Abstract
Neural connectivity requires proper polarization of neurons, guidance to appropriate target locations, and establishment of synaptic connections. From when neurons are born to when they finally reach their synaptic partners, neurons undergo constant rearrangment of the cytoskeleton to achieve appropriate shape and polarity. Of particular importance to neuronal guidance to target locations is the growth cone at the tip of the axon. Growth-cone steering is also dictated by the underlying cytoskeleton. All these changes require spatiotemporal control of the cytoskeletal machinery. This review summarizes the proteins that are involved in modulating the actin and microtubule cytoskeleton during the various stages of neuronal development.
1. INTRODUCTION
The development and function of the vertebrate nervous system is founded upon the formation of appropriate synaptic connections between neurons that are often located in distant regions of the organism. The complex neural network, in which each neuron synapses onto multiple postsynaptic partners and receives inputs from multiple presynaptic partners, is established by directional migration of nascent neurons from sites of neurogenesis to specific locations in the organism, the directional extension and branching of the presynaptic axon toward distal synaptic sites, and the ramification of postsynaptic dendrites and formation of dendritic spines. A failure to establish proper neural connectivity due to specific genetic mutations is associated with many neurological and neuropsychiatric conditions including multiple congenital cranial dysinnvervation disorders, mirror movement disorders, L1 syndrome, Joubert syndrome, and Kallmann syndrome, among others (Engle, 2010). Furthermore, variation in a number of genes associated with polygenic complex trait conditions including schizophrenia, bipolar disorder, and autism are also associated with improper connectivity (Connor et al., 2011; Mingorance-Le Meur and O’Connor, 2009; Wolff et al., 2012). Therefore a thorough understanding of the molecular mechanisms dictating proper formation of a functional nervous system is not only an exciting area of fundamental scientific research, but also a critical area of scientific investigation to improve the human condition.
2. CYTOSKELETAL DYNAMICS AND ORGANIZATION DURING NEURONAL DEVELOPMENT
The shape, polarization, and motility of all cell types are dictated by intracellular filamentous networks that constitute the cytoskeleton, including microfilaments or filamentous actin (F-actin) composed of polymerized globular actin (G-actin), intermediate filaments, which in the nervous system are neurofilaments composed of intermediate filament proteins, and the microtubule (MT) cytoskeleton comprising protofilaments of αβ-tubulin heterodimers. The polymerization and depolymerization dynamics of these cytoskeletons, their organization into higher-order structures or architecture, and the carefully orchestrated interplay between the cytoskeletons are driving forces behind cell membrane protrusion and neuronal shape change and stability. Pharmacological inhibitors that disrupt the polymerization or depolymerization of F-actin or MTs have demonstrated that dynamics of F-actin and MTs are essential for proper polarization, migration and guidance of neurons, and steering of axons toward target locations. For example, perturbing actin dynamics with cytochalasin D, which prevents actin polymerization at the fast growing “barbed” ends, prevents neurite initiation. Similarly jasplakinolide treatment, which blocks F-actin depolymerization, induces axon retraction (Dent et al., 2007; Gallo et al., 2002). Similarly altering MT stability using low concentrations of taxol, which promotes polymerization at the MT plus end, facilitates axon regeneration, whereas colchicine or colcemid treatment, which prevents MT polymerization, prevents axon elongation (Bamburg et al., 1986; Gomez and Letourneau, 2013; Sengottuvel et al., 2011).
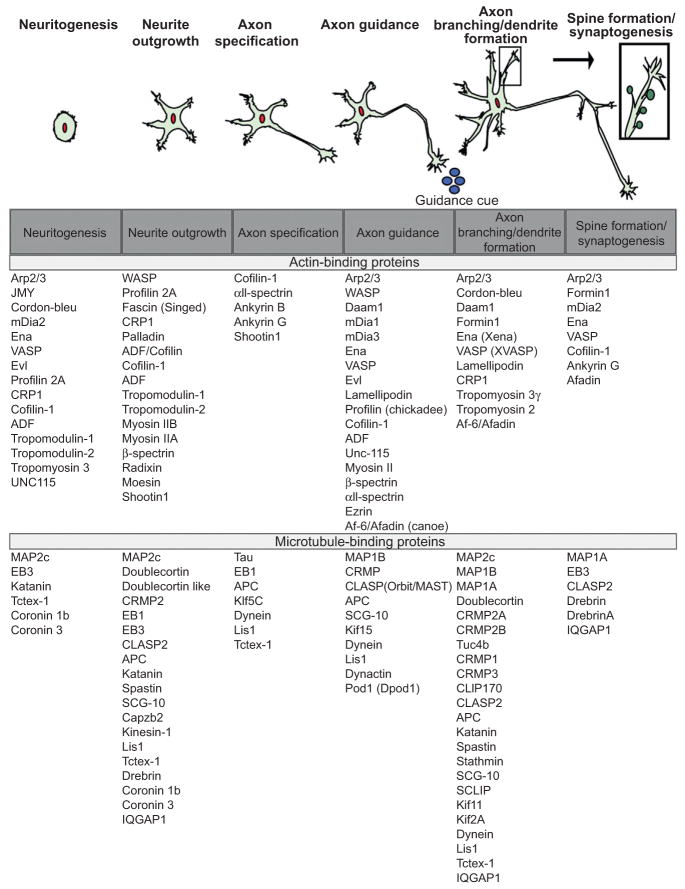
In vivo newly born neurons are spherical shaped, whereas dissociated neurons cultured on a 2D surface in vitro are initially asymmetrical, but flattened onto the substrate in a pancake-like shape (stage 1). Cultured neurons undergo a stereotypical progression of shape changes during developmental neurogenesis (Bradke and Dotti, 2000; Dotti et al., 1988) (Fig. 1). F-actin rich sheet-like lamellipodial protrusions and finger-like filopodial protrusions decorate the periphery of immature neurons. The lamellipodia and filopodia coalesce into thin minor neurites (stage 2) (Dehmelt and Halpain, 2004; Dent et al., 2007; Mingorance-Le Meur and O’Connor, 2009). Whereas initially each neurite process is of similar length and composed of nearly equivalent amounts of F-actin and MTs and similar cytoskeletal regulatory proteins, eventually one neurite establishes polarity, accumulates axonal-specific proteins, and elongates at a faster rate during axon specification (stage 3). The remaining neurites subsequently acquire dendritic markers.
Figure 1.
Developmental stages of a neuron. (A) Representation of a neuron as it progresses through developmental stages: neuritogenesis, neurite outgrowth, axon specification, axon guidance in response to guidance cues, axon branching and dendrite formation, spine formation followed by synaptogenesis. (B) List of actin and MT-regulatory proteins involved during the different stages of development.
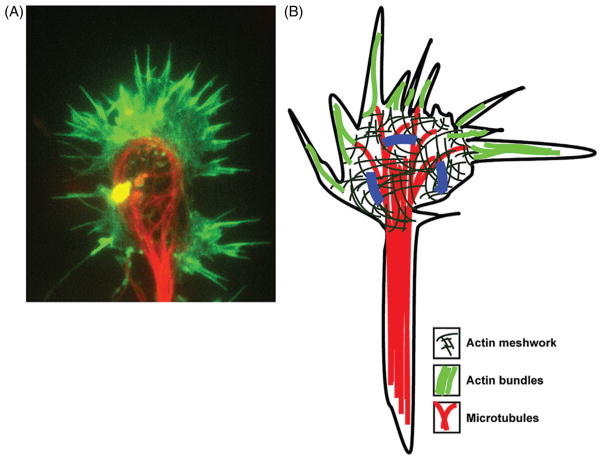
The growth cone at the tip of the elongating axon is a specialized cytoskeletal-based motile structure, which responds to extracellular cues to guide the axon toward postsynaptic partners (Fig. 2). The axonal growth cone is organized into three domains: the central domain, the peripheral domain, and the transition zone (Lowery and Van Vactor, 2009). The central domain, or palm of the growth cone contains MTs extending from the axonal shaft. The F-actin rich lamellipodial veils in the peripheral domain in contrast, are studded with bundled F-actin rich filopodial protrusions and relatively few “pioneering” MTs. In nonneuronal cells, filopodial bundles are thought to arise from branched F-actin networks, but whether this is the case in the growth cone is less clear. In the peripheral zone, barbed filament ends are oriented toward the growth-cone periphery, whereas the depolymerizing “pointed” ends of F-actin lie in the transition zone. When F-actin is engaged to the substrate through adhesion complexes, polymerization at the barbed ends of F-actin is thought to provide the force for filopodial and lamellipodial protrusion during growth-cone translocation (Gomez and Letourneau, 2013). In contrast, incomplete engagement of F-actin to the substrate results in polymerization-dependent retrograde flow of F-actin away from the plasma membrane. As the branched F-actin network moves retrogradely toward the transition zone, it becomes a substrate for nonmuscle myosin II-mediated contraction. Actomyosin contraction further enhances retrograde flow, leading to contraction and reorganization of F-actin into an arc in the transition zone. MTs polymerize into this region, pushing the F-actin arc toward the growth-cone periphery. F-actin depolymerization in the proximal zone and contraction-mediated bundling of MTs reduces the area of the rear of the growth cone as it becomes a part of the axon shaft (Burnette et al., 2008). During axon guidance, extracellular cues modulate cytoskeletal dynamics and F-actin retrograde flow in the growth cone to dictate the rate and directionality of axonal protrusion (Dent et al., 2011).
Figure 2.
Axonal growth cone. (A) A typical mouse cortical neuron growth cone with F-actin labeled with fluorescent phalloidin and MTs labeled with an antibody against neuron specific βIII-tubulin. (B) Representation of a growth cone with numerous filopodia protruding from the lamellipodial veil. Marked in dark gray are MTs that extend through the axon shaft and into the central domain. A few pioneering MTs extend into the peripheral domain of the growth cone. A meshwork of F-actin (thin dashed lines) is seen in the peripheral domain, which is studded with numerous filopodia that have bundled F-actin in them.
The human brain contains approximately 1000-fold more synapses than neurons, indicating that branching of the axon is required to achieve full synaptic capacity. Axon branching similarly requires cytoskeletal rearrangements. Just like in the transition zone of growth cones, F-actin and MTs colocalize and interact at the axon branch points. Pharmacological inhibitors of actin or MT dynamics blocked axon branching and directed axon outgrowth of cortical neurons in vitro (Dent and Kalil, 2001).
F-actin and MT dynamics during dendrite specification and branching differ from axon specification, growth, and branching. Dendritic growth requires strong adhesion to the substrate to counteract actin-driven tensile forces (Chamak and Prochiantz, 1989). The strong substrate adhesion helps to compensate the lack of contractile forces produced by the less densely packed MTs in the dendrites. MTorientation in the dendrites is still a study in progress with reports suggesting that more than 90% of MTs in the dendrites have their minus-end pointed and oriented away from the soma or oriented biaxially. In contrast, MTs in the axons have their plus-end pointed outward (Georges et al., 2008; Stone et al., 2008). Invasion of MTs into dendritic growth-cone filopodia is essential to stabilize the filopodia, which then becomes a dendritic branch. F-actin localizes to the cortex of dendrites and dendritic spines. Dendritic plasticity involves turnover of F-actin (Georges et al., 2008).
Due to the extended cytoskeletal-based processes of neurons, the F-actin, neurofilament, and MT cytoskeletons comprise a significant portion of neuronal cell material. The unique morphology of neurons is accomplished through the activity of numerous cytoskeletal regulatory proteins, which dynamicallyorganize the neuronal cytoskeletal during neuronal morphogenesis (Fig. 1). These regulatory proteins can promote or repress polymerization and/ or depolymerization or organize cytoskeletons into higher-order structures. In this section of the review we summarize the numerous cytoskeletal-associated proteins known to alter the F-actin and MT cytoskeletons to precisely orchestrate cytoskeletal organization and dynamics in developing neurons. In contrast, our understanding of the regulation of the NF cytoskeleton is considerably more rudimentary and requires extensive investigation.
2.1 Actin-Binding Proteins
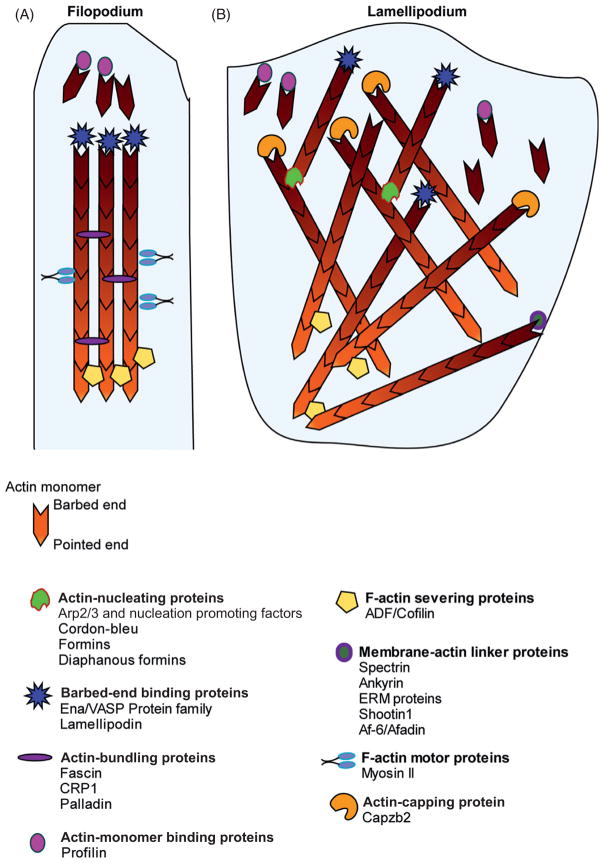
F-actin is a polar, 9-nm wide right-hand coiled filament containing polymerized G-actin. The rate-limiting step of F-actin polymerization is the formation of a stable oligomer of actin comprising three to four G-actin subunits, termed nucleation. Once nucleated, actin filaments can elongate on both ends. However, the ends of the filament have distinct biochemical properties and critical concentrations of G-actin at which they polymerize. The barbed end continues to polymerize at lower concentrations of G-actin (0.1 μM), whereas the critical concentration for the minus end is 0.8 μM. Due to this difference, at G-actin concentrations between these values, barbed ends polymerize while the pointed ends depolymerize, creating an actin treadmill. This treadmill promotes turnover of F-actin in the growth cone. In the following sections we will review the actin-binding proteins that regulate actin dynamics and organization (Fig. 3).
Figure 3.
F-actin and actin-binding proteins. (A) Shows a filopodium with bundled F-actin with the barbed-end pointing outward and the pointed-end toward the inside. Actin monomers, with the aid of actin monomer-binding proteins, are added to the barbed end of F-actin, which is protected by barbed-end binding proteins. Bundling proteins hold the F-actin filaments as bundles. Motor proteins transport cargo along F-actin bundles. Severing proteins disassemble F-actin at the pointed end. (B) Shows a lamellipodial veil decorated with a meshwork of F-actin. Actin-nucleator proteins and capping proteins help form the dendritic actin network.
2.1.1 Actin-Nucleating Proteins
2.1.1.1 Arp2/3 and Nucleation Promoting Factors
In cells the rate-limiting step of F-actin nucleation is accelerated by actin-nucleating proteins. The nucleating Arp2/3 complex is composed of seven proteins, in which actin-related proteins Arp2 and Arp3 mimic a dimer of G-actin and participate in the nucleation of a new “daughter” filament at a ~70 degree angle branch on the side of an existing “mother” filament (Mullins et al., 1998). The Arp2/3 complex stabilizes the pointed end of the new filament to the existing filament and creates a new barbed end for polymerization. In nonneuronal cells, the Arp2/3 complex is involved in the formation of lamellipodia and filopodia, suggesting it would likely be critical for morphology changes of developing neurons (Svitkina and Borisy, 1999; Svitkina et al., 2003). However, work from several labs has indicated that the role of Arp2/3 during different stages of neuronal development is more complex, and likely dependent upon the extracellular environment of the neuron and type of neuron.
Based on the evidence that filopodia and lamellipodia are required precursors of neuritogenesis (Dent et al., 2007), involvement of the Arp2/3 complex in neuritogenesis may be expected. In embryonic mouse cortical neurons this requirement appears to be context dependent, inhibition of Arp2/3 complex by expression of the inhibitory peptide CA only disrupted neuritogenesis stimulated by laminin (Gupton and Gertler, 2010). In contrast, depletion of either the Arp3 or p34 subunit of Arp2/3 in primary neuronal cultures using siRNA reduced the number of lamellipodial and filopodial protrusions, and decreased F-actin retrograde flow during neuritogenesis. This was associated with supernumary neurite formation and decreased neurite length (Korobova and Svitkina, 2008). One explanation for this may be that inhibition of Arp2/3 decreases F-actin stability, thus allowing MTeasier access to the periphery, as has been seen in other contexts to promote neuritogenesis (Flynn et al., 2012). Similarly, mutations in the Arp2/3 genes (Arx genes) in Caenorhabditis elegans are associated with decreased filopodia number and growth-cones size in the PQR neuron (Norris et al., 2009). In contrast, the Lanier group observed that Arp2/3 is predominantly located in the central domain of the growth cone of embryonic mouse hippocampal neurons, and that dominant negative-based inhibition of Arp2/3 function did not inhibit lamellipodia protrusion or filopodia formation, but did increase axon outgrowth and increase aberrant axon crossing of an inhibitory semaphorin 3a substrate (Strasser et al., 2004), suggesting that the inhibition of Arp2/3 complex function disrupts axon guidance in some contexts. In chicken sensory axons, axonal filopodia arising from actin patches along the axon serve as precursors for collateral axon branches (Spillane et al., 2011). Dominant negative inhibition of the Arp2/3 complex impaired the formation of actin patches, and subsequent filopodia formation and collateral branching. Furthermore, platinum replica EM and immunostaining studies in hippocampal neurons indicate that Arp2/3 complex localizes in spine heads and presynaptic boutons, where the F-actin network is in a branched as opposed to bundled architecture (Korobova and Svitkina, 2010). Supporting a role for Arp2/3 in spine formation, siRNA-mediated depletion of the p34 subunit or the Arp3 of Arp2/3 reduced the number of spine heads in dissociated hippocampal cultures (Hotulainen et al., 2009; Wegner et al., 2008). Cre-mediated deletion of the ArpC3 subunit of Arp2/3 in postnatal forebrain excitatory neurons resulted in a gradual loss of spines in vivo preceded by a decrease in the structural plasticity of spines and an accumulation of filopodia-like spines (Kim et al., 2013). Whereas these studies indicate that Arp2/3 functions distinctly in the variety of plasma membrane protrusions in neurons, further studies exploiting conditional deletion of Arp2/3 components or acute inhibition of Arp2/3 function using the pharmacological inhibitor CK-666 are required to better define the role of Arp2/3 in the stages of neuronal morphogenesis and ameliorate the controversies in the field.
Arp2/3-mediated F-actin nucleation requires Arp2/3 activation by one of many nucleation promoting factors (NPFs) such as Wiskott–Aldrich syndrome protein (WASP) family members and/or suppressor of cAMP receptor (Scar) (Rotty et al., 2012). NPFs modulate actin filament nucleation by competing with profilin to bind to G-actin and delivering G-actin to Arp2/3 bound to a preexisting filament. Although many studies have established the function of NPFs in motility and cell shape change in nonneuronal cells, very little is known about NPFs in neuron morphology establishment. Suetsugu et al. suggest that a balance between WASP phosphorylation and degradation is essential to neurite extension and required for axonal growth-cone filopodia formation (Chacon et al., 2012; Suetsugu et al., 2002). Junction-mediating and regulatory protein (JMY) promotes actin polymerization either by functioning as an NPF for the Arp2/3 complex or through its tandem-monomer-binding property independent of Arp2/3 activity. Its function independent of Arp2/3 likely increases the number of available mother filaments to form a branched actin network via Arp2/3 activity (Zuchero et al., 2009). Depletion of JMY via siRNA in Neuro 2a cells increases the number of cells that form neurite-like extensions, suggesting JMY is a negative regulator of neuritogenesis (Firat-Karalar et al., 2011). While WASP and Scar are the well-characterized NPFs in nonneuronal cells, their function in neuronal morphogenesis remains to be determined. It is intriguing to speculate that the multitude of NPFs may function to regulate Arp2/3 function during distinct phases of neuronal development or in response to different extracellular cues. For example, N-WASP is implicated downstream of netrin-1 stimulation (Shekarabi, 2005).
2.1.1.2 Cordon-Bleu
Cordon-bleu (Cobl) is a brain-enriched actin-nucleating protein (Ahuja et al., 2007). Cobl contains three WH2 (WASP homology 2) domains, that tightly bind G-actin and decrease the lag phase of actin polymerization in vitro, indicating that Cobl promotes nucleation of new filaments (Ahuja et al., 2007). Filaments formed by Cobl nucleation are long, unbundled, and unbranched. Overexpression of full-length Cobl or a Cobl construct containing all three WH2 domains, but not constructs containing individual WH2 domain or the C-terminal proline-rich domains lead to a marked increase in both the number of dendrites and the branching along axons and dendrites in primary rat hippocampal neurons (Ahuja et al., 2007). Analogously, RNAi-mediated knockdown of Cobl reduced neurite number and loss of branching (Ahuja et al., 2007). Therefore Cobl-dependent actin nucleation likely promotes neurite formation and branching. Similarly, RNAi-mediated depletion of Cobl in vivo resulted in a significant decrease in dendritic arborization (DA) in Purkinje neurons (Haag et al., 2012), indicating that Cobl is also involved in terminal DA. Whereas the function of Arp2/3 is critical in lamellipodial protrusion in nonneuronal cells, Cobl may function in neurons as a critical actin-nucleating protein for filopodia-dense neurons. Cobl function may also explain why loss of Arp2/3 function in neurons is not always associated with dramatic neuronal morphology change.
2.1.1.3 Formins
Formins are a large family of actin-regulatory proteins that nucleate unbranched actin filaments and subsequently bind processively to the barbed end and promote F-actin polymerization. Several formin family members are implicated in neuronal development. Loss of function of the Drosphila formin DAAM results in loss of filopodia formation in axonal growth cones in the fly embryo (Matusek et al., 2008). Maternal and zygotic null daam1 mutant flies exhibit several gross neuroanatomical defects, including misrouted axons, breaks in the connectives and commissures, a disorganized nerve cord, and in some cases, a complete lack of axon bundles. When cultured in vitro, daam1 mutant neurons show a marked decrease in the number and length of axonal growth-cone filopodia (Matusek et al., 2008), further supporting the hypothesis that filopodia are critical structures in axonal development. Since expression of murine Daam1 could rescue loss of drosophila daam1, this suggests functional conservation of Daam1 between fly and mouse. The role of Daam1 in axonal development in the vertebrate has not yet been established. However, in mouse hippocampal neurons, Daam1 localizes to the shaft of dendrites and to dendritic spines (Salomon et al., 2008). In this study, viral-mediated overexpression of Daam1 in hippocampal slices did not influence dendritic numbers but did decrease the number of spines, suggesting that Daam1 also controls dendritic spine density. In mouse hippocampal neurons, overexpression and siRNA-mediated knockdown experiments suggest that another formin family member, formin1 mediates dendritogenesis and synaptogenesis downstream of the Neurogenin3 transcription factor (Simon-Areces et al., 2011).
2.1.1.3.1 Diaphanous Formins
The three actin-nucleating mammalian formins of the Diaphanous family (mDia1, mDia2, and mDia3) are activated downstream of Rho GTPases (Wasserman, 1998). mDia1 and mDia3 are enriched in the developing and adult brain (Shinohara et al., 2012), and mDia2 expression increases during the later stages of neuronal morphogenesis (Hotulainen et al., 2009). Ectopic expression of mDia2 in embryonic cortical neurons rescues filopodia formation and neuritogenesis caused by deletion of members of the Ena/vasodilator stimulated phosphoprotein (VASP) family of actin-regulatory proteins (Dent et al., 2007). This suggests that although filopodia are a prerequisite of neurite formation, the specific mechanism of their formation may not be critical. Evidence gathered thus far indicates that mDia1 functions in axon elongation in mouse cerebellar granule cells downstream of the neural cytokine SDF-1. siRNA-mediated knockdown of mDia1 or expression of a dominant negative mDia1 inhibits axon elongation stimulated by SDF-1 (Arakawa et al., 2003). Analogously, expression of an active mutant of mDia1 induces axon elongation. Deletion of the genes encoding mDia1 and mDia3 impairs tangential migration of precursors of interneurons from the subventricular zone (Shinohara et al., 2012). Axons of spinal cord neurons from these mice aberrantly cross the midline in vivo (Toyoda et al., 2013). In vitro their axons failed to retract in response to the repulsive cues Ephrin-A5, Ephrin-B3, or Sema3A, suggesting that diaphanous formins are required for collapse responses to these repulsive cues (Toyoda et al., 2013). mDia2 expression increases during the emergence of dendritic spines. mDia2 overexpression reduces the number of spine heads, whereas depleting mDia2 expression leads to the formation of stubby spines (Hotulainen et al., 2009). This suggests that formins are critical to both axonal elongation and dendrite morphology including dendritic spine formation and synaptic transmission.
2.1.2 Barbed-End Binding Proteins
2.1.2.1 Ena/VASP Protein Family
The Ena/VASP family of actin-regulatory proteins comprises three vertebrate family members: mena (mEnabled), VASP, and EVL (Ena/VASP-like protein) that bind the barbed end of F-actin and promote polymerization. There is a single ortholog in Drosophila(enabled) and C.elegans(unc-34) (Bear and Gertler, 2009). This family is ubiquitously expressed and involved in numerous physiological processes including morphogenesis, endothelial barrier function, and cancer cell invasion. In nonneuronal cell types, Ena/VASP proteins localize to focal adhesions, tips of filopodia, and/or at the leading edge of lamellipodia. They promote actin polymerization by interacting with the free barbed ends of F-actin and to profilin: G-actin complexes, thereby facilitating the transfer of monomeric actin to the barbed end, as well as by preventing termination of elongation by F-actin capping proteins (Barzik et al., 2005).
In neurons, Ena/VASP proteins are critical for the formation of filopodia (Dent et al., 2007), which are crucial throughout neuronal development. Genetic loss of all three mammalian family members: ENAH (gene-encoding Mena), VASP, and EVL in mouse embryonic cortical neurons results in a failure in both filopodia formation and subsequently neuritogenesis in vitro. This corresponds to a block in cortical axon formation in vivo as well. Inhibition of neuritogenesis is rescued by expression of other actin-regulatory proteins that promote filopodia formation, including Myo10 or mDia2 (Dent et al., 2007), suggesting filopodia are prerequisite of neuritogenesis. In order to study the function of Ena/VASP proteins in the later stages of neuronal development; therefore, genetic deletion could not be used. The role of Ena/VASP in growth-cone filopodia formation was established by exploiting the binding of the Ena/VASP Homology Domain 1 (EVH1) to a specific proline-rich motif (abbreviated as FP4) attached to a mitochondrial targeting sequence (FP4Mito). Expression of FP4Mito mistargets Ena/VASP proteins to mitochondria, sequestering Ena/VASP function (Bear et al., 2000). FP4Mito expression prior to neuritogenesis blocks neurite initiation, phenocopying genetic deletion of the family (Gupton and Gertler, 2010). Expression subsequent to neurite outgrowth revealed that Ena/VASP proteins are also required for growth-cone filopodia formation and filopodial response to the soluble axon-guidance cue netrin-1 (Lebrand et al., 2004), suggesting Ena/VASP proteins are likely important in axon guidance. This is supported by several experiments in C.elegans, where loss of functional unc-34, disrupts proper guidance in response to both netrin and slit axon-guidance cues (Norris et al., 2009; Yu et al., 2002). Whereas in retinal ganglion cells (RGCs) isolated from Xenopus, sequestering the function of XEna/XVASP orthologs to the mitochondrial surface reduced filopodia formation, the number of axons extending out of the eye, and terminal axon arborization, but surprisingly did not cause an axon-guidance defect along the optic tract (Dwivedy et al., 2007), suggesting that the Ena/VASP proteins are not essential for axonal guidance in the Xenopus optic nerve. In contrast in mice genetically null for all three Ena/VASP proteins, the optic nerves were thinner and failed to extend to the midline to form the x-shaped optic chiasm (Dent et al., 2007).
These apparent differences are likely due to a number of factors including neuronal subtype and Ena/VASP responses to distinct extracellular cues and intracellular signaling pathways. Indeed the requirement for Ena/VASP proteins in cortical neuron neuritogenesis can also be bypassed by attachment to the ECM component laminin-1 and activation of integrin signaling pathways (Gupton and Gertler, 2010). This differential response occurred in vivo as well: in mice genetically null for Ena/VASP genes, the intralayer cortical positioning and cortical fiber tract formation were disrupted within the cortex, suggesting that neuronal migration and axon-guidance defects occur in the absence of Ena/VASP proteins. However, ectopic neurite formation occurred in laminin-rich regions of the brain (Dent et al., 2007). Genetic loss of function studies performed in vivo in Drosophila DA sensory neurons indicated Enabled is a positive regulator of dendritic branching and spine-like protrusions (Li et al., 2005a). While there are instances of unique functions of the vertebrate Ena/VASP proteins (Gupton et al., 2012; Lanier et al., 1999; Philippar et al., 2008; Worth et al., 2010), expression of any of the Ena/VASP proteins rescues loss of filopodia caused by deletion of all three genes (Dent et al., 2007) and genetic loss of all three is required for the gross neuroanatomical anomalies. However a recent study from the Webb lab suggests that VASP may have unique functions in spine formation (Lin et al., 2010). siRNA-mediated knockdown experiments in murine hippocampal cultures suggested that VASP regulates spine density, size, and morphology as well as synaptic strength, likely through its modulation of actin dynamics in dendritic spines (Lin et al., 2010). What the unique functions of VASP are in this instance, and why Mena or EVL were not sufficient to maintain dendritic spine morphology in the absence of VASP is unclear.
2.1.2.2 Lamellipodin
Lamellipodin was originally identified as an Ena/VASP ligand that independently localizes to lamellipodial and filopodial protrusions via a pleckstrin homology domain that specifically binds PI(3,4)P2 (Krause et al., 2004). Although lamellipodin does not directly interact with actin, in nonneuronal cells lamellipodin recruits Ena/VASP proteins to the leading edge of a cell and modulates lamellipodial protrusions (Michael et al., 2010). In utero electroporation of shRNA against lamellipodin disrupts radial migration of newly born cortical neurons in vivo (Pinheiro et al., 2011). siRNA-mediated knockdown of lamellipodin in primary hippocampal neurons in vitro reduced axon length without affecting dendrites (Michael et al., 2010). In C. elegans, single mutants of unc-34 or the lamellipodin ortholog MIG-10 exhibited minor axon-guidance defects, whereas severe axon-guidance defects occurred in response to both netrin and slit in double mutants (Chang et al., 2006). These defects were associated with decreased filopodia numbers and reduced axon outgrowth. While knocking down lamellipodin had no effect on basal dendrite length or number in hippocampal or cortical neuronal cultures (Michael et al., 2010; Tasaka et al., 2011), it did blunt the increase in dendrite length and number downstream of active Ras (Tasaka et al., 2011), suggesting that lamellipodin may also affect dendrite morphology in some instances. Lamellipodin also regulates dendrite development downstream of Slit-Robo-Ras signaling (Tasaka et al., 2012). Knockdown of lamellipodin by shRNA decreases dendritic outgrowth and branching in dissociated rat cortical neurons. The phenotypes associated with deletion of lamellipodin or its paralog RIAM, which is expressed later in neuronal development have not yet been described.
2.1.3 Actin-Monomer Binding Proteins
2.1.3.1 Profilin
The profilin family of proteins promotes F-actin polymerization by binding and making G-actin polymerization competent. Profilin 2A is a brain-specific isoform that increases the ratio of F/G-actin, and thus is suggested to increase the stability of F-actin (Da Silva et al., 2003). Deletion of the profilin 2A gene (PFN2) or depletion of profilin 2A using antisense oligonucleotides induces supernumerary neurites and increases neurite length in dissociated hippocampal neuronal cultures (Da Silva et al., 2003). One possible explanation for this is that, decreasing the stability of F-actin promotes neurite sprouting and elongation by allowing increased MT entry into the F-actin based protrusions that were maintained, which is a theme revisited throughout this review and may explain why loss of Arp2/3 function also increases neurite number. Genetic mutants of chickadee (chick), the Drosophila ortholog of profilin, exhibit a failure in ISNb axon outgrowth to their target locations, and these stalled growth cones were characterized by increased filopodia formation (Wills et al., 1999). The multiple profilins present in the mammalian genome likely overlap in function in the developing brain. Future studies must determine how blocking both profilin1 and profilin2 function affects axon outgrowth and guidance, to determine if this phenocopies loss of the single drosophila ortholog.
2.1.4 Actin-Bundling Proteins
2.1.4.1 Fascin
Fascin bundles parallel F-actin and is involved in the assembly of structures such as filopodia, microspikes, and lamellipodial ribs in nonneuronal cells (Vignjevic et al., 2006). These structures in neuronal growth cones are regions of fascin enrichment (Cohan et al., 2001), suggesting that fascin is a likely player in neuritogenesis and neurite outgrowth. However, experiments to address this have not been reported. A genetic deficiency in the drosophila homolog of fascin (singed) leads to an increase in F-actin within the neurites and growth cones, and aberrant curling of the neurites of mushroom body γ neurons in vitro (Kraft et al., 2006), suggesting that fascin function may be important for cytoskeletal tensegrity. In sensory neurons of the s larvae, singed is enriched in class III neurons, and singed loss of function mutants are characterized by a loss of filopodial protrusions (Nagel et al., 2012). Interestingly fascin expression correlates with a morphological distinction between class III and class IV neurons, as well as the curvature of the dendritic arbor. The function of fascin in vertebrate neurons remains to be explored, and as the three fascin genes may have redundant functions; individual function may be difficult to determine.
2.1.4.2 CRP1
CRP1 (cysteine-rich protein) is the only known member of the CRP actin-bundling/crosslinking protein family expressed in the CNS, where it localizes to growth-cone filopodia (Ma et al., 2011). CRP family proteins interact with both α-actinin and zyxin (Crawford et al., 1994; Harper et al., 2000; Sadler et al., 1992). CRP1 mediates F-actin bundling either by binding to α-actinin or by binding to F-actin directly. Overexpression of CRP1 in cultured hippocampal neurons increases filopodia number, whereas decreasing CRP1 expression using shRNA decreases the number of primary neurites and filopodia, and the degree of neurite branching (Ma et al., 2011). Since the length of the longest neurite, presumably the axon, was unaffected, CRP1 was suggested to specifically modulate dendritic growth.
2.1.4.3 Palladin
Palladin is an actin-bundling phosphoprotein critical in organizing the actin cytoskeleton in fibroblast cells (Goicoechea et al., 2008). Multiple palladin isoforms are expressed differentially based on tissue type and developmental stages. The brain-specific isoform is shorter than the protein expressed in other cell types, raising the possibility that it performs a function unique to neuronal cells. Localization studies reveal that palladin predominantly localizes to the axonal growth cone and is absent from dendrites (Boukhelifa et al., 2001). Antisense RNA-mediated knockdown of palladin in dissociated cortical neurons results in decreased neurite outgrowth (Boukhelifa et al., 2001). However, more recent studies found no defects in neurite outgrowth in cortical neurons isolated from palladin knockout mice (Shu et al., 2009). Whether the lack of effect in outgrowth observed in the palladin knockout is due to compensation by another actin-bundling protein that does not occur following acute reduction of palladin levels has not yet been determined.
2.1.5 F-Actin-Severing Proteins
2.1.5.1 ADF/Cofilin
The three proteins of the actin-depolymerizing factor (ADF)/cofilin family, cofilin-1 (or n-cofilin), cofilin-2 (or m-cofilin), and ADF, bind to F-actin, increase F-actin torsional dynamics, and subsequently sever F-actin. This produces a new barbed end, for actin polymerization as well as an unprotected pointed end for F-actin depolymerization (Bamburg and Berstein, 2010). ADF and cofilin-1 are expressed in the adult and developing mammalian brain (Bellenchi et al., 2007). ADF/cofilin activity is repressed by phosphorylation at Ser3, mediated by LIM Kinase. ADF/ cofilin is reactivated by the phosphatase Slingshot. Based on immunocytochemistry in mouse hippocampal neurons using antibodies against total cofilin and inactive, Ser3 phosphorylated cofilin, Garvalov et al. suggest that active cofilin is concentrated in the axonal growth cone (Garvalov et al., 2007). Supporting a role for ADF/cofilin in axon outgrowth, gain of function experiments employing overexpression of wildtype or nonphosphorylatable Xenopus ADF/cofilin in rat cortical neurons and mouse hippocampal neurons increases neurite length (Garvalov et al., 2007; Meberg and Bamburg, 2000). However, expression of a pseudophosphory-lated Xenopus ADF/cofilin does not reduce neurite length, indicating it does not act as a dominant negative. In contrast, siRNA-mediated knockdown of cofilin-1 in mouse hippocampal neurons reduced both the number of neurons containing Tau-positive axons and the length of the longest neurite (Garvalov et al., 2007; Meberg and Bamburg, 2000), suggesting that acute depletion of cofilin-1 blocks axon outgrowth and specification. Deletion of CFCL1 (the gene encoding cofilin-1) in newly born neurons causes defects in radial migration of cortical neurons and impairs neurite outgrowth of cortical neurons in vitro and in vivo but does not inhibit neuritogenesis. However, deletion of ADF alone fails to cause defects in neuritogenesis or subsequent stages of neuronal development (Bellenchi et al., 2007). This indicates that there are some nonoverlapping functions of cofilin-1 and ADF; however, whether redundant functions exist was not confirmed in this study. To address this, Flynn et al. crossed the constitutive ADF−/− mice with Nestin-Cre CFCL1fl/fl mice to produce brain-targeted deletion of ADF/cofilin-1. Unlike the individual knockout mice, the double-knockout mice died within 12 h of birth and displayed severe brain defects at embryonic day 17 including disrupted cortical lamination, enlarged ventricles, and no obvious axonal fiber tracts (Flynn et al., 2012). Correspondingly, dissociated cortical neurons from the double mutants did not initiate or extend neurites. EM revealed a gross disorganization of F-actin arranged circumferentially to the cell periphery as opposed to the tight bundles in filopodia and a meshwork of F-actin observed in the lamellipodia of wild-type neurons. Live cell imaging showed a decrease in F-actin retrograde flow in mutant neurons (Flynn et al., 2012), suggesting that ADF/cofilin promote breaking the symmetry of the neuronal spherical shape by reorganizing the F-actin cytoskeleton and permitting F-actin retrograde flow. These severe disruptions to actin dynamics and neuronal morphology that occurred in the absence of ADF/cofilin function underscore the importance of turnover of F-actin based structures in the developing neurons, as discussed earlier with Arp2/3 and profilin2. Interestingly, the failure of neuritogenesis in vitro can be partially rescued by pharmacological destabilization of F-actin, which promoted MT entry into the periphery (Flynn et al., 2012). Similar conclusions were made in cerebellar granule neurons, in which unphosphorylated cofilin is required for neurite outgrowth in response to the neural cell adhesion molecule L1 (Figge et al., 2012). In contrast to genetic deletion, this study utilized the phosphatase calcineurin that functions upstream of cofilin and by using peptide inhibitors of cofilin phosphoryation and dephosphorylation.
Cofilin also functions in axonal growth-cone guidance in response to both attractive and repulsive cues. Peptide inhibitors of cofilin dephosphorylation block growth-cone repulsion from Sema3A in DRG neurons (Aizawa et al., 2001). Manipulations that decrease unphosphorylated, active cofilin block DRG and RGC guidance toward NGF and netrin, respectively, whereas inducing a gradient of cell permeable active cofilin was sufficient to induce attractive turning (Marsick et al., 2010). EM data indicates that cofilin also localizes at the periphery of dendritic spines in rat hippocampal neurons (Racz and Weinberg, 2006). During spine formation, siRNA-mediated knockdown of cofilin-1 results in spines that are long, thin, and more branched, indicating that ADF/cofilin and actin turnover are important in dendritic spine morphology (Hotulainen et al., 2009). This is corroborated by a number of studies in which manipulating cofilin levels or activity was shown to modulate spine morphology and alter synaptic transmission (Shi et al., 2009; Zhou et al., 2004, 2007).
2.1.6 Other F-Actin-Binding Proteins
2.1.6.1 Tropomodulin
Tropomodulin (Tmod) family proteins bind to the pointed end of F-actin, and block both elongation and depolymerization (Weber et al., 1994), and thus regulate the length of actin filaments. The two neuronal isoforms Tmod1 and Tmod2 exhibit distinct localization patterns in dissociated hippocampal neurons. Tmod1 localizes to growth-cone lamellipodia and Tmod2 predominantly localizes to the soma (Fath et al., 2011). shRNA-mediated knockdown of Tmod2 in a neuroblastoma line increases the proportion of cells extending neurites and increases the neurite length. In contrast shRNA-mediated knockdown of Tmod1 increases the number of neurites per cell and slightly decreases neurite length (Fath et al., 2011). This indicates that proper control of pointed-end capping is critical to control of neurite initiation and elongation in neuronal-like cells. Further supporting distinct roles for these isoforms, overexpression of one isoform is unable to compensate for the loss of the other (Fath et al., 2011). While interpretations of these experiments are complicated by the fact that knockdown of a single Tmod isoform increases the expression of the other, one possible difference in the function of Tmod isoforms in neuronal morphology may be due to their relative affinities for both the pointed end and actin monomers (Yamashiro et al., 2010). Tmod2−/− mice exhibit defects in behavior, learning, memory, and synaptic function (Cox et al., 2003), but whether this is due to changes in the formation, morphology, or plasticity of dendritic spines remains to be seen. Further elucidation of the roles for Tmods in neuronal development are warranted.
2.1.6.2 Tropomyosins
Tropomyosin (Tm) isoforms bind along the major grove of F-actin as head-to-tail dimers. They are derived from four genes, and via alternative splicing there are >40 isoforms (El-Mezgueldi, 2014; Marston and Gautel, 2013), a specific subset of which are expressed in neurons (Schevzov et al., 1997). Early studies indicate that Tm4, Tm5a, and Tm5b isoforms are enriched in growth cones of developing neurons, whereas Tm5NM1 (tropomyosin 3, gamma) localizes to the axon hillock and proximal regions of axons and dendrites, and TmBr3 to presynaptic terminals (Had et al., 1994; Schevzov et al., 1997; Weinberger et al., 1996). Overexpression of Tm5NM1 in mouse cortical neurons significantly enlarges growth cones and increases dendrite number and axon branching (Schevzov et al., 2005). In contrast, overexpression of Tm3 inhibits neurite outgrowth. Loss of the tropomyosin 2 gene in Drosophila increases the size of dendritic fields (Li and Gao, 2003), indicative of evolutionarily conserved functions of this gene family. The expression patterns and isoform diversity of the tropomyosin family complicate their functional studies. Conditional knockouts will be required to define the neurological functions of this gene family.
2.1.6.3 UNC-115/abLIM Proteins
The actin-binding LIM (abLIM) protein family comprises three vertebrate members: abLIM1, abLIM2, and abLIM3, and a single ortholog in C.elegans, unc-115. abLIM proteins directly bind F-actin and localize to actin stress fibers in nonneuronal cells. Although they are suggested to regulate the actin cytoskeleton through scaffolding other regulatory proteins, their effects on actin dynamics and organization has not been defined. abLIM 1 was originally identified in human retina and murine cardiac tissue sarcomeres (Roof, 1997; Yang and Lundquist, 2005); abLIM2 and abLIM3 are enriched in neuronal and muscle tissue (Barrientos et al., 2007), however, literature regarding the neuronal function of vertebrate abLIMs is scant. In contrast, by using unc-115 deleted C. elegans mutants and unc-115 minigenes to rescue their mutant phenotypes, the Lundquist group implicates unc-115 in axon guidance in the worm (Gitai et al., 2003; Lin et al., 1996; Lundquist et al., 1998; Struckhoff and Lundquist, 2003; Yang and Lundquist, 2005). Similarly, mutation of the drosophila ortholog also results in axon projection defects (Garcia et al., 2007). Overexpression of unc-115 leads to the formation of ectopic neurites tipped with lamellipodial and filopodial-rich growth cones in PDE neurons, which requires the F-actin-binding domain of unc-115 (Yang and Lundquist, 2005). However, the biochemical function of abLIM proteins has yet to be identified. Therefore future work that defines the mechanisms abLIMs in regulating the actin cytoskeleton, as well as whether they have conserved functions in the developing vertebrate nervous system are critical.
2.1.7 F-Actin Motor Proteins
2.1.7.1 Myosin II
Myosin II minifilaments bind to F-actin via motor head domains, forming contractile structures important to cell shape change and motility of muscle and nonmuscle cells. The three vertebrate isoforms: myosin IIA, myosin IIB, myosin IIC, contain a heavy chain (MHC) comprising a motor domain and a cargo-binding tail domain. MHC-A and MHC-B are highly expressed isoforms in neurons and localize to the central domain of growth cones, with MHC-B exhibiting a slightly more peripheral localization (Rochlin et al., 1995). Seminal experiments from the Forscher lab utilized nonspecific inhibitors of myosin ATPase activity to implicate myosin II activity in F-actin retrograde flow in axonal growth cones and thus growth-cone shape and motility (Lin et al., 1996), leading to the formulation of the “clutch hypothesis.” More recently, these experiments have been revisited with the specific myosin II inhibitor, blebbistatin (Medeiros et al., 2006; Yang et al., 2012). Blebbistatin treatment similarly revealed that myosin II activity was required for floor plate chemorepulsion of chick cranial motor neurons downstream of slit and netrin-1 (Murray et al., 2010). Myosin II contractility is regulated by the phosphorylation of its myosin regulatory light chain (MRLC). Phosphorylation regulates the spatiotemporal organization of the actomyosin network in response to both intra- and extracellular cues. Inhibition of myosin II activity in vivo, by electroporating chick embryos with dominant negative or constitutively active MRLC results in misguided axonal pathfinding of the cranial motor axons and a loss of turning toward exit points (Murray et al., 2010). These results suggest that myosin II-dependent regulation of F-actin retrograde flow in the growth cone is important for axonal pathfinding. Although blebbistatin treatment can inhibit all myosin II isoforms, there is conflicting evidence of isoform-specific functions during neuronal development. Antisense nucleotides against myosin IIB-blocked neurite outgrowth and elongation, whereas antisense nucleotides against myosin IIA-reduced neurite retraction in neuroblastoma cells (Wylie et al., 1998). In line with a positive role for myosin IIB in axon outgrowth, superior cervical ganglia neurons cultured from MHC IIB knockout mice exhibited decreased rates of neurite outgrowth, and significantly smaller growth cones, and decreased filopodial traction force (Tullio et al., 2001). In contrast, in cerebellar granule neurons the blebbistatin-mediated block in RGM-dependent neurite retraction was phenocopied by siRNA specific to myosin IIA, but not myosin IIB, implicating myosin IIA as a negative regulator of axon outgrowth (Kubo et al., 2008). In DRG neurons, however, overexpression of myosin IIA but not myosin IIB could prevent growth-cone collapse and growthcone retraction in response to Sema3A. Inhibiting all myosin II isoforms with blebbistatin completely abolished retraction in response to Sema3A; and deletion of myosin IIB alone did not block retraction, solidifying a role for other isoforms (Brown et al., 2009). Myosin IIB is shown to further enhance the process of retraction by localizing to the neck and rear of these growth cones. SCG neurons isolated from myosin IIB knockout mice exhibited reduced growth cone size, filopodia number, and traction forces generated at these growth cones (Bridgman et al., 2001). Interestingly, these growth cones exhibited faster F-actin retrograde flow, suggesting that different myosin II isoforms cooperate to control retrograde flow (Brown, 2003). Clearly myosin II is a critical player in the growth cone, however, there may be cell type-specific uses of the different isoforms.
2.1.8 Membrane-Actin Linker Proteins
2.1.8.1 Spectrin
Spectrins are multiunit protein scaffold complexes first identified in red blood cells that stabilize membrane microdomains. 2α-spectrin genes and 5β-spectrin genes have been identified in mammals, each encoding multiple splice variants. 2α, 2β, and 2μ tetramerize to form an actin-crosslinking protein, with different isoforms localizing to the soma and neurite processes (Lazarides and Nelson, 1983). More recently, superresolution microscopy revealed distinct localization of spectrin within the axon compared to dendrites (Xu et al., 2013). In the axon, spectrin forms periodic ring-like structures, whereas in dendrites spectrin was organized longitudinally along F-actin within the dendritic shaft (Xu et al., 2013). A brain-specific β-spectrin, fodrin, relocalizes from the cortical plasma membrane to intermediate filaments upon neurite formation in PC12 cells (Takemura et al., 1993) and is transported down the axon in RGCs and SGC, possibly in a KIF3-dependent manner (Takeda et al., 2000; Willard and Simon, 1983) and localizes in the central domain of the growth cone (Sobue and Kanda, 1989). β1-Spectrins may link neural cell adhesion molecules (NCAM) to the actin cytoskeleton, and are involved in connecting the actin cytoskeleton to the extracellular environment, and establishing neuronal morphology. Specifically, interrupting the binding of β1-spectrin to NCAM with dominant negative β1-spectrin subunits 2–3 in hippocampal neurons blocked neurite outgrowth downstream of NCAM (Leshchyns’ka et al., 2003). Axon formation and guidance is also hindered in the absence of αII-spectrin as seen in the ventricular zone of mice genetically lacking αII-spectrin (Stankewich et al., 2011). Loss of function of β-spectrin but not α-spectrin in Drosophila causes axon-guidance defects at the midline (Hulsmeier et al., 2007). Similarly loss of unc-70, a β-spectrin ortholog in C. elegans is associated with inhibited axon outgrowth as well as spontaneous axon breaking (Hammarlund et al., 2000; Hammarlund et al., 2007). The localization of spectrin in vertebrate neurons, and the phenotypes observed in invertebrates, suggest that the function for multiple spectrin variants is likely important in vertebrate neuronal morphogenesis. Due to the large number of genes and splice variants, these functions will likely be difficult to parse.
2.1.8.2 Ankyrin
Ankyrin family proteins (ankyrins-R, ankyrins-B, ankyrins-G) link the spectrin-actin cortical cytoskeleton to the cytoplasmic domains of integral membrane proteins, including cell adhesion molecules and ion channels (Mohler et al., 2002). Ankyrin B, for example, interacts with L1CAM and modulates L1CAM mobility (Gil et al., 2003), perhaps acting as a clutch and modulating axon outgrowth. Ankyrin G localizes to the axonal initial segment (AIS) and nodes of Ranvier (Kordeli et al., 1995). In vivo, cerebellar loss of ankyrin-G in mice causes disorganized axon initial segments and decreased ability to fire action potentials (Jenkins and Bennett, 2001; Zhou et al., 1998).
2.1.8.3 ERM Proteins
The Ezrin, radixin, and moesin (ERM) family of proteins links the actin cytoskeleton to the plasma membrane, which stabilizes membrane protrusions. Subcellular fractionation and immunocytochemistry experiments in rat hippocampal neurons indicate radixin and moesin mainly localize to the growth cones of developing neurons (Paglini et al., 1998). Suppression of these two ERM proteins, but not ezrin by antisense oligonucleotides caused a reduction in growth-cone size, retraction of growth-cone lamellipodia, increased filopodial protrusive activity, and decreased rate of neurite elongation, resulting in stunted neurites (Paglini et al., 1998). In contrast, a more recent study suggests that ezrin interacts with the netrin receptor DCC and localizes to growth-cone filopodia upon netrin stimulation. Expression of dominant negative ezrin mutants inhibited netrin-1 mediated axon outgrowth in rat cortical neurons, but not basal axon outgrowth (Antoine-Bertrand et al., 2011). Chromophore-assisted laser inactivation of radixin in the growth cones of chick DRG neurons reduced lamellipodial area (Castelo and Jay, 1999). Additionally inhibiting ERM function, either by using dominant negative ERM construct that competes with endogenous ERM protein thereby preventing membrane-actin cytoskeleton linkage or by using a siRNA mixture against radixin and moesin, resulting in disorganized actin filaments within a smaller and less motile growth cone (Marsick et al., 2012). Together these experiments point to a role for ERM proteins in establishing the structural integrity of the growth cone.
2.1.8.4 Shootin1
Brain-specific Shootin1 was identified in a proteomics screen for proteins upregulated during neuronal polarization that were specifically enriched in the axon of hippocampal neurons (Toriyama et al., 2006). Shootin1 localizes to all neurite growth cones in stage 2 dissociated hippocampal neurons, whereas it becomes polarized and localized specifically to the axonal growth cone in stage 3 hippocampal neurons. When overexpressed, Shootin1 failed to polarize in only one neurite, resulting in the formation of more than one Tau-positive axon per neuron (Toriyama et al., 2006). In the growth cones of hippocampal neurons, Shootin1 links the cell adhesion molecule L1 to F-actin retrograde flow (Shimada et al., 2008). This was observed by fluorescent speckle microscopy with L1-CAM-Fc coated beads placed on axonal growth cones. shRNA-mediated depletion of Shootin1 decreased the velocity of the retrograde flow of the L-Fc coated beads, suggesting the linkage between the L1-CAM and F-actin had been disrupted. Since shRNA-mediated silencing of Shootin1 also inhibited axonal outgrowth and overexpression of Shootin1 increased neurite outgrowth, shootin1 is a plausible link or clutch molecule between F-actin retrograde flow and adhesion.
2.1.8.5 Af-6/Afadin
Af-6/Afadin and the drosophila homolog Canoe (cno) bind to F-actin and localize to cadherin-based cell-to-cell adherens junctions (Mandai et al., 1997; Xie et al., 2005). In the Drosophila larva, cno is expressed in midline longitudinal axonal tracts of the CNS. In cno loss-of-function mutants, several axonal defects were observed in a subset of Robo-expressing axons normally repelled from the midline. These include defasciculation, axon stalling, and aberrant axon crossing of the midline (Slovakova et al., 2012). Cno forms a complex with the slit receptor, robo and regulates the filopodial localization of robo. Genetic loss of afadin also results in defasciculation, axon stalling, and misguidance thus suggesting that afadin regulation of robo is required for axon response to slit. This is further supported since these axonal phenotypes are slightly alleviated in slit/cno or robo/cno double mutants (Slovakova et al., 2012). In mouse cortical neurons, afadin interacts with the small GTPase Ras in a GTP-bound state to promote axon branching (Iwasawa et al., 2012). Afadin overexpression increases axon branch density, whereas reduction of afadin expression using shRNA reduces axon branching. Afadin is an effector of the small GTPase Rap1 (Xie et al., 2005). Activated Rap1 recruits afadin to dendritic spine heads in cortical neurons and this modulates spine morphogenesis. Afadin overexpression increased the spine neck length and area. Deletion of afadin (mllt1) in postmitotic hippocampal neurons reduced adhesion density, spine density, and excitatory synaptic transmission (Beaudoin et al., 2012). This family of actin-binding proteins impart structural integrity to the extending axon and dendritic spine.
2.2 MT-Binding Proteins
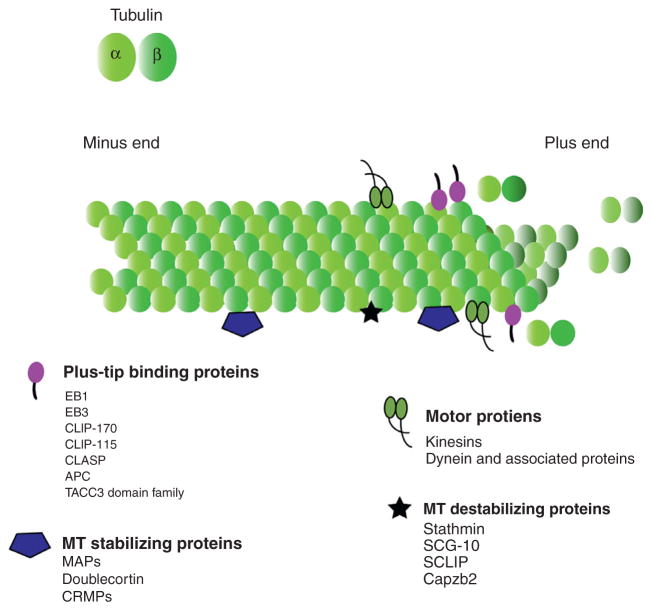
MTs are polar, hollow tubes composed of 13 protofilaments of obligate α/β-tubulin heterodimers polymerized in a head-to-tail fashion. In nonneuronal cells, the plus ends of MTs are arranged distally to the nucleus and undergo frequent switches between states of polymerization and depolymerization, an inherent behavior known as “dynamic instability” (Mitchison and Kirschner, 1984). The minus ends of MTs are typically anchored near the nucleus in the centrosomes in most cell types, however in neurons most MTs are noncentrosomal (Akhmanova and Hoogenraad, 2015). In axons MTs are oriented with plus-ends oriented outward, whereas in dendrites MTs are oriented with either plus-end or minus-ends outward (Baas et al., 1988). At the distal dip of the axon near the growth cone, there is an abrupt switch from MTs composed of stable MTs in which α-tubulin has been posttranslationally detyrosinated and/or acetylated (glu), to dynamic MTs rich in tyrosinated α-tubulin (tyr) (Tarrade et al., 2006). Since MTs comprise the structural components of both axonal and dendritic processes, they are required for neuronal morphology acquisition. Neuritogenesis is inhibited by treatment with nocodazole, which depolymerizes MTs, or by taxol treatment, which stabilizes MTs (Dent et al., 2007). MTs act as tracks for motor proteins to move cargo in and out of neurites, important in specifying and maintaining axonal and dendritic identity and function. Mutations in the neuronalspecific βIII-tubulin gene (TUBB3) in humans result in a variety of TUBB3 syndromes, which entail ocular motility disorders, intellectual and behavioral impairments, facial paralysis, and axonal sensorimotor polyneuropathy (Tischfield et al., 2010). These mutations disrupt MT dynamics and the ability of kinesin motors to bind MTs, and result in severe axon-guidance defects. Pioneering studies in Xenopus growth cones from the Kirschner lab noted that MTs orient toward the direction of axon outgrowth (Sabry et al., 1991; Tanaka and Kirschner, 1991), suggesting they directed axonal navigation. This hypothesis was supported by experiments in which local uncaging of drugs that alter MT polymerization and depolymerization was sufficient to induce growth-cone turning (Buck and Zheng, 2002). Thus control of MT organization, dynamics, and transport are integral in every stage of neuronal morphological development. In the following sections, we will review the myriad of MT-associated proteins (MAPs) that alter MT dynamics, organization, and MT-based transport (Fig. 4). There are numerous types of MAPs including MT motors, MT plus-end binding proteins, structural and enzymatically active MAPs, and centrosome-associated proteins.
Figure 4.
MT and MT-binding proteins. α- and β-Tubulin polymer with the plus end serving as a site for addition of more monomers and also for depolymerization. Plus-end binding proteins bind to MTs and along with other stabilizing proteins help stabilize the polymer. Destabilizing proteins aid in depolymerization. Motor proteins transport cargo along the polymer.
2.2.1 MT-Stabilizing Proteins
2.2.1.1 MAPs
The “classical” and most abundant MAPs were discovered in the brain and bind along the MT lattice where they stabilize MTs. These include MAP2/ Tau and MAP1A/1B family members. These MAPs are highly expressed in the brain and have distinct localization and expression patterns. For example, MAP2 is restricted to dendrites, whereas Tau localizes specifically to the axon (Cáceres et al., 1984; Kosik and Finch, 1987). MAP1B is expressed at higher-levels developmentally, and MAP1A expression rises in the mature nervous system (Schoenfeld et al., 1989).
2.2.1.2 MAP2
MAP2 increases the rigidity of MTs and induces MT bundling. In addition, MAP2c interacts with F-actin (Roger et al., 2004), and thus may crosslink MTs and F-actin during neuronal development. MAP2 has alternatively spliced isoforms with distinct expression patterns. MAP2c is expressed early and downregulated after development, at which time MAP2a expression increases. MAP2b is expressed both developmentally and in the adult (Dehmelt and Halpain, 2005). Expression of a dominant negative MAP2c mutant, which impairs the ability of MAP2c to bind and stabilize MTs, reduced neurite number in hippocampal neurons (Dehmelt et al., 2003), suggesting MAP2c-mediated MT stability promotes neuritogenesis. This corroborates prior studies that utilized antisense nucleotides against MAP2, which blocked neurite initiation in cerebellar macroneurons (Caceres et al., 1992). Furthermore, an interaction between MAP2c and L1-CAM may be important for neurite outgrowth on L1-CAM (Poplawski et al., 2012). c-Jun N-terminal kinase 1 (JNK1) mediated phosphorylation of MAP2 promotes DA (Björkblom et al., 2005).
2.2.1.3 Tau
Like MAP2, Tau binds to the MT lattice and alters MT rigidity and organization. Additionally, Tau may protect MTs from katanin-mediated severing (Qiang et al., 2006). Tau specifically accumulates in the axon upon specification, suggesting Tau plays a functional role in differentiating the axon from dendrites. In cerebellar macroneurons plated on poly-D-lysine, suppression of Tau expression using antisense oligonucleotides inhibited axon/neurite elongation but not initiation (Caceres et al., 1992; DiTella et al., 1996); however, when cultured on laminin the longest neurite, presumably the axon, continues to elongate at a similar rate (Caceres et al., 1992; DiTella et al., 1996) This may have been due to the compensatory function of MAP1B, which increased in expression on laminin. Several groups have generated Tau-deficient mice, which surprisingly are viable and lack any overt phenotypes until aged (Dawson et al., 2001; Harada et al., 1994; Ke et al., 2012). Cultured hippocampal neurons from one of theTau-deficient lines exhibit growth cones characterized by reduced MT numbers but normal morphological progression (Harada et al., 1994). In this line, deletion of MAP1B, however, did retard neuronal development in vitro and caused more striking phenotypes in vivo (Takei et al., 2000), further suggesting redundant or compensatory functions of these two MAPs. In contrast in anotherTau-deficient line, cultured hippocampal neurons exhibited a delay in morphological progression without additional deletion of MAP1B (Dawson et al., 2001). The difference between these results may be due to different culture conditions or differential MAP expression in distinct genetic backgrounds. The difference between genetic deletion of Tau and acute suppression of Tau expression also suggests that compensation by another MAP can reduce the severity of the phenotype associated with Tau deletion. In several of the Tau-deficient lines, MAP1A expression was upregulated (Dawson et al., 2001; Harada et al., 1994).
2.2.1.4 MAP1B
MAP1B is highly expressed during neural development in actively extending axons (Fischer and Romano-Clarke, 1991; Gordon-Weeks and Fischer, 2000), suggestive of a role in axon extension. Antisense oligonucleotides expressed in cerebellar macroneurons specifically inhibit axon elongation promoted by laminin (DiTella et al., 1996), suggesting that in some contexts MAP1B function is required for axon elongation. Phosphorylated MAP1B is highly concentrated in the distal axon and growth cone of chick RGCs. When growing along the border of nonpermissive substrate, phosphoMAP1B is restricted to stable regions of the growth cone (Mack et al., 2000), suggesting it may play a role in growth-cone directionality. Microscale chromophore-assisted laser inactivation (CALI) of phosphorylated MAP1B in one half of the growth-cone induced turning and altered motility (Mack et al., 2000). Supporting a role for MAP1B during netrin-dependent axonal guidance, hippocampal and dorsal spinal cord explants from MAP1B null mice lack chemoattractive guidance toward netrin-1 (Del Río et al., 2004). Furthermore, RNAi of MAP1B in cultured rat embryonic cortical neurons suggests that MAP1B also modulates axon growth and inhibits axonal branching. These neurons exhibit highly branched axons and slower axonal growth (Edelmann et al., 1996; Tymanskyj et al., 2011). This is attributed to a reduction in the speed of MT growth at the proximal and distal ends of axons but not in growth-cone filopodia. Similar results corroborate the role of MAP1B in axonal regeneration in dorsal root ganglia neurons (Bouquet et al., 2004). MAP1B expression drops in the CNS following neuronal maturation, but persists in peripheral neurons such as DRGs. In regenerating DRG neurons isolated from MAP1B−/− mice, growth-cone directionality was impaired and there was elevated terminal branching (Bouquet et al., 2007). In addition to a developmental role, these results suggest that MAP1B is required for the regenerative capacity of adult DRGs.
Like Tau knockout mice, several lines of MAP1B mutant mice have been generated, which exhibit phenotypes of varying severity (Edelmann et al., 1996; Meixner et al., 2000; Takei et al., 1997). In contrast to the Tau knockout mice, MAP1B-deficient mice exhibited more striking defects. In some lines, overall brain size was reduced, mice lacked a corpus callosum, neuronal morphology was affected, and development was delayed (Edelmann et al., 1996). That the corpus callosum and the hippocampal commissures are disrupted is reminiscent of NTN1 (the gene-encoding netrin-1) and DCC knockouts (Fazeli et al., 1997; Serafini et al., 1996), further supporting a role for MAP1B in netrin-mediated axon guidance.
2.2.1.5 MAP1A
Unlike MAP1B, MAP1A is expressed and localized within the dendrites of matureand cultured neurons (Halpain and Dehmelt, 2006; Szebenyi et al., 2005). In cultured hippocampal neurons, MAP1A protein expression increased at 4 days in vitro, when maximum dendritic growth and branching occurs. MAP1A expression is restricted to dendrites by 2–3 weeks in vitro, when dendritic spines are forming (Szebenyi et al., 2005). MAP1A-specific siRNA decreased activity-dependent increases in dendrite length and branching (Szebenyi et al., 2005), indicating a role for MAP1A-mediated modulation of MT dynamics in the final stages of neuronal maturation.
2.2.1.6 Doublecortin
Doublecortin (DCX) and doublecortin-like (DCL) are nonclassical MAPs lacking sequence homology to the classical Map2/Tau family members. In vitro DCX nucleates MTs, promotes polymerization by cooperatively binding to the lattice of MTs, crosslinking protofilaments, preventing outward splaying of protofilaments, and thus catastrophe (Bechstedt and Brouhard, 2012; Gleeson et al., 1999). DCX is highly expressed during corticogenesis and adult neurogenesis (Brown et al., 2003; Francis et al., 1999), where it localizes both to the soma and leads to the process of newly born neurons, and eventually to the nascent axon. Mutations in X-linked DCX in humans causes lissencephaly in hemizygous males, whereas heterozygous females exhibit a heterotopic band of neurons beneath the cortex, referred to as double cortex syndrome (Gleeson et al., 1998; Portes et al., 1998). Hemizygous male mice carrying a mutated DCX exhibit normal cortical lamination, but disrupted hippocampal lamination (Corbo et al., 2002). DCX RNAi results in disrupted radial migration of cortical neurons, however, deletion of DCX induced a milder phenotype with aberrant tangential migration of interneurons and exuberant unstable branching of leading processes (Bai et al., 2003; Kappeler et al., 2006). The difference in phenotypes from acute knockdown versus genetic deletion suggests compensation occurs in the knockout. DCL is also expressed in the developing cortex. Deletion of DCL caused no observable phenotype in mice, but DCL and DCX double-knockout mice exhibit abnormal brain architecture, including impaired cortical lamination that was suggestive of aberrant cortical neuron migration and defective axon-projection patterns (Deuel et al., 2006). RNAi knockdown of DCX in DCL−/− neurons resulted in shorter axons and dendrites in cultured hippocampal neurons, indicating overlapping function in neurite outgrowth (Deuel et al., 2006).
2.2.1.7 Collapsin Response Mediator Proteins
The collapsin response mediator protein (CRMP) family of cytosolic phosphoproteins was originally identified as Sema3A signaling mediators. In vitro, CRMP proteins bind tubulin heterodimers as well as MTs and promote MT polymerization (Fukata et al., 2002b; Lin et al., 2011). The family comprises five known vertebrate isoforms, CRMP1–5, that are expressed in the developing and adult nervous system, CRMP2 being best understood. CRMP2 localizes preferentially to growing axons; overexpression of CRMP2 in hippocampal neurons induces supernumarary axon formation, whereas expression of truncated CRMP2 blocks axon formation (Inagaki et al., 2001). While this likely involves CRMP-mediated MT regulation, work from the Kaibuchi lab suggests CRMP-mediated axon formation depends on WAVE-mediated actin regulation as well, and that CRMP2 interacts with a kinesin motor to transport the WAVE complex into the extending axon (Kawano et al., 2005). A role for polarized sorting of cargo between axon and dendrites was similarly observed for the single CRMP ortholog in C.elegans, unc-33 (Maniar et al., 2011), which is required for axon elongation and guidance (Li et al., 1992). Thus CRMP-mediated modulation of MT dynamics and/or transport during axon elongation is evolutionarily conserved. Expression of the CRMP2B isoform in chick RGCs promotes axon branching at the expense of axon length, and this activity is blocked by coexpression of CRMP2A isoform (Yuasa-Kawada et al., 2003). These authors showed that CRMP2 isoforms differentially regulate MTorientation.
Differential effects are also observed for alternate isoforms of the CRMP4/TUC4 family (Quinn et al., 2003). Whereas Tuc4a is expressed constitutively, Tuc4b is expressed only in developing neurons and localizes to vesicles, suggesting Tuc4b may regulate vesicular transport (Quinn et al., 2003). In this case overexpression of Tuc4b but not Tuc4a induces axon branching and extension. Although little is known about CRMP1 and CRMP3, genetic deletion of either gene specifically inhibits hippocampal form and function in vivo, and has phenotypes that are suggestive of a role in dendritic morphology (Quach et al., 2007; Su et al., 2007). CRMP1−/− hippocampal neurons exhibited altered MAP2 organization and disrupted dendrites (Su et al., 2007). Deletion of CRMP3 caused wavy and abnormally thick dendrites in the CA1 region of the hippocampus (Quach et al., 2007). Due to the high similarity of CRMP genes, there are likely to be redundant functions that will complicate analysis of single mutants.
2.2.2 Plus-End Tracking Proteins
The frequent occurrence of plus-end catastrophes and rescues that define MT dynamic instability are hypothesized to allow MTs to more rapidly explore and sample the cellular environment (Mimori-Kiyosue and Tsukita, 2003). The plus-end tracking proteins (+TIPs) are a family of MAPs that specifically associate with polymerizing plus ends of MTs, and thus this family of proteins is thought to probe the cytoplasmic environment (van Haren et al., 2009) and alter MT dynamics during neuronal development. Use of fluorescently tagged +TIPs indicates that MT polymerization is slower in neurons than in other cell types, and confirms the distinct orientation of MTs in axons and dendrites (Stepanova et al., 2003).
2.2.2.1 End-Binding Proteins, EB1 and EB3
The end-binding (EB) family, contains EB1, EB2, and EB3, with EB1 ubiquitously expressed and EB3 enriched in neurons (Nakagawa et al., 2000). EB1 and EB3 track the growing end of MTs autonomously, promoting persistent MT polymerization by suppressing catastrophe occurrence (Komarova et al., 2009). In contrast EB2 is lattice associated, unless EB1 levels are decreased, suggesting these proteins compete for the plus end. The N-terminal domain of EB1 and EB3 associate with the growing plus ends of MTs; the C-terminal domain interacts with other +TIPs, recruiting them to polymerizing plus ends. Mechanistic studies indicate that EB1 proteins preferentially bind to the hydrolyzable GTP cap at MT plus ends and promote incorporation of lateral subunits. EB1 and EB3 suppress catastrophe and promote stable MT growth. Both accumulate at the plus ends of growing MTs throughout the neuron and promote neurite growth (Geraldo et al., 2008; Stepanova et al., 2003, 2010). Furthermore, overexpression of EB1 in map1b null mouse hippocampal neurons rescues defects in axonogenesis (Jiménez-Mateos et al., 2005), suggesting EB1 has a role complementary to MAP1B, likely in promoting MT stability (Tortosa et al., 2013).
In addition to promoting MT growth in neurons, EB3 interacts with drebrin, an actin-binding protein. Presumably this interaction occurs when MT tips enter filopodia (Geraldo et al., 2008), and promote neurite outgrowth and axonal growth-cone formation. Perturbation of the interaction between drebrin and endogenous EB3 by expression of an EB3 mutant that binds drebrin but not MT plus ends, disrupts neuritogenesis in cortical neurons (Geraldo et al., 2008). Later in neuronal development, reduction of EB3 expression by shRNA resulted in a loss of mushroom-shaped spines and increased the number of long, thin filopodia-like spines (Jaworski et al., 2009). This EB3 function also appears to modulate actin dynamics through p140Cap, a regulator of Src kinase that modulates cortactin-dependent actin dynamics. By disrupting the interaction between EB3 and p140Cap using dominant negative mutants of both EB3 and p140Cap they show that EB3–p140Cap interaction is necessary for the formation of mushroom-headed spines. Thereby this highly regulated actin and MT dynamics result in proper spine morphogenesis and plasticity.
2.2.2.2 Cytoplasmic Linker Proteins CLIP-170 and CLIP-115
Cytoplasmic linker protein-170 (CLIP-170) was the first +TIP identified (Perez et al., 1999). CLIP-170 and CLIP-115 localize to the MT plus end through an interaction with EB1. CLIP-170 also mediates crosslinking between MTs and F-actin by interacting with IQGAP1, an actin-binding protein (Fukata et al., 2002a). The role of this interaction is described in the crosslinking section later in the chapter. shRNA-mediated depletion of CLIP-170 in hippocampal cultures reduces the number of dendritic tips and total dendritic length (Swiech et al., 2011). Using a mutant of CLIP-170 that does not bind MTs also alters DA. CLIP-115 is a paralog of CLIP-170 specifically expressed in the nervous system and enriched in dendrites, where it associates with the dendritic lamellar body, a membranous organelle located at dendrodendritic gap junctions (De Zeeuw et al., 1995, 1997). Compared to CLIP-170, CLIP-115 has a divergent carboxy terminus and differentially affects MT organization when expressed at high levels (Hoogenraad et al., 2000). The human CLIP-115 gene, (CYLN2) lies within the chromosomal region deleted in the neurodevelopmental disorder Williams syndrome. Targeted deletion of CYLN2 in mice recapitulates some of the symptoms of Williams syndrome, including brain abnormalities and hippocampal dysfunction (Hoogenraad et al., 2002). Interestingly although deletion of CYLN2 did cause brain anatomy anomalies and disrupted hippocampal-associated behavior, there was also increased CLIP-170 localization to MT plus ends in CYLN2−/− fibroblasts, suggesting these proteins likely have redundant functions (Hoogenraad et al., 2002). However, more studies are warranted to determine how acute reduction of CLIP-115 and/or CLIP-170 or genetic deletion of both genes affects neuronal development. Although double-knockout mice have been reported, there has been no description of any phenotypes associated with loss of this family of proteins in neurons (Akhmanova et al., 2001; Dragestein et al., 2008).
2.2.2.3 Cytoplasmic Linker Associated Protein
CLIP-associated protein (CLASP)1 and CLASP2 are +TIPs that interact with CLIP-170, CLIP-115, EB1, and the cell cortex (Akhmanova et al., 2001; Beffert et al., 2012; Lansbergen et al., 2006; Mimori-Kiyosue et al., 2005). They localize to various intracellular structures including kinetochores, the mitotic spindle midzone, the cell cortex, and the Golgi and function during cell motility and mitosis. CLASPs are recruited to MT plus ends via an interaction with EB1, and in mammalian cells they additionally bind directly to the lattice of MTs in a spatially restricted fashion via two TOG domains in their N-terminal and C-terminal domains (Al-Bassam et al., 2010; Wittmann and Waterman-Storer, 2005). The TOG domains of yeast ortholog Cls1p also bind tubulin heterodimers, thus it is thought that CLASPs promote MT rescue and suppress catastrophe by delivering tubulin heterodimers to the plus end (Al-Bassam et al., 2010). CLASP1 is ubiquitously expressed, whereas CLASP2 is enriched in the nervous system (Akhmanova et al., 2001). CLASP2 expression increases throughout neuronal development and CLASP2 protein localizes to growth cones (Beffert et al., 2012). As in nonneuronal cells, CLASPs exhibit two distinct MT-binding patterns in neurons: plus-end binding and distal lattice-binding (Hur et al., 2011). In embryonic cortical neurons, expression of shRNA targeting CLASP2 but not CLASP1 promoted axonal growth (Hur et al., 2011), suggesting that CLASP2 plays a negative role in axon growth. In contrast to its role in axons, CLASP2 depletion impaired dendritic growth. In contrast another study suggested that inhibition of CLASP2 expression in dissociated hippocampal cultures by shRNA reduced both axonal and dendritic length (Beffert et al., 2012). Alternately upon overexpression of CLASP2 dendritic branching increased and supernumarary axons formed (Beffert et al., 2012). The disparity between these two studies based on CLASP2 depletion may be due to differences in the functions and relative levels of the two distinctly localized CLASP populations. For example, mutants of CLASP2 only able to display MT-lattice binding prevent MT extension into the growth cone and were associated with reduced axon growth, whereas expression of CLASP mutants only capable of tip-tracking promoted axon elongation, presumably by stabilizing pioneering MTs (Hur et al., 2011).
A screen for axon formation and axon-guidance defects identified Orbit/ MAST (the drosophila ortholog of CLASP) loss-of-function mutants (Lee et al., 2004). Whereas typically the repulsive cue Slit blocks aberrant midline crossing, Orbit/MAST mutants were characterized by ectopic midline crossing associated with both a fasciculation defect and a defect in axonal growth-cone orientation. This study went on to demonstrate that Xenopus CLASP (Xorbit) specifically binds to a subset of MTs that interact with the F-actin rich axonal growth-cone periphery, suggesting CLASP spatially modulates MT dynamics during growth-cone guidance. Interestingly CLASP2 gene deletion in mice have been reported (Pereira et al., 2006), but whether they exhibit a neuronal phenotype has not. CLASP2 overexpression in mouse hippocampal neurons increases the number and size of synapses and altered excitatory but not inhibitory event frequencies (Beffert et al., 2012), suggesting CLASP2 involvement in later stages of neuronal morphogenesis as well.
2.2.2.4 Adenomatous Polyposis Coli
The +TIP adenomatous polyposis coli (APC) binds MTs directly as well as indirectly via EB1 and kinesin-2. In vitro binding of APC to MTs promotes MT assembly, bundling, and stabilization (Munemitsu et al., 1994; Zumbrunn et al., 2001). In stage 2 neurons, APC localizes to the tips of multiple neurites, however prior to axon elongation, APC localizes to the future axon (Votin et al., 2005), suggesting that APC may play a role in axon specification. Furthermore, APC associates with Par3/Par6/aPKC polarity complex in the axon, and dominant-negative APC expressed in cultured hippocampal neurons results in an impairment of polarized localization of the polarity complex and a failure in axonal specification (Shi et al., 2004; Chen et al., 2011), suggesting that APC is critical in establishing neuronal polarity. However, deletion of all APC genes in drosophila failed to induce defect in axon specification, outgrowth, or guidance (Rusan et al., 2008), calling into question the interpretation of dominant negative expression results. However, conditional deletion of APC in mice leads to defective axonal guidance in the cerebral cortex (Yokota et al., 2009). In this study, APC-deficient neurons fail to produce axonal outgrowths at embryonic stage E14 and by E16 these mice show defects in the organization of the major fiber tracts in the cortex in vivo. In vitro dissociated cortical neurons isolated from APC-deficient mice exhibit excessive axonal branching and the tips of the axons curl aberrantly. Consistent with a role in MT stabilization, the authors found a reduction in acetylated tubulin, a marker of stable MTs, in cortices from APC-deficient mice (Yokota et al., 2009). However, neuronal polarization and axon specification occur in the absence of APC. Exuberant axon branching of cortical neurons was associated with disorganized and misoriented MTs in the axon and increase in the growth-cone size and splitting (Chen et al., 2011). Expression of the N-terminal of APC, which contains both the oligomerization motif and the armadillo repeats that bind several cytoskeletal regulatory proteins including ASEF, IQGAP, and the KAP3 subunit of kinesin-2, was sufficient to rescue both branching and MT-debundling defect. However the C-terminus, which directly binds MTs and EB1, only partially rescues the aberrant axon branching and MT phenotypes. These results suggest that dimerization of the armadillo repeats promotes APC interaction with actin and MT-regulatory proteins, thereby functioning as a scaffolding protein (Chen et al., 2011). Inactivation of the N-terminal oligomerization domain of APC in one-half of the growth cone of chick RGCs causes the inactivated region to collapse and the rest of the growth cone to turn away from the laser-treated region. In contrast, inactivating the 20-mer region that disrupts APC–MT interaction expands the growth-cone region and induces turning toward the laser treated area (Koester et al., 2007). This study suggests that APC regulates growth-cone dynamics by crosslinking the MT cytoskeleton via its dimerization.
2.2.2.5 Transforming Acidic Coiled-Coil Domain Family
A recent study in Xenopuslaevis growth cones showed that the MT growth-promoting protein TACC3 acts as a novel +TIP protein, which localizes at the very plus-end tip, distal of EB1 (Nwagbara et al., 2014). Knockdown of TACC3 slows MT polymerization in these growth cones, whereas overexpression increases MT polymerization rates, as assayed by EB1 comet velocities (Nwagbara et al., 2014). Future studies will be required to determine the functional role of TACC3 in neuronal morphogenesis.
2.2.3 MT-Severing Protein
2.2.3.1 Katanin
Katanin hydrolyses ATP in order to sever MTs, which produces multiple MTs with new ends available for polymerization (McNally and Vale, 1993). As small, dynamic MTs, which are observed at sites of outgrowth, are more readily transported throughout the neuron and growth cone (Dent et al., 1999), katanin is well poised to modulate neurite outgrowth. Indeed neurons express higher levels of katanin than other cell types, particularly during phases of rapid neurite outgrowth (Karabay et al., 2004). Katanin comprises two subunits, a p60 subunit that has enzymatic activity and often localizes at neurite tips, and a p80 subunit that localizes to the centrosome and regulates p60 (Yu et al., 2005). MT-severing releases MTs from the centrosomes to be transported throughout the cell. However, katanin localizes throughout the neuron, suggesting it may sever noncentrosomal MTs also. Inactivation of katanin in rat superior cervical ganglion neurons (SGC) using antibody microinjection results in an accumulation of MTs attached to the centrosome observed by EM (Ahmad et al., 1999). Additionally, katanin inhibition reduces axonal length (Ahmad et al., 1999), suggesting that MT severing is required for prompt delivery of small MT filaments to the growing neurites. This is corroborated by expression of dominant negative p60 reducing axonal outgrowth (Karabay et al., 2004). However, overexpression of p60 in SCGs or hippocampal neurons results in excess MT severing that impedes axon outgrowth as well (Karabay et al., 2004; Yu et al., 2005), suggesting that precise calibration of katanin expression and activity is required for optimal axon outgrowth. Interestingly MTs in axons are more resistant to increased katanin overexpression (Yu et al., 2005), likely due to the ability of Tau to protect these MTs from severing (Qiang, 2006). Accordingly p60 overexpression only induces axon branching in rat hippocampal neurons when Tau expression is simultaneously reduced by RNAi (Yu et al., 2008). Work in Drosophila indicates loss-of-function mutants of katanin p60-like1 reduces dendrite arborization and dendrite pruning in class IV DA neurons (Lee et al., 2009; Stewart et al., 2012). Katanin, however, stops axonal growth when the growing axon is presented with the target it was supposed to reach and innervate (Karabay et al., 2004).
2.2.3.2 Spastin
Like Katanin, spastin is an ATPase capable of severing and disassembling MTs in vitro and in cells (Roll-Mecak and Vale, 2005, 2008). Spastin is commonly mutated in the neurodegenerative disorder hereditary spastic paraplegia (Hazan et al., 1999). During hereditary spastic paraplegia, there is progressive retrograde degeneration of corticospinal axons, which are the longest axons in the human body. This suggests that spastin maintains axonal processes through regulation of MT dynamics or transport along MTs. Deletion of the spastin gene in mice (Sp) is associated with progressive CNS axonal degeneration, with axonal swelling containing accumulated organelles and cytoskeletal proteins indicative of a trafficking defect (Tarrade et al., 2006). Cortical neurons from these mice exhibit swellings along neurites near the growth cone when cultured, at regions where MTs switch from glu-tubulin to tyr-tubulin (Tarrade et al., 2006). Interestingly these swellings occur where posttranslational modification of tubulin changes from a mixture of glu and tyr MTs to only tyr MTs and is associated with an impairment of MT dynamics (Fassier et al., 2013; Tarrade et al., 2006). Morpholinos directed toward spastin block motor axon outgrowth in zebrafish (Wood, 2006). Similarly depletion of spastin from mouse hippocampal neurons reduced axonal length (Riano et al., 2009). Spastin localizes to sites of axon-branch formation (Yu et al., 2008). Spastin overexpression increases the formation of axon branches in rat hippocampal neurons (Yu et al., 2008). In contrast loss of spastin in class IV dendritic arbor neurons in drosophila caused a reduction in DA, although this was not associated with a change in MT polymerization as with katinin (Stewart et al., 2012).
2.2.4 MT-Destabilizing Proteins
Stathmin family proteins include stathmin, SCG-10, SCLIP, and RB3, with stathmin ubiquitously expressed, and the remainder expressed exclusively in the nervous system at different times and locations. Each contain a domain with homology to stathmin, and neuronal proteins also contain N-terminal domains that affect their localization. All family members sequester two tubulin heterodimers, although their affinity and kinetics of binding are distinct (Charbaut et al., 2001). In cells these proteins destabilize MTs by both binding tubulin dimers as well as directly binding MTs and promoting depolymerization. Drosophila has a single stathmin-like gene, which is required to maintain axonal MTs (Duncan et al., 2013). The phenotypes of stathmin mutant flies suggest that stathmin-mediated maintenance of axonal MTs is necessary for long-distance axonal transport.
2.2.4.1 Stathmin
Loss of the stathmin gene in mice does not induce obvious developmental phenotypes, however, these mice suffer axonopathy of myelinated axons in motor tracts in the CNS and PNS (Liedtke et al., 2002). Interestingly, SCG-10 expression increased in these mice, suggesting compensation may occur. In the cerebellum stathmin may play a negative role in DA. Stathmin expression decreases during cerebellar development and overexpression of stathmin dramatically reduces dendritc growth of cultured Purkinje cells (Ohkawa et al., 2007).
2.2.4.2 SCG-10
Superior cervical ganglia neural-specific 10 protein (SCG-10) is a membrane-associated stathmin family member that is highly expressed in the developing nervous system and predominantly localizes to the growth cone (Grenningloh et al., 2003; Mori and Morii, 2002). Like stathmin, in vitro SCG-10 potently inhibits MT polymerization and promotes MT depolymerization (Riederer et al., 1997). SCG-10 destabilizes MTs at the minus end by binding along the length of MTs and increasing catastrophe frequency and shortening rate. However, in contrast to stathmin, SCG-10 also binds the plus end and promotes MT stability by increasing the rate and extent of polymerization (Manna et al., 2007). siRNA-mediated depletion of SCG-10 reduces neurite outgrowth in rat hippocampal cultures, suggesting that SCG-10 positively regulates neurite outgrowth (Morii et al., 2006). This was confirmed by increased outgrowth following SCG-10 overexpression. This study also suggests that optimal levels of SCG-10 are required for growth-cone motility: decreased SCG-10 increased growth-cone stability, whereas SCG-10 overexpression increased growth-cone dynamics. In dissociated RGCs cultured in the presence of both laminin and L1-CAM, exposure to the axon-guidance cue EphB induces a pause in growth-cone motility, a reorganization of growth-cone MTs, and a reduction in SGC-10 protein. The splayed MT phenotype and growth-cone pausing were phenocopied by injection of a SGC-10 function-blocking antibody (Suh et al., 2004), suggesting that SCG-10 may play a role in axon navigation. Like stathmin, overexpression of SCG-10 in cultured Purkinje cells reduced DA (Ohkawa et al., 2007).
2.2.4.3 SCG-10-Like Protein
SCG-10-like protein, SCLIP, is a neuronal member of the stathmin family of MT-destabilizing proteins that closely resemble SCG-10 and has similar tubulin-binding properties (Charbaut et al., 2001). Like SCG-10, SCLIP is expressed in early stages of hippocampal neuron development in culture, where it localizes to vesicular structures along MTs, particularly in the growth cone (Poulain and Sobel, 2007). In contrast to SCG-10, when SCLIP expression is inhibited by siRNA in hippocampal neurons, axonal branching increases significantly, indicating that SCLIP negatively regulates axon branching. Although all members of the stathmin family destabilize MTs, based on the differences in SCLIP and SCG-10 knockdown, they perform distinct functions during neuronal development. This is supported by their overlapping but distinct expression profiles in different neuronal types (Nakazawa et al., 2000).
2.2.4.4 Capzb2
Capzb2 is the brain-specific isoform of the β subunit of the heterodimeric F-actin capping protein (CP). In addition to capping F-actin and blocking actin polymerization, Capzb2 has been reported to bind βIII-tubulin and slow MT polymerization in vitro (Davis et al., 2009). shRNA-mediated depletion of Capzb2 in hippocampal neurons resulted in short neurites and improperly formed growth cones with increased MT invasion. Whereas expression of full-length Capzb2 could rescue these phenotypes, expression of a mutant that maintained actin-capping function, but not MT-binding function could not (Davis et al., 2009). This indicates that MT binding is necessary, but whether it is sufficient, or if F-actin:MT crosslinking is required has not been addressed.
2.2.5 MT-Motor Proteins
The unique elongated size of neurons creates special requirements on the transport of materials throughout neurites. In addition, to maintain the functional differences and thus identity of axons and dendrites, cargos must be differentially directed to these compartments. MTs serve as tracks for kinesin and dynein motors to generate force and transport cargo throughout the neuron (Goldstein and Yang, 2000; Maday et al., 2014), and indeed the first MT motor protein identified was isolated from the axoplasm of the squid giant axon (Vale et al., 1985). A number of degenerative diseases result from mutations in motor proteins that result in problems in cargo transport along MTs, and subsequently axonal integrity (Roy et al., 2005).
2.2.5.1 Kinesins
The vast majority of members of the kinesin family of molecular motors move toward the MT plus end, although there are some minus-end directed and nonmotile kinesins. Therefore kinesins comprise the major motors for anterograde fast axonal transport (Brady et al., 1990; Gindhart, 1998; Martin et al., 1999). The motile kinesins function through intracellular transport of specific cargos and/or by altering MT dynamics. Kinesins alter MT dynamics by changing the conformation of tubulin heterodimers or by transporting proteins that influence MT dynamics along the lattice to the tips of the MTs (Drummond, 2011). The nonmotile kinesin family member, kinesin-13 functions as MT depolymerase. Plus-end directed motors are typically localized at neurite tips (Jacobson et al., 2006). Various members of the kinesin superfamily have been implicated in different aspects of neuronal development. Interestingly in cultured drosophila neurons, pharmacologically blocking F-actin polymerization or MT polymerization did not block axon outgrowth (Lu et al., 2013). However, reduction of kinesin-1 levels in cultured neurons from hypomorophic lines revealed an associated loss of MT sliding and reduced neurite outgrowth (Lu et al., 2013). Since kinesin-1 is highly conserved across species it is highly likely that MT sliding is a feasible mechanism for initiating neurite outgrowth. In contrast, reduction of kine-sin-5 (Kif11) levels by RNAi in rat SCG neurons dramatically increases axon length, axon branching, and growth-cone size (Myers and Baas, 2007). This increased axonal growth is associated with a decrease in axon retraction and increased transport of short MTs (Myers and Baas, 2007). Similar increases in axon growth and MT invasion of filopodia occurs following reduction of kinesin-12 (Kif15) in rat cortical neurons, although this is not associated with increased axon branching and growth-cone size (Liu et al., 2010). Whereas SGC neurons typically prefer a laminin substrate and avoid PDL, following Kif15 knockdown, PDL avoidance is disrupted (Liu et al., 2010), indicating Kif15 may also regulate axon guidance. Prior to axon outgrowth the kinesin-1 family member Kif5C localizes equally to all neurites (Jacobson et al., 2006). Stable accumulation of Kif5C at a neurite tip correlates with axon specification, whereas this is not the case with the kinesin-3 family member Kif1A. This suggests that Kif5C may be involved in axon specification, via selective transport of axon-specific cargos. The M-kinesin family including Kif2A depolymerize MTs at their plus tips in vitro and in growth cones (Desai et al., 1999; Homma et al., 2003). Deletion of Kif2A in mice induces gross neuroanatomical defects and perinatal lethality (Homma et al., 2003). Genetic loss of Kif2A in hippocampal neurons results in increased length of axon collateral branches without an increase in branch number, suggesting Kif2a suppresses collateral branches during axon extension.
2.2.5.2 Dynein and Dynein-Associated Proteins
Compared to kinesin motors, dynein is a considerably larger complex of proteins containing two heavy-chain subunits, intermediate chains, light-intermediate chains, and light chains. Additionally, dynein activity is regulated by accessory proteins including lissencephaly 1 (LIS1), nuclear distribution E (NudE), and the dynactin complex (Levy et al., 2006). The MT minus-end directed motor dynein and its regulatory factor Lis1 play an important role in axonogenesis by modulating MT transport and penetration into the peripheral zone during axon specification and elongation (Grabham et al., 2007; Roossien et al., 2014). Lis1 is enriched in neurons compared to other cell types (Smith et al., 2000). Mutations in human LIS1 cause lissencephaly, a disease characterized by an almost complete lack of gyri, enlarged ventricles, and thickened cortex. Dynein, dynactin, and Lis1 accumulate in the growth cones of actively extending axons (Grabham et al., 2007). Genetic deletion of LIS1 in mice reduces neuronal migration and neurite length in vivo (Youn et al., 2009). Antibody-based inhibition of dynein or LIS1 in chick DRGs blocks growth-cone dynamics and MT reorganization. This is not due to defective MT polymerization, but rather an inability of MTs to resist F-actin retrograde flow. LIS1 RNAi also blocked axon elongation (Grabham et al., 2007). Similarly, CALI of the growth-cone pool of dynamitin, a component of the dynactin complex, decreases growth-cone motility (Abe et al., 2008). Further suggesting dynein helps MTresist F-actin retrograde flow, grow into the peripheral zone, and modulate growth-cone direction, the knockdown of dynein heavy chain in rat SGC causes MTs to remain within the central domain of the growth cone and axons to retract further and fail to efficiently turn in response to substrate borders (Myers et al., 2006). Suppression of the dynein light chain Tctex-1 by RNAi in cultured hippocampal neurons delays neuritogenesis, whereas Tctex-1 overexpression induces supernumarary axons, indicative of roles in neurite outgrowth and axon specification (Chuang et al., 2005), however, these phenotypes are not phenocopied by suppression of dynein heavy-chain expression, suggesting they may not be dynein related. In DA neurons in Drosophila, dynein controls the orientation of axonal MTs and transport of Golgi outposts into dendrites (Zheng et al., 2008). The axons in dynein mutant neurons contain both MTs with minus-ends and plus-ends distal, and aberrantly contain Golgi outposts, which are normally restricted to dendrites. Additionally neurons with mutated dynein have shorter, less arborized dendrites (Satoh et al., 2008; Zheng et al., 2008). Loss of Lis1 in Drosophila also blocks dendritic growth, branching, and maturation (Luo et al., 2000). Additionally, Kapitein et al. identified that dynein helps to sort cargo between the axons and the dendrites to maintain a polarized morphology. These studies were performed in rat hippocampal neurons by examining the trajectories of peroxisomes and their delivery to the dendrites (Kapitein et al., 2010).
2.3 Actin/MT-Linking Actin-Binding Proteins
2.3.1 Drebrin
Drebrin (developmentally regulated brain protein) is a side-binding F-actin-binding protein that also directly interacts with the MT + Tip EB3 (Geraldo et al., 2008). Drebrin binding to F-actin prevents binding of tropomyosin and alpha-actinin, resulting in thick, curved F-actin bundles (Hayashi, 1999; Ishikawa and Kohama, 2007). Drebrin localizes within the transition zone of growth cones at the base of filopodia, between the F-actin rich periphery and MT-rich central domain (Geraldo et al., 2008; Mizui et al., 2009). Similarly drebrin localizes within dendritic spines, another site of MT and F-actin interactions (Hayashi et al., 1996; Merriam et al., 2013). This localization allows drebrin to interact with EB3 and promote MT extension along F-actin bundles. Expression of a mutant of EB3 that maintains interactions with drebrin but not MTs results in a loss of neurite extension and failure to form growth cones. Similarly, siRNA-mediated knockdown of drebrin also impedes neurite extension and growth-cone formation (Geraldo et al., 2008). In line with this, overexpression of drebrin promotes axon outgrowth in cultured hippocampal neurons (Mizui et al., 2009). shRNA-mediated knockdown and overexpression studies in chick oculomotor neurons demonstrate that drebrin is required for appropriate axonal morphology, but not growth-cone guidance (Dun et al., 2012). Drebrin A is a major drebrin isoform expressed in the adult brain that localizes to dendritic spines (Hayashi and Shirao, 1999). Overexpression of drebrin A in DIV7–9 immature hippocampal neurons enlarges filopodial protrusions that are rich in F-actin and PSD95 but negative for MAP2, but does not promote early spine maturation (Mizui et al., 2005). Another study shows that overexpressed drebrin A localizes to spines in cultured cortical neurons, which were dependent on its ability to bind F-actin. Cortical-neuron spine length also increases upon drebrin A overexpression (Hayashi and Shirao, 1999). Drebrin knockown by siRNA inhibits MTentry into spines, whereas increased drebrin expression promotes MTentry (Merriam et al., 2013), suggesting that similar actin–MT crosslinking occurs in spines as in the extending axonal growth cone. Therefore during dendritic spine formation drebrin A plays an important role by controlling the size of filopodia spines and enhancing F-actin/MT interactions in the spines.
2.3.2 TcTex-1
TcTex-1 is a light-chain subunit of the cytoplasmic dynein motor complex that is expressed in postmitotic hippocampal neurons and accumulates in growing axons (Chuang et al., 2005). Biochemical and microscopy-based evidence suggests that Tctex-1 can function separately from the dynein complex and closely controls both F-actin and MT dynamics. Hippocampal neurons depleted of TcTex-1 by siRNA or antisense RNA develop segmented lamellipodia but fail to develop neurites (Chuang et al., 2005). Conversely, neurons overexpressing Tctex-1 form multiple axons, an increased number of MAP2 positive dendrites, and elevated dendrite branching. The authors of this study suggested that TcTex-1 regulates neuronal morphogenesis independent of its motor protein activity, as expression of the dynactin subunit p50 dynamitin, which interferes with dynein activity, or downregulation of dynein heavy chain, did not phenocopy Tctex-1 depletion. Furthermore expression of a phosphomimetic version of Tctex-1 that does not incorporate with the dynein complex mimicked overexpression axonal and dendritic phenotypes, whereas an unphosphorylatable mutant could not (Chuang et al., 2005). The dynein-independent function of Tctex-1 is proposed to be through modulation of activity of Rho GTPases and thus the actin cytoskeleton (Chuang et al., 2005; Conde et al., 2010; Rothenberg et al., 2003). Loss-of-function studies indicate it is essential during multiple stages of neuronal morphogenesis.
2.3.3 Pod1
Pod1 (polarity osmotic defective-1) is an F-actin-binding protein with homology to the mammalian Type III Coronin, Coro7 (Chan et al., 2011). In both nonneuronal cells and neurons, Drosophila Pod-1 (Dpod1) localizes to lamellar sites of F-actin polymerization as well as with a subset of MTs polymerizing into this region, including at the tips of growing axons (Rothenberg et al., 2003). In vitro, Dpod1 crosslinks F-actin and MTs. Whereas RNAi-mediated knockdown of Dpod1 in nonneuronal S2 cells failed to disrupt F-actin and MT organization, overexpression of Dpod1 induced dramatic dose-dependent changes in cell shape, producing neurite-like projections (Rothenberg et al., 2003). The phenotypes of dpod1 null flies suggest that Dpod1-mediated crosslinking between actin and MTs is not required for neurite outgrowth but instead regulates growth-cone guidance in the ventral nerve cord. These flies exhibit multiple axonal defects including midline crossing defects, thinning of longitudinals, wandering trajectories, axon breaks, thinning of the commissures, and axon tangles in the ventral nerve cord (Rothenberg et al., 2003). Interestingly, loss of Dpod1 disrupts guidance downstream of filopodia formation; the number of filopodia at these growth cones was unchanged. Further indication that precise levels of Dpod1 are required for appropriate crosslinking of the actin and MT cytoskeletons, overexpression of Dpod1 using the UAS-Gal4 driver system induces defects similar to those observed upon loss of Dpod1. Whether the mammalian Coro7, which is highly expressed in the cortex, is involved in neuronal morphogenesis has yet to be established. Since Coro7 localizes to the Golgi apparatus as opposed to F-actin based structures in mammalian cells and lacks a MT-binding domain (Rybakin et al., 2004), if it does affect neuronal morphogenesis, it will likely be through a distinct mechanism. Of the other mammalian coronins, coronin 1b and coronin 3 are expressed in the brain but their role in vivo has not been established. However, acute knockdown of coronin 1b or overexpression of truncated coronin 3 in PC-12 cells decreases neurite number (Di Giovanni, 2004; Hasse et al., 2005). These results therefore suggest a possible divergence in coronin protein function with evolution or divergent functions within the coronin family.
2.3.4 IQGAP1
IQGAP1 (IQ domain-containing GTPase-activating protein 1) is a 190-kDa phosphoprotein involved in cell–matrix interactions and F-actin dynamics at the leading edge of nonneuronal cells. In vitro purified IQGAP1 directly binds F-actin and promotes F-actin crosslinking (Bashour et al., 1997). IQGAP1 mediates crosslinking between MTs and F-actin via an interaction with the MT +Tip CLIP-170. Phosphorylation of IQGAP1 at Ser residues 1441 and 143 promote neurite outgrowth in the neuroblastoma cell line N1E-115 as seen by overexpression studies using either the wild-type or a phosphomimetic mutant, but not nonphorphorylatable mutants (Li et al., 2005b). Several lines of evidence implicate IQGAP1 in the formation of dendritic spines: IQGAP1 depletion by shRNA and IQGAP1 overexpression decreases and increases dendritic complexity and dendritic spines in cultured hippocampal neurons, respectively (Jausoro et al., 2013; Swiech et al., 2011). Deletion of IQGAP1 also decreases hippocampal spine numbers in vivo and was associated with specific cognitive deficits (Gao et al., 2011). These functions appear to rely on the function of IQGAP to interact with a number of cytoskeletal proteins. To demonstrate interplay between IQGAP1 and CLIP-170 maintains dendritic arbor morphology, Sweich et al. performed reciprocal rescue experiments; shRNA-mediated knockdown of one protein could be rescued by overexpressing a GFP-tagged construct of the other protein in hippocampal neurons (Swiech et al., 2011). Similarly, Jausoro et al. show that the N-terminal of IQGAP1 mediates spine head formation possibly by modulating the N-WASP/Arp2/3 complex function, whereas the region-binding Cdc42 and Rac is required for stalk-neck formation in spines by overexpressing either wild-type or domain deletion constructs of IQGAP-1 in dissociated hippocampal cultures (Jausoro et al., 2013). Lack of the N-WASP binding domain of IQGAP-1 results in filopodia-like spines without a distinct head region whereas inability to bind Cdc42 and Rac resulted in short stubby spines.
2.4 Molecular Switches: Rho GTPases and Their Downstream Effectors
Rho GTPases are master regulators of both the F-actin and MT cytoskeletons. Their functions in neuronal development have recently been reviewed (Hall and Lalli, 2010; Spillane and Gallo, 2014).
3. CONCLUDING REMARKS
The cytoskeleton of the neuron continues to be an area of intense research. The last few decades have identified many of the important players of the actin and MT cytoskeleton that are sufficient or sometimes essential in establishing neuronal polarity, establishing and maintaining neuronal morphology, steering axons to their correct target locations, and lastly forming synaptic connections that are required for proper functioning of the brain. In contrast, the field of neurofilaments is relatively far behind, and has room for more advances. From the numerous studies presented in this chapter, an overarching theme is the redundancy in protein function, either from the same protein family or from compensatory signaling pathways and cytoskeletal regulatory proteins. Another recurring concept throughout the review is the evolutionary divergence of brain-specific isoforms of a number of actin and MT-regulatory proteins. But critical to defining the function of these players is a more thorough understanding of how acute versus stable loss or gain of function can alter phenotypes both in vivo and in vitro. Additionally, another contributing factor modulating the phenotypes observed experimentally is the extracellular matrix on which neurons are grown. As evident from the studies summarized here, the extracellular matrix protein or peptides or axon-guidance cues used for experimental interrogations can lead to significantly divergent results. What is not discussed here but is another area of neuronal research is the influence of substrate compliance and dimensionality when performing in vitro experiments (Koch et al., 2012; Moore et al., 2009). Neurons in the brain are in a more compliant 3D substrate as compared to when neurons are cultured in vitro on glass or plastic 2D surfaces. Therefore the choice of extracellular ligand, the type of substrate neurons are cultured on and genetic manipulation methodologies can lead to distinct results and observable phenotypes. In conclusion, we mention that this chapter provides an insight into the multitude of actin and MT-binding/ regulatory proteins, but the field is still in its infancy and requires more research into dissecting redundant pathways, and alternate strategies to culture and manipulate neurons.
Acknowledgments
Stephanie Gupton was supported by the National Institutes of Health grant, GM108970, and Shalini Menon was supported by American Heart Association Fellowship, 14POST20450085.
References
- Abe TK, Honda T, Takei K, Mikoshiba K, Hoffman-Kim D, Jay DG, Kuwano R. Dynactin is essential for growth cone advance. Biochem Biophys Res Commun. 2008;372:418–422. doi: 10.1016/j.bbrc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Ahmad FJ, Yu W, McNally FJ, Baas PW. An essential role for katanin in severing microtubules in the neuron. J Cell Biol. 1999;145:305–315. doi: 10.1083/jcb.145.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahuja R, Pinyol R, Reichenbach N, Custer L, Klingensmith J, Kessels MM, Qualmann B. Cordon-bleu is an actin nucleation factor and controls neuronal morphology. Cell. 2007;131:337–350. doi: 10.1016/j.cell.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4:367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- Akhmanova A, Hoogenraad CC. Microtubule minus-end-targeting proteins. Curr Biol. 2015;25:R162–R171. doi: 10.1016/j.cub.2014.12.027. [DOI] [PubMed] [Google Scholar]
- Akhmanova A, Hoogenraad CC, Drabek K, Stepanova T, Dortland B, Verkerk T, Vermeulen W, Burgering BM, De Zeeuw CI, Grosveld F, Galjart N. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 2001;104:923–935. doi: 10.1016/s0092-8674(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Al-Bassam J, Kim H, Brouhard G, van Oijen A, Harrison SC, Chang F. CLASP promotes microtubule rescue by recruiting tubulin dimers to the microtubule. Dev Cell. 2010;19:245–258. doi: 10.1016/j.devcel.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine-Bertrand J, Ghogha A, Luangrath V, Bedford FK, Lamarche-Vane N. The activation of ezrin-radixin-moesin (ERM) proteins is regulated by netrin-1 through Src kinase and RhoA/Rho kinase activities and mediates netrin-1-induced axon outgrowth. Mol Biol Cell. 2011;22:3734–3746. doi: 10.1091/mbc.E10-11-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa Y, Bito H, Furuyashiki T, Tsuji T, Takemoto-Kimura S, Kimura K, Nozaki K, Hashimoto N, Narumiya S. Control of axon elongation via an SDF-1alpha/ Rho/mDia pathway in cultured cerebellar granule neurons. J Cell Biol. 2003;161:381–391. doi: 10.1083/jcb.200210149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Black MM, Banker GA. Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. J Cell Biol. 1989;109:3085–3094. doi: 10.1083/jcb.109.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–1283. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- Bamburg JR, Bernstein BW. Roles of ADF/cofilin in actin polymerization and beyond. F1000 Biol Rep. 2010;2:62. doi: 10.3410/B2-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamburg JR, Bray D, Chapman K. Assembly of microtubules at the tip of growing axons. Nature. 1986;8(6072):788–790. doi: 10.1038/321788a0. [DOI] [PubMed] [Google Scholar]
- Barrientos T, Frank D, Kuwahara K, Bezprozvannaya S, Pipes GCT, Bassel-Duby R, Richardson JA, Katus HA, Olson EN, Frey N. Two novel members of the ABLIM protein family, ABLIM-2 and -3, associate with STARS and directly bind F-actin. J Biol Chem. 2007;282:8393–8403. doi: 10.1074/jbc.M607549200. [DOI] [PubMed] [Google Scholar]
- Barzik M, Kotova TI, Higgs HN, Hazelwood L, Hanein D, Gertler FB, Schafer DA. Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins. J Biol Chem. 2005;280:28653–28662. doi: 10.1074/jbc.M503957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashour AM, Fullerton AT, Hart MJ, Bloom GS. IQGAP1, a Rac- and Cdc42-binding protein, directly binds and cross-links microfilaments. J Cell Biol. 1997;137:1555–1566. doi: 10.1083/jcb.137.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Gertler FB. Ena/VASP: towards resolving a pointed controversy at the barbed end. J Cell Sci. 2009;122:1947–1953. doi: 10.1242/jcs.038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Loureiro JJ, Libova I, Fässler R, Wehland J, Gertler FB. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 2000;101:717–728. doi: 10.1016/s0092-8674(00)80884-3. [DOI] [PubMed] [Google Scholar]
- Beaudoin GMJ, Schofield CM, Nuwal T, Zang K, Ullian EM, Huang B, Reichardt LF. Afadin, a Ras/Rap effector that controls cadherin function, promotes spine and excitatory synapse density in the hippocampus. J Neurosci. 2012;32:99–110. doi: 10.1523/JNEUROSCI.4565-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechstedt S, Brouhard GJ. Doublecortin recognizes the 13-protofilament microtubule cooperatively and tracks microtubule ends. Dev Cell. 2012;23:181–192. doi: 10.1016/j.devcel.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U, Dillon GM, Sullivan JM, Stuart CE, Gilbert JP, Kambouris JA, Ho A. Microtubule plus-end tracking protein CLASP2 regulates neuronal polarity and synaptic function. J Neurosci. 2012;32:13906–13916. doi: 10.1523/JNEUROSCI.2108-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellenchi GC, Gurniak CB, Perlas E, Middei S, Ammassari-Teule M, Witke W. N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex. Genes Dev. 2007;21:2347–2357. doi: 10.1101/gad.434307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkblom B, Ostman N, Hongisto V, Komarovski V, Filén JJ, Nyman TA, Kallunki T, Courtney MJ, Coffey ET. Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: role of microtubule-associated protein 2 as an effector. J Neurosci. 2005;25:6350–6361. doi: 10.1523/JNEUROSCI.1517-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhelifa M, Parast MM, Valtschanoff JG, LaMantia AS, Meeker RB, Otey CA. A role for the cytoskeleton-associated protein palladin in neurite outgrowth. Mol Biol Cell. 2001;12:2721–2729. doi: 10.1091/mbc.12.9.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouquet C, Ravaille-Veron M, Propst F, Nothias F. MAP1B coordinates microtubule and actin filament remodeling in adult mouse Schwann cell tips and DRG neuron growth cones. Mol Cell Neurosci. 2007;36:235–247. doi: 10.1016/j.mcn.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Bouquet C, Soares S, von Boxberg Y, Ravaille-Veron M, Propst F, Nothias F. Microtubule-associated protein 1B controls directionality of growth cone migration and axonal branching in regeneration of adult dorsal root ganglia neurons. J Neurosci. 2004;24:7204–7213. doi: 10.1523/JNEUROSCI.2254-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. Establishment of neuronal polarity: lessons from cultured hippocampal neurons. Curr Opin Neurobiol. 2000;10:574–581. doi: 10.1016/s0959-4388(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Brady ST, Pfister KK, Bloom GS. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc Natl Acad Sci. 1990;87:1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman PC, Dave S, Asnes CF, Tullio AN, Adelstein RS. Myosin IIB is required for growth cone motility. J Neurosci. 2001;21:6159–6169. doi: 10.1523/JNEUROSCI.21-16-06159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Wysolmerski RB, Bridgman PC. Dorsal root ganglion neurons react to semaphorin 3A application through a biphasic response that requires multiple myosin II isoforms. Mol Biol Cell. 2009;20:1167–1179. doi: 10.1091/mbc.E08-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres SB, Cooper-Kuhn CM, Winkler JR, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Brown ME. Retrograde flow rate is increased in growth cones from myosin IIB knockout mice. J Cell Sci. 2003;116:1087–1094. doi: 10.1242/jcs.00335. [DOI] [PubMed] [Google Scholar]
- Buck KB, Zheng JQ. Growth cone turning induced by direct local modification of microtubule dynamics. J Neurosci. 2002;22:9358–9367. doi: 10.1523/JNEUROSCI.22-21-09358.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette DT, Ji L, Schaefer AW, Medeiros NA, Danuser G, Forscher P. Myosin II activity facilitates microtubule bundling in the neuronal growth cone neck. Dev Cell. 2008;15:163–169. doi: 10.1016/j.devcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres A, Banker G, Steward O, Binder L, Payne M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Dev Brain Res. 1984;13:314–318. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- Caceres A, Mautino J, Kosik KS. Suppression of MAP2 in cultured cerebellar macroneurons inhibits minor neurite formation. Neuron. 1992;9:607–618. doi: 10.1016/0896-6273(92)90025-9. [DOI] [PubMed] [Google Scholar]
- Castelo L, Jay DG. Radixin is involved in lamellipodial stability during nerve growth cone motility. Mol Biol Cell. 1999;10:1511–1520. doi: 10.1091/mbc.10.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon MR, Navarro AI, Cuesto G, del Pino I, Scott R, Morales M, Rico B. Focal adhesion kinase regulates actin nucleation and neuronal filopodia formation during axonal growth. Development. 2012;139:3200–3210. doi: 10.1242/dev.080564. [DOI] [PubMed] [Google Scholar]
- Chamak B, Prochiantz A. Influence of extracellular matrix proteins on the expression of neuronal polarity. Development. 1989;106:483–491. doi: 10.1242/dev.106.3.483. [DOI] [PubMed] [Google Scholar]
- Chan KT, Creed SJ, Bear JE. Unraveling the enigma: progress towards understanding the coronin family of actin regulators. Trends Cell Biol. 2011;21:481–488. doi: 10.1016/j.tcb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Adler CE, Krause M, Clark SG, Gertler FB, Tessier-Lavigne M, Bargmann CI. MIG-10/lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr Biol. 2006;16:854–862. doi: 10.1016/j.cub.2006.03.083. [DOI] [PubMed] [Google Scholar]
- Charbaut E, Curmi PA, Ozon S, Lachkar S, Redeker V, Sobel A. Stathmin family proteins display specific molecular and tubulin binding properties. J Biol Chem. 2001;276:16146–16154. doi: 10.1074/jbc.M010637200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Tian X, Kim WY, Snider WD. Adenomatous polyposis coli regulates axon arborization and cytoskeleton organization via its N-terminus. PLoS ONE. 2011;6:e24335. doi: 10.1371/journal.pone.0024335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JZ, Yeh TY, Bollati F, Conde C, Canavosio F, Cáceres A, Sung CH. The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev Cell. 2005;9:75–86. doi: 10.1016/j.devcel.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohan CS, Welnhofer EA, Zhao L, Matsumura F, Yamashiro S. Role of the actin bundling protein fascin in growth cone morphogenesis: localization in filopodia and lamellipodia. Cell Motil Cytoskeleton. 2001;48:109–120. doi: 10.1002/1097-0169(200102)48:2<109::AID-CM1002>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Cecilia C, Arias C, Robin M, Li A, Saito M, Chuang JZ, Nairn AC, Sung CH, Cáceres A. Evidence for the involvement of Lfc and Tctex-1 in axon formation. J Neurosci. 2010;30:6793–6800. doi: 10.1523/JNEUROSCI.5420-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CM, Crawford BC, Akbarian S. White matter neuron alterations in schizophrenia and related disorders. Int J Dev Neurosci. 2011;29:325–334. doi: 10.1016/j.ijdevneu.2010.07.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo JC, Deuel TA, Long JM, LaPorte P, Tsai E, Wynshaw-Boris A, Walsh CA. Doublecortin is required in mice for lamination of the hippocampus but not the neocortex. J Neurosci. 2002;22:7548–7557. doi: 10.1523/JNEUROSCI.22-17-07548.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox PR, Fowler V, Xu B, Sweatt JD, Paylor R, Zoghbi HY. Mice lacking Tropomodulin-2 show enhanced long-term potentiation, hyperactivity, and deficits in learning and memory. Mol Cell Neurosci. 2003;23:1–12. doi: 10.1016/s1044-7431(03)00025-3. [DOI] [PubMed] [Google Scholar]
- Crawford AW, Pino JD, Beckerle MC. Biochemical and molecular characterization of the chicken cysteine-rich protein, a developmentally regulated LIM-domain protein that is associated with the actin cytoskeleton. J Cell Biol. 1994;124:117–127. doi: 10.1083/jcb.124.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva JS, Medina M, Zuliani C, Di Nardo A, Witke W, Dotti CG. RhoA/ ROCK regulation of neuritogenesis via profilin IIa-mediated control of actin stability. J Cell Biol. 2003;162:1267–1279. doi: 10.1083/jcb.200304021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DA, Wilson MH, Giraud J, Xie Z, Tseng HC, England C, Herscovitz H, Tsai LH, Delalle I. Capzb2 interacts with beta-tubulin to regulate growth cone morphology and neurite outgrowth. PLoS Biol. 2009;7:e1000208. doi: 10.1371/journal.pbio.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Hertzberg EL, Mugnaini E. The dendritic lamellar body: a new neuronal organelle putatively associated with dendrodendritic gap junctions. J Neurosci. 1995;15:1587–1604. doi: 10.1523/JNEUROSCI.15-02-01587.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw CI, Hoogenraad CC, Goedknegt E, Hertzberg E, Neubauer A, Grosveld F, Galjart N. CLIP-115, a novel brain-specific cytoplasmic linker protein, mediates the localization of dendritic lamellar bodies. Neuron. 1997;19:1187–1199. doi: 10.1016/s0896-6273(00)80411-0. [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. Actin and microtubules in neurite initiation: are MAPs the missing link? J Neurobiol. 2004;58:18–33. doi: 10.1002/neu.10284. [DOI] [PubMed] [Google Scholar]
- Dehmelt L, Halpain S. The MAP2/Tau family of microtubule-associated proteins. Genome Biol. 2005;6:204. doi: 10.1186/gb-2004-6-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L, Smart FM, Ozer RS, Halpain S. The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J Neurosci. 2003;23:9479–9490. doi: 10.1523/JNEUROSCI.23-29-09479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Río JA, González-Billault C, Ureña JM, Jiménez EM, Barallobre MJ, Pascual M, Pujadas L, Simó S, La Torre A, Wandosell F, Avila J, Soriano E. MAP1B is required for Netrin 1 signaling in neuronal migration and axonal guidance. Curr Biol. 2004;14:840–850. doi: 10.1016/j.cub.2004.04.046. [DOI] [PubMed] [Google Scholar]
- Dent EW, Callaway JL, Szebenyi G, Baas PW, Kalil K. Reorganization and movement of microtubules in axonal growth cones and developing interstitial branches. J Neurosci. 1999;19:8894–8908. doi: 10.1523/JNEUROSCI.19-20-08894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Gupton SL, Gertler FB. The Growth Cone Cytoskeleton in Axon Outgrowth and Guidance. Cold Spring Harb Perspect Biol. 2011;3:a001800. doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Kalil K. Axon branching requires interactions between dynamic microtubules and actin filaments. J Neurosci. 2001;21:9757–9769. doi: 10.1523/JNEUROSCI.21-24-09757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Kwiatkowski AV, Mebane LM, Philippar U, Barzik M, Rubinson DA, Gupton S, Van Veen JE, Furman C, Zhang J, Alberts AS, Mori S, Gertler FB. Filopodia are required for cortical neurite initiation. Nat Cell Biol. 2007;9:1347–1359. doi: 10.1038/ncb1654. [DOI] [PubMed] [Google Scholar]
- Desai A, Verma S, Mitchison TJ, Walczak CE. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 1999;96:69–78. doi: 10.1016/s0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Deuel TAS, Liu JS, Corbo JC, Yoo SY, Rorke-Adams LB, Walsh CA. Genetic interactions between doublecortin and doublecortin-like kinase in neuronal migration and axon outgrowth. Neuron. 2006;49:41–53. doi: 10.1016/j.neuron.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Di Giovanni S. In vivo and in vitro characterization of novel neuronal plasticity factors identified following spinal cord injury. J Biol Chem. 2004;280:2084–2091. doi: 10.1074/jbc.M411975200. [DOI] [PubMed] [Google Scholar]
- DiTella MC, Feiguin F, Carri N, Kosik KS, Caceres A. MAP-1B/TAU functional redundancy during laminin-enhanced axonal growth. J Cell Sci. 1996;109:467–477. doi: 10.1242/jcs.109.2.467. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragestein KA, van Cappellen WA, van Haren J, Tsibidis GD, Akhmanova A, Knoch TA, Grosveld F, Galjart N. Dynamic behavior of GFP-CLIP-170 reveals fast protein turnover on microtubule plus ends. J Cell Biol. 2008;180:729–737. doi: 10.1083/jcb.200707203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DR. Regulation of microtubule dynamics by kinesins. Semin Cell Dev Biol. 2011;22:927–934. doi: 10.1016/j.semcdb.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Dun XP, de Lima TB, Allen J, Geraldo S, Gordon-Weeks P, Chilton JK. Drebrin controls neuronal migration through the formation and alignment of the leading process. Mol Cell Neurosci. 2012;49:341–350. doi: 10.1016/j.mcn.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JE, Lytle NK, Zuniga A, Goldstein LSB. The microtubule regulatory protein stathmin is required to maintain the integrity of axonal microtubules in Drosophila. PLoS ONE. 2013;8:e68324. doi: 10.1371/journal.pone.0068324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedy A, Gertler FB, Miller J, Holt CE, Lebrand C. Ena/VASP function in retinal axons is required for terminal arborization but not pathway navigation. Development. 2007;134:2137–2146. doi: 10.1242/dev.002345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann W, Zervas M, Costello P, Roback L, Fischer I, Hammarback JA, Cowan N, Davies P, Wainer B, Kucherlapati R. Neuronal abnormalities in microtubule-associated protein 1B mutant mice. Proc Natl Acad Sci USA. 1996;93:1270–1275. doi: 10.1073/pnas.93.3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mezgueldi M. Tropomyosin dynamics. J Muscle Res Cell Motil. 2014;35:203–210. doi: 10.1007/s10974-014-9377-x. [DOI] [PubMed] [Google Scholar]
- Engle EC. Human genetic disorders of axon guidance. Cold Spring Harb Perspect Biol. 2010;2:a001784. doi: 10.1101/cshperspect.a001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassier C, Tarrade A, Peris L, Courageot S, Mailly P, Dalard C, Delga S, Roblot N, Lefèvre J, Job D, Hazan J, Curmi PA, Melki J. Microtubule-targeting drugs rescue axonal swellings in cortical neurons from spastin knockout mice. Dis Model Mech. 2013;6:72–83. doi: 10.1242/dmm.008946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath T, Fischer RS, Dehmelt L, Halpain S, Fowler VM. Tropomodulins are negative regulators of neurite outgrowth. Eur J Cell Biol. 2011;90:291–300. doi: 10.1016/j.ejcb.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA. Phenotype of mice lacking functional deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Figge C, Loers G, Schachner M, Tilling T. Neurite outgrowth triggered by the cell adhesion molecule L1 requires activation and inactivation of the cytoskeletal protein cofilin. Mol Cell Neurosci. 2012;49:196–204. doi: 10.1016/j.mcn.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Firat-Karalar EN, Hsiue PP, Welch MD. The actin nucleation factor JMY is a negative regulator of neuritogenesis. Mol Biol Cell. 2011;22:4563–4574. doi: 10.1091/mbc.E11-06-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer I, Romano-Clarke G. Association of microtubule-associated protein (MAP1B) with growing axons in cultured hippocampal neurons. J Mol Cell Neurosci. 1991;2:39–51. doi: 10.1016/1044-7431(91)90038-p. [DOI] [PubMed] [Google Scholar]
- Flynn KC, Hellal F, Neukirchen D, Jacob S, Tahirovic S, Dupraz S, Stern S, Garvalov BK, Gurniak C, Shaw AE, Meyn L, Wedlich-Söldner R, Bamburg JR, Small JV, Witke W, Bradke F. ADF/cofilin-mediated actin retrograde flow directs neurite formation in the developing brain. Neuron. 2012;76:1091–1107. doi: 10.1016/j.neuron.2012.09.038. [DOI] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002a;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Itoh TJ, Kimura T, Ménager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H, Kaibuchi K. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 2002b;4:583–591. doi: 10.1038/ncb825. [DOI] [PubMed] [Google Scholar]
- Gallo G, Yee HF, Letourneau PC. Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J Cell Biol. 2002;158:1219–1228. doi: 10.1083/jcb.200204140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Frausto SF, Guedea AL, Tronson NC, Jovasevic V, Leaderbrand K, Corcoran KA, Guzman YF, Swanson GT, Radulovic J. IQGAP1 regulates NR2A signaling, spine density, and cognitive processes. J Neurosci. 2011;31:8533–8542. doi: 10.1523/JNEUROSCI.1300-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MC, Abbasi M, Singh S, He Q. Role of Drosophila gene dunc-115 in nervous system. Invert Neurosci. 2007;7:119–128. doi: 10.1007/s10158-007-0047-1. [DOI] [PubMed] [Google Scholar]
- Garvalov BK, Flynn KC, Neukirchen D, Meyn L, Teusch N, Wu X, Brakebusch C, Bamburg JR, Bradke F. Cdc42 regulates cofilin during the establishment of neuronal polarity. J Neurosci. 2007;27:13117–13129. doi: 10.1523/JNEUROSCI.3322-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges PC, Hadzimichalis NM, Sweet ES, Firestein BL. The yin-yang of dendrite morphology: unity of actin and microtubules. Mol Neurobiol. 2008;38:270–284. doi: 10.1007/s12035-008-8046-8. [DOI] [PubMed] [Google Scholar]
- Geraldo S, Khanzada UK, Parsons M, Chilton JK, Gordon-Weeks PR. Targeting of the F-actin-binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nat Cell Biol. 2008;10:1181–1189. doi: 10.1038/ncb1778. [DOI] [PubMed] [Google Scholar]
- Gil OD, Sakurai T, Bradley AE, Fink MY, Cassella MR, Kuo JA, Felsenfeld DP. Ankyrin binding mediates L1CAM interactions with static components of the cytoskeleton and inhibits retrograde movement of L1CAM on the cell surface. J Cell Biol. 2003;162:719–730. doi: 10.1083/jcb.200211011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindhart JG. Kinesin light chains are essential for axonal transport in Drosophila. J Cell Biol. 1998;141:443–454. doi: 10.1083/jcb.141.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitai Z, Yu TW, Lundquist EA, Tessier-Lavigne M, Bargmann CI. The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron. 2003;37:53–65. doi: 10.1016/s0896-6273(02)01149-2. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Goicoechea S, Arneman D, Otey C. The role of palladin in actin organization and cell motility. Eur J Cell Biol. 2008;87:517–525. doi: 10.1016/j.ejcb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LSB, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu Rev Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Letourneau PC. Actin dynamics in growth cone motility and navigation. J Neurochem. 2013;129:221–234. doi: 10.1111/jnc.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Weeks PR, Fischer I. MAP1B expression and microtubule stability in growing and regenerating axons. PLoS ONE. 2000;48:63–74. doi: 10.1002/(SICI)1097-0029(20000115)48:2<63::AID-JEMT2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Grabham PW, Seale GE, Bennecib M, Goldberg DJ, Vallee RB. Cytoplasmic dynein and LIS1 are required for microtubule advance during growth cone remodeling and fast axonal outgrowth. J Neurosci. 2007;27:5823–5834. doi: 10.1523/JNEUROSCI.1135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G, Soehrman S, Bondallaz P, Ruchti E, Cadas H. Role of the microtubule destabilizing proteins SCG10 and stathmin in neuronal growth. J Neurobiol. 2003;58:60–69. doi: 10.1002/neu.10279. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Gertler FB. Integrin signaling switches the cytoskeletal and exocytic machinery that drives neuritogenesis. Dev Cell. 2010;18:725–736. doi: 10.1016/j.devcel.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton SL, Riquelme D, Hughes-Alford SK, Tadros J, Rudina SS, Hynes RO, Lauffenburger D, Gertler FB. Mena binds α5 integrin directly and modulates α5β1 function. J Cell Biol. 2012;198:657–676. doi: 10.1083/jcb.201202079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag N, Schwintzer L, Ahuja R, Koch N, Grimm J, Heuer H, Qualmann B, Kessels MM. The actin nucleator Cobl is crucial for Purkinje cell development and works in close conjunction with the F-actin binding protein Abp1. J Neurosci. 2012;32:17842–17856. doi: 10.1523/JNEUROSCI.0843-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Had L, Faivre-Sarrailh C, Legrand C, Mery J, Brugidou J, Rabie A. Tropomyosin isoforms in rat neurons: the different developmental profiles and distributions of TM-4 and TMBr-3 are consistent with different functions. J Cell Sci. 1994;107(Pt 10):2961–2973. doi: 10.1242/jcs.107.10.2961. [DOI] [PubMed] [Google Scholar]
- Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S, Dehmelt L. The MAP1 family of microtubule-associated proteins. Genome Biol. 2006;7:224. doi: 10.1186/gb-2006-7-6-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M, Davis WS, Jorgensen EM. Mutations in beta-spectrin disrupt axon outgrowth and sarcomere structure. J Cell Biol. 2000;149:931–942. doi: 10.1083/jcb.149.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M, Jorgensen EM, Bastiani MJ. Axons break in animals lacking-spectrin. J Cell Biol. 2007;176:269–275. doi: 10.1083/jcb.200611117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- Harper BD, Beckerle MC, Pomiès P. Fine mapping of the alpha-actinin binding site within cysteine-rich protein. Biochem J. 2000;350(Pt 1):269–274. [PMC free article] [PubMed] [Google Scholar]
- Hasse A, Rosentreter A, Spoerl Z, Stumpf M, Noegel AA, Clemen CS. Coronin 3 and its role in murine brain morphogenesis. Eur J Neurosci. 2005;21:1155–1168. doi: 10.1111/j.1460-9568.2005.03917.x. [DOI] [PubMed] [Google Scholar]
- Hayashi K. Domain analysis of the actin-binding and actin-remodeling activities of drebrin. Exp Cell Res. 1999;253:673–680. doi: 10.1006/excr.1999.4663. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ishikawa R, Ye LH, He XL, Takata K, Kohama K, Shirao T. Modulatory role of drebrin on the cytoskeleton within dendritic spines in the rat cerebral cortex. J Neurosci. 1996;16:7161–7170. doi: 10.1523/JNEUROSCI.16-22-07161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Shirao T. Change in the shape of dendritic spines caused by overexpres-sion of drebrin in cultured cortical neurons. J Neurosci. 1999;19:3918–3925. doi: 10.1523/JNEUROSCI.19-10-03918.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan J, Fonknechten N, Mavel D, Paternotte C, Samson D, Artiguenave F, Davoine CS, Cruaud C, Dürr A, Wincker P, Brottier P, Cattolico L, Barbe V, Burgunder JM, Prud’homme JF, Brice A, Fontaine B, Heilig R, Weissenbach J. Spastin, a new AAA protein, is altered in the most frequent form of autosomal dominant spastic paraplegia. Nat Genet. 1999;23:296–303. doi: 10.1038/15472. [DOI] [PubMed] [Google Scholar]
- Homma N, Takei Y, Tanaka Y, Nakata T, Terada S, Kikkawa M, Noda Y, Hirokawa N. Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell. 2003;114:229–239. doi: 10.1016/s0092-8674(03)00522-1. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Akhmanova A, Grosveld F, De Zeeuw CI, Galjart N. Functional analysis of CLIP-115 and its binding to microtubules. J Cell Sci. 2000;113(Pt 12):2285–2297. doi: 10.1242/jcs.113.12.2285. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Koekkoek B, Akhmanova A, Krugers H, Dortland B, Miedema M, van Alphen A, Kistler WM, Jaegle M, Koutsourakis M, Van Camp N, Verhoye M, van der Linden A, Kaverina I, Grosveld F, De Zeeuw CI, Galjart N. Targeted mutation of Cyln2 in the Williams syndrome critical region links CLIP-115 haploinsufficiency to neurodevelopmental abnormalities in mice. Nat Genet. 2002;32:116–127. doi: 10.1038/ng954. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Llano O, Smirnov S, Tanhuanpää K, Faix J, Rivera C, Lappalainen P. Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J Cell Biol. 2009;185:323–339. doi: 10.1083/jcb.200809046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsmeier J, Pielage J, Rickert C, Technau GM, Klambt C, Stork T. Distinct functions of α-spectrin and β-spectrin during axonal pathfinding. Development. 2007;134:713–722. doi: 10.1242/dev.02758. [DOI] [PubMed] [Google Scholar]
- Hur EM, Saijilafu, Lee BD, Kim S-J, Xu W-L, Zhou F-Q. GSK3 controls axon growth via CLASP-mediated regulation of growth cone microtubules. Genes Dev. 2011;25:1968–1981. doi: 10.1101/gad.17015911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Chihara K, Arimura N, Ménager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4:781–782. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- Ishikawa R, Kohama K. Actin-binding proteins in nerve cell growth cones. J Pharmacol Sci. 2007;105:6–11. doi: 10.1254/jphs.cp0070071. [DOI] [PubMed] [Google Scholar]
- Iwasawa N, Negishi M, Oinuma I. R-Ras controls axon branching through afadin in cortical neurons. Mol Biol Cell. 2012;23:2793–2804. doi: 10.1091/mbc.E12-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson C, Schnapp B, Banker GA. A change in the selective translocation of the Kinesin-1 motor domain marks the initial specification of the axon. Neuron. 2006;49:797–804. doi: 10.1016/j.neuron.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Jausoro I, Mestres I, Quassollo G, Masseroni L, Heredia F, Cáceres A. Regulation of spine density and morphology by IQGAP1 protein domains. PLoS ONE. 2013;8:e56574. doi: 10.1371/journal.pone.0056574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA, Di Stefano P, Demmers J, Krugers H, Defilippi P, Akhmanova A, Hoogenraad CC. Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron. 2009;61:85–100. doi: 10.1016/j.neuron.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Mateos EM, Paglini G, González-Billault C, Cáceres A, Avila J. End binding protein-1 (EB1) complements microtubule-associated protein-1B during axono-genesis. J Neurosci Res. 2005;80:350–359. doi: 10.1002/jnr.20453. [DOI] [PubMed] [Google Scholar]
- Kapitein LC, Schlager MA, Kuijpers M, Wulf PS, van Spronsen M, MacKintosh FC, Hoogenraad CC. Mixed microtubules steer dynein-driven cargo transport into dendrites. Curr Biol. 2010;20:290–299. doi: 10.1016/j.cub.2009.12.052. [DOI] [PubMed] [Google Scholar]
- Kappeler C, Saillour Y, Baudoin JP, Tuy FPD, Alvarez C, Houbron C, Gaspar P, Hamard G, Chelly J, Métin C, Francis F. Branching and nucleokinesis defects in migrating interneurons derived from doublecortin knockout mice. Hum Mol Genet. 2006;15:1387–1400. doi: 10.1093/hmg/ddl062. [DOI] [PubMed] [Google Scholar]
- Karabay A, Yu W, Solowska JM, Baird DH, Baas PW. Axonal growth is sensitive to the levels of katanin, a protein that severs microtubules. J Neurosci. 2004;24:5778–5788. doi: 10.1523/JNEUROSCI.1382-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Yoshimura T, Tsuboi D, Kawabata S, Kaneko-Kawano T, Shirataki H, Takenawa T, Kaibuchi K. CRMP-2 is involved in kinesin-1-dependent transport of the Sra-1/WAVE1 complex and axon formation. Mol Cell Biol. 2005;25:9920–9935. doi: 10.1128/MCB.25.22.9920-9935.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke YD, Suchowerska AK, van der Hoven J, De Silva DM, Wu CW, van Eersel J, Ittner A, Ittner LM. Lessons from tau-deficient mice. Int J Alzheimer Dis. 2012;2012:1–8. doi: 10.1155/2012/873270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IH, Racz B, Wang H, Burianek L, Weinberg R, Yasuda R, Wetsel WC, Soderling SH. Disruption of Arp2/3 results in asymmetric structural plasticity of dendritic spines and progressive synaptic and behavioral abnormalities. J Neurosci. 2013;33:6081–6092. doi: 10.1523/JNEUROSCI.0035-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch D, Rosoff WJ, Jiang J, Geller HM, Urbach JS. Strength in the periphery: growth cone biomechanics and substrate rigidity response in peripheral and central nervous system neurons. Biophys J. 2012;102:452–460. doi: 10.1016/j.bpj.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester MP, Müller O, Pollerberg GE. Adenomatous polyposis coli is differentially distributed in growth cones and modulates their steering. J Neurosci. 2007;27:12590–12600. doi: 10.1523/JNEUROSCI.2250-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova Y, De Groot CO, Grigoriev I, Gouveia SM, Munteanu EL, Schober JM, Honnappa S, Buey RM, Hoogenraad CC, Dogterom M, Borisy GG, Steinmetz MO, Akhmanova A. Mammalian end binding proteins control persistent microtubule growth. J Cell Biol. 2009;184:691–706. doi: 10.1083/jcb.200807179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordeli E, Lambert S, Bennett V. Ankyrin (G) J Biol Chem. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Arp2/3 complex is important for filopodia formation, growth cone motility, and neuritogenesis in neuronal cells. Mol Biol Cell. 2008;19:1561–1574. doi: 10.1091/mbc.E07-09-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korobova F, Svitkina T. Molecular architecture of synaptic actin cytoskeleton in hippocampal neurons reveals a mechanism of dendritic spine morphogenesis. Mol Biol Cell. 2010;21:165–176. doi: 10.1091/mbc.E09-07-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Finch EA. MAP2 and tau segregate into dendritic and axonal domains after the elaboration of morphologically distinct neurites: an immunocytochemical study of cultured rat cerebrum. J Neurosci. 1987;7:3142–3153. doi: 10.1523/JNEUROSCI.07-10-03142.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft R, Escobar MM, Narro ML, Kurtis JL, Efrat A, Barnard K, Restifo LL. Phenotypes of Drosophila brain neurons in primary culture reveal a role for fascin in neurite shape and trajectory. J Neurosci. 2006;26:8734–8747. doi: 10.1523/JNEUROSCI.2106-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Leslie JD, Stewart M, Lafuente EM, Valderrama F, Jagannathan R, Strasser GA, Rubinson DA, Liu H, Way M, Yaffe MB, Boussiotis VA, Gertler FB. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev Cell. 2004;7:571–583. doi: 10.1016/j.devcel.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Kubo T, Endo M, Hata K, Taniguchi J, Kitajo K, Tomura S, Yamaguchi A, Mueller BK, Yamashita T. Myosin IIA is required for neurite outgrowth inhibition produced by repulsive guidance molecule. J Neurochem. 2008;105:113–126. doi: 10.1111/j.1471-4159.2007.05125.x. [DOI] [PubMed] [Google Scholar]
- Lanier LM, Gates MA, Witke W, Menzies AS, Wehman AM, Macklis JD, Kwiatkowski D, Soriano P, Gertler FB. Mena is required for neurulation and commissure formation. Neuron. 1999;22:313–325. doi: 10.1016/s0896-6273(00)81092-2. [DOI] [PubMed] [Google Scholar]
- Lansbergen G, Grigoriev I, Mimori-Kiyosue Y, Ohtsuka T, Higa S, Kitajima I, Demmers J, Galjart N, Houtsmuller AB, Grosveld F, Akhmanova A. CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5beta. Dev Cell. 2006;11:21–32. doi: 10.1016/j.devcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Lazarides E, Nelson WJ. Erythrocyte and brain forms of spectrin in cerebellum: distinct membrane-cytoskeletal domains in neurons. Science. 1983;220:1295–1296. doi: 10.1126/science.6190228. [DOI] [PubMed] [Google Scholar]
- Lebrand C, Dent EW, Strasser GA, Lanier LM, Krause M, Svitkina TM, Borisy GG, Gertler FB. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron. 2004;42:37–49. doi: 10.1016/s0896-6273(04)00108-4. [DOI] [PubMed] [Google Scholar]
- Lee H, Engel U, Rusch J, Scherrer S, Sheard K, Van Vactor D. The microtubule plus end tracking protein Orbit/MAST/CLASP acts downstream of the tyrosine kinase Abl in mediating axon guidance. Neuron. 2004;42:913–926. doi: 10.1016/j.neuron.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Lee HH, Jan LY, Jan YN. Drosophila IKK-related kinase Ik2 and Katanin p60-like 1 regulate dendrite pruning of sensory neuron during metamorphosis. Proc Natl Acad Sci. 2009;106:6363–6368. doi: 10.1073/pnas.0902051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshchyns’ka I, Sytnyk V, Morrow JS, Schachner M. Neural cell adhesion molecule (NCAM) association with PKCbeta2 via betaI spectrin is implicated in NCAM-mediated neurite outgrowth. J Cell Biol. 2003;161:625–639. doi: 10.1083/jcb.200303020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JR, Sumner CJ, Caviston JP, Tokito MK, Ranganathan S, Ligon LA, Wallace KE, LaMonte BH, Harmison GG, Puls I, Fischbeck KH, Holzbaur ELF. A motor neuron disease-associated mutation in p150Glued perturbs dynactin function and induces protein aggregation. J Cell Biol. 2006;172:733–745. doi: 10.1083/jcb.200511068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Gao FB. Actin filament-stabilizing protein tropomyosin regulates the size of dendritic fields. J Neurosci. 2003;23:6171–6175. doi: 10.1523/JNEUROSCI.23-15-06171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Herman RK, Shaw JE. Analysis of the Caenorhabditiselegans axonal guidance and outgrowth gene unc-33. Genetics. 1992;132:675–689. doi: 10.1093/genetics/132.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Li Y, Gao FB. Abelson, enabled, and p120catenin exert distinct effects on dendritic morphogenesis in Drosophila. Dev Dyn. 2005a;234:512–522. doi: 10.1002/dvdy.20496. [DOI] [PubMed] [Google Scholar]
- Li Z, McNulty DE, Marler KJM, Lim L, Hall C, Annan RS, Sacks DB. IQGAP1 promotes neurite outgrowth in a phosphorylation-dependent manner. J Biol Chem. 2005b;280:13871–13878. doi: 10.1074/jbc.M413482200. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Leman EE, Fyffe REW, Raine CS, Schubart UK. Stathmin-deficient mice develop an age-dependent axonopathy of the central and peripheral nervous systems. Am J Pathol. 2002;160:469–480. doi: 10.1016/S0002-9440(10)64866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Espreafico EM, Mooseker MS, Forscher P. Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron. 1996;16:769–782. doi: 10.1016/s0896-6273(00)80097-5. [DOI] [PubMed] [Google Scholar]
- Lin PC, Chan PM, Hall C, Manser E. Collapsin response mediator proteins (CRMPs) are a new class of microtubule-associated protein (MAP) that selectively interacts with assembled microtubules via a taxol-sensitive binding interaction. J Biol Chem. 2011;286:41466–41478. doi: 10.1074/jbc.M111.283580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WH, Nebhan CA, Anderson BR, Webb DJ. Vasodilator-stimulated phosphoprotein (VASP) induces actin assembly in dendritic spines to promote their development and potentiate synaptic strength. J Biol Chem. 2010;285:36010–36020. doi: 10.1074/jbc.M110.129841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Nadar VC, Kozielski F, Kozlowska M, Yu W, Baas PW. Kinesin-12, a mitotic microtubule-associated motor protein, impacts axonal growth, navigation, and branching. J Neurosci. 2010;30:14896–14906. doi: 10.1523/JNEUROSCI.3739-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Fox P, Lakonishok M, Davidson MW, Gelfand VI. Initial neurite outgrowth in Drosophila neurons is driven by kinesin-powered microtubule sliding. Curr Biol. 2013;23:1018–1023. doi: 10.1016/j.cub.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist EA, Herman RK, Shaw JE, Bargmann CI. UNC-115, a conserved protein with predicted LIM and actin-binding domains, mediates axon guidance in C. elegans. Neuron. 1998;21:385–392. doi: 10.1016/s0896-6273(00)80547-4. [DOI] [PubMed] [Google Scholar]
- Luo L, Liu Z, Steward R. Drosophila Lis1 is required for neuroblast proliferation, dendritic elaboration and axonal transport. Nat Cell Biol. 2000;2:776–783. doi: 10.1038/35041011. [DOI] [PubMed] [Google Scholar]
- Ma L, Greenwood JA, Schachner M. CRP1, a protein localized in filopodia of growth cones, is involved in dendritic growth. J Neurosci. 2011;31:16781–16791. doi: 10.1523/JNEUROSCI.2595-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack TG, Koester MP, Pollerberg GE. The microtubule-associated protein MAP1B is involved in local stabilization of turning growth cones. PLoS ONE. 2000;15:51–65. doi: 10.1006/mcne.1999.0802. [DOI] [PubMed] [Google Scholar]
- Maday S, Twelvetrees AE, Moughamian AJ, Holzbaur ELF. Axonal transport: cargo-specific mechanisms of motility and regulation. Neuron. 2014;84:292–309. doi: 10.1016/j.neuron.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniar TA, Kaplan M, Wang GJ, Shen K, Wei L, Shaw JE, Koushika SP, Bargmann CI. UNC-33 (CRMP) and ankyrin organize microtubules and localize kinesin to polarize axon-dendrite sorting. Nat Neurosci. 2011;15:48–56. doi: 10.1038/nn.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna T, Grenningloh G, Miller HP, Wilson L. Stathmin family protein SCG10 differentially regulates the plus and minus end dynamics of microtubules at steady state in vitro: implications for its role in neurite outgrowth. Biochemistry. 2007;46:3543–3552. doi: 10.1021/bi061819d. [DOI] [PubMed] [Google Scholar]
- Marsick BM, Flynn KC, Santiago-Medina M, Bamburg JR, Letourneau PC. Activation of ADF/cofilin mediates attractive growth cone turning toward nerve growth factor and netrin-1. Dev Neurobiol. 2010;70:565–588. doi: 10.1002/dneu.20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsick BM, San Miguel-Ruiz JE, Letourneau PC. Activation of ezrin/radixin/ moesin mediates attractive growth cone guidance through regulation of growth cone actin and adhesion receptors. J Neurosci. 2012;32:282–296. doi: 10.1523/JNEUROSCI.4794-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S, Gautel M. Introducing a special edition of the Journal of Muscle Research and Cell Motility on tropomyosin: form and function. J Muscle Res Cell Motil. 2013;34:151–153. doi: 10.1007/s10974-013-9361-x. [DOI] [PubMed] [Google Scholar]
- Martin MAE, Hurd DD, Saxton WM. Kinesins in the nervous system. Cell Mol Life Sci. 1999;56:200–216. doi: 10.1007/s000180050422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusek T, Gombos R, Szécsényi A, Sánchez-Soriano N, Czibula A, Pataki C, Gedai A, Prokop A, Raskó I, Mihály J. Formin proteins of the DAAM subfamily play a role during axon growth. J Neurosci. 2008;28:13310–13319. doi: 10.1523/JNEUROSCI.2727-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- Meberg PJ, Bamburg JR. Increase in neurite outgrowth mediated by overexpression of actin depolymerizing factor. J Neurosci. 2000;20:2459–2469. doi: 10.1523/JNEUROSCI.20-07-02459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8:215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- Meixner A, Haverkamp S, Wassle H, Fuhrer S, Thalhammer J, Kropf N, Bittner RE, Lassmann H, Wiche G, Propst F. MAP1B is required for axon guidance and is involved in the development of the central and peripheral nervous system. J Cell Biol. 2000;151:1169–1178. doi: 10.1083/jcb.151.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam EB, Millette M, Lumbard DC, Saengsawang W, Fothergill T, Hu X, Ferhat L, Dent EW. Synaptic regulation of microtubule dynamics in dendritic spines by calcium, F-actin, and drebrin. J Neurosci. 2013;33:16471–16482. doi: 10.1523/JNEUROSCI.0661-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael M, Vehlow A, Navarro C, Krause M. c-Abl, lamellipodin, and Ena/VASP proteins cooperate in dorsal ruffling of fibroblasts and axonal morphogenesis. Curr Biol. 2010;20:783–791. doi: 10.1016/j.cub.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Grigoriev I, Lansbergen G, Sasaki H, Matsui C, Severin F, Galjart N, Grosveld F, Vorobjev I, Tsukita S, Akhmanova A. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J Cell Biol. 2005;168:141–153. doi: 10.1083/jcb.200405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Tsukita S. “Search-and-capture” of microtubules through plus-end-binding proteins (+TIPs) J Biochem. 2003;134:321–326. doi: 10.1093/jb/mvg148. [DOI] [PubMed] [Google Scholar]
- Mingorance-Le Meur A, O’Connor TP. Neurite consolidation is an active process requiring constant repression of protrusive activity. EMBO J. 2009;28:248–260. doi: 10.1038/emboj.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Mizui T, Kojima N, Yamazaki H, Katayama M, Hanamura K, Shirao T. Drebrin E is involved in the regulation of axonal growth through actin-myosin interactions. J Neurochem. 2009;109:611–622. doi: 10.1111/j.1471-4159.2009.05993.x. [DOI] [PubMed] [Google Scholar]
- Mizui T, Takahashi H, Sekino Y, Shirao T. Overexpression of drebrin A in immature neurons induces the accumulation of F-actin and PSD-95 into dendritic filopodia, and the formation of large abnormal protrusions. Mol Cell Neurosci. 2005;30:630–638. [PubMed] [Google Scholar]
- Mohler PJ, Gramolini AO, Bennett V. Ankyrins. J Cell Sci. 2002;115:1565–1566. doi: 10.1242/jcs.115.8.1565. [DOI] [PubMed] [Google Scholar]
- Moore SW, Biais N, Sheetz MP. Traction on immobilized netrin-1 is sufficient to reorient axons. Science. 2009;325:166. doi: 10.1126/science.1173851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morii H, Shiraishi-Yamaguchi Y, Mori N. SCG10, a microtubule destabilizing factor, stimulates the neurite outgrowth by modulating microtubule dynamics in rat hippocampal primary cultured neurons. J Neurobiol. 2006;66:1101–1114. doi: 10.1002/neu.20295. [DOI] [PubMed] [Google Scholar]
- Mori N, Morii H. SCG10-related neuronal growth-associated proteins in neural development, plasticity, degeneration, and aging. J Neurosci Res. 2002;70:264–273. doi: 10.1002/jnr.10353. [DOI] [PubMed] [Google Scholar]
- Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci USA. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S, Souza B, Müller O, Albert I, Rubinfeld B, Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994;54:3676–3681. [PubMed] [Google Scholar]
- Murray A, Naeem A, Barnes SH, Drescher U, Guthrie S. Slit and netrin-1 guide cranial motor axon pathfinding via Rho-kinase, myosin light chain kinase and myosin II. Neural Dev. 2010;5:16. doi: 10.1186/1749-8104-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KA, Baas PW. Kinesin-5 regulates the growth of the axon by acting as a brake on its microtubule array. J Cell Biol. 2007;178:1081–1091. doi: 10.1083/jcb.200702074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KA, Tint I, Nadar CV, He Y, Black MM, Baas PW. Antagonistic forces generated by cytoplasmic dynein and myosin-ii during growth cone turning and axonal retraction. Traffic. 2006;7:1333–1351. doi: 10.1111/j.1600-0854.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- Nagel J, Delandre C, Zhang Y, Förstner F, Moore AW, Tavosanis G. Fascin controls neuronal class-specific dendrite arbor morphology. Development. 2012;139:2999–3009. doi: 10.1242/dev.077800. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Koyama K, Murata Y, Morito M, Akiyama T, Nakamura Y. EB3, a novel member of the EB1 family preferentially expressed in the central nervous system, binds to a CNS-specific APC homologue. Oncogene. 2000;19:210–216. doi: 10.1038/sj.onc.1203308. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Nakano I, Furuyama T, Morii H, Tamai M, Mori N. The SCG10-related gene family in the developing rat retina: persistent expression of SCLIP and stathmin in mature ganglion cell layer. Brain Res. 2000;861:399–407. doi: 10.1016/s0006-8993(00)02056-4. [DOI] [PubMed] [Google Scholar]
- Norris AD, Dyer JO, Lundquist EA. The Arp2/3 complex, UNC-115/abLIM, and UNC-34/Enabled regulate axon guidance and growth cone filopodia formation in Caenorhabditiselegans. Neural Dev. 2009;4:38. doi: 10.1186/1749-8104-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwagbara BU, Faris AE, Bearce EA, Erdogan B, Ebbert PT, Evans MF, Rutherford EL, Enzenbacher TB, Lowery LA. TACC3 is a microtubule plus end-tracking protein that promotes axon elongation and also regulates microtubule plus end dynamics in multiple embryonic cell types. Mol Biol Cell. 2014;25:3350–3362. doi: 10.1091/mbc.E14-06-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa N, Fujitani K, Tokunaga E, Furuya S, Inokuchi K. The microtubule destabilizer stathmin mediates the development of dendritic arbors in neuronal cells. J Cell Sci. 2007;120:1447–1456. doi: 10.1242/jcs.001461. [DOI] [PubMed] [Google Scholar]
- Paglini G, Kunda P, Quiroga S, Kosik K, Caceres A. Suppression of radixin and moesin alters growth cone morphology, motility, and process formation in primary cultured neurons. J Cell Biol. 1998;143:443–455. doi: 10.1083/jcb.143.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AL, Pereira AJ, Maia ARR, Drabek K, Sayas CL, Hergert PJ, Lince-Faria M, Matos I, Duque C, Stepanova T, Rieder CL, Earnshaw WC, Galjart N, Maiato H. Mammalian CLASP1 and CLASP2 cooperate to ensure mitotic fidelity by regulating spindle and kinetochore function. Molecular biology of the cell. 2006;17:4526–4542. doi: 10.1091/mbc.E06-07-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez F, Diamantopoulos GS, Stalder R, Kreis TE. CLIP-170 highlights growing microtubule ends in vivo. Cell. 1999;96:517–527. doi: 10.1016/s0092-8674(00)80656-x. [DOI] [PubMed] [Google Scholar]
- Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, Sahai E, Condeelis JS, Gertler FB. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15:813–828. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro EM, Xie Z, Norovich AL, Vidaki M, Tsai LH, Gertler FB. Lpd depletion reveals that SRF specifies radial versus tangential migration of pyramidal neurons. Nat Cell Biol. 2011;13:989–995. doi: 10.1038/ncb2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poplawski GHD, Tranziska AK, Leshchyns’ka I, Meier ID, Streichert T, Sytnyk V, Schachner M. L1CAM increases MAP2 expression via the MAPK pathway to promote neurite outgrowth. Mol Cell Neurosci. 2012;50:169–178. doi: 10.1016/j.mcn.2012.03.010. [DOI] [PubMed] [Google Scholar]
- des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrié A, Gelot A, Dupuis E, Motte J, Berwald-Netter Y, Catala M, Kahn A, Beldjord C, Chelly J. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Poulain FE, Sobel A. The “SCG10-Like protein” SCLIP is a novel regulator of axonal branching in hippocampal neurons, unlike SCG10. Mol Cell Neurosci. 2007;34:137–146. doi: 10.1016/j.mcn.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Qiang L, Yu W, Andreadis A, Luo M, Baas PW. Tau protects microtubules in the axon from severing by katanin. Journal of Neuroscience. 2006;26:3120–3129. doi: 10.1523/JNEUROSCI.5392-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach TT, Massicotte G, Belin MF, Honnorat J, Glasper ER, Devries AC, Jakeman LB, Baudry M, Duchemin AM, Kolattukudy PE. CRMP3 is required for hippocampal CA1 dendritic organization and plasticity. FASEB J. 2007;22:401–409. doi: 10.1096/fj.07-9012com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CC, Chen E, Kinjo TG, Kelly G, Bell AW, Elliott RC, McPherson PS, Hockfield S. TUC-4b, a novel TUC family variant, regulates neurite outgrowth and associates with vesicles in the growth cone. J Neurosci. 2003;23:2815–2823. doi: 10.1523/JNEUROSCI.23-07-02815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz B, Weinberg RJ. Spatial organization of cofilin in dendritic spines. Neuroscience. 2006;138:447–456. doi: 10.1016/j.neuroscience.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Riano E, Martignoni M, Mancuso G, Cartelli D, Crippa F, Toldo I, Siciliano G, Di Bella D, Taroni F, Bassi MT, Cappelletti G, Rugarli EI. Pleiotropic effects of spastin on neurite growth depending on expression levels. J Neurochem. 2009;108:1277–1288. doi: 10.1111/j.1471-4159.2009.05875.x. [DOI] [PubMed] [Google Scholar]
- Riederer BM, Pellier V, Antonsson B, Di Paolo G, Stimpson SA, Lütjens R, Catsicas S, GRENNINGLOH G. Regulation of microtubule dynamics by the neuronal growth-associated protein SCG10. Proc Natl Acad Sci USA. 1997;94:741–745. doi: 10.1073/pnas.94.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlin MW, Itoh K, Adelstein RS, Bridgman PC. Localization of myosin II A and B isoforms in cultured neurons. J Cell Sci. 1995;108(Pt 12):3661–3670. doi: 10.1242/jcs.108.12.3661. [DOI] [PubMed] [Google Scholar]
- Roger B, Al-Bassam J, Dehmelt L, Milligan RA, Halpain S. MAP2c, but not tau, binds and bundles F-actin via its microtubule binding domain. Curr Biol. 2004;14:363–371. doi: 10.1016/j.cub.2004.01.058. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A, Vale RD. The Drosophila homologue of the hereditary spastic paraplegia protein, spastin, severs and disassembles microtubules. Curr Biol. 2005;15:650–655. doi: 10.1016/j.cub.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A, Vale RD. Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature. 2008;451:363–367. doi: 10.1038/nature06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof DJ. Molecular characterization of abLIM, a novel actin-binding and double zinc finger protein. J Cell Biol. 1997;138:575–588. doi: 10.1083/jcb.138.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossien DH, Lamoureux P, Miller KE. Cytoplasmic dynein pushes the cytoskeletal meshwork forward during axonal elongation. J Cell Sci. 2014;127:3593–3602. doi: 10.1242/jcs.152611. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Rogers SL, Vale RD, Jan LY, Jan YN. Drosophila pod-1 crosslinks both actin and microtubules and controls the targeting of axons. Neuron. 2003;39:779–791. doi: 10.1016/s0896-6273(03)00508-7. [DOI] [PubMed] [Google Scholar]
- Rotty JD, Wu C, Bear JE. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat Rev Mol Cell Biol. 2012;14:7–12. doi: 10.1038/nrm3492. [DOI] [PubMed] [Google Scholar]
- Roy S, Zhang B, Lee VMY, Trojanowski JQ. Axonal transport defects: a common theme in neurodegenerative diseases. Acta Neuropathol. 2005;109:5–13. doi: 10.1007/s00401-004-0952-x. [DOI] [PubMed] [Google Scholar]
- Rusan NM, Akong K, Peifer M. Putting the model to the test: are APC proteins essential for neuronal polarity, axon outgrowth, and axon targeting? J Cell Biol. 2008;183:203–212. doi: 10.1083/jcb.200807079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakin V, Stumpf M, Schulze A, Majoul IV, Noegel AA, Hasse A. Coronin 7, the mammalian POD-1 homologue, localizes to the Golgi apparatus. FEBS Lett. 2004;573:161–167. doi: 10.1016/j.febslet.2004.07.066. [DOI] [PubMed] [Google Scholar]
- Sabry JH, O’Connor TP, Evans L, Toroian-Raymond A, Kirschner M, Bentley D. Microtubule behavior during guidance of pioneer neuron growth cones in situ. J Cell Biol. 1991;115:381–395. doi: 10.1083/jcb.115.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler I, Crawford AW, Michelsen JW, Beckerle MC. Zyxin and cCRP: two interactive LIM domain proteins associated with the cytoskeleton. J Cell Biol. 1992;119:1573–1587. doi: 10.1083/jcb.119.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon SN, Haber M, Murai KK, Dunn RJ. Localization of the diaphanous-related formin Daam1 to neuronal dendrites. Neurosci Lett. 2008;447:62–67. doi: 10.1016/j.neulet.2008.09.051. [DOI] [PubMed] [Google Scholar]
- Satoh D, Sato D, Tsuyama T, Saito M, Ohkura H, Rolls M, Ishikawa F, Uemura T. Spatial control of branching within dendritic arbors by dynein-dependent transport of Rab5-endosomes. Nat Cell Biol. 2008;10:1164–1171. doi: 10.1038/ncb1776. [DOI] [PubMed] [Google Scholar]
- Schevzov G, Bryce NS, Almonte-Baldonado R, Joya J, Lin JJC, Hardeman E, Weinberger R, Gunning P. Specific features of neuronal size and shape are regulated by tropomyosin isoforms. Mol Biol Cell. 2005;16:3425–3437. doi: 10.1091/mbc.E04-10-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevzov G, Gunning P, Jeffrey PL, Temm-Grove C, Helfman DM, Lin JJ, Weinberger RP. Tropomyosin localization reveals distinct populations of microfilaments in neurites and growth cones. Mol Cell Neurosci. 1997;8:439–454. doi: 10.1006/mcne.1997.0599. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, McKerracher L, Obar R, Vallee RB. MAP 1A and MAP 1B are structurally related microtubule associated proteins with distinct developmental patterns in the CNS. J Neurosci. 1989;9:1712–1730. doi: 10.1523/JNEUROSCI.09-05-01712.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengottuvel V, Leibinger M, Pfreimer M, Andreadaki A, Fischer D. Taxol facilitates axon regeneration in the mature CNS. J Neurosci. 2011;31:2688–2699. doi: 10.1523/JNEUROSCI.4885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Shekarabi M. Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits cdc42, rac1, pak1, and n-wasp into an intracellular signaling complex that promotes growth cone expansion. J Neurosci. 2005;25:3132–3141. doi: 10.1523/JNEUROSCI.1920-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Cheng T, Jan LY, Jan YN. APC and GSK-3β are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol. 2004;14:2025–2032. doi: 10.1016/j.cub.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Shi Y, Pontrello CG, DeFea KA, Reichardt LF, Ethell IM. Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity. J Neurosci. 2009;29:8129–8142. doi: 10.1523/JNEUROSCI.4681-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Toriyama M, Uemura K, Kamiguchi H, Sugiura T, Watanabe N, Inagaki N. Shootin1 interacts with actin retrograde flow and L1-CAM to promote axon outgrowth. J Cell Biol. 2008;181:817–829. doi: 10.1083/jcb.200712138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara R, Thumkeo D, Kamijo H, Kaneko N, Sawamoto K, Watanabe K, Takebayashi H, Kiyonari H, Ishizaki T, Furuyashiki T, Narumiya S. A role for mDia, a Rho-regulated actin nucleator, in tangential migration of interneuron precursors. Nat Neurosci. 2012;15:373–380. doi: 10.1038/nn.3020. [DOI] [PubMed] [Google Scholar]
- Shu RZ, Zhang F, Liu XS, Li CL, Wang L, Tai YL, Wu XL, Yang X, Liao XD, Jin Y, Gu MM, Huang L, Pang XF, Wang ZG. Target deletion of the cytoskeleton-associated protein palladin does not impair neurite outgrowth in mice. PLoS ONE. 2009;4:e6916. doi: 10.1371/journal.pone.0006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Areces J, Dopazo A, Dettenhofer M, Rodriguez-Tebar A, Garcia-Segura LM, Arevalo MA. Formin1 mediates the induction of dendritogenesis and synaptogenesis by neurogenin3 in mouse hippocampal neurons. PLoS ONE. 2011;6:e21825. doi: 10.1371/journal.pone.0021825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slovakova J, Speicher S, Sanchez-Soriano N, Prokop A, Carmena A. The actin-binding protein Canoe/AF-6 forms a complex with robo and is required for slit-robo signaling during axon pathfinding at the CNS midline. J Neurosci. 2012;32:10035–10044. doi: 10.1523/JNEUROSCI.6342-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Niethammer M, Ayala R, Zhou Y, Gambello MJ, Wynshaw-Boris A, Tsai LH. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat Cell Biol. 2000;2:767–775. doi: 10.1038/35041000. [DOI] [PubMed] [Google Scholar]
- Sobue K, Kanda K. Alpha-actinins, calspectin (brain spectrin or fodrin), and actin participate in adhesion and movement of growth cones. Neuron. 1989;3:311–319. doi: 10.1016/0896-6273(89)90255-9. [DOI] [PubMed] [Google Scholar]
- Spillane M, Gallo G. Involvement of Rho-family GTPases in axon branching. Small GTPases. 2014;5:e27974. doi: 10.4161/sgtp.27974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillane M, Ketschek A, Jones SL, Korobova F, Marsick B, Lanier L, Svitkina T, Gallo G. The actin nucleating Arp2/3 complex contributes to the formation of axonal filopodia and branches through the regulation of actin patch precursors to filopodia. Dev Neurobiol. 2011;71:747–758. doi: 10.1002/dneu.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankewich MC, Cianci CD, Stabach PR, Ji L, Nath A, Morrow JS. Cell organization, growth, and neural and cardiac development require αII-spectrin. J Cell Sci. 2011;124:3956–3966. doi: 10.1242/jcs.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova T, Slemmer J, Hoogenraad CC, Lansbergen G, Dortland B, De Zeeuw CI, Grosveld F, van Cappellen G, Akhmanova A, Galjart N. Visualization of microtubule growth in cultured neurons via the use of EB3-GFP (end-binding protein 3-green fluorescent protein) J Neurosci. 2003;23:2655–2664. doi: 10.1523/JNEUROSCI.23-07-02655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova T, Smal I, van Haren J, Akinci U, Liu Z, Miedema M, Limpens R, van Ham M, van der Reijden M, Poot R, Grosveld F, Mommaas M, Meijering E, Galjart N. History-dependent catastrophes regulate axonal microtubule behavior. Curr Biol. 2010;20:1023–1028. doi: 10.1016/j.cub.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Stewart A, Tsubouchi A, Rolls MM, Tracey WD, Sherwood NT. Katanin p60-like1 promotes microtubule growth and terminal dendrite stability in the larval class IV sensory neurons of Drosophila. J Neurosci. 2012;32:11631–11642. doi: 10.1523/JNEUROSCI.0729-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone MC, Roegiers F, Rolls MM. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol Biol Cell. 2008;19:4122–4129. doi: 10.1091/mbc.E07-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser GA, Rahim NA, VanderWaal KE, Gertler FB, Lanier LM. Arp2/3 is a negative regulator of growth cone translocation. Neuron. 2004;43:81–94. doi: 10.1016/j.neuron.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Struckhoff EC, Lundquist EA. The actin-binding protein UNC-115 is an effector of Rac signaling during axon pathfinding in C.elegans. Development. 2003;130:693–704. doi: 10.1242/dev.00300. [DOI] [PubMed] [Google Scholar]
- Su KY, Chien WL, Fu WM, Yu IS, Huang HP, Huang PH, Lin SR, Shih JY, Lin YL, Hsueh YP, Yang PC, Lin SW. Mice deficient in collapsin response mediator protein-1 exhibit impaired long-term potentiation and impaired spatial learning and memory. J Neurosci. 2007;27:2513–2524. doi: 10.1523/JNEUROSCI.4497-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetsugu S, Hattori M, Miki H, Tezuka T, Yamamoto T, Mikoshiba K, Takenawa T. Sustained activation of N-WASP through phosphorylation is essential for neurite extension. Dev Cell. 2002;3:645–658. doi: 10.1016/s1534-5807(02)00324-6. [DOI] [PubMed] [Google Scholar]
- Suh LH, Oster SF, Soehrman SS, Grenningloh G, Sretavan DW. L1/Laminin modulation of growth cone response to EphB triggers growth pauses and regulates the microtubule destabilizing protein SCG10. J Neurosci. 2004;24:1976–1986. doi: 10.1523/JNEUROSCI.1670-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol. 1999;145:1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima SI, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol. 2003;160:409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiech L, Blazejczyk M, Urbanska M, Pietruszka P, Dortland BR, Malik AR, Wulf PS, Hoogenraad CC, Jaworski J. CLIP-170 and IQGAP1 cooperatively regulate dendrite morphology. J Neurosci. 2011;31:4555–4568. doi: 10.1523/JNEUROSCI.6582-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebenyi G, Bollati F, Bisbal M, Sheridan S, Faas L, Wray R, Haferkamp S, Nguyen S, Cáceres A, Brady ST. Activity-driven dendritic remodeling requires microtubule-associated protein 1A. Curr Biol. 2005;15:1820–1826. doi: 10.1016/j.cub.2005.08.069. [DOI] [PubMed] [Google Scholar]
- Takeda S, Yamazaki H, Seog DH, Kanai Y, Terada S, Hirokawa N. Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building. J Cell Biol. 2000;148:1255–1265. doi: 10.1083/jcb.148.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Kondo S, Harada A, Inomata S, Noda T, Hirokawa N. Delayed development of nervous system in mice homozygous for disrupted microtubule-associated protein 1B (MAP1B) gene. J Cell Biol. 1997;137:1615–1626. doi: 10.1083/jcb.137.7.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Teng J, Harada A, Hirokawa N. Defects in axonal elongation and neuronal migration in mice with disrupted tau and map1b genes. J Cell Biol. 2000;150:989–1000. doi: 10.1083/jcb.150.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura R, Okabe S, Kobayashi N, Hirokawa N. Reorganization of brain spectrin (fodrin) during differentiation of PC12 cells. Neuroscience. 1993;52:381–391. doi: 10.1016/0306-4522(93)90165-c. [DOI] [PubMed] [Google Scholar]
- Tanaka EM, Kirschner MW. Microtubule behavior in the growth cones of living neurons during axon elongation. J Cell Biol. 1991;115:345–363. doi: 10.1083/jcb.115.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrade A, Fassier C, Courageot S, Charvin D, Vitte J, Peris L, Thorel A, Mouisel E, Fonknechten N, Roblot N, Seilhean D, Dierich A, Hauw JJ, Melki J. A mutation of spastin is responsible for swellings and impairment of transport in a region of axon characterized by changes in microtubule composition. Hum Mol Genet. 2006;15:3544–3558. doi: 10.1093/hmg/ddl431. [DOI] [PubMed] [Google Scholar]
- Tasaka G, Oinuma I, Negishi M. Lamellipodin, a novel effector of M-Ras, regulates dendrite development by reconstructing actin network. Neurosci Res. 2011;71:e132. [Google Scholar]
- Tasaka GI, Negishi M, Oinuma I. Semaphorin 4D/Plexin-B1-mediated M-Ras GAP activity regulates actin-based dendrite remodeling through lamellipodin. J Neurosci. 2012;32:8293–8305. doi: 10.1523/JNEUROSCI.0799-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischfield MA, Baris HN, Wu C, Rudolph G, Van Maldergem L, He W, Chan WM, Andrews C, Demer JL, Robertson RL, Mackey DA, Ruddle JB, Bird TD, Gottlob I, Pieh C, Traboulsi EI, Pomeroy SL, Hunter DG, Soul JS, Newlin A, Sabol LJ, Doherty EJ, de Uzcátegui CE, de Uzcátegui N, Collins MLZ, Sener EC, Wabbels B, Hellebrand H, Meitinger T, de Berardinis T, Magli A, Schiavi C, Pastore-Trossello M, Koc F, Wong AM, Levin AV, Geraghty MT, Descartes M, Flaherty M, Jamieson RV, Møller HU, Meuthen I, Callen DF, Kerwin J, Lindsay S, Meindl A, Gupta ML, Pellman D, Engle EC. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell. 2010;140:74–87. doi: 10.1016/j.cell.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriyama M, Shimada T, Kim KB, Mitsuba M, Nomura E, Katsuta K, Sakumura Y, Roepstorff P, Inagaki N. Shootin1: a protein involved in the organization of an asymmetric signal for neuronal polarization. J Cell Biol. 2006;175:147–157. doi: 10.1083/jcb.200604160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortosa E, Galjart N, Avila J, Sayas CL. MAP1B regulates microtubule dynamics by sequestering EB1/3 in the cytosol of developing neuronal cells. EMBO J. 2013;32:1293–1306. doi: 10.1038/emboj.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda Y, Shinohara R, Thumkeo D, Kamijo H, Nishimaru H, Hioki H, Kaneko T, Ishizaki T, Furuyashiki T, Narumiya S. EphA4-dependent axon retraction and midline localization of Ephrin-B3 are disrupted in the spinal cord of mice lacking mDia1 and mDia3 in combination. Genes Cells. 2013;18:873–885. doi: 10.1111/gtc.12081. [DOI] [PubMed] [Google Scholar]
- Tullio AN, Bridgman PC, Tresser NJ, Chan CC, Conti MA, Adelstein RS, Hara Y. Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscle myosin II-B heavy chain. J Comp Neurol. 2001;433:62–74. doi: 10.1002/cne.1125. [DOI] [PubMed] [Google Scholar]
- Tymanskyj SR, Scales TME, Gordon-Weeks PR. MAP1B enhances microtubule assembly rates and axon extension rates in developing neurons. Mol Cell Neurosci. 2011;49:110–119. doi: 10.1016/j.mcn.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Vale R, Reese T, Sheetz M. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haren J, Draegestein K, Keijzer N, Abrahams JP, Grosveld F, Peeters PJ, Moechars D, Galjart N. Mammalian navigators are microtubule plus-end tracking proteins that can reorganize the cytoskeleton to induce neurite-like extensions. Cell Motil Cytoskeleton. 2009;66:824–838. doi: 10.1002/cm.20370. [DOI] [PubMed] [Google Scholar]
- Vignjevic D, Kojima SI, Aratyn Y, Danciu O, Svitkina T, Borisy GG. Role of fascin in filopodial protrusion. J Cell Biol. 2006;174:863–875. doi: 10.1083/jcb.200603013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votin V, Nelson WJ, Barth AIM. Neurite outgrowth involves adenomatous polyposis coli protein and beta-catenin. J Cell Sci. 2005;118:5699–5708. doi: 10.1242/jcs.02679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S. FH proteins as cytoskeletal organizers. Trends Cell Biol. 1998;8:111–115. doi: 10.1016/s0962-8924(97)01217-8. [DOI] [PubMed] [Google Scholar]
- Weber A, Pennise CR, Babcock GG, Fowler VM. Tropomodulin caps the pointed ends of actin filaments. J Cell Biol. 1994;127:1627–1635. doi: 10.1083/jcb.127.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner AM, Nebhan CA, Hu L, Majumdar D, Meier KM, Weaver AM, Webb DJ. N-WASP and the Arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J Biol Chem. 2008;283:15912–15920. doi: 10.1074/jbc.M801555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger R, Schevzov G, Jeffrey P, Gordon K, Hill M, Gunning P. The molecular composition of neuronal microfilaments is spatially and temporally regulated. J Neurosci. 1996;16:238–252. doi: 10.1523/JNEUROSCI.16-01-00238.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard M, Simon C. Modulations of neurofilament axonal transport during the development of rabbit retinal ganglion cells. Cell. 1983;35:551–559. doi: 10.1016/0092-8674(83)90189-7. [DOI] [PubMed] [Google Scholar]
- Wills Z, Marr L, Zinn K, Goodman CS, Van Vactor D. Profilin and the Abl tyrosine kinase are required for motor axon outgrowth in the Drosophila embryo. Neuron. 1999;22:291–299. doi: 10.1016/s0896-6273(00)81090-9. [DOI] [PubMed] [Google Scholar]
- Wittmann T, Waterman-Storer CM. Spatial regulation of CLASP affinity for microtubules by Rac1 and GSK3beta in migrating epithelial cells. J Cell Biol. 2005;169:929–939. doi: 10.1083/jcb.200412114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, Evans AC, Hazlett HC, Kostopoulos P, McKinstry RC, Paterson SJ, Schultz RT, Zwaigenbaum L, Piven J, Network IBIS. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD. The microtubule-severing protein Spastin is essential for axon outgrowth in the zebrafish embryo. Hum Mol Genet. 2006;15:2763–2771. doi: 10.1093/hmg/ddl212. [DOI] [PubMed] [Google Scholar]
- Worth DC, Hodivala-Dilke K, Robinson SD, King SJ, Morton PE, Gertler FB, Humphries MJ, Parsons M. Alpha v beta3 integrin spatially regulates VASP and RIAM to control adhesion dynamics and migration. J Cell Biol. 2010;189:369–383. doi: 10.1083/jcb.200912014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SR, Wu PJ, Patel H, Chantler PD. A conventional myosin motor drives neurite outgrowth. Proc Natl Acad Sci. 1998;95:12967–12972. doi: 10.1073/pnas.95.22.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Huganir RL, Penzes P. Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron. 2005;48:605–618. doi: 10.1016/j.neuron.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Xu K, Zhong G, Zhuang X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science. 2013;339:452–456. doi: 10.1126/science.1232251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S, Speicher KD, Speicher DW, Fowler VM. Mammalian tropomodulins nucleate actin polymerization via their actin monomer binding and filament pointed end-capping activities. J Biol Chem. 2010;285:33265–33280. doi: 10.1074/jbc.M110.144873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Zhang XF, Pollard TD, Forscher P. Arp2/3 complex-dependent actin networks constrain myosin II function in driving retrograde actin flow. J Cell Biol. 2012;197:939–956. doi: 10.1083/jcb.201111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lundquist EA. The actin-binding protein UNC-115/abLIM controls formation of lamellipodia and filopodia and neuronal morphogenesis in Caenorhabditisele-gans. Mol Cell Biol. 2005;25:5158–5170. doi: 10.1128/MCB.25.12.5158-5170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Kim WY, Chen Y, Wang X, Stanco A, Komuro Y, Snider W, Anton ES. The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron. 2009;61:42–56. doi: 10.1016/j.neuron.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn YH, Pramparo T, Hirotsune S, Wynshaw-Boris A. Distinct dose-dependent cortical neuronal migration and neurite extension defects in Lis1 and Ndel1 mutant mice. J Neurosci. 2009;29:15520–15530. doi: 10.1523/JNEUROSCI.4630-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TW, Hao JC, Lim W, Tessier-Lavigne M, Bargmann CI. Shared receptors in axon guidance: SAX-3/Robo signals via UNC-34/Enabled and a Netrin-independent UNC-40/DCC function. Nat Neurosci. 2002;5:1147–1154. doi: 10.1038/nn956. [DOI] [PubMed] [Google Scholar]