Abstract
In acute organ injuries, mitochondria are often dysfunctional, and recent research has revealed that recovery of mitochondrial and renal functions is accelerated by induction of mitochondrial biogenesis (MB). We previously reported that the nonselective 5-HT2 receptor agonist DOI [1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine] induced MB in renal proximal tubular cells (RPTCs). The goal of this study was to determine the role of 5-HT2 receptors in the regulation of mitochondrial genes and oxidative metabolism in the kidney. The 5-HT2C receptor agonist CP-809,101 [2-[(3-chlorophenyl)methoxy]-6-(1-piperazinyl)pyrazine] and antagonist SB-242,084 [6-chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride] were used to examine the induction of renal mitochondrial genes and oxidative metabolism in RPTCs and in mouse kidneys in the presence and absence of the 5-HT2C receptor. Unexpectedly, both CP-809,101 and SB-242,084 increased RPTC respiration and peroxisome proliferator–activated receptor γ coactivator-1α (PGC-1α) mRNA expression in RPTCs at 1–10 nM. In addition, CP-809,101 and SB-242,084 increased mRNA expression of PGC-1α and the mitochondrial proteins NADH dehydrogenase subunit 1 and NADH dehydrogenase (ubiquinone) β subcomplex 8 in mice. These compounds increased mitochondrial genes in RPTCs in which the 5-HT2C receptor was downregulated with small interfering RNA and in the renal cortex of mice lacking the 5-HT2C receptor. By contrast, the ability of these compounds to increase PGC-1α mRNA and respiration was blocked in RPTCs treated with 5-HT2A receptor small interfering RNA or the 5-HT2A receptor antagonist eplivanserin. In addition, the 5-HT2A receptor agonist NBOH-2C-CN [4-[2-[[(2-hydroxyphenyl)methyl]amino]ethyl]-2,5-dimethoxybenzonitrile] increased RPTC respiration at 1–100 nM. These results suggest that agonism of the 5-HT2A receptor induces MB and that the classic 5-HT2C receptor agonist CP-809,101 and antagonist SB-242,084 increase mitochondrial genes and oxidative metabolism through the 5-HT2A receptor. To our knowledge, this is the first report that links 5-HT2A receptor agonism to mitochondrial function.
Introduction
Mitochondrial dysfunction is a pathologic state underlying many diseases, including chronic diseases such as diabetes, Alzheimer’s disease, Huntington’s disease, and amyotrophic lateral sclerosis, and acute injuries to the heart, liver, kidney, and brain (Butterfield et al., 2001; Lifshitz et al., 2004; Ballinger, 2005; Chen et al., 2011; Da Cruz et al., 2012; Jaeschke et al., 2012). The kidney requires high levels of ATP to drive the processes necessary for active tubular transport and is at risk for mitochondrial dysfunction caused by acute ischemia/reperfusion, drugs, toxicants, and diabetes (Ozbek, 2012). It has been demonstrated that recovery of mitochondrial function precedes recovery of cellular structure and function in a renal proximal tubule cell (RPTC) model of oxidant injury (Weinberg et al., 1997; Nowak et al., 1998; Weinberg et al., 2000; Feldkamp et al., 2005). Our laboratory has previously reported that mitochondrial proteins are suppressed up to 144 hours after ischemic acute kidney injury, which indicates that promoting the recovery of mitochondrial function may be a viable therapeutic strategy for the treatment of acute kidney injury (Rasbach and Schnellmann, 2007; Funk and Schnellmann, 2012).
One strategy for improving mitochondrial function is promotion of mitochondrial biogenesis (MB). MB is an intricate process that drives coordinated transcription of both mitochondrial DNA and nuclear-encoded genes to increase cellular mitochondrial content. Mitochondrial health and homeostasis is maintained through MB, mitochondrial fission and fusion, and mitophagy; under physiologic conditions, these mechanisms function together to remove unhealthy mitochondria (Seo et al., 2010). Central to this process is the induction of peroxisome proliferator–activated receptor γ coactivator-1α (PGC-1α), the “master regulator of MB” (Kelly and Scarpulla, 2004). Increases in PGC-1α lead directly and indirectly to the transcription of genes necessary for mitochondrial function, including nuclear respiratory factor, mitochondrial transcription factor A, and genes that encode proteins of the electron transport chain (Ventura-Clapier et al., 2008). The net result of increased PGC-1α is the upregulation of electron transport chain proteins, resulting in greater electron flux and increased ATP production. PGC-1α expression can be induced by a number of physiologic and pathologic stimuli, resulting in the amelioration of mitochondrial dysfunction in several model systems, including acute oxidant injury in RPTCs, amyotrophic lateral sclerosis, and Huntington’s disease (Puigserver et al., 1998; Rasbach and Schnellmann, 2007; Da Cruz et al., 2012; Tsunemi et al., 2012).
Our drug discovery program in MB has previously identified several pharmacological targets that activate PGC-1α and induce MB, including the β2 adrenergic receptor, phosphodiesterases inhibitors, and the 5-HT2 class of receptors (Rasbach et al., 2010; Wills et al., 2012). Of these targets, the role of the 5-HT2 receptor in mitochondrial signaling has been explored the least.
The 5-HT2 family of receptors is composed of three receptor subtypes: 5-HT2A, 5-HT2B, and 5-HT2C. All 5-HT2 receptors are G protein–coupled receptors traditionally described as being coupled to Gq/11 protein for their signal transduction, although some groups have suggested that at least some of the receptor subtypes in this class may also couple to Gi/0 (Garnovskaya et al., 1995; Roth, 2006; Alexander et al., 2008). Signaling of these receptors through Gq/11 second messengers can lead to activation of pathways previously implicated in the regulation of PGC-1α, and 5-HT has been identified as a survival factor for mitochondria in cardiomyocytes through 5-HT2B receptor signaling (Nebigil et al., 2003). Several studies examining 5-HT2 receptors in the kidney show 5-HT2A, 5-HT2B, and 5-HT2C mRNA expression in cultured renal cells, in addition to 5-HT2A protein in renal mesangial cells (Garnovskaya et al., 1995; Xu et al., 2007).
Consequently, previous investigations into the role of the renal 5-HT2 receptor in mitochondria demonstrated that DOI [1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine], a nonselective 5-HT2 receptor agonist, induced MB and repaired mitochondrial dysfunction caused by acute oxidant injury in RPTCs (Rasbach et al., 2010). However, because DOI is a 5-HT2 receptor pan-agonist, the MB activity could not be assigned to a specific 5-HT2 receptor. Thus, the goal of this study was to determine the role of 5-HT2C receptors in mitochondrial gene expression.
Unexpectedly, we found that the 5-HT2C receptor agonist CP-809,101 and antagonist SB-242,084 both increased cellular respiration and genetic markers of MB through the 5-HT2A receptor, in vitro and in vivo. To our knowledge, this is the first report that 5-HT2A agonism is linked to a mitochondrial function.
Materials and Methods
Reagents.
CP-809,101 [2-[(3-chlorophenyl)methoxy]-6-(1-piperazinyl)pyrazine] (Siuciak et al., 2007), SB-242,084 [6-chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride] (Bromidge et al., 1997), eplivanserin (Rinaldi-Carmona et al., 1992), and NBOH-2C-CN [4-[2-[[(2-hydroxyphenyl)methyl]amino]ethyl]-2,5-dimethoxybenzonitrile] (Hansen et al., 2014) were purchased from Tocris Bioscience (Ellisville, MO). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Animal Care and Use.
All experiments were performed in strict accordance with the guidelines in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina or Columbia University and appropriate efforts were made to reduce animal suffering. This study adhered to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) standards previously detailed by Kilkenny et al. (2010).
Isolation and Culture of Proximal Tubules.
Female New Zealand white rabbits (1.5–2.0 kg) were purchased from Charles River Laboratories (Wilmington, MA). RPTC isolation was performed using the iron oxide perfusion method, and RPTCs were cultured under improved conditions as previously described (Nowak and Schnellmann, 1995, 1996). Three days after initial plating, dedifferentiated RPTCs were trypsinized and replated on XF-96 polystyrene cell culture microplates (Seahorse Bioscience, North Bellerica, MA) at a density of 18,000 cells/well and were maintained at 37°C for 3 days before pharmacological manipulation (Beeson et al., 2010). For other RPTC experiments, isolated renal proximal tubules were plated in 35-mm dishes and used 8 days after initial plating. RPTCs were treated with experimental compounds for 24 hours.
Oxygen Consumption.
The oxygen consumption rate (OCR) of RPTCs was measured using the Seahorse Bioscience XF-96 Extracellular Flux Analyzer as previously described (Beeson et al., 2010). Each 96-well assay plate was treated with vehicle control (dimethylsulfoxide < 0.5%) or 1-nM, 10-nM, or 100-nM concentrations of the experimental compounds. Basal OCR was measured before injection of carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) (0.5 μM), which allows for the measurement of uncoupled OCR (FCCP-OCR), a marker of MB (Beeson et al., 2010).
5-HT Receptor Gene Expression.
Total mRNAs were extracted from RPTCs using TRIzol RNA extract reagent (Thermo Fisher Scientific, Waltham, MA). For quantitative reverse-transcription (RT) polymerase chain reaction (PCR) analysis, cDNA was synthesized using the iScript Advanced cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) with 4 μg RNA. PCR products were amplified from 5 μl cDNA template using SsoAdvanced Universal SYBR Green Supermix reagent (Bio-Rad Laboratories) and 400-nM concentrations of each primer (Integrated DNA Technologies, Inc., Coralville, IA). Primer sequences for 5-HT2A, 5-HT2B, and 5-HT2C were previously described (Rasbach et al., 2010).
5-HT2C Receptor Protein Expression.
5-HT receptor proteins were isolated from RPTCs as previously described (Morabito et al., 2010). A 30-µg sample of the resulting protein was then treated with either N-glycosidase F (PNGase) (New England BioLabs, Ipswich, MA) according to the manufacturer’s instructions or temperature-matched control conditions for 2 hours, then loaded onto an SDS-PAGE gel. After electrophoretic transfer to nitrocellulose, 5-HT2C protein levels were determined by immunoblot analysis using an anti-5HT2C receptor monoclonal antibody [SR-2C (D-12), 1:250; Santa Cruz Biotechnology, Dallas, TX], followed by a horseradish peroxidase–labeled anti-mouse secondary antibody [1:20,000; Santa Cruz Biotechnology]. The secondary antibody was detected with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific).
5-HT2A Receptor Protein Expression.
RPTCs were suspended in 150 µl protein lysis buffer (1% Triton X-100, 150 mM NaCl, and 10 mM Tris-HCl, pH 7.4; 1 mM EDTA; 1 mM EGTA; 2 mM sodium orthovanadate; 0.2 mM phenylmethylsulfonyl fluoride; 1 mM HEPES, pH 7.6; 1 μg/ml leupeptin; and 1 μg/ml aprotinin) and sonicated. The homogenate was stored on ice for 10 minutes and then centrifuged at 10,000 × g for 10 minutes at 4°C. The supernatant was collected, and protein was determined using a bicinchoninic acid kit (Sigma-Aldrich) with bovine serum albumin as the standard. Proteins (50–75 μg) were separated on 4%–20% gradient SDS-polyacrylamide gels and electrophoretically transferred to nitrocellulose membranes. 5-HT2A protein levels were determined by immunoblot analysis using an anti-5-HT2A receptor monoclonal antibody (1:500; ImmunoStar Inc., Hudson, WI), then with horseradish peroxidase–labeled anti-rabbit secondary antibody (1:2000; Abcam, Cambridge, MA). The secondary antibody was detected as described above.
In Vivo Mouse Studies.
Male C57/Bl6 mice (aged 6–8 weeks) were purchased from the National Cancer Institute (Bethesda, MD). Mice were individually housed in a temperature-controlled room under a 12-hour light/dark cycle and were randomly assigned to either a vehicle control group or one of two treatment groups. Mice were administered a single intraperitoneal dose of either diluent [40% (2-hydroxypropyl)-β-cyclodextrin in 0.9% saline], CP-809,101 [1 mg/kg], or SB-242,084 [1 mg/kg]. Doses of CP-809,101 and SB-242,084 were chosen based on previously published studies (Kennett et al., 1997; Siuciak et al., 2007; Strong et al., 2009). At 24 hours after treatment, mice were euthanized by CO2 asphyxiation followed by cervical dislocation. Kidneys were isolated and snap frozen for quantitative polymerase chain reaction (qPCR) analysis.
Generation of 5-HT2C Transgenic Mice.
5-HT2C transgenic mice on a 129SvEv/Tac background were bred as described previously (Xu et al., 2008). Briefly, 5-HT2C is an X-linked gene; therefore, female mice heterozygotic for the transgene were bred with wild-type (WT) male mice, generating WT and heterozygote female mice, and WT and 5-HT2C null male offspring. Tail clips were taken and lysed overnight for PCR genotyping identification of the animals. Mice were weaned at 3 to 4 weeks and single-sex group housed under standard conditions until adulthood.
5-HT2C Transgenic Mouse Experiments.
Animals were housed on a 12-hour light/dark cycle with food and water available ad libitum. At age 6–9 weeks, 5-HT2C WT and knockout (KO) mice were randomly assigned to vehicle control, CP-809,101 (1 mg/kg), or SB-242,084 (1 mg/kg) treatment groups and treated as described above. At 24 hours after a single injection of either diluent or drug, mice were euthanized by cervical dislocation, and kidneys were isolated and snap frozen for qPCR analysis.
5-HT2C and 5-HT2A Knockdown Experiments in RPTCs.
Rabbit mRNA sequences for 5-HT2A and 5-HT2C receptors were obtained from Ensembl (Ensembl Project, Hinxton, England). The BLOCK-iT RNAi Designer (Thermo Fisher Scientific) was used to design small interfering RNA (siRNA) to these sequences. Two days postconfluence, RPTCs were treated with either 200 nM scramble siRNA (siGenome nontargeting siRNA 3; Dharmacon RNAi Technologies, Lafayette, CO), 100 nM of both 5-HT2A siRNAs 1 and 2 (siRNA 1: 5′-C AAG TCG CTC CAG AAA GAA GCT ACT-3′; siRNA 2: 5′-GAG AAC AAG AAA CCG TTG CAG TTA A-3′) or 100 nM of both 5-HT2C siRNAs 1 and 2 (siRNA 1: 5′-GA GAT GAA GAC AAA GTG TTC GTG AA-3′; siRNA 2: 5′-CCA GCA CTG TCA ATC GTG GTA ATA AT-3′). To optimize gene silencing prior to measuring a pharmacological response, RPTCs were treated with the experimental drugs 72 hours after initial siRNA exposure. After 24 hours of exposure to the experimental drugs, the cells were harvested for RT-PCR analysis.
Real-Time RT-PCR.
Total RNA was extracted from RPTCs or renal cortex samples using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. cDNA was synthesized via RT using the RevertAid First Strand cDNA kit (Thermo Fisher Scientific) with 1 to 2 μg RNA. PCR products were amplified from 5 μl cDNA template using 2× Maxima SYBR green qPCR master mix (Thermo Fisher Scientific) and 400-nM concentrations of each primer (Integrated DNA Technologies, Inc.). Primer sequences for PGC-1α, NADH dehydrogenase subunit 1 (ND1), NADH dehydrogenase (ubiquinone) β subcomplex 8 (NDUFB8), tubulin, and β-actin were described previously (Smith et al., 2015). All measurements within a species used the same housekeeping gene (e.g., tubulin for rabbit, actin for mouse), and individual PCR runs were considered invalid if the average threshold cycle value for the housekeeping gene varied more than 0.5 cycles between treatment groups.
Statistical Analyses.
Data are presented as means ± S.E.M. Single comparisons were performed using a t test, whereas data found to not have a normal distribution were subjected to a Mann–Whitney U test. Multiple comparisons for normal data were performed using one-way analysis of variance with an appropriate post hoc test to compare multiple means. Kruskal–Wallis one-way analysis of variance was used to perform multiple comparisons for non-normal data, and a Holm–Sidak’s post test was used to compare multiple means. Single and multiple comparison data were considered statistically different at P ≤ 0.05. RPTCs isolated from a single rabbit represented an individual experiment (n = 1) and were repeated until n ≥ 4 was obtained. Rodent studies were repeated until n ≥ 3 was obtained.
Results
The 5-HT2C Receptor Is Expressed in the Kidney and in RPTCs.
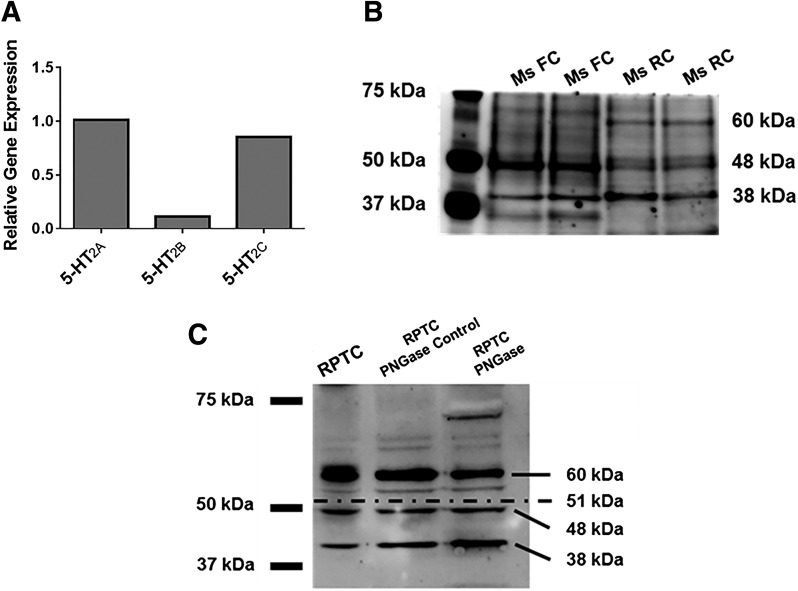
5-HT2A, 5-HT2B, and 5-HT2C receptor mRNAs were identified in RPTCs (Fig. 1A). Relative expression of 5-HT2A and 5-HT2C mRNA was similar and was approximately 10-fold greater than 5-HT2B. The most commonly reported molecular mass of the 5-HT2c receptor is 48 kDa; this molecular mass corresponds to the receptor after it has undergone one N-glycosylation (Abramowski and Staufenbiel, 1995). In addition, it has been reported that the receptor is also expressed as a 60-kDa protein N-glycosylated at two sites and as a 38-kDa protein with no N-glycosylations (Abramowski and Staufenbiel, 1995). In the mouse frontal cortex, the 48-kDa receptor was the most prominent form of the 5-HT2c receptor; the 38-kDa form was also detected, but there was no expression of the 60-kDa form (Fig. 1B). Conversely, the most prominent isoform detected in the mouse kidney was the 38-kDa nonglycosylated protein, with lower levels of the 60-kDa and 48-kDa glycosylated proteins (Fig. 1B).
Fig. 1.
5-HT2 receptors are expressed in renal tissue. 5-HT2A, 5-HT2B, and 5-HT2C receptor mRNA expression was measured in RPTCs using RT-qPCR. (A) Tubulin was used as a control gene, and 5-HT2B and 5-HT2C expression was normalized to 5-HT2A. (B) 5-HT2C receptor protein expression was analyzed by immunoblot in the mouse frontal cortex and mouse renal cortex. (C) 5-HT2C receptor protein expression was analyzed by immunoblot in rabbit RPTCs in the presence and absence of PNGase. The expected bands are 60 kDa (receptor with two N-glycosylations), 48 kDa (receptor with one N-glycosylation), 38 kDa (nonglycosylated receptor), and 70 kDa (nonglycosylated receptor dimer). FC, frontal cortex; Ms, mouse; RC, renal cortex.
In RPTCs, all three isoforms of the receptor were identified, with the 60-kDa receptor being most prominent. Previous investigations into the glycosylation state of the 5-HT2C receptor examined lysates treated with PNGase F, an enzyme that cleaves an N-glycosyl group from an asparagine residue, and described the loss of the 60-kDa band with corresponding increases observed in the 48-kDa and 38-kDa bands (Abramowski and Staufenbiel, 1995). Accordingly, in our study, 2-hour PNGase F treatment of our enriched protein decreased the quantity of the 60-kDa receptor and increased the quantity of the 38-kDa receptor (Fig. 1C). In addition, a band was detected at 70 kDa after PNGase F treatment, which corresponds to a homodimer formed by two nonglycosylated 5-HT2C receptor proteins as previously described (Herrick-Davis et al., 2015).
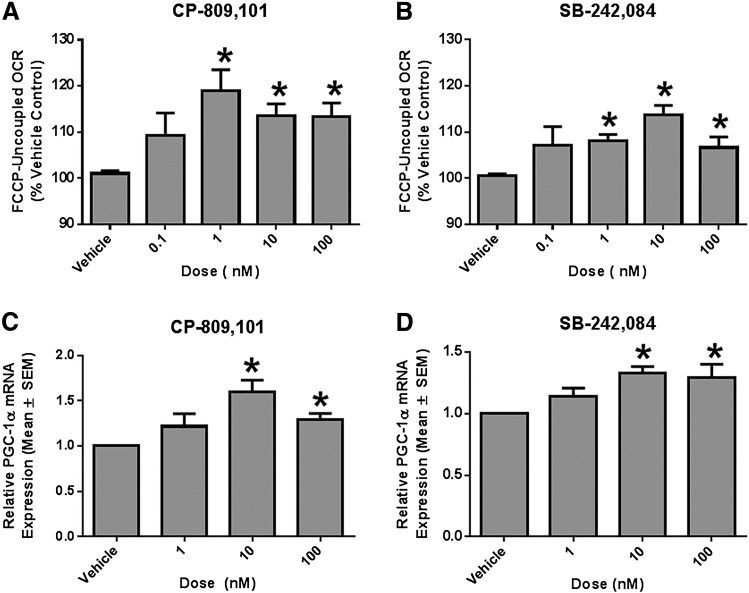
5-HT2C Receptor Agonists and Antagonists Increase FCCP-Uncoupled Respiration and PGC-1α mRNA Expression in RPTCs.
RPTCs were plated in XF-96 plates and grown under improved culture conditions that maintain polarity, differentiated function, and respiration rates similar to in vivo RPTCs (Nowak and Schnellmann, 1995, 1996; Wills et al., 2012). RPTCs were treated for 24 hours with either vehicle control (0.5% dimethylsulfoxide), CP-809,101 (selective 5-HT2C agonist), or SB-242,084 (selective 5-HT2C antagonist). FCCP-OCR is a stress test to measure maximal respiratory capacity and mitochondrial function and is a marker of MB. CP-809,101 and SB-242,084 increased FCCP-OCR relative to vehicle controls at concentrations ranging from 1 to 100 nM (Fig. 2, A and B). It should be noted that the pKi values for CP-809,101 and SB-242,084 for the 5-HT2C receptor are 0.1 nM and 10 nM, respectively (Table 1). CP-809101 is 1,000-fold more selective at the 5-HT2C receptor compared with 5-HT2A and 5-HT2B receptors (Table 1) (Siuciak et al., 2007). Similarly, SB-242,084 is 10-100-fold more selective at the 5-HT2C receptor compared with 5-HT2A and 5-HT2B receptors, respectively (Table 1) (Kennett et al., 1997).
Fig. 2.
A 5-HT2C agonist and antagonist induce FCCP-uncoupled respiration in RPTCs. FCCP-uncoupled respiration is a screening assay for MB. RPTCs were treated with either CP-809,101 (A and C) or SB-242,084 (B and D) for 24 hours. FCCP-uncoupled mitochondrial respiration was measured using the Seahorse XF-96 instrument. RPTC PGC-1α mRNA expression was determined by RT-qPCR using tubulin as a control. Data were analyzed using Kruskal–Wallis one-way analysis of variance with Dunn’s multiple comparison test. Values are reported as means ± S.E.M. (n ≥ 3). *P < 0.05 versus vehicle control.
TABLE 1.
Receptor binding data for 5-HT2 receptor ligands
| Drug Name | 5-HT2A | 5-HT2B | 5-HT2C |
|---|---|---|---|
| DOI | 7.4–9.2 | 7.6 – 7.7 | 7.2–8.6 |
| CP-809,101 | 6.8 | 7.2 | 10.0 |
| SB-242,084 | 6.1–6.8 | 6.8–7.0 | 8.2–9.0 |
| Eplivanserin | 9.4 | — | 6.13 |
| NBOH-2C-CN | 8.9 | — | 6.9 |
pKi values for DOI and SB-242,084 are reported by the IUPHAR Database. pEC50 values for CP-809,101 are reported by Siuciak et al. (2007). Whole-cell pKi values for eplivanserin are reported by Vanover and Davis (2010). Whole-cell pKi values for NBOH-2C-CN are reported by Hansen et al. (2014).
To demonstrate that these increased FCCP-OCR rates resulted from changes in mitochondrial homeostasis, PGC-1α mRNA was measured using RT-qPCR. PGC-1α mRNA increased at 10 and 100 nM CP-809,101 and at 10 and 100 nM SB-242,084 (Fig. 2, C and D).
5-HT2C Receptor Agonists and Antagonists Increase Mitochondrial Gene Expression in Mouse Renal Cortex.
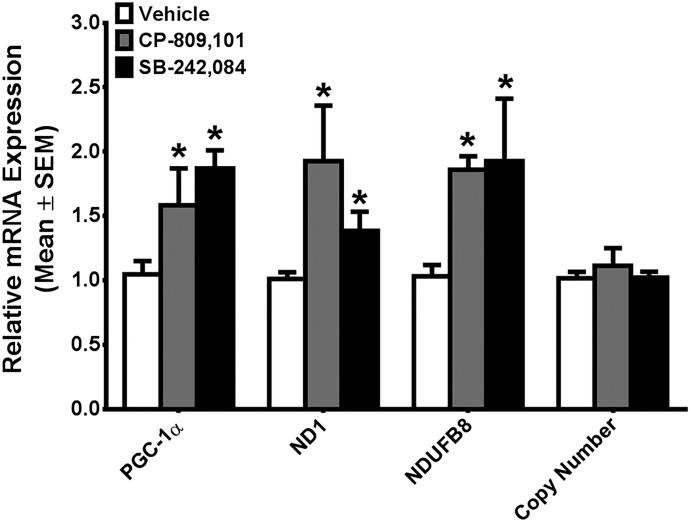
Mice treated with 1 mg/kg CP-809,101 for 24 hours exhibited increased PGC-1α mRNA (1.6-fold), whereas mRNA expression of the mitochondrial-encoded gene ND1 and nuclear-encoded gene NDUFB8 increased 1.9-fold (Fig. 3). Mice treated with 1 mg/kg SB-242,084 for 24 hours revealed a 1.9-fold increase in renal cortical PGC-1α mRNA, with concomitant 1.4- and 1.9-fold increases in ND1 and NDUFB8 mRNA, respectively (Fig. 3). Despite these changes in mRNA levels, no changes in mtDNA copy number were detected between vehicle and either 1 mg/kg CP-809,101 or 1 mg/kg SB-242,084 at this time point (Fig. 3).
Fig. 3.
A 5-HT2C agonist and antagonist induce mitochondrial gene expression in naïve mouse kidney cortex. C57/Bl6 mice were treated with a single intraperitoneal 1-mg/kg dose of CP-809,101, SB-242,084, or vehicle. PGC-1α, ND1, and NDUFB8 mRNA expression in the renal cortex was determined by RT-qPCR using actin as a control. mtDNA copy number was determined by qPCR, using ND1 for the mtDNA gene and actin for the nuclear control gene. Values are reported as means ± S.E.M. The t test was used to determine significance (n ≥ 3). *P < 0.05 versus vehicle group.
5-HT2C Receptor Agonists and Antagonists Increase Mitochondrial Gene Expression in Mice Lacking the 5-HT2C Receptor.
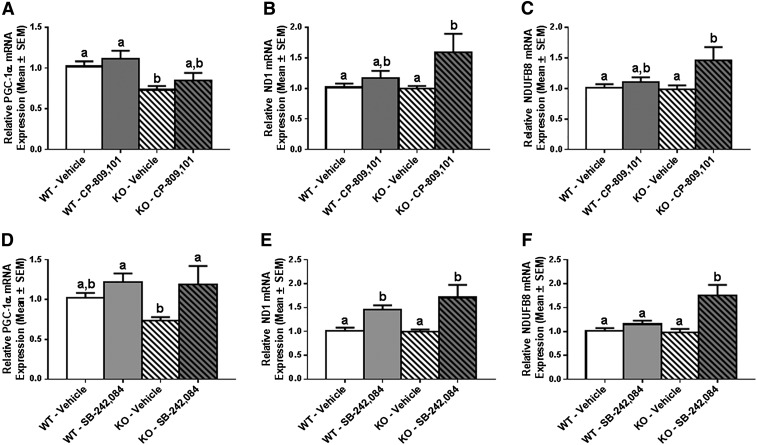
At baseline, mice lacking the 5-HT2C receptor had 20% less PGC-1α mRNA compared with WT control mice, but there was no difference in either ND1 or NDUFB8 mRNA between the two groups (Fig. 4). Treatment of WT mice with CP-809,101 did not increase PGC-1α, ND1, or NDUFB8 mRNA over vehicle (Fig. 4, A–C), whereas treatment of these mice with SB-242,084 increased ND1 mRNA 1.5-fold but did not increase PGC-1α or NDUFB8 mRNA over vehicle treatment (Fig. 4, D–F). Notably, these results in the WT 129/sv mice are different from those observed in the WT C57bl/6 mice used in the previously described naïve mouse experiments. However, treatment of 5-HT2C receptor KO mice with CP-809,101 induced a 1.5- and 1.4-fold increase in ND1 and NDUFB8 mRNA, respectively, although PGC-1α mRNA expression remained unchanged (Fig. 4, A–C). Treatment with SB-242,084 increased PGC-1α mRNA 1.5-fold over vehicle-treated 5-HT2C KO mice (no change over vehicle-treated WT mice), with 1.5-fold changes in both ND1 and NDUFB8 mRNA (Fig. 4, D–F). These results provide strong evidence that CP-809,101 and SB-242,084 induce mitochondrial genes but not through the 5-HT2C receptor.
Fig. 4.
The role of 5-HT2C loss on agonist- and antagonist-induced renal mitochondrial gene expression. 5-HT2C receptor WT and KO 129Sv mice were treated with a single intraperitoneal 1-mg/kg dose of CP-809,101 or SB-242,084 and euthanized 24 hours postinjection. PGC-1α (A and D), ND1 (B and E), and NDUFB8 (C and F) mRNA expression in renal cortex was determined by RT-qPCR using actin as a control gene. Fold expression of change in each group is reported relative to the WT/vehicle group. Data were analyzed using Kruskal–Wallis one-way analysis of variance with Bonferroni’s multiple comparison test. Values are reported as means ± S.E.M. Bars with different superscript letters are significantly different from one another (n ≥ 5). *P < 0.05.
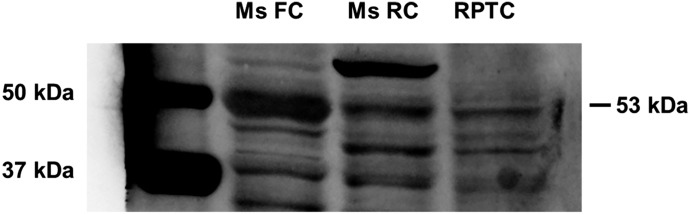
The 5-HT2A Receptor Is Expressed in the Kidney and in RPTCs.
Because the above studies indicated that the 5-HT2C receptor is not responsible for the increased mitochondrial gene expression, we hypothesized that the observed effects of CP-809,101 and SB-242,084 in RPTCs and in naïve mice were mediated by the 5-HT2A receptor. Although protein expression of this receptor has been reported in the mesangial cells of the kidney, its expression has not previously been demonstrated in the tubular epithelium (Garnovskaya et al., 1995). Immunoblot analysis demonstrated that the 5-HT2A receptor is expressed in the mouse frontal cortex, mouse renal cortex, and primary RPTCs (Fig. 5).
Fig. 5.
The 5-HT2A receptor is expressed in renal tissue. 5-HT2A receptor protein expression was analyzed by immunoblot in the mouse frontal cortex, mouse renal cortex, and rabbit RPTCs. The expected molecular mass is 53 kDa. FC, frontal cortex; Ms, mouse; RC, renal cortex.
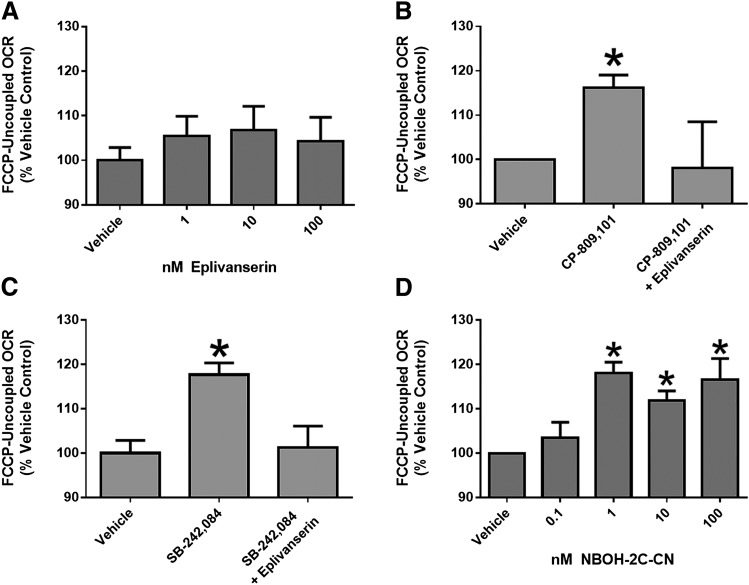
A 5-HT2A Receptor Antagonist Blocks CP-809,101– and SB-242,084–Induced FCCP-Uncoupled Respiration in RPTCs.
RPTCs treated with 10 nM CP-809,101 or SB-242,084 for 24 hours had a 1.15-fold increase in FCCP-uncoupled OCR over vehicle-treated RPTC (Figs. 2 and 6). When RPTCs were pretreated with 1 nM eplivanserin, a 5-HT2A receptor antagonist, neither CP-809,101 nor SB-242,084 increased FCCP-uncoupled respiration (Fig. 6, B and C). It should be noted that eplivanersin alone had no effect on FCCP-uncoupled OCR at any concentration (Fig. 6A). These results provide evidence that CP-809,101 and SB-242,084 act through 5-HT2A receptors with respect to mitochondrial changes.
Fig. 6.
A 5-HT2A antagonist blocks CP-809,101– and SB-242,084–induced FCCP-uncoupled respiration in RPTCs and a 5-HT2A agonist increases FCCP-uncoupled respiration in RPTCs. (A) FCCP-uncoupled mitochondrial respiration was measured in RPTCs treated with the 5-HT2A receptor antagonist eplivanserin at 1, 10, and 100 nM for 24 hours. (B and C) RPTCs were then pretreated with eplivanserin (1 nM) 1 hour before exposure to 10 nM CP-809,101 (B) or 10 nM SB-242,084 (C) for 24 hours. (D) RPTCs were also treated with 5-HT2A receptor agonist NBOH-2C-CN at 0.1, 1, 10, and 100 nM for 24 hours. FCCP-OCR was measured using the Seahorse XF-96 instrument. Data were analyzed using Kruskal–Wallis one-way analysis of variance with Dunn’s multiple comparison test. Values are reported as means ± S.E.M. (n ≥ 4). *P < 0.05 versus vehicle treatment group.
A 5-HT2A Receptor Agonist Increases FCCP-Uncoupled Respiration in RPTCs.
RPTCs treated with 1, 10, and 100 nM NBOH-2C-CN had increased FCCP-uncoupled OCR over vehicle-treated RPTCs, whereas treatment with 0.1 nM NBOH-2C-CN had no effect on FCCP-uncoupled OCR (Fig. 6D). These results further suggest that agonism of the 5-HT2A receptor mediates renal MB.
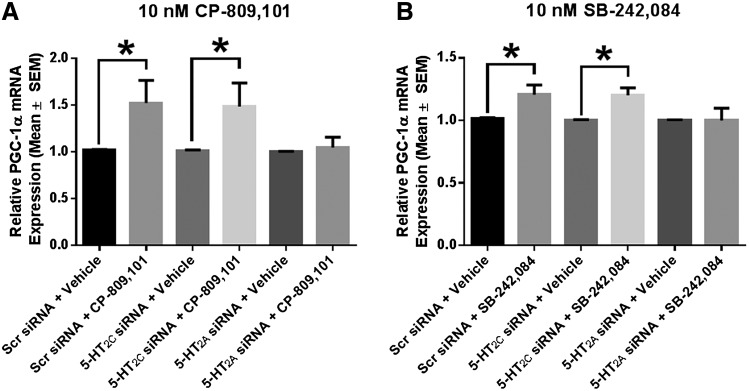
5-HT2C Receptor Agonists and Antagonists Increase PGC-1α mRNA Expression in RPTCs Treated with 5-HT2C siRNA But Not in RPTCs Treated with 5-HT2A siRNA.
The next set of experiments was designed to further confirm that CP-809,101 and SB-242,084 act through the 5-HT2A receptor. In RPTCs pretreated for 72 hours with 200 nM scrambled siRNA, treatment with 10 nM CP-809,101 increased PGC-1α mRNA 1.5-fold. RPTCs pretreated with siRNA directed toward 5-HT2C had a 1.5-fold increase in PGC-1α mRNA 24 hours after treatment with 10 nM CP-809,101, a response that was similar to that observed with parallel treatment of matched scrambled siRNA- or vehicle-treated RPTCs with 10 nM CP-809,101 (Fig. 7A). By contrast, RPTCs pretreated with 100 nM concentrations of two pooled siRNAs directed toward 5-HT2A mRNA did not result in an increase in PGC-1α mRNA 24 hours after treatment with 10 nM CP-809,101, compared with matched scrambled siRNA- and vehicle-treated RPTCs (Fig. 7A). Similar effects were observed in RPTCs treated with 10 nM SB-242,084 after 72-hour pretreatment with either scrambled siRNA or siRNA directed toward 5-HT2C or 5-HT2A (Fig. 7B). These results confirm that CP-809,101 and SB-242,084 act through the 5-HT2A receptor to induce transcription of mitochondrial genes.
Fig. 7.
A 5-HT2C agonist and antagonist induce MB in RPTCs treated with 5-HT2C siRNA but not in RPTCs treated with 5-HT2A siRNA. (A and B) RPTCs were pretreated with negative control or siRNA directed toward the 5-HT2C or 5-HT2A receptor for 72 hours and then treated with vehicle, 10 nM CP-809,101 (A), or 10 nM SB-242,084 (B) for 24 hours. PGC-1α mRNA expression was measured using tubulin as a control gene. Data were analyzed using a Mann–Whitney U test between each siRNA plus vehicle group and its corresponding treatment group. Values are reported as means ± S.E.M. (n ≥ 5). *P < 0.05 versus siRNA-matched vehicle control.
Discussion
Disruption of mitochondrial homeostasis is a pathologic process common to a multitude of diseases, and early intervention to correct mitochondrial dysregulation is an attractive therapeutic strategy for a variety of acute and chronic injuries. Therefore, pharmacological inducers of MB represent a novel class of drugs to preserve or restore mitochondrial and cellular function.
An early examination of the effects of a 5-HT2 pan-agonist, DOI, revealed that it induced MB and promoted recovery of cellular respiration in RPTCs after acute oxidant injury (Rasbach et al., 2010). In this study, we sought to further define the role of the 5-HT2 receptors in renal mitochondrial gene expression and oxidative metabolism. We first examined gene expression of the 5-HT2A, 5-HT2B, and 5-HT2C receptors in RPTCs and demonstrated that 5-HT2A and 5-HT2C had similar quantities of gene product that were approximately 10-fold greater than 5-HT2B. We then determined that the 5-HT2C receptor protein was expressed in both the mouse renal cortex and in primary cultures of rabbit RPTCs. A protein isolation protocol previously shown to enrich the quantity of 5-HT2 receptors and immunoblot analysis identified a 60-kDa 5-HT2C protein in both RPTCs and mouse renal cortex. This 60-kDa band was determined to be a 5-HT2C receptor with two N-glycosylations, verified in RPTCs by treatment with PNGase, which decreased levels of the 60-kDa band and increased the 38-kDa band that conforms with the reported nonglycosylated 5-HT2C receptor (Abramowski and Staufenbiel, 1995). The presence of the 60-kDa N-glycosylated band, previously identified in vivo in the hippocampus and choroid plexus, is significant because it indicates the presence of the mature 5-HT2C receptor in both the mouse renal cortex and in rabbit RPTCs. The presence of the mature 5-HT2C receptor in the kidney, previously thought to be expressed almost exclusively in the central nervous system, suggests a novel role for this receptor in renal cellular homeostasis.
CP-809,101 is reported to be a potent 5-HT2C agonist, with a pEC50 of 9.96 for 5-HT2C and 1000-fold selectivity over the 5-HT2A and 5-HT2B receptors (Table 1) (Siuciak et al., 2007). SB-242,084 is reported to be a potent 5-HT2C antagonist, with a pKi of 8.2–9.0 for 5-HT2C and 100-fold selectivity over the 5-HT2A and 5-HT2B receptors (Kennett et al., 1997). We expected that CP-809,101 but not SB-242,084 would induce MB in RPTCs. However, both of these compounds increased FCCP-OCR at nanomolar concentrations and increased PGC-1α mRNA at 10-nM concentrations in vitro, which indicates that these compounds induce MB at concentrations previously reported to be specific for the 5-HT2C receptor.
CP-809,101 and SB-242,084 were administered to naïve C57Bl/6 mice at 1 mg/kg. This dose is consistent with those previously administered to animals, CP-809,101 (0.3–56 mg/kg) and SB-242,084 (0.01–10 mg/kg) (Kennett et al., 1997; Siuciak et al., 2007; Strong et al., 2009). Both CP-809,101 and SB-242,084 increased PGC-1α mRNA and mRNA for the mitochondrial DNA-encoded gene ND1 and the nuclear DNA-encoded gene NDUFB8. Thus, the in vitro and in vivo findings are consistent.
We next sought to verify that these two compounds were inducing PGC-1α, ND1, and NDUFB8 mRNA through the 5-HT2C receptor. Our first observation was that 5-HT2C KO mice had a 20% decrease in PGC-1α mRNA compared with WT mice, indicating a role for the 5-HT2C receptor in renal physiologic mitochondrial homeostasis. It should be noted that mitochondrial-encoded ND1 and nuclear-encoded NDUFB8 did not decrease in 5-HT2C KO mice, providing evidence that a compensatory pathway is present under physiologic conditions to maintain mitochondrial homeostasis.
Treatment of 5-HT2C KO mice with CP-809,101 led to increases in both ND1 and NDUFB8 mRNA and treatment of 5-HT2C KO mice with SB-242,084 increased PGC-1α, ND1, and NDUFB8 mRNA expression. In addition, treatment of RPTCs with either CP-809,101 or SB-242,084 after pretreatment with siRNA directed toward the 5-HT2C receptor still increased PGC-1α mRNA to the same magnitude as that observed after treatment of these compounds in negative control siRNA-treated cells. In summary, these results indicate that these compounds do not induce MB-associated mRNA expression through the 5-HT2C receptor.
We next hypothesized that these compounds were not specific for the 5-HT2C receptor in primary renal cells at the doses used in the previous experiments and that these compounds instead increase cellular FCCP-uncoupled OCR and renal PGC-1α, ND1, and NDUFB8 mRNA expression through the 5-HT2A receptor, previously reported to be expressed in mouse renal mesangial cells (Garnovskaya et al., 1995). The 5-HT2A receptor was expressed in both the mouse renal cortex and primary rabbit RPTCs using immunoblot analysis, and the 5-HT2A receptor antagonist eplivanserin blocked the increases in FCCP-uncoupled OCR previously observed in RPTCs after 24-hour treatment with either CP-809,101 or SB-242,084. These results were confirmed, in that no increases in PGC-1α mRNA were detected in RPTCs treated with either of these compounds after pretreatment with 5-HT2A receptor siRNA. Finally, the 5-HT2A receptor agonist NBOH-2C-CN increased FCCP-uncoupled OCR at concentrations as low as 1 nM, further supporting our conclusion that the 5-HT2A receptor mediates MB in RPTCs.
Although the concentrations of CP-809,101 and SB-242,084 used in the initial experiments are reported to be specific for the 5-HT2C receptor, our results strongly indicate that these compounds most likely increase multiple markers of MB in both rabbit RPTCs and in the mouse renal cortex via signaling through the 5-HT2A receptor. It is notable that these compounds increase these markers of MB at concentrations below their reported pKi or pEC50 values for the 5-HT2A receptor. It has been previously demonstrated that 5-HT2 receptor ligands can have different affinities for the same receptor in different tissues, so it is possible that the pKi values for these compounds may differ in renal tissue (Killam et al., 1990).
Another explanation for these paradoxical results is that the presumed action (i.e., agonist/antagonist), potency, and/or downstream signaling of these compounds on the 5-HT2A receptor was determined in neuronal cells or cell lines overexpressing the receptor. By contrast, we used primary cultures of renal epithelial cells that have differentiated functions and physiologic receptor expression to probe a unique mitochondrial target. Thus, it is possible that agonism of the 5-HT2A receptor in the kidney by either CP-809,101 or SB-242,084 at the doses used in our studies can result in release of calcium and subsequent increase in cGMP, previously demonstrated to be important for pharmacological induction of renal MB (Whitaker et al., 2013). This hypothesis is supported by evidence that SB-242,084 can act as an agonist for the calcium-mediated signaling pathway of the 5-HT2C receptor (Roth, 2006; Neve, 2009). Future studies will focus on determining the relative specificity of these compounds for the three 5-HT2 receptors in RPTCs and elucidating the signaling pathways by which these drugs increase gene expression of several MB markers.
This study is limited in that we did not have a consistent response to either CP-809,101 or SB-242,084 between two different mouse strains: C57Bl/6 and 129Sv. Although this inconsistency between strains is unfortunate in that it limited our ability to use available KO animals to further investigate our drugs of interest, it is not unexpected. Furthermore, previous work in another laboratory demonstrated that the C56Bl/6 and 129Sv strains had different behavioral outcomes when exposed to the 5-HT2A/5-HT2C agonist (±)-2,5-dimethoxy-4-methylamphetamine, indicating that these two strains differ in serotonergic drug response (Dulawa and Geyer, 2000).
In summary, our findings indicate that modulation of 5-HT2A signaling in the kidney may promote MB. Furthermore, these studies support 5-HT2A receptor agonists as viable candidates for the treatment of kidney disease.
Acknowledgments
The authors thank Gyda Beeson for help with RPTC culture and the Seahorse XF instrument, Tamilselvan Jayavelu for help with mRNA expression of the 5-HT2 receptors, Sara Garrett for help with the initial oxygen consumption studies, and Dr. Bryan Roth for guidance in the selection of screening compounds.
Abbreviations
- CP-809,101
2-[(3-chlorophenyl)methoxy]-6-(1-piperazinyl)pyrazine
- DOI
1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine
- FCCP
4-(trifluoromethoxy)phenylhydrazone
- KO
knockout
- MB
mitochondrial biogenesis
- NBOH-2C-CN
4-[2-[[(2-hydroxyphenyl)methyl]amino]ethyl]-2,5-dimethoxybenzonitrile
- ND1
NADH dehydrogenase subunit 1
- NDUFB8
NADH dehydrogenase (ubiquinone) β subcomplex 8
- OCR
oxygen consumption rate
- PCR
polymerase chain reaction
- PGC-1α
peroxisome proliferator–activated receptor γ coactivator-1α
- PNGase
N-glycosidase F
- qPCR
quantitative polymerase chain reaction
- RPTC
renal proximal tubule cell
- RT
reverse transcription
- SB-242,084
6-chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride
- siRNA
small interfering RNA
- WT
wild type
Authorship Contributions
Participated in research design: Harmon, Beeson, Schnellmann.
Conducted experiments: Harmon, Wills, McOmish.
Contributed new reagents or analytic tools: McOmish, Demireva, Gingrich.
Performed data analysis: Harmon, Wills, Schnellmann.
Wrote or contributed to the writing or the manuscript: Harmon, McOmish, Schnellmann.
Footnotes
This research was supported in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants F30DK091107 and T32DK083262 (to J.L.H.)]; the National Health and Medical Research Council [C.J. Martin Overseas Biomedical Fellowship (to C.E.M.)]; the Brain and Behavior Research Foundation [Young Investigator Award (to C.E.M.)]; the National Institutes of Health National Institute of Mental Health [Grants R21MH099458 and R01MH080116 (to J.A.G.)]; the National Institutes of Health National Institute of General Medical Sciences [Grants R01GM084147 (to R.G.S) and P20GM103542-02 (to South Carolina COBRE in Oxidants, Redox Balance, and Stress Signaling)]; the National Institutes of Health National Center for Research Resources [Grant UL1RR029882]; the Biomedical Laboratory Research and Development Program of the U.S. Department of Veterans Affairs [Grant 5I01 BX-000851 (to R.G.S.)]; and the South Carolina Clinical and Translational Research Institute at the Medical University of South Carolina. Animal facilities were funded by the National Institutes of Health National Center for Research Resources [Grant C06RR015455].
References
- Abramowski D, Staufenbiel M. (1995) Identification of the 5-hydroxytryptamine2C receptor as a 60-kDa N-glycosylated protein in choroid plexus and hippocampus. J Neurochem 65:782–790. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. (2008) Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol 153 (Suppl 2):S1–S209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger SW. (2005) Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med 38:1278–1295. [DOI] [PubMed] [Google Scholar]
- Beeson CC, Beeson GC, Schnellmann RG. (2010) A high-throughput respirometric assay for mitochondrial biogenesis and toxicity. Anal Biochem 404:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromidge SM, Duckworth M, Forbes IT, Ham P, King FD, Thewlis KM, Blaney FE, Naylor CB, Blackburn TP, Kennett GA, et al. (1997) 6-Chloro-5-methyl-1-[[2-[(2-methyl-3-pyridyl)oxy]-5-pyridyl]carbamoyl]-indoline (SB-242084): the first selective and brain penetrant 5-HT2C receptor antagonist. J Med Chem 40:3494–3496. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Howard BJ, LaFontaine MA. (2001) Brain oxidative stress in animal models of accelerated aging and the age-related neurodegenerative disorders, Alzheimer’s disease and Huntington’s disease. Curr Med Chem 8:815–828. [DOI] [PubMed] [Google Scholar]
- Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. (2011) Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci 12:7199–7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cruz S, Parone PA, Lopes VS, Lillo C, McAlonis-Downes M, Lee SK, Vetto AP, Petrosyan S, Marsala M, Murphy AN, et al. (2012) Elevated PGC-1α activity sustains mitochondrial biogenesis and muscle function without extending survival in a mouse model of inherited ALS. Cell Metab 15:778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Geyer MA. (2000) Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology 39:2170–2179. [DOI] [PubMed] [Google Scholar]
- Feldkamp T, Kribben A, Weinberg JM. (2005) Assessment of mitochondrial membrane potential in proximal tubules after hypoxia-reoxygenation. Am J Physiol Renal Physiol 288:F1092–F1102. [DOI] [PubMed] [Google Scholar]
- Funk JA, Schnellmann RG. (2012) Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol 302:F853–F864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnovskaya MN, Nebigil CG, Arthur JM, Spurney RF, Raymond JR. (1995) 5-Hydroxytryptamine2A receptors expressed in rat renal mesangial cells inhibit cyclic AMP accumulation. Mol Pharmacol 48:230–237. [PubMed] [Google Scholar]
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL. (2014) Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chem Neurosci 5:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Lindsley T, Teitler M, Mancia F, Cowan A, Mazurkiewicz JE. (2015) Native serotonin 5-HT2C receptors are expressed as homodimers on the apical surface of choroid plexus epithelial cells. Mol Pharmacol 87:660–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. (2012) Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev 44:88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DP, Scarpulla RC. (2004) Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 18:357–368. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, et al. (1997) SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology 36:609–620. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, NC3Rs Reporting Guidelines Working Group (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160:1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killam AL, Nikam SS, Lambert GM, Martin AR, Nelson DL. (1990) Comparison of two different arterial tissues suggests possible 5-hydroxytryptamine2 receptor heterogeneity. J Pharmacol Exp Ther 252:1083–1089. [PubMed] [Google Scholar]
- Lifshitz J, Sullivan PG, Hovda DA, Wieloch T, McIntosh TK. (2004) Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion 4:705–713. [DOI] [PubMed] [Google Scholar]
- Morabito MV, Abbas AI, Hood JL, Kesterson RA, Jacobs MM, Kump DS, Hachey DL, Roth BL, Emeson RB. (2010) Mice with altered serotonin 2C receptor RNA editing display characteristics of Prader-Willi syndrome. Neurobiol Dis 39:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebigil CG, Etienne N, Messaddeq N, Maroteaux L. (2003) Serotonin is a novel survival factor of cardiomyocytes: mitochondria as a target of 5-HT2B receptor signaling. FASEB J 17:1373–1375. [DOI] [PubMed] [Google Scholar]
- Neve K. (2009) Functional Selectivity of G Protein–Coupled Receptor Ligands, Humana, New York. [Google Scholar]
- Nowak G, Aleo MD, Morgan JA, Schnellmann RG. (1998) Recovery of cellular functions following oxidant injury. Am J Physiol 274:F509–F515. [DOI] [PubMed] [Google Scholar]
- Nowak G, Schnellmann RG. (1995) Improved culture conditions stimulate gluconeogenesis in primary cultures of renal proximal tubule cells. Am J Physiol 268:C1053–C1061. [DOI] [PubMed] [Google Scholar]
- Nowak G, Schnellmann RG. (1996) L-ascorbic acid regulates growth and metabolism of renal cells: improvements in cell culture. Am J Physiol 271:C2072–C2080. [DOI] [PubMed] [Google Scholar]
- Ozbek E. (2012) Induction of oxidative stress in kidney. Int J Nephrol 2012:465897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839. [DOI] [PubMed] [Google Scholar]
- Rasbach KA, Funk JA, Jayavelu T, Green PT, Schnellmann RG. (2010) 5-hydroxytryptamine receptor stimulation of mitochondrial biogenesis. J Pharmacol Exp Ther 332:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasbach KA, Schnellmann RG. (2007) PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun 355:734–739. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Congy C, Santucci V, Simiand J, Gautret B, Neliat G, Labeeuw B, Le Fur G, Soubrie P, Breliere JC. (1992) Biochemical and pharmacological properties of SR 46349B, a new potent and selective 5-hydroxytryptamine2 receptor antagonist. J Pharmacol Exp Ther 262:759–768. [PubMed] [Google Scholar]
- Roth BL, editor (2006) The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics, Humana Press, Totowa, NJ. [Google Scholar]
- Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. (2010) New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci 123:2533–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Chapin DS, McCarthy SA, Guanowsky V, Brown J, Chiang P, Marala R, Patterson T, Seymour PA, Swick A, et al. (2007) CP-809,101, a selective 5-HT2C agonist, shows activity in animal models of antipsychotic activity. Neuropharmacology 52:279–290. [DOI] [PubMed] [Google Scholar]
- Smith JA, Stallons LJ, Collier JB, Chavin KD, Schnellmann RG. (2015) Suppression of mitochondrial biogenesis through toll-like receptor 4-dependent mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling in endotoxin-induced acute kidney injury. J Pharmacol Exp Ther 352:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong PV, Greenwood BN, Fleshner M. (2009) The effects of the selective 5-HT(2C) receptor antagonist SB 242084 on learned helplessness in male Fischer 344 rats. Psychopharmacology (Berl) 203:665–675. [DOI] [PubMed] [Google Scholar]
- Tsunemi T, Ashe TD, Morrison BE, Soriano KR, Au J, Roque RA, Lazarowski ER, Damian VA, Masliah E, La Spada AR. (2012) PGC-1α rescues Huntington’s disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci Transl Med 4:142ra97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R, Garnier A, Veksler V. (2008) Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res 79:208–217. [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Roeser NF, Davis JA, Venkatachalam MA. (1997) Glycine-protected, hypoxic, proximal tubules develop severely compromised energetic function. Kidney Int 52:140–151. [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. (2000) Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci USA 97:2826–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker RM, Wills LP, Stallons LJ, Schnellmann RG. (2013) cGMP-selective phosphodiesterase inhibitors stimulate mitochondrial biogenesis and promote recovery from acute kidney injury. J Pharmacol Exp Ther 347:626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, Schnellmann RG. (2012) The β2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J Pharmacol Exp Ther 342:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yao B, Fan X, Langworthy MM, Zhang MZ, Harris RC. (2007) Characterization of a putative intrarenal serotonergic system. Am J Physiol Renal Physiol 293:F1468–F1475. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, et al. (2008) 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 60:582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]