Abstract
Nociceptin/orphanin FQ receptor (NOP) agonists have been reported to produce antinociceptive effects in rhesus monkeys with comparable efficacy to μ-opioid receptor (MOP) agonists, but without their limiting side effects. There are also known to be species differences between rodents and nonhuman primates (NHPs) in the behavioral effects of NOP agonists. The aims of this study were the following: 1) to determine if the NOP agonist Ro 64-6198 could be trained as a discriminative stimulus; 2) to evaluate its pharmacological selectivity as a discriminative stimulus; and 3) to establish the order of potency with which Ro 64-6198 produces discriminative stimulus effects compared with analgesic effects in NHPs. Two groups of rhesus monkeys were trained to discriminate either fentanyl or Ro 64-6198 from vehicle. Four monkeys were trained in the warm-water tail-withdrawal procedure to measure antinociception. Ro 64-6198 produced discriminative stimulus effects that were blocked by the NOP antagonist J-113397 and not by naltrexone. The discriminative stimulus effects of Ro 64-6198 partially generalized to diazepam, but not to fentanyl, SNC 80, ketocyclazocine, buprenorphine, phencyclidine, or chlorpromazine. Fentanyl produced stimulus effects that were blocked by naltrexone and not by J-113397, and Ro 64-6198 did not produce fentanyl-appropriate responding in fentanyl-trained animals. In measures of antinociception, fentanyl, but not Ro 64-6198, produced dose-dependent increases in tail-withdrawal latency. Together, these results demonstrate that Ro 64-6198 produced stimulus effects in monkeys that are distinct from other opioid receptor agonists, but may be somewhat similar to diazepam. In contrast to previous findings, Ro 64-6198 did not produce antinociception in the majority of animals tested even at doses considerably greater than those that produced discriminative stimulus effects.
Introduction
The nociceptin/orphanin FQ receptor (NOP) is a seven-transmembrane receptor that was first cloned in 1994, and was noted to share significant sequence and structural homology with the classic opioid receptors μ, κ, and δ [μ-opioid receptor (MOP), κ-opioid receptor (KOP), and δ-opioid receptor (DOP)] (Mollereau et al., 1994). Despite these similarities, the canonical endogenous opioid peptides have negligible affinity for NOP, as does the opioid antagonist naloxone. One year later, two separate groups identified a 17 amino acid peptide that bound with high affinity to NOP as the endogenous ligand (Meunier et al., 1995; Reinscheid et al., 1995). This peptide was given two names, nociceptin and orphanin FQ, and was found to share considerable sequence homology with the KOP-selective peptide dynorphin A. Functional experiments in vitro demonstrated that NOP, MOP, DOP, and KOP all coupled predominantly to the Gαi/o class of G-proteins, and produced analogous signal transduction following agonist stimulation (inhibition of adenylyl cyclase, and Ca2+ conductance; stimulation of K+ conductance).
Given its classification, the initial behavioral pharmacology focused on evaluating the role of NOP in modifying pain-related behaviors. The results from many of these experiments were nuanced relative to MOP agonist effects, and there were considerable differences noted between rodents and nonhuman primates. In rodents, the analgesic response to NOP agonists varied depending on the route of administration, and the type of pain stimulus. There is now general agreement that NOP agonists produce analgesia in rodents when given i.t. across a variety of pain assays. Systemic or supraspinal administration in rodents does not produce antinociception against an acute thermal stimulus (tail flick, hot plate), but may have positive effects in inflammatory, neuropathic, and chronic pain states (for reviews, see Mogil and Pasternak, 2001; Lambert, 2008; Schröder et al., 2014).
However, in rhesus monkeys NOP agonists given systemically or spinally were shown to be antinociceptive, antiallodynic, and anti-inflammatory (Ko et al., 2009; Ko and Naughton, 2009; Kangas and Bergman, 2014). When administered i.t., nociceptin and orphanin FQ produced antinociception that was less potent, but similar in magnitude, to morphine. However, unlike morphine, or other NOP agonists given i.t., nociceptin and orphanin FQ did not produce respiratory depression or pruritus. The first small molecule agonist to be tested systemically in monkeys, Ro 64-6198, was reported to produce acute thermal antinociception and to be antiallodynic (Ko et al., 2009). When compared with alfentanil, Ro 64-6198 was equipotent in producing antinociception, but was not self-administered, and did not produce respiratory depression or itch. The antinociceptive effects of Ro 64-6198 were selectively blocked by the NOP antagonist J-113397 and not by the opioid antagonist naltrexone. Although not systematically evaluated, NOP agonists were reported to produce analgesia in the absence of sedation, unlike agonists at MOP and KOP (Ko and Naughton, 2009; Podlesnik et al., 2011). Furthermore, whereas the preclinical literature in rodents showed a complex relationship between the NOP system and analgesia, the data in monkeys appeared to be more straightforward.
To our knowledge, other than analgesia, there have been no other reports identifying behaviors that are modified by NOP agonists in nonhuman primates (most of the studies have noted their relative lack of effects on a target behavior). Drug discrimination procedures are widely used to study the interoceptive properties of central nervous system drugs, and are well suited for establishing the in vivo selectively of ligands from a broad array of drug classes. Previous research has established that agonist stimulation of MOP, DOP, and KOP produced discriminative stimulus effects that are pharmacologically selective (Woods et al., 1988; Colpaert, 1999). Previous drug discrimination studies with Ro 64-6198 in rats showed that it possessed stimulus properties that were unlike opioid agonists at MOP, DOP, and KOP (Recker and Higgins, 2004). These effects were selectively blocked with the NOP antagonist, J-113397, but not with the opioid antagonist naltrexone. This study successfully established that, in rodents, the discriminative stimulus effects of Ro 64-6198 were selectively mediated through the NOP receptor, and were pharmacologically and behaviorally distinct from the stimulus effects produced through traditional opioid receptors. However, these experiments did not examine whether the stimulus effects of Ro 64-6198 were distinguishable from drugs in other pharmacological classes. Furthermore, the apparent species differences in terms of other NOP agonist effects raised the possibility that these findings may not be generalizable to nonhuman primates.
The purpose of the present experiments was to determine if rhesus monkeys could be trained to discriminate Ro 64-6198 and to compare the relative potencies of Ro 64-6198 and the MOP agonist fentanyl in measures of analgesia, discriminative stimulus effects, and the ability to suppress rates of responding. We also wanted to test whether the stimulus effects of Ro 64-6198 were similar to other opioids and drugs from different classes.
Materials and Methods
Subjects.
In the drug discrimination studies, six adult rhesus monkeys (one female, weight, 8 kg; five males, weight, 9–12.5 kg) were individually housed in steel cages (83.3 cm high × 76.2 cm wide × 91.4 cm deep) on a 12-hour light/12-hour dark schedule. Their diet consisted of Laboratory Fiber Plus Monkey Diet (PMI Nutrition Intl., LLC, St. Louis, MO) that was supplemented with fresh fruit daily. Water and enrichment toys were continuously available in the home cage. All of the monkeys used in this experiment had served as subjects in other studies, and had prior drug histories. In the antinociception experiments, three separate adult male rhesus monkeys (weight, 10–11.4 kg) were employed and housed under the same conditions as the drug discrimination animals. One female monkey that was trained to discriminate fentanyl (weight, 8 kg) was also used in the antinociception studies. The antinociception experiments in this animal commenced 118 days after drug discrimination training was discontinued. All animals were maintained and experiments were performed, in accordance with the Institutional Animal Care and Use Committee at the University of Michigan.
Apparatus.
Drug discrimination experiments were conducted in the home cage 7 days a week beginning at 12pm. A metal panel was mounted on either side of the cage (20 cm wide × 28 cm high) that housed three response levers with three stimulus lights located 5 cm above each lever. A food dispenser was located on the same side as the operant levers. In an adjacent room, computers with MED-PC software controlled all of the experimental procedures (Med-Associates, Georgia, VA).
For warm-water tail-withdrawal studies, monkeys were trained to sit in Plexiglas primate chairs that were 1.5 m in height. A tea kettle with hot water was maintained at approximately 100°C. Cold tap water and hot water were mixed together in a Thermos and calibrated with a total immersion thermometer to obtain the desired temperatures. Tail-withdrawal latencies were measured using a digital stopwatch.
Drug Discrimination Procedure.
Monkeys were trained to discriminate either fentanyl 0.01 mg/kg from vehicle (n = 3; monkeys BC, MI, and BU) or Ro 64-6198 (0.18 mg/kg, monkey FA; 0.1 mg/kg, monkeys IE and ST) from vehicle in a two-lever, single-component drug discrimination procedure. Monkey FA had no history of operant conditioning, while monkey IE had previous experience responding for i.v. drug administration and food, and monkey ST had a history of responding for food. Monkeys MI and BU had no previous operant training before this experiment, and monkey BC had prior experience responding both for food and i.v. drug administration.
The session began with the illumination of two green stimulus lights above the right and left lever. The monkeys were trained to respond on a fixed-ratio (FR) 30 schedule for a single 300 mg food pellet (Bio Serv, Flemington, NJ) (Dustless Precision Pellets Primate; purified: 300 mg, banana flavor). Drug and vehicle/sham-paired levers were randomly assigned to each monkey. Pretreatment time varied by drug condition; fentanyl animals were given an injection 20 minutes before the beginning of the session and Ro 64-6198 animals were injected 30 minutes prior to the session. Following administration of the training drug or sham/vehicle injection, completion of a FR 30 on the condition-appropriate lever extinguished the green stimulus lights and illuminated the center red stimulus light that signaled the delivery of a food pellet. Completion of a FR 30 on the inappropriate lever extinguished the green stimulus lights and initiated a 10-second time out during which responses had no scheduled consequence. Any responses emitted on the incorrect lever reset the response requirement on the condition-appropriate key. The session ended when the monkey received 75 food pellets or after 60 minutes had elapsed.
To meet criteria for drug testing, subjects were required to emit no less than 85% of their total responses on the condition-appropriate lever, and to complete the first FR in 45 responses or less. Stimulus control was deemed adequate for testing when monkeys met criteria for six consecutive sessions out of 7 days of training. Test conditions were identical to training sessions except that completion of a FR 30 on either key produced a food pellet. Subsequent test sessions were carried out following two consecutive training sessions during which the monkey met the criteria. If the subject failed to meet criteria during one of these sessions, testing was suspended until the criteria were again satisfied for two consecutive sessions.
Drug substitution studies were performed using selective opioid agonists and other drugs that are known to produce stimulus effects from different pharmacological classes. The MOP agonists fentanyl and buprenorphine, the KOP agonist ketocyclazocine, and the DOP agonist SNC 80 were used to determine if Ro 64-6198 had interoceptive effects in common with drugs that act at these opioid sites. Ketocyclazocine’s KOP-mediated effects have been demonstrated in discrimination studies in rhesus monkeys (Hein et al., 1981); SNC 80’s DOP-mediated effects have likewise been shown in rhesus monkeys (Brandt et al., 1999). The nonopioid drugs diazepam, phencyclidine (PCP), and chlorpromazine were used to further test the selectivity of Ro 64-6198s interoceptive effects. These drugs were tested up to doses that suppressed rates of responding or where the limits in drug solubility were reached. Drugs were considered to have partially substituted for the training stimulus if responses on the drug-appropriate lever were in excess of 50%, and full generalization was interpreted as greater than 85% responding on the drug-paired lever. The ability of antagonists to alter the stimulus effects of fentanyl and Ro 64-6198 was investigated by pretreating animals with antagonists 40 and 50 minutes, respectively, prior to the beginning of the session.
Acute Thermal Antinociception.
For measurements of antinociception, the monkeys were trained to sit in a primate chair and their tails were periodically immersed in water heated to 38, 42, 46, or 50°C. Temperatures were tested randomly at each time point with approximately 30–60 seconds between each measurement. The session began with baseline measurements recorded at each temperature; this was followed by a series of injections (drug or saline) in 30-minute cycles. Two different experimenters, who were blind to the water temperature, tested each temperature once 20 minutes following the injection. Water heated to 50°C reliably produced nociception and the subject typically withdrew its tail from the water in 2–5 seconds. Effective opioid analgesics raise the nociceptive threshold at this temperature and increase the tail-withdrawal latency compared with saline treatment.
Data Analysis.
Drug discrimination data are presented as a percent of drug-appropriate lever responding and plotted as a function of dose. If a test compound was administered to a monkey more than once, an average was calculated for drug-appropriate responding in that condition. Mean data are presented as an average of all the monkeys in the group.
In the warm-water tail-withdrawal study, each point represents an average of two sessions with a single monkey. The averages for each monkey were then converted to percentage of maximum possible effect using the following calculation: percentage of maximum possible effect = [(test latency − control latency)/(20-second cutoff latency − control latency)] × 100.
Drugs.
All drugs were administered i.m. in volumes between 0.1 and 2 ml. Fentanyl (Sigma Aldrich, St. Louis, MO), ketocyclazocine (Sterling-Winthrop Research Institute, Rensselaer, NY), PCP (Warner-Lambert/Parke-Davis, Ann Arbor, MI), naltrexone (National Institute on Drug Abuse, Bethesda, MD; National Institutes of Health, Bethesda, MD), and chlorpromazine (Sigma Aldrich) were all dissolved in sterile water. Ro 64-6198 (Hoffman-La Roche, Basel, Switzerland) was dissolved in 10% dimethylsulfoxide, 10% Tween 80, and 80% sterile water for a final ratio of 1:1:8. J-113397 (K. Rice, National Institute on Drug Abuse and National Institutes of Health) was dissolved in sterile water with 1.1 Eq of 1M HCl. Diazepam and flumazenil (Hoffman-La Roche) were dissolved in 50% ethanol, 30% Alkamul, and 20% sterile water. SNC 80 (K. Rice, National Institute on Drug Abuse and National Institutes of Health) was dissolved in 0.5% HCl.
Results
Monkeys trained to discriminate Ro 64-6198 from vehicle required an average of 75 sessions (range, 56–95) to acquire stimulus control. Monkey FA, the first monkey to be trained, met criteria after 95 sessions and three different changes in dose (0.03, 0.32, and 0.18 mg/kg). The remaining monkeys, IE and ST, were trained to discriminate 0.1 mg/kg of Ro 64-6198, which required 56 and 75 sessions, respectively. The three monkeys in the fentanyl group were all trained to discriminate 0.01 mg/kg of fentanyl from saline and required an average of 97 sessions (range, 91–105) to reach criteria. All monkeys in the fentanyl group started training at 0.0056 mg/kg before the dose was increased.
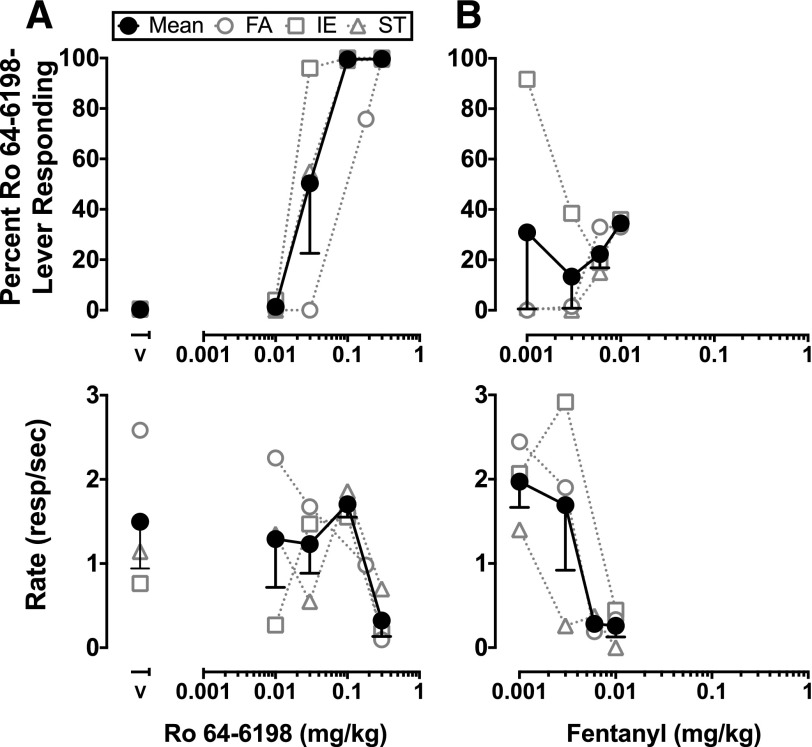
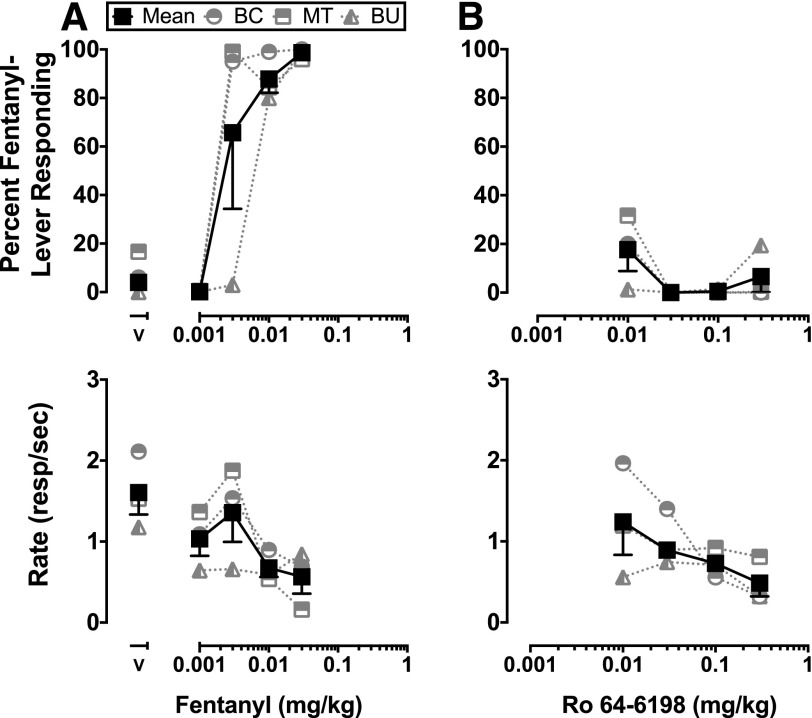
In Ro 64-6198-trained animals, increasing doses of the training drug produced increases in responding on the drug-appropriate lever (Fig. 1A). On average, fentanyl substitution in Ro 64-6198-trained animals did not produce significant increases in responding on the drug-appropriate key that satisfied the a priori criteria for stimulus generalization (Fig. 1B). Doses of fentanyl between 0.0056 and 0.01 mg/kg produced slight increases (range, 15%–36%) in the number of responses on the drug lever in all three Ro 64-6198-trained animals, while simultaneously producing large decreases in the rates of responding. Peculiarly, the lowest dose of fentanyl tested (0.001 mg/kg) produced full generalization in monkey IE, but elicited responding only on the vehicle-appropriate lever in both monkeys ST and FA. In fentanyl-trained animals, fentanyl produced dose-dependent increases in drug-lever responding (Fig. 2A). Tests of stimulus generalization with Ro 64-6198 did not engender responding on the drug-appropriate lever up to doses that suppressed rates of responding (Fig. 2B).
Fig. 1.
Discriminative stimulus and response rate effects of Ro 64-6198 in Ro 64-6198-trained animals (A) and drug substitution studies with fentanyl (B). Data from individual monkeys (FA, IE, and ST) and their mean (± S.E.M.) are plotted (n = 3). FA was trained to discriminate Ro 64-6198 0.18 mg/kg, and IE and ST were trained to discriminate Ro 64-6198 0.1 mg/kg. Abscissae: dose in milligrams per kilogram, and vehicle responding (V). Ordinates: percent Ro 64-6198-lever responding and response rate (responses/s).
Fig. 2.
Discriminative stimulus and response rate effects of fentanyl in fentanyl-trained animals (A) and drug substitution studies with Ro-64-6198 (B). Data from individual monkeys (BC, MT, and BU) and their mean (± S.E.M.) are plotted (n = 3). All animals were trained to discriminate fentanyl 0.01 mg/kg. Abscissae: dose in milligrams per kilogram, and vehicle responding (V). Ordinates: percent fentanyl-lever responding and response rate (responses/s).
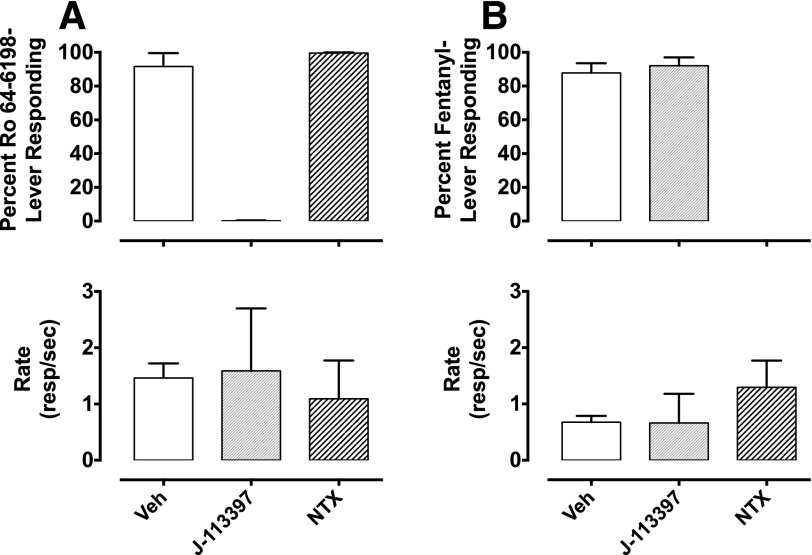
When Ro 64-6198-trained animals were pretreated with 1 mg/kg of the NOP selective antagonist J-113397, responding on the drug-paired lever was completely abolished. However, pretreatment with a μ-selective dose of naltrexone (0.03 mg/kg) did not alter responding on the Ro 64-6198-paired lever (Fig. 3A). In fentanyl-trained animals, responding on the drug-paired lever was blocked by pretreatment with 0.03 mg/kg of naltrexone but was not altered by pretreatment with J-113397 (Fig. 3B). No significant effects were found on rates of responding in either experiment.
Fig. 3.
The effect of J-113397 (1 mg/kg) and naltrexone (0.03 mg/kg) on the discriminative stimulus and response rate effects of the training dose in Ro 64-6198-trained animals (A) and fentanyl-trained animals (B). Data are presented as the mean and (± S.E.M.) for each group of monkeys (n = 3). Abscissae: vehicle (Veh), J-113397, and naltrexone (NTX) in the presence of the training drug. Ordinates: percent Ro 64-6198-lever responding (A), percent fentanyl-lever responding (B), and response rate (responses/s).
To further verify that Ro 64-6198 did not produce MOP-mediated stimulus effects in vivo, fentanyl-trained animals were pretreated with a dose of J-113397 (1 mg/kg) that completely abolished the stimulus effect of Ro 64-1698 in Ro 64-6198-trained animals at the highest dose tested (0.32 mg/kg). The rationale for this experiment was to test if eliminating the stimulus effects produced through NOP would unmask any MOP-mediated stimulus effects of Ro 64-6198. Under these conditions, fentanyl-trained animals responded only on the vehicle-appropriate lever (data not shown).
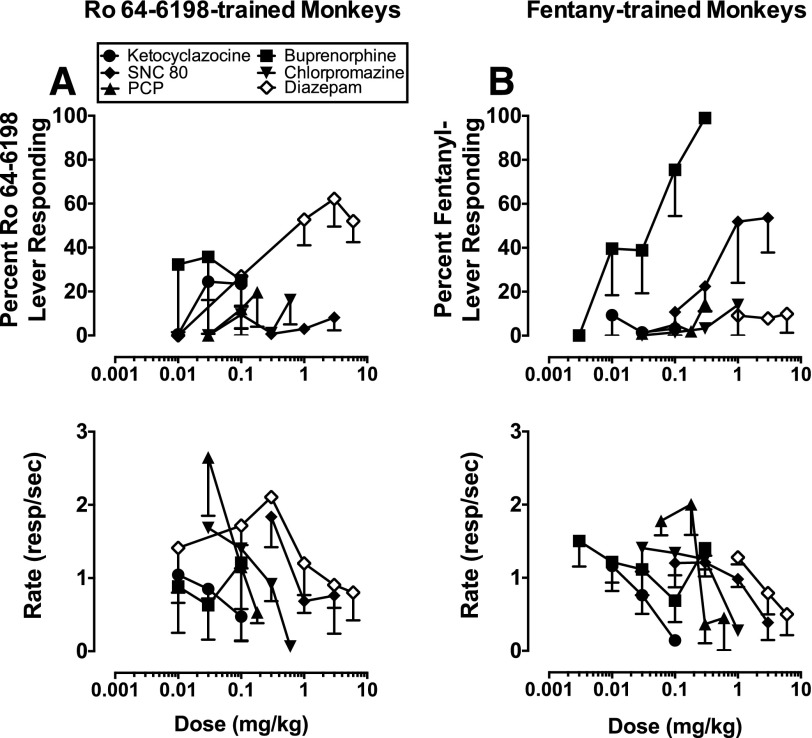
The in vivo pharmacological selectivity of Ro 64-6198 was further tested with the KOP agonist (ketocyclazocine), DOP agonist (SNC 80), MOP agonist (buprenorphine), N-methyl-D-aspartate receptor antagonist (PCP), nonselective dopamine antagonist (chlorpromazine), and gamma-aminobutryic acid A receptor (GABAA) allosteric modulator (diazepam). Up to doses that suppressed rates of responding, ketocyclazocine, SNC 80, PCP, and chlorpromazine did not produce drug-lever responding in animals trained to discriminate Ro 64-6198 (Fig. 4A). Overall, buprenorphine did not produce significant increases in drug-lever responding in Ro 64-6198-trained animals. However, IE, the monkey that generalized to fentanyl at the lowest dose tested, also exhibited complete stimulus generalization to the lowest dose of buprenorphine (0.01 mg/kg), and partial generalization at the middle and high doses.
Fig. 4.
Discriminative stimulus and response rate effects of drugs that did not substitute for a Ro 64-6198 stimulus (A) and discriminative stimulus and response rate effects of drugs that did not substitute for a fentanyl stimulus (B). Data are presented as the mean of three animals (± S.E.M.). Abscissae: dose in milligrams per kilogram. Ordinates: percent Ro 64-6198-lever responding (A), percent fentanyl-lever responding (B), and response rate (responses/s).
In fentanyl-trained animals, ketocyclazocine, PCP, chlorpromazine, and diazepam did not produce increases in drug-lever responding (Fig. 4B). The MOP agonist buprenorphine produced dose-dependent increases in drug lever responding, and fully generalized to a fentanyl cue. SNC 80 also produced dose-dependent increases in the drug-lever responding in fentanyl-trained animals, and at the two highest doses tested produced partial generalization to a fentanyl stimulus.
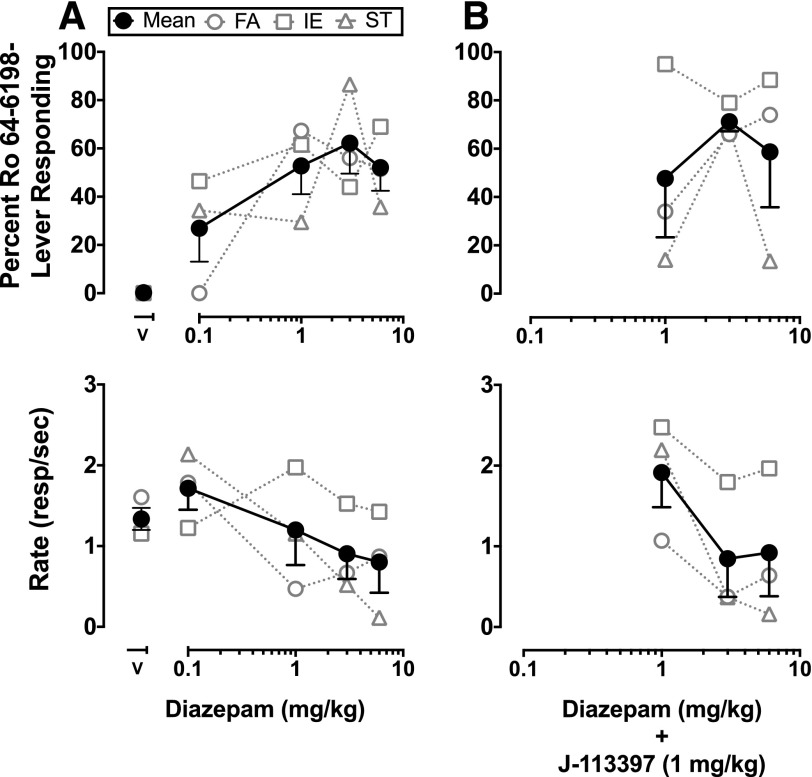
Drug substitution studies with diazepam produced dose-dependent increases in Ro 64-6198-lever responding that, on average, met criteria for partial generalization (Fig. 5A). All three subjects partially or fully generalized to at least one dose of diazepam, and at every dose tested two out of three animals made 50% or more of their responses on the drug-appropriate lever. Ro 64-6198-lever responding following diazepam was unaltered by J-113397 1 mg/kg (Fig. 5B), and the potency of the Ro 64-6198 training dose was similarly unaffected by flumazenil 1 mg/kg (data not shown).
Fig. 5.
Discriminative stimulus and response rate effects of diazepam in Ro 64-6198-trained monkeys. Diazepam alone (A) and following pretreatment with J-113397 (1 mg/kg) (B). Data from individual monkeys and the mean (± S.E.M.) are presented. Abscissae: dose in milligrams per kilogram of diazepam and vehicle responding (V). Ordinates: percent Ro 64-6198-lever responding and response rate (responses/s).
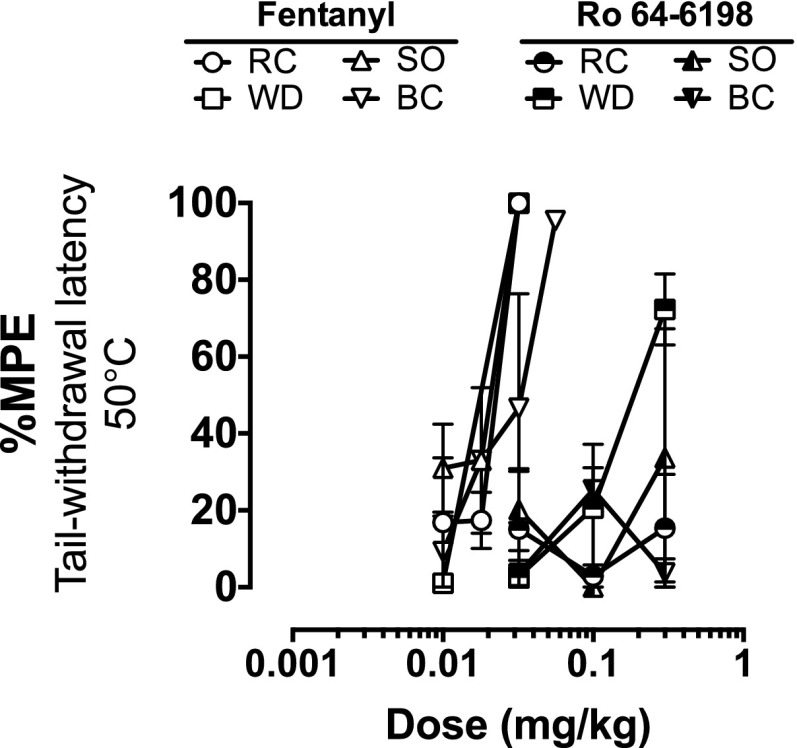
In the warm-water tail-withdrawal procedure, fentanyl produced dose-dependent increases in tail-withdrawal latency in all four subjects, and in the same animals doses of Ro 64-6198 up to 0.32 mg/kg did not increase tail-withdrawal latency in 3 out of 4 animals. In one animal (monkey WD), there was an increase in tail-withdrawal latency from 2.7 seconds at baseline to 15.2 seconds, or 72% of maximum percent effect, at the highest dose tested (0.32 mg/kg) (Fig. 6).
Fig. 6.
Effects of fentanyl and Ro 64-6198 in the warm-water tail-withdrawal assay at 50°C. Data are presented from individual monkeys as the mean of two observations (± S.E.M.) and were converted to maximum percent effect (MPE).
Discussion
This is the first study to demonstrate that a NOP agonist, Ro 64-6198, can be trained as a discriminative stimulus in rhesus monkeys. The interoceptive effects of Ro 64-6198 were distinct from those mediated through other opioid receptors as evidenced by the lack of stimulus generalization to selective opioid agonists, and the ability of selective NOP antagonists to block the Ro 64-6198-induced cue. Additional drug substitution studies with chlorpromazine and PCP, which have both been trained as discriminative stimuli (Goas and Boston, 1978; Solomon et al., 1982), also failed to generalize, indicating that these drugs and the receptors they bind do not contribute to a Ro 64-6198 stimulus.
While Ro 64-6198 was shown to be 100-fold more selective for NOP in vitro, it possesses reasonable affinity for MOP (approximately 50 nM), where it functions as a full agonist (Jenck et al., 2000; Wichmann et al., 2000). Generally, studies evaluating the effects of Ro 64-6198 on analgesia, anxiety, and locomotion in rodents, and on analgesia in monkeys, have shown these effects are not sensitive to the opioid antagonists, naloxone and naltrexone (Higgins et al., 2001; Varty et al., 2005; Ko et al., 2009). The absence of stimulus generalization to a fentanyl cue in animals trained to discriminate Ro 64-6198, and likewise the absence of stimulus generalization to a Ro 64-6198 cue in animals trained to fentanyl, supports previous findings in rats and monkeys that Ro 64-6198 has no appreciable efficacy at MOP in vivo. To test this further, we attempted to unmask the MOP agonist effects of Ro 64-6198 by pretreating fentanyl-trained animals with J-113397 before administering a large dose of Ro 64-6198. Even under conditions where NOP activity was silent and the dose of Ro 64-6198 was high compared with doses that produce other behavioral effects, the animals responded only on the vehicle-appropriate lever, further confirming that Ro 64-6198 lacks MOP effects. While it is possible that even higher doses of Ro 64-6198 may display some MOP-like stimulus effects, the dose tested here was high enough to produce significant behavioral disruption. Thus, it is unlikely that Ro 64-6198 has any behavioral effects mediated through MOP.
Curiously, the Ro 64-6198-trained monkey IE reported that the lowest dose of fentanyl (0.001 mg/kg) fully generalized to the training drug and this effect decreased as the dose of fentanyl was increased. Consistent with this pattern of responding, monkey IE completely generalized to the MOP partial agonist buprenorphine at low doses, while larger doses only produced partial generalization. Monkey IE had an extensive i.v. drug history that included working for cocaine, remifentanil, and methylphenidate, as well as for food, under a variety of different schedules. While we cannot rule out prior experimental history as a reason for this unusual pattern of stimulus generalization, it is not apparent what specific aspects are impacting these data. Individual differences in the pharmacodynamics and/or pharmacokinetics of drugs are common, and may be caused by genetic differences in neurobiological makeup and in how the drug is absorbed, distributed, and metabolized. If significant overlap between the behavioral effects of Ro 64-6198 and MOP agonists continue to be reported in rhesus monkeys or any other species, then pharmacogenetic studies looking at genetic polymorphisms in the MOP and NOP receptor, as well as the enzymes known to metabolize Ro 64-6198, may help provide an explanation for these findings.
The current study found that Ro 64-6198-trained animals partially generalized to the benzodiazepine diazepam. Several studies in rats have reported that Ro 64-6198 produced anxiolytic-like effects in the elevated plus-maze, fear-potentiated startle test and the modified Geller-Seifter conflict test (Jenck et al., 2000; Varty et al., 2005; Goeldner et al., 2012). These anxiolytic-like effects were similar in magnitude to those produced with diazepam and alprazolam, but unlike the benzodiazepines Ro 64-6198 was anxiolytic-like at doses that did not disrupt motor or cognitive performance. In rhesus monkeys, we found that diazepam produced partial generalization to Ro 64-6198 and that these effects were blocked with flumazenil but not with J-113397. Additionally, the potency of the Ro 64-6198 training dose was unaltered by pretreatment with flumazenil. This suggests that Ro 64-6198 and diazepam share components of their interoceptive effects, but that they are not mediated through a common receptor. The in vitro binding data support that Ro 64-6198 has no significant affinity for the GABAA channel or the benzodiazepine-binding site on GABAA (Wichmann et al., 2000). To the extent that the interoceptive effects produced with diazepam are related to its therapeutic efficacy, these data may support the use of Ro 64-6198 as a novel anxiolytic.
Consistent with the previous literature (France et al., 1992), monkeys readily learned to discriminate between fentanyl and vehicle. The effects of fentanyl were antagonized with MOP-selective doses of naltrexone but not with J-113397. Ro 64-6198, diazepam, chlorpromazine, ketocyclazocine, and PCP did not produce stimulus generalization in fentanyl-trained animals, while the MOP agonist buprenorphine produced full generalization. Surprisingly, the DOP-selective agonist SNC 80 produced partial generalization to a fentanyl cue. To our knowledge this is the first time that a DOP agonist has partially generalized to a MOP stimulus in monkeys, although rats and monkeys trained to discriminate SNC 80 and other DOP agonists have shown stimulus generalization to drugs from other pharmacological classes such as ketamine, cocaine, and amphetamine (Suzuki et al., 1997a,b; Brandt et al., 1999). These results, taken in the context of the previous literature, suggest that the stimulus effects of SNC 80 may be more complex than previously thought. However, in general these findings confirm that the stimulus effects of MOP agonists are selective.
Overall, in contrast to the apparent species differences in the antinociceptive effects, there is good concordance between the stimulus effects of Ro 64-6198 in rats and monkeys. Rats trained to discriminate Ro 64-6198 from saline, morphine 6 mg/kg, and buprenorphine 0.1 mg/kg, produced 40%–50% responding on the drug-appropriate lever, but these doses significantly suppressed rates of responding, and there was considerable variability among subjects (Recker and Higgins, 2004). This is consistent with the present study in that Ro 64-6198-trained monkeys responded on the drug-appropriate lever when tested with doses of fentanyl and buprenorphine that also suppressed rates of responding, except that neither drug met criteria for generalization. Likewise, in both rats and monkeys, stimulus effects of Ro 64-6198 were blocked only with J-113397 and not naloxone or naltrexone, and drug substitution studies with selective opioid agonists at KOP and DOP did not produce stimulus generalization in these animals.
In the present study, Ro 64-6198 did not produce antinociception against an acute thermal nociceptive stimulus (50°C water) in three out of four monkeys tested. One monkey showed increases in tail-withdrawal latency at the highest dose of Ro 64-6198 (0.32 mg/kg), which produced 72% of the maximum possible effect. In contrast, the MOP agonist fentanyl produced maximum antinociception in four out of four animals tested. The analgesic potency and efficacy of fentanyl in this experiment are consistent with previously published studies, and the order of potency with which fentanyl was discriminated, suppressed rates of responding, and produced analgesia is consistent with the pharmacodynamic profile of this drug across the literature (i.e., potency order of discrimination > rate suppression > analgesia) (Dykstra et al., 1988; France et al., 1992; Stevenson et al., 2003). If this same order of potency held true for NOP agonists, analgesia should have been observed at doses of 0.32 mg/kg of Ro 64-6198. However, very limited analgesia was observed even at this large dose of Ro 64-6198. Interestingly, in previous studies with rhesus monkeys, a dose of 0.03 mg/kg Ro 64-6198 was reported to produce analgesia in the same thermal nociception assay as that used here (e.g., Ko et al., 2009; Ko and Naughton, 2009; Sukhtankar et al., 2014). This is an order of magnitude less than would be expected to produce analgesia if the commonly observed order of behavioral potency held true with this NOP agonist.
The reasons for the differences in the antinociceptive efficacy of Ro 64-6198 between studies are not clear. Limits in drug solubility prevented increasing the dose of Ro 64-6198 above 0.32 mg/kg, but this dose was more than an order of magnitude greater than the reported ED50 for antinociception published in previous studies using analogous procedures (Ko et al., 2009; Cremeans et al., 2012). When monkeys self-administering remifentanil were pretreated with Ro 64-6198 0.32 mg/kg i.v., there was a comparable decrease in rates of responding and the animals reportedly displayed signs of sedation (Podlesnik et al., 2011). Even when accounting for differences in potency as a function of route of administration, there appears to be little difference in the potency of Ro 64-6198 to produce sedation in the present study and in studies where Ro 64-6198 was found to be antinociceptive. These findings suggest that the antinociceptive effects of Ro 64-6198 may be more variable than previously described.
Since additional NOP agonists and antagonists were not characterized in these procedures, the results from this study may not be broadly generalizable to the whole class of compounds. This is a limitation of the present investigation. Other small molecule NOP agonists, such as SCH 221510, have also been shown to produce antinociception in rhesus monkeys using the warm-water tail-withdrawal assay (Cremeans et al., 2012). SCH 221510 was subsequently found to produce analgesia in a novel food-reinforced antinociceptive assay where squirrel monkeys were trained to pull a lever heated at different temperatures for various periods of time (Kangas and Bergman, 2014). This procedure appeared to be quite sensitive to different analgesics when compared with assays that use warm water as the thermal stimulus, and not all drugs that were found to be antinociceptive in warm-water tail-withdrawal studies were antinociceptive in this procedure. It will be interesting to learn whether Ro 64-6198 is antinociceptive in this assay. The results from these studies illustrate the importance of testing potential therapeutics in a variety of species, with different procedures, across different laboratories.
In summary, this study establishes that Ro 64-6198 can be trained as a discriminative stimulus in rhesus monkeys and that these effects are selectively mediated through the NOP receptor. The stimulus properties of Ro 64-6198 are not like other opioid agonists, but may share similarities with diazepam. Future studies should examine the extent to which drugs that act at the GABAA receptor generalize to Ro 64-6198, and whether the stimulus effects produced with Ro 64-6198 are characteristic of all NOP-selective ligands. Finally, Ro 64-6198 failed to produce antinociception in the present study, suggesting that more research is needed to assess Ro 64-6198 and other NOP agonists as novel agents for pain control.
Acknowledgments
The authors thank Matthew Zaks and Yong-Gong Shi for excellent technical assistance, as well as Hoffman-La Roche for providing Ro 64-6198. The authors also thank Gail D. Winger for reading and editing the manuscript.
Abbreviations
- DOP
δ-opioid receptor
- FR
fixed ratio
- GABAA
gamma-aminobutryic acid A receptor
- KOP
κ-opioid receptor
- MOP
μ-opioid receptor
- NOP
nociceptin/orphanin FQ receptor
- PCP
phencyclidine
Authorship Contributions
Participated in research design: Saccone, Woods.
Conducted experiments: Saccone, Zelenock, Lindsey.
Contributed new reagents or analytic tools: Prinssen, Wichmann, Sulima, Rice.
Performed data analysis: Saccone.
Wrote or contributed to the writing of the manuscript: Saccone, Woods.
Footnotes
This study was supported by the National Institutes of Health National Institute on Drug Abuse [Grants F31-DA038488 and T32-DA007281]. A portion of this work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism and the National Institutes of Health National Institute on Drug Abuse Intramural Research Programs. The work of the Drug Design and Synthesis Section, Chemical Biology Research Branch, National Institute on Drug Abuse, and National Institute of Alcohol Abuse and Alcoholism was supported by the National Institutes of Health Intramural Research Programs of the National Institute on Drug Abuse and National Institute of Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute on Drug Abuse.
References
- Brandt MR, Negus SS, Mello NK, Furness MS, Zhang X, Rice KC. (1999) Discriminative stimulus effects of the nonpeptidic δ-opioid agonist SNC80 in rhesus monkeys. J Pharmacol Exp Ther 290:1157–1164. [PubMed] [Google Scholar]
- Colpaert FC. (1999) Drug discrimination in neurobiology. Pharmacol Biochem Behav 64:337–345. [DOI] [PubMed] [Google Scholar]
- Cremeans CM, Gruley E, Kyle DJ, Ko MC. (2012) Roles of μ-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J Pharmacol Exp Ther 343:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra LA, Bertalmio AJ, Woods JH. (1988) Discriminative and analgesic effects of mu and kappa opioids: in vivo pA2 analysis. Psychopharmacol Ser 4:107–121. [DOI] [PubMed] [Google Scholar]
- France CP, Winger G, Seggel MR, Rice KC, Woods JH. (1992) Pharmacological profile of a potent, efficacious fentanyl derivative in rhesus monkeys. Psychopharmacology (Berl) 109:291–298. [DOI] [PubMed] [Google Scholar]
- Goas JA, Boston JE., Jr (1978) Discriminative stimulus properties of clozapine and chlorpromazine. Pharmacol Biochem Behav 8:235–241. [DOI] [PubMed] [Google Scholar]
- Goeldner C, Spooren W, Wichmann J, Prinssen EP. (2012) Further characterization of the prototypical nociceptin/orphanin FQ peptide receptor agonist Ro 64-6198 in rodent models of conflict anxiety and despair. Psychopharmacology (Berl) 222:203–214. [DOI] [PubMed] [Google Scholar]
- Hein DW, Young AM, Herling S, Woods JH. (1981) Pharmacological analysis of the discriminative stimulus characteristics of ethylketazocine in the rhesus monkey. J Pharmacol Exp Ther 218:7–15. [PubMed] [Google Scholar]
- Higgins GA, Grottick AJ, Ballard TM, Richards JG, Messer J, Takeshima H, Pauly-Evers M, Jenck F, Adam G, Wichmann J. (2001) Influence of the selective ORL1 receptor agonist, Ro64-6198, on rodent neurological function. Neuropharmacology 41:97–107. [DOI] [PubMed] [Google Scholar]
- Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, Lundstrom K, Cesura AM, Poli SM, Roever S, et al. (2000) A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci USA 97:4938–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J. (2014) Operant nociception in nonhuman primates. Pain 155:1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN. (2009) Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys. J Pain 10:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP. (2009) Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology 34:2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DG. (2008) The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov 7:694–710. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377:532–535. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. (2001) The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev 53:381–415. [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. (1994) ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett 341:33–38. [DOI] [PubMed] [Google Scholar]
- Podlesnik CA, Ko MC, Winger G, Wichmann J, Prinssen EP, Woods JH. (2011) The effects of nociceptin/orphanin FQ receptor agonist Ro 64-6198 and diazepam on antinociception and remifentanil self-administration in rhesus monkeys. Psychopharmacology (Berl) 213:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recker MD, Higgins GA. (2004) The opioid receptor like-1 receptor agonist Ro 64-6198 (1S,3aS-8-2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one) produces a discriminative stimulus in rats distinct from that of a μ, κ, and δ opioid receptor agonist cue. J Pharmacol Exp Ther 311:652–658. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. (1995) Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270:792–794. [DOI] [PubMed] [Google Scholar]
- Schröder W, Lambert DG, Ko MC, Koch T. (2014) Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists. Br J Pharmacol 171:3777–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RE, Herling S, Domino EF, Woods JH. (1982) Discriminative stimulus effects of N-substituted analogs of phencyclidine in rhesus monkeys. Neuropharmacology 21:1329–1336. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Folk JE, Linsenmayer DC, Rice KC, Negus SS. (2003) Opioid interactions in rhesus monkeys: effects of δ + μ and δ + κ agonists on schedule-controlled responding and thermal nociception. J Pharmacol Exp Ther 307:1054–1064. [DOI] [PubMed] [Google Scholar]
- Sukhtankar DD, Lee H, Rice KC, Ko MC. (2014) Differential effects of opioid-related ligands and NSAIDs in nonhuman primate models of acute and inflammatory pain. Psychopharmacology (Berl) 231:1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Mori T, Tsuji M, Maeda J, Kishimoto Y, Misawa M, Nagase H. (1997a) Differential effects of μ-, δ- and κ-opioid receptor agonists on the discriminative stimulus properties of cocaine in rats. Eur J Pharmacol 324:21–29. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Mori T, Tsuji M, Misawa M, Nagase H. (1997b) The role of δ-opioid receptors in the discriminative stimulus properties of a low dose of methamphetamine. Eur J Pharmacol 331:1–8. [DOI] [PubMed] [Google Scholar]
- Varty GB, Hyde LA, Hodgson RA, Lu SX, McCool MF, Kazdoba TM, Del Vecchio RA, Guthrie DH, Pond AJ, Grzelak ME, et al. (2005) Characterization of the nociceptin receptor (ORL-1) agonist, Ro64-6198, in tests of anxiety across multiple species. Psychopharmacology (Berl) 182:132–143. [DOI] [PubMed] [Google Scholar]
- Wichmann J, Adam G, Röver S, Hennig M, Scalone M, Cesura AM, Dautzenberg FM, Jenck F. (2000) Synthesis of (1S,3aS)-8-(2,3,3a,4,5, 6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one, a potent and selective orphanin FQ (OFQ) receptor agonist with anxiolytic-like properties. Eur J Med Chem 35:839–851. [DOI] [PubMed] [Google Scholar]
- Woods JH, Bertalmio AJ, Young AM, Essman WD, Winger G. (1988) Receptor mechanisms of opioid drug discrimination. Psychopharmacol Ser 4:95–106. [DOI] [PubMed] [Google Scholar]