Abstract
Background
Previous studies have relied on international spirometry criteria to diagnose COPD in patients with lung cancer without considering the effect lung cancer might have on spirometric results. The aim of this study was to examine the prevalence of COPD and emphysema at the time of primary lung cancer diagnosis and to examine factors associated with survival.
Materials and methods
Medical records, pulmonary function tests, and computed tomography scans were used to determine the presence of COPD and emphysema in patients diagnosed with primary lung cancer at the University Hospital of North Norway in 2008–2010.
Results
Among the 174 lung cancer patients, 69% had COPD or emphysema (39% with COPD, 59% with emphysema; male:female ratio 101:73). Neither COPD nor emphysema were significantly associated with lung cancer mortality, whereas patients with non-small-cell lung cancer other than adenocarcinoma and squamous cell carcinoma had a risk of lung cancer mortality that was more than four times higher than that of patients with small-cell lung cancer (hazard ratio [HR] 4.19, 95% confidence interval [CI] 1.56–11.25). Females had a lower risk of lung cancer mortality than males (HR 0.63, 95% CI 0.42–0.94), and patients aged ≥75 years had a risk that was twice that of patients aged <75 years (HR 2.48, 95% CI 1.59–3.87). Low partial arterial oxygen pressure (4.0–8.4 kPa) increased the risk of lung cancer mortality (HR 2.26, 95% CI 1.29–3.96). So did low partial arterial carbon dioxide pressure (3.0–4.9 kPa) among stage IV lung cancer patients (HR 2.23, 95% CI 1.29–3.85). Several patients with respiratory failure had previously been diagnosed with COPD.
Conclusion
The observed prevalence of COPD was lower than that in previous studies. Neither COPD nor emphysema were significantly associated with lung cancer mortality.
Keywords: lung cancer, COPD, emphysema, computed tomography
Introduction
Lung cancer is the leading cause of cancer death worldwide, accounting for more than a million deaths annually.1,2 A Canadian study using life-table methodology found that 172 in 1,000 males and 116 in 1,000 females who currently smoke will eventually develop lung cancer, in addition to 13 in 1,000 male and 14 in 1,000 female nonsmokers.3 Another study reported that 16% of male and 10% of female cigarette smokers die of lung cancer.4 Overall 5-year lung cancer survival is poor, at approximately 15%.5,6
COPD is characterized by airflow obstruction in the lungs and symptoms related to decreased expiratory volume.7 One study reported that about 26% of heavy smokers develop clinically significant COPD.8 Another study reported that COPD affects 15%–20% of smokers and 50%–80% of lung cancer patients with a substantial smoking history.9 Indeed, 40%–70% of lung cancer patients also have COPD, and the risk of COPD is sixfold higher in lung cancer patients than in matched smokers, leading to the conclusion that COPD and lung cancer must share risk factors other than tobacco exposure.10–14 Such factors as airflow limitation, smoking, and genotype can predispose a person to COPD and lung cancer,10 and smokers have a host susceptibility to both these diseases.4,8,9,15 However, the association between COPD and lung cancer is largely explained by smoking habits and the timing of COPD diagnosis. Powell et al reported that 23% of lung cancer cases had a prior diagnosis of COPD, compared with only 6% of controls.16
Pulmonary emphysema is defined morphologically as the enlargement of air spaces distal to the terminal bronchiole, due to dilatation or destruction of alveolar walls.7,17 Computed tomography (CT)-detected emphysema has been shown to be associated with an increased risk of lung cancer,15,18–20 and even nonsmokers with emphysema have an elevated risk of lung cancer.12,13
Inflammatory processes may also play a central role in carcinogenesis,11 and COPD and emphysema are major causes of inflammation in lung tissue.12 However, precise details of the relationship between COPD, emphysema, and lung cancer remain uncertain. Whether airflow obstruction predisposes to lung cancer or whether both arise from a common factor is unclear and beyond the scope of this study.6
Previous studies either relied on international spirometry criteria to diagnose COPD in patients with lung cancer without considering the effect lung cancer might have on the spirometric results or used variable spirometric criteria for pulmonary function testing (PFT) that did not conform to revised standard guidelines. The aim of this study was to examine the prevalence of COPD and emphysema at the time of primary lung cancer diagnosis using a combination of medical records, PFT (including spirometry and blood gases), and CT scans of the lungs. We also examined factors associated with lung cancer mortality.
Materials and methods
Data
We retrospectively reviewed the medical records of the 174 patients (101 males and 73 females between 31 and 90 years of age) diagnosed with primary lung cancer from 2008 to 2010 at the University Hospital of North Norway. Information on age, sex, smoking status, body mass index (BMI), lung cancer diagnosis, histologic type, tumor size, and cancer stage was taken from medical records, as were PFT results like spirometry (forced vital capacity [FVC] and forced expiratory volume in 1 second [FEV1]), arterial blood gases (partial arterial oxygen pressure [PaO2], PaCO2, and carboxyhemoglobin [COHb] in arterial blood plasma), and CT scans of the lungs. Smoking status was recorded as only two categories: nonsmoker and smoker/ex-smoker. An anonymized version of the data set is available in Table S1. The study was approved by the Regional Committee of Research Ethics of North Norway. Patient consent was not obtained as this is a retrospective study.
Lung cancer
Histologic lung cancer was categorized as small-cell lung cancer (SCLC) and non-SCLC (NSCLC). NSCLC was then further categorized as adenocarcinoma, squamous cell carcinoma (SCC), or other, which consisted primarily of large-cell carcinoma and undifferentiated carcinoma of the lungs. Patients with bronchial carcinoids were excluded. Cancer stage was recorded using the TNM classification and the staging I–IV.21–24
Assessment of COPD
Spirometry values at lung cancer diagnosis (Jaeger Master-Screen PFT; BD, Franklin Lakes, NJ, USA) were recorded using European reference values. FVC and FEV1 were recorded in medical records in liters and percentage predicted values, as well as FEV1/FVC%. The GOLD (Global initiative for chronic Obstructive Lung Disease) criteria were used to assign a grade of clinical severity to COPD based on FEV1 and FEV1/FVC%:7 patients with an FEV1/FVC ratio ≤70% were classified as having COPD in all grades; grade 1 was defined as having an FEV1 ≥80%; grade 2 as 50% ≤ FEV1 < 80%; grade 3 as 30% ≤ FEV1 < 50%; and grade 4 as FEV1 <30%.
Patients were classified as having COPD at lung cancer diagnosis if they had a previous spirometric diagnosis of COPD in their medical records (known COPD), or if they fulfilled the spirometric criteria, including the authentic flow-volume curve seen in obstructive lung disease (undiagnosed COPD). Patients with bronchial asthma and a normal PFT shortly before lung cancer diagnosis were classified as not having COPD (no COPD), as were patients with an obvious explanation for their spirometric findings, such as a central tumor or atelectasis. Patients with missing data were categorized as “missing – spirometry”.
Assessment of emphysema
The presence of emphysema at lung cancer diagnosis was determined based on information from CT scans (Sensation 16; Siemens AG, Munich, Germany) contained in medical records. All CT scans were reviewed at diagnosis by one radiologist and two pulmonologists, using previously published methods. When emphysema was detected visually in the CT scan (ie, with a score ≥1), the patient was classified as having emphysema.15,25,26
Statistical analysis
Survival times were recorded in days from diagnosis of lung cancer, and were analyzed using the Cox proportional hazard model. Each patient was followed from the date of lung cancer diagnosis until death from primary lung cancer or the end of the study period (October 1, 2014), whichever came first. The following variables were also recorded at diagnosis and considered time-independent covariates in the Cox model: age, sex, smoking status, BMI, histologic type, tumor size, cancer stage, COPD, PaO2, PaCO2, COHb, and emphysema.
Preliminary analyses were performed on subsets of the data, in order to identify possible interactions between covariates. The only significant interaction was between cancer stage and PaCO2. As part of the preliminary analyses, we categorized age into four groups: 31–64, 65–69, 70–74, and 75–90 years. The three youngest age-groups were found to have an equal effect on lung cancer mortality, and age was thus reduced to a dichotomous variable (31–74 and 75–90 years). PaO2 was divided into three categories (4–8.4, 8.5–9.9, and ≥10 kPa), as was PaCO2 (3–4.9, 5–5.9, and 6–10 kPa).
It is common to include cancer stage as a categorical covariate in a Cox proportional hazard regression model, but this must be done with care, because a mixture of individuals with cancer stages ranging from I to IV may give rise to nonproportionality in hazard ratios (HRs). This phenomenon is thoroughly discussed in the literature.27 We tested proportionality, and did not find any significant violations of the proportional hazard assumption. Another possible feature of the covariate cancer stage is heterogeneity within each stage, where some individuals have a more seriously developed cancer than others within the same stage. Such heterogeneity can be modeled as a frailty covariate.27 We considered this in the preliminary analyses, and found only minor changes in the coefficient estimates compared to a model without frailty.
In addition to the traditional Cox model, we modeled the data as a randomized Wiener process27 to identify whether a covariate was of one of two types: 1) a measure of how far the cancer had advanced; or 2) a causal influence on the development of the disease.
Results are reported as HRs with 95% confidence intervals (CIs), and P-values are given. Calculations were performed in the statistical computing language R28 using the R package Survival.29 A test for violation of the proportional hazard assumption in the Cox model was performed with the R-routine cox.zph29 in the Survival package. For the Wiener process, we used the R package “invGauss”.30
Results
Of the 174 patients, 120 (69%) had COPD and/or emphysema (67 [38.5%] with COPD and 102 [58.6%] with emphysema). Of the 67 patients with COPD, only 18 (26.9%) did not have coexisting emphysema. The male:female ratios were 101 (58%):73 (42%) for all patients, 40 (59.7%):27 (40.3%) for patients with COPD, and 65 (63.7%):37 (36.3%) for patients with emphysema (Table 1). Of all the patients, 138 (80%) had stage III–IV lung cancer at the time of diagnosis. Only eight (23%) of the 35 patients with stage I–II lung cancer had neither COPD nor emphysema (Table 1). Of the 18 patients with COPD without concomitant emphysema, all but two had stage III–IV lung cancer (data not shown). Only five patients were nonsmokers, and two had missing data on smoking status (data not shown).
Table 1.
Baseline characteristics, means, and standard deviation of lung cancer patients diagnosed at the University Hospital of North Norway in 2008–2010 (n=174) according to presence of COPD and emphysema
| Patient group | All | COPD | Emphysema without COPD | Neither COPD nor emphysema | ||||
|---|---|---|---|---|---|---|---|---|
| n | 174 | 67 | 53 | 54 | ||||
| % male | 58 | 59.7 | 66 | 48.1 | ||||
| n, cancer stage I + II | 35 | 16 | 11 | 8 | ||||
| n, cancer stage III + IV | 138 | 51 | 42 | 45 | ||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years), n=174 | 67.92 | 10.17 | 70.73 | 8.52 | 67.58 | 9.47 | 64.76 | 11.80 |
| BMI (kg/m2), n=155 | 24.97 | 5.01 | 25.31 | 5.23 | 23.35 | 4.47 | 25.93 | 4.92 |
| FVC (L), n=144 | 2.62 | 0.84 | 2.40 | 0.75 | 2.74 | 0.94 | 2.81 | 0.81 |
| FVC (%) predicted | 78.70 | 20.21 | 73.38 | 17.29 | 80.56 | 22.52 | 84.43 | 20.40 |
| FEV1 (L), n=144 | 1.83 | 0.67 | 1.48 | 0.50 | 1.99 | 0.65 | 2.18 | 0.67 |
| FEV1 (%) predicted | 69.05 | 21.08 | 57.50 | 16.75 | 74.59 | 20.87 | 80.41 | 18.77 |
| FEV1/FVC (%), n=144 | 70.17 | 12.68 | 62.46 | 12.05 | 73.85 | 11.15 | 77.61 | 8.29 |
| PaO2 (kPa, air), n=151 | 9.93 | 1.58 | 9.56 | 1.34 | 9.70 | 1.56 | 10.65 | 1.68 |
| PaCO2 (kPa, air), n=151 | 5.10 | 0.68 | 5.22 | 0.57 | 5.14 | 0.86 | 4.89 | 0.62 |
| COHb (%), n=144 | 1.49 | 1.12 | 1.40 | 0.82 | 1.68 | 1.40 | 1.44 | 1.21 |
| Tumor size (cm) | ||||||||
| All patients, n=167 | 5.13 | 2.46 | 5.32 | 2.55 | 5.27 | 2.69 | 4.73 | 2.04 |
| Females, n=68 | 4.73 | 2.26 | 4.84 | 2.16 | 4.64 | 2.68 | 4.67 | 2.12 |
Abbreviations: BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; PaO2, partial arterial oxygen pressure; COHb, carboxyhemoglobin (in arterial blood plasma); PaCO2, partial arterial carbon dioxide pressure.
Six patients had missing histologic type, and were excluded from the analyses. Of the remaining 168 patients, 27% were diagnosed with SCLC, 37% with adenocarcinoma, 32% with SCC, and 4% with other – NSCLC (Figure 1). We further excluded 23 patients with missing data on at least one of the significant factors for lung cancer mortality: one patient with missing cancer stage, and 22 patients with missing PaO2/PaCO2 values. Finally, we excluded one male patient with missing PFT who had a strong influence on the Cox-model estimates. Therefore, the final analyses included 144 patients.
Figure 1.
Prevalence of COPD and emphysema by histologic type of primary lung cancer (n=168).
Abbreviations: NSCLC, non-small-cell lung cancer; SCC, squamous cell carcinoma; SCLC, small-cell lung cancer.
Smoking status, BMI, tumor size, COHb, and emphysema were not significant covariates in the Cox model. The risk of lung cancer mortality was not significantly different for known-COPD/undiagnosed-COPD patients compared to no-COPD patients, and missing-spirometry patients had a significantly higher risk of lung cancer mortality (HR 6.33, 95% CI 2.69–14.93). The randomized Wiener process identified missing-spirometry patients as a group with more advanced cancer. In a separate analysis, we also categorized COPD into the four GOLD grades,7 but found no significant differences in survival between these grades of COPD (data not shown).
Among the histologic types, other – NSCLC patients had a risk of lung cancer mortality that was four times higher than that of SCLC patients (HR 4.19, 95% CI 1.56–11.25). The Wiener process identified other – NSCLC as having poorer survival, meaning a more advanced cancer. The risk of lung cancer mortality among patients with adenocarcinoma and SCC was not significantly different from that of SCLC patients (Table 2).
Table 2.
Impact of evaluated variables on survival of patients with primary lung cancer according to Cox regression analysis (n=144)
| Variables | Category | HR | 95% CI | P-value |
|---|---|---|---|---|
| Age (reference <75 years) | ≥75 years | 2.48 | 1.59–3.87 | 0.0001 |
| Sex | Female | 0.63 | 0.42–0.94 | 0.0239 |
| COPD (reference no – COPD) | Known – COPD | 1.02 | 0.65–1.60 | 0.9388 |
| Undiagnosed – COPD | 1.02 | 0.47–2.23 | 0.9609 | |
| Missing – spirometry | 6.33 | 2.69–14.93 | <0.0001 | |
| Histologic type (reference SCLC) | Adenocarcinoma – NSCLC | 1.30 | 0.78–2.15 | 0.3157 |
| SCC – NSCLC | 0.99 | 0.57–1.71 | 0.9679 | |
| Other – NSCLC | 4.19 | 1.56–11.25 | 0.0045 | |
| PaO2 (kPa, air), reference ≥10 | 4.0–8.4 | 2.26 | 1.29–3.96 | 0.0045 |
| 8.5–9.9 | 1.44 | 0.91–2.28 | 0.1172 | |
| PaCO2 (kPa, air), reference 5.0–5.9 | ||||
| Stage I | 3–4.9 | 1.69 | 0.31–9.34 | 0.5456 |
| 6–10 | 11.24 | 2.64–47.85 | 0.0011 | |
| Stage II | 3–4.9 | 1.70 | 0.47–6.21 | 0.4226 |
| 6–10 | 19.97 | 1.92–208.06 | 0.0123 | |
| Stage III | 3–4.9 | 1.58 | 0.77–3.22 | 0.2096 |
| 6–10 | 0.76 | 0.22–2.64 | 0.6644 | |
| Stage IV | 3–4.9 | 2.23 | 1.29–3.85 | 0.0040 |
| 6–10 | 0.28 | 0.07–1.06 | 0.0611 |
Note: Patients with missing data were categorized as “missing – spirometry”.
Abbreviations: HR, hazard ratio; CI, confidence interval; NSCLC, non-small-cell lung cancer; SCC, squamous cell carcinoma; PaO2, partial arterial oxygen pressure; SCLC, small-cell lung cancer; PaCO2, partial arterial carbon dioxide pressure.
We found a weak but significant effect of sex on lung cancer mortality, with females showing a lower risk than males (HR 0.63, 95% CI 0.42–0.94). In contrast, advanced age was found to increase the risk of lung cancer mortality (Table 2). Further results from the Cox model showed that patients with a low PaO2 (4.0–8.4), compared to those with a high PaO2 (≥10), had an HR of 2.26 (95% CI 1.29–3.96). Among the 28 patients with low PaO2, 16 were known-COPD patients, while only five were no-COPD patients (data not shown). Among patients with stage IV lung cancer, having a low PaCO2 (3.0–4.9) increased the risk of lung cancer mortality (HR 2.23, 95% CI 1.29–3.85). Among the 33 patients with stage IV lung cancer and a low PaCO2, there were 19 no-COPD patients and seven known-COPD patients (data not shown). The Wiener process showed that the effect of having stage IV lung cancer and a low PaCO2 was due to the presence of advanced cancer at time of diagnosis.
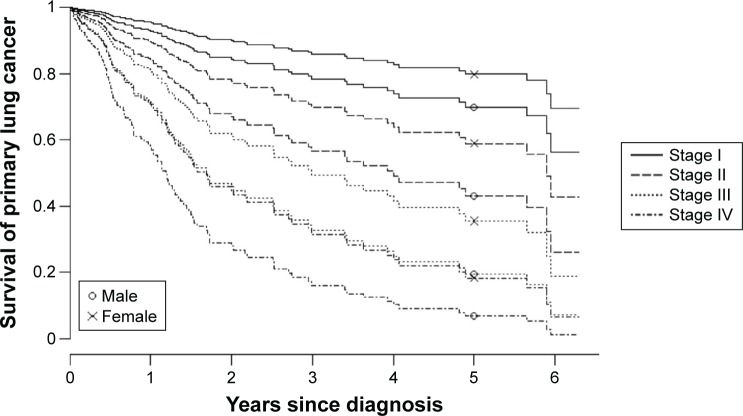
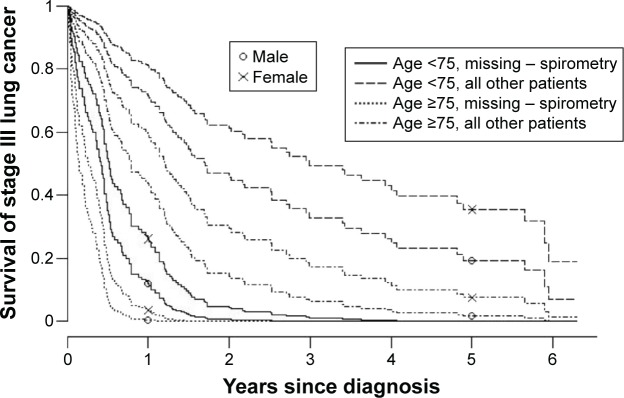
Among patients below 75 years of age, 5-year survival with stage I lung cancer was 80% in females and 70% in males. Two-year survival with stage III lung cancer was 60% in females and 45% in males (Figure 2). Patients (except those with other – NSCLC) with missing spirometry showed the poorest 2-year survival with stage III lung cancer, at 6% or less, whereas 2-year survival among all other patients was 60% and 45% for young females and males, respectively, and 28% and 14% in females and males aged 75 years and older (Figure 3).
Figure 2.
Survival curves for primary lung cancer (except other – NSCLC) by stage among males and females younger than 75 years.
Note: PaO2 fixed at ≥10, PaCO2 at 5.0–5.9 kPa.
Abbreviations: NSCLC, non-small-cell lung cancer; PaO2, partial arterial oxygen pressure; PaCO2, partial arterial carbon dioxide pressure.
Figure 3.
Survival curve for primary lung cancer (except other – NSCLC) stage III by age, sex, and spirometry.
Notes: PaO2 fixed at ≥10, PaCO2 at 5.0–5.9 kPa. Patients with missing data were categorized as “missing – spirometry”.
Abbreviations: NSCLC, non-small-cell lung cancer; PaO2, partial arterial oxygen pressure; PaCO2, partial arterial carbon dioxide pressure.
Discussion
About 80% of patients with primary lung cancer are diagnosed with stage III–IV cancer,1,2,5 which is consistent with our findings. However, whereas previous studies used history of sputum production and spirometry as diagnostic criteria for COPD,7,17,18,31 we chose to classify patients as having COPD if a diagnosis was present in their medical records, or if they had spirometric results, arterial blood-gas results, and visual detection of emphysema in their CT scan that indicated COPD. Patients with a central tumor with or without coexisting atelectasis as the only known cause of decreased FEV1 were categorized as not having COPD, thus reducing the possibility of overdiagnosis of COPD. We found a prevalence of COPD of 39%, which is one of the lowest published so far.12,15,19,32 The prevalence of emphysema that we observed was similar to that in previous studies.15,19 In our study, all but two patients with COPD and no concomitant emphysema had stage III–IV lung cancer. However, it is important to note that emphysema may be less visible on the CT scans of patients with advanced lung cancer. Moreover, the PFT of patients with lung cancer may be lower, which can mask COPD or lead to misdiagnosis.15
This retrospective, descriptive study of patients with primary lung cancer included a 5-year survival study. We found that lung cancer patients with missing spirometry data had the poorest survival. Indeed, many of these patients had stage III–IV lung cancer and were too ill to perform PFT at time of diagnosis. However, we did not find any significant difference in survival between patients with and without COPD. The majority of our lung cancer patients with both COPD and emphysema had moderate COPD, and due to the short survival time of lung cancer patients with advanced-stage disease, neither COPD nor emphysema had a significant impact on survival in this study.
Other-NSCLC patients had poorer survival than those with adenocarcinoma, SCC, and SCLC, which is in agreement with previous knowledge, as the other histologic types of NSCLC have increased malignancy and a lower treatment effect.1,2,5 Survival was age-independent until 75 years of age, and we found weak significantly better survival in females compared to males. Lung cancer accounts for 25% of all cancer deaths among females, and this percentage continues to increase, possibly due to a lower decrease in smoking frequency among females.31 In contrast, lung cancer mortality is decreasing in males, although previous studies have shown that males generally smoke more pack-years than females.31
Respiratory failure affects the prognosis of COPD survival severely. The information on arterial blood gases in our study showed that lung cancer patients with severe hypoxemia had significantly poorer survival. Moreover, a majority of these patients had COPD. Hypercapnia predicted significantly poorer survival in patients with stage I lung cancer, but there were few patients in this group. A low PaCO2 level indicates hyperventilation provoked by the sensation of dyspnea caused by the severity of the lung cancer itself, and predicted poorer survival in patients with stage IV lung cancer in our study.
This study represents a time period immediately before 2011, when the Lean method was implemented as a clinical pathway facilitator in patients with lung cancer. After the introduction of the Lean method, the workup time for lung cancer patients decreased from a mean of 64 days to 16 days, and the median time from diagnosis to surgery went from 26.5 days to 15 days,33 both of which could lead to increased survival rates.
A previous study showed that there is an association between COPD and lung cancer, and that the combination of these two diseases leads to a worse outcome. It is reported that there are fewer thoracic surgeries performed in patients with COPD than in those without, and that PFT, cancer stage, and age at diagnosis are associated with the decision to go forward with surgery. Older lung cancer patients with COPD can be treated with chemotherapy and/or radiation therapy, but a systematic, comprehensive assessment of COPD at time of bronchoscopy allows us to implement the optimum management for lung cancer patients.34,35 For example, patients with primary, stage I lung cancer who are ineligible for surgery due to COPD are now treated by stereotactic radiation therapy with curative intention, and thus achieve improved survival.36 EGFR-targeted therapy may also increase survival in some lung cancer patients with COPD.37
Coexistence of lung cancer and emphysema can be assessed by a CT scan. Indeed, low-dose CT scans of the chest as screening for lung cancer have reduced the number of lung cancer deaths significantly, but costs have to be reduced.25,37 Preventive interventions should focus on smoking cessation and screening for early diagnosis.4,37,38 Future screening programs should focus on those 60–74 years of age, smokers, identifying patients with COPD, and early detection of lung cancer based on low-dose CT scans of the chest.37
Conclusion
The observed prevalence of COPD was lower than that in previous studies. Neither COPD nor emphysema were significantly associated with lung cancer mortality. However, at an early lung cancer stage, diagnosis and grading of COPD may have the potential to improve treatment decision and prognosis.
Supplementary material
Table S1.
Survival of primary lung cancer of all 174 patients diagnosed at University Hospital of North Norway 2008–2010
| ID | Sex | Age-group, years | COPD diagnosis | Emph | PaO2, range | PaCO2, range | Cancer | Stage | Time | D |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | III | 377 | 1 |
| 2 | M | 30–74 | Missing – spirometry | 0 | NA | NA | SCC – NSCLC | IV | 340 | 1 |
| 3 | M | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | SCLC | IV | 157 | 1 |
| 4 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | SCLC | IV | 547 | 1 |
| 5 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | Ad-NSCLC | IV | 2,067 | 1 |
| 6 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCC – NSCLC | III | 1,243 | 1 |
| 7 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | SCC – NSCLC | III | 279 | 1 |
| 8 | M | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCLC | III | 365 | 1 |
| 9 | F | 75–91 | Known COPD | 0 | 10–15 | 5–5.9 | SCLC | III | 620 | 1 |
| 10 | M | 75–91 | Known COPD | 0 | 8.5–9.9 | 3–4.9 | SCLC | IV | 16 | 1 |
| 11 | F | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Other – NSCLC | I | 803 | 1 |
| 12 | M | 75–91 | No COPD | 1 | 4–8.4 | 5–5.9 | SCLC | IV | 77 | 1 |
| 13 | F | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | Ad-NSCLC | I | 353 | 1 |
| 14 | M | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | SCLC | IV | 188 | 1 |
| 15 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCC – NSCLC | II | 289 | 1 |
| 16 | F | 30–74 | No COPD | 1 | 4–8.4 | 6–10 | SCC – NSCLC | III | 2,175 | 1 |
| 17 | M | 30–74 | Missing – spirometry | 0 | 10–15 | 5–5.9 | SCC – NSCLC | IV | 104 | 1 |
| 18 | M | 75–91 | Missing – spirometry | 1 | 4–8.4 | 3–4.9 | NA | IV | 10 | 1 |
| 19 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Other – NSCLC | IV | 41 | 1 |
| 20 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | SCLC | NA | 155 | 1 |
| 21 | F | 30–74 | No COPD | 0 | 8.5–9.9 | 5–5.9 | Ad-NSCLC | IV | 245 | 1 |
| 22 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCC – NSCLC | IV | 116 | 1 |
| 23 | F | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 131 | 1 |
| 24 | M | 30–74 | Known COPD | 1 | 4–8.4 | 3–4.9 | Ad-NSCLC | IV | 287 | 1 |
| 25 | M | 75–91 | No COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | IV | 45 | 1 |
| 26 | F | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | III | 1,787 | 1 |
| 27 | M | 75–91 | Undiagnosed COPD | 1 | 8.5–9.9 | 5–5.9 | SCC – NSCLC | IV | 52 | 1 |
| 28 | F | 30–74 | Missing – spirometry | 1 | 4–8.4 | 5–5.9 | SCC – NSCLC | IV | 54 | 1 |
| 29 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | NA | IV | 162 | 1 |
| 30 | M | 75–91 | Known COPD | 1 | 4–8.4 | 6–10 | SCC – NSCLC | IV | 319 | 1 |
| 31 | F | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 561 | 1 |
| 32 | M | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCLC | IV | 1,076 | 1 |
| 33 | M | 30–74 | Missing – spirometry | 1 | NA | NA | SCC – NSCLC | III | 91 | 1 |
| 34 | M | 75–91 | Known COPD | 0 | 10–15 | 5–5.9 | SCLC | III | 27 | 1 |
| 35 | M | 75–91 | No COPD | 0 | 10–15 | 3–4.9 | SCLC | IV | 7 | 1 |
| 36 | F | 30–74 | No COPD | 0 | 4–8.4 | 5–5.9 | Ad-NSCLC | IV | 76 | 1 |
| 37 | F | 75–91 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | II | 192 | 1 |
| 38 | F | 75–91 | No COPD | 1 | 10–15 | 3–4.9 | SCC – NSCLC | II | 370 | 1 |
| 39 | F | 30–74 | No COPD | 1 | 8.5–9.9 | 6–10 | Ad-NSCLC | I | 377 | 1 |
| 40 | M | 75–91 | Missing – spirometry | 0 | 10–15 | 5–5.9 | SCC – NSCLC | IV | 36 | 1 |
| 41 | F | 30–74 | Known COPD | 0 | 10–15 | 5–5.9 | Other – NSCLC | III | 33 | 1 |
| 42 | M | 30–74 | Known COPD | 0 | 4–8.4 | 5–5.9 | SCLC | III | 1,461 | 1 |
| 43 | M | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | Other – NSCLC | IV | 141 | 1 |
| 44 | F | 30–74 | Missing – spirometry | 1 | NA | NA | SCC – NSCLC | III | 28 | 1 |
| 45 | F | 30–74 | No COPD | 0 | 8.5–9.9 | 5–5.9 | Ad-NSCLC | IV | 576 | 1 |
| 46 | F | 30–74 | Missing – spirometry | 1 | 4–8.4 | 5–5.9 | SCLC | IV | 130 | 1 |
| 47 | M | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | Ad-NSCLC | III | 38 | 1 |
| 48 | F | 75–91 | No COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 44 | 1 |
| 49 | M | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | I | 2,298 | 0 |
| 50 | M | 30–74 | No COPD | 0 | 10–15 | 6–10 | Ad-NSCLC | III | 132 | 1 |
| 51 | M | 30–74 | Undiagnosed COPD | 1 | 8.5–9.9 | 5–5.9 | Ad-NSCLC | III | 83 | 1 |
| 52 | M | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | III | 204 | 1 |
| 53 | M | 30–74 | No COPD | 1 | 8.5–9.9 | 5–5.9 | SCLC | IV | 166 | 1 |
| 54 | F | 75–91 | Undiagnosed COPD | 0 | 8.5–9.9 | 6–10 | SCC – NSCLC | II | 50 | 1 |
| 55 | F | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCLC | IV | 175 | 1 |
| 56 | M | 75–91 | No COPD | 1 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | III | 28 | 1 |
| 57 | M | 30–74 | Undiagnosed COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | I | 2,155 | 1 |
| 58 | M | 30–74 | Known COPD | 0 | 10–15 | 3–4.9 | Other – NSCLC | IV | 168 | 1 |
| 59 | F | 30–74 | Missing – spirometry | 0 | 4–8.4 | 3–4.9 | Ad-NSCLC | IV | 2 | 1 |
| 60 | M | 75–91 | Known COPD | 0 | 10–15 | 5–5.9 | SCC – NSCLC | III | 415 | 1 |
| 61 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | Ad-NSCLC | IV | 917 | 1 |
| 62 | M | 30–74 | Known COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | III | 475 | 1 |
| 63 | F | 30–74 | No COPD | 0 | 8.5–9.9 | 5–5.9 | SCLC | IV | 1,485 | 1 |
| 64 | M | 30–74 | Known COPD | 1 | 4–8.4 | 3–4.9 | SCLC | III | 13 | 1 |
| 65 | M | 75–91 | Undiagnosed COPD | 1 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | III | 460 | 1 |
| 66 | F | 30–74 | No COPD | 0 | NA | NA | Ad-NSCLC | III | 698 | 1 |
| 67 | M | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | Ad-NSCLC | IV | 226 | 1 |
| 68 | M | 30–74 | No COPD | 0 | 8.5–9.9 | 5–5.9 | Ad-NSCLC | IV | 184 | 1 |
| 69 | F | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | I | 2,196 | 0 |
| 70 | F | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | Ad-NSCLC | III | 265 | 1 |
| 71 | F | 30–74 | No COPD | 1 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | II | 2,189 | 0 |
| 72 | M | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | SCC – NSCLC | III | 287 | 1 |
| 73 | M | 30–74 | Known COPD | 1 | 8.5–9.9 | 5–5.9 | SCLC | IV | 492 | 1 |
| 74 | M | 30–74 | Missing – spirometry | 0 | 8.5–9.9 | 6–10 | SCC – NSCLC | IV | 501 | 1 |
| 75 | F | 30–74 | No COPD | 0 | 8.5–9.9 | 5–5.9 | Ad-NSCLC | II | 1,758 | 1 |
| 76 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCC – NSCLC | III | 432 | 1 |
| 77 | F | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCLC | III | 2,142 | 0 |
| 78 | M | 30–74 | No COPD | 1 | NA | NA | SCC – NSCLC | II | 2,141 | 0 |
| 79 | M | 30–74 | No COPD | 1 | 8.5–9.9 | 3–4.9 | SCLC | IV | 18 | 1 |
| 80 | F | 30–74 | Missing – spirometry | 0 | 10–15 | 3–4.9 | SCLC | IV | 19 | 1 |
| 81 | M | 75–91 | Missing – spirometry | 0 | NA | NA | SCLC | IV | 35 | 1 |
| 82 | F | 30–74 | Missing – spirometry | 0 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | IV | 135 | 1 |
| 83 | F | 30–74 | Known COPD | 1 | 4–8.4 | 3–4.9 | SCLC | IV | 111 | 1 |
| 84 | F | 30–74 | Known COPD | 0 | NA | NA | SCLC | IV | 58 | 1 |
| 85 | F | 30–74 | Undiagnosed COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | III | 2,114 | 0 |
| 86 | F | 30–74 | No COPD | 0 | NA | NA | Ad-NSCLC | III | 254 | 1 |
| 87 | M | 75–91 | Missing – spirometry | 1 | NA | NA | SCLC | IV | 35 | 1 |
| 88 | F | 75–91 | No COPD | 0 | 4–8.4 | 3–4.9 | Ad-NSCLC | IV | 18 | 1 |
| 89 | M | 75–91 | Known COPD | 1 | 10–15 | 3–4.9 | SCLC | II | 417 | 1 |
| 90 | M | 30–74 | Known COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | I | 249 | 1 |
| 91 | F | 30–74 | Known COPD | 1 | 4–8.4 | 6–10 | Ad-NSCLC | III | 630 | 1 |
| 92 | F | 30–74 | Undiagnosed COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 22 | 1 |
| 93 | M | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | III | 728 | 1 |
| 94 | M | 75–91 | No COPD | 1 | 8.5–9.9 | 3–4.9 | NA | III | 171 | 1 |
| 95 | F | 30–74 | Known COPD | 0 | 8.5–9.9 | 5–5.9 | SCLC | IV | 512 | 1 |
| 96 | F | 30–74 | Missing – spirometry | 1 | NA | NA | Ad-NSCLC | IV | 455 | 1 |
| 97 | M | 30–74 | Known COPD | 0 | 10–15 | 5–5.9 | SCLC | III | 441 | 1 |
| 98 | F | 75–91 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCC – NSCLC | I | 1,329 | 1 |
| 99 | M | 75–91 | Known COPD | 1 | 4–8.4 | 3–4.9 | SCC – NSCLC | III | 163 | 1 |
| 100 | F | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | SCLC | I | 1,902 | 0 |
| 101 | F | 75–91 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 537 | 1 |
| 102 | F | 75–91 | No COPD | 0 | 8.5–9.9 | 5–5.9 | NA | IV | 345 | 1 |
| 103 | F | 75–91 | Undiagnosed COPD | 0 | 4–8.4 | 5–5.9 | Ad-NSCLC | IV | 159 | 1 |
| 104 | M | 75–91 | Missing – spirometry | 0 | NA | NA | NA | IV | 14 | 1 |
| 105 | M | 30–74 | No COPD | 0 | 8.5–9.9 | 5–5.9 | SCLC | I | 1,983 | 0 |
| 106 | M | 75–91 | Known COPD | 1 | 8.5–9.9 | 5–5.9 | SCC – NSCLC | II | 526 | 1 |
| 107 | M | 30–74 | Missing – spirometry | 1 | NA | NA | Ad-NSCLC | IV | 222 | 1 |
| 108 | F | 75–91 | Known COPD | 1 | NA | NA | Ad-NSCLC | IV | 82 | 1 |
| 109 | M | 75–91 | Known COPD | 0 | 8.5–9.9 | 5–5.9 | Ad-NSCLC | IV | 168 | 1 |
| 110 | F | 75–91 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCC – NSCLC | III | 197 | 1 |
| 111 | M | 30–74 | No COPD | 0 | NA | NA | Ad-NSCLC | IV | 1,412 | 1 |
| 112 | F | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCC – NSCLC | IV | 1,092 | 1 |
| 113 | M | 75–91 | No COPD | 1 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | II | 195 | 1 |
| 114 | M | 30–74 | Known COPD | 1 | 10–15 | 5–5.9 | SCLC | IV | 990 | 1 |
| 115 | F | 75–91 | No COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | I | 1,254 | 1 |
| 116 | M | 30–74 | No COPD | 1 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | III | 789 | 1 |
| 117 | F | 30–74 | Undiagnosed COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | II | 1,892 | 0 |
| 118 | F | 75–91 | Known COPD | 1 | 8.5–9.9 | 3–4.9 | SCLC | I | 921 | 1 |
| 119 | F | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCC – NSCLC | III | 618 | 1 |
| 120 | M | 30–74 | Undiagnosed COPD | 0 | 10–15 | 5–5.9 | SCLC | I | 1,875 | 0 |
| 121 | F | 30–74 | Known COPD | 1 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | III | 69 | 1 |
| 122 | M | 30–74 | Undiagnosed COPD | 1 | 4–8.4 | 3–4.9 | Ad-NSCLC | IV | 16 | 1 |
| 123 | F | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 181 | 1 |
| 124 | M | 75–91 | Known COPD | 1 | 10–15 | 3–4.9 | SCC – NSCLC | IV | 81 | 1 |
| 125 | M | 30–74 | Known COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | III | 195 | 1 |
| 126 | M | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | SCC – NSCLC | III | 471 | 1 |
| 127 | F | 30–74 | Known COPD | 1 | 8.5–9.9 | 6–10 | SCC – NSCLC | III | 92 | 1 |
| 128 | M | 30–74 | No COPD | 1 | 8.5–9.9 | 3–4.9 | SCLC | IV | 3 | 1 |
| 129 | M | 30–74 | No COPD | 1 | 8.5–9.9 | 5–5.9 | SCC – NSCLC | IV | 153 | 1 |
| 130 | M | 30–74 | Known COPD | 1 | 10–15 | 6–10 | SCC – NSCLC | I | 555 | 1 |
| 131 | M | 30–74 | Known COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | III | 148 | 1 |
| 132 | M | 75–91 | No COPD | 1 | NA | NA | Ad-NSCLC | I | 1,847 | 0 |
| 133 | M | 30–74 | Known COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | III | 417 | 1 |
| 134 | M | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | I | 1,840 | 0 |
| 135 | M | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | II | 1,875 | 0 |
| 136 | F | 30–74 | No COPD | 1 | NA | NA | Ad-NSCLC | III | 776 | 1 |
| 137 | M | 30–74 | No COPD | 1 | 4–8.4 | 3–4.9 | Ad-NSCLC | IV | 14 | 1 |
| 138 | F | 30–74 | Undiagnosed COPD | 1 | 10–15 | 3–4.9 | SCLC | IV | 437 | 1 |
| 139 | F | 30–74 | Known COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | III | 1,818 | 0 |
| 140 | M | 75–91 | No COPD | 1 | 8.5–9.9 | 5–5.9 | SCLC | III | 924 | 1 |
| 141 | M | 75–91 | Known COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | I | 1,797 | 0 |
| 142 | M | 30–74 | Known COPD | 0 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | IV | 214 | 1 |
| 143 | F | 30–74 | Known COPD | 1 | 8.5–9.9 | 5–5.9 | SCC – NSCLC | I | 1,744 | 0 |
| 144 | M | 75–91 | Missing – spirometry | 1 | 8.5–9.9 | 5–5.9 | SCC – NSCLC | III | 701 | 1 |
| 145 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 278 | 1 |
| 146 | M | 75–91 | Known COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 397 | 1 |
| 147 | M | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | SCLC | III | 176 | 1 |
| 148 | M | 30–74 | Missing – spirometry | 1 | 4–8.4 | 5–5.9 | Ad-NSCLC | IV | 37 | 1 |
| 149 | M | 30–74 | Known COPD | 0 | 10–15 | 5–5.9 | Ad-NSCLC | IV | 290 | 1 |
| 150 | F | 30–74 | Known COPD | 1 | 8.5–9.9 | 5–5.9 | NA | III | 249 | 1 |
| 151 | M | 30–74 | Missing – spirometry | 1 | NA | NA | Ad-NSCLC | IV | 131 | 1 |
| 152 | F | 30–74 | No COPD | 1 | NA | NA | SCLC | IV | 288 | 1 |
| 153 | M | 75–91 | Known COPD | 1 | 10–15 | 6–10 | SCC – NSCLC | I | 98 | 1 |
| 154 | F | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | Ad-NSCLC | III | 1,747 | 0 |
| 155 | M | 30–74 | Known COPD | 1 | 8.5–9.9 | 5–5.9 | SCC – NSCLC | III | 741 | 1 |
| 156 | M | 75–91 | Missing – spirometry | 1 | NA | NA | SCLC | IV | 184 | 1 |
| 157 | F | 30–74 | No COPD | 1 | 8.5–9.9 | 5–5.9 | SCLC | IV | 398 | 1 |
| 158 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCLC | IV | 449 | 1 |
| 159 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | II | 1,437 | 1 |
| 160 | F | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 166 | 1 |
| 161 | M | 30–74 | No COPD | 1 | 8.5–9.9 | 3–4.9 | Ad-NSCLC | IV | 141 | 1 |
| 162 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | SCC – NSCLC | III | 1,001 | 1 |
| 163 | F | 30–74 | Missing – spirometry | 1 | 10–15 | 5–5.9 | SCLC | IV | 181 | 1 |
| 164 | F | 75–91 | Known COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | II | 335 | 1 |
| 165 | F | 30–74 | Missing – spirometry | 0 | NA | NA | SCC – NSCLC | IV | 236 | 1 |
| 166 | F | 75–91 | Known COPD | 1 | 8.5–9.9 | 5–5.9 | Other – NSCLC | IV | 68 | 1 |
| 167 | M | 30–74 | Known COPD | 1 | 8.5–9.9 | 6–10 | Ad-NSCLC | IV | 9 | 1 |
| 168 | M | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCLC | I | 445 | 1 |
| 169 | F | 30–74 | No COPD | 0 | NA | NA | SCLC | III | 514 | 1 |
| 170 | F | 30–74 | Known COPD | 0 | NA | NA | SCLC | IV | 333 | 1 |
| 171 | F | 30–74 | Known COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | II | 243 | 1 |
| 172 | F | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCLC | IV | 244 | 1 |
| 173 | F | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | III | 632 | 1 |
| 174 | M | 30–74 | Missing – spirometry | 1 | NA | NA | Ad-NSCLC | IV | 57 | 1 |
Notes: Time represents survival time (days) from diagnosis of primary lung cancer; D represents a censoring variable (1 if dead, 0 if alive at the end of the study period); PaO2 and PaCO2 in kPa. Patients with missing data were categorized as “missing – spirometry”.
Abbreviations: Ad-NSCLC, adenocarcinoma non-small-cell lung cancer; Emph, emphysema; F, female; M, male; NA, not available; PaO2, partial arterial oxygen pressure; SCC, squamous cell carcinoma; SCLC, small-cell lung cancer; PaCO2, partial arterial carbon dioxide pressure.
Acknowledgments
The authors thank Inger Sperstad at the Clinical Research Center for preparing the data for scanning into the SPSS. We also thank Monica Linea Vold for performing part of the data registration. This work was funded by the Department of Clinical Medicine, UiT Arctic University of Norway. No writing assistance outside the authors listed was used in preparing this manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Cancer [webpage on the Internet] World Health Organization; [Accessed February 9, 2016]. [cited February 2015]. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/ [Google Scholar]
- 3.Villeneuve PJ, Mao Y. Lifetime probability of developing lung cancer, by smoking status, Canada. Can J Public Health. 1994;85:385–388. [PubMed] [Google Scholar]
- 4.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321:323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP Evidence-Based Clinical Practice Guidelines (2nd edition) Chest. 2007;132:29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 6.Midthun DE, Jett RJ. Lung tumors. In: Albert RK, Spiro SG, Jett JR, editors. Clinical Respiratory Medicine. 3rd ed. Philadelphia: Mosby Elsevier; 2008. pp. 605–631. [Google Scholar]
- 7.Global Strategy for Diagnosis Management Prevention of COPD - 2016. [webpage on the Internet] Global Initiative for Chronic Obstructive Lung Disease; [Accessed February 9, 2016]. Available from: www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. [Google Scholar]
- 8.Fletcher C, Peto R, Tinker C, Speizer FE. The Natural History of Chronic Bronchitis and Emphysema: An Eight-year Study of Early Chronic Obstructive Lung Disease in Working Men in London. New York: Oxford University Press; 1976. [Google Scholar]
- 9.El-Zein RA, Young RP, Hopkins RJ, Etzel CJ. Genetic predisposition to chronic obstructive pulmonary disease and/or lung cancer: important considerations when evaluating risk. Cancer Prev Res (Phila) 2012;5:522–527. doi: 10.1158/1940-6207.CAPR-12-0042. [DOI] [PubMed] [Google Scholar]
- 10.Takiguchi Y, Sekine I, Iwasawa S, Kurimoto R, Tatsumi K. Chronic obstructive pulmonary disease as a risk factor for lung cancer. World J Clin Oncol. 2014;5:660–666. doi: 10.5306/wjco.v5.i4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peek RM, Jr, Mohla S, DuBois RN. Inflammation in the genesis and perpetuation of cancer: summary and recommendations from a National Cancer Institute-sponsored meeting. Cancer Res. 2005;65:8583–8586. doi: 10.1158/0008-5472.CAN-05-1777. [DOI] [PubMed] [Google Scholar]
- 12.Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS One. 2011;6:e17479. doi: 10.1371/journal.pone.0017479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med. 2007;176:285–290. doi: 10.1164/rccm.200612-1792OC. [DOI] [PubMed] [Google Scholar]
- 14.Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34:380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 15.de Torres JP, Bastarrika G, Wisnivesky JP, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132:1932–1938. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 16.Powell HA, Iyen-Omofoman B, Baldwin DR, Hubbard RB, Tata LJ. Chronic obstructive pulmonary disease and risk of lung cancer: the importance of smoking and timing of diagnosis. J Thorac Oncol. 2013;8:6–11. doi: 10.1097/JTO.0b013e318274a7dc. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society Chronic bronchitis, asthma and pulmonary emphysema: a statement by the Committee on Diagnostic Standards for Nontuberculous Respiratory Diseases. Am Rev Respir Dis. 1962;85:762–768. [Google Scholar]
- 18.Lindberg A, Jonsson AC, Rönmark E, Lundgren R, Larsson LG, Lundbäck B. Prevalence of chronic obstructive pulmonary disease to BTS, ERS, GOLD and ATS criteria in relation to doctor’s diagnosis, symptoms, age, gender, and smoking habits. Respiration. 2005;72:471–479. doi: 10.1159/000087670. [DOI] [PubMed] [Google Scholar]
- 19.Smith BM, Pinto L, Ezer N, Sverzellati N, Muro S, Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77:58–63. doi: 10.1016/j.lungcan.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 20.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–1723. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 23.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 24.Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260–271. doi: 10.1378/chest.08-0978. [DOI] [PubMed] [Google Scholar]
- 25.Wilson DO, Leader JK, Fuhrman CR, Reilly JJ, Sciurba FC, Weissfeld JL. Quantitative computed tomography analysis, airflow obstruction, and lung cancer in the Pittsburg Lung Screening Study. J Thorac Oncol. 2011;6:1200–1205. doi: 10.1097/JTO.0b013e318219aa93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Emphysema Treatment Trial Research Group Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med. 2001;345:1075–1083. doi: 10.1056/NEJMoa11798. [DOI] [PubMed] [Google Scholar]
- 27.Aalen OO, Borgan O, Gjessing HK. Survival and Event History Analysis: A Process Point of View. New York: Springer; 2008. [Google Scholar]
- 28.Therneau T. Survival: survival analysis. 2012. [Accessed February 11, 2016]. Available from: https://cran.r-project.org/web/packages/survival/index.html.
- 29.R Foundation The R Project for statistical computing. [Accessed February 11, 2016]. Available from: https://www.r-project.org.
- 30.Gjessing HK. invGauss: Threshold regression that fits the (randomized drift) inverse Gaussian distribution to survival data. 2014. [Accessed February 11, 2016]. Available from: http://cran.r-project.org/package=invGauss.
- 31.Loganathan RS, Stover DE, Shi W, Venkatraman E. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest. 2006;129:1305–1312. doi: 10.1378/chest.129.5.1305. [DOI] [PubMed] [Google Scholar]
- 32.Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax. 2005;60:570–575. doi: 10.1136/thx.2004.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aasebø U, Strøm HH, Postmyr M. The Lean method as a clinical pathway facilitator in patients with lung cancer. Clin Respir J. 2012;6:169–174. doi: 10.1111/j.1752-699X.2011.00271.x. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto N, Matsuzaki A, Okada Y, et al. Clinical impact of prevalence and severity of COPD on the decision-making process for therapeutic management of lung cancer patients. BMC Pulm Med. 2014;14:14. doi: 10.1186/1471-2466-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34:17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 36.Palma D, Lagerwaard F, Rodrigues G, Haasbeek C, Senan S. Curative treatment of stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys. 2012;82:1149–1156. doi: 10.1016/j.ijrobp.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Field JK, Hansell DM, Duffy SW, Baldwin DR. CT screening for lung cancer: countdown to implementation. Lancet Oncol. 2013;14:e591–e600. doi: 10.1016/S1470-2045(13)70293-6. [DOI] [PubMed] [Google Scholar]
- 38.Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. JAMA. 2005;294:1505–1510. doi: 10.1001/jama.294.12.1505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Survival of primary lung cancer of all 174 patients diagnosed at University Hospital of North Norway 2008–2010
| ID | Sex | Age-group, years | COPD diagnosis | Emph | PaO2, range | PaCO2, range | Cancer | Stage | Time | D |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | III | 377 | 1 |
| 2 | M | 30–74 | Missing – spirometry | 0 | NA | NA | SCC – NSCLC | IV | 340 | 1 |
| 3 | M | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | SCLC | IV | 157 | 1 |
| 4 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | SCLC | IV | 547 | 1 |
| 5 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | Ad-NSCLC | IV | 2,067 | 1 |
| 6 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCC – NSCLC | III | 1,243 | 1 |
| 7 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | SCC – NSCLC | III | 279 | 1 |
| 8 | M | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCLC | III | 365 | 1 |
| 9 | F | 75–91 | Known COPD | 0 | 10–15 | 5–5.9 | SCLC | III | 620 | 1 |
| 10 | M | 75–91 | Known COPD | 0 | 8.5–9.9 | 3–4.9 | SCLC | IV | 16 | 1 |
| 11 | F | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Other – NSCLC | I | 803 | 1 |
| 12 | M | 75–91 | No COPD | 1 | 4–8.4 | 5–5.9 | SCLC | IV | 77 | 1 |
| 13 | F | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | Ad-NSCLC | I | 353 | 1 |
| 14 | M | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | SCLC | IV | 188 | 1 |
| 15 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCC – NSCLC | II | 289 | 1 |
| 16 | F | 30–74 | No COPD | 1 | 4–8.4 | 6–10 | SCC – NSCLC | III | 2,175 | 1 |
| 17 | M | 30–74 | Missing – spirometry | 0 | 10–15 | 5–5.9 | SCC – NSCLC | IV | 104 | 1 |
| 18 | M | 75–91 | Missing – spirometry | 1 | 4–8.4 | 3–4.9 | NA | IV | 10 | 1 |
| 19 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Other – NSCLC | IV | 41 | 1 |
| 20 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | SCLC | NA | 155 | 1 |
| 21 | F | 30–74 | No COPD | 0 | 8.5–9.9 | 5–5.9 | Ad-NSCLC | IV | 245 | 1 |
| 22 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCC – NSCLC | IV | 116 | 1 |
| 23 | F | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 131 | 1 |
| 24 | M | 30–74 | Known COPD | 1 | 4–8.4 | 3–4.9 | Ad-NSCLC | IV | 287 | 1 |
| 25 | M | 75–91 | No COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | IV | 45 | 1 |
| 26 | F | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | III | 1,787 | 1 |
| 27 | M | 75–91 | Undiagnosed COPD | 1 | 8.5–9.9 | 5–5.9 | SCC – NSCLC | IV | 52 | 1 |
| 28 | F | 30–74 | Missing – spirometry | 1 | 4–8.4 | 5–5.9 | SCC – NSCLC | IV | 54 | 1 |
| 29 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | NA | IV | 162 | 1 |
| 30 | M | 75–91 | Known COPD | 1 | 4–8.4 | 6–10 | SCC – NSCLC | IV | 319 | 1 |
| 31 | F | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 561 | 1 |
| 32 | M | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCLC | IV | 1,076 | 1 |
| 33 | M | 30–74 | Missing – spirometry | 1 | NA | NA | SCC – NSCLC | III | 91 | 1 |
| 34 | M | 75–91 | Known COPD | 0 | 10–15 | 5–5.9 | SCLC | III | 27 | 1 |
| 35 | M | 75–91 | No COPD | 0 | 10–15 | 3–4.9 | SCLC | IV | 7 | 1 |
| 36 | F | 30–74 | No COPD | 0 | 4–8.4 | 5–5.9 | Ad-NSCLC | IV | 76 | 1 |
| 37 | F | 75–91 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | II | 192 | 1 |
| 38 | F | 75–91 | No COPD | 1 | 10–15 | 3–4.9 | SCC – NSCLC | II | 370 | 1 |
| 39 | F | 30–74 | No COPD | 1 | 8.5–9.9 | 6–10 | Ad-NSCLC | I | 377 | 1 |
| 40 | M | 75–91 | Missing – spirometry | 0 | 10–15 | 5–5.9 | SCC – NSCLC | IV | 36 | 1 |
| 41 | F | 30–74 | Known COPD | 0 | 10–15 | 5–5.9 | Other – NSCLC | III | 33 | 1 |
| 42 | M | 30–74 | Known COPD | 0 | 4–8.4 | 5–5.9 | SCLC | III | 1,461 | 1 |
| 43 | M | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | Other – NSCLC | IV | 141 | 1 |
| 44 | F | 30–74 | Missing – spirometry | 1 | NA | NA | SCC – NSCLC | III | 28 | 1 |
| 45 | F | 30–74 | No COPD | 0 | 8.5–9.9 | 5–5.9 | Ad-NSCLC | IV | 576 | 1 |
| 46 | F | 30–74 | Missing – spirometry | 1 | 4–8.4 | 5–5.9 | SCLC | IV | 130 | 1 |
| 47 | M | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | Ad-NSCLC | III | 38 | 1 |
| 48 | F | 75–91 | No COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 44 | 1 |
| 49 | M | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | I | 2,298 | 0 |
| 50 | M | 30–74 | No COPD | 0 | 10–15 | 6–10 | Ad-NSCLC | III | 132 | 1 |
| 51 | M | 30–74 | Undiagnosed COPD | 1 | 8.5–9.9 | 5–5.9 | Ad-NSCLC | III | 83 | 1 |
| 52 | M | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | III | 204 | 1 |
| 53 | M | 30–74 | No COPD | 1 | 8.5–9.9 | 5–5.9 | SCLC | IV | 166 | 1 |
| 54 | F | 75–91 | Undiagnosed COPD | 0 | 8.5–9.9 | 6–10 | SCC – NSCLC | II | 50 | 1 |
| 55 | F | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCLC | IV | 175 | 1 |
| 56 | M | 75–91 | No COPD | 1 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | III | 28 | 1 |
| 57 | M | 30–74 | Undiagnosed COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | I | 2,155 | 1 |
| 58 | M | 30–74 | Known COPD | 0 | 10–15 | 3–4.9 | Other – NSCLC | IV | 168 | 1 |
| 59 | F | 30–74 | Missing – spirometry | 0 | 4–8.4 | 3–4.9 | Ad-NSCLC | IV | 2 | 1 |
| 60 | M | 75–91 | Known COPD | 0 | 10–15 | 5–5.9 | SCC – NSCLC | III | 415 | 1 |
| 61 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | Ad-NSCLC | IV | 917 | 1 |
| 62 | M | 30–74 | Known COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | III | 475 | 1 |
| 63 | F | 30–74 | No COPD | 0 | 8.5–9.9 | 5–5.9 | SCLC | IV | 1,485 | 1 |
| 64 | M | 30–74 | Known COPD | 1 | 4–8.4 | 3–4.9 | SCLC | III | 13 | 1 |
| 65 | M | 75–91 | Undiagnosed COPD | 1 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | III | 460 | 1 |
| 66 | F | 30–74 | No COPD | 0 | NA | NA | Ad-NSCLC | III | 698 | 1 |
| 67 | M | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | Ad-NSCLC | IV | 226 | 1 |
| 68 | M | 30–74 | No COPD | 0 | 8.5–9.9 | 5–5.9 | Ad-NSCLC | IV | 184 | 1 |
| 69 | F | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | I | 2,196 | 0 |
| 70 | F | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | Ad-NSCLC | III | 265 | 1 |
| 71 | F | 30–74 | No COPD | 1 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | II | 2,189 | 0 |
| 72 | M | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | SCC – NSCLC | III | 287 | 1 |
| 73 | M | 30–74 | Known COPD | 1 | 8.5–9.9 | 5–5.9 | SCLC | IV | 492 | 1 |
| 74 | M | 30–74 | Missing – spirometry | 0 | 8.5–9.9 | 6–10 | SCC – NSCLC | IV | 501 | 1 |
| 75 | F | 30–74 | No COPD | 0 | 8.5–9.9 | 5–5.9 | Ad-NSCLC | II | 1,758 | 1 |
| 76 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCC – NSCLC | III | 432 | 1 |
| 77 | F | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCLC | III | 2,142 | 0 |
| 78 | M | 30–74 | No COPD | 1 | NA | NA | SCC – NSCLC | II | 2,141 | 0 |
| 79 | M | 30–74 | No COPD | 1 | 8.5–9.9 | 3–4.9 | SCLC | IV | 18 | 1 |
| 80 | F | 30–74 | Missing – spirometry | 0 | 10–15 | 3–4.9 | SCLC | IV | 19 | 1 |
| 81 | M | 75–91 | Missing – spirometry | 0 | NA | NA | SCLC | IV | 35 | 1 |
| 82 | F | 30–74 | Missing – spirometry | 0 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | IV | 135 | 1 |
| 83 | F | 30–74 | Known COPD | 1 | 4–8.4 | 3–4.9 | SCLC | IV | 111 | 1 |
| 84 | F | 30–74 | Known COPD | 0 | NA | NA | SCLC | IV | 58 | 1 |
| 85 | F | 30–74 | Undiagnosed COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | III | 2,114 | 0 |
| 86 | F | 30–74 | No COPD | 0 | NA | NA | Ad-NSCLC | III | 254 | 1 |
| 87 | M | 75–91 | Missing – spirometry | 1 | NA | NA | SCLC | IV | 35 | 1 |
| 88 | F | 75–91 | No COPD | 0 | 4–8.4 | 3–4.9 | Ad-NSCLC | IV | 18 | 1 |
| 89 | M | 75–91 | Known COPD | 1 | 10–15 | 3–4.9 | SCLC | II | 417 | 1 |
| 90 | M | 30–74 | Known COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | I | 249 | 1 |
| 91 | F | 30–74 | Known COPD | 1 | 4–8.4 | 6–10 | Ad-NSCLC | III | 630 | 1 |
| 92 | F | 30–74 | Undiagnosed COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 22 | 1 |
| 93 | M | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | III | 728 | 1 |
| 94 | M | 75–91 | No COPD | 1 | 8.5–9.9 | 3–4.9 | NA | III | 171 | 1 |
| 95 | F | 30–74 | Known COPD | 0 | 8.5–9.9 | 5–5.9 | SCLC | IV | 512 | 1 |
| 96 | F | 30–74 | Missing – spirometry | 1 | NA | NA | Ad-NSCLC | IV | 455 | 1 |
| 97 | M | 30–74 | Known COPD | 0 | 10–15 | 5–5.9 | SCLC | III | 441 | 1 |
| 98 | F | 75–91 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCC – NSCLC | I | 1,329 | 1 |
| 99 | M | 75–91 | Known COPD | 1 | 4–8.4 | 3–4.9 | SCC – NSCLC | III | 163 | 1 |
| 100 | F | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | SCLC | I | 1,902 | 0 |
| 101 | F | 75–91 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 537 | 1 |
| 102 | F | 75–91 | No COPD | 0 | 8.5–9.9 | 5–5.9 | NA | IV | 345 | 1 |
| 103 | F | 75–91 | Undiagnosed COPD | 0 | 4–8.4 | 5–5.9 | Ad-NSCLC | IV | 159 | 1 |
| 104 | M | 75–91 | Missing – spirometry | 0 | NA | NA | NA | IV | 14 | 1 |
| 105 | M | 30–74 | No COPD | 0 | 8.5–9.9 | 5–5.9 | SCLC | I | 1,983 | 0 |
| 106 | M | 75–91 | Known COPD | 1 | 8.5–9.9 | 5–5.9 | SCC – NSCLC | II | 526 | 1 |
| 107 | M | 30–74 | Missing – spirometry | 1 | NA | NA | Ad-NSCLC | IV | 222 | 1 |
| 108 | F | 75–91 | Known COPD | 1 | NA | NA | Ad-NSCLC | IV | 82 | 1 |
| 109 | M | 75–91 | Known COPD | 0 | 8.5–9.9 | 5–5.9 | Ad-NSCLC | IV | 168 | 1 |
| 110 | F | 75–91 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCC – NSCLC | III | 197 | 1 |
| 111 | M | 30–74 | No COPD | 0 | NA | NA | Ad-NSCLC | IV | 1,412 | 1 |
| 112 | F | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCC – NSCLC | IV | 1,092 | 1 |
| 113 | M | 75–91 | No COPD | 1 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | II | 195 | 1 |
| 114 | M | 30–74 | Known COPD | 1 | 10–15 | 5–5.9 | SCLC | IV | 990 | 1 |
| 115 | F | 75–91 | No COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | I | 1,254 | 1 |
| 116 | M | 30–74 | No COPD | 1 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | III | 789 | 1 |
| 117 | F | 30–74 | Undiagnosed COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | II | 1,892 | 0 |
| 118 | F | 75–91 | Known COPD | 1 | 8.5–9.9 | 3–4.9 | SCLC | I | 921 | 1 |
| 119 | F | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCC – NSCLC | III | 618 | 1 |
| 120 | M | 30–74 | Undiagnosed COPD | 0 | 10–15 | 5–5.9 | SCLC | I | 1,875 | 0 |
| 121 | F | 30–74 | Known COPD | 1 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | III | 69 | 1 |
| 122 | M | 30–74 | Undiagnosed COPD | 1 | 4–8.4 | 3–4.9 | Ad-NSCLC | IV | 16 | 1 |
| 123 | F | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 181 | 1 |
| 124 | M | 75–91 | Known COPD | 1 | 10–15 | 3–4.9 | SCC – NSCLC | IV | 81 | 1 |
| 125 | M | 30–74 | Known COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | III | 195 | 1 |
| 126 | M | 30–74 | No COPD | 1 | 10–15 | 3–4.9 | SCC – NSCLC | III | 471 | 1 |
| 127 | F | 30–74 | Known COPD | 1 | 8.5–9.9 | 6–10 | SCC – NSCLC | III | 92 | 1 |
| 128 | M | 30–74 | No COPD | 1 | 8.5–9.9 | 3–4.9 | SCLC | IV | 3 | 1 |
| 129 | M | 30–74 | No COPD | 1 | 8.5–9.9 | 5–5.9 | SCC – NSCLC | IV | 153 | 1 |
| 130 | M | 30–74 | Known COPD | 1 | 10–15 | 6–10 | SCC – NSCLC | I | 555 | 1 |
| 131 | M | 30–74 | Known COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | III | 148 | 1 |
| 132 | M | 75–91 | No COPD | 1 | NA | NA | Ad-NSCLC | I | 1,847 | 0 |
| 133 | M | 30–74 | Known COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | III | 417 | 1 |
| 134 | M | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | I | 1,840 | 0 |
| 135 | M | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | II | 1,875 | 0 |
| 136 | F | 30–74 | No COPD | 1 | NA | NA | Ad-NSCLC | III | 776 | 1 |
| 137 | M | 30–74 | No COPD | 1 | 4–8.4 | 3–4.9 | Ad-NSCLC | IV | 14 | 1 |
| 138 | F | 30–74 | Undiagnosed COPD | 1 | 10–15 | 3–4.9 | SCLC | IV | 437 | 1 |
| 139 | F | 30–74 | Known COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | III | 1,818 | 0 |
| 140 | M | 75–91 | No COPD | 1 | 8.5–9.9 | 5–5.9 | SCLC | III | 924 | 1 |
| 141 | M | 75–91 | Known COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | I | 1,797 | 0 |
| 142 | M | 30–74 | Known COPD | 0 | 8.5–9.9 | 3–4.9 | SCC – NSCLC | IV | 214 | 1 |
| 143 | F | 30–74 | Known COPD | 1 | 8.5–9.9 | 5–5.9 | SCC – NSCLC | I | 1,744 | 0 |
| 144 | M | 75–91 | Missing – spirometry | 1 | 8.5–9.9 | 5–5.9 | SCC – NSCLC | III | 701 | 1 |
| 145 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 278 | 1 |
| 146 | M | 75–91 | Known COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 397 | 1 |
| 147 | M | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | SCLC | III | 176 | 1 |
| 148 | M | 30–74 | Missing – spirometry | 1 | 4–8.4 | 5–5.9 | Ad-NSCLC | IV | 37 | 1 |
| 149 | M | 30–74 | Known COPD | 0 | 10–15 | 5–5.9 | Ad-NSCLC | IV | 290 | 1 |
| 150 | F | 30–74 | Known COPD | 1 | 8.5–9.9 | 5–5.9 | NA | III | 249 | 1 |
| 151 | M | 30–74 | Missing – spirometry | 1 | NA | NA | Ad-NSCLC | IV | 131 | 1 |
| 152 | F | 30–74 | No COPD | 1 | NA | NA | SCLC | IV | 288 | 1 |
| 153 | M | 75–91 | Known COPD | 1 | 10–15 | 6–10 | SCC – NSCLC | I | 98 | 1 |
| 154 | F | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | Ad-NSCLC | III | 1,747 | 0 |
| 155 | M | 30–74 | Known COPD | 1 | 8.5–9.9 | 5–5.9 | SCC – NSCLC | III | 741 | 1 |
| 156 | M | 75–91 | Missing – spirometry | 1 | NA | NA | SCLC | IV | 184 | 1 |
| 157 | F | 30–74 | No COPD | 1 | 8.5–9.9 | 5–5.9 | SCLC | IV | 398 | 1 |
| 158 | M | 30–74 | No COPD | 0 | 10–15 | 5–5.9 | SCLC | IV | 449 | 1 |
| 159 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | II | 1,437 | 1 |
| 160 | F | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | Ad-NSCLC | IV | 166 | 1 |
| 161 | M | 30–74 | No COPD | 1 | 8.5–9.9 | 3–4.9 | Ad-NSCLC | IV | 141 | 1 |
| 162 | M | 30–74 | No COPD | 0 | 10–15 | 3–4.9 | SCC – NSCLC | III | 1,001 | 1 |
| 163 | F | 30–74 | Missing – spirometry | 1 | 10–15 | 5–5.9 | SCLC | IV | 181 | 1 |
| 164 | F | 75–91 | Known COPD | 1 | 10–15 | 5–5.9 | Ad-NSCLC | II | 335 | 1 |
| 165 | F | 30–74 | Missing – spirometry | 0 | NA | NA | SCC – NSCLC | IV | 236 | 1 |
| 166 | F | 75–91 | Known COPD | 1 | 8.5–9.9 | 5–5.9 | Other – NSCLC | IV | 68 | 1 |
| 167 | M | 30–74 | Known COPD | 1 | 8.5–9.9 | 6–10 | Ad-NSCLC | IV | 9 | 1 |
| 168 | M | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCLC | I | 445 | 1 |
| 169 | F | 30–74 | No COPD | 0 | NA | NA | SCLC | III | 514 | 1 |
| 170 | F | 30–74 | Known COPD | 0 | NA | NA | SCLC | IV | 333 | 1 |
| 171 | F | 30–74 | Known COPD | 1 | 10–15 | 3–4.9 | Ad-NSCLC | II | 243 | 1 |
| 172 | F | 30–74 | Known COPD | 1 | 4–8.4 | 5–5.9 | SCLC | IV | 244 | 1 |
| 173 | F | 30–74 | No COPD | 1 | 10–15 | 5–5.9 | SCC – NSCLC | III | 632 | 1 |
| 174 | M | 30–74 | Missing – spirometry | 1 | NA | NA | Ad-NSCLC | IV | 57 | 1 |
Notes: Time represents survival time (days) from diagnosis of primary lung cancer; D represents a censoring variable (1 if dead, 0 if alive at the end of the study period); PaO2 and PaCO2 in kPa. Patients with missing data were categorized as “missing – spirometry”.
Abbreviations: Ad-NSCLC, adenocarcinoma non-small-cell lung cancer; Emph, emphysema; F, female; M, male; NA, not available; PaO2, partial arterial oxygen pressure; SCC, squamous cell carcinoma; SCLC, small-cell lung cancer; PaCO2, partial arterial carbon dioxide pressure.