potent when coupling to Gαo1 or Gαo2 (Table 3). Dopamine and norepinephrine showed higher potencies for the D4R than for the D2R variants when coupled to Gαi1 and Gαi2 proteins but similar potencies when coupled to Gαo1 or Gαo2. When coupling to Gαi3, dopamine was more potent for D4R than for D2R, but norepinephrine showed similar potencies. Dopamine and norepinephrine showed higher potency for D3R than for the other D2-like receptors with all Gαi/o protein subunits with the exception of Gαi2, which seemed to be completely insensitive to dopamine or norepinephrine-induced D3R activation.
No statistically significant differences between D4R variants were observed. On the other hand, a significantly higher potency of dopamine and norepinephrine could be observed at D2SR compared with D2LR with all Gαi/o protein subunits but Gαi2. The same as for the adenylyl cyclase signaling experiments, for most Gαi/o protein subunits the Emax values for both dopamine and norepinephrine were significantly higher for D2SR and lower for D3R compared with D2L, and no major differences were observed among the D4R variants (Table 3).
Norepinephrine Promotes β Arrestin-2 Recruitment to the D2-Like Receptor in Transiently Transfected HEK-293T Cells.
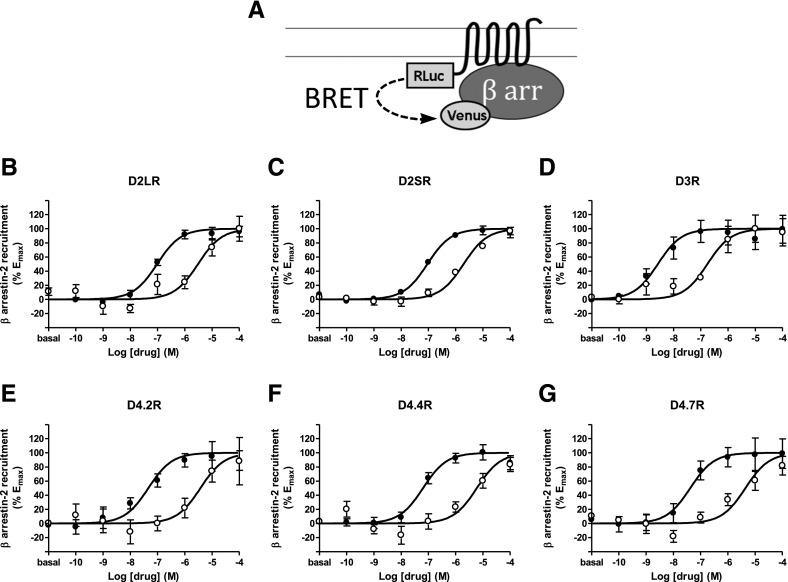
Receptor activation also leads to β arrestin-mediated receptor recruitment and its own signaling events (Shenoy and Lefkowitz, 2011), which are major effector-triggered events besides the G protein-mediated signal transduction. Hence, dopamine and norepinephrine-induced β arrestin recruitment across all the D2-like receptors were also analyzed. RLuc8-fused receptor constructs (Urizar et al., 2011) were transfected with Venus-fused β arrestin-2 and GRK2, whose phosphorylation precedes and assists β arrestin recruitment (Evron et al., 2012). Although right-shifted compared with the G protein-related assays, the EC50 values were in agreement with previous reports (Namkung et al., 2009; Clayton et al., 2014). Notably, consistent with the radioligand-binding and G protein–related experiments, the rank order of potency was D3R > D4R > D2R for both dopamine and norepinephrine, and no variant differences were observed (Fig. 3, Table 4). The Emax values for both dopamine and norepinephrine were very similar across all dopamine receptor subtypes (Table 4).
Fig. 3.
(A) Scheme of the constructs used for β arrestin-2 recruitment-BRET experiments with different D2-like receptors fused to RLuc8 and β arrestin-2 fused to mVenus. (B–G) Dose–response experiments of β arrestin-2 recruitment by dopamine (●) or norepinephrine (○) mediated by (B) D2LR, (C) D2SR, (D) D3R, (E) D4.2R, (F) D4.4R, or (G) D4.7R in HEK-293T cells transiently expressing the indicated D2-like receptor fused to RLuc8, β arrestin-2-mVenus, and GRK2. Cells were treated with coelenterazine H followed by increasing concentrations of dopamine or norepinephrine. After 20 minutes, BRET was measured as described in Materials and Methods. BRET values in the absence of ligands were subtracted from the BRET values for each agonist concentration. Data were fit by nonlinear regression to a sigmoidal dose–response relationship against the agonist concentration and are shown as a percentage of the maximal effect. Data are the mean ± S.E.M. of 5 to 10 experiments performed in triplicate (see Table 4 for EC50 and Emax values and statistical comparisons).
TABLE 4.
β arrestin-2 recruitment induced by dopamine and norepinephrine mediated by D2LR, D2SR, D3R, D4.2R, D4.4R, and D4.7R
Results were obtained from β arrestin-2 recruitment-BRET experiments in HEK-293T cells transiently transfected with different D2-like receptors fused to RLuc8 and β arrestin-2 fused to mVenus. Data of individual experiments were fit by nonlinear regression to a sigmoidal dose–response relationship against the agonist concentration. The EC50 and Emax values are the mean ± S.E.M. of 5 to 15 experiments performed in triplicate. Emax values are expressed as 1000 × [BRETmax − BRETbasal].
| Receptor | Dopamine |
Norepinephrine |
NE/DA (EC50) | ||
|---|---|---|---|---|---|
| EC50 (nM) | Emax | EC50 (nM) | Emax | ||
| D2LR | 96 ± 16 | 11 ± 1 | 4800 ± 1720 | 11 ± 2 | 50 |
| D2SR | 105 ± 21 | 18 ± 2a | 2880 ± 1150 | 17 ± 2 | 27.4 |
| D3R | 3 ± 1b | 15 ± 2c | 32 ± 5c | 13 ± 2 | 10.7 |
| D4.2R | 38 ± 13c | 9 ± 1 | 3750 ± 1620 | 10 ± 2 | 100 |
| D4.4R | 66 ± 13 | 8 ± 1 | 2830 ± 666 | 7 ± 1 | 43 |
| D4.7R | 44 ± 12c | 9 ± 1 | 1840 ± 889 | 8 ± 2 | 41.8 |
NE/DA, ratio between EC50 values of norepinephrine and dopamine for each receptor.
P < 0.01, compared with D2LR (one-way analysis of variance, followed by post hoc Newman-Keuls test).
P < 0.001, compared with D2LR (one-way analysis of variance, followed by post hoc Newman-Keuls test).
P < 0.05, compared with D2LR (one-way analysis of variance, followed by post hoc Newman-Keuls test).
Norepinephrine Activates G Protein, Inhibits Adenylyl Cyclase, and Promotes β Arrestin-2 Recruitment via α2AR at Concentrations 20-Fold Lower Than D2-Like Receptors.
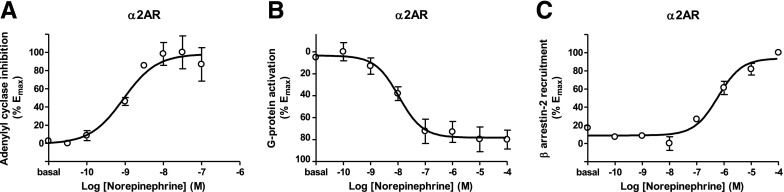
The α2AR is a canonical Gi-coupled receptor for norepinephrine that is highly expressed in the prefrontal cortex (Wang et al., 1996) where D2R and D4R also are expressed (Missale et al., 1998). The EC50 values of norepinephrine for the G protein activation and adenylyl cyclase inhibition assays were both at the nanomolar range and just about 20-fold lower compared with those of D3R and D4R (Fig. 4, Table 5). Similarly, the potency of norepinephrine for β arrestin-2 recruitment with α2AR was 4-fold higher than with D2-like receptors, in agreement with their potency separation on G protein activation (Fig. 4, Table 5).
Fig. 4.
(A–C) Dose–response experiments of norepinephrine on α2AR function. (A) Adenylyl cyclase inhibition assay using CAMYEL biosensor. (B) G protein activation assay using Gαi1 and γ2 subunits. (C) β Arrestin-2 recruitment assay using β arrestin-2-mVenus and GRK2. Conditions for each assay are identical to the ones described in Figures 1–3. Basal BRET values for forskolin alone (A) or in the absence of ligands (B, C) were subtracted from the BRET values for each agonist concentration. Data were fit by nonlinear regression to a sigmoidal dose–response relationship against the agonist concentration and are shown as a percentage of the maximal effect. Data are the mean ± S.E.M. of three to four experiments performed in triplicate (see Table 5 for EC50 and Emax values and statistical comparisons).
TABLE 5.
Adenylyl cyclase inhibition, G-protein activation and β arrestin-2 recruitment induced by norepinephrine mediated by α2AR
Data of individual experiments were fit by nonlinear regression to a sigmoidal dose–response relationship against the agonist concentration. The EC50 and Emax values are the mean ± S.E.M. of 3 to 4 experiments performed in triplicate. Emax values are expressed as 1000 × [BRETmax − BRETbasal].
| BRET Assay | Norepinephrine |
|
|---|---|---|
| EC50 (nM) | Emax | |
| Adenylyl cyclase inhibition | 1 ± 0.2 | 76 ± 10 |
| G-protein activation (Gi1) | 12 ± 2 | 24 ± 2 |
| β Arrestin-2 recruitment | 687 ± 247 | 15 ± 1 |
Discussion
From all D2-like receptor subtypes, to our knowledge, only D4R has been suggested to be a “promiscuous” receptor that can be activated by both dopamine and norepinephrine and function as an adrenergic receptor in the brain (Lanau et al., 1997; Newman-Tancredi et al., 1997; Czermak et al., 2006; Cummings et al., 2010; Root et al., 2015). Our present study indicates that all D2-like receptor subtypes can bind with high affinity and be activated by norepinephrine and that their potencies, particularly for D3R and D4R, are only ∼20-fold lower than for α2AR activation.
Application of BRET assays that allow measuring activation of specific G protein subtypes has provided evidence for a significant differential dependence on Gαi/o protein subunits on the ability of both catecholamines to activate D2-like receptors. Relative potencies obtained with the four assays used in this study were largely consistent, verifying the validity of potencies for the norepinephrine-mediated G protein activation. Furthermore, the study does not support differences in the ability of dopamine or norepinephrine to activate the three main D4R polymorphic variants but does support differences in the two D2R isoform variants for their ability to couple with specific Gαi/o protein subunits.
Several studies have reported that norepinephrine can bind with high affinity and activate D4R with lower potency compared with dopamine in mammalian transfected cells. The difference in agonist potency varied significantly depending on the study, ranging from 2 to more than 50 times (Lanau et al., 1997; Newman-Tancredi et al., 1997; Czermak et al., 2006; Cummings et al., 2010 ). Part of this variability could certainly be related to methodologic differences in radioligand binding, G protein activation, or effector activation assays used. But our study highlights the importance of the Gαi/o protein subunit involved in D4R activation. Thus, with the three main human D4R polymorphic variants—D4.2R, D4.4R, and D4.7R—dopamine was approximately 10 times more potent than norepinephrine at activating G proteins containing Gαi1, Gαi2, or Gαi3 subunits; it was only twice as potent at activating G proteins containing Gαo1 or Gαo2 subunits. No statistically significant differences with the potencies of dopamine or norepinephrine could be observed among the three D4R polymorphic variants coupled to any of the Gi/o protein subtypes. This extends previous results showing no differences in the ability of dopamine to activate the three different D4R variants reconstituted with purified G proteins containing Gαi1, Gαi2, or Gαi3 subunits (Kazmi et al., 2000), which questions the studies that have suggested D4R variant-dependent differences in the potency of dopamine or norepinephrine to activate G protein ([35S]GTPγS binding; Czermak et al., 2006) or to inhibit adenylyl cyclase (Asghari et al., 1995). As expected, we did not find any difference in the ability of dopamine or norepinephrine to inhibit adenylyl cyclase or to promote β arrestin recruitment between cells transfected with D4.2R, D4.4R, or D4.7R.
Previous studies reported a low potency of norepinephrine to bind and activate D2R (Lanau et al., 1997; Newman-Tancredi et al., 1997); however, after analyzing agonist binding, G protein activation, adenylyl cyclase inhibition, and β arrestin recruitment, we provide clear evidence for a similar catecholamine “promiscuity” of D2R and D3R as for D4R. The same as for D4R, Gαi/o protein subunit-dependent differences were demonstrated in the potencies of dopamine and norepinephrine for D2SR, D2LR, and D3R. The most striking finding was the selective inability of dopamine or norepinephrine to activate G proteins containing the Gαi2 subunit in D3R-transfected cells, which agrees with previous results from experiments with receptor-G protein fusion proteins and pertussis toxin-resistant mutants of G proteins containing different Gαi or Gαo subunits (Lane et al., 2008). Lane and colleagues reported a very efficient agonist-induced coupling of D3R to Gαo1 and an inability of D3R to couple not only to Gαi2 but also to Gαi1 or Gαi3. In our study we were also able to confirm a high potency of dopamine (and norepinephrine) to induce D3R-mediated coupling to Gαo1 and Gαo2 as well as to Gαi1 and Gαi3. Different methodologies should explain the discrepancies between both studies, but in support of our results not only dopamine but also norepinephrine were more potent at D3R than at D2LR or D2SR to induce Gαi1 and Giα3 activation.
Furthermore, our results with G protein activation are congruent with our signaling experiments because HEK-293 cells have been reported to express predominantly Gαi1, Gαi2, and Gαi3 and statistically nonsignificant levels of Gαo1 and Gαo2 (Atwood et al., 2011). Thus, both dopamine and norepinephrine were more potent at D3R than at D2LR or D2SR to inhibit adenylyl cyclase or recruit β arrestin. Even though G proteins of the Gαs-Gαolf family do show contrasting brain expression pattern (Hervé 2011), to our knowledge no clear region-specific pattern of mRNA expression for Gαi/o protein subtypes has been reported. Detailed characterization of expression patterns for Gαi/o protein subtypes would be central to determine their stoichiometric relationship with the different D2-like receptors and thus the possible contribution of dopamine and norepinephrine in their activation.
Although still a matter of intense debate, as for the human D4R polymorphic variants there is no consensus about the main functional differences between the two D2R isoform variants (Gardner et al., 1996; Xu et al., 2002). Nevertheless, two research groups have independently reported a differential dependence of D2LR and D2SR on G protein subtypes for agonist-induced signaling. Specifically, D2LR has been reported to interact efficiently with the Gαi2 subunit and poorly with the Gαi1 and Gαi3 subunits, while D2SR does not discriminate between these three G protein subunits (Montmayeur et al., 1993; Liu et al., 1994; Guiramand et al., 1995). It has also been suggested that the differential G protein subunit dependence is also effector dependent. Thus, G proteins containing Gαi1, Gαi2, or Gαi3 subunits would selectively couple and inhibit adenylyl cyclase whereas G proteins containing Gαo subunits would couple and close calcium channels (Liu et al., 1994). In our study, we did not find an absolute selective ability of D2LR to activate Gαi2-containing G protein, but a relative Gαi2 preference compared with D2SR and a higher potency of dopamine and norepinephrine at D2SR compared with D2LR were observed with most Gαi/αo subunits. The concomitant expression of Gαi1, Gαi2, and Gαi3 subunits in HEK-293 could explain the higher potency of dopamine at D2SR to inhibit adenylyl cyclase, compared with D2LR (Montmayeur et al., 1993).
The α2AR is functionally relevant to the D2-like receptors among the canonical norepinephrine adrenergic receptors because it is coupled to Gi/o proteins and highly expressed in the prefrontal cortex (Wise et al., 1997) where D2-like receptors are also expressed, especially D2R and D4R (Missale et al., 1998). The α2AR is considered a high-affinity receptor for norepinephrine with a reported Ki value of as low as 15 nM in transfected cells (O’Rourke et al., 1994). This is only 3- to 5-fold higher affinity compared with the Ki values of norepinephrine for D2-like receptor reported herein. Our functional readouts of Gi activation, adenylyl cyclase inhibition, and β arrestin recruitment showed greater but still relatively small separation between the potencies of norepinephrine and dopamine for α2AR and D2-like receptors (20-fold), verifying D2-like receptors as potential signal transducers for norepinephrine.
With our findings on relative potencies, the previously reported so-called cross reactivity phenomenon between dopamine and norepinephrine (Guiard et al., 2008; Gonzalez et al., 2012a; Lei, 2014) can now be better explained at the receptor-effector levels and considered as likely biologically occurring events. Furthermore, dopamine and norepinephrine concentrations in prefrontal cortex are comparable (Koob et al., 1975; Blank et al., 1979; Li et al., 1998), suggesting that actions of norepinephrine at cortical D2-like receptors are likely functionally significant.
In conclusion, norepinephrine is a potent agonist for D2-like receptors and, like dopamine, follows a general rank order of potency of D3R > D4R ≥ D2SR ≥ D2L in most functional assays. If D4R functions as an adrenergic receptor in some brain regions (Root et al., 2015), the other D2-like receptors—which in fact can be expressed with higher density in some of those areas—should also be considered as functional adrenergic receptors. Some intriguing differences in G protein activation have emerged, which depend on the Gαi and Gαo subunits involved. Although the framework of similarity among different effectors as well as between the two catecholamines may serve as a foundation for future studies on modulatory effects such as protein–protein interactions, the dissimilarities may be potentially pursued for further pharmacologic investigations.
Supplementary Material
Acknowledgments
The authors thank Dr. Céline Galés (INSERM) for Gαi-Rluc constructs, Dr. Jonathan Javitch (Columbia University) for dopamine receptor and β arrestin constructs, Dr. Thomas Keck for developing the [3H]7-OH DPAT binding assays, and Dr. Bruce Hope (NIDA, NIH) for the use of FACS CantoII (BD Biosciences).
Abbreviations
- α2AR
α2A adrenergic receptor
- BRET
bioluminescence resonance energy transfer
- CAMYEL
cAMP sensor with yellow fluorescence protein-Epac-luciferase
- D2R
D2 receptor
- D2SR
D2 receptor short isoform
- D2LR
D2 receptor long isoform
- D3R
D3 receptor
- D4R
D4 receptor
- D4.2R
D4.4R, D4.7R, D4 receptor variant with 2-,4-,7-tandem repeats
- FACS
fluorescence-activated cell sorting
- DMSO
dimethylsulfoxide
- GPCR
G protein-coupled receptor
- GRK2
G protein-coupled receptor kinase 2
- HEK
human embryonic kidney
- L745,870
3-{[4-(4-chlorophenyl)piperazin-1-yl]methyl}-1H-pyrrolo[2,3-b]pyridine
- RLuc8
Renilla luciferase 8
- 7-OH-DPAT
7-hydroxy-N,N-di-n-propyl-2-aminotetralin
- YFP
yellow fluorescence protein
Authorship Contributions
Participated in research design: Sánchez-Soto, Ferré, Yano.
Conducted experiments: Sánchez-Soto, Bonifazi, Cai, Ellenberger, Yano.
Contributed new reagents or analytic tools: Yano.
Performed data analysis: Sánchez-Soto, Bonifazi, Cai, Ellenberger, Newman, Ferré, Yano.
Wrote or contributed to the writing of the manuscript: Sánchez-Soto, Bonifazi, Newman, Ferré, Yano.
Footnotes
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Drug Abuse (All authors) and a fellowship from the Japan Society for the Promotion of Science (H.Y.).
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. (2010) Understanding wiring and volume transmission. Brain Res Brain Res Rev 64:137–159. [DOI] [PubMed] [Google Scholar]
- Antoni FA. (2012) New paradigms in cAMP signalling. Mol Cell Endocrinol 353:3–9. [DOI] [PubMed] [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HHM. (1995) Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem 65:1157–1165. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Lopez J, Wager-Miller J, Mackie K, Straiker A. (2011) Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J-M, Gainetdinov RR. (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217. [DOI] [PubMed] [Google Scholar]
- Blank CL, Sasa S, Isernhagen R, Meyerson LR, Wassil D, Wong P, Modak AT, Stavinoha WB. (1979) Levels of norepinephrine and dopamine in mouse brain regions following microwave inactivation--rapid post-mortem degradation of striatal dopamine in decapitated animals. J Neurochem 33:213–219. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martín AB, Pisani A, Tognazzi N, Bernardi G, Moratalla R, et al. (2003) Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci 23:6245–6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton CC, Donthamsetti P, Lambert NA, Javitch JA, Neve KA. (2014) Mutation of three residues in the third intracellular loop of the dopamine D2 receptor creates an internalization-defective receptor. J Biol Chem 289:33663–33675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costagliola S, Rodien P, Many M-C, Ludgate M, Vassart G. (1998) Genetic immunization against the human thyrotropin receptor causes thyroiditis and allows production of monoclonal antibodies recognizing the native receptor. J Immunol 160:1458–1465. [PubMed] [Google Scholar]
- Cummings DF, Ericksen SS, Goetz A, Schetz JA. (2010) Transmembrane segment five serines of the D4 dopamine receptor uniquely influence the interactions of dopamine, norepinephrine, and Ro10-4548. J Pharmacol Exp Ther 333:682–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czermak C, Lehofer M, Liebmann PM, Traynor J. (2006) [35S]GTPγS binding at the human dopamine D4 receptor variants hD4.2, hD4.4 and hD4.7 following stimulation by dopamine, epinephrine and norepinephrine. Eur J Pharmacol 531:20–24. [DOI] [PubMed] [Google Scholar]
- Descarries L, Lemay B, Doucet G, Berger B. (1987) Regional and laminar density of the dopamine innervation in adult rat cerebral cortex. Neuroscience 21:807–824. [DOI] [PubMed] [Google Scholar]
- Devoto P, Flore G. (2006) On the origin of cortical dopamine: is it a co-transmitter in noradrenergic neurons? Curr Neuropharmacol 4:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evron T, Daigle TL, Caron MG. (2012) GRK2: multiple roles beyond G protein-coupled receptor desensitization. Trends Pharmacol Sci 33:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Casadó V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin J-P, Guitart X. (2014) G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev 66:413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick AL, Yano H, Trifilieff P, Vishwasrao HD, Biezonski D, Mészáros J, Urizar E, Sibley DR, Kellendonk C, Sonntag KC, et al. (2015) Evidence against dopamine D1/D2 receptor heteromers. Mol Psychiatry 20:1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galés C, Rebois RV, Hogue M, Trieu P, Breit A, Hébert TE, Bouvier M. (2005) Real-time monitoring of receptor and G-protein interactions in living cells. Nat Methods 2:177–184. [DOI] [PubMed] [Google Scholar]
- Galés C, Van Durm JJJ, Schaak S, Pontier S, Percherancier Y, Audet M, Paris H, Bouvier M. (2006) Probing the activation-promoted structural rearrangements in preassembled receptor-G protein complexes. Nat Struct Mol Biol 13:778–786. [DOI] [PubMed] [Google Scholar]
- Gardner B, Hall DA, Strange PG. (1996) Pharmacological analysis of dopamine stimulation of [35S]-GTP γ S binding via human D2short and D2long dopamine receptors expressed in recombinant cells. Br J Pharmacol 118:1544–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith SK, Joyce JN. (1994) Dopamine D2 receptor expression in hippocampus and parahippocampal cortex of rat, cat, and human in relation to tyrosine hydroxylase-immunoreactive fibers. Hippocampus 4:354–373. [DOI] [PubMed] [Google Scholar]
- González S, Moreno-Delgado D, Moreno E, Pérez-Capote K, Franco R, Mallol J, Cortés A, Casadó V, Lluís C, Ortiz J, et al. (2012a) Circadian-related heteromerization of adrenergic and dopamine D₄ receptors modulates melatonin synthesis and release in the pineal gland. PLoS Biol 10:e1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González S, Rangel-Barajas C, Peper M, Lorenzo R, Moreno E, Ciruela F, Borycz J, Ortiz J, Lluís C, Franco R, et al. (2012b) Dopamine D4 receptor, but not the ADHD-associated D4.7 variant, forms functional heteromers with the dopamine D2S receptor in the brain. Mol Psychiatry 17:650–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XM, Kobilka TS, Kobilka BK. (1992) Enhancement of membrane insertion and function in a type IIIb membrane protein following introduction of a cleavable signal peptide. J Biol Chem 267:21995–21998. [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Blier P. (2008) Cross-talk between dopaminergic and noradrenergic systems in the rat ventral tegmental area, locus ceruleus, and dorsal hippocampus. Mol Pharmacol 74:1463–1475. [DOI] [PubMed] [Google Scholar]
- Guiramand J, Montmayeur J-P, Ceraline J, Bhatia M, Borrelli E. (1995) Alternative splicing of the dopamine D2 receptor directs specificity of coupling to G-proteins. J Biol Chem 270:7354–7358. [DOI] [PubMed] [Google Scholar]
- Guitart X, Navarro G, Moreno E, Yano H, Cai N-S, Sánchez-Soto M, Kumar-Barodia S, Naidu YT, Mallol J, Cortés A, et al. (2014) Functional selectivity of allosteric interactions within G protein-coupled receptor oligomers: the dopamine D1-D3 receptor heterotetramer. Mol Pharmacol 86:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, Filizola M, Javitch JA. (2008) Dopamine D2 receptors form higher order oligomers at physiological expression levels. EMBO J 27:2293–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervé D. (2011) Identification of a specific assembly of the G protein golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front Neuroanat 5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LI, Collins J, Davis R, Lin K-M, DeCamp D, Roach T, Hsueh R, Rebres RA, Ross EM, Taussig R, et al. (2007) Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J Biol Chem 282:10576–10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Spicher K, Boulay G, Wang Y, Birnbaumer L. (2001) Most central nervous system D2 dopamine receptors are coupled to their effectors by Go. Proc Natl Acad Sci USA 98:3577–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic V, Guan HC, Van Tol HHM. (1999) Comparative pharmacological and functional analysis of the human dopamine D4.2 and D4.10 receptor variants. Pharmacogenetics 9:561–568. [PubMed] [Google Scholar]
- Kazmi MA, Snyder LA, Cypess AM, Graber SG, Sakmar TP. (2000) Selective reconstitution of human D4 dopamine receptor variants with Gi α subtypes. Biochemistry 39:3734–3744. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, Goldman-Rakic PS. (1998) Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci USA 95:7731–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Balcom GJ, Meyerhoff JL. (1975) Dopamine and norepinephrine levels in the nucleus accumbens, olfactory tubercle and corpus striatum following lesions in the ventral tegmentalarea. Brain Res 94:45–55. [DOI] [PubMed] [Google Scholar]
- Lanau F, Zenner M-T, Civelli O, Hartman DS. (1997) Epinephrine and norepinephrine act as potent agonists at the recombinant human dopamine D4 receptor. J Neurochem 68:804–812. [DOI] [PubMed] [Google Scholar]
- Lane JR, Powney B, Wise A, Rees S, Milligan G. (2008) G protein coupling and ligand selectivity of the D2L and D3 dopamine receptors. J Pharmacol Exp Ther 325:319–330. [DOI] [PubMed] [Google Scholar]
- Lei S. (2014) Cross interaction of dopaminergic and adrenergic systems in neural modulation. Int J Physiol. Pathol Pharmacol 6:137–142. [PMC free article] [PubMed] [Google Scholar]
- Lévesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P. (1992) Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci USA 89:8155–8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-M, Perry KW, Wong DT, Bymaster FP. (1998) Olanzapine increases in vivo dopamine and norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacology (Berl) 136:153–161. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Rakic P, Innis RB. (1989) Dopamine D2 receptors in the cerebral cortex: distribution and pharmacological characterization with [3H]raclopride. Proc Natl Acad Sci USA 86:6412–6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren N, Usiello A, Goiny M, Haycock J, Erbs E, Greengard P, Hokfelt T, Borrelli E, Fisone G. (2003) Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. Proc Natl Acad Sci USA 100:4305–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YF, Jakobs KH, Rasenick MM, Albert PR. (1994) G protein specificity in receptor-effector coupling. Analysis of the roles of G0 and Gi2 in GH4C1 pituitary cells. J Biol Chem 269:13880–13886. [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. (1998) Dopamine receptors: from structure to function. Physiol Rev 78:189–225. [DOI] [PubMed] [Google Scholar]
- Montmayeur JP, Guiramand J, Borrelli E. (1993) Preferential coupling between dopamine D2 receptors and G-proteins. Mol Endocrinol 7:161–170. [DOI] [PubMed] [Google Scholar]
- Namkung Y, Dipace C, Javitch JA, Sibley DR. (2009) G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor. J Biol Chem 284:15038–15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A, Audinot-Bouchez V, Gobert A, Millan MJ. (1997) Noradrenaline and adrenaline are high affinity agonists at dopamine D4 receptors. Eur J Pharmacol 319:379–383. [DOI] [PubMed] [Google Scholar]
- O’Rourke MF, Blaxall HS, Iversen LJ, Bylund DB. (1994) Characterization of [3H]RX821002 binding to alpha-2 adrenergic receptor subtypes. J Pharmacol Exp Ther 268:1362–1367. [PubMed] [Google Scholar]
- Orford M, Mazurkiewicz D, Milligan G, Saggerson D. (1991) Abundance of the α-subunits of Gi1, Gi2 and Go in synaptosomal membranes from several regions of the rat brain is increased in hypothyroidism. Biochem J 275:183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. (1989) Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neuroscience 30:767–777. [DOI] [PubMed] [Google Scholar]
- Rivera A, Peñafiel A, Megías M, Agnati LF, López-Téllez JF, Gago B, Gutiérrez A, de la Calle A, Fuxe K. (2008) Cellular localization and distribution of dopamine D(4) receptors in the rat cerebral cortex and their relationship with the cortical dopaminergic and noradrenergic nerve terminal networks. Neuroscience 155:997–1010. [DOI] [PubMed] [Google Scholar]
- Root DH, Hoffman AF, Good CH, Zhang S, Gigante E, Lupica CR, Morales M. (2015) Norepinephrine activates dopamine D4 receptors in the rat lateral habenula. J Neurosci 35:3460–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguéla P, Watkins KC, Geffard M, Descarries L. (1990) Noradrenaline axon terminals in adult rat neocortex: an immunocytochemical analysis in serial thin sections. Neuroscience 35:249–264. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. (2011) β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci 32:521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urizar E, Yano H, Kolster R, Galés C, Lambert N, Javitch JA. (2011) CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat Chem Biol 7:624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Gimenez LE, Francis DJ, Hanson SM, Hubbell WL, Klug CS, Gurevich VV. (2011) Few residues within an extensive binding interface drive receptor interaction and determine the specificity of arrestin proteins. J Biol Chem 286:24288–24299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Macmillan LB, Fremeau RT, Jr, Magnuson MA, Lindner J, Limbird LE. (1996) Expression of alpha 2-adrenergic receptor subtypes in the mouse brain: evaluation of spatial and temporal information imparted by 3 kb of 5′ regulatory sequence for the alpha 2A AR-receptor gene in transgenic animals. Neuroscience 74:199–218. [DOI] [PubMed] [Google Scholar]
- Wędzony K, Chocyk A, Maćkowiak M, Fijał K, Czyrak A. (2000) Cortical localization of dopamine D4 receptors in the rat brain—immunocytochemical study. J Physiol Pharmacol 51:205–221. [PubMed] [Google Scholar]
- Wise A, Grassie MA, Parenti M, Lee M, Rees S, Milligan G. (1997) A cysteine-3 to serine mutation of the G-protein Gi1 α abrogates functional activation by the α 2A-adrenoceptor but not interactions with the β γ complex. Biochemistry 36:10620–10629. [DOI] [PubMed] [Google Scholar]
- Xu R, Hranilovic D, Fetsko LA, Bucan M, Wang Y. (2002) Dopamine D2S and D2L receptors may differentially contribute to the actions of antipsychotic and psychotic agents in mice. Mol Psychiatry 7:1075–1082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.