Abstract
Ethanol alters GABAA receptor trafficking and function through activation of protein kinases, and these changes may underlie ethanol dependence and withdrawal. In this study, we used subsynaptic fraction techniques and patch-clamp electrophysiology to investigate the biochemical and functional effects of protein kinase A (PKA) and protein kinase C (PKC) activation by ethanol on synaptic GABAA α4 receptors, a key target of ethanol-induced changes. Rat cerebral cortical neurons were grown for 18 days in vitro and exposed to ethanol and/or kinase modulators for 4 hours, a paradigm that recapitulates GABAergic changes found after chronic ethanol exposure in vivo. PKA activation by forskolin or rolipram during ethanol exposure prevented increases in P2 fraction α4 subunit abundance, whereas inhibiting PKA had no effect. Similarly, in the synaptic fraction, activation of PKA by rolipram in the presence of ethanol prevented the increase in synaptic α4 subunit abundance, whereas inhibiting PKA in the presence of ethanol was ineffective. Conversely, PKC inhibition in the presence of ethanol prevented the ethanol-induced increases in synaptic α4 subunit abundance. Finally, we found that either activating PKA or inhibiting PKC in the presence of ethanol prevented the ethanol-induced decrease in GABA miniature inhibitory postsynaptic current decay τ1, whereas inhibiting PKA had no effect. We conclude that PKA and PKC have opposing effects in the regulation of synaptic α4 receptors, with PKA activation negatively modulating, and PKC activation positively modulating, synaptic α4 subunit abundance and function. These results suggest potential targets for restoring normal GABAergic functioning in the treatment of alcohol use disorders.
Introduction
Ethanol causes adaptations in GABAA receptors that are associated with alcohol dependence and withdrawal (Kumar et al., 2009). GABAA receptors are ligand-gated chloride channels mediating the majority of inhibitory neurotransmission in the brain through both phasic and tonic currents (Farrant and Nusser, 2005). These channels consist of five subunits typically composed of two α subunits (1–6), two β subunits (1–3), and a γ (1–2) or δ subunit (Tretter and Moss, 2008). Two important targets of ethanol regulation in particular are the synaptic α4βγ2 and extrasynaptic α4βδ GABAA receptors mediating a portion of phasic and tonic inhibition, respectively (Olsen and Sieghart, 2009). The extrasynaptic α4 receptors have been the subject of much study, owing to their responses to relatively low doses of ethanol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003; Wei et al., 2004); however, surprisingly little is known about the regulation of synaptic α4 receptors.
Although early studies showed that ethanol regulates the abundance of α4 subunit mRNA and protein expression (Devaud et al., 1996, 1997; Papadeas et al., 2001; Cagetti et al., 2003), the question of whether this regulation reflected synaptic or extrasynaptic receptor adaptations depended on subsequent functional studies of synaptic α4 receptor kinetics. Synaptic α4 receptors are upregulated after both acute (Liang et al., 2007) and chronic (Liang et al., 2006; Werner et al., 2011) ethanol exposure in the rat hippocampus and cortex. More recently, we demonstrated ethanol regulation of synaptic α4 subunits in C57BL/6J mice after an acute injection of ethanol, using subcellular fractionation to isolate synaptic versus subsynaptic receptors (Carlson et al., 2014). Isolating the precise physiologic and behavioral ramifications of these changes in synaptic α4 receptors is difficult because there are no selective pharmacological agents targeting these receptors. The α4βγ2 receptors are benzodiazepine insensitive, although they show relatively high affinity for the GABA inverse agonist Ro15-4513 (ethyl-8-azido-5,6-dihydro-5-methyl-6-oxo-4H-imidazo-1,4-benzodiazepine-3-carboxylate) (Knoflach et al., 1996). However, Ro15-4513 also antagonizes effects of ethanol on α4βδ receptors (Hanchar et al., 2006) that likely mediate effects of ethanol on tonic inhibition.
Recent studies from our laboratory have uncovered that kinase activation by ethanol plays a major role in GABAA receptor regulation, and that protein kinase A (PKA) and protein kinase C (PKC) may have opposing effects on GABAergic trafficking and function. PKA activity positively regulates synaptic GABAA α1 subunit abundance and function in cortical neurons, whereas PKCγ activity negatively regulates these receptors (Kumar et al., 2010; Carlson et al., 2013). PKA activation also positively regulates extrasynaptic GABAA α4 and δ subunits in cortical neurons, whereas PKC is not involved in regulating these receptors in this brain region (Carlson et al., 2016). Finally, PKCγ activation by ethanol causes an increase in GABAA α4 subunits, although it is unclear whether this effect is specific to the synaptic population of receptors (Werner et al., 2011). It is also unclear what role PKA activity may play in regulating this subset of α4 receptors.
This study elucidates the role of PKA and PKC in ethanol regulation of synaptic α4-containing GABAA receptors in cerebral cortical cultured neurons. We used a 4-hour ethanol exposure paradigm that recapitulates many of the GABAergic adaptations observed after chronic ethanol exposure in vivo (Devaud et al., 1997; Kumar et al., 2003). We measured changes in subunit abundance using a subcellular fractionation technique that enriches synaptic proteins combined with western blot analysis, whereas functional changes were measured using whole-cell patch-clamp analysis of GABA miniature inhibitory postsynaptic currents (mIPSCs).
Materials and Methods
Cultured Cerebral Cortical Neurons.
All experiments were conducted in accordance with guidelines from the National Institutes of Health and Institutional Animal Care and Use Committee at the University of North Carolina. Mixed-sex rat pups from Sprague-Dawley breeding pairs (Harlan, Indianapolis, IN) were decapitated on postnatal day 0–1. Brains were rapidly dissected and the cerebral cortices were isolated. Cortical halves were minced into fine pieces and tissue was incubated in CO2-independent media containing papain (50 U/ml; Worthington, Lakewood, NJ) and l-cysteine and DNase (both from Sigma-Aldrich, St. Louis, MO) for 30 minutes at 37°C. Tissue pieces were gently washed, followed by gentle trituration in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY) containing 10% horse serum, penicillin-streptomycin, and DNase. Cells used for biochemistry were plated onto poly(d-lysine)–coated flasks, whereas cells used for electrophysiology were plated onto poly(d-lysine)–coated coverslips in 12-well plates. Cells were maintained in a 5% CO2 humidified incubator. After day 3, cells were fed with serum-free medium containing B27 and penicillin-streptomycin (10,000 U/ml; final concentration 50 U per flask). Media were changed twice per week with no more than one-third of the media being removed during exchanges. For all experiments, penicillin-streptomycin was removed from cultures on day 14 to prevent interactions with GABAA receptors. Cultures were maintained for 18 days before conducting experiments, because prior studies determined that GABAA receptor expression was stable between 15 and 19 days in vitro.
Ethanol and Drug Exposure.
Cultured cells were exposed to 50 mM ethanol and placed in a plastic vapor chamber within the incubator. This concentration was chosen because it produces changes in GABAergic inhibition consistent with in vivo models (Devaud et al., 1997; Kumar et al., 2003). A beaker of water with 50 mM ethanol was used to maintain stable ethanol concentrations in the chamber. Control cells were exposed to an equivalent amount of water and placed in a plastic vapor chamber with a beaker containing water. Cells were exposed to ethanol for 4 hours. To examine PKA involvement, the PKA inhibitor Rp-adenosine 3′,5′-cyclic monophosphothioate triethylamine (Rp-cAMP; 50 µM), the adenylyl cyclase activator forskolin (10 µM; Tocris Bioscience, Minneapolis, MN), or the phosphodiesterase inhibitor rolipram (10 µM; Sigma-Aldrich) was added to the cell media. To examine PKC involvement, the PKC inhibitor calphostin C (CalC; 0.3 µM in 0.1% dimethylsulfoxide, final concentrations; Sigma-Aldrich) or the activator phorbol-12,13-dibutyrate (0.1 µM; Sigma-Aldrich) was added to the cell media. The concentrations of PKA and PKC modulators were chosen based on previous studies (Zhang and Pandey, 2003; Carlson et al., 2013).
Subcellular Fractionation.
After experiments, the reactions were stopped by placing the flasks on ice. Cells were washed with cold phosphate-buffered saline, scraped, centrifuged at 1000g for 18 minutes, and stored at −80°C until fractionation. Cell pellets were homogenized in 0.32 M sucrose and centrifuged at 1000g for 10 minutes. The supernatant was then centrifuged twice for 30 minutes at 12,000g to yield the P2 fraction pellet. For experiments examining the synaptic fraction, the P2 fraction was further purified into the synaptic fraction according to the methods of Goebel-Goody et al. (2009) as previously described (Carlson et al., 2014, 2016). The fractions were separated by 30-minute incubation in 0.5% Triton-X, followed by two centrifugations at 32,000g for 30 minutes. The resulting pellet was resuspended to yield the synaptic fraction. Protein concentrations for isolated P2 fraction or synaptic fractions were calculated using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Waltham, MA). Samples were then subjected to gel electrophoresis and western blot analysis.
Western Blot Analysis.
GABAA receptor α4 subunit abundance was analyzed by western blot. Protein samples were subjected to SDS-PAGE using Novex Tris-Glycine (8%–16%) gels and were transferred to polyvinylidene fluoride (PVDF) membranes (Invitrogen, Carlsbad, CA). Membranes were probed with GABAA receptor α4 antibody (1:500 dilution; Abcam, Cambridge, MA), anti-GABAA δ (1:750 dilution; Novus, St. Louis, MO), or anti-GABAA γ2 (1:1000 dilution; Novus), followed by β-actin antibody (1:3000 dilution; Millipore, Billerica, MA) for normalization. Proteins were detected with enhanced chemiluminescence (GE Healthcare, Amersham, UK). Membranes were imaged using a LAS-4000 (GE Healthcare), and densitometric analysis was conducted using GE ImageQuant software. Comparisons were made within blots and expressed as a percentage of averaged control values.
Electrophysiology.
Whole-cell voltage clamp recordings were used to assess mIPSCs. Electrodes were pulled using a PP-830 device (Narishige, Tokyo, Japan) and were fire polished to a resistance of 2 to 3 MΩ. Intracellular solution contained 150 mM KCl, 3.1 mM MgCl2, 15 mM HEPES, 5 mM KATP, 5 mM EGTA, and 15 mM phosphocreatine, adjusted to pH 7.4 with KOH. Extracellular solution contained 145 mM NaCl, 5 mM KCl, 10 mM HEPES, 2 mM CaCl2, 1 mM MgCl2, 5 mM sucrose, and 10 mM glucose, adjusted to pH 7.4 with NaOH. For mIPSC recordings, the external solution also contained 6-cyno-7-nitroquinoxaline-2,3-dione (10 μM; Sigma-Aldrich), d-2-amino-5-phosphonopentanoic acid (40 μM; Tocris Bioscience), and tetrodotoxin (1 μM; Sigma-Aldrich). Membrane potential was held at −60 mV and currents were recorded with an Axopatch ID amplifier (Axon Instruments, Union City, CA). Data were collected using Clampex software (Axon Instruments). mIPSCs were analyzed using miniAnalysis software (version 5.6.4; Synaptosoft, Decatur, GA). mIPSCs were recorded for a minimum of 3 minutes. Minimum threshold detection was set to 5 pA. Frequency was determined using automatic detection of each recording. To assess mIPSC kinetics, the recording trace was visually inspected and only events with a stable baseline, sharp rising phase, and single peak were used. Only recordings with a minimum of 25 events fitting these criteria were analyzed. Decay time constants were obtained by using a double exponential fit for the average of the mIPSCs in a single recording.
Statistical Analysis.
Numerical data are presented as means ± S.E.M. Analyses were conducted using analysis of variance (ANOVA) and the Bonferroni post-test or t test.
Results
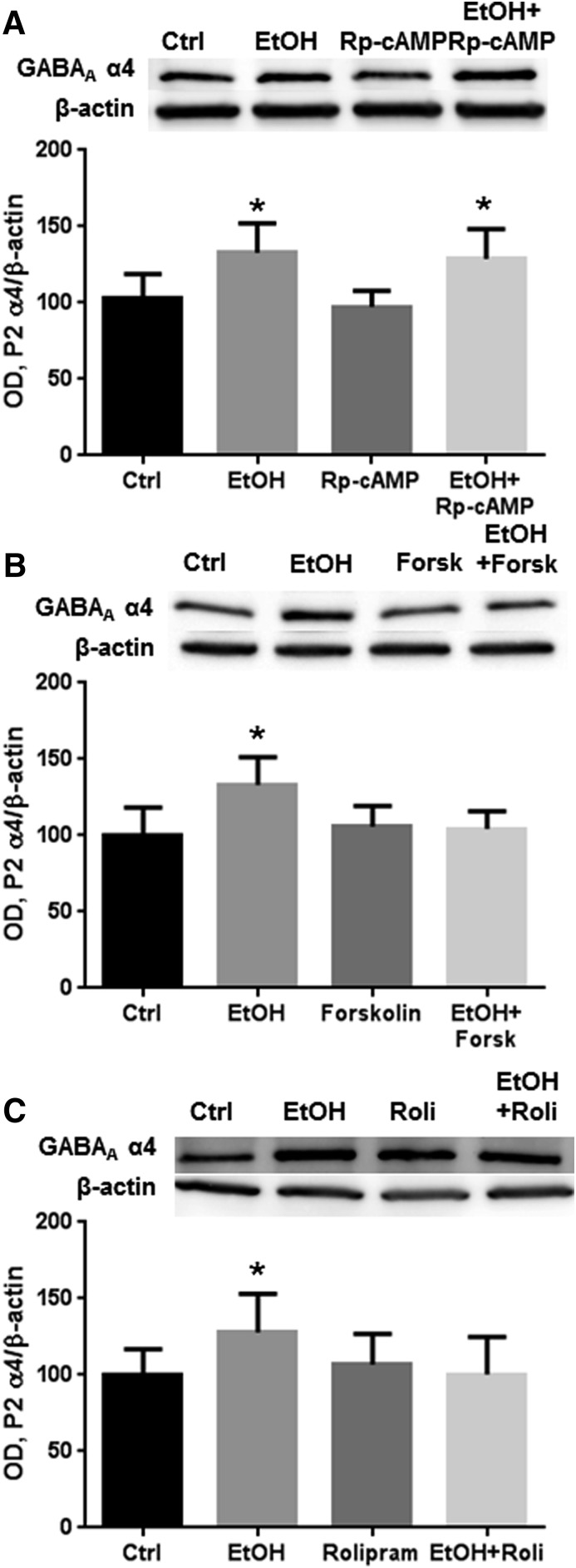
Direct PKA Activation Prevents the Effects of 4-Hour Ethanol Exposure on Total GABAA α4 Abundance in the P2 Fraction.
We first examined the effect of PKA modulation on GABAA α4 subunits during ethanol exposure. Exposure to ethanol for 4 hours increased GABAA α4 subunit levels in the P2 fraction of cerebral cortical neurons as expected based on previous studies (Fig. 1). Inhibition of PKA by Rp-cAMP did not affect ethanol-induced increases in α4 abundance (Fig. 1A). Conversely, activation of PKA during ethanol exposure by either the adenylyl cyclase activator forskolin (10 μM; Fig. 1B, P < 0.01, one-way ANOVA, F = 6.330, P < 0.01, Bonferroni post-test, n = 6 to 7 per group) or the phosphodiesterase inhibitor rolipram (10 μM; Fig. 1C, P < 0.05, one-way ANOVA, F = 3.107, P < 0.05, Bonferroni post-test, n = 7 to 8 per group) prevented the increase of α4 subunit abundance. None of the PKA modulators alone had any effect on GABAA α4 subunit abundance.
Fig. 1.
PKA activation prevents ethanol-induced increases in P2 fraction GABAA α4 subunits. Cortical neurons were exposed to vehicle, ethanol (50 mM), Rp-cAMP (50 μM), forskolin (10 μM), and/or rolipram (10 μM) for 4 hours, followed by P2 fractionation and western blot analysis. (A) Exposure to ethanol for 4 hours increased P2 fraction levels of the GABAA α4 subunit, which was not affected by coexposure with Rp-cAMP. (B and C) Exposure to the adenylyl cyclase activator forskolin (B) or the phosphodiesterase inhibitor rolipram (C) with ethanol prevented the increase in P2 fraction levels of α4 subunits induced by ethanol. *P < 0.05 (one-way ANOVA, Bonferroni post-test, n = 6–8 per group). Ctrl, control; EtOH, ethanol; Forsk, forskolin; OD, optical density; Roli, rolipram.
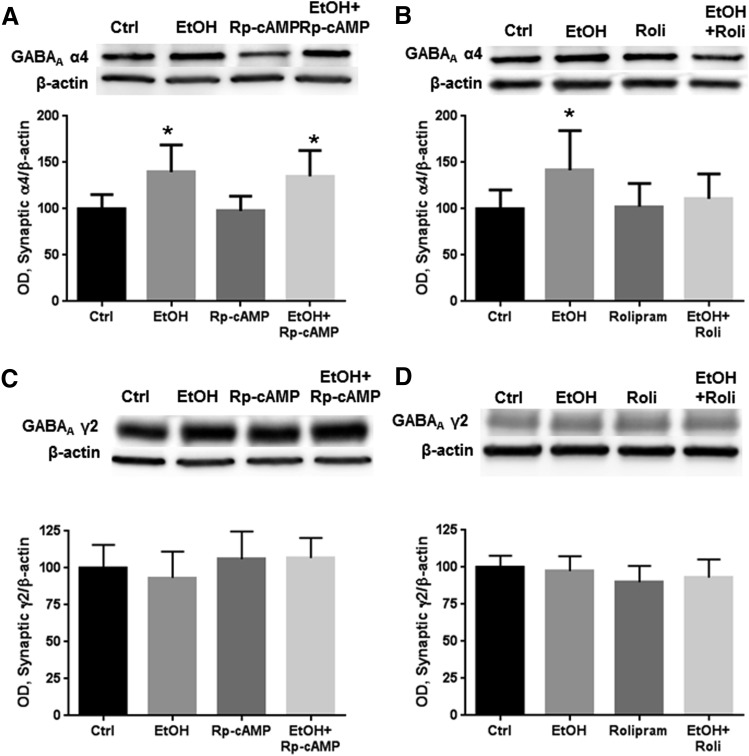
PKA Activation Prevents the Effects of 4-Hour Ethanol Exposure on Synaptic Fraction GABAA α4 Abundance.
To resolve whether the effects of ethanol and PKA on GABAA α4 subunits in the P2 fraction represent synaptic receptor regulation, we expanded the study shown in Fig. 1 to determine whether these effects occur within the synaptic fraction purified by subcellular fractionation (Carlson et al., 2014, 2016; Bohnsack et al., 2016). Coexposure of Rp-cAMP with ethanol did not prevent ethanol-induced increases in synaptic GABAA α4 abundance (Fig. 2A, P < 0.01, one-way ANOVA, F = 5.776, P < 0.05, Bonferroni post-test, n = 6–8 per group). Conversely, coexposure of rolipram with ethanol prevented increases in synaptic GABAA α4 abundance (Fig. 2B, P < 0.05, one-way ANOVA, F = 3.107, P < 0.05, Bonferroni post-test, n = 7 to 8 per group). No changes in GABAA γ2 subunit abundance were observed in response to ethanol or PKA modulators (Fig. 2, C and D). No δ subunits were detected in the synaptic fraction (data not shown), although δ subunit abundance is known to decrease after 4-hour ethanol exposure in the extrasynaptic fraction (Carlson et al., 2016).
Fig. 2.
PKA activation prevents the increased abundance of synaptic α4 receptors induced by ethanol. Cortical neurons were exposed to vehicle, ethanol (50 mM), Rp-cAMP (50 μM), and/or rolipram (10 μM) for 4 hours, followed by synaptic fractionation and western blot analysis. (A) Ethanol decreased synaptic GABAA α4 abundance, an effect that was not altered by coexposure with Rp-cAMP. (B) Coexposure with rolipram prevented ethanol-induced increases in synaptic α4 abundance. (C and D) GABAA γ2 abundance was not altered by exposure to ethanol or either Rp-cAMP (C) or rolipram (D). *P < 0.05 (one-way ANOVA, Bonferroni post-test, n = 6–10). Ctrl, control; EtOH, ethanol; OD, optical density; Roli, rolipram.
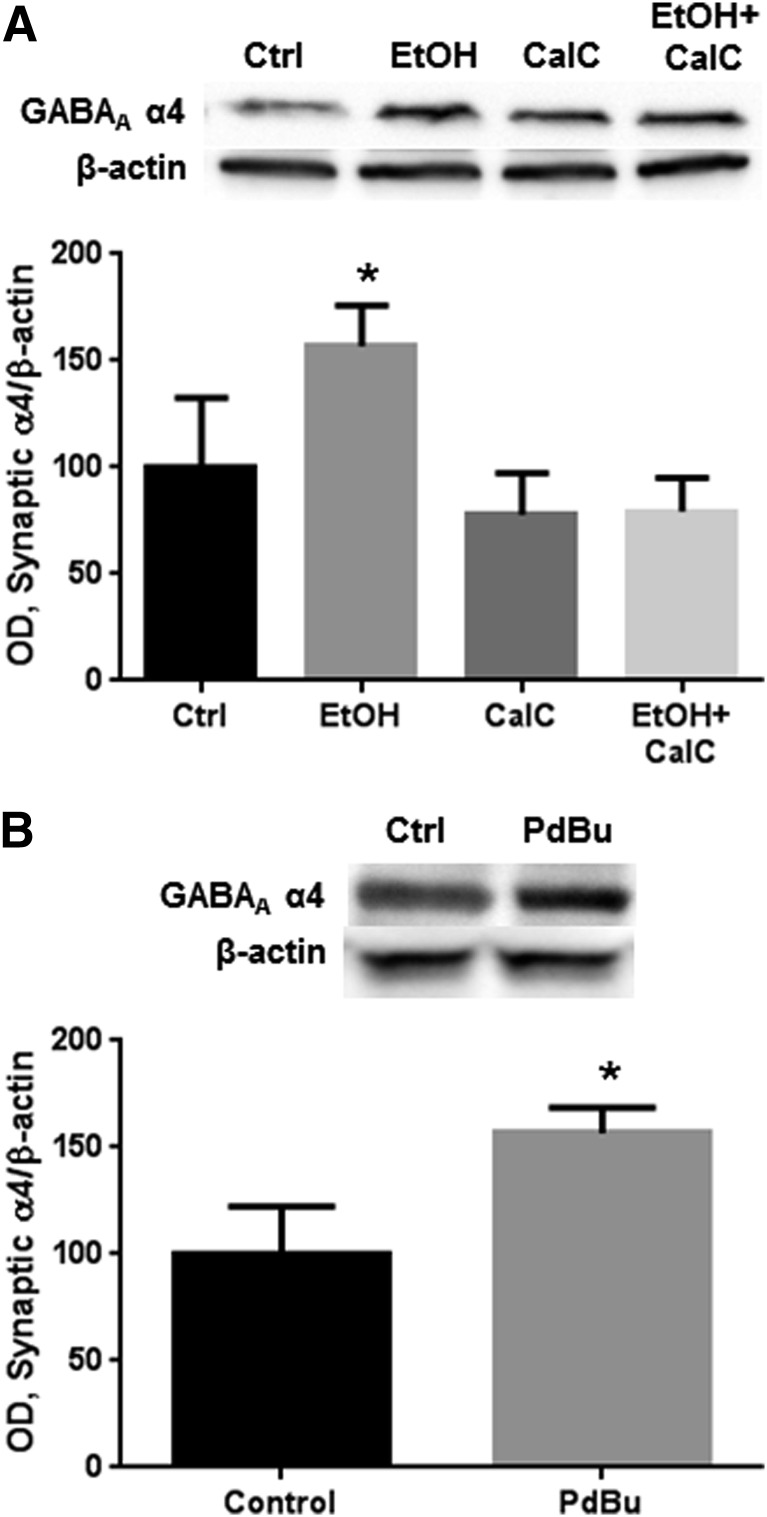
PKC Activity Mediates Ethanol-Induced Increases in Synaptic GABAA α4 Subunits.
Although we previously found increases in P2 fraction levels of GABAA α4 to be caused by PKC activity (Werner et al., 2011), we next used subcellular fractionation to confirm that these changes occur in the synaptic fraction. Ethanol increased synaptic GABAA α4 levels, an effect that was prevented by inhibiting PKC with CalC (0.3 µM; Fig. 3A, P < 0.01, one-way ANOVA, F = 12.53, P < 0.01, Bonferroni post-test, n = 6 per group). CalC alone had no effect on GABAA α4 subunit abundance. Direct activation of PKC with the phorbol ester phorbol-12,13-dibutyrate mimicked the effect of ethanol in increasing synaptic GABAA α4 abundance (Fig. 3B, P < 0.01, t test, n = 5 to 6 per group).
Fig. 3.
PKC activation by ethanol increases GABAA α4 subunit levels in the synaptic fraction. Cortical neurons were exposed to vehicle, ethanol (50 mM), CalC (0.3 μM), and/or PdBu (0.1 µM) for 4 hours followed by synaptic fractionation and western blot analysis. (A) Exposure to ethanol for 4 hours increased synaptic fraction levels of the GABAA α4 subunit, which was prevented by coexposure with CalC. (B) Exposure to PdBu mimicked the effect of ethanol on synaptic GABAA α4 levels. *P < 0.05 (t test or one-way ANOVA, Bonferroni post-test, n = 5 to 6 per group). Ctrl, control; EtOH, ethanol; OD, optical density; PdBu, phorbol-12,13-dibutyrate; CalC, Calphostin C.
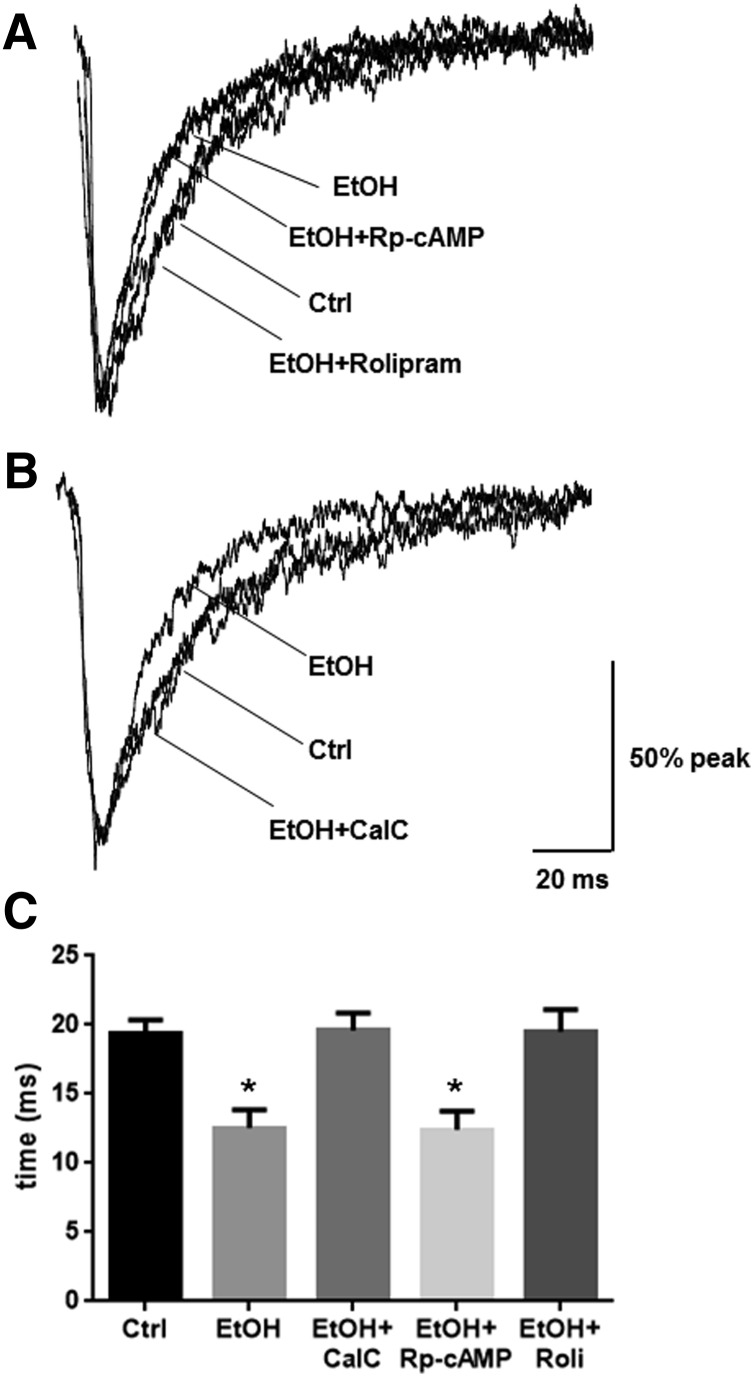
PKC Inhibition and PKA Activation Prevent Ethanol-Induced Changes in GABA mIPSCs.
Finally, we investigated the ramifications of PKA and PKC activity on mIPSC kinetics during 4-hour ethanol exposure. We previously demonstrated that GABA mIPSC decay τ1 is decreased after 4-hour ethanol exposure (Werner et al., 2011). Coexposure of ethanol with Rp-cAMP had no effect on the ethanol-induced decrease in GABA mIPSC decay τ1, whereas coexposure with rolipram prevented the decrease (Fig. 4, A and C, P < 0.001, one-way ANOVA, F = 9.955, P < 0.001, Bonferroni post-test, n = 11–14 per group). Coexposure of ethanol with CalC prevented the decrease in GABA mIPSC decay τ1 (Fig. 4, B and C, P < 0.001, one-way ANOVA, F = 11.78, P < 0.001, Bonferroni post-test, n = 11–14 per group). There were no other effects on mIPSC kinetics (Table 1).
Fig. 4.
PKA activation or PKC inhibition mitigate ethanol-induced alterations in mIPSC responses. Whole-cell patch-clamp recordings of cortical neurons were made in the presence of TTX (1 μM), CNQX (10 μM), and AP-5 (40 μM) to isolate GABA mIPSCs after 4-hour exposure to ethanol and/or kinase modulatory drugs. (A) Decay time (decay τ1) was significantly decreased by ethanol exposure, an affect that was not affected by coexposure of ethanol with Rp-cAMP; however, coexposure of ethanol with rolipram prevented this decrease. (B) Coexposure of ethanol with CalC also prevented the decrease in decay rate induced by ethanol alone. (C) Quantification of changes in decay τ1 is shown. (*P < 0.05 compared with control, EtOH plus CalC, or EtOH plus rolipram groups; one-way ANOVA, Bonferroni post-test). Summarized mIPSC metrics are presented in Table 1. AP-5, d-2-amino-5-phosphonopentanoic acid; CNQX, 6-cyno-7-nitroquinoxaline-2,3-dione; Ctrl, control; EtOH, ethanol; Roli, rolipram; TTX, tetrodotoxin.
TABLE 1.
GABA mIPSC decay kinetics after exposure to PKA modulators and ethanol
Data are presented as means ± S.E.M.
| Measure | Control | Ethanol | Ethanol Plus CalC | Ethanol Plus Rp-cAMP | Ethanol Plus Rolipram |
|---|---|---|---|---|---|
| Rise time (ms) | 2.4 ± 1.0 | 3.1 ± 1.4 | 2.8 ± 0.9 | 1.9 ± 1.0 | 2.6 ± 0.6 |
| Amplitude (pA) | 22.1 ± 1.2 | 23.5 ± 1.0 | 22.8 ± 1.9 | 21.6 ± 0.9 | 22.5 ± 1.1 |
| Frequency (Hz) | 1.9 ± 0.4 | 1.5 ± 0.8 | 2.1 ± 0.6 | 1.8 ± 0.4 | 2.0 ± 0.5 |
| Decay τ1 | 19.4 ± 1.0 | 12.5 ± 1.3* | 19.6 ± 1.3 | 12.4 ± 1.4* | 19.5 ± 1.6 |
| Decay τ2 | 30.1 ± 2.3 | 32.1 ± 2.6 | 31.2 ± 2.5 | 32.0 ± 1.9 | 31.6 ± 1.4 |
| Number | 14 | 11 | 11 | 11 | 11 |
P < 0.05 (compared with control, ethanol plus CalC, or ethanol plus rolipram groups, one-way ANOVA, Bonferroni post-test).
Discussion
In this study, we demonstrate that PKA and PKC have opposing effects on synaptic GABAA α4 subunit abundance and function. We elucidate that PKC activation by ethanol upregulates synaptic GABAA α4 abundance to reduce the mIPSC decay rate, whereas maintaining PKA activation through phosphodiesterase inhibition prevents these effects. These data further characterize the regulation of a poorly understood GABAA receptor that may be an important mediator of the chronic effects of ethanol, and these results enhance our understanding of kinase regulation of GABAA receptors by ethanol (Table 2). Thus, these findings implicate potential therapeutic methods for restoring normal GABAergic functioning after chronic alcohol misuse.
TABLE 2.
Summary of findings on modulation of GABAA α1 and α4 subunits by ethanol and kinases
Data are presented as means ± S.E.M. Downward arrows indicate negative modulation, whereas upward arrows indicate positive modulation. The dash indicates no change.
| Subunit | Chronic Ethanol | PKC | PKA |
|---|---|---|---|
| GABAA α1 | ↓ (Kumar et al., 2003) | ↓ (Kumar et al., 2010) | ↑ (Carlson et al., 2013) |
| Extrasynaptic GABAA α4 | ↓ (Liang et al., 2006)a | — (Carlson et al., 2016) | ↑ (Carlson et al., 2016) |
| Synaptic GABAA α4 | ↑ (Liang et al., 2006)a | ↑ (This study) | ↓ (This study) |
Note that these studies are in the hippocampus.
We used a 4-hour in vitro ethanol exposure paradigm that recapitulates changes observed after chronic ethanol consumption and withdrawal (Devaud et al., 1997; Kumar et al., 2003; Carlson et al., 2013, 2016). It was not surprising that PKA inhibition during 4-hour ethanol exposure had no effect, because we previously found that PKA abundance (Carlson et al., 2013) and activity (Carlson et al., 2016) are not altered by ethanol exposure for 4 hours, despite the effects of ethanol at 1 hour. Nonetheless, it was possible that earlier PKA activation might produce downstream effects and this possibility was ruled out by testing the effect of PKA inhibition. Since PKA inhibition had no effect on synaptic GABAA α4 receptor expression, it appears that constitutive PKA activity does not regulate synaptic GABAA α4 receptors. The data suggest that PKA activation downregulates synaptic GABAA α4 receptors, because either maintaining PKA activity through phosphodiesterase inhibition or activating PKA through adenylyl cyclase activation prevented ethanol-induced increases in GABAA α4 subunits. Because GABAA α4βγ2 receptors are upregulated in other pathologic conditions, including seizure disorders (González and Brooks-Kayal, 2011) and progesterone withdrawal (Gulinello et al., 2002), PKA regulation of these receptors likely has broad implications deserving further exploration.
Our laboratory previously demonstrated that PKC activation by ethanol leads to an upregulation of the abundance of GABAA α4 subunits in vitro after 4 hours, purportedly owing to an increase in the synaptic population of GABAA α4 receptors (Werner et al., 2011). However, these studies did not isolate synaptic α4 receptors since δ subunits were detected in the P2 fraction. Our current results, combined with recent findings demonstrating a lack of effect of PKC on extrasynaptic GABAA α4δ receptors (Bohnsack et al., 2016; Carlson et al., 2016), support the hypothesis that PKC effects are specific for the synaptic GABAA α4 receptors in cortical neurons and that synaptic and extrasynaptic populations of receptors are subject to different methods of regulation. The similarity of ethanol effects in the synaptic fraction and P2 fraction suggests that the P2 fraction largely consists of synaptic components and that the subsynaptic fraction, although functionally relevant (Carlson et al., 2016), may not be present in sufficient quantities to confound these results.
Our results suggest that PKA and PKC play oppositional roles in synaptic GABAA α4 receptor regulation, similar to our previous findings on GABAA α1 receptor regulation (Carlson et al., 2013). These results are consistent with other studies demonstrating oppositional roles of PKC and PKA activity on GABA receptor functioning (Poisbeau et al., 1999; Brandon et al., 2000; Bohnsack et al., 2016). The observation that PKA activation decreases synaptic α4 abundance could explain why we previously found no difference in whole-cell GABA-evoked current amplitude or GABA dose response after 1 hour of PKA activation, despite an increase in α1 receptor abundance (Carlson et al., 2013). Similarly, the lack of change in GABAA γ2 subunit abundance observed in this study is likely attributable to opposing changes in α4βγ2 vs. α1βγ2 receptors (Kumar et al., 2010). The observation that kinase inhibition in the absence of ethanol had no effect on synaptic α4 subunits suggests that these receptors do not undergo constitutive regulation by these pathways but are only altered after a physiologic insult such as high concentrations of ethanol. These findings are consistent with previous studies in which the PKA RIIβ subunit did not constitutively regulate α4 subunits in vivo (Carlson et al., 2014).
The more rapid mIPSC decay constants after 4-hour ethanol exposure were consistent with previous findings from our laboratory (Werner et al., 2011). The synaptic GABAA α4 receptors display more rapid decay times than GABAA α1 receptors in recombinant systems (Whittemore et al., 1996; Brown et al., 2002) and α4 knockout mice have longer decay times compared with wild-type mice (Chandra et al., 2006). Thus, the faster decay time after chronic ethanol exposure is consistent with a higher proportion of GABAA α4 receptors in the synaptic GABA receptor population; however, it will be important to confirm this conclusion in recombinant systems that can isolate synaptic α4 receptors physiologically. These results mirror similar studies uncovering reduced decay times after chronic ethanol exposure in the hippocampus (Cagetti et al., 2003; Liang et al., 2006) and during withdrawal from the neuroactive steroid allopregnanolone (Hsu et al., 2003). These changes are correlated with a reduction in the anxiolytic and sleep-inducing effects of ethanol associated with withdrawal and dependence. Thus, the finding that PKC inhibition or PKA activation in the presence of ethanol prevented the faster GABAA mIPSC decay time suggests two possible methods of preventing pathologic changes associated with ethanol dependence. Finally, the correspondence of the functional changes in mIPSCs with the change in α4 subunit expression detected by synaptic fractionation technique further validates the viability of this method of isolating synaptic proteins.
Our results underscore the potential therapeutic relevance for phosphodiesterase inhibition using drugs such as rolipram. Recent studies in rodent models have demonstrated decreased drinking behavior in animals given phosphodiesterase inhibitors (Hu et al., 2011; Wen et al., 2012; Blednov et al., 2014; Franklin et al., 2015). Together, these studies suggest that phosphodiesterase inhibition provides a promising target for the treatment of alcohol use disorders. Future in vivo studies examining effects of coadministration of ethanol and rolipram (or administration of rolipram after chronic ethanol) on GABAergic trafficking and GABA-related behavior would be a logical extension of these data.
This study expands our understanding of kinase signaling in modulating the GABAergic effects of ethanol. The data suggest that PKA activity may prevent alterations of GABAergic inhibition associated with chronic alcohol misuse, whereas PKC activity may facilitate them. These second messenger pathways could provide important targets for treatments to prevent or restore normal GABAA receptor functioning associated with alcohol tolerance, dependence, and withdrawal.
Acknowledgments
The authors thank Todd K. O’Buckley, Raechel McKinley, and Danielle Morrow for technical assistance.
Abbreviations
- ANOVA
analysis of variance
- CalC
calphostin C
- mIPSC
miniature inhibitory postsynaptic current
- PdBu
phorbol-12,13-dibutyrate
- PKA
protein kinase A
- PKC
protein kinase C
- Ro15-4513
ethyl-8-azido-5,6-dihydro-5-methyl-6-oxo-4H-imidazo-1,4-benzodiazepine-3-carboxylate
- Rp-cAMP
Rp-adenosine 3′,5′-cyclic monophosphothioate triethylamine
Authorship Contributions
Participated in research design: Carlson, Bohnsack, Morrow.
Conducted experiments: Carlson, Bohnsack.
Performed data analysis: Carlson, Bohnsack.
Wrote or contributed to the writing of the manuscript: Carlson, Bohnsack, Morrow.
Footnotes
This research was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants P60-AA11605 and T32-AA007573] and the University of North Carolina Bowles Center for Alcohol Studies.
References
- Blednov YA, Benavidez JM, Black M, Harris RA. (2014) Inhibition of phosphodiesterase 4 reduces ethanol intake and preference in C57BL/6J mice. Front Neurosci 8:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack JP, Carlson SL, Morrow AL. (2016) Differential regulation of synaptic and extrasynaptic α4 GABA(A) receptor populations by protein kinase A and protein kinase C in cultured cortical neurons. Neuropharmacology 105:124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Delmas P, Kittler JT, McDonald BJ, Sieghart W, Brown DA, Smart TG, Moss SJ. (2000) GABAA receptor phosphorylation and functional modulation in cortical neurons by a protein kinase C-dependent pathway. J Biol Chem 275:38856–38862. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. (2002) Pharmacological characterization of a novel cell line expressing human α(4)β(3)δ GABA(A) receptors. Br J Pharmacol 136:965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. (2003) Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol 63:53–64. [DOI] [PubMed] [Google Scholar]
- Carlson SL, Bohnsack JP, Patel V, Morrow AL. (2016) Regulation of extrasynaptic GABAA α4 receptors by ethanol-induced protein kinase A, but not protein kinase C activation in cultured rat cerebral cortical neurons. J Pharmacol Exp Ther 356:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Kumar S, Werner DF, Comerford CE, Morrow AL. (2013) Ethanol activation of protein kinase A regulates GABAA α1 receptor function and trafficking in cultured cerebral cortical neurons. J Pharmacol Exp Ther 345:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, O’Buckley TK, Thomas R, Thiele TE, Morrow AL. (2014) Altered GABAA receptor expression and seizure threshold following acute ethanol challenge in mice lacking the RIIβ subunit of PKA. Neurochem Res 39:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, et al. (2006) GABAA receptor α 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA 103:15230–15235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Fritschy JM, Sieghart W, Morrow AL. (1997) Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem 69:126–130. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. (1996) Sensitization of γ-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther 278:510–517. [PubMed] [Google Scholar]
- Farrant M, Nusser Z. (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6:215–229. [DOI] [PubMed] [Google Scholar]
- Franklin KM, Hauser SR, Lasek AW, McClintick J, Ding ZM, McBride WJ, Bell RL. (2015) Reduction of alcohol drinking of alcohol-preferring (P) and high-alcohol drinking (HAD1) rats by targeting phosphodiesterase-4 (PDE4). Psychopharmacology (Berl) 232:2251–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. (2009) Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience 158:1446–1459. [DOI] [PubMed] [Google Scholar]
- González MI, Brooks-Kayal A. (2011) Altered GABA(A) receptor expression during epileptogenesis. Neurosci Lett 497:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Smith SS. (2002) Progesterone withdrawal increases the alpha4 subunit of the GABA(A) receptor in male rats in association with anxiety and altered pharmacology - a comparison with female rats. Neuropharmacology 43:701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW. (2006) Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to alpha4/6beta3delta GABAA receptors. Proc Natl Acad Sci USA 103:8546–8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FC, Waldeck R, Faber DS, Smith SS. (2003) Neurosteroid effects on GABAergic synaptic plasticity in hippocampus. J Neurophysiol 89:1929–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Lu T, Chen A, Huang Y, Hansen R, Chandler LJ, Zhang HT. (2011) Inhibition of phosphodiesterase-4 decreases ethanol intake in mice. Psychopharmacology (Berl) 218:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scheurer L, Lüddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. (1996) Pharmacological modulation of the diazepam-insensitive recombinant gamma-aminobutyric acidA receptors alpha 4 beta 2 gamma 2 and alpha 6 beta 2 gamma 2. Mol Pharmacol 50:1253–1261. [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O’Buckley TK, Grobin AC, Morrow AL. (2003) Chronic ethanol consumption enhances internalization of α1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem 86:700–708. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. (2009) The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 205:529–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Suryanarayanan A, Boyd KN, Comerford CE, Lai MA, Ren Q, Morrow AL. (2010) Ethanol reduces GABAA alpha1 subunit receptor surface expression by a protein kinase Cγ-dependent mechanism in cultured cerebral cortical neurons. Mol Pharmacol 77:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. (2007) Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci 27:12367–12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. (2006) Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci 26:1749–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. (2009) GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadeas S, Grobin AC, Morrow AL. (2001) Chronic ethanol consumption differentially alters GABA(A) receptor alpha1 and alpha4 subunit peptide expression and GABA(A) receptor-mediated 36 Cl(-) uptake in mesocorticolimbic regions of rat brain. Alcohol Clin Exp Res 25:1270–1275. [PubMed] [Google Scholar]
- Poisbeau P, Cheney MC, Browning MD, Mody I. (1999) Modulation of synaptic GABAA receptor function by PKA and PKC in adult hippocampal neurons. J Neurosci 19:674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. (2002) Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci 5:721–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Moss SJ. (2008) GABA(A) receptor dynamics and constructing GABAergic synapses. Front Mol Neurosci 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. (2003) Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA 100:15218–15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. (2004) Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci 24:8379–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen RT, Zhang M, Qin WJ, Liu Q, Wang WP, Lawrence AJ, Zhang HT, Liang JH. (2012) The phosphodiesterase-4 (PDE4) inhibitor rolipram decreases ethanol seeking and consumption in alcohol-preferring Fawn-Hooded rats. Alcohol Clin Exp Res 36:2157–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner DF, Kumar S, Criswell HE, Suryanarayanan A, Fetzer JA, Comerford CE, Morrow AL. (2011) PKCγ is required for ethanol-induced increases in GABA(A) receptor α4 subunit expression in cultured cerebral cortical neurons. J Neurochem 116:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore ER, Yang W, Drewe JA, Woodward RM. (1996) Pharmacology of the human gamma-aminobutyric acidA receptor alpha 4 subunit expressed in Xenopus laevis oocytes. Mol Pharmacol 50:1364–1375. [PubMed] [Google Scholar]
- Zhang H, Pandey SC. (2003) Effects of PKA modulation on the expression of neuropeptide Y in rat amygdaloid structures during ethanol withdrawal. Peptides 24:1397–1402. [DOI] [PubMed] [Google Scholar]