Abstract
There is increasing evidence that bone and vascular calcification share common pathogenesis. Little is known about potential links between bone and valvular calcification. The purpose of this study was to determine the association between spine bone mineral density (BMD) and vascular and valvular calcification. Participants included 1317 participants (689 women, 628 men) in the Framingham Offspring Study (mean age 60 years). Integral, trabecular, and cortical volumetric bone density (vBMD) and arterial and valvular calcification were measured from computed tomography (CT) scans and categorized by sex-specific quartiles (Q4 = high vBMD). Calcification of the coronary arteries (CAC), abdominal aorta (AAC), aortic valve (AVC), and mitral valve (MVC) were quantified using the Agatston Score (AS). Prevalence of any calcium (AS >0) was 69% for CAC, 81% for AAC, 39% for AVC, and 20% for MVC. In women, CAC increased with decreasing quartile of trabecular vBMD: adjusted mean CAC = 2.1 (Q4), 2.2 (Q3), 2.5 (Q2), 2.6 (Q1); trend p = 0.04. However, there was no inverse trend between CAC and trabecular vBMD in men: CAC = 4.3 (Q4), 4.3 (Q3), 4.2 (Q2), 4.3 (Q1); trend p = 0.92. AAC increased with decreasing quartile of trabecular vBMD in both women (AAC = 4.5 [Q4], 4.8 [Q3], 5.4 [Q2], 5.1 [Q1]; trend p = 0.01) and men (AAC = 5.5 [Q4], 5.8 [Q3], 5.9 [Q2], 6.2 [Q1]; trend p = 0.01). We observed no association between trabecular vBMD and AVC or MVC in women or men. Finally, cortical vBMD was unrelated to vascular calcification and valvular calcification in women and men. Women and men with low spine vBMD have greater severity of vascular calcification, particularly at the abdominal aorta. The inverse relation between AAC and spine vBMD in women and men may be attributable to shared etiology and may be an important link on which to focus treatment efforts that can target individuals at high risk of both fracture and cardiovascular events.
Keywords: BONE, AGING, QCT, OSTEOPOROSIS, VASCULAR CALCIFICATION, VALVULAR CALCIFICATION, EPIDEMIOLOGY
Introduction
Atherosclerosis, heart valve sclerosis, and osteoporosis are widespread disorders that contribute to serious morbidity and mortality in our aging population. Osteoporosis is a skeletal disease characterized by reduced bone mineral density (BMD), bone structure deterioration, and increased frequency of fractures. Atherosclerosis is characterized by the deposition of calcified plaques in the vascular bed, whereas heart valve sclerosis is preceded by valvular calcification and fibrosis.(1) Growing evidence suggests cardiovascular calcification is inversely associated with BMD independent of age and other clinical risk factors shared by both disorders.(2,3) Further, there is some evidence to suggest a similar relationship between valvular calcification and BMD.(4–6)
Several molecular mechanisms have been suggested for a potential link between cardiovascular calcification and bone metabolism.(7,8) Vascular calcification has been shown to be an active process that resembles bone mineralization.(7–9) Minerals in calcified plaques in blood vessel walls and valve leaflets have been identified as calcium hydroxyapatite. In addition, bone marrow cells such as osteoblasts, osteoclasts, chondrocytes, and hematopoietic cells have been identified in calcified plaques.(10) Moreover, alkaline phosphatase (APL)-containing matrix vesicles and several bone matrix proteins, such as osteopontin and osteocalcin, are also found at the site of cardiovascular mineralization.(11–13) In knockout mouse models, deletion of matrix Gla protein and osteoprotegerin causes both cardiovascular calcification and osteoporosis.(14,15)
Prior studies of bone density and vascular calcification often include women only,(16–20) use plain radiographs to evaluate calcification of a single vascular bed, the abdominal aorta,(2,16,18–21) and rely on dual-energy X-ray absorptiometry (DXA) measurements of areal BMD (aBMD).(16,19–24) DXA poses limitations for studies of bone and vascular calcification, particularly aBMD measures at the lumbar spine. As a 2D measure, aBMD is confounded by bone size.(25) In addition, DXA is not able to distinguish between trabecular and cortical compartments of bone. These issues are important limitations because the individual compartments differ with respect to the influence of several factors, including hormones, loading, and aging. At the lumbar spine, the skeletal site frequently evaluated in the clinical setting, DXA aBMD is overestimated in the presence of aortic calcification(26) and features of spinal degeneration, such as osteophytes. In contrast to DXA, computed tomography (CT) allows quantification of volumetric bone density, separately for the trabecular and cortical compartments, not confounded by aortic calcification and degenerative disease.
To our knowledge, no prior investigations have used multidetector computed tomography (MDCT) to assess both bone and vascular calcification, as well as valvular calcification, in a population-based study of women and men. Therefore, the purpose of our study was to determine the association between CT-derived volumetric BMD (vBMD) at the lumbar spine, including both trabecular vBMD and cortical vBMD, and vascular and valvular calcification, in women and men.
Materials and Methods
Participants
The current study included 1317 participants of the Framingham Offspring Study (689 women, 628 men, mean age 60 years, range 36 to 83 years), who underwent CT examinations in 2002 to 2005, and had complete information for calcium scores, volumetric bone mineral density, and covariates. One hundred five individuals were excluded because of uninterpretable CT images (n = 54) or missing covariate data (n = 51). Boston University Institutional Review Board approved the study, and participants provided written informed consent.
CT imaging
Participants underwent cardiac imaging using an 8-slice multidetector CT scanner (Lightspeed Ultra; General Electric Medical Systems, Milwaukee, WI, USA) in 2002 to 2005. Two scans were obtained for each individual using a sequential scan protocol with a slice collimation of 8 × 2.5 mm (120 KVp, 320/400 mA for <220 and >220 lb body weight, respectively) during a single end-inspiratory breath hold (typical duration, 18 seconds). Image acquisition (330 ms) was prospectively initiated at 50% of the cardiac cycle. For the chest, 2.5-mm slices were acquired from the carina to the diaphragm. For the abdomen, 2.5-mm slices were obtained of a 125-mm abdominal segment above S1.(27)
Vascular and valvular calcium scoring
Vascular and valvular calcium measurements were performed using an offline workstation (Acquarius, Terarecon, San Mateo, CA, USA) by four experienced readers, who independently analyzed the axial images. A calcified lesion was defined as an area of ≥3 connected pixels with a CT attenuation of ≥130 Hounsfield units, with the use of 3-dimensional connectivity criteria (6 points). Scans for each individual were evaluated for coronary artery calcification (CAC), aortic artery calcification (AAC), aortic valve calcification (AVC), and mitral valve calcification (MVC).
The Agatston Score was used to quantify the level of calcification. For each individual, the Agatston Score is calculated by multiplying the area of each calcified lesion by a density factor, dependent on the maximal attenuation (HU) within the lesion, and summing each of these values for a total calcification score.(28,29) The density factor, ranging from zero to four, is determined as follows: 1 = 130 to 199 HU, 2 = 200 to 299 HU, 3 = 300 to 399 HU, and 4 > 400 HU. Aortic valve calcium was defined as calcium deposits of the aortic cusps or nodular deposits at the coaptation points of the aortic cusps. Calcium deposits restricted to the aortic wall were excluded in scoring aortic valve calcium. Mitral valve calcium was defined as calcium deposits in the region of the annulus and/or the mitral valve leaflets. Interobserver reliability of calcium measurements was high, with intraclass correlation coefficients (ICCs) greater than 0.96 for both vascular and valvular calcium scores.(1,27) Prevalence of any calcification was defined as Agatston Score greater than zero (Agatston Score >0).
Volumetric bone mineral density
Integral, trabecular, and cortical volumetric bone density (vBMD; g/cm3) of L3 was measured from the CT scans using previously published algorithms.(30,31) The volume of interest for integral vBMD included the entire vertebral body (both cortical and trabecular compartments) but excluded the transverse and posterior processes. The volume of interest for trabecular vBMD measurements was an elliptical region encompassing the anterior vertebral body, centered at the midvertebral level and encompassing 70% of the volume between vertebral endplates. The volume of interest for cortical vBMD measurements encompassed approximately the outer 1 mm of the peripheral bone of the vertebral body.(30)
Covariates
Information on clinical risk factors was obtained from study visits, conducted in 1998 to 2001, that included physical examinations, laboratory testing, and questionnaires. Height, to the nearest one-quarter inch, and weight, to the nearest half pound, were measured using a stadiometer and balance-beam scale, respectively. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). Information on cigarette smoking, physical activity (Framingham Physical Activity Index), (32) and, in women, menopause status (cessation of menses for at least 1 year) and use of hormone-replacement therapy were determined by questionnaire.
Statistical analysis
Multivariable linear regression was used to model mean Agatston Score as a function of sex-specific quartile of vBMD (Q1 = low vBMD). Because of a large proportion of participants with Agatston Score = 0, we performed a log transformation, calculated as: log (Agatston Score + 1). Separate models were used for integral, trabecular, and cortical vBMD. We performed the analysis separately for women and men. Models included the following covariates: age (years), height (inches), BMI (kg/m2), Framingham physical activity index, current smoking (yes/no), current hormone-replacement therapy (yes/no), and menopause status (post-/premenopausal). We repeated our analyses considering vBMD as a continuous variable and found no differences in the interpretation of results based on vBMD quartiles, presented herein.
Results
The study included 1317 participants. Age ranged from 36 to 83 years, and mean age was 60 years (Table 1). Prevalence of any calcification (Agatston Score > 0) was higher for vascular calcification than valve calcification in both women and men (Table 2). Women had lower prevalence of any calcification than men for CAC, AAC, and AVC, but prevalence was similar in women and men for MVC.
Table 1.
Characteristics of Participants in CT Study of Volumetric Bone Mineral Density of the Lumbar Spine and Vascular and Valvular Calcification, Framingham Offspring Cohort, 1998 to 2001
| Women (n=689) |
Men (n=628) |
|||
|---|---|---|---|---|
|
|
|
|
||
| Characteristics | Mean or % |
SD or n |
Mean or % |
SD or n |
| Age (years) | 60 | 9 | 60 | 9 |
| Height (inches) | 64 | 2 | 69 | 3 |
| Weight (lb) | 159 | 33 | 195 | 33 |
| Body mass index (kg/m2) | 28 | 6 | 29 | 5 |
| Physical activity index | 37 | 5 | 39 | 7 |
| Current smoker (%) | 10 | 68 | 11 | 67 |
| Postmenopausal (%) | 84 | 578 | – | – |
| Current hormone-replacement therapy use (%) |
35 | 241 | – | – |
Table 2.
Distribution of Vascular and Valvular Calcification (Agatston Scores)a
| Coronary artery calcification (CAC) |
Aortic artery calcification (AAC) |
Aortic valve calcification (AVC) |
Mitral valve calcification (MVC) |
|||||
|---|---|---|---|---|---|---|---|---|
| Agatston Score >0 |
Mean (SD) |
Agatston Score >0 |
Mean (SD) |
Agatston Score >0 |
Mean (SD) |
Agatston Score >0 |
Mean (SD) |
|
| Women (n=689) |
57% | 2.3 (2.4) | 77% | 5.0 (3.2) | 31% | 1.1 (1.9) | 20% | 0.9 (2.0) |
| Men (n=628) |
83% | 4.3 (2.6) | 86% | 5.8 (3.0) | 47% | 1.9 (2.3) | 21% | 0.9 (1.9) |
Log (Agatston Score+1).
Integral vBMD and vascular and valvular calcification
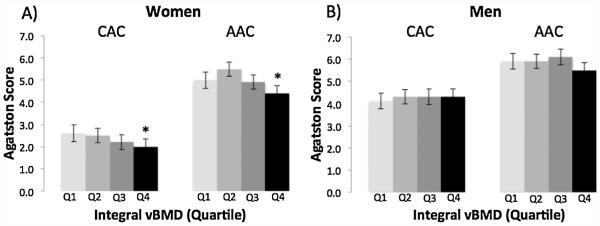
CAC increased in women from 2.0 to 2.6 with decreasing quartile of integral vBMD (trend, p = 0.02; Fig. 1). AAC similarly increased in women from 4.4 to 5.0 with decreasing quartile of integral vBMD (trend, p = 0.01). In contrast, no inverse trend between CAC (p = 0.69) or AAC (p = 0.14) and integral vBMD was observed in men.
Fig. 1.
Multivariable-adjusted mean Agatston Score for coronary artery calcification (CAC) and aortic artery calcification (AAC) by quartile of integral vBMD (Q1=low) in women (A) and men (B).
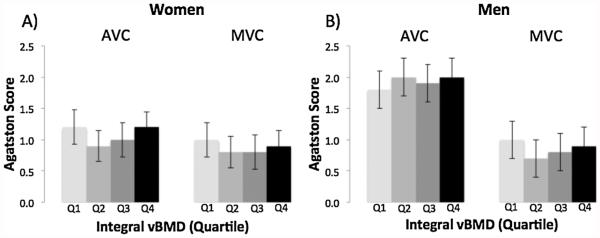
There was no association between integral vBMD and valvular calcification (AVC, MVC) in women or men (Fig. 2). For example, mean AVC was 1.2 for women in the lowest quartile of integral vBMD as well as women in the highest quartile of integral vBMD (trend, p = 0.77), and mean AVC was 1.8 for men in the lowest quartile of integral vBMD and 2.0 for the highest quartile of integral vBMD (trend, p = 0.63).
Fig. 2.
Multivariable-adjusted mean Agatston Score for aortic valve calcification (AVC) and mitral valve calcification (MVC) by quartile of integral vBMD (Q1 = low) in women (A) and men (B).
Trabecular vBMD and vascular and valvular calcification
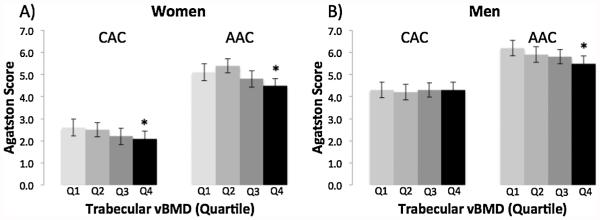
Similar to results for integral vBMD, women with the lowest trabecular vBMD had the greatest mean CAC (trend, p = 0.04) and AAC (trend, p = 0.01; Fig. 3). In men, CAC was unrelated to trabecular vBMD (trend, p = 0.92), whereas mean AAC increased from 5.5 for the highest quartile to 6.2 for the lowest quartile of trabecular vBMD (trend, p = 0.01).
Fig. 3.
Multivariable-adjusted mean Agatston Score for coronary artery calcification (CAC) and aortic artery calcification (AAC) by quartile of trabecular vBMD (Q1 =low) in women (A) and men (B).
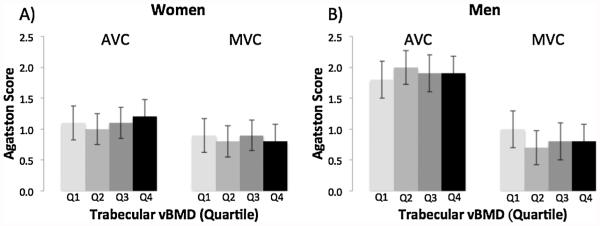
There was no association between trabecular vBMD and valvular calcification in women or men (Fig. 4).
Fig. 4.
Multivariable-adjusted mean Agatston Score for aortic valve calcification (AVC) and mitral valve calcification (MVC) by quartile of trabecular vBMD (Q1 = low) in women (A) and men (B).
Cortical vBMD and vascular and valvular calcification
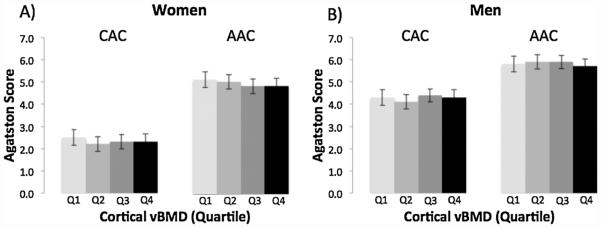
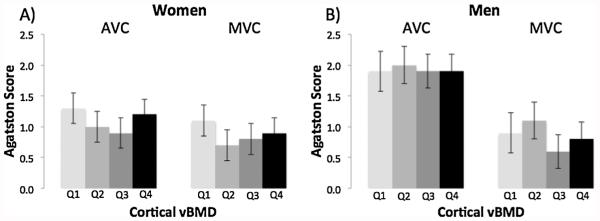
Finally, we found no association between cortical vBMD and vascular calcification (CAC, AAC; Fig. 5) or valvular calcification (AVC, MVC; Fig. 6) in women or men.
Fig. 5.
Multivariable-adjusted mean Agatston Score for coronary artery calcification (CAC) and aortic artery calcification (AAC) by quartile of cortical vBMD (Q1 = low) in women (A) and men (B).
Fig. 6.
Multivariable-adjusted mean Agatston Score for aortic valve calcification (AVC) and mitral valve calcification (MVC) by quartile of cortical vBMD (Q1 = low) in women (A) and men (B).
Discussion
In this large, population-based cohort of women and men, we used CT scans to evaluate trabecular and cortical vBMD and severity of vascular and valvular calcium of the coronary arteries, abdominal aorta, and mitral and aortic valves. We found: 1) CAC increased with decreasing trabecular vBMD in women but not in men; 2) AAC increased with decreasing trabecular vBMD in both women and men; 3) calcification of the mitral and aortic valves was not related to vBMD.
Our findings are consistent with prior work on bone and vascular calcification; however, only one investigation including both women and men employed methods similar to ours, using CT to evaluate CAC, AAC, and volumetric bone density.(2) Identical to our results, the Multi-Ethnic Study of Atherosclerosis (MESA) observed an inverse association between trabecular vBMD and CAC in women but not men, and an inverse association between trabecular vBMD and AAC in both women and men.(2) Studies limited to women, including SWAN (Study of Women’s Health Across the Nation)(17) and a clinically based study,(18) also found that women with the lowest trabecular vBMD had the highest AAC. In contrast, the Rochester Epidemiology Project(33) and a study in the Amish(24) reported no association between trabecular vBMD and AAC in women or men. Reasons for discrepant findings between studies are not readily apparent. Differences between studies in characteristics of participants, imaging modalities, and other factors may explain, at least in part, discordant results. For example, the Amish study used electron beam computed tomography to evaluate calcium scores and DXA to evaluate areal BMD. In the Rochester study, prevalence of any AAC was higher in women but lower in men compared with those our study.
This work extends results of prior studies in which measurements of vascular calcification were limited to radiographic assessments of the abdominal aorta, and measurements of bone density were based on DXA or radiographic assessments. A 25-year longitudinal study of the original cohort of the Framingham Heart Study found that women, but not men, with the greatest loss of metacarpal cortical area, evaluated from hand radiographs, had the greatest increase in abdominal aortic calcification, also evaluated from plain radiographs.(3) Reasons for sex differences in results are not readily apparent but may indicate potential differences between women and men in the underlying pathophysiology of bone mineralization, vascular calcification, or both. Differences in methodologies across studies may also account for differences in results for women and men.
There are several potential mechanisms that link vascular calcification with osteoporosis. One plausible explanation is inflammation and lipids.(34–37) In atherosclerosis, deposition and oxidation of lipids in the subendothelial space leads to inflammation, which results in further progression of disease.(38) Osteolysis has also been shown to accompany inflammation, and lipid deposits have also been found in the subendothelial spaces of osteoporotic bone tissues.(39) Through Msx2 and Wnt signaling pathways, inflammatory cytokines, such as TNF-alpha, are strong regulators of osteochondrogenic differentiation in osteoblastic and vascular cells, which subsequently exert effects on biomineralization.(40–42) The seemingly paradoxical effects of cytokines on reducing bone formation in the skeleton, yet upregulating bone formation in the vascular wall, are not well understood. Towler and colleagues(40) have demonstrated, for example, that TNF-alpha upregulates the MSX2-WNT signaling pathway to promote vascular calcification in diabetic mice. Yet, in the skeleton, TNF-alpha has been shown to potently suppress Smad signaling, induced by TGF-beta and BMP-2, downregulate BMP-2-mediated Runx2 expression, and inhibit mineralization of osteoblasts.(43) Thus, the same cytokine may signal differently in the two tissue microenvironments, although the mechanisms are not clear. Additional potential mechanisms linking vascular calcification with osteoporosis include fat intake, as an association between lipid level or saturated fat intake and bone density is supported by some but not all studies.(44–46) Shared endogenous regulatory factors, such as transglutaminase 2, fetuin, and osteoprotegerin,(34,47,48) may play a role in the link between bone and vascular calcification.
We observed no association between vBMD and aortic or mitral valve calcification. In contrast to numerous investigations of bone density and vascular calcification, few studies have investigated associations between bone density and valvular calcification. Small studies, based on referral samples, found an association between lower T-score and aortic valve calcification, evaluated by echocardiography examination.(4–6) These findings are concordant with our results for bone and vascular calcification, however, not with our null findings for bone and valvular calcification. Of note, prevalence of valvular calcification was low relative to prevalence of vascular calcification. For example, prevalence of any calcification was 20% for mitral valves compared with 82% for the abdominal aorta. Thus, it is possible that the power of our study was not adequate to detect a potential association between valve calcium and vBMD, particularly an association of small magnitude. Vascular and valvular calcification share similar underlying pathophysiology. Hemodynamic stress and reactive oxygen species trigger both processes.(38,49,50) Both valvular myofibroblast-like cells and vascular smooth muscle cells respond similarly to macrophage-derived proteinases,(51) and both cell types could be converted into osteoblastic cells.(52,53)
We found that cortical vBMD was unrelated to vascular calcification in women and men. Griffith and colleagues illustrated that decreasing marrow perfusion leads to increasing fat content with trabecular bone thinning.(54) In women, the rapid loss of trabecular bone in early menopause corresponds with the period of increased development of atherosclerosis of the aorta among women.(55) It is thought that the loss of trabecular elements is more common in women because of a menopause-associated increase in osteoclastic activity that leads to a more discontinuous trabecular network.(56)
There are several strengths of our study. First, we used CT imaging to measure both calcification and vBMD. Second, Agatston Scores measured from CT scans are sensitive markers of vascular calcification and have been shown to successfully track progression of coronary artery calcification in patients with coronary artery disease.(57,58) However, the use of density factors, truncated to single digits (1 to 4), to calculate Agatston Scores may reduce both accuracy and precision. We would expect such misclassification to be random, thereby reducing our ability to detect associations. Third, we examined both vascular and valvular calcification, providing a comprehensive evaluation of calcification. Fourth, we controlled for important confounders, including risk factors for both osteoporosis and cardiovascular disease, collected using highly standardized, rigorous data collection methods. Finally, our study included a large number of both women and men from a community-based cohort, allowing us to generalize our results to similar populations.
A limitation of this study is the observational design. Results may have been affected by uncontrolled confounding and causation cannot be inferred. In addition, members of the Framingham Study largely represent the predominantly white sociodemographic makeup of the town of Framingham, MA, at the time of enrollment of the original cohort in 1949.(59) Therefore, the results of our study may not be directly applicable to other race groups. However, the similarity of our results to those found in the Multi-Ethnic Study of Atherosclerosis (MESA)(2) suggests that our findings may be applicable to individuals with race and ethnic backgrounds that differ from our study cohort.
In conclusion, women and men with low spine vBMD have greater severity of vascular calcification, particularly at the abdominal aorta. The inverse relation between abdominal aortic calcification and spine vBMD in women and men may be an important link on which to focus prevention efforts that can target individuals at high risk of both fracture and cardiovascular events.
Acknowledgments
This work was supported by National Institutes of Health Grant K01 AR053118; Medical Student Training in Aging Research Grants 1T35AG038027-03, R01 AR053986, and R01 AR41398; and National Heart, Lung, and Blood Institute’s Framingham Heart Study Grant N01-HC-25195.
Authors’ roles: Study design: EJS, DPK. Data collection: EJS, DPK, CJO, UH. Data analysis: JC, LAC, EJS. Data interpretation: ALL AUTHORS. Drafting manuscript: JC, EJS. Revising manuscript content: ALL AUTHORS. Approving final version of manuscript: All authors take responsibility for the integrity of the data analysis.
Footnotes
Disclosures
All authors state that they have no conflicts of interest.
References
- 1.Thanassoulis G, Massaro JM, Cury R, et al. Associations of long-term and early adult atherosclerosis risk factors with aortic and mitral valve calcium. J Am Coll Cardiol. 2010;55(22):2491–8. doi: 10.1016/j.jacc.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyder JA, Allison MA, Wong N, et al. Association of coronary artery and aortic calcium with lumbar bone density: the MESA Abdominal Aortic Calcium Study. Am J Epidemiol. 2009;169(2):186–94. doi: 10.1093/aje/kwn303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O’Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68(5):271–6. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 4.Choi HS, Rhee Y, Hur NW, Chung N, Lee EJ, Lim SK. Association between low bone mass and aortic valve sclerosis in Koreans. Clin Endocrinol (Oxf) 2009;71(6):792–7. doi: 10.1111/j.1365-2265.2009.03543.x. [DOI] [PubMed] [Google Scholar]
- 5.Aksoy Y, Yagmur C, Tekin GO, et al. Aortic valve calcification: association with bone mineral density and cardiovascular risk factors. Coronary Artery Dis. 2005;16(6):379–83. doi: 10.1097/00019501-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Davutoglu V, Yilmaz M, Soydinc S, et al. Mitral annular calcification is associated with osteoporosis in women. Am Heart J. 2004;147(6):1113–6. doi: 10.1016/j.ahj.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Persy V, D’Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med. 2009;15(9):405–16. doi: 10.1016/j.molmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Shroff RC, Shanahan CM. The vascular biology of calcification. Semin Dial. 2007;20(2):103–9. doi: 10.1111/j.1525-139X.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- 9.Thompson B, Towler DA. Arterial calcification and bone physiology: role of the bone-vascular axis. Nat Rev Endocrinol. 2012;8(9):529–43. doi: 10.1038/nrendo.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eastell R, Newman C, Crossman DC. Cardiovascular disease and bone. Arch Biochem Biophys. 2010;503(1):78–83. doi: 10.1016/j.abb.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Bostrom KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ Res. 2011;109(5):564–77. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Idelevich A, Rais Y, Monsonego-Ornan E. Bone Gla protein increases HIF-1alpha-dependent glucose metabolism and induces cartilage and vascular calcification. Arterioscler Thromb Vasc Biol. 2011;31(9):e55–71. doi: 10.1161/ATVBAHA.111.230904. [DOI] [PubMed] [Google Scholar]
- 13.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27(11):2302–9. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 14.Bucay N, Sarosi I, Dunstan CR, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12(9):1260–8. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo G, Ducy P, McKee M, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 16.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. Radiographic measure of aorta calcification is a site-specific predictor of bone loss and fracture risk at the hip. J Intern Med. 2006;259(6):598–605. doi: 10.1111/j.1365-2796.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- 17.Farhat GN, Cauley JA, Matthews KA, et al. Volumetric BMD and vascular calcification in middle-aged women: the Study of Women’s Health Across the Nation. J Bone Miner Res. 2006;21(12):1839–46. doi: 10.1359/jbmr.060903. [DOI] [PubMed] [Google Scholar]
- 18.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89(9):4246–53. doi: 10.1210/jc.2003-030964. [DOI] [PubMed] [Google Scholar]
- 19.Tanko LB, Bagger YZ, Christiansen C. Low bone mineral density in the hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int. 2003;73(1):15–20. doi: 10.1007/s00223-002-2070-x. [DOI] [PubMed] [Google Scholar]
- 20.Vogt MT, San Valentin R, Forrest KY, Nevitt MC, Cauley JA. Bone mineral density and aortic calcification: the Study of Osteoporotic Fractures. J Am Geriatr Soc. 1997;45(2):140–5. doi: 10.1111/j.1532-5415.1997.tb04498.x. [DOI] [PubMed] [Google Scholar]
- 21.Naves M, Rodriguez-Garcia M, Diaz-Lopez JB, Gomez-Alonso C, Cannata-Andia JB. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int. 2008;19(8):1161–6. doi: 10.1007/s00198-007-0539-1. [DOI] [PubMed] [Google Scholar]
- 22.Bandeira E, Neves AP, Costa C, Bandeira F. Association between vascular calcification and osteoporosis in men with type 2 diabetes. J Clin Densitom. 2012;15(1):55–60. doi: 10.1016/j.jocd.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Szulc P, Samelson EJ, Sornay-Rendu E, Chapurlat R, Kiel DP. Severity of aortic calcification is positively associated with vertebral fracture in older men—a densitometry study in the STRAMBO cohort. Osteoporos Int. 2013;24(4):1177–84. doi: 10.1007/s00198-012-2101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen H, Bielak LF, Streeten EA, et al. Relationship between vascular calcification and bone mineral density in the Old-order Amish. Calcif Tissue Int. 2007;80(4):244–50. doi: 10.1007/s00223-007-9006-4. [DOI] [PubMed] [Google Scholar]
- 25.Bruno AG, Broe KE, Zhang X, et al. Vertebral size, bone density, and strength in men and women matched for age and areal spine BMD. J Bone Miner Res. 2014;29(3):562–9. doi: 10.1002/jbmr.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N, Li XM, Xu L, Sun WJ, Cheng XG, Tian W. Comparison of QCT and DXA: osteoporosis detection rates in postmenopausal women. Int J Endocrinol. 2013;2013:895474. doi: 10.1155/2013/895474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann U, Siebert U, Bull-Stewart A, et al. Evidence for lower variability of coronary artery calcium mineral mass measurements by multi-detector computed tomography in a community-based cohort—consequences for progression studies. Eur J Radiol. 2006;57(3):396–402. doi: 10.1016/j.ejrad.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham Heart Study) Am J Cardiol. 2008;102(9):1136–41. doi: 10.1016/j.amjcard.2008.06.038. 1141 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 30.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19(6):1006–12. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 31.Samelson EJ, Christiansen BA, Demissie S, et al. QCT measures of bone strength at the thoracic and lumbar spine: the Framingham Study. J Bone Miner Res. 2012;27(3):654–63. doi: 10.1002/jbmr.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kannel WB, Belanger A, D’Agostino R, Israel I. Physical activity and physical demand on the job and risk of cardiovascular disease and death: the Framingham Study. Am Heart J. 1986;112(4):820–5. doi: 10.1016/0002-8703(86)90480-1. [DOI] [PubMed] [Google Scholar]
- 33.Chow JT, Khosla S, Melton LJ, 3rd, Atkinson EJ, Camp JJ, Kearns AE. Abdominal aortic calcification, BMD, and bone microstructure: a population-based study. J Bone Miner Res. 2008;23(10):1601–12. doi: 10.1359/JBMR.080504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demer LL, Tintut Y. Mechanisms linking osteoporosis with cardiovascular calcification. Curr Osteoporos Rep. 2009;7(2):42–6. doi: 10.1007/s11914-009-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parhami F, Morrow AD, Balucan J, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17(4):680–7. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 36.Parhami F, Bostrom K, Watson K, Demer LL. Role of molecular regulation in vascular calcification. J Atheroscler Thromb. 1996;3(2):90–4. doi: 10.5551/jat1994.3.90. [DOI] [PubMed] [Google Scholar]
- 37.Parhami F, Garfinkel A, Demer LL. Role of lipids in osteoporosis. Arterioscler Thromb Vasc Biol. 2000;20(11):2346–8. doi: 10.1161/01.atv.20.11.2346. [DOI] [PubMed] [Google Scholar]
- 38.Aikawa E, Nahrendorf M, Figueiredo JL, et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116(24):2841–50. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 39.Tintut Y, Morony S, Demer LL. Hyperlipidemia promotes osteoclastic potential of bone marrow cells ex vivo. Arterioscler Thromb Vasc Biol. 2004;24(2):e6–10. doi: 10.1161/01.ATV.0000112023.62695.7f. [DOI] [PubMed] [Google Scholar]
- 40.Al-Aly Z, Shao JS, Lai CF, et al. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2007;27(12):2589–96. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 41.Cheng SL, Shao JS, Cai J, Sierra OL, Towler DA. Msx2 exerts bone anabolism via canonical Wnt signaling. J Biol Chem. 2008;283(29):20505–22. doi: 10.1074/jbc.M800851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai CF, Seshadri V, Huang K, et al. An osteopontin-NADPH oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ Res. 2006;98(12):1479–89. doi: 10.1161/01.RES.0000227550.00426.60. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Li A, Strait K, Zhang H, Nanes MS, Weitzmann MN. Endogenous TNFalpha lowers maximum peak bone mass and inhibits osteo-blastic Smad activation through NF-kappaB. J Bone Miner Res. 2007;22(5):646–55. doi: 10.1359/jbmr.070121. [DOI] [PubMed] [Google Scholar]
- 44.Adami S, Braga V, Zamboni M, et al. Relationship between lipids and bone mass in 2 cohorts of healthy women and men. Calcif Tissue Int. 2004;74(2):136–42. doi: 10.1007/s00223-003-0050-4. [DOI] [PubMed] [Google Scholar]
- 45.Samelson EJ, Cupples LA, Hannan MT, et al. Long-term effects of serum cholesterol on bone mineral density in women and men: the Framingham Osteoporosis Study. Bone. 2004;34(3):557–61. doi: 10.1016/j.bone.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 46.Solomon DH, Avorn J, Canning CF, Wang PS. Lipid levels and bone mineral density. Am J Med. 2005;118(12):1414. doi: 10.1016/j.amjmed.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 47.Fiore CE, Celotta G, Politi GG, et al. Association of high alpha2-Heremans-Schmid glycoprotein/fetuin concentration in serum and intima-media thickness in patients with atherosclerotic vascular disease and low bone mass. Atherosclerosis. 2007;195(1):110–5. doi: 10.1016/j.atherosclerosis.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 48.Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102(5):529–37. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aikawa E, Aikawa M, Libby P, et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009;119(13):1785–94. doi: 10.1161/CIRCULATIONAHA.108.827972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aikawa E, Nahrendorf M, Sosnovik D, et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115(3):377–86. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 51.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. 2001;104(21):2525–32. doi: 10.1161/hc4601.099489. [DOI] [PubMed] [Google Scholar]
- 52.Rattazzi M, Bennett BJ, Bea F, et al. calcification of advanced atherosclerotic lesions in the innominate arteries of ApoE-deficient mice: potential role of chondrocyte-like cells. Arterioscler Thromb Vasc Biol. 2005;25(7):1420–5. doi: 10.1161/01.ATV.0000166600.58468.1b. [DOI] [PubMed] [Google Scholar]
- 53.Steitz SA, Speer MY, Curinga G, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89(12):1147–54. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 54.Griffith JF, Yeung DK, Antonio GE, et al. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236(3):945–51. doi: 10.1148/radiol.2363041425. [DOI] [PubMed] [Google Scholar]
- 55.Riggs BL, Melton LJ, 3rd, Robb RA, et al. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19(12):1945–54. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 56.Parfitt AM, Mathews CH, Villanueva AR, Kleerekoper M, Frame B, Rao DS. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest. 1983;72(4):1396–409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Almasi A, Pouraliakbar H, Sedghian A, Karimi MA, Firouzi A, Tehrai M. The value of coronary artery calcium score assessed by dual-source computed tomography coronary angiography for predicting presence and severity of coronary artery disease. Pol J Radiol. 2014;79:169–74. doi: 10.12659/PJR.890809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma ES, Yang ZG, Li Y, Dong ZH, Zhang L, Qian LL. Correlation of calcium measurement with low dose 64-slice CT and angiographic stenosis in patients with suspected coronary artery disease. Int J Cardiol. 2010;140(2):249–52. doi: 10.1016/j.ijcard.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 59.Dawber TR, Meadors GF, Moore FE. Epidemiological approaches to heart disease: the Framingham Study. Am J Pub Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]