Introduction
Mycosis fungoides (MF), the most common form of cutaneous T-cell lymphoma, is composed of skin-homing mature effector T lymphocytes.1 Limited patch or plaque disease generally follows an indolent course with little, if any, effect on overall survival; therefore, skin-directed therapies are preferred and include corticosteroids, nitrogen mustard, phototherapy, and radiotherapy.2 Systemic interferon alfa has long been used in the treatment of more generalized disease by enhancing cytotoxic helper T cell (Th)1 lymphocyte and natural killer cell responses and suppressing Th2 differentiation, but is not used for limited disease secondary to its side-effect profile and method of administration.3 Imiquimod is a topical immunomodulator that stimulates a Th1 response through activation of toll-like receptor 7 on plasmacytoid dendritic cells, leading to production of interferon alpha, tumor necrosis factor alfa, and interleukin-12.4 Production of this cytotoxic cytokine milieu is thought to be the driving force in the treatment of condyloma, molluscum contagiosum, superficial basal cell carcinomas, lentigo maligna, and cutaneous lymphomas.5 Several reports were published on the effectiveness of topical imiquimod in early-stage MF.6, 7 We present 2 cases highlighting the effectiveness of topical imiquimod in the treatment of folliculotropic and tumor MF.
Case 1
A woman in her 80s with a 15-year history of stage IB MF presented to the dermatology clinic for a 6-year history of progressive involvement of the periocular skin, despite prior use of class I topical corticosteroids, topical carmustine, topical bexarotene, psoralen plus ultraviolet A, and systemic methotrexate. Physical examination found infiltrated plaques involving the left upper and lower eyelids and right nasal sidewall with variable alopecia (Fig 1, A). Biopsies confirmed MF with follicular involvement (folliculotropic MF). Localized radiotherapy had been proposed, but we recommended a trial of monotherapy with topical imiquimod 5% cream. Treatment was initiated at twice weekly and titrated to 3 times weekly, which resulted in complete clinical clearance after 6 months of treatment. During therapy, she reported a mildly symptomatic erythema at the sites of application that occurred primarily during the first several weeks of initiating treatment. No other adverse effects were reported. Recurrence has not developed at these sites for 10 months (Fig 1, B), but new lesions developed in previously noninvolved sites that have responded similarly with topical imiquimod monotherapy.
Fig 1.
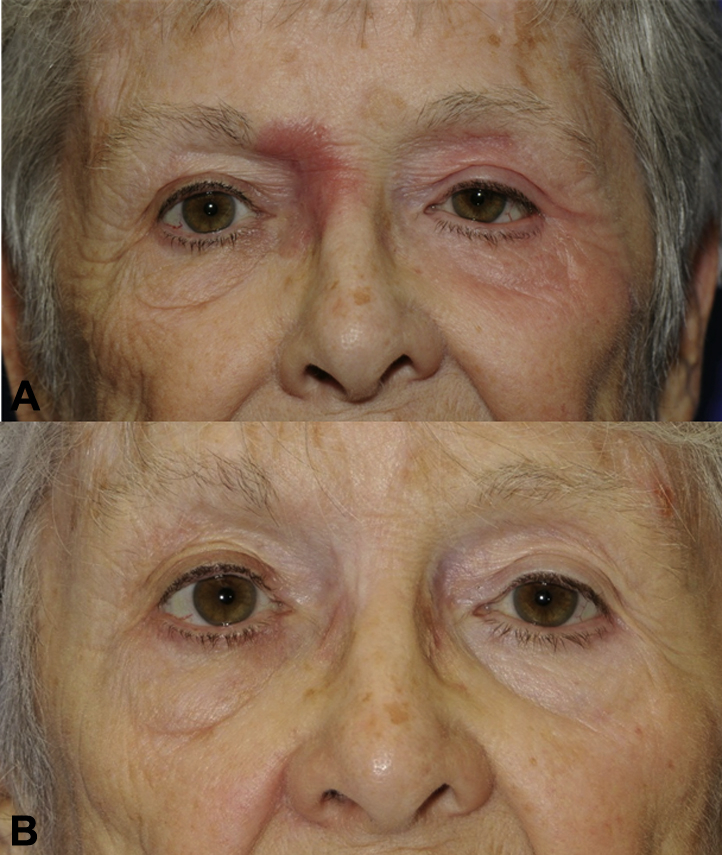
A, Patient 1 with infiltrated plaques of the left upper and lower eyelid and right nasal sidewall with variable alopecia. B, Same patient 1 year later after 6 months of topical imiquimod. Subtle hypopigmentation in areas of prior involvement is present.
Case 2
A white man in his 60s with a 4-year history of nummular dermatitis, previously controlled with topical betamethasone and occasional oral prednisone tapers, presented with a progressive indurated plaque on the right ankle. Punch biopsies found an atypical dermal lymphocytic infiltrate with variable epidermotropism. Biopsies of the other grossly eczematous plaques on the lower extremities confirmed subacute dermatitis with rare eosinophils. Peripheral blood flow cytometry was unremarkable, and a diagnosis of stage IB MF was made. One month of class I corticosteroids improved the spongiotic dermatitis but did not clear the mycosis fungoides. Despite the addition of narrow-band ultraviolet B phototherapy 3 times a week, the plaque ulcerated and a tumor developed (Fig 2, A). A repeat 4-mm punch biopsy confirmed tumor MF but, the result was negative for large cell transformation. Topical steroids and phototherapy were then discontinued, and a trial of topical imiquimod 5% cream as monotherapy was initiated once daily, 5 days a week. A brisk inflammatory response developed and cleared after 2 months of therapy (Fig 2, B). This treatment site has remained clear for 9 months, although the patient subsequently had new dyshidrotic papules elsewhere on the foot that showed an atypical epidermotropic T-cell infiltrate clonally identical to the presenting ankle lesion. This vesicular MF was also treated with a second course of imiquimod, once daily for 5 days a week, with complete clearance after 3 months of therapy.
Fig 2.
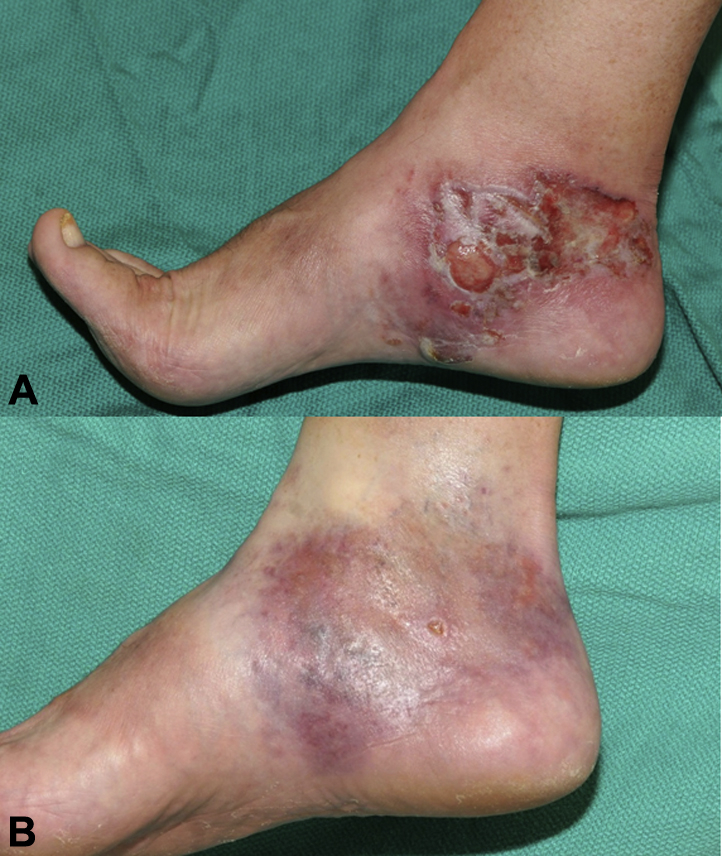
A, Patient 2 with indurated eroded plaque of the right ankle with new-onset tumor. B, Same patient after 2 months of topical imiquimod.
Discussion
We describe 2 cases of topical imiquimod 5% cream causing complete clinical clearance of MF subsets that traditionally have been refractive to conventional therapies.8 Case 1 highlights the use of imiquimod as monotherapy in folliculotropic MF lesions in a difficult-to-treat area, without the risk of skin atrophy associated with topical corticosteroid use. Case 2 highlights imiquimod as an effective therapy in a case of plaque disease with tumoral progression to stage IIB MF otherwise refractory to topical corticosteroids and narrowband ultraviolet B phototherapy. Later new-onset vesicular MF also responded to imiquimod treatment. In both cases, imiquimod was well tolerated, with macular erythema noted within 2 weeks of initiation of therapy, and maintenance therapy was not required after resolution of the lesions. Clearance of the folliculotropic MF was comparatively slower than for the tumor MF.
Application site reactions similar to those reported for treatment of nonmelanoma skin cancers should be expected, including erythema, scabbing, erosion, edema, and possibly ulceration with accompanying itching, pain, and burning especially with longer term use.8 These signs and symptoms reflect the suspected mechanism of activation of the immune system. In our experience, the frequency of application must be titrated for each patient to achieve the desired localized cutaneous reaction. If imiquimod is not applied at the necessary frequency, and patients do not have the expected inflammatory response, it is less likely to have an adequate response to therapy. Compared with a prior series using 5% imiquimod cream in the treatment of patch/plaque MF, the treatment duration in these cases was longer and/or imiquimod application more frequent, which may explain the higher response rate.9
In this series of 2 patients, imiquimod was a safe, well-tolerated, and effective skin-directed therapy for skin-limited MF. Our cases highlight its potential value in limited tumor stage and folliculotropic disease.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
Presented at the American Academy of Dermatology Annual Meeting, Denver, CO, March 2014.
References
- 1.Jawed S.I., Myskowski P.L., Horwitz S., Moskowitz A., Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70(2):205.e1–205.e16. doi: 10.1016/j.jaad.2013.07.049. quiz 221-2. [DOI] [PubMed] [Google Scholar]
- 2.Jawed S.I., Myskowski P.L., Horwitz S., Moskowitz A., Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70(2):223.e1–223.e17. doi: 10.1016/j.jaad.2013.08.033. quiz 240-2. [DOI] [PubMed] [Google Scholar]
- 3.Olsen E.A. Interferon in the treatment of cutaneous T-cell lymphoma. Dermatol Ther. 2003;16(4):311–321. doi: 10.1111/j.1396-0296.2003.01643.x. [DOI] [PubMed] [Google Scholar]
- 4.Sauder D.N. Immunomodulatory and pharmacologic properties of imiquimod. J Am Acad Dermatol. 2000;43(1 Pt 2):S6–S11. doi: 10.1067/mjd.2000.107808. [DOI] [PubMed] [Google Scholar]
- 5.Huen A.O., Rook A.H. Toll receptor agonist therapy of skin cancer and cutaneous T-cell lymphoma. Curr Opin Oncol. 2014;26(2):237–244. doi: 10.1097/CCO.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 6.Coors E.A., Schuler G., Von Den Driesch P. Topical imiquimod as treatment for different kinds of cutaneous lymphoma. Eur J Dermatol. 2006;16(4):391–393. [PubMed] [Google Scholar]
- 7.Dummer R., Urosevic M., Kempf W., Kazakov D., Burg G. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207(1):116–118. doi: 10.1159/000070962. [DOI] [PubMed] [Google Scholar]
- 8.Marks R., Gebauer K., Shumack S. Imiquimod 5% cream in the treatment of superficial basal cell carcinoma: results of a multicenter 6-week dose-response trial. J Am Acad Dermatol. 2001;44(5):807–813. doi: 10.1067/mjd.2001.113689. [DOI] [PubMed] [Google Scholar]
- 9.Deeths M.J., Chapman J.T., Dellavalle R.P., Zeng C., Aeling J.L. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52(2):275–280. doi: 10.1016/j.jaad.2004.04.049. [DOI] [PubMed] [Google Scholar]


