Abstract
The primary psychoactive ingredient of marijuana, Δ9-tetrahydrocannabinol (Δ9-THC), has medicinal value but also produces unwanted deleterious effects on cognitive function, promoting the search for improved cannabinergic therapeutics. The present studies used a battery of touchscreen procedures in squirrel monkeys to compare the effects of different types of cannabinergic drugs on several measures of performance including learning (repeated acquisition), cognitive flexibility (discrimination reversal), short-term memory (delayed matching-to-sample), attention (psychomotor vigilance), and motivation (progressive ratio). Drugs studied included the cannabinoid agonist Δ9-THC, fatty acid amide hydrolase (FAAH) inhibitor cyclohexylcarbamic acid 3-carbamoylbiphenyl-3-yl ester (URB597), and endocannabinoid anandamide and its stable synthetic analog methanandamide [(R)-(+)-arachidonyl-1′-hydroxy-2′-propylamide]. The effects of Δ9-THC and anandamide after treatment with the cannabinoid receptor type 1 inverse agonist/antagonist rimonabant [5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1Hpyrazole-3-carboxamide] and the FAAH inhibitor URB597, respectively, also were examined. The results showed the following: 1) Δ9-THC produced dose-related impairments of discrimination-based cognitive behavior with potency that varied across tasks (discriminative capability < learning < flexibility < short-term memory); 2) anandamide alone and URB597 alone were without effect on all endpoints; 3) anandamide following URB597 pretreatment and methanandamide had negligible effects on discriminative capability, learning, and reversal, but following large doses affected delayed matching-to-sample performance in some subjects; 4) all drugs, except anandamide and URB597, disrupted attention; and 5) progressive ratio breakpoints were generally unaffected by all drugs tested, suggesting little to no effect on motivation. Taken together, these data indicate that metabolically stable forms of anandamide may have lesser adverse effects on cognitive functions than Δ9-THC, possibly offering a therapeutic advantage in clinical settings.
Introduction
The cannabinoid receptor type 1 (CB1) agonist Δ9-tetrahydrocannabinol (Δ9-THC) is the primary psychoactive ingredient in marijuana, the most commonly used illicit drug in the United States. Recent surveys estimate approximately 20 million current (past month) users (Substance Abuse and Mental Health Services Administration; (http://www.samhsa.gov/data/sites/default/files/NSDUHresultsPDFWHTML2013/Web/NSDUHresults2013.pdf). In addition, Δ9-THC has apparent medicinal value and there appears to be growing acceptance of its therapeutic, as well as recreational, use. For example, Δ9-THC, formulated as Marinol for oral delivery, is employed as an appetite stimulant and can serve as an antinausea and antiemetic agent (reviewed in Sharkey et al., 2014). While the full therapeutic value of Δ9-THC or other CB1 agonists is not yet understood, such cannabinergic effects are of known benefit in the palliative care of anorectic patients undergoing chemotherapy or suffering debilitating conditions such as AIDS or Alzheimer’s disease (reviewed in Cridge and Rosengren, 2013).
The apparent medicinal benefits of Δ9-THC have led to a broadening interest in the clinical utility of drugs that target the endocannabinoid system. However, in this regard Δ9-THC also is generally acknowledged to produce some unwanted effects in humans. These include adverse effects on several types of cognitive function and, especially, behavior thought to be mediated in the prefrontal cortex (reversal learning and attention) and hippocampus (short-term memory) (reviewed in Iversen 2005; Egerton et al., 2006; Crean et al., 2011; Zanettini et al., 2011). Laboratory studies with nonhuman primates comparing the relative impact of Δ9-THC across several cognitive endpoints have confirmed that these aspects of cognitive function appear particularly vulnerable to Δ9-THC (e.g., Schulze et al., 1988; Winsauer et al., 1999; Taffe, 2012; Wright et al., 2013).
Concern regarding such adverse effects of Δ9-THC on cognitive function has led to efforts to develop cannabinergic drugs that retain medicinal value, yet produce lesser adverse effects on cognition. One recent approach that has yielded encouraging preclinical results does not target the development of novel CB1 agonists but instead involves enhancing endogenous cannabinergic activity by pharmacologically inhibiting the rapid metabolism of anandamide by fatty acid amide hydrolase (FAAH) (e.g., Kathuria et al., 2003; Seierstad and Breitenbucher, 2008; Gaetani et al., 2009; Pertwee, 2014). An emerging literature in which this approach is explored provides some preclinical evidence of efficacy in animal models of nausea, vomiting, and appetite (e.g., Williams and Kirkham, 1999; Cross-Mellor et al., 2007; Rock et al., 2008; Parker et al., 2009; Limebeer et al., 2014). However, the effects of anandamide on cognitive function in nonhuman primates have not yet been fully delineated, and consequently it is unclear whether anandamide (or other endocannabinoids) offer a therapeutic advantage over CB1 agonists such as Δ9-THC. The present studies were conducted to address this by examining the relative impact of the cannabinoid agonist Δ9-THC and both the endocannabinoid anandamide (administered alone and following FAAH inhibition) and its metabolically stable analog methanandamide [(R)-(+)-arachidonyl-1′-hydroxy-2′-propylamide] on performance across a range of touchscreen-based assays of cognitive function in nonhuman primates.
Materials and Methods
Subjects
Nine adult male squirrel monkeys (Saimiri sciureus) were used in the present studies. Six of the subjects engaged in two tasks, two subjects engaged in three tasks, and one subject engaged in one task. All subjects previously served in behavioral studies of dopamine-related drugs or opioids, but had not received drug treatments for at least 6 months prior to the present studies. In addition, no subject had received cannabinoid receptor–related drugs or had touchscreen experience prior to the present studies.
All subjects were individually housed in a temperature- and humidity-controlled vivarium with a 12-hour light/12-hour dark cycle (7 AM to 7 PM), and had unlimited access to water in the home cage. Subjects were maintained at approximate free-feeding weights by postsession feedings of a nutritionally balanced diet of high-protein, banana-flavored biscuits (Purina Monkey Chow, St. Louis, MO). In addition, fresh fruit and environmental enrichment were provided daily. Experimental sessions were conducted 5 days a week (Monday to Friday). The experimental protocol for the present studies was approved by the Institutional Animal Care and Use Committee at McLean Hospital. Subjects were maintained in a facility licensed by the U.S. Department of Agriculture and in accordance with guidelines provided by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animals Resources, Commission on Life Sciences, National Research Council (www.ncbi.nlm.nih.gov/books/n/nap12910/pdf).
Apparatus
Details, schematics, and photographs of the touch-sensitive experimental chamber can be found in Kangas and Bergman (2012). Briefly, a custom-built Plexiglas chamber (38 × 40 × 60 cm) was situated in a sound- and light-attenuating enclosure (50 × 60 × 70 cm). A 17-inch touch-sensitive screen (1739L, ELO TouchSystems, Menlo Park, CA) was mounted on an inside wall of the enclosure and fit into a cutout in the chamber’s front wall (34 × 27 cm). An infusion pump (PHM-100-10, Med Associates, St. Albans, VT) outside the enclosure was used to deliver pulses of 0.15 ml of a 30% sweetened condensed milk solution into the shallow reservoir (diameter: 2.5 cm) of a custom-designed Plexiglas receptacle (5 × 3.5 × 1.27 cm). Both touchscreen and fluid reservoir were easily accessible to the subject. A speaker bar (NQ576AT, Hewlett-Packard, Palo Alto, CA) mounted above the touchscreen (i.e., at the top of the inside wall of the enclosure) was used to emit an audible feedback click each time the subject touched a stimulus presented on the screen. All experimental events and data collection were programmed in E-Prime Professional 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA). During all behavioral procedures described subsequently, subjects were placed within the experimental chamber prior to the daily session and were not restrained or restricted in their movement.
Procedures
Repeated Acquisition.
Previously established methods were used to train subjects to repeatedly discriminate novel visual discriminations (Kangas and Bergman, 2014). Briefly, each session began with concurrent presentation of two 7 × 7 cm digital photographs, each in a different randomly selected quadrant of the screen (white background). A touch response on one stimulus initiated the delivery of milk into the reservoir (S+) paired with an 880-millisecond yellow screen flash, and followed by a 10- second intertrial interval (ITI) blackout; a touch response to the other stimulus immediately initiated the 10-second ITI (S−). The same two stimuli were presented during each of 200 trials comprising the day’s session, and a new S+/S− pair was introduced each session. Photographs for each session were randomly selected from our laboratory bank of >10,000 images. Thus, the subject was required to learn a new S+/S− discrimination each session based on distinguishing features of two visual stimuli that had not been previously viewed (i.e., repeated acquisition). Subjects were exposed to the repeated acquisition task for 30 sessions prior to introduction of the discrimination reversal task (described subsequently).
Discrimination Reversal.
In the discrimination reversal task, a variant of discrimination learning for examining cognitive flexibility (Mackintosh et al., 1968; Easton 2005), the programmed consequences of responding reliably to the S+ (and not S−) stimulus were reversed after the subject learned the initial discrimination in daily sessions. Briefly, from the 31st session onward, the first 100 trials in the daily session were conducted exactly as described previously, i.e., subjects learned a novel discrimination each session. However, on trial 101 the relationship between S+ and S− was reversed without signal, i.e., during trials 101–200 the stimulus that was initially S+ was made S−, and vice versa. Prior to any drug testing, subjects were exposed to this discrimination reversal condition for a minimum of 30 sessions (see Kangas and Bergman, 2014) and until stability was observed in both steady-state acquisition and reversal performance. Stability was defined as 10 consecutive sessions in which, for both initial acquisition and reversal, the number of trials required for mastery (i.e., responding to S+ in nine out of 10 consecutive trials) was within ±20% of the average of the 10-session mean for each type of learning.
Discriminative Capability Trials.
After steady-state discrimination was observed under the repeated acquisition and reversal tasks, discriminative capability trials were introduced to permit evaluation of the effects of drugs on the basic discriminative behavior required in repeated acquisition and discrimination reversal procedures. First, subjects were allowed to acquire a novel discrimination (here, a picture of an apple was S+ and a picture of an orange was S−) for five consecutive 200-trial sessions to provide a history of high accuracy in discriminating this stimulus pair. Subsequently, this stimulus pair appeared in every 10th trial of all sessions and served to identify nonselective motoric effects versus selective effects on learning when the effects of drugs were studied (cf. Galizio et al., 2009).
Delayed Matching-to-Sample.
Previously established methods were used to train subjects under delayed matching-to-sample (DMTS) conditions (e.g., Blough, 1959; McCarthy and White, 1985; Kangas et al., 2011). Each DMTS session consisted of 60 trials (12 trials of each delay, presented in a quasi-random order). Each trial began with presentation of one of two 7 × 7 cm pictorial stimuli (sample stimulus; either a dog or a box of crayons), which remained in the center of the screen (silver background) until 20 touchscreen responses were completed. Upon completion of the 20th fixed ratio (FR) response (FR20), the sample stimulus disappeared, initiating a delay of 0, 2, 4, 8, or 16 seconds. Following the delay, both stimuli were presented left and right of center, and a single touch response on the stimulus that matched the previously presented sample initiated milk presentation paired with an 880-millisecond yellow screen flash, followed by a 10-second ITI; a touch response on the other pictorial stimulus immediately initiated only the 10-second ITI. Steady-state performance in individual subjects was obtained prior to drug testing and was defined as 10 consecutive sessions with average accuracies under each retention interval within ±10% of the average of the 10-session mean.
Psychomotor Vigilance.
A psychomotor vigilance task was used to evaluate attention, reaction time, and underlying motoric behavior over time (e.g., Mackworth, 1948; Weed and Gold, 1998). Briefly, a 7 × 7 cm stimulus (pink box) appeared on the screen (blue background) in one of nine screen locations (evenly spaced 3 × 3 matrix). The duration of stimulus presentation was 2 seconds on the first trial of the session, and thereafter both the intermittency of the stimulus presentation (after 5-, 10-, or 15-second ITI; no blackout during ITI) and the stimulus location were randomized across trials. If the subject successfully touched the stimulus within 2 seconds, milk was delivered and the duration of stimulus presentation decreased by 0.25 seconds on the subsequent trial; if the subject failed to respond within 2 seconds, the stimulus was extinguished and, following a timeout period, the duration of the stimulus presentation increased by 0.25 seconds on the subsequent trial. These titrating contingencies were designed to capture the subject’s reaction time and ability to maintain task performance across a 100-trial session (i.e., focused vigilance). The primary dependent measure under these conditions was mean titrated duration value. Steady-state performance in individual subjects was obtained prior to drug administration and was defined as five consecutive session means within ±20% of the average mean of the five sessions.
Progressive Ratio.
A progressive ratio procedure (Hodos, 1961) was used to measure motivational value in the present study by determining the maximum number of responses that were emitted for milk delivery. Briefly, subjects first learned to touch a 7 × 7 cm green box (purple background) in the middle of the screen to produce milk delivery. Next, a progressive ratio schedule of milk presentation was introduced, and each reinforcer delivery led to an increase in the number of touchscreen responses required for subsequent presentation. The progressive ratio requirement was programmed with a log 2 step size (i.e., 1, 2, 4, 8, 16, 32, 64, 128, 256, 512, and 1024). Each milk reinforcer was paired with an 880-millisecond yellow screen flash and followed by a 10-second ITI blackout. Each session was terminated following either 5 minutes without a response or 45 minutes, whichever came first. Steady-state performance in individual subjects was obtained prior to drug testing and was defined as five consecutive sessions yielding a breakpoint within one of two adjacent step sizes.
Drug Testing
After stable performance was obtained in all tasks, test sessions were conducted to determine the dose-related effects of the CB1 agonist Δ9-THC, the endocannabinoid anandamide alone, and after treatment with the FAAH inhibitor URB597 (cyclohexylcarbamic acid 3-carbamoylbiphenyl-3-yl ester), as well as its metabolically stable analog methanandamide. The effects of Δ9-THC on discriminative capability trials, repeated acquisition, discrimination reversal, and DMTS were also assessed following pretreatment with the CB1 selective inverse agonist/antagonist rimonabant [5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1Hpyrazole-3-carboxamide]. Drugs were tested in a quasi-random order.
Each dose of each drug was studied on each task individually across sessions with at least three intervening control (no drug) or saline sessions to minimize the development of tolerance. For repeated acquisition, DMTS, psychomotor vigilance, and progressive ratio, subjects were injected, placed in a holding chamber for the drug’s pretreatment interval (described subsequently), and then placed in the touchscreen chamber for the session. To assess drug effects on discrimination reversal, subjects first learned a novel discrimination during a 100-trial acquisition session. Next, subjects were removed from the touchscreen chamber, injected, and placed in a holding chamber for the pretreatment interval, and then returned to the touchscreen chamber with the contingencies reversed. Previous research in our laboratory has indicated that such intervals between acquisition and reversal have no effect on reversal learning (see Kangas and Bergman, 2014). With the exception of progressive ratio, all experimental sessions were terminated if 15 minutes elapsed without a response. However, in the present studies, this limit was only contacted in the instances highlighted subsequently when relatively large doses of drugs tested abolished performance.
Data Analysis
The effects of all drugs on discriminative capability trials, repeated acquisition, and discrimination reversal were expressed as changes in accuracy. Drug effects on DMTS performance similarly were expressed as changes in accuracy as a function of delay value (i.e., forgetting function). Accuracy was calculated using the following equation: [number of correct trials/total number of trials] × 100. In addition, latency to respond (seconds) was calculated for discriminative capability trials, repeated acquisition, and discrimination reversal as the interval between trial onset and response to either S+ or S−. For DMTS, response rate (responses/second) during the FR20 sample stimulus response requirement was calculated by dividing 20 by the interval between the onset and offset of the sample stimulus. For repeated acquisition, discrimination reversal, and DMTS, if 15 minutes elapsed without a response the session was terminated and accuracy was considered indeterminate. The effects of drugs on psychomotor vigilance were measured as changes in mean titrated duration. Overall mean titrated duration values were calculated by averaging the 100 titrated duration values recorded in the session. The primary dependent measure used to evaluate dose effects on progressive ratio was mean change in breakpoint expressed as step-size deviation averaged across individual subjects.
A repeated-measures one-way analysis of variance was conducted to evaluate the statistical significance of each drug treatment in all tasks except DMTS. When appropriate, analysis of variance was followed by a Dunnett’s test to evaluate the statistical significance of dose-related changes from group-average control values. The criterion for significance was set at P < 0.05. Given the relatively small sample size in the DMTS task (n = 3), performance was assessed by visual inspection.
Drugs
Anandamide, methanandamide, and URB597 were synthesized by the present authors (V.G.S., S.O.A., S.P.N., and A.M.) in the Center for Drug Discovery at Northeastern University. ∆9-THC and rimonabant were provided by the National Institute on Drug Abuse Drug Supply Program (Rockville, MD). All drugs were prepared for administration in a 20:20:60 mixture of 95% ethanol, Polysorbate-80 (Tween-80; Sigma-Aldrich, St. Louis, MO), and saline. All drug solutions were refrigerated and protected from light. Injections of drug or saline were prepared in volumes of 0.3 ml/kg body weight or less and administered i.m. in calf or thigh muscle. Saline was delivered prior to injection control sessions because previous studies in our laboratory have found no behavioral effects of this vehicle and we wanted to preclude potential tissue damage associated with i.m. ethanol injections. The session pretreatment times were selected on the basis of pilot experiments and were 5 minutes for anandamide, 30 minutes for Δ9-THC and methanandamide, and 60 minutes for URB597.
Results
Repeated Acquisition, Discrimination Reversal, and Discriminative Capability Trials.
All subjects effectively engaged in the repeated acquisition and discrimination reversal tasks following 30 training sessions under each task. A summary of the baseline performance described previously is plotted as control values in Figs. 1 and 2. Data are plotted in terms of accuracy, i.e., the percentage of trials that were correctly completed instead of trials to mastery because discrimination and/or reversal mastery during drug testing were not fully achieved following administration of some behaviorally active doses. Stable baseline performance under the repeated acquisition task was observed by the end of the 30-session training condition with subjects requiring, on average, approximately 14 (±4) trials to master the novel discrimination. The open circles show the baseline accuracy of repeated acquisition during control sessions. The group mean of 94% correct represents the group average of 14 trials to master a novel discrimination (with intermittent correct responses during the initially chance performance). Stable baseline performance was also observed under the discrimination reversal task by the end of the 30-session training condition; however, more trials in each session were needed to achieve the same level of mastery [group average of 46 (±6) trials]. The open triangles in Figs. 1 and 2 show baseline accuracy of discrimination reversal during control sessions. The group mean of 81% correct represents the group average of 46 trials to master the reversal (again with intermittent correct responses during initially chance performance). Thus, on average the reacquisition of stimulus control by reversed stimuli in the second half of the daily session required more than three times the number of trials needed to establish the initial discrimination. We have previously observed this difference in the steady-state rate of learning and reversal performance (for additional details on the development of learning and reversal performance, see Kangas and Bergman, 2014). Discriminative capability trials were quickly mastered and near-perfect accuracy was observed in control sessions throughout the present studies, shown by the open diamonds in Figs 1 and 2 (group mean of 99% correct). In all three tasks, saline administration had no systematic effect on accuracies when compared with noninjection control values.
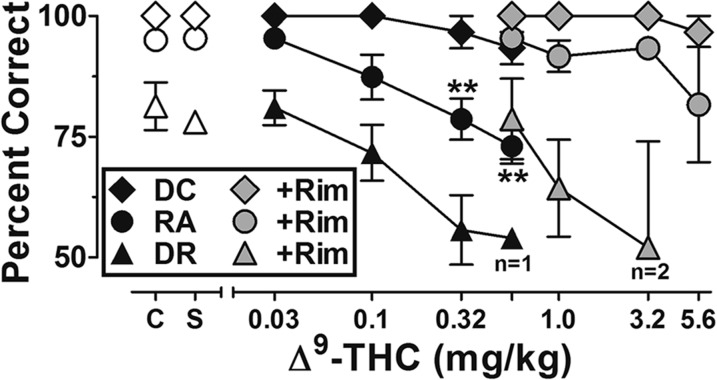
Fig. 1.
Dose-effect functions for Δ9-THC alone (black symbols) and following treatment of 1.0 mg/kg of rimonabant (shaded symbols) on repeated acquisition (RA) trials (circles), discrimination reversal (DR) trials (triangles), and discriminative capability (DC) trials (diamonds). Abscissa, cumulative dose, log scale; ordinate, mean percent correct. Symbols left of abscissa break indicate performance during noninjection control (C) and saline (S) sessions. Points represent averages (±S.E.M.) for the groups of subjects; n = 5, *P < 0.05, **P < 0.01.
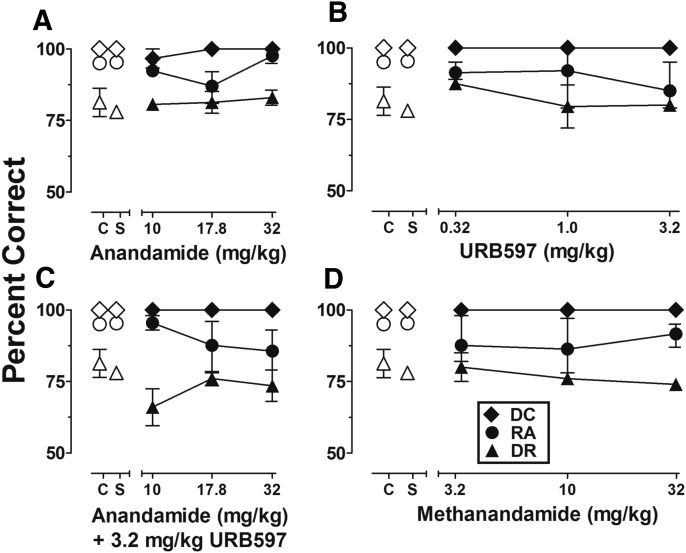
Fig. 2.
Dose-effect functions for anandamide (A), URB597 (B), anandamide following treatment of 3.2 mg/kg of URB597 (C), and methanandamide (D) on repeated acquisition (RA) trials (circles), discrimination reversal (DR) trials (triangles), and discriminative capability (DC) trials (diamonds). Abscissae, cumulative dose, log scale; ordinate, mean percent correct. Symbols left of abscissae breaks indicate performance during noninjection control (C) and saline (S) sessions. Points represent averages (±S.E.M.) for the groups of subjects; n = 5.
Figure 1 presents the dose-response functions for Δ9-THC alone (black symbols) and following pretreatment with 1.0 mg/kg rimonabant (shaded symbols). Accuracy under the discriminative capability trials remained near-perfect until the dose of 1.0 mg/kg Δ9-THC abolished performance. Modest, but orderly and significant, dose-related decreases in accuracy occurred under the repeated acquisition task following administration of 0.32 mg/kg (f = 4.41, P < 0.01) and 0.56 mg/kg (f = 5.91, P < 0.01), and again the dose of 1.0 mg/kg abolished responding in all subjects. The dose-response function for accuracy during the discrimination reversal task reveals larger dose-related effects on reversal performance. In particular, 0.32 mg/kg of Δ9-THC produced a group-average decrease in reversal performance accuracy (55% correct) that only approached statistical significance (f = 2.94, P = 0.06). A dose of 0.56 mg/kg of Δ9-THC abolished reversal performance in four out of five subjects and resulted in poor accuracy (54%) in the fifth subject.
As the shaded symbols in Fig. 1 indicate, pretreatment with 1.0 mg/kg of rimonabant shifted all three dose-response functions—discriminative capability, acquisition, and reversal—to the right. Thus, in the presence of rimonabant, 5.6 mg/kg of Δ9-THC was required to abolish discrimination reversal performance in all subjects, reflecting a three-quarter log-unit shift rightward in the dose-response function. Latency to respond under repeated acquisition, discrimination reversal, and discriminative capability trials was, on average, stable and relatively quick under control conditions [1.7 seconds (±0.4)]. Group-average latencies following 0.03–0.32 mg/kg Δ9-THC remained consistently less than 3 seconds. However, the one subject able to engage in discrimination reversal following 0.56 mg/kg Δ9-THC had an average session-wide latency of 5.45 seconds. Latency measures were either comparable or slightly increased relative to control values following Δ9-THC with rimonabant pretreatment. Session–wide group averages remained under 3 seconds across the range of doses, with the exception of the two subjects able to engage in discrimination reversal following 3.2 mg/kg Δ9-THC (4.9 and 12.2 seconds).
Figure 2, A–D shows discriminative capability trials (filled diamonds), repeated acquisition (filled circles), and discrimination reversal (filled triangles) following administration of anandamide, the FAAH inhibitor URB597, anandamide following pretreatment with 3.2 mg/kg URB597, and methanandamide. As Fig. 2 shows, neither anandamide nor URB597 up to doses of 32.0 and 3.2 mg/kg, respectively, significantly disturbed discriminative capability trials, repeated acquisition, or discrimination reversal. In addition, following pretreatment with 3.2 mg/kg URB597, doses of anandamide up to 32.0 mg/kg failed to adversely affect discriminative capability trials, repeated acquisition, or discrimination reversal. A small decrease in the group-average reversal accuracy was observed following 3.2 mg/kg URB597 + 10.0 mg/kg anandamide, but this effect was largely due to data in one subject and was not statistically significant (f = 2.14, P > 0.05). Finally, doses up to 32.0 mg/kg methanandamide also failed to disturb discriminative capability trials, repeated acquisition, or discrimination reversal. Latency to respond was relatively insensitive to the range of URB597, anandamide (with or without URB597 pretreatment), and methanandamide doses tested. Some minor variability in session-wide latencies were observed; however, group averages in all cases did not exceed 3 seconds, and most often approximated control values.
Delayed Matching-to-Sample.
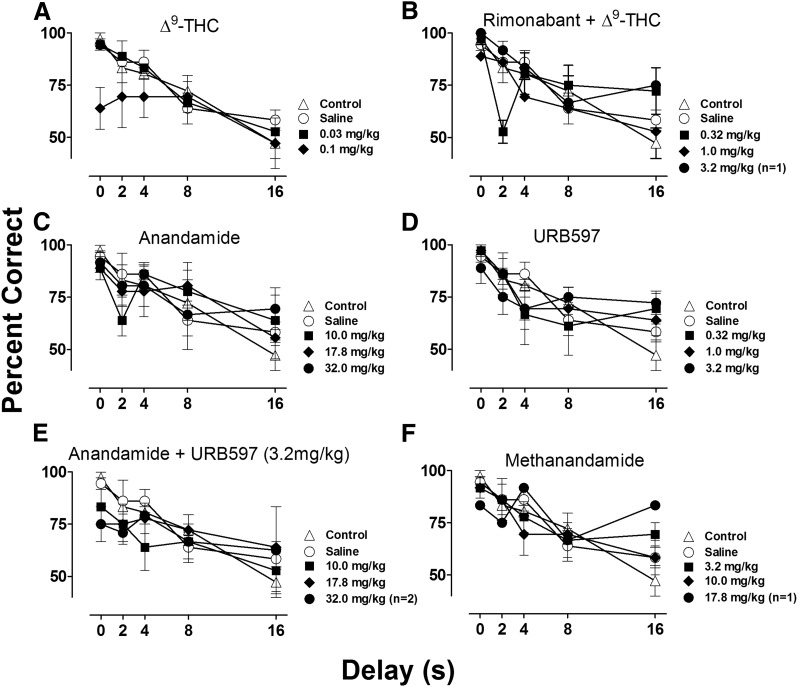
Figure 3, A–F shows grouped data for DMTS forgetting functions for Δ9-THC alone and following pretreatment of 1.0 mg/kg rimonabant, anandamide, URB597, anandamide following pretreatment with 3.2 mg/kg URB597, and methanandamide. A dose of 0.03 mg/kg Δ9-THC produced no changes from the control forgetting function, whereas 0.1 mg/kg produced decreases in accuracy under the smaller delay values [0, 2, and 4 seconds, where high accuracy (>75%) was previously observed under control conditions] but not under longer delay values (Fig. 3A). Reasons for this difference in performance following shorter delays are currently unclear. It may be that performance approaching chance levels under control conditions (here, 50% correct) is less sensitive to the effects of Δ9-THC. A higher dose of 0.32 mg/kg Δ9-THC was tested and abolished all responding in all subjects. These effects of Δ9-THC on DMTS performance were antagonized by rimonabant (Fig. 3B); following treatment with 1.0 mg/kg of rimonabant, 0.32 mg/kg of Δ9-THC was generally without effect, whereas a 10-fold higher dose (3.2 mg/kg Δ9-THC) abolished responding in two subjects and was without effect in the third subject. Neither anandamide (Fig. 3C) nor URB597 (Fig. 3D) alone produced any decreases in DMTS performance under a wide range of doses; however, moderate increases in accuracy were observed under the 16-second delay following the largest dose of each drug tested. Following administration of 32.0 mg/kg of anandamide after treatment with 3.2 mg/kg of URB597 (Fig. 3E), performance was abolished in one subject and average accuracy was reduced under the 0- and 2-second delays to <75% in the other two subjects. Treatment with 17.8 mg/kg methanandamide abolished responding in two subjects and, in the third subject, reduced accuracy under the 0- and 2-second delay and increased accuracy to under the 16-second delay (Fig. 3F).
Fig. 3.
DMTS forgetting functions across several doses of Δ9-THC (A), Δ9-THC following treatment of 1.0 mg/kg rimonabant (B), anandamide (C), URB597 (D), anandamide following treatment of 3.2mg/kg URB597 (E), and methanandamide (F). Abscissae, delay value (s); ordinate, mean percent correct. Noninjection control (open triangles) and saline (open circles) values are represented in all graphs. Points represent averages (±S.E.M.) for the groups of subjects. A larger dose of Δ9-THC (0.32 mg/kg) was tested, but performance was abolished making accuracy indeterminate; n = 3, *P < 0.05.
Table 1 presents response rate data during the FR20 sample stimulus response requirement. Response rates were stable and relatively consistent among individual subjects [2.6 responses/second (±0.19)] under control conditions. Relatively moderate dose-related decreases in response rate were observed following administration of Δ9-THC (with and without rimonabant pretreatment), but average rates remained consistently above 1 response/second. Similarly modest decreases were also observed with the largest doses of anandamide (with URB597 pretreatment) and methanandamide tested.
TABLE 1.
Group-average response rate (±S.E.M.) during the FR20 sample response requirement in DMTS
| Control [2.6 (±0.19)] |
Saline [2.7 (±0.19)] |
||||
|---|---|---|---|---|---|
| Drug |
Responses/Second |
Mean (S.E.M.) |
Drug |
Responses/Second |
Mean (S.E.M.) |
| mg/kg | mg/kg | ||||
| Δ9-THC | 0.03 | 3.17 (±0.41) | Rimonabant + Δ9-THC | 0.32 | 2.80 (±0.26) |
| 0.1 | 1.3 (±0.26) | 1.0 | 1.51 (±0.48) | ||
| 3.2 (n = 1) | 1.84 | ||||
| Anandamide | 10.0 | 2.87 (±0.57) | URB597 | 0.32 | 2.40 (±0.65) |
| 17.8 | 2.34 (±0.41) | 1.0 | 3.53 (±0.42) | ||
| 32.2 | 2.59 (±0.36) | 3.2 | 3.03 (±0.28) | ||
| URB597 + Anandamide | 10.0 | 2.1 (±0.50) | Methanandamide | 3.2 | 2.80 (±0.49) |
| 17.8 | 2.77 (±0.31) | 10.0 | 2.95 (±0.34) | ||
| 32.0 (n = 2) | 1.60 (±0.87) | 17.8 (n = 1) | 2.10 | ||
Psychomotor Vigilance.
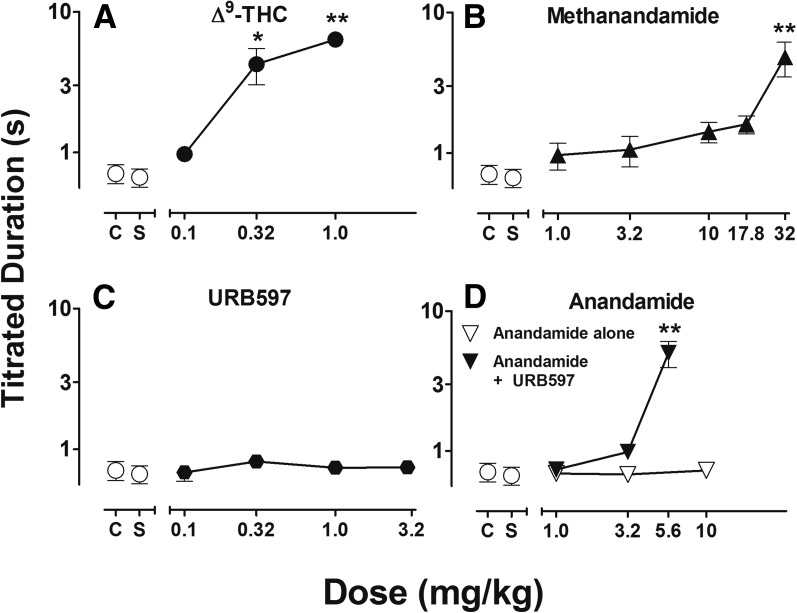
Figure 4, A–D presents mean titrated delay values under the psychomotor vigilance task following treatment with Δ9-THC, methanandamide, URB597, and anandamide alone (open triangles) and following treatment with 3.2 mg/kg URB597 (filled triangles). All subjects were able to maintain focused vigilance with mean titrated durations of <1 second throughout the 100-trial control sessions. As Fig. 4 shows, Δ9-THC and methanandamide produced dose-related increases in reaction time. No statistically significant effects were observed on reaction time following low doses of each agonist, whereas the largest doses of Δ9-THC and methanandamide produced marked and significant response disruptions in reaction time, yielding mean titrated duration values approaching 10 seconds (f = 5.66, P < 0.01 and f = 5.02, P < 0.01, respectively). Intermediate doses produced moderate increases in mean titrated duration values in all subjects but failed to reach statistical significance, with the exception of 0.32 mg/kg Δ9-THC (f = 3.47, P < 0.05). URB597 and anandamide alone up to doses of 3.2 and 10.0 mg/kg, respectively, did not increase mean titrated durations. However, 5.6 mg/kg anandamide following treatment with 3.2 mg/kg URB597 significantly increased mean titrated duration value to approximately 5 seconds (f = 5.51, P < 0.01).
Fig. 4.
Dose-effect functions for Δ9-THC (A), methanandamide (B), URB597 (C), and anandamide alone (open inverted-triangle) or following treatment of 3.2mg/kg URB597 (filled inverted-triangle) (D) on titrated duration values. Abscissae, cumulative dose, log scale; ordinate, mean titrated duration (s). Symbols left of abscissae breaks indicate performance during noninjection control (C) and saline (S) sessions. Points represent averages (±S.E.M.) for the groups of subjects; n = 5, *P < 0.05, **P < 0.01.
Progressive Ratio.
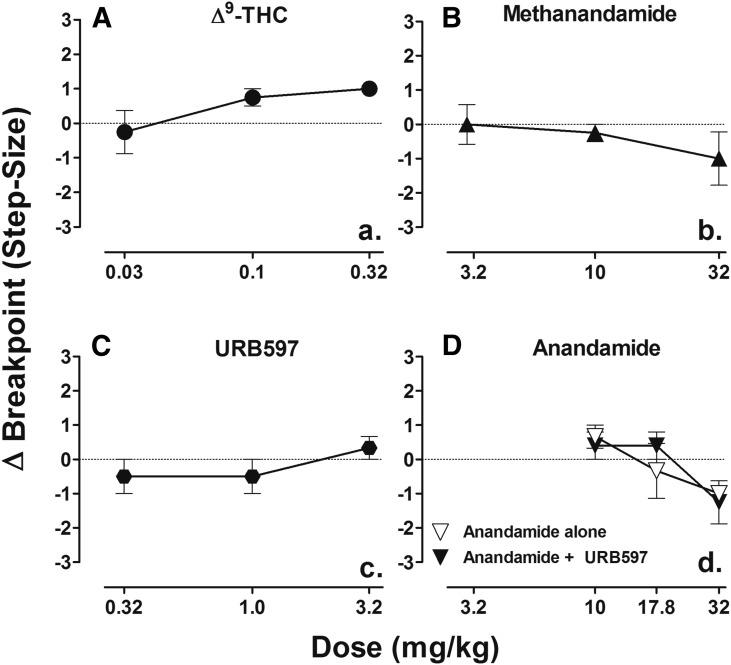
Steady-state progressive ratio performance developed in fewer than 10 sessions in all six subjects. Modal response breakpoints varied among subjects and were 32 (n = 2), 64 (n = 2), and 128 (n = 2). Figure 5, A–D shows mean step-size changes in the progressive ratio breakpoint following Δ9-THC, methanandamide, URB597, and anandamide alone (open triangles) and following treatment with 3.2 mg/kg URB597 (filled triangles). No statistically significant changes in breakpoint were observed for any drug across the range of doses shown in Fig. 5; doses of Δ9-THC one-half log unit higher than those shown in Fig. 5 consistently abolished responding, making breakpoints indeterminate.
Fig. 5.
Dose-effect functions for Δ9-THC (A), methanandamide (B), URB597 (C), and anandamide alone (open inverted-triangle) or following treatment of 3.2mg/kg URB597 (filled inverted-triangle) (D) on progressive ratio. Abscissae, cumulative dose, log scale; ordinate, mean change in breakpoint. Points represent averages (±S.E.M.) for the groups of subjects. A larger dose of Δ9-THC (1.0 mg/kg) was tested, but performance was abolished making breakpoints indeterminate; n = 6.
Discussion
In the present experiments, the effects of cannabinergic drugs were studied using a battery of touchscreen procedures to assay different facets of cognition-related behavior in nonhuman primates. The use of such a battery to provide multiple complex behavioral endpoints has been highlighted in previous research in human and nonhuman subjects (e.g., Sahakian and Owen, 1992; Nagahara et al., 2010) and is considered to provide a meaningful assessment of the varied cognition-related effects of psychoactive drugs. In the present experiments, this approach permitted the identification of key qualitative distinctions between the behavioral effects of the CB1 receptor agonist Δ9-THC and those of anandamide, whether administered alone, in the presence of a FAAH inhibitor, or as its stable analog methanandamide.
Δ9-THC produced dose-related decrements in the performance of each task in the present studies, with the exception of progressive ratio. The largely null results obtained with the progressive ratio procedure (Fig. 5) provide evidence that the dose-related effects observed in the other assays likely are due to the effects on cognitive behavior rather than changes in motivation to respond. Moreover, the capacity of rimonabant to antagonize the effects of Δ9-THC is consistent with the view that such decrements in cognitive behavior are due to its CB1-mediated actions. These effects of Δ9-THC administration systematically replicate previously observed findings in nonhuman primates (e.g., Schulze et al., 1988; Winsauer et al., 1999; Taffe, 2012; Wright et al., 2013).
Anandamide alone was without effect on all endpoints; this was almost certainly due to its rapid metabolic inactivation (e.g., Willoughby et al., 1997; Stewart and McMahon, 2011). Large doses of methanandamide, the stable analog of anandamide, or anandamide administered after treatment with the FAAH inhibitor URB597 also did not affect measures of discriminative capability, learning, or cognitive flexibility, but in some subjects abolished responding in the short-term memory task. Comparing the four discrimination tasks in the present studies (discriminative capability, repeated acquisition, discrimination reversal, and DMTS), short-term memory assayed by the DMTS task was most vulnerable to drug action, followed by discrimination reversal, repeated acquisition, and discriminative capability. Although differences in baseline accuracies make potency comparisons across tasks difficult, examination of drug doses that eliminated discriminative performance reveals the differences in relative potency. For example, a dose of 0.32 mg/kg Δ9-THC had moderate effects on repeated acquisition performance; however, none of the subjects were able to initiate the DMTS task. Likewise, although doses of 32 mg/kg anandamide (after URB597 pretreatment) and 17.8 mg/kg methanandamide failed to perturb acquisition or reversal performance, these same doses abolished performance in the DMTS task in some subjects. The memorial vulnerability revealed by these comparisons across discrimination-based tasks is consistent with previous suggestions that cannabis has particularly deleterious effects on short-term memory in humans (reviewed in Ranganathan and D’Souza, 2006; Solowij and Battisti, 2008) and systematically replicates previous findings with nonhuman primates (Schulze et al., 1988; Taffe, 2012).
The basis for the observed differences in cannabinoid potency across tasks is uncertain but may be related to differences in sensitivity to drug action across the different neural substrates involved in these complex behavioral performances. The present studies were not designed to assess localization of function under the different cognitive tasks, but previous research may inform the current findings. For example, the control of discrimination reversal performance appears to be highly localized in the orbital prefrontal cortex (reviewed in Chudasama, 2011), whereas DMTS is thought to be closely controlled by hippocampal mechanisms (reviewed in Eichenbaum et al., 1992). Although CB1 receptors are widely expressed throughout the brain (Freund et al., 2003), recent studies have indicated that CB1 receptor dynamics (e.g., desensitization and downregulation) differ across brain regions (see Lazenka et al., 2013). For example, Sim-Selley et al. (2006) treated mice chronically with Δ9-THC or WIN55,212-2 [R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo-[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate] and showed that following discontinuation cannabinoid-induced decreases in CB1 receptor function persisted relatively longer and CB1 receptor signaling recovered more quickly in the striatum compared with the hippocampus. These findings are consistent with recent data from positron emission tomography imaging studies in human marijuana users that showed slower recovery of CB1 receptors in hippocampus than in other brain regions (Hirvonen et al., 2012). Although not well understood at present, such differences in regional CB1 receptor dynamics and signaling possibly are also reflected in the different potencies of CB1 agonists across cognitive tasks that are mediated in different brain areas.
Alternatively, it may be that differences in the relative potencies of the cannabinergic drugs across tasks most directly reflect differences in the difficulty of the tasks, i.e., more difficult tasks may be more vulnerable to cannabinergic drug action, resulting in higher potency. For example, early research by Branch et al. (1980) showed that squirrel monkeys trained to emit a 5-key response sequence were more sensitive to acute doses of Δ9-THC than when emitting a 2-key sequence. In this regard, performances under the four tasks represented in Figs. 1–3 all require discriminative behavior but differ in complexity. That is, discriminative capability trials are a simple and well-learned discrimination, repeated acquisition adds complexity with the daily presentation of novel stimuli, and discrimination reversal is made even more complex due to the unsignaled shift in contingency. DMTS is perhaps the most complex task among the four procedures because accuracy depends on a conditional discrimination (i.e., S+ and S− contingencies are conditional on the previously presented sample) and, moreover, a delay between the presentation of sample and comparison stimuli. Thus, although the extent to which a drug’s potency depends on task complexity may be difficult to determine a priori, the present results support the view that this factor at least contributed to differences in the potency of cannabinergic drugs in cognition-related studies.
Whether differences in potency in the present studies were based on neural mechanisms and/or behavioral complexity, anandamide (delivered exogenously with or without a FAAH inhibitor) and its metabolically stable analog methanandamide up to large doses had less disruptive effects on performance of all tasks than observed with Δ9-THC. Although solubility issues precluded testing doses of anandamide and methanandamide larger than 32.0 mg/kg, data from the psychomotor vigilance task (Fig. 4) provide evidence that the highest doses of anandamide and methanandamide in the present studies were behaviorally active and simply not very effective in other tasks. For example, following pretreatment with 3.2 mg/kg URB597, a dose of 5.6 mg/kg anandamide significantly increased mean titrated duration values, whereas doses up to 32.0 mg/kg anandamide failed to perturb acquisition or reversal performance. Likewise, 32.0 mg/kg of methanandamide significantly disrupted psychomotor vigilance but had no effect on acquisition or reversal performance. In contrast, the doses of Δ9-THC that significantly disrupted psychomotor vigilance also reliably disrupted reversal performance. These data are somewhat surprising given the highly stable latency measures observed in repeated acquisition and discrimination reversal performance following the same dose ranges. Dose-related effects on response rate during the DMTS task corresponded to the relative dose-related effects on accuracy, but were nevertheless fairly modest in magnitude. Therefore, it appears that maintaining short titrating duration values across a 100-trial session (i.e., focused vigilance) is more sensitive to cannabinergic action than reaction time or response rate during discriminative performance. However, it should be noted that this psychomotor vigilance task was not designed to tease apart the effect of a drug on the subject’s ability to detect a signal (vigilance) versus the ability to respond repeatedly (psychomotor effect).
While it is possible that larger doses of anandamide following FAAH inhibition or methanandamide might have led to decreases in discriminative performance, the present results support the view that metabolically stable forms of anandamide may activate the cannabinoid system with less profound effects on cognitive behavior. Explanations for such lesser effects of the endocannabinoid are presently uncertain. Based on data from previous studies, anandamide may have lower efficacy at CB1 receptors than other cannabinergic drugs, and thus its lesser effects on cognitive function may reflect its partial agonist activity (Mackie et al., 1993; Järbe et al., 1998; Desai et al., 2013). In this regard, Δ9-THC also is usually considered to be a CB1 partial agonist; however, its relative efficacy at CB1 receptors that mediate its effects in these tasks may be greater than that of anandamide. This idea is consistent with findings in rats that methanandamide disrupted the learning of response chains more than anandamide (alone) but less than Δ9-THC (Brodkin and Moerschbaecher, 1997). From a clinical perspective, the present findings may become especially meaningful if FAAH inhibitors and/or metabolically stable forms of anandamide can provide medicinal (e.g., antinauseant, antiemetic, or appetite stimulant) effects in humans that have been reported in other species (e.g., Williams and Kirkham, 1999; Cross-Mellor et al., 2007; Rock et al., 2008; Parker et al., 2009; Limebeer et al., 2014; Sharkey et al., 2014). In that case, the present data support the view that the activation of CB1 receptors by endocannabinoids—and in particular anandamide—may provide a therapeutic avenue with fewer deleterious effects on cognitive performance than produced by Δ9-THC.
Acknowledgments
The authors thank Drs. R. I. Desai, S. J. Kohut, C. A. Paronis, and R. D. Spealman for comments on a previous version of this manuscript.
Abbreviations
- CB1
cannabinoid receptor type 1
- Δ9-THC
Δ9-tetrahydrocannabinol
- DMTS
delayed matching-to-sample
- FAAH
fatty acid amide hydrolase
- FR
fixed ratio
- ITI
intertrial interval
- methanandamide (AM356)
(R)-(+)-arachidonyl-1′-hydroxy-2′-propylamide
- rimonabant (SR141716A)
5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1Hpyrazole-3-carboxamide
- SEM
standard error of the mean
- URB597
cyclohexylcarbamic acid 3-carbamoylbiphenyl-3-yl ester
Authorship Contributions
Participated in research design: Kangas, Bergman.
Conducted experiments: Kangas, Leonard.
Contributed new reagents or analytic tools: Shukla, Nikas, Alapafuja, Makriyannis.
Performed data analysis: Kangas, Leonard.
Wrote or contributed to the writing of the manuscript: Kangas, Bergman.
Footnotes
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants K01-DA035974 (to B.D.K.) and R01-DA031020 (to J.B.)]
References
- Blough DS. (1959) Delayed matching in the pigeon. J Exp Anal Behav 2:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch MN, Dearing ME, Lee DM. (1980) Acute and chronic effects of Δ9-tetrahydrocannabinol on complex behavior of squirrel monkeys. Psychopharmacology (Berl) 71:247–256. [DOI] [PubMed] [Google Scholar]
- Brodkin J, Moerschbaecher JM. (1997) SR141716A antagonizes the disruptive effects of cannabinoid ligands on learning in rats. J Pharmacol Exp Ther 282:1526–1532. [PubMed] [Google Scholar]
- Chudasama Y. (2011) Animal models of prefrontal-executive function. Behav Neurosci 125:327–343. [DOI] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. (2011) An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med 5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cridge BJ, Rosengren RJ. (2013) Critical appraisal of the potential use of cannabinoids in cancer management. Cancer Manag Res 5:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Mellor SK, Ossenkopp KP, Piomelli D, Parker LA. (2007) Effects of the FAAH inhibitor, URB597, and anandamide on lithium-induced taste reactivity responses: a measure of nausea in the rat. Psychopharmacology (Berl) 190:135–143. [DOI] [PubMed] [Google Scholar]
- Desai RI, Thakur GA, Vemuri VK, Bajaj S, Makriyannis A, Bergman J. (2013) Analysis of tolerance and behavioral/physical dependence during chronic CB1 agonist treatment: effects of CB1 agonists, antagonists, and noncannabinoid drugs. J Pharmacol Exp Ther 344:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A. (2005) Behavioural flexibility, social learning, and the frontal cortex, in The Cognitive Neuroscience of Social Behavior (Easton A, Emery NJ. eds) pp 59–80, Psychology Press, New York. [Google Scholar]
- Egerton A, Allison C, Brett RR, Pratt JA. (2006) Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neurosci Biobehav Rev 30:680–695. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. (1992) The hippocampus—what does it do? Behav Neural Biol 57:2–36. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. (2003) Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83:1017–1066. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Dipasquale P, Romano A, Righetti L, Cassano T, Piomelli D, Cuomo V. (2009) The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. Int Rev Neurobiol 85:57–72. [DOI] [PubMed] [Google Scholar]
- Galizio M, McKinney P, Cerutti DT, Pitts RC. (2009) Effects of MDMA, methamphetamine and methylphenidate on repeated acquisition and performance in rats. Pharmacol Biochem Behav 94:305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB. (2012) Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 17:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. (1961) Progressive ratio as a measure of reward strength. Science 134:943–944. [DOI] [PubMed] [Google Scholar]
- Iversen L. (2005) Long-term effects of exposure to cannabis. Curr Opin Pharmacol 5:69–72. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Makriyannis A, Lin S, Goutopoulos A. (1998) Delta9-THC training dose as a determinant for (R)-methanandamide generalization in rats. Psychopharmacology (Berl) 140:519–522. [DOI] [PubMed] [Google Scholar]
- Kangas BD, Bergman J. (2012) A novel touch-sensitive apparatus for behavioral studies in unrestrained squirrel monkeys. J Neurosci Methods 209:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J. (2014) Repeated acquisition and discrimination reversal in the squirrel monkey (Saimiri sciureus). Anim Cogn 17:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Berry MS, Branch MN. (2011) On the development and mechanics of delayed matching-to-sample performance. J Exp Anal Behav 95:221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, et al. (2003) Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 9:76–81. [DOI] [PubMed] [Google Scholar]
- Lazenka MF, Selley DE, Sim-Selley LJ. (2013) Brain regional differences in CB1 receptor adaptation and regulation of transcription. Life Sci 92:446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limebeer CL, Abdullah RA, Rock EM, Imhof E, Wang K, Lichtman AH, Parker LA. (2014) Attenuation of anticipatory nausea in a rat model of contextually elicited conditioned gaping by enhancement of the endocannabinoid system. Psychopharmacology (Berl) 231:603–612. [DOI] [PubMed] [Google Scholar]
- Mackie K, Devane WA, Hille B. (1993) Anandamide, an endogenous cannabinoid, inhibits calcium currents as a partial agonist in N18 neuroblastoma cells. Mol Pharmacol 44:498–503. [PubMed] [Google Scholar]
- Mackintosh NJ, McGonigle B, Holgate V, Vanderver V. (1968) Factors underlying improvement in serial reversal learning. Can J Psychol 22:85–95. [DOI] [PubMed] [Google Scholar]
- Mackworth NH. (1948) The breakdown of vigilance during prolonged visual search. Q J Exp Psychol 1:6–21. [Google Scholar]
- McCarthy D, White KG. (1985) Behavioral models of delayed detection and their applications to the study of memory, in Quantitative Analyses of Behavior: The Effect of Delay and Intervening Events (Commons ML, Mazur JE, Nevin JA, Rachlin H. eds) vol. 5, pp 29–54, Ballinger, Cambridge, MA. [Google Scholar]
- Nagahara AH, Bernot T, Tuszynski MH. (2010) Age-related cognitive deficits in rhesus monkeys mirror human deficits on an automated test battery. Neurobiol Aging 31:1020–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL, Rock EM, Litt DL, Kwiatkowska M, Piomelli D. (2009) The FAAH inhibitor URB-597 interferes with cisplatin- and nicotine-induced vomiting in the Suncus murinus (house musk shrew). Physiol Behav 97:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. (2014) Elevating endocannabinoid levels: pharmacological strategies and potential therapeutic applications. Proc Nutr Soc 73:96–105. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D’Souza DC. (2006) The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl) 188:425–444. [DOI] [PubMed] [Google Scholar]
- Rock EM, Limebeer CL, Mechoulam R, Piomelli D, Parker LA. (2008) The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmacology (Berl) 196:389–395. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Owen AM. (1992) Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med 85:399–402. [PMC free article] [PubMed] [Google Scholar]
- Schulze GE, McMillan DE, Bailey JR, Scallet A, Ali SF, Slikker W, Jr, Paule MG. (1988) Acute effects of delta-9-tetrahydrocannabinol in rhesus monkeys as measured by performance in a battery of complex operant tests. J Pharmacol Exp Ther 245:178–186. [PubMed] [Google Scholar]
- Seierstad M, Breitenbucher JG. (2008) Discovery and development of fatty acid amide hydrolase (FAAH) inhibitors. J Med Chem 51:7327–7343. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, Darmani NA, Parker LA. (2014) Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system. Eur J Pharmacol 722:134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, Selley DE. (2006) Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol 70:986–996. [DOI] [PubMed] [Google Scholar]
- Solowij N, Battisti R. (2008) The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev 1:81–98. [DOI] [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. (2011) The fatty acid amide hydrolase inhibitor URB 597: interactions with anandamide in rhesus monkeys. Br J Pharmacol 164:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA. (2012) Δ9Tetrahydrocannabinol impairs visuo-spatial associative learning and spatial working memory in rhesus macaques. J Psychopharmacol 26:1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed MR, Gold LH. (1998) The effects of dopaminergic agents on reaction time in rhesus monkeys. Psychopharmacology (Berl) 137:33–42. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC. (1999) Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 143:315–317. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Moore SF, Martin BR, Ellis EF. (1997) The biodisposition and metabolism of anandamide in mice. J Pharmacol Exp Ther 282:243–247. [PubMed] [Google Scholar]
- Winsauer PJ, Lambert P, Moerschbaecher JM. (1999) Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behav Pharmacol 10:497–511. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Vandewater SA, Parsons LH, Taffe MA. (2013) Δ9Tetrahydrocannabinol impairs reversal learning but not extra-dimensional shifts in rhesus macaques. Neuroscience 235:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S. (2011) Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci 5:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]