Abstract
Kisspeptin neurones located in the arcuate nucleus (ARC) and preoptic area (POA) are critical mediators of gonadal steroid feedback onto GnRH neurones. ARC kisspeptin cells that co-localize neurokinin B (NKB) and dynorphin (Dyn), are collectively referred to as KNDy (Kisspeptin/NKB/Dyn) neurones, and have been shown to also co-express the glutamatergic marker, vGlut2, in mice. The ARC in rodents has long been known as a site of hormone-induced neuroplasticity, and changes in synaptic inputs to ARC neurones in rodents occur over the oestrous cycle. Based on this evidence, the goal of this study was to examine possible changes across the ovine oestrous cycle in synaptic inputs onto kisspeptin cells in the ARC (KNDy) and POA, and inputs onto GnRH neurones. Gonadal-intact breeding season ewes were perfused using 4% paraformaldehyde during either the luteal or follicular phase of the oestrous cycle, the latter group sacrificed at the time of the luteinising (LH) surge. Hypothalamic sections were processed for triple-label immunodetection of kisspeptin/vGlut2/synaptophysin or kisspeptin/vGlut2/GnRH. The total numbers of synaptophysin- and vGlut2-positive inputs to ARC KNDy neurones were significantly increased at the time of the LH surge compared to luteal phase; as these did not contain kisspeptin they do not arise from KNDy neurons. In contrast to the ARC, the total number of synaptophysin-positive inputs onto POA kisspeptin neurones did not differ between luteal phase and surge animals. The total number of kisspeptin and vGlut2 inputs onto GnRH neurones in both the POA and mediobasal hypothalamus was also increased during the LH surge. Taken together, these results provide novel evidence of synaptic plasticity at the level of inputs onto KNDy and GnRH neurones during the ovine oestrous cycle, changes which may contribute to the generation of the preovulatory GnRH/LH surge.
Keywords: kisspeptin, vGlut2, GnRH, KNDy, arcuate, plasticity
INTRODUCTION
Kisspeptin is a member of the RFamide-related peptide family that has been firmly established as a key messenger in conveying the feedback influence of gonadal steroids onto gonadotrophin-releasing hormone (GnRH) cells (1–2). Kisspeptin neurones are direct targets for the actions of oestradiol (E2), progesterone and androgens (3–4), and kisspeptin itself is a potent stimulator of GnRH and luteinising hormone (LH) release in a wide range of mammals, including mice (5–6), rats (7–10), sheep (11–12), monkeys (13) and humans (14). There is now also strong evidence that endogenous kisspeptin is important for both tonic GnRH/LH secretion and the preovulatory GnRH/LH surge in females because receptor antagonists disrupt both modes of LH secretion in rats (15–16) and sheep (17–18) and GnRH release in pubertal monkeys (17, 19).
Kisspeptin neurones are present in two major neuronal populations, a rostral one located in the preoptic region, and a caudal population in the arcuate nucleus of the hypothalamus (ARC) (1). In rodents, the rostral population, located specifically in the rostral periventricular area of the third ventricle (RP3V), plays a key role in the positive feedback actions of E2 leading to the GnRH/LH surge (15, 20), while the caudal population in the ARC is functionally involved in the negative feedback actions of E2 (7, 21–22). However, in sheep, guinea pigs and primates, there is evidence that ARC kisspeptin neurons may also mediate E2 positive feedback. In all three species the positive feedback action of E2 occurs in the MBH (23) and kisspeptin expression (mRNA and/or protein) in the ARC increases during the follicular phase or LH surge (24–27). Moreover, ovine ARC kisspeptin neurons express cFos in response to a surge-inducing dose of E2 (26) and cFos expression is elevated at the time of the LH surge (28).
A distinguishing feature of ARC kisspeptin neurons is their colocalisation with two other peptides implicated in central control of reproduction: the tachykinin, neurokinin B (NKB) and the endogenous opioid peptide, dynorphin (29). NKB has now been recognized as another important stimulatory peptide in the control of GnRH secretion with functional roles in puberty (30–31) and the control of GnRH pulses (30, 32). Dynorphin is an inhibitory peptide that has been shown to play a key role in the negative feedback influence of progesterone on GnRH pulses (33). Based on this colocalisation, the ARC kisspeptin population has been referred to as the KNDy (Kisspeptin/Neurokinin B/Dynorphin) cell group. KNDy cells appear to be conserved across multiple mammalian species, including mice (34), rats (35–36), goats (37), sheep, and female humans (38), although their presence in human males is controversial (39). Anatomically, another distinguishing feature of the KNDy population is that they are reciprocally interconnected, reflected by the presence of KNDy peptide-containing terminals in synaptic contact with KNDy cell bodies in sheep and rodents (35, 40–41). In addition to containing these three neuropeptides, ARC kisspeptin cells in mice may also contain glutamate, based on expression of the mRNA for the vesicular glutamate transporter, vGlut2 (42–43). However, this has not yet been demonstrated in other species. Therefore the first objective of this work was to determine whether synaptic terminals from ovine kisspeptin neurones contained glutamate, and if some or all of these were derived from the KNDy population. We focused on synaptic terminals because of evidence that the vesicular glutamate transporters, vGlut1 and vGlut2, are established as markers of presynaptic glutamatergic terminals, localized to synaptic terminals rather than cell bodies (44–45).
Earlier studies in rodents have shown that ARC neurones undergo significant synaptic plasticity across the oestrous cycle (46–49), although the precise identity of ARC cells exhibiting neuroplasticity was not determined in those studies. Based on the involvement of ovine KNDy cells in both negative and positive feedback (21, 25–26, 28), the second objective of the present study was to investigate potential neuroplasticity in synaptic inputs to KNDy cells, as well as to rostral (POA) kisspeptin neurones and GnRH neurones, across the ovine oestrous cycle. Specifically, we tested the hypothesis that the activation of GnRH (50) and kisspeptin (28, 51) neurons that occurs at the time of the LH surge may reflect, in part, changes in synaptic input to these neurons.
MATERIALS AND METHODS
Animals
Adult blackface ewes with regular oestrous cycles were maintained under normal conditions in an open barn with free access to food and water during the breeding season (September through February). Ewes were moved to an indoor facility 3–5 d before any experimental procedures. In this facility, they were exposed to photoperiod simulating natural outdoor day length, had free access to water and a mineral lick, and were fed a pelleted maintenance diet daily. Blood samples (3–5 ml) were taken by jugular venipuncture and placed into heparinised tubes, and plasma was collected and stored at −20 C. All experimental procedures involving animals were approved by the West Virginia Animal Care and Use Committee, and in accordance to the National Institutes of Health guidelines for animal research.
Animal Protocol
For this work, we used tissue collected for a previous study that examined Fos expression in kisspeptin neurons at different times of the estrous cycle (28). Briefly, cycles were synchronised with injections of prostaglandin F2α (10 mg; im; PGF2α; Lutalyse; Upjohn, Kalamazoo, MI) to induce regression of the corpus luteum. Once cycles had been synchronised, on d9-d10 of the luteal phase, brain tissue was collected or PGF2α was again injected and two Controlled Internal Drug Releasing (CIDR) devices were inserted (52). The CIDRs were removed 10 d later, and blood samples were collected every 4 h starting 12 h after CIDR removal until perfusion. Tissue was collected 4 h after onset of oestrous behavior and, based on LH concentrations, animals were classified into surge and pre-surge groups; only tissues from animals in the luteal phase or showing an LH surge were used in this study. Mean LH levels at perfusion were: 2.43 ± 1.02 and 18.52 ± 6.28 ng/ml for luteal and surge groups, respectively.
Tissue Collection
The collection and processing of sheep tissue was performed as previously described (29). Ewes were euthanised via an iv overdose of sodium pentobarbital (~2g in 7ml saline; Sigma, St. Louis, MO) after two iv injections of heparin (25,000 U), given 10 and 0 min prior to the administration of pentobarbital. The heads were then removed, both internal carotids cannulated and the head perfused at a rate of 200–250 mL/min with 6 liters of fixative (4% paraformaldehyde containing 10 U/ml heparin and sodium nitrite) using a peristaltic pump. After perfusion, brains were removed and a tissue block containing POA and hypothalamic tissue was dissected out. The tissue was then infiltrated with 30% sucrose, and 6 parallel series of coronal sections (45 μm thick) were cut on a freezing microtome and stored at −20 C in cryoprotectant for later processing.
Assays
LH concentrations in plasma samples were measured in duplicate aliquots of 50–200 μl, using a previously validated RIA (53), and expressed in terms of NIH-LH-S12. The minimal detectable concentration of LH in these assays averaged 0.077 ng/tube; inter- and intra-assay coefficients of variation were 3.8% and 1.7%, respectively. Circulating progesterone concentration was measured in duplicate aliquots of 150 μl plasma using a commercially available solid- phase RIA kit (Coat-A-Count P4, Diagnostic Products, Corp., Los Angeles, CA), which has been validated for use in sheep (54). In the luteal phase group, progesterone concentrations were greater than 2 ng/ml, while in the surge group circulating progesterone concentrations were less than 0.2 ng/ml.
Immunocytochemistry
All immunocytochemistry was carried out on free-floating sections at room temperature (RT), and washed with 0.1 M phosphate buffered saline (PBS) between incubations. For all experiments, sections were incubated in 10% hydrogen peroxide (10 min in PBS; EMD Chemicals, Inc., Gibbstown, NJ) to block endogenous peroxidase activity, followed by incubation in a solution containing 20% normal goat serum (NGS; Jackson Immunoresearch Laboratories, Inc., West Grove, PA) in PBS containing 0.4% Triton X-100 (Fisher Scientific, Pittsburgh, PA) for 1 h to minimize nonspecific binding. Information on the specificity of all antibodies used is shown in Supplemental Table S1. Whenever two primary antibodies from the same species (i.e. in rabbits: kisspeptin, vGlut1, vGlut2, or Dynorphin) were needed, the kisspeptin antisera was used at a high dilution (1:200,000) and amplified using TSA; this procedure has been previously validated to prevent cross-reactivity of second antibodies under these circumstances (29, 55).
Kisspeptin, vGlut1, Synaptophysin
To examine the expression of vGlut1 within kisspeptin terminals and presence of inputs to kisspeptin cell bodies, triple- label immunofluorescence was conducted on a series of every sixth POA and ARC section from luteal (n=3) and surge (n=3) animals for kisspeptin, vGlut1 and synaptophysin. First, sections were incubated for 17 h in polyclonal rabbit anti- kisspeptin10 serum (1:200,000; No. 564, A. Caraty, Université Tours, Nouzilly, France), which has been previously characterised as specific for kisspeptin10 in sheep tissue (29, 56). Next, sections were incubated with biotinylated goat anti- rabbit IgG (1:500 in PBS containing 0.4% Triton X-100 and 4% NGS; 1 h, Jackson Immunoresearch Laboratories, Inc.), ABC-elite (1:500 in PBS; 1 h, Vector Laboratories, Burlingame, CA), TSA (1:250 in PBS containing 3% hydrogen peroxide/ml; 10 min, Perkin Elmer LAS, Inc., Boston, MA) and Alexa 488- Streptavidin (1:100 in PBS; 30 minutes; S32354, Invitrogen, Carlsbad, CA). Subsequently, sections were co- incubated in polyclonal rabbit anti-vGlut1 serum (1:1,000; Synaptic Systems, 135002, Goettingen, Germany) and monoclonal mouse anti-synaptophysin serum (1:200; Sigma, S5768) for 17 h. Next, sections were incubated sequentially for 30 minutes with fluorescent antibodies Alexa 555 goat anti-rabbit (1:100; A21428, Invitrogen) and Cy5- donkey anti- mouse (1:100; 715-175-151, Jackson Immunoresearch Laboratories, Inc.). Sections were mounted onto Superfrost slides, dried and coverslipped with gelvatol (40).
Kisspeptin, vGlut2, Synaptophysin
Triple label immunofluorescence for kisspeptin/synaptophysin, and vGlut2 was conducted on luteal (n=4) and surge (n=4) tissue from a series of every sixth POA and ARC section using the identical protocol as described above, but with the substitution of rabbit anti- vGlut2 serum (1:2000; Synaptic Systems, 135403).
Kisspeptin, vGlut2, Dynorphin
KNDy neurones and glutamate terminals in ARC sections of luteal and surge animals (n=3) were detected using the identical protocol as above using rabbit anti- kisspeptin10 serum (1:200,000; no. 564, A. Caraty), monoclonal mouse anti- vGlut2 (1:500; MAB5504, Chemicon, Billerica, MA), and rabbit anti-Dynorphin A (1:1,000; H-021-03, Phoenix Pharmaceuticals, Inc, Burlingame, CA). The kisspeptin signal was detected using TSA and Cy5- Streptavidin (1:100; 016-170-084, Jackson Immunoresearch Laboratories, Inc.), while vGlut2 and dynorphin were visualised using Alexa 488 goat anti-mouse IgG (1:100; Invitrogen) and Alexa555-goat anti-rabbit IgG (1:100; Invitrogen), respectively.
Kisspeptin, vGlut2, GnRH
To examine changes in kisspeptin and glutamatergic inputs to GnRH neurones across the oestrous cycle, POA and MBH tissue sections of luteal (n=4) and surge (n=4) animals were processed for triple-label immunodetection of kisspeptin, vGlut2 and GnRH, using the same protocol as above. Kisspeptin was visualised using TSA and CY5-steptavidin. GnRH neurones were detected using monoclonal mouse anti-GnRH (1:400; SMI41R, Sternberger Monoclonals, Inc., Princeton, NJ), and Alexa 488-goat anti-mouse IgG (1:100; A11001, Invitrogen). vGlut2 was visualised using Alexa555-goat anti-rabbit IgG (1:100; A21428, Invitrogen).
ICC Controls
Specificity for kisspeptin and dynorphin antibodies has previously been determined (29, 40). Preabsorption of the rabbit vGlut2 antibody (1:2,000, Synaptic Systems) with vGlut2 peptide (1, 10, 25 and 50 μg/ml; Synaptic Systems, 135-4P) resulted in a complete abolishment of all vGlut2 staining in hypothalamic sections and western blot analysis showed a single band using sheep hypothalamic protein. To control for specificity of the Mouse anti-vGlut 2 antibody (Chemicon), a dual- label immunofluorescence experiment was performed with mouse anti- vGlut2 (Chemicon) and rabbit anti-vGlut2 (Synaptic Systems). Confocal microscopy analysis confirmed that both vGlut2 antibodies exhibited complete overlap of the same terminals. To confirm that the rabbit vGlut2 antibody (Synaptic Systems) does not crossreact with kisspeptin, rabbit vGlut2 antiserum (1:2,000; Synaptic Systems) was preincubated with kisspeptin peptide (10, 50 and 100 ug/ml; kisspeptin-10/Metastin 45–54 amide; human, 048-56, Phoenix Pharmaceuticals, Inc). Preabsorption of the vGlut2 antibody with kisspeptin peptide did not interfere with vGlut2 immunostaining or co-expression of kisspeptin and vGlut2 in synaptic terminals for any peptide concentration. Dual-label immunofluorescence was carried out to confirm that vGlut1 and vGlut2 are not co-expressed in the same varicosities in the sheep MBH. The distribution and expression patterns of vGlut1 and vGlut2 are primarily complementary, present in different brain regions (57–59), however, some terminals in the rat have been shown to express both (60). In addition, although expression profiles differ, these transporters retain very similar functional properties (44, 58). Tissue sections were stained for both vGlut1 (1:80,000; Synaptic Systems) and vGlut2 (1:2,000; Synaptic Systems) using the amplification protocol described above (vGlut1 amplified with TSA), as both antibodies were raised in rabbit. Confocal microscopy revealed no instances of colocalisation of vGlut1 and vGlut2. Finally, for each triple- and dual-label experiment, omission of one or more of the primary antibodies resulted in a lack of staining for the corresponding antigen, demonstrating lack of cross-reactivity of secondary antibodies.
Confocal Analyses
All confocal analyses were conducted with an LSM510 laser- scanning confocal microscope (Zeiss, Thornwood, NY). Alexa 488 was visualised with a 505 nm emission filter and Argon laser, while Alexa 555 and Cy5 were imaged with HeNe lasers and a 560 and 650 nm emission filters, respectively. Neurones in which complete cell bodies were visible were selected for analysis and images were taken in 1 μm intervals along the z- plane. For each animal, close contacts onto kisspeptin or GnRH cells were defined as an immunolabeled terminal in close apposition (no intervening pixels) to a cell body or proximal dendrite. When analyzing images through the entire z-stack, markers were placed on putative terminals, so that terminals in flanking optical sections were not counted twice. Furthermore, orthogonal views confirmed contacts so that only labeled terminals contacting the neurone in all planes were accepted as close contacts. Minor brightness and level adjustments were made to the image using Adobe Photoshop (San Jose, CA).
For all analyses, the percentage of either kisspeptin or GnRH neurones receiving one or more specific close contacts was determined on a sub-population of these cells (~20–30 neurones/ewe). A subset of neurones (7–9/ewe) that received at least one immunoreactive close contact was then selected randomly to quantify the number and type of synaptic close contacts in tissue from luteal phase and preovulatory surge groups. To ensure comparisons were not confounded by cell size, optical thickness measurements were taken of each cell in this analysis by counting the number of 1 μm optical sections containing the cell in the z-stack. No significant differences were identified in optical thickness between luteal and surge groups, or within groups between POA and ARC (data not shown).
To investigate whether dual-labeled kisspeptin+vGlut2 terminals expressed dynorphin, all kisspeptin boutons in single 1 μm optical sections taken from the ARC and POA were counted and analyzed for the presence of vGlut2 and/or dynorphin, independent of whether they contact a labeled neurone. The percentage of kisspeptin/vGlut2 dual-labelled varicosities that also expressed dynorphin, and the percentage of dual-labelled kiss/dynorphin varicosities that expressed vGlut2, were calculated.
Statistical Analysis
As the examination of colocalisation of vGlut1 and vGlut2 with kisspeptin was descriptive, no statistical analysis was performed on these data and tissue from only 3 animals was used. Statistical significance for all confocal analyses were determined using two-way ANOVAs, with group (luteal vs surge, n=4/group) and region (POA vs ARC) as factors because these were normally distributed. All pairwise comparisons that were normally distributed were done using the Holm-Sidak method with a 95% confidence level; if this criterion was not met, data were analyzed with the non-parametric Kruskal-Wallis test, with the Tukey test used for pairwise comparisons. All results are reported as mean ± SEM, and statistical significance was considered as P < 0.05.
RESULTS
vGlut2 as a marker for KNDy neurones and their terminals
Because of evidence in rodents that a majority of ARC Kiss1 cells are glutamatergic (42), we first examined kisspeptin cells and fibers for colocalisation of vGlut1 and vGlut2. There was no colocalisation of kisspeptin and vGlut1 in cell bodies or fibers in either luteal and surge animals, and the majority (>95%) of kisspeptin cell bodies in the POA and ARC showed at least one apposition by dual- labelled vGlut1/synaptophysin presynaptic terminals. Because preliminary data indicated colocalsation of vGlut2 with kisspeptin, subsequent analyses focused on colocalisation of this transporter in KNDy cells and their projections, identified by colocalised dynorphin and kisspeptin in individual cells, fibers and boutons.
In the ARC and POA, a large majority (>90%) of dual-labelled kisspeptin/dynorphin terminals colocalised vGlut2 (Fig. 1). Conversely, very few instances of kisspeptin/vGlut2 dual-labeled terminals that did not colocalise dynorphin were noted (<2% and <8% in ARC and POA, respectively). The high degree of colocalisation of vGlut2 with two KNDy peptides (kisspeptin + dynorphin) thus suggests that vGlut2 serves as an additional marker for fibers originating from ARC kisspeptin cells in the sheep. As seen previously (1, 40), KNDy terminals in the ARC were frequently observed in direct contact with KNDy cell bodies and dendrites. Almost all (98%) of these KNDy-KNDy contacts also colocalised vGlut2 (arrows in Fig. 1), indicating that these interconnections, like other KNDy projections, are glutamatergic.
Figure 1.
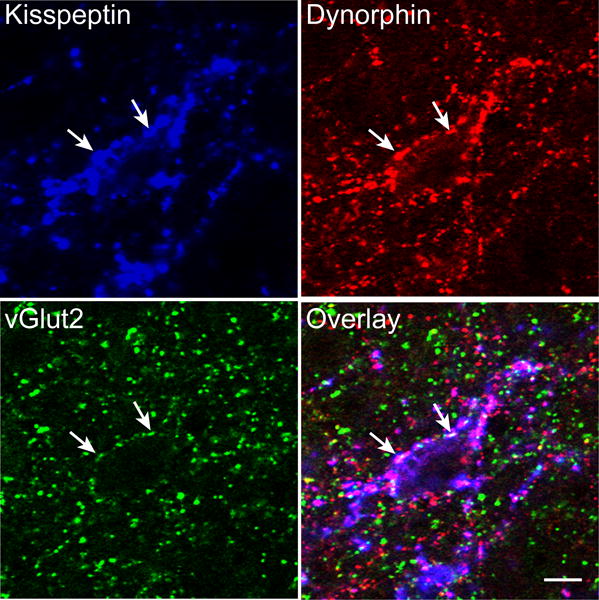
Confocal images (1 μm thickness; 63×) of a section immunolabeled for kisspeptin (blue), dynorphin (red) and vGlut2 (green) in the ARC. White arrows indicate examples of triple-labelled kisspeptin/vGlut2/dynorphin terminals in close apposition to a dual-labeled kisspeptin/dynorphin (KNDy) cell body. Scale bar, 10 μm.
Changes in inputs onto kisspeptin cells across the oestrous cycle
To unambiguously identify close contacts between kisspeptin/vGlut2 terminals and kisspeptin cells as presynaptic terminals, synaptophysin was used as a marker (56). The total number of synaptophysin-positive contacts onto kisspeptin cells of the ARC (KNDy cells) and POA, as well as the number of triple-labeled kisspeptin/vGlut2/synaptophysin inputs between luteal and surge groups were analyzed (Fig. 2).
Figure 2.
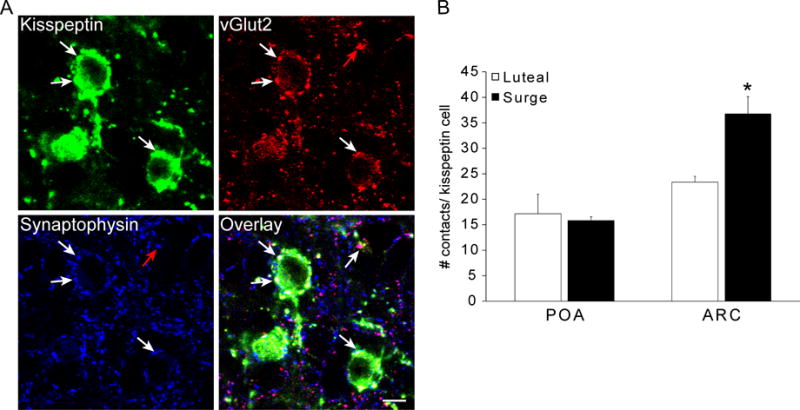
Synaptic inputs to kisspeptin neurones in the POA and ARC. A: Confocal images (1 μm thickness; 63×) of triple-labeling for kisspeptin (green), vGlut2 (red) and synaptophysin (blue) in the ARC of a luteal phase ewe. White arrows indicate examples of a triple-labelled kisspeptin/vGlut2/synaptophysin terminal in close contact with a kisspeptin soma, and red arrows indicate an example of a dual-labeled vGlut2/synaptophysin terminal. Scale bar, 10 μm. B: Total number of synaptophysin-positive contacts onto kisspeptin neurones in luteal (n=4) and surge (n=4) animals. No differences were seen between groups in the total number of synaptic inputs to POA kisspeptin neurones, but ARC kisspeptin neurones received significantly more synaptic inputs in surge animals. *, P < 0.05. Data presented as means ± SEM.
Kisspeptin neurones of the POA and ARC, in both luteal and surge animals, received triple-labeled kisspeptin/vGlut2/synaptophysin-positive (Kiss + vGlut2 + Syn) inputs, as well as dual-labeled vGlut2/synaptophysin (vGlut2 + Syn), and single-labeled synaptophysin (Syn only) inputs (Table 1). Statistical analysis showed significant main effects of estrous cycle stage for the total number of synaptic inputs (all synaptophysin-positive inputs; F1,12= 5.378; P = 0.039), vGlut2 + Syn (F1,12= 5.189; P = 0.042), and Syn only (F1,12= 5.694; P = 0.034) inputs. Post hoc analyses revealed these effects of experimental group on numbers of synaptic inputs to be restricted to the ARC. The total number of synaptic inputs (all synaptophysin-positive contacts) onto ARC kisspeptin neurones was significantly higher during the surge compared to the luteal phase (P < 0.01), while no differences were seen between groups in contacts onto POA kisspeptin neurones (Fig. 2). This difference in inputs onto ARC kisspeptin cells was not due to changes in the number of Kiss + vGlut2 + Syn inputs, which did not change across the oestrous cycle, but rather to a near doubling in the numbers of vGlut2 + Syn (P < 0.01) and Syn-only contacts (P < 0.01) onto ARC kisspeptin neurones in surge animals compared to luteal phase (Table 1). By contrast, Kiss + Syn inputs were rarely seen in close apposition to kisspeptin neurones in either the POA or ARC (Table 1) (Wilcoxon-Mann-Whitney test).
Table 1.
Number of contacts onto Kisspeptin Neurones
| POA | Luteal (n=4) | Surge (n=4) |
|---|---|---|
| Kiss + vGlut2 + Syn | 1.94 ± 0.29 | 1.77 ± 0.18 |
| vGlut2 + Syn | 7.52 ± 1.53 | 6.99 ± 0.40 |
| Kiss + Syn | 0.03 ± 0.033 | 0 ± 0 |
| Syn Only | 7.67 ± 2.19 | 7.08 ± 0.25 |
|
| ||
| ARC | Luteal (n=4) | Surge (n=4) |
|
| ||
| Kiss + vGlut2 + Syn | 7.33 ± 1.31# | 7.12 ± 0.59# |
| vGlut2 + Syn | 7.30 ± 0.40 | 13.21 ± 1.73*# |
| Kiss + Syn | 0.03 ± 0.03 | 0.13 ± 0.09 |
| Syn Only | 8.71 ± 0.93 | 16.35 ± 1.74*# |
Significant difference between luteal and surge groups, within brain region (P < 0.05)
Significant difference between ARC and POA, within luteal or surge groups (P < 0.05). Data are shown as mean ± SEM.
Statistical analyses detected a significant main effect of region for total inputs, (F1,12= 27.030; P < 0.01), vGlut2 + Syn (F1,12= 6.437; P = 0.026), Kiss + vGlut2 + Syn (F1,12= 53.053; P < 0.01) and Syn-only (F1,12= 12.174; P < 0.01) inputs. Post hoc analyses revealed that ARC kisspeptin cells in surge animals receive significantly more total inputs (P < 0.001), vGlut2 + Syn (P < 0.01), and Syn only (P < 0.001) inputs than POA kisspeptin cells, whereas no regional differences were detected during the luteal phase for these types of inputs. In addition, ARC kisspeptin cells receive significantly more triple-labeled Kiss + vGlut2 + Syn inputs than POA kisspeptin cells (P < 0.001), regardless of phase of the oestrous cycle. Interestingly, the proportion of Kiss + vGlut2 + Syn inputs as a function of total inputs showed a significant effect using the Kruskal-Wallis non-parametric test (H= 12.110; P < 0.01), and post hoc analysis (Tukey test) revealed that these triple-labeled inputs represent a greater proportion of inputs onto ARC kisspeptin cells than POA kisspeptin cells during luteal phase (31.07 ± 4.36% ARC vs. 11.93 ± 1.47% POA; q= 4.096, P < 0.05).
Changes in inputs to GnRH cells across the oestrous cycle
GnRH neurones located in both the POA and MBH were contacted by dual-labelled kisspeptin/vGlut2 (Kiss + vGlut2) terminals, as well as by single-labeled vGlut2 and kisspeptin terminals (Table 2). Almost all (>95%) GnRH neurones in the POA and MBH received at least one single-labeled vGlut2 input to their cell body, and between 45% and 60% of GnRH neurones received at least one dual-labeled Kiss + vGlut2 input in the POA and MBH, respectively. In contrast to kisspeptin cells which received almost no single-labeled kisspeptin inputs, 55–65% of GnRH neurones in the POA and MBH were contacted by at least one single-labeled kisspeptin terminal in both luteal and surge groups. To summarize, 76–80% of MBH GnRH neurones received at least one kisspeptin input (single or dual-labeled) in luteal and surge groups, and in the POA, 61–70% of GnRH cells received at least one kisspeptin input (from single or dual-labeled terminals).
Table 2.
Number of contacts onto GnRH Neurones
| POA | Luteal (n=4) | Surge (n=4) |
|---|---|---|
| Kiss + vGlut2 | 1.57 ± 0.15 | 1.91 ± 0.16 |
| vGlut2 | 4.67 ± 0.48 | 6.63 ± 0.60 |
| Kiss | 2.06 ± 0.30# | 2.13 ± 0.39# |
|
| ||
| MBH | Luteal (n=4) | Surge (n=4) |
|
| ||
| Kiss + vGlut2 | 2.05 ± 0.28 | 4.85 ± 0.53*# |
| vGlut2 | 5.65 ± 0.80 | 8.67 ± 2.46 |
| Kiss | 1.18 ± 0.16 | 1.45 ± 0.44 |
Significant difference between luteal and surge groups, within brain region (P < 0.05)
Significant difference between POA and MBH, within luteal or surge groups (P < 0.05). Data are shown as mean ± SEM.
Statistical analyses revealed significant effects of estrous cycle stage on total kisspeptin and vGlut2 inputs (both single- and dual-labeled; Kruskal Wallis test, H= 11.890, P = 0.008), and dual Kiss + vGlut2 inputs (F1,12= 24.636; P < 0.01) to GnRH neurones. Post hoc analyses using the Tukey test revealed that the total kisspeptin and vGlut2 inputs (both single- and dual-labeled) per GnRH neurone was greater during the surge than the luteal phase, in the MBH (q=3.886, P < 0.05) (Fig. 3). increase appeared to be due to an increase in the number of dual-labeled Kiss + vGlut2 inputs (P < 0.01), as single vGlut2 and single kiss inputs did not differ (Table 2).
Figure 3.
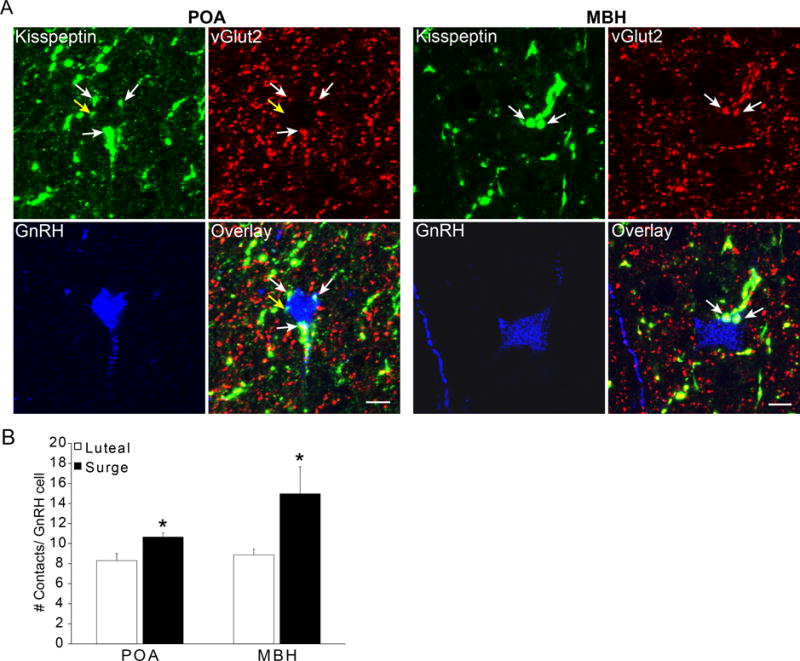
Kisspeptin- and vGlut2-positive inputs onto GnRH neurones located in the POA and MBH. A: Confocal images (1 μm thickness; 63×) triple-labelled for kisspeptin (green), vGlut2 (red) and GnRH (blue) in the POA and MBH. White arrows indicate examples of dual labelled kisspeptin/vGlut2 terminal in close contact with a GnRH soma. Yellow arrow indicates an example of a single-labeled kiss input to a GnRH neurone in the POA. Scale bar, 10 μm. B: Total number of contacts onto GnRH neurones from kisspeptin and vGlut2 labelled terminals (single and dual-labelled). GnRH neurones in the MBH receive significantly more kisspeptin and vGlut2 inputs during the surge than the luteal phase. *, P < 0.05. Data presented as means ± SEM.
Statistical analysis also showed a main effect of region for single Kiss (F1,12= 5.399; P = 0.039) and dual-labeled Kiss + vGlut2 (F1,12= 29.179; P < 0.01) inputs. Post hoc comparisons revealed that POA GnRH neurones receive significantly more single-labeled kisspeptin inputs than MBH GnRH cells, regardless of phase of the oestrous cycle (P = 0.039; Table 2), and that MBH GnRH neurones receive significantly more Kiss + vGlut2 inputs during the surge (P < 0.01) than during luteal phase (Table 2).
In addition to dual-labeled Kiss + vGlut2 inputs onto GnRH cell bodies, we also observed Kiss + vGlut2 fibers within the internal and external zones of the median eminence (Fig. 4). Dual-labeled Kiss + vGlut2 fibers were often seen in close proximity to GnRH fibers and terminals (Fig. 4). Single-labeled vGlut2-positive terminals were also seen in the median eminence, frequently in close proximity to GnRH fibers and terminals.
Figure 4.
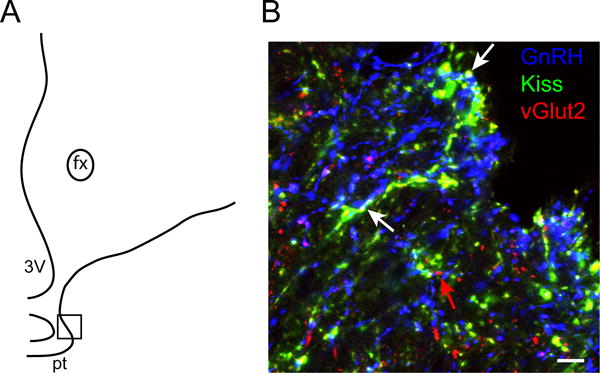
Dual-labeled kisspeptin- and vGlut2-positive fibers in close association to GnRH fibers in the median eminence. A: Schematic drawing of a section through the ovine median eminence, showing the approximate location of the image (boxed area) shown in B. B: Confocal image (1 μm thickness; 63×) of a section through the ovine median eminence triple-labelled for kisspeptin (green), vGlut2 (red) and GnRH (blue). White arrows indicate examples of dual-labelled (yellow) kisspeptin/vGlut2 fibers and terminals adjacent to GnRH fibers (blue), and the red arrow indicates a single labelled vGlut2 terminal. fx = fornix; pt= pars tuberalis; 3V= third ventricle. Scale bar, 10 μm.
DISCUSSION
The results of this study provide novel evidence for neuroplasticity of synaptic inputs to KNDy cells and GnRH neurones across the oestrous cycle in sheep (Fig. 5). Specifically, we found that KNDy neurones in animals perfused during the LH surge had nearly twice the number of total (synaptophysin-positive) inputs per cell as did KNDy cells in luteal phase animals. By contrast, we saw no evidence for changes across the oestrous cycle in synaptic inputs onto POA kisspeptin cells despite evidence that these cells, like KNDy neurones, are activated during the preovulatory LH surge in sheep (28, 51). In parallel to the changes in inputs to KNDy neurones, we also saw increases in the number of inputs to GnRH neurones in the MBH at the time of the LH surge compared to those in the luteal phase, although with the limited markers employed (kisspeptin and vGlut2) we were not able to assess all (e.g., synaptophysin-positive) inputs. As evidence for oestrous cycle-related neuroplasticity, our results complement and extend earlier observations in rodents and reinforce the long held notion that the ARC is a focal region for morphological changes that accompany female reproductive cyclicity (48–49, 61).
Figure 5.
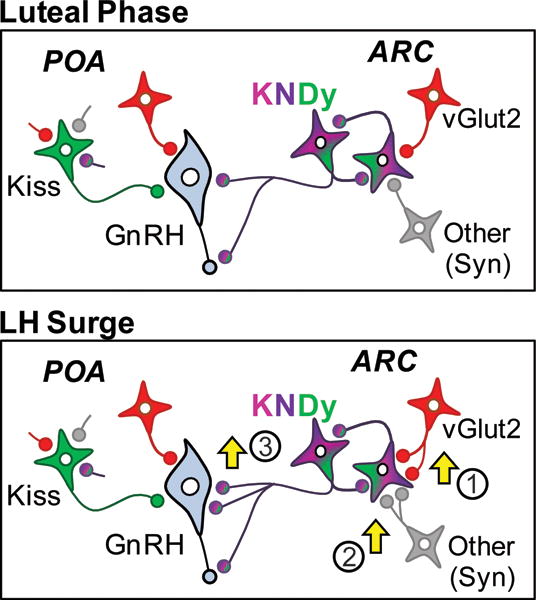
Changes in synaptic inputs to ARC KNDy neurons and MBH GnRH neurons during the preovulatory surge. The present data shows that glutamatergic (as shown by vGlut2 inputs; 1) and other inputs, as represented by synaptophysin-labeled terminals (2), to ARC KNDy neurons are increased at the time of the preovulatory surge. In addition, KNDy (kisspeptin/vGlut2) labeled inputs onto MBH GnRH neurons are increased at the time of the surge (3). The present data also shows that although POA kisspeptin neurones receive vGlut2, synaptophysin and KNDy inputs to their cell bodies, the numbers of these inputs are not significantly different between luteal phase and the surge.
Our results also suggest that, in addition to the three KNDy neuropeptides, this ARC subpopulation in the ewe also contains the excitatory neurotransmitter, glutamate (based on the presence of vGlut2). Our conclusion that KNDy cells are glutamatergic was based on the high percentage (>90%) of dual-labelled kisspeptin/dynorphin terminals that colocalised vGlut2. Two other lines of evidence also support this contention. First, vGlut2 and ER-α are colocalised in the same neurones in the sheep ARC, as shown using dual immunohistochemistry (62). Given that KNDy neurones possess the highest degree of co-localization of ER-α of any neuropeptide cell group of the ARC (1), it is likely that many of these vGlut2/ER-α cells were KNDy neurones. Second, studies in rodents have shown that ARC NKB neurones express vGlut2 protein (43), and ARC kisspeptin cells express vGlut2 mRNA (42), indicating that at least in rodents, these neurones are glutamatergic. One interesting corollary of the observation that KNDy neurones contain vGlut2 is that kisspeptin terminals that do not contain vGlut2 must originate from other kisspeptin neurones. One likely source is the POA kisspeptin neurones, although small populations of kisspeptin neurones have also been described in the dorsomedial and ventromedial hypothalamic nuclei of sheep (1, 4, 25). It is also possible that a subset of KNDy neurones are not glutamatergic, since a small number of KNDy (kisspeptin + dynorphin) terminals in the ARC and POA were not labelled with vGlut2. Ultimately, confirmation of the cellular origin of vGlut2/kisspeptin terminals in sheep awaits the analysis of Kiss1 mRNA and vGlut2 mRNA co-expression in the same neurones.
A collection of evidence supports a direct effect of kisspeptin on GnRH neurones: kisspeptin stimulates GnRH electrophysiological activity (6, 63), GnRH neurones express the Kiss1 receptor (6–7, 64–65), and confocal studies in diverse mammalian species show kisspeptin contacts onto GnRH cell bodies (1). An intriguing finding from our work is that POA and MBH GnRH neurones receive single-labeled kisspeptin inputs as well as dual-labeled vGlut2/kisspeptin inputs. In the POA, single-labeled kisspeptin terminals make up a larger proportion of the total inputs to these neurones, while in the MBH, glutamatergic kisspeptin inputs comprise a larger proportion of the total inputs. This regional difference suggests that kisspeptin inputs to MBH GnRH neurones arise primarily from nearby KNDy cells, whereas the kisspeptin contacts onto POA GnRH neurones likely originate mostly from POA kisspeptin neurones. Interestingly, in rodents, where GnRH neurones are found in the POA but not in the MBH, the primary source of kisspeptin inputs to GnRH neurones is similarly from the rostral kisspeptin population in the RP3V (66).
While the majority of kisspeptin inputs to GnRH cells in the POA appeared as single-labeled terminals, there were a considerable number that colocalised vGlut2 and kisspeptin, suggesting that ARC KNDy cells do provide some input to POA GnRH cells. Moreover, we have shown using other markers of KNDy terminals that KNDy neurones provide direct inputs to GnRH neurones in the POA, as well as in the anterior hypothalamic area and MBH (67). This is supported by tract tracing evidence in the sheep showing retrogradely-labeled glutamatergic cells in the ARC after Flouro-Gold injection into the ventral POA (62). However, injections of anterograde tracers in the sheep ARC have revealed only very few fibers projecting to the POA (68) and contacting GnRH neurones (69). This discrepancy may be due to the limitations of the tracing techniques, since anterograde tracer injections only label a small number of cell bodies in comparison to retrograde tracers that are taken up by terminals arising from many cell bodies. It is likely that our use of dual immunocytochemical markers for KNDy cells is also a more sensitive method of labelling projections than anterograde tracer injections. Conversely, studies in rodents using dual-immunolabeling to identify KNDy projections also show only a small number of fibers in the POA (36, 70–71) however in most of these studies contacts onto GnRH neurones were not analyzed. Our data also support a projection from KNDy neurones to GnRH terminals in the median eminence because GnRH-immunoreactive axons in the external zone of the median eminence are frequently juxtaposed by glutamatergic KNDy axonal fibers (Figs. 4 and 5). These observations are consistent with other data in sheep (65) and support a proposed stimulatory action of kisspeptin (and KNDy) cells on GnRH nerve terminals located in the neurosecretory zone of the median eminence in a variety of mammals (1).
Previous electron microscopic and confocal studies demonstrated that vGlut2-containing terminals provide synaptic inputs onto POA GnRH cells in both rodents and sheep (72–76) and that these inputs increase on the day of the LH surge in rats (72, 74) and during the breeding season in the ewe (76). Our data extends previous findings of seasonal plasticity in sheep to show that the number of glutamatergic inputs to MBH GnRH neurones, and specifically that of dual-labeled glutamatergic/kisspeptin inputs, increases during the preovulatory surge. This change is consistent with evidence in rats and mice implicating glutamate in the preovulatory LH surge: glutamate receptor antagonists block the LH surge (77), glutamate neurotransmission to GnRH is increased during positive feedback (78–79), and the expression and activation of glutamate receptors on GnRH neurones are increased in the presence of E2 and during the preovulatory surge (80–81). These data are also consistent with the hypothesis that KNDy neurones play a key role in the activation of GnRH neurones at the time of the LH surge (50, 82–83).
Our findings extend previous observations of neurochemical (29) and morphological (84) differences between ARC and POA kisspeptin neurones to include differences in synaptic input. In luteal phase animals, ARC kisspeptin (KNDy) cells received more vGlut2/kisspeptin (KNDy) inputs than POA kisspeptin cells (see Table 1). Moreover, synaptic inputs to ARC kisspeptin cells, but not those in the POA, increased during the preovulatory surge. Interestingly, this increase in total synaptic inputs reflected an increase in glutamatergic terminals (and other unidentified synaptic terminals), but not kisspeptin-containing synapses. Although there is evidence for inhibitory inputs to ARC kisspeptin neurons in other species (85–87), these unidentified synaptic terminals most likely reflect excitatory inputs because Fos expression is markedly elevated in ovine KNDy neurons during the LH surge (28). In rodents, synaptic plasticity occurs across the oestrous cycle, with an increase in axosomatic synapses onto RP3V neurones during proestrous (47, 88) that is oestrogen-dependent (49); however the phenotype of the neurones that are targets of this plasticity has not been described. In rodents the RP3V is the site of E2 positive feedback (20), while in sheep, this site lies within the MBH (89). These anatomical and temporal correlations in both rodents and sheep raise the possibility that this increase in the number of glutamatergic synaptic inputs may be an important mechanism in the control of GnRH and gonadotrophin secretion leading to the generation of the preovulatory surge. In future work it will be of interest to test this hypothesis by determining if these changes are produced by the follicular phase rise in E2 concentrations by comparing inputs in estrogen-treated animals with those in the follicular phase.
Our observation of colocalised kisspeptin and glutamate in terminals contacting GnRH neurones is consistent with the hypothesis that these two stimulatory neurotransmitters act synergistically. Studies in middle aged female rats reveal that delayed and attenuated LH surges are correlated with both a decrease in glutamatergic neurotransmission and Kiss1 mRNA, and that infusion of kisspeptin restores surge amplitude and glutamate levels in these rats (79, 90–91). Interestingly, blockade of glutamate receptor activation can prevent this restoration of surge amplitude by kisspeptin, while still maintaining heightened glutamate levels (90). These data do not eliminate the possibility that glutamate is acting at least in part, in an autoregulatory fashion upon the same kisspeptin neurones from which it is released, but also suggests that kisspeptin neurones may provide afferent input to other glutamatergic neurones. Electrophysiological studies demonstrate both a direct and an indirect effect of kisspeptin on GnRH, suggesting that glutamate release may be occurring downstream of kisspeptin neurones (63, 90). Thus, the action of glutamate is most likely occurring at various levels of the system (i.e. on kisspeptin and KNDy neurones, within the KNDy-KNDy circuitry, and downstream of kisspeptin), and the physiological role at each level remains to be determined.
In summary, our results suggest that KNDy neurones in the female sheep are glutamatergic, and that glutamatergic markers are present within terminals that constitute reciprocal circuitry among KNDy neurones, as well as in their inputs to GnRH cells. In addition, we found that inputs to both KNDy and MBH GnRH neurones are increased during the preovulatory surge compared to those during the luteal phase, suggesting a role for the ARC KNDy neurones and glutamatergic synaptic inputs in positive feedback regulation of GnRH. Finally, the results reveal neuroplasticity of the kisspeptin-GnRH circuit across the oestrous cycle, and thus highlight the complexity of this network in the feedback regulation of reproduction.
Acknowledgments
We thank Heather Bungard and Jennifer Lydonat (West Virginia University Food and Animal Research Facility) for care of animals, and Paul Harton for his technical assistance in sectioning tissue. We also thank Dr. Al Parlow and the National Hormone and Peptide Program for reagents used to measure LH.
Supported by: NIH RO1 HD039916 (M.N.L. and R.L.G.)
Footnotes
Disclosure Summary: the authors have nothing to disclose.
References
- 1.Lehman MN, Merkley CM, Coolen LM, Goodman RL. Anatomy of the kisspeptin neural network in mammals. Brain Research. 2010:136490–102. doi: 10.1016/j.brainres.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oakley AE, Clifton DK, Steiner RA. Kisspeptin Signaling in the Brain. Endocr Rev. 2009;30(6):713–43. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K-I. Involvement of Anteroventricular Periventricular Matastin/Kisspeptin Neurons in Estrogen Positive Feedback Action on Leutentizing Hormone Release in Female Rats. Journal of Reproduction and Development. 2007;53(2):367–78. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 4.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neuroscience Letters. 2006;401(3):225–30. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A Role for Kisspeptins in the Regulation of Gonadotropin Secretion in the Mouse. Endocrinology. 2004;145(9):4073–7. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 6.Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of Gonadotropin-Releasing Hormone Neurons by Kisspeptin as a Neuroendocrine Switch for the Onset of Puberty. J Neurosci. 2005;25(49):11349–56. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin Activation of Gonadotropin Releasing Hormone Neurons and Regulation of KiSS-1 mRNA in the Male Rat. Neuroendocrinology. 2004;80(4):264–72. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K-I. Involvement of Central Metastin in the Regulation of Preovulatory Luteinizing Hormone Surge and Estrous Cyclicity in Female Rats. Endocrinology. 2005;146(10):4431–6. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 9.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and Hormonally Regulated Messenger Ribonucleic Acid Expression of KiSS-1 and Its Putative Receptor, GPR54, in Rat Hypothalamus and Potent Luteinizing Hormone-Releasing Activity of KiSS-1 Peptide. Endocrinology. 2004;145(10):4565–74. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 10.Thompson E, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and Peripheral Administration of Kisspeptin-10 Stimulates the Hypothalamic-Pituitary-Gonadal Axis. Journal of Neuroendocrinology. 2004;16(10):850–8. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- 11.Caraty A, Smith JT, Lomet D, Ben Said S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. Kisspeptin Synchronizes Preovulatory Surges in Cyclical Ewes and Causes Ovulation in Seasonally Acyclic Ewes. Endocrinology. 2007;148(11):5258–67. doi: 10.1210/en.2007-0554. [DOI] [PubMed] [Google Scholar]
- 12.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MBL, Colledge WH, Caraty A, Aparicio SAJR. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(5):1761–6. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(6):2129–34. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayasena CN, Nijher GMK, Chaudhri OB, Murphy KG, Ranger A, Lim A, Patel D, Mehta A, Todd C, Ramachandran R, Salem V, Stamp GW, Donaldson M, Ghatei MA, Bloom SR, Dhillo WS. Subcutaneous Injection of Kisspeptin-54 Acutely Stimulates Gonadotropin Secretion in Women with Hypothalamic Amenorrhea, But Chronic Administration Causes Tachyphylaxis. J Clin Endocrinol Metab. 2009;94(11):4315–23. doi: 10.1210/jc.2009-0406. [DOI] [PubMed] [Google Scholar]
- 15.Maeda K, Adachi S, Inoue K, Ohkura S, Tsukamura H. Metastin/Kisspeptin and control of the estrous cycle in rats. Reviews in Endocrine & Metabolic Disorders. 2007;8(1):21–9. doi: 10.1007/s11154-007-9032-6. [DOI] [PubMed] [Google Scholar]
- 16.Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, Morgan K, Pinilla L, Millar RP, Tena-Sempere M. Critical Roles of Kisspeptins in Female Puberty and Preovulatory Gonadotropin Surges as Revealed by a Novel Antagonist. Endocrinology. 2010;151(2):722–30. doi: 10.1210/en.2009-0803. [DOI] [PubMed] [Google Scholar]
- 17.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of Potent Kisspeptin Antagonists Delineate Physiological Mechanisms of Gonadotropin Regulation. J Neurosci. 2009;29(12):3920–9. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001–12. doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- 19.Guerriero KA, Keen KL, Millar RP, Terasawa E. Developmental Changes in GnRH Release in Response to Kisspeptin Agonist and Antagonist in Female Rhesus Monkeys (Macaca mulatta): Implication for the Mechanism of Puberty. Endocrinology. 2012;153(2):825–36. doi: 10.1210/en.2011-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: The case for the rostral periventricular area of the third ventricle (RP3V) Brain Research Reviews. 2008;57(2):277–87. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 Messenger Ribonucleic Acid Expression in the Hypothalamus of the Ewe Is Regulated by Sex Steroids and Season. Endocrinology. 2007;148(3):1150–7. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- 22.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 Gene Expression in the Brain of the Female Mouse. Endocrinology. 2005;146(9):3686–92. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 23.Goodman RL, Inskeep EK. Neuroendocrine Control of Gonadotropin Secretion: Comparative Aspects. In: Plant TM, Zeleznik AJ, editors. Knobil and Neill’s Physiology of Reproduction. 4th. Amsterdam: Elsevier; 2014. pp. 1537–74. [Google Scholar]
- 24.Bosch MA, Xue C, Rønnekleiv OK. Kisspeptin expression in guinea pig hypothalamus: Effects of 17β-estradiol. The Journal of Comparative Neurology. 2012;520(10):2143–62. doi: 10.1002/cne.23032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. Elevated KiSS-1 Expression in the Arcuate Nucleus Prior to the Cyclic Preovulatory Gonadotrophin-Releasing Hormone/Lutenising Hormone Surge in the Ewe Suggests a Stimulatory Role for Kisspeptin in Oestrogen-Positive Feedback. Journal of Neuroendocrinology. 2006;18(10):806–9. doi: 10.1111/j.1365-2826.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith JT, Li Q, Pereira A, Clarke IJ. Kisspeptin Neurons in the Ovine Arcuate Nucleus and Preoptic Area Are Involved in the Preovulatory Luteinizing Hormone Surge. Endocrinology. 2009;150(12):5530–8. doi: 10.1210/en.2009-0712. [DOI] [PubMed] [Google Scholar]
- 27.Smith JT, Shahab M, Pereira A, Pau KY, Clarke IJ. Hypothalamic expression of KISS1 and gonadotropin inhibitory hormone genes during the menstrual cycle of a non-human primate. Biol Reprod. 2010;83(4):568–77. doi: 10.1095/biolreprod.110.085407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, Goodman RL, Lehman MN. KNDy (Kisspeptin/Neurokinin B/Dynorphin) Neurons Are Activated during Both Pulsatile and Surge Secretion of LH in the Ewe. Endocrinology. 2012;153(11):5406–14. doi: 10.1210/en.2012-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin Neurons in the Arcuate Nucleus of the Ewe Express Both Dynorphin A and Neurokinin B. Endocrinology. 2007;148(12):5752–60. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 30.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B Stimulates GnRH Release in the Male Monkey (Macaca mulatta) and Is Colocalized with Kisspeptin in the Arcuate Nucleus. Endocrinology. 2010;151(9):4494–503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topaloglu AK. Neurokinin B signaling in puberty: Human and animal studies. Molecular and Cellular Endocrinology. 2010;324(1–2):64–9. doi: 10.1016/j.mce.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology. 2012;153(6):2756–65. doi: 10.1210/en.2011-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence That Dynorphin Plays a Major Role in Mediating Progesterone Negative Feedback on Gonadotropin-Releasing Hormone Neurons in Sheep. Endocrinology. 2004;145(6):2959–67. doi: 10.1210/en.2003-1305. [DOI] [PubMed] [Google Scholar]
- 34.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of Gonadotropin-Releasing Hormone Secretion by Kisspeptin/Dynorphin/Neurokinin B Neurons in the Arcuate Nucleus of the Mouse. J Neurosci. 2009;29(38):11859–66. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. The Journal of Comparative Neurology. 2006;498(5):712–26. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- 36.True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of Arcuate Nucleus Kisspeptin/Neurokinin B Neuronal Projections and Regulation during Lactation in the Rat. Journal of Neuroendocrinology. 2011;23(1):52–64. doi: 10.1111/j.1365-2826.2010.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K-I, Steiner RA, Okamura H. Neurokinin B and Dynorphin A in Kisspeptin Neurons of the Arcuate Nucleus Participate in Generation of Periodic Oscillation of Neural Activity Driving Pulsatile Gonadotropin-Releasing Hormone Secretion in the Goat. J Neurosci. 2010;30(8):3124–32. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hrabovszky E, Ciofi P, Vida B, Horvath MC, Keller E, Caraty A, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z, Kallo I. The kisspeptin system of the human hypothalamus: sexual dimorphism and relationship with gonadotropin-releasing hormone and neurokinin B neurons. European Journal of Neuroscience. 2010;31(11):1984–98. doi: 10.1111/j.1460-9568.2010.07239.x. [DOI] [PubMed] [Google Scholar]
- 39.Hrabovszky E, Sipos MT, Molnár CS, Ciofi P, Borsay BÁ, Gergely P, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Low Degree of Overlap Between Kisspeptin, Neurokinin B, and Dynorphin Immunoreactivities in the Infundibular Nucleus of Young Male Human Subjects Challenges the KNDy Neuron Concept. Endocrinology. 2012;153(10):4978–89. doi: 10.1210/en.2012-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of Dynorphin A and Neurokinin B Immunoreactivity in the Arcuate Nucleus and Median Eminence of the Sheep. Journal of Neuroendocrinology. 2006;18(7):534–41. doi: 10.1111/j.1365-2826.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- 41.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166(2):680–97. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazão R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011:17337–56. doi: 10.1016/j.neuroscience.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ciofi P, Leroy D, Tramu G. Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience. 2006;141(4):1731–45. doi: 10.1016/j.neuroscience.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 44.Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The Expression of Vesicular Glutamate Transporters Defines Two Classes of Excitatory Synapse. Neuron. 2001;31(2):247–60. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- 45.Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neuroscience Research. 2002;42(4):243–50. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Segura LM, Hernandez P, Olmos G, Tranque PA, Naftolin F. Neuronal membrane remodelling during the oestrus cycle: a freeze-fracture study in the arcuate nucleus of the rat hypothalamus. J Neurocytol. 1988;17(3):377–83. doi: 10.1007/BF01187859. [DOI] [PubMed] [Google Scholar]
- 47.Olmos G, Naftolin F, Perez J, Tranque PA, Garcia-Segura LM. Synaptic remodeling in the rat arcuate nucleus during the estrous cycle. Neuroscience. 1989;32(3):663–7. doi: 10.1016/0306-4522(89)90288-1. [DOI] [PubMed] [Google Scholar]
- 48.Naftolin F, Garcia-Segura LM, Horvath TL, Zsarnovszky A, Demir N, Fadiel A, Leranth C, Vondracek-Klepper S, Lewis C, Chang A, Parducz A. Estrogen-Induced Hypothalamic Synaptic Plasticity and Pituitary Sensitization in the Control of the Estrogen-Induced Gonadotrophin Surge. Reproductive Sciences. 2007;14(2):101–16. doi: 10.1177/1933719107301059. [DOI] [PubMed] [Google Scholar]
- 49.Naftolin F, Mor G, Horvath TL, Luquin S, Fajer AB, Kohen F, Garcia-Segura LM. Synaptic remodeling in the arcuate nucleus during the estrous cycle is induced by estrogen and precedes the preovulatory gonadotropin surge. Endocrinology. 1996;137(12):5576–80. doi: 10.1210/endo.137.12.8940386. [DOI] [PubMed] [Google Scholar]
- 50.Moenter SM, Karsch FJ, Lehman MN. Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology. 1993;133(2):896–903. doi: 10.1210/endo.133.2.8344224. [DOI] [PubMed] [Google Scholar]
- 51.Hoffman GE, Le WW, Franceschini I, Caraty A, Advis JP. Expression of Fos and in Vivo Median Eminence Release of LHRH Identifies an Active Role for Preoptic Area Kisspeptin Neurons in Synchronized Surges of LH and LHRH in the Ewe. Endocrinology. 2011;152(1):214–22. doi: 10.1210/en.2010-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Cleef J, Karsch FJ, Padmanabhan V. Characterization of endocrine events during the periestrous period in sheep after estrous synchronization with controlled internal release (CIDR) device. Domestic Animal Endocrinology. 1998;15(1):23–34. doi: 10.1016/s0739-7240(97)00059-3. [DOI] [PubMed] [Google Scholar]
- 53.Niswender G, Reichert LE, Jr, Midgley AR, Jr, Nalbandov AV. Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology. 1969:841166–73. doi: 10.1210/endo-84-5-1166. [DOI] [PubMed] [Google Scholar]
- 54.Padmanabhan V, Evans NP, Dahl GE, McFadden KL, Mauger DT, Karsch FJ. Evidence for short or ultrashort loop negative feedback of GnRH secretion. Neuroendocrinology. 1995:62242–58. doi: 10.1159/000127011. [DOI] [PubMed] [Google Scholar]
- 55.Hunyady B, Krempels K, Harta G, Mezey E. Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J Histochem Cytochem. 1996;44(12):1353–62. doi: 10.1177/44.12.8985127. [DOI] [PubMed] [Google Scholar]
- 56.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in Kisspeptin and RFamide-Related Peptide (RFRP) Expression and Terminal Connections to Gonadotropin-Releasing Hormone Neurons in the Brain: A Novel Medium for Seasonal Breeding in the Sheep. Endocrinology. 2008;149(11):5770–82. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407(6801):189–94. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- 58.Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of Differentiation-Associated Brain-Specific Phosphate Transporter as a Second Vesicular Glutamate Transporter (VGLUT2) The Journal of Neuroscience. 2001;21(22):RC182. doi: 10.1523/JNEUROSCI.21-22-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herzog E, Bellenchi GC, Gras C, Bernard V, Ravassard P, Bedet C, Gasnier B, Giros B, El Mestikawy S. The Existence of a Second Vesicular Glutamate Transporter Specifies Subpopulations of Glutamatergic Neurons. The Journal of Neuroscience. 2001;21(22):RC181. doi: 10.1523/JNEUROSCI.21-22-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakata-Haga H, Kanemoto M, Maruyama D, Hoshi K, Mogi K, Narita M, Okado N, Ikeda Y, Nogami H, Fukui Y, Kojima I, Takeda J, Hisano S. Differential localization and colocalization of two neuron-types of sodium-dependent inorganic phosphate cotransporters in rat forebrain. Brain Research. 2001;902(2):143–55. doi: 10.1016/s0006-8993(01)02290-9. [DOI] [PubMed] [Google Scholar]
- 61.Parducz A, Zsarnovszky A, Naftolin F, Horvath TL. Estradiol affects axo-somatic contacts of neuroendocrine cells in the arcuate nucleus of adult rats. Neuroscience. 2003;117(4):791–4. doi: 10.1016/s0306-4522(02)00967-3. [DOI] [PubMed] [Google Scholar]
- 62.Pompolo S, Pereira A, Scott CJ, Fujiyma F, Clarke IJ. Evidence for estrogenic regulation of gonadotropin-releasing hormone neurons by glutamatergic neurons in the ewe brain: An immunohistochemical study using an antibody against vesicular glutamate transporter-2. The Journal of Comparative Neurology. 2003;465(1):136–44. doi: 10.1002/cne.10805. [DOI] [PubMed] [Google Scholar]
- 63.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin Acts Directly and Indirectly to Increase Gonadotropin-Releasing Hormone Neuron Activity and Its Effects Are Modulated by Estradiol. Endocrinology. 2008;149(4):1979–86. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herbison AE, d’Anglemont de Tassigny X, Doran J, Colledge WH. Distribution and Postnatal Development of Gpr54 Gene Expression in Mouse Brain and Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2010;151(1):312–21. doi: 10.1210/en.2009-0552. [DOI] [PubMed] [Google Scholar]
- 65.Smith JT, Li Q, Sing Yap K, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin Is Essential for the Full Preovulatory LH Surge and Stimulates GnRH Release from the Isolated Ovine Median Eminence. Endocrinology. 2011en:2010–1225. doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- 66.Clarkson J, Herbison AE. Postnatal Development of Kisspeptin Neurons in Mouse Hypothalamus; Sexual Dimorphism and Projections to Gonadotropin-Releasing Hormone Neurons. Endocrinology. 2006;147(12):5817–25. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Merkley CM, Coolen LM, Goodman RL, Lehman MN. Direct projections of arcuate KNDy (Kisspeptin/Neurokinin B/Dynorphin) neurons to GnRH neurons in the ewe. Ann Meeting Soc Neurosci. 2011 Abstract 712. [Google Scholar]
- 68.Backholer K, Smith J, Clarke IJ. Melanocortins May Stimulate Reproduction by Activating Orexin Neurons in the Dorsomedial Hypothalamus and Kisspeptin Neurons in the Preoptic Area of the Ewe. Endocrinology. 2009;150(12):5488–97. doi: 10.1210/en.2009-0604. [DOI] [PubMed] [Google Scholar]
- 69.Pompolo S, Rawson JA, Clarke IJ. Projections from the arcuate/ventromedial region of the hypothalamus to the preoptic area and bed nucleus of stria terminalis in the brain of the ewe; lack of direct input to gonadotropin-releasing hormone neurons. Brain Research. 2001;904(1):1–12. doi: 10.1016/s0006-8993(01)02372-1. [DOI] [PubMed] [Google Scholar]
- 70.Kalló I, Vida B, Deli L, Molnár CS, Hrabovszky E, Caraty A, Ciofi P, Coen CW, Liposits Z. Co-Localisation of Kisspeptin with Galanin or Neurokinin B in Afferents to Mouse GnRH Neurones. Journal of Neuroendocrinology. 2012;24(3):464–76. doi: 10.1111/j.1365-2826.2011.02262.x. [DOI] [PubMed] [Google Scholar]
- 71.Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A, Franceschini I. Mapping of Kisspeptin Fibres in the Brain of the Pro-Oestrous Rat. Journal of Neuroendocrinology. 2010;22(10):1101–12. doi: 10.1111/j.1365-2826.2010.02053.x. [DOI] [PubMed] [Google Scholar]
- 72.Khan M, De Sevilla L, Mahesh VB, Brann DW. Enhanced Glutamatergic and Decreased Gabaergic Synaptic Appositions to GnRH Neurons on Proestrus in the Rat: Modulatory Effect of Aging. PLoS ONE. 2010;5(4):e10172. doi: 10.1371/journal.pone.0010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kiss J, Kocsis K, Csáki Á, Halász B. Evidence for vesicular glutamate transporter synapses onto gonadotropin-releasing hormone and other neurons in the rat medial preoptic area. European Journal of Neuroscience. 2003;18(12):3267–78. doi: 10.1111/j.1460-9568.2003.03085.x. [DOI] [PubMed] [Google Scholar]
- 74.Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-Phenotype GABA/Glutamate Neurons in Adult Preoptic Area: Sexual Dimorphism and Function. J Neurosci. 2004;24(37):8097–105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pompolo S, Periera A, Kaneko T, Clarke IJ. Seasonal Changes in the Inputs to Gonadotropin-Releasing Hormone Neurones in the Ewe Brain: An Assessment by Conventional Fluorescence and Confocal Microscopy. Journal of Neuroendocrinology. 2003;15(5):538–45. doi: 10.1046/j.1365-2826.2003.01030.x. [DOI] [PubMed] [Google Scholar]
- 76.Sergeeva S, Jansen HT. Neuroanatomical plasticity in the gonadotropin-releasing hormone system of the ewe: Seasonal variation in glutamatergic and gamma-aminobutyric acidergic afferents. The Journal of Comparative Neurology. 2009;515(6):615–28. doi: 10.1002/cne.22087. [DOI] [PubMed] [Google Scholar]
- 77.Brann DW, Mahesh VB. Endogenous Excitatory Amino Acid Involvement in the Preovulatory and Steroid-Induced Surge of Gonadotropins in the Female Rat. Endocrinology. 1991;128(3):1541–7. doi: 10.1210/endo-128-3-1541. [DOI] [PubMed] [Google Scholar]
- 78.Christian CA, Moenter SM. Critical Roles for Fast Synaptic Transmission in Mediating Estradiol Negative and Positive Feedback in the Neural Control of Ovulation. Endocrinology. 2008;149(11):5500–8. doi: 10.1210/en.2008-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neal-Perry GS, Zeevalk GD, Shu J, Etgen AM. Restoration of the Luteinizing Hormone Surge in Middle-Aged Female Rats by Altering the Balance of GABA and Glutamate Transmission in the Medial Preoptic Area. Biol Reprod. 2008;79(5):878–88. doi: 10.1095/biolreprod.108.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adjan V, Centers A, Jennes L. Expression and Activation of N-Methyl-d-Aspartate Receptor Subunit-1 Receptor Subunits in Gonadotrophin-Releasing Hormone Neurones of Young and Middle-Aged Mice During the Luteinising Hormone Surge. Journal of Neuroendocrinology. 2008;20(10):1147–54. doi: 10.1111/j.1365-2826.2008.01775.x. [DOI] [PubMed] [Google Scholar]
- 81.Bailey J, Centers A, Jennes L. Expression of AMPA Receptor Subunits (GluR1–GluR4) in Gonadotrophin-Releasing Hormone Neurones of Young and Middle-Aged Persistently Oestrous Rats During the Steroid-Induced Luteinising Hormone Surge. Journal of Neuroendocrinology. 2006;18(1):1–12. doi: 10.1111/j.1365-2826.2005.01361.x. [DOI] [PubMed] [Google Scholar]
- 82.Lehman MN, Coolen LM, Goodman RL. Minireview: Kisspeptin/Neurokinin B/Dynorphin (KNDy) Cells of the Arcuate Nucleus: A Central Node in the Control of Gonadotropin-Releasing Hormone Secretion. Endocrinology. 2010;151(8):3479–89. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hoffman GE, Lee WS, Attardi B, Yann V, Fitzsimmons MD. Luteinizing hormone-releasing hormone neurons express c-fos antigen after steroid activation. Endocrinology. 1990;126(3):1736–41. doi: 10.1210/endo-126-3-1736. [DOI] [PubMed] [Google Scholar]
- 84.Ducret E, Gaidamaka G, Herbison AE. Electrical and Morphological Characteristics of Anteroventral Periventricular Nucleus Kisspeptin and Other Neurons in the Female Mouse. Endocrinology. 2010;151(5):2223–32. doi: 10.1210/en.2009-1480. [DOI] [PubMed] [Google Scholar]
- 85.Poling MC, Quennell JH, Anderson GM, Kauffman AS. Kisspeptin Neurones do not Directly Signal to RFRP–3 Neurones but RFRP–3 may Directly Modulate a Subset of Hypothalamic Kisspeptin Cells in Mice. Journal of Neuroendocrinology. 2013;25(10):876–86. doi: 10.1111/jne.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurian JR, Keen KL, Guerriero KA, Terasawa E. Tonic Control of Kisspeptin Release in Prepubertal Monkeys: Implications to the Mechanism of Puberty Onset. Endocrinology. 2012;153(7):3331–6. doi: 10.1210/en.2012-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frazão R, Cravo RM, Donato J, Ratra DV, Clegg DJ, Elmquist JK, Zigman JM, Williams KW, Elias CF. Shift in Kiss1 Cell Activity Requires Estrogen Receptor α. The Journal of Neuroscience. 2013;33(7):2807–20. doi: 10.1523/JNEUROSCI.1610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Langub JMC, Maley BE, Watson JRE. Estrous cycle-associated axosomatic synaptic plasticity upon estrogen receptive neurons in the rat preoptic area. Brain Research. 1994;641(2):303–10. doi: 10.1016/0006-8993(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 89.Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A. Evidence That the Mediobasal Hypothalamus Is the Primary Site of Action of Estradiol in Inducing the Preovulatory Gonadotropin Releasing Hormone Surge in the Ewe. Endocrinology. 1998;139(4):1752–60. doi: 10.1210/endo.139.4.5904. [DOI] [PubMed] [Google Scholar]
- 90.Neal-Perry G, Lebesgue D, Lederman M, Shu J, Zeevalk GD, Etgen AM. The Excitatory Peptide Kisspeptin Restores the Luteinizing Hormone Surge and Modulates Amino Acid Neurotransmission in the Medial Preoptic Area of Middle-Aged Rats. Endocrinology. 2009;150(8):3699–708. doi: 10.1210/en.2008-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neal-Perry GS, Zeevalk GD, Santoro NF, Etgen AM. Attenuation of Preoptic Area Glutamate Release Correlates with Reduced Luteinizing Hormone Secretion in Middle-Aged Female Rats. Endocrinology. 2005;146(10):4331–9. doi: 10.1210/en.2005-0575. [DOI] [PubMed] [Google Scholar]


