Abstract
Background
Diabetes mellitus (DM) is a risk factor for pancreatic cancer (PC), but its prognostic value in PC is still unclear. To elucidate this issue, we systematically reviewed the evidence concerning the association between diabetes status and PC.
Methods
Medline and EMBASE databases were searched to identify the eligible studies. Overall and subgroup analyses were performed to detect the discrepancy of prognosis according to diabetes status. Hazard ratios (HRs) with 95% CI were used to estimate the effect size.
Results
Eighteen studies including 16,181 patients with sample size ranging from 113 to 4,658 were pooled in this meta-analysis. Results showed that patients with DM had worse survival (HR 1.19, 95% CI: 1.07–1.32). In view of the impact of diabetes duration and tumor stage on the outcomes, we classified the studies into different groups. The results indicated that DM was associated with survival in both long-standing diabetes (HR 1.26, 95% CI: 1.14–1.40) and recent-onset diabetes (HR 1.29, 95% CI: 1.09–1.51). Data regarding localized disease (HR 1.57, 95% CI: 1.00–2.46) and nonlocalized (locally advanced and metastatic) disease (HR 1.42, 95% CI: 1.16–1.73) verified that the prognostic value was independent of tumor stage.
Conclusion
Our results suggested that patients with DM were associated with worse survival than those without DM. Diabetes may be a predictive factor of survival in patients with PC. Surveillance of diabetes status and antidiabetes medication administration after the diagnosis of PC is of clinical importance.
Keywords: diabetes mellitus, pancreatic cancer, survival, meta-analysis
Introduction
Pancreatic cancer (PC) is the fourth leading cause of cancer-related death. It is estimated that 227,000 deaths per year are related to PC.1 In western countries, >80% of patients with PC have distant metastatic diseases at initial presentation. Radical surgery is restricted to these patients as their best chance of a cure. Moreover, the efficacy of chemotherapy and radiotherapy is limited, and the overall 5-year survival rate among patients is <5%.2 The prognosis of patients with PC is affected by numerous factors, such as the number of metastatic lymph nodes, the infiltration of peripancreatic blood vessels, histologic grade, and positive margins after surgery,1,3 all of which can be evaluated only after resection. The ability to find an optimal prognostic indicator prior to treatment would greatly improve management.
Diabetes mellitus (DM) is a common endocrine disease worldwide. Epidemic data show that the incidence of DM is increasing among the population aged from 20 years to 79 years.4 It is well established that DM is one of the significant risk factors for PC, besides alcohol consumption, tobacco smoking, and obesity.5,6 This may contribute to hormonal and metabolic alterations brought by insulin resistance or compensatory hyperinsulinemia. Long-term existence of insulin resistance-related metabolic and inflammatory components can be involved in the formation of the microenvironment for tumorigenesis and tumor progression. Accumulating evidence has demonstrated that people with diabetes may develop PC in the long run.7,8 Furthermore, diabetes may also affect the survival of patients with PC. Several clinical studies confirm that patients with diabetes tend to have worse overall survival compared to patients without diabetes and the use of preoperative insulin will reduce the survival time.9–11 However, Beg et al12 from the University of Texas Southwestern Medical Center investigated 4,658 patients from the Veterans Affairs Central Cancer Registry and found that DM had no effect on the overall survival of PC.
So far, the association between diabetes and increased risk of several common cancers has reached a consensus. Nevertheless, the role of diabetes in PC prognosis is still uncertain. A previous meta-analysis tried to review the prognostic value of preoperative diabetes on the survival of patients.13 However, it only enrolled patients with curative resection, which accounted for a small part of the population diagnosed with PC, and did not consider the discrepancy of different durations of diabetes. In addition, several new prospective cohorts are published recently. These data provide an excellent opportunity for us to determine the role of diabetes in the progression of PC. Therefore, we conduct this meta-analysis and hope to transform the results into clinical application.
Materials and methods
Search strategies
Using Medline and EMBASE databases, we conducted a literature search of studies published before May 2015 that evaluated the prognostic value of DM in PC. We also manually searched bibliographic reviews and associated abstracts. There was no restriction of language. Our research strategy included keywords of “diabetes mellitus” (eg, “diabetes,” “glucose intolerance,” “hyperglycemia,” and “hyperglycemia”), “pancreatic cancer” (eg, “pancreatic carcinoma” and “pancreatic adenocarcinoma”), and “survival” (eg, “prognosis” and “outcome”). The complete search strategy is shown in Supplementary Material. All included records were added to an EndNote (Version X6) library.
Study inclusion and exclusion criteria
Studies were included in the meta-analysis if they met the following criteria: 1) studies published as an original series that evaluated the survival or outcomes of patients according to diabetes status after PC diagnosis, information of diabetes status acquired from patients’ self-report on questionnaires, blood glucose tests, or medical records, and studies reported before May 2015 and 2) studies providing hazard ratios (HRs) with corresponding 95% CIs of overall survival (OS) or having sufficient information to reconstruct them.
Exclusion criteria were as follows: 1) studies with no sufficient data or consistent data; 2) literature reporting only the mortality of patients in hospital or after surgery; and 3) studies without enough information to estimate HR and 95% CI associated with diabetes.
Data extraction and quality assessment
All potential studies were independently reviewed by two reviewers (HS and MZ). Results were compared and consensus was reached. The following variables were recorded: first author, year of publication, median age-to-sex ratio of included patients, geographical region, duration of follow-up, adjustment variables, tumor stage, and treatment. If the patients mainly received surgical treatment, the study was classified into surgical therapy group. If part or all of the patients cannot undergo surgery, it was divided into multiple therapy group. We used the TNM staging system or metastatic status to represent the tumor stage. When important data was not reported, we tried to contact the authors. The definitions of long-standing and recent-onset diabetes are not the same in different studies. The cutoff in study by Yuan et al14 was 4 years. Study by Hwang et al15 defined DM >5 years as long-standing diabetes. The cutoff was 2 years in the studies by Ben et al16 and Chu et al.17
The meta-analysis followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement.18 The study quality was scored by HS and MZ using the Newcastle-Ottawa Scale.19 Of the 18 studies, 17 obtain scores of ≥6. The Newcastle-Ottawa Scale is frequently used for nonrandom studies (case–control and cohort studies), and scores of ≥6 are identified as high-quality studies.
Data synthesis and analysis
HRs with 95% CIs were directly obtained from included studies. When multivariate and univariate analyses were available to obtain, multivariate data were extracted. Study-specific HR estimates were combined using a random- or fixed-effects model.20 I2 values were adopted for the quantification of statistical inconsistency, described as the percentage of variation between studies due to heterogeneity.21 Publication bias was assessed by Begg’s funnel plot22 and Egger’s bias indicator test.23 The trim-and-fill method by Duval and Tweedie24 was applied to estimate the influence of publication bias on the overall effect. The stability of the results was evaluated by sensitivity analysis. We used Stata 12.0 (StataCorp LP, College Station, TX, USA) commercial software with meta-analysis commands to perform all statistical analyses.
Results
Literature search and study characteristics
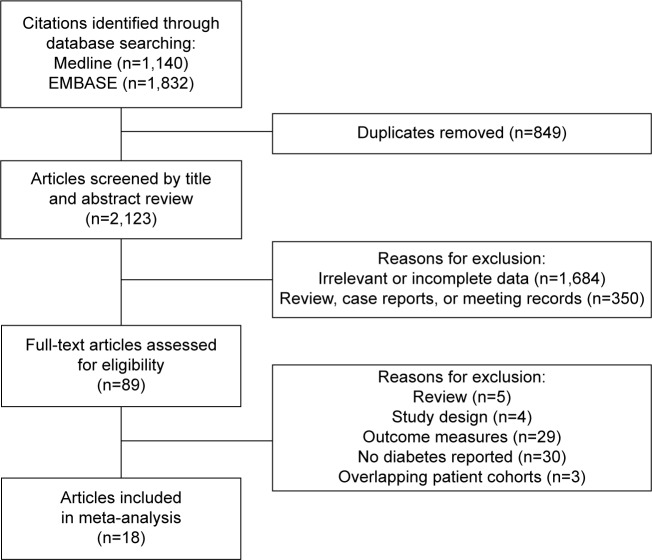
By searching the dataset, 18 studies were included initially. A Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow diagram depicting the selection process is shown in Figure 1. After excluding the studies that did not meet requirements, 89 identified studies concerning the prognostic value of diabetes in PC were further evaluated. By further review, 30 studies were not relative to diabetes. Twenty-nine studies did not provide the survival time of patients with diabetes. Three studies had the overlapping patient cohorts with other larger studies.25–27 Five studies were review of previous studies. Three studies focused on the impact of diabetes on the mortality of general populations, not patients with PC,9,28,29 and one study provided HR for patients with fasting serum glucose ≥126 mg/dL compared to 0–109 mg/dL group.30 All of the studies mentioned above were excluded. Finally, 18 studies including 16,181 patients with sample size ranging from 113 to 4,658 were pooled in this meta-analysis.12,14–17,31–43
Figure 1.
Search strategy of eligible studies.
The general characteristics of included studies are summarized in Table 1. The study by Yuan et al14 included three cohorts, so we divided them into three groups, Yuan(NHS), Yuan(HPFS), and Yuan(DFCI). The study by Olson et al39 was classified into two cohorts, resected group and nonresected group. There were 16 retrospective studies (17 cohorts) and two prospective studies (four cohorts) identified. There were ten studies (13 cohorts) from USA, two studies from the People’s Republic of China and two from Italy, and one study from each of the UK, Germany, the Netherlands, and France. Nine of these studies (11 cohorts) enrolled <500 people, and nine studies enrolled >500. Five studies only enrolled pancreatic ductal adenocarcinoma,16,17,33,34,41 and others included all the exocrine pancreas cancer. HRs in only two of the 18 studies (21 cohorts) were produced by univariate analysis.
Table 1.
Data from studies included in this meta-analysis
| Author | Year | Location | Study design | Study period | Follow-up (months) | Population source | Diabetes ascertainment | Adjustment variables | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Yuan et al14 | 2015 | USA | Prospective | NHS/HPFS, 1986–2010; DFCI, 2004–2013 | NR | NHS/HPFS: population based; DFCI: hospital based | Self-reported | Age, sex, race, smoking status, year of diagnosis, and cancer stage | 7 |
| Dong et al34 | 2014 | People’s Republic of China | Retrospective | 2009–2011 | 15.2 (1.4–52.0) | Hospital based | Medical records/blood glucose test | Age, sex, jaundice, tumor location, treatment, tumor diameter, tumor stage, differentiation, surgical margins, and perineural invasion | 6 |
| Toriola et al31 | 2014 | USA | Prospective | 1993–2001 | NR | Population based | Self-reported | Age, sex, BMI, race, smoking, and tumor stage | 7 |
| Pelucchi et al32 | 2014 | Italy | Retrospective | 1983–2008 | NR | Hospital based | Self-reported | Age and calendar period at diagnosis, study center, and sex | 6 |
| Hart et al33 | 2014 | USA | Retrospective | 2000–2010 | NR | Hospital based | Self-reported/blood glucose test | Age, BMI, weight loss percentage, smoking status, family history of DM, DM treatment, tumor size, tumor grade, number of positive lymph nodes, margin status, adjuvant chemotherapy, and tumor stage | 7 |
| Beg et al12 | 2014 | USA | Retrospective | 1995–2008 | 3.6 (1.3–7.4) | Population based | Medical records | Age, sex, race, alcohol, tobacco, stage, tumor site, and treatment | 7 |
| Hwang et al15 | 2013 | UK | Retrospective | 2003–2010 | NR | Population based | Medical records | Age, sex, history of pancreatic resection, pancreatitis, and Charlson index | 7 |
| Sahin et al35 | 2012 | USA | Retrospective | 1996–2011 | NR | Hospital based | Self-reported | Perineural invasion, margin status, node status, and differentiation | 6 |
| Ben et al16 | 2012 | People’s Republic of China | Retrospective | 2005–2010 | 20 (4–62) | Hospital based | Self-reported/blood glucose test | Age, tumor stage, neural invasion, CA19-9 levels, and node involvement | 7 |
| Gong et al42 | 2012 | USA | Retrospective | 1995–2008 | 121.2 | Hospital based | Self-reported | Age, sex, race, education, body mass index, smoking status, diabetes, stage, tumor grade, tumor site, and primary treatment | 7 |
| Barbas et al36 | 2012 | USA | Retrospective | 1996–2008 | NR | Hospital based | Medical records | Age, sex, race, comorbidity, positive lymph node status, margin status, histological grade, vascular invasion, perineural invasion, and treatment | 7 |
| Hartwig et al37 | 2011 | Germany | Retrospective | 2001–2009 | 17 (1–92) | Hospital based | Medical records | Age, ASA score, CEA, CA19-9, histological grade, tumor stage, lymph node ratio, and margin status | 7 |
| Dandona et al38 | 2011 | USA | Retrospective | 1995–2009 | NR | Hospital based | Self-reported | None | 6 |
| Dehayem et al40 | 2011 | France | Retrospective | 2002–2004 | NR | Hospital based | Medical records/blood glucose test | NR | 7 |
| Olson et al39 | 2010 | USA | Retrospective | 2004–2008 | NR | Hospital based | Self-reported | None | 6 |
| van de Poll-Franse et al43 | 2007 | the Netherlands | Retrospective | 1995–2005 | NR | Hospital based | Medical records | Age, sex, stage, treatment, and cardiovascular disease | 7 |
| Chu et al17 | 2010 | USA | Retrospective | 2000–2007 | NR | Hospital based | Medical records | Age, sex, BMI, ethnicity, tumor location, and histopathologic variables | 7 |
| Sperti et al41 | 1996 | Italy | Retrospective | 1970–1992 | NR | Hospital based | Medical records | NR | 5 |
Abbreviations: ASA, American Society of Anesthesiologists; CEA, carcino-embryonic antigen; BMI, body mass index; DM, diabetes mellitus; NOS, Newcastle-Ottawa Scale; NR, not reported; NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-Up Study; DFCI, Dana-Farber Cancer Institute.
DM and OS
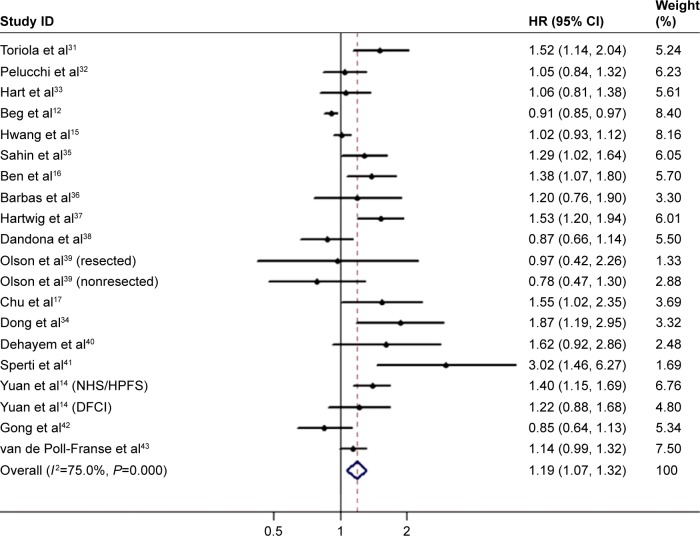
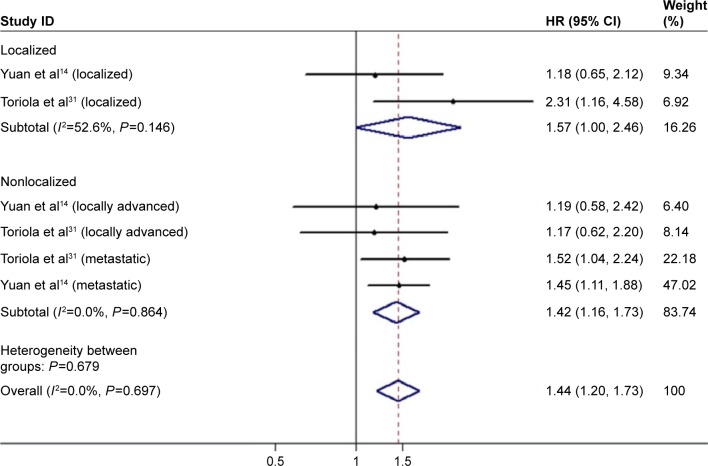
There were 18 studies (21 cohorts) presenting the HRs for the OS. The pooled HR was 1.19 (95% CI: 1.07–1.32; Figure 2) with remarkable heterogeneity (I2=75.0%, P<0.001). Results of the combined analysis showed that patients with diabetes may have shorter OS. Due to the presence of heterogeneity, subgroup analysis was performed based on the different study types (prospective or retrospective), study regions (USA, Europe, or Asia), sample size (<500 or ≥500), and therapeutic interventions (curative resection or multiple treatment) (Table 2). Subgroup analysis by therapeutic interventions indicated that diabetes status was significantly associated with a poorer outcome in curative resection patients but not in multiple treatment patients. While the subgroup analysis failed to figure out the underlying source of heterogeneity, study types, study regions, sample size, and therapeutic interventions were not the main reasons for heterogeneity. Because tumor stage is one of the predominant factors for prognosis and knowledge about the role of tumor stage in the relationship between diabetes and PC is little, we pooled the results of two relevant studies.14,31 Results showed that diabetes was associated with survival in both localized disease (HR 1.57, 95% CI: 1.00–2.46; Figure 3) and nonlocalized (locally advanced and metastatic) disease (HR 1.42, 95% CI: 1.16–1.73).
Figure 2.
Meta-analysis of the effect of diabetes mellitus on overall survival.
Abbreviations: HR, hazard ratio; NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-Up Study; DFCI, Dana-Farber Cancer Institute.
Table 2.
Subgroup analysis of the studies reporting the association between diabetes mellitus and overall survival
| Stratified analysis | Number of cohorts | Pooled HRs (95% CI)
|
Heterogeneity
|
||
|---|---|---|---|---|---|
| Fixed | Random | I2 (%) | P-value | ||
| Study type | |||||
| Prospective | 3 | 1.39 (1.20–1.60) | 1.39 (1.20–1.60) | 0 | 0.609 |
| Retrospective | 17 | 1.02 (0.98–1.07) | 1.15 (1.03–1.28) | 73 | <0.001 |
| Study region | |||||
| USA | 11 | 1.00 (0.94–1.05) | 1.12 (0.95–1.32) | 75.5 | <0.001 |
| Europe | 7 | 1.10 (1.03–1.18) | 1.20 (1.03–1.40) | 69.1 | 0.004 |
| Asia | 2 | 1.49 (1.19–1.87) | 1.52 (1.16–1.99) | 21.3 | 0.26 |
| Sample size | |||||
| <500 | 11 | 1.21 (1.08–1.35) | 1.26 (1.05–1.51) | 55.9 | 0.012 |
| >500 | 9 | 1.03 (0.98–1.08) | 1.15 (1.01–1.31) | 82.9 | <0.001 |
| Treatment | |||||
| Curative resection | 9 | 1.33 (1.19–1.48) | 1.37 (1.14–1.65) | 57.9 | 0.015 |
| Multiple treatment | 11 | 1.01 (0.97–1.06) | 1.09 (0.98–1.22) | 72.9 | <0.001 |
Abbreviation: HRs, hazard ratios.
Figure 3.
Meta-analysis of the pooled estimates stratified by different tumor stages. Abbreviation: HR, hazard ratio.
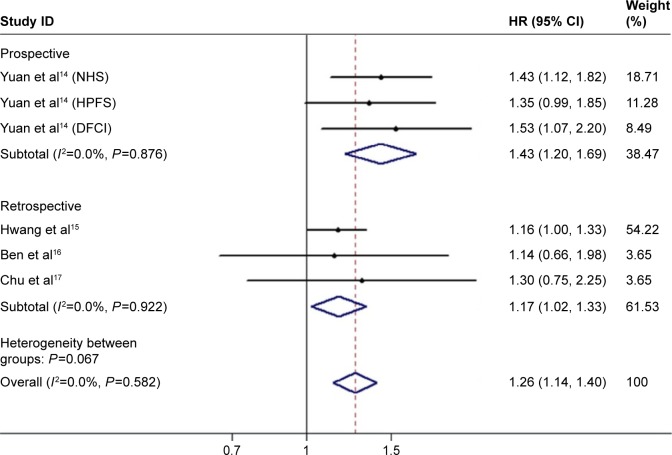
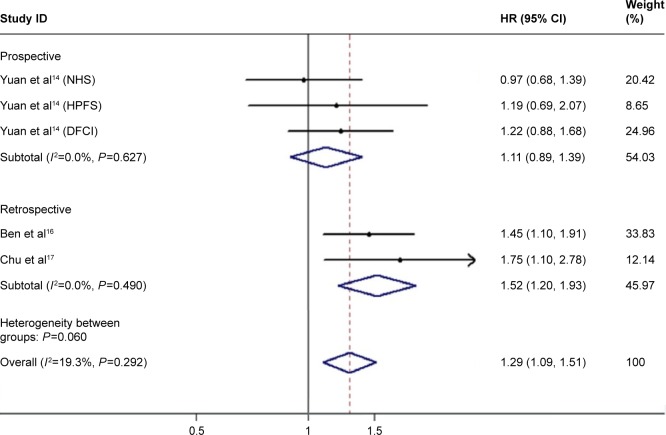
In consideration of the impact of diabetes duration on the outcomes of PC, patients with DM were classified into long-standing and recent-onset groups. In the group of long-standing diabetes, the results of four studies (six cohorts) showed a pooled HR of 1.26 (95% CI: 1.14–1.40; Figure 4) with no heterogeneity (I2=0.0%, P=0.582). There were three studies (five cohorts) providing data associated with recent-onset diabetes. The pooled estimate of HR was 1.29 (95% CI: 1.09–1.51; Figure 5) with no heterogeneity (I2=19.3%, P=0.292), but data from three prospective cohorts showed an HR of 1.11 (95% CI: 0.89–1.39). The results indicated both long-standing and recent-onset diabetes tended to be related with poor survival of PC.
Figure 4.
Meta-analysis of the effect of long-standing diabetes on overall survival.
Abbreviations: HR, hazard ratio; NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-Up Study; DFCI, Dana-Farber Cancer Institute.
Figure 5.
Meta-analysis of the effect of recent-onset diabetes on overall survival.
Abbreviations: NHS, Nurses’ Health Study; HPFS, Health Professionals Follow-Up Study; DFCI, Dana-Farber Cancer Institute; HR, hazard ratio.
Publish bias and sensitivity analysis
In addition, Begg’s funnel plot and Egger’s test were used to evaluate the publication bias of included studies. The statistical results (Begg’s test, P=0.49; Egger’s test, P=0.003) showed evidence of publication bias, and the shape of the funnel plot was unsymmetrical (Figure 6). Then, trim-and-fill analysis was performed to deduce the potential unpublished studies. The results indicated that seven studies were missing. The filled analysis showed an HR of 2.79 (95% CI: 2.50–3.14), which was in accord with the previous result. Sensitivity analysis showed that the pooled results and heterogeneity could not be changed substantially by deleting a single study each time. All these analyses demonstrated the stable quality of our study.
Figure 6.
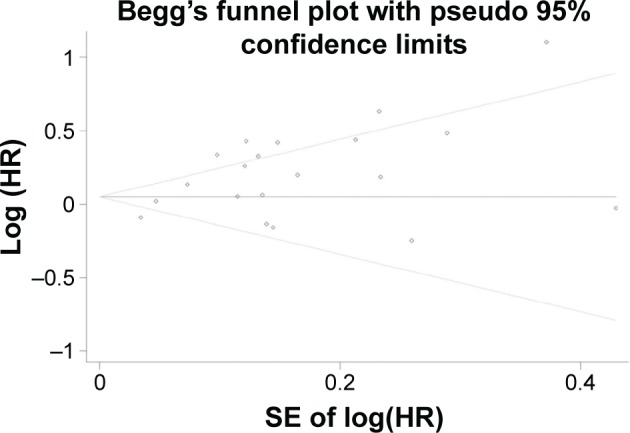
Begg’s funnel plot of publication bias.
Abbreviations: SE, standard error; HR, hazard ratio.
Discussion
Although the guidelines for standardized treatment of PC are enacted, prognostication in advanced cancer relies heavily on the intuition and experience of clinicians. But the estimate by clinicians is often inaccurate according to previous studies.44 The decision whether to give potent anticancer treatments with side effect (eg, chemotherapy) or not is often hard to make without the support of scientific assessment system. As a result, accurate prognostication is important, especially for patients nearing the end of life.
In this meta-analysis, we reported evidence from 18 studies (21 cohorts) about the effect of DM on the survival of PC investigated in a total of 16,181 patients. The results demonstrated the predictive value of diabetes on survival. It is known that some pancreatic tumors can secret excess insulin and lead to hyperglycemia.45 Studies have shown that this PC-induced DM frequently happens within 3 years before PC diagnosis in 20%–30% of patients, and half of this new-onset DM will be cured after surgical resection of the tumors.46 Therefore, we classified DM into long-standing and recent-onset groups according to the different duration. Further analysis verified that both long-standing and recent-onset diabetes were associated with shorter OS. But data from three prospective studies by Yuan et al14 showed nonsignificant results for the recent-onset group.14 The authors suggested that the chronic alterations in metabolic components brought by long-term glucose intolerance can lead to some genetic mutations, and the proto-oncogene mutation would make the tumor a more aggressive one.
The underlying mechanism is confusing and may be connected to the hormonal and metabolic alterations brought by diabetes. The compensatory hyperinsulinemia induced by reduced insulin sensitivity can increase the bioavailability of circulating insulin-like growth factors (IGFs).47 Experimental studies have shown that both IGF-1 and IGF-1 receptor are highly expressed in PC. Once insulin or IGF-1 receptors interact with their ligands, multiple signaling pathways involved in proliferation, invasion, metastasis, angiogenesis, and antiapoptosis are activated. Increased oxidative stress and inflammatory responses also play an important role in this pathological process. Studies found that oxidative stress and inflammation state may be the first step of pathological process of insulin resistance, which can be suppressed by antioxidants.4 Increased oxidative stress and inflammatory factors, such as nuclear factor-κB and signal transducer and activator of transcription protein, can activate the signaling pathway and then enhance the progression of cancer.48 Metformin, the most commonly used medication in patients with diabetes, has been found to suppress cell proliferation and reduce cell cycle arrest by activation of adenosine 5′-monophosphate-activated protein kinase.49 Experiments verified the antitumor effect of metformin on animals with high-energy diet.50 The results suggest that treatment with metformin may reduce the mortality of cancer.
Heterogeneity within studies was observed, but subgroup analysis did not change the heterogeneity substantially. Study types, study regions, sample size, and therapeutic interventions had no contribution to the heterogeneity. The different forms of diabetes ascertainment in the recruited studies may be one of the reasons. Some studies acquired the information of diabetes status from patients’ self-report on questionnaires. Others adopted the information from blood glucose tests or medical records. Patients’ self-report can provide the whole history of diabetes status, but it is not so reliable, while the credible blood glucose tests or medical records only give the ongoing status. From another point of view, the potential publication bias may partially explain the source of heterogeneity, though trim-and-fill and sensitive analyses verified the reliability of the pooled results. It is recognized that studies with negative results are less likely to be published, and even though these results are reported, they are more frequently published in native languages.51 As this meta-analysis only enrolled fully published studies in Medline or EMBASE, conference abstracts and studies with no sufficient data were excluded. Moreover, the study by Sperti et al41 was conducted in the 20th century and involved only 113 patients. Because of the defective design and small sample size, it showed a result quite different from others. It decreased the heterogeneity in some degree by deleting the study.
To the best of our knowledge, this is the first study to discuss the impact of diabetes on the prognosis of PC in early or late stage. In this study, patients with surgical or nonsurgical cancer are all recruited. Two studies with four prospective cohorts published recently enhance the strength of the evidence. Moreover, in view of the bidirectional relationship between diabetes and PC, long-standing and recent-onset diabetes are analyzed separately with the help of pertinent studies. We also admitted some other limitations existing in this meta-analysis. First, with only two prospective studies (four cohorts), limitations are inherent to the biases brought by the retrospective studies included. Second, the studies in our review were done mainly in clinical centers from USA and population of white people. The differences in outcomes observed might reflect geographic differences among populations. Third, diabetes is often accompanied by cigarette smoking, obesity, and other unhealthy lifestyle habits, which were related to prognosis. The relevant confounding factors should be discussed. A study by Yuan et al14 verified that sex, smoking status, and body mass index did not affect the association between diabetes status and PC. But the data of Toriola et al31 suggested that the correlation may be more evident in the groups of male. Although the data adopted in our analysis excluded the interference of other multivariance, such as sex, smoking status, and body mass index, with the limit of stratified analysis, we cannot make a proper judgment.
Conclusion
In conclusion, this meta-analysis indicated that diabetes was associated with worse survival. DM may be a predictive factor for survival in patients with PC. Surveillance of diabetes status and antidiabetes medication administration after the diagnosis of PC is of clinical importance. Meanwhile, more prospective and large sample studies are still needed to confirm these results.
Acknowledgments
This work was supported by the National Science Foundation of China (81072011, 81272748, and 81472240), the Foundation of Science and Technology Commission of Shanghai Municipality (12XD1403400), and the Foundation of Shanghai Municipal Health Bureau (XBR2011035).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Zhang D-X, Dai Y-D, Yuan S-X, Tao L. Prognostic factors in patients with pancreatic cancer. Exp Ther Med. 2012;3(3):423–432. doi: 10.3892/etm.2011.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D. Diabetes and pancreatic cancer. Mol Carcinog. 2012;51(1):64–74. doi: 10.1002/mc.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAuliffe JC, Christein JD. Type 2 diabetes mellitus and pancreatic cancer. Surg Clin North Am. 2013;93(3):619–627. doi: 10.1016/j.suc.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an update. Dig Dis. 2010;28(4–5):645–656. doi: 10.1159/000320068. [DOI] [PubMed] [Google Scholar]
- 7.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011;47(13):1928–1937. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Batabyal P, Vander Hoorn S, Christophi C, Nikfarjam M. Association of diabetes mellitus and pancreatic adenocarcinoma: a meta-analysis of 88 studies. Ann Surg Oncol. 2014;21(7):2453–2462. doi: 10.1245/s10434-014-3625-6. [DOI] [PubMed] [Google Scholar]
- 9.Fedeli U, Zoppini G, Gennaro N, Saugo M. Diabetes and cancer mortality: a multifaceted association. Diabetes Res Clin Pract. 2014;106(3):E86–E89. doi: 10.1016/j.diabres.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Raghavan SR, Ballehaninna UK, Chamberlain RS. The impact of perioperative blood glucose levels on pancreatic cancer prognosis and surgical outcomes an evidence-based review. Pancreas. 2013;42(8):1210–1217. doi: 10.1097/MPA.0b013e3182a6db8e. [DOI] [PubMed] [Google Scholar]
- 11.Nakata B, Ishikawa T, Amano R, Kimura K, Hirakawa K. Impact of preoperative diabetes mellitus on clinical outcome after pancreatectomy. Int J Surg. 2013;11(9):757–761. doi: 10.1016/j.ijsu.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Beg MS, Dwivedi AK, Ahmad SA, Ali S, Olowokure O. Impact of diabetes mellitus on the outcome of pancreatic cancer. PLoS One. 2014;9(5):e98511. doi: 10.1371/journal.pone.0098511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter U, Kohlert T, Rahbari NN, Weitz J, Welsch T. Impact of preoperative diabetes on long-term survival after curative resection of pancreatic adenocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol. 2014;21(4):1082–1089. doi: 10.1245/s10434-013-3415-6. [DOI] [PubMed] [Google Scholar]
- 14.Yuan C, Rubinson DA, Qian ZR, et al. Survival among patients with pancreatic cancer and long-standing or recent-onset diabetes mellitus. J Clin Oncol. 2015;33(1):29–35. doi: 10.1200/JCO.2014.57.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang A, Narayan V, Yang Y-X. Type 2 diabetes mellitus and survival in pancreatic adenocarcinoma. Cancer. 2013;119(2):404–410. doi: 10.1002/cncr.27731. [DOI] [PubMed] [Google Scholar]
- 16.Ben Q, Xu M, Jiang Y, et al. Clinical profiles and long-term outcomes of patients with pancreatic ductal adenocarcinoma and diabetes mellitus. Diabetes Metab Res Rev. 2012;28(2):169–176. doi: 10.1002/dmrr.1284. [DOI] [PubMed] [Google Scholar]
- 17.Chu CK, Mazo AE, Goodman M, et al. Preoperative diabetes mellitus and long-term survival after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2010;17(2):502–513. doi: 10.1245/s10434-009-0789-6. [DOI] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Statistics in Medicine. 2001;20(4):641–654. doi: 10.1002/sim.698. [DOI] [PubMed] [Google Scholar]
- 23.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.He XY, Li JF, Yao WY, Yuan YZ. Resolution of new-onset diabetes after radical pancreatic resection predicts long-term survival in patients with pancreatic ductal cell adenocarcinoma. Ann Surg Oncol. 2013;20(12):3809–3816. doi: 10.1245/s10434-013-3095-2. [DOI] [PubMed] [Google Scholar]
- 26.Cannon RM, LeGrand R, Chagpar RB, et al. Multi-institutional analysis of pancreatic adenocarcinoma demonstrating the effect of diabetes status on survival after resection. HPB. 2012;14(4):228–235. doi: 10.1111/j.1477-2574.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McWilliams RR, Matsumoto ME, Burch PA, et al. Obesity adversely affects survival in pancreatic cancer patients. Cancer. 2010;116(21):5054–5062. doi: 10.1002/cncr.25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng C-H. Diabetes, insulin use, smoking, and pancreatic cancer mortality in Taiwan. Acta Diabetol. 2013;50(6):879–886. doi: 10.1007/s00592-013-0471-0. [DOI] [PubMed] [Google Scholar]
- 29.Calle EE, Murphy TK, Rodriguez C, Thun MJ, Heath CW. Diabetes mellitus and pancreatic cancer mortality in a prospective cohort of United States adults. Cancer Causes Control. 1998;9(4):403–410. doi: 10.1023/a:1008819701485. [DOI] [PubMed] [Google Scholar]
- 30.Park SM, Lim MK, Shin SA, Yun YH. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol. 2006;24(31):5017–5024. doi: 10.1200/JCO.2006.07.0243. [DOI] [PubMed] [Google Scholar]
- 31.Toriola AT, Stolzenberg-Solomon R, Dalidowitz L, Linehan D, Colditz G. Diabetes and pancreatic cancer survival: a prospective cohort-based study. Br J Cancer. 2014;111(1):181–185. doi: 10.1038/bjc.2014.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelucchi C, Galeone C, Polesel J, et al. Smoking and body mass index and survival in pancreatic cancer patients. Pancreas. 2014;43(1):47–52. doi: 10.1097/MPA.0b013e3182a7c74b. [DOI] [PubMed] [Google Scholar]
- 33.Hart PA, Law RJ, Frank RD, et al. Impact of diabetes mellitus on clinical outcomes in patients undergoing surgical resection for pancreatic cancer: a retrospective, cohort study. Am J Gastroenterol. 2014;109(9):1484–1492. doi: 10.1038/ajg.2014.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong Q, Jing W, Yang X, Liu Y, Qu X. Type 2 diabetes mellitus is a prognostic predictor in patients with resectable pancreatic ductal adenocarcinoma. Chin J Clin Oncol. 2014;41(15):979–983. doi: 10.1016/j.clinre.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Sahin IH, Shama MA, Tanaka M, et al. Association of diabetes and perineural invasion in pancreatic cancer. Cancer Med. 2012;1(3):357–362. doi: 10.1002/cam4.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbas AS, Turley RS, Ceppa EP, et al. Comparison of outcomes and the use of multimodality therapy in young and elderly people undergoing surgical resection of pancreatic cancer. J Am Geriatr Soc. 2012;60(2):344–350. doi: 10.1111/j.1532-5415.2011.03785.x. [DOI] [PubMed] [Google Scholar]
- 37.Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254(2):311–319. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 38.Dandona M, Linehan D, Hawkins W, Strasberg S, Gao F, Wang-Gillam A. Influence of obesity and other risk factors on survival outcomes in patients undergoing pancreaticoduodenectomy for pancreatic cancer. Pancreas. 2011;40(6):931–937. doi: 10.1097/MPA.0b013e318215a9b1. [DOI] [PubMed] [Google Scholar]
- 39.Olson SH, Chou JF, Ludwig E, et al. Allergies, obesity, other risk factors and survival from pancreatic cancer. Int J Cancer. 2010;127(10):2412–2419. doi: 10.1002/ijc.25240. [DOI] [PubMed] [Google Scholar]
- 40.Dehayem YM, Phelip JM, Kengne AP, et al. Impact of diabetes mellitus on clinical presentation and prognosis of pancreatic cancer. Ann Endocrinol. 2011;72(1):24–29. doi: 10.1016/j.ando.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Sperti C, Pasquali C, Piccoli A, Choukem SP, Benhamou PY, Halimi S. Survival after resection for ductal adenocarcinoma of the pancreas. Br J Surg. 1996;83(5):625–631. doi: 10.1002/bjs.1800830512. [DOI] [PubMed] [Google Scholar]
- 42.Gong Z, Holly EA, Bracci PM. Obesity and survival in population-based patients with pancreatic cancer in the San Francisco Bay Area. Cancer Causes Control. 2012;23(12):1929–1937. doi: 10.1007/s10552-012-0070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van de Poll-Franse LV, Houterman S, Janssen-Heijnen MLG, Dercksen MW, Coebergh JWW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. 2007;120(9):1986–1992. doi: 10.1002/ijc.22532. [DOI] [PubMed] [Google Scholar]
- 44.Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ. 2003;327(7408):195–198. doi: 10.1136/bmj.327.7408.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10(7):423–433. doi: 10.1038/nrgastro.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersen DK, Andren-Sandberg A, Duell EJ, et al. Pancreatitis-diabetes-pancreatic cancer: summary of an NIDDK-NCI workshop. Pancreas. 2013;42(8):1227–1237. doi: 10.1097/MPA.0b013e3182a9ad9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon D, Balkau B. Diabetes mellitus, hyperglycaemia and cancer. Diabetes Metab. 2010;36(3):182–191. doi: 10.1016/j.diabet.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Mardilovich K, Pankratz SL, Shaw LM. Expression and function of the insulin receptor substrate proteins in cancer. Cell Commun Signal. 2009;7:14. doi: 10.1186/1478-811X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66(21):10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 50.Algire C, Zakikhani M, Blouin MJ, Shuai JH, Pollak M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer. 2008;15(3):833–839. doi: 10.1677/ERC-08-0038. [DOI] [PubMed] [Google Scholar]
- 51.Egger M, Zellweger-Zahner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet. 1997;350(9074):326–329. doi: 10.1016/S0140-6736(97)02419-7. [DOI] [PubMed] [Google Scholar]