Introduction
Cutaneous infiltration in the skin is rare in patients with pre–B-cell acute lymphoblastic leukemia (pre–B-ALL). We describe the case of a patient with leukemia cutis (LC) who presented with nodules predominantly on the face. To our knowledge, there are no previously reported cases of LC in adults with pre–B-ALL.
Case report
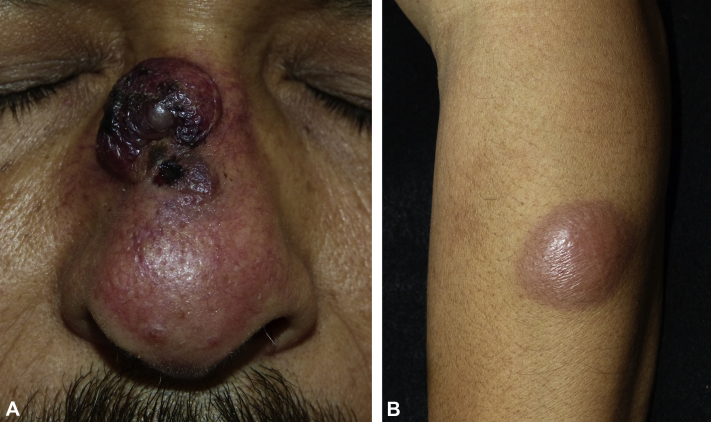
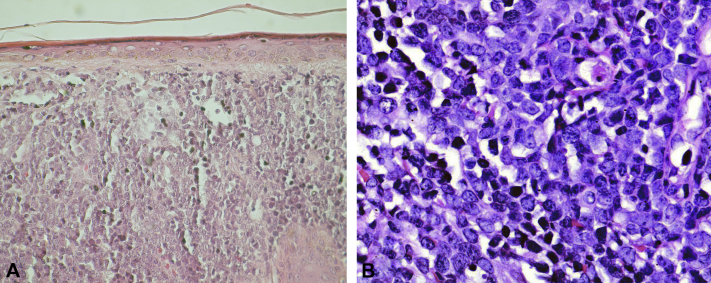
A 46-year-old man, who was a farmer without a significant medical history, presented to the emergency department for acute lower chest pain and a 3 month history of a 2- × 1-cm dome-shaped, indurated, erythematous-purplish nodule on the nose surrounded by atrophic and telangiectatic skin. He also complained of a 5 kg weight loss in the last month. He also had forehead and left forearm nodules that were larger, firm in consistency, and homogeneously erythematous (Fig 1). All lesions were asymptomatic. Examination of his oral cavity, regional lymph nodes, and the rest of his skin was unremarkable. Blood tests found a total leukocyte count of 17,700/mm3, with 3,600 lymphocytes without blasts, 12,900/mm3 neutrophils, 800/mm3 monocites, 300/mm3 eosinophils and 100/mm3 basophils, serum hemoglobin of 9.3 g/dL, platelet count of 35,000/mm3, and lactic acid dehydrogenase level of 1,569 (IU/L). The first diagnostic impression was cutaneous lymphoma, and a skin biopsy was performed. The histopathologic examination of the skin biopsy specimen found an atrophic epidermis and a massive proliferation of leukemic cells with marked nuclear pleomorphism characterized by cerebriform nuclei, atypical mitoses, microvacuolated cytoplasm within the papillary, and reticular dermis surrounding the vessels as well as cutaneous appendages without vasculitis (Fig 2).
Fig 1.
Leukemia cutis. A, A 2- × 1-cm, dome-shaped, indurated, erythematous-purplish nodule in the nose surrounded by atrophic and telangiectatic skin. B, A 5- × 3-cm asymptomatic erythematous firm nodule on the left forearm.
Fig 2.
A, Massive proliferation of leukemic cells invading papillary and reticular dermis. B, Marked nuclear pleomorphism characterized by cerebriform nuclei, atypical mitoses, and microvacuolated cytoplasm. (A and B, Hematoxylin-eosin stain; original magnifications: A, ×100; B, ×400.)
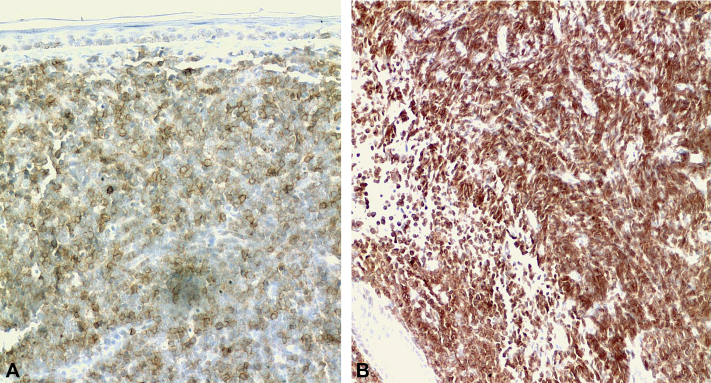
Immunohistochemical examination found neoplastic cells positive for CD20 and CD79, indicating an infiltrate of B-cell precursors (Fig 3). Additional immunolabeling of lesions by flow cytometry was positive for CD10, CD20, and CD19 and negative for surface immunoglobulin. This pattern was compatible with pre–B-ALL according to the current World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues.1 The bone marrow aspirate found 30% of blasts in the smear, and flow cytometric analysis found the expression of CD20, CD22, and CD79a as markers of B cells and the CD19 antigen as a primitive precursor of B cells. There was no expression of T-cell antigens such as CD2, CD3, CD5, or CD7. According to the WHO classification system, the patient presented features of poor prognosis, such as age older than 40 years and an immature cell immunophenotype.2
Fig 3.
A, Immunohistochemical study that shows leukemic cells positive for CD20 and B, cells positive for CD79. These markers are usually positive in pre–B-ALL. (Avidin-biotin-peroxidase complex staining. Original magnifications: A and B, ×200.)
After the diagnosis, he received the induction phase of treatment for ALL in adults with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone. Cutaneous lesions evolved with partial involution, but the patient's general condition worsened after 3 months. He refused further treatment and was lost to follow-up.
Discussion
LC is an extramedullary manifestation of leukemia, defined as cutaneous infiltration by neoplastic leukemic cells resulting in clinically recognizable skin lesions.3 The clinical and morphologic features of LC are variable and can present as violaceous, erythematous, or hemorrhagic nodules, papules, vesicles, bullae, or plaques of varying sizes.3 LC is more frequently associated with acute myeloid leukemia than with ALL.4 The prevalence of LC in ALL is 1% to 3%, but it is rare in cases of pre–B-ALL.5
Apparently, there have only been 2 cases reported in the literature related to LC and pre–B-ALL. One patient presented LC in the absence of systemic leukemia and exhibited a solitary, dome-shaped, erythematous, indurated nodule on his left cheek.6 Another case was in a child who manifested disseminated nodules that became edematous, pruritic, and erythematous after stroking them (Darier's sign).7 This case is unique, as it may represent, to our knowledge, the first reported case of LC in an adult with pre–B-ALL. This subtype of leukemia is clinically and morphologically indistinguishable from the classic ALL, but differs in terms of the surface antigens expressed on cells, whose presence confers a different prognostic value in this setting. Pre–B-ALL is usually more aggressive, showing a median survival rate of 14 months after diagnosis.6, 8
On clinical examination we considered a natural killer T-cell lymphoma because of location and morphology; however, immunohistochemical examination found neoplastic cells positive for CD20 and CD79, indicating an infiltrate of B-cell precursors. Additionally, lymphoblastic lymphoma was ruled out after imaging studies demonstrated no evidence of lymph nodes or mass lesions and based on the morphologic characteristics of bone marrow cellularity (>30% of blasts cells with a prominent nucleolus).
Pre–B-ALL accounts for approximately 2% of acute leukemias; it occurs most frequently in childhood but can also be seen in adulthood, with an overall median age of 40 years. Hispanics have the highest incidence of any ethnic group, where hepatomegaly, splenomegaly, and lymphadenopathy can be seen in up to half of adults on presentation. In these cases, the central nervous system is the most common extramedullary site involved.8 Cutaneous infiltration is more frequently implicated in precursor T-cell lymphoblastic acute leukemias, but in pre–B-ALL, this phenomenon is a rarity, and skin involvement would be the expression of advanced disease.9 According to the WHO classification for hematologic neoplasms, patient age older than 40 years, immunophenotype and genetic features such as traslocations, numerical changes, and IKZF gene expression are poor prognosis factors in acute leukemias. LC in adults with ALL usually predicts an accelerated disease progression with worse prognosis, as studies indicate a survival rate of approximately of 15% at 6 months after its recognition.10
LC is an infrequent finding in ALL, but should always be considered in the differential diagnosis of asymptomatic rapidly growing cutaneous nodules, especially if they are associated with hematologic alterations. Thus, the suspicion and early intervention of dermatologists, assisted by further histologic studies of cutaneous lesions, plays an important role in diagnosis, discriminating between variants and in establishing early treatment.
Footnotes
Funding sources: None
Conflicts of interest: None declared.
References
- 1.Swerdlow S.H., Campo E., Harris N.L. IARC Press; Lyon, France: 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 2.Boucheix C., David B., Sebban C. Immunophenotype of adult acute lymphoblastic leukemia, clinical parameters, and outcome: an analysis of a prospective trial including 562 tested patients (LALA87). French Group on Therapy for Adult Acute Lymphoblastic Leukemia. Blood. 1994;84:1603–1612. [PubMed] [Google Scholar]
- 3.Wagner G., Fenchel K., Back W. Leukemia cutis: Epidemiology, clinical presentation, and differential diagnosis. J Dtsch Dermatol Ges. 2012;10:27–36. doi: 10.1111/j.1610-0387.2011.07842.x. [DOI] [PubMed] [Google Scholar]
- 4.Jasim Z.F., Cooke N., Somerville J.E., Hay R.J. Chronic lymphocytic leukaemia skin infiltrates affecting prominent parts of the face and the scalp. Br J Dermatol. 2006;154:981–982. doi: 10.1111/j.1365-2133.2006.07129.x. [DOI] [PubMed] [Google Scholar]
- 5.Su W.P. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;3:223–230. [PubMed] [Google Scholar]
- 6.Ansell L.H., Mehta J., Cotliar J. Recurrent aleukemic leukemia cutis in a patient with pre-B-cell acute lymphoblastic leukemia. J Clin Oncol. 2013;31:e353–e355. doi: 10.1200/JCO.2012.46.1939. [DOI] [PubMed] [Google Scholar]
- 7.Cho-Vega J.H., Medeiros J., Prieto V.G. Leukemia cutis. Am J Clin Pathol. 2008;129:130–142. doi: 10.1309/WYACYWF6NGM3WBRT. [DOI] [PubMed] [Google Scholar]
- 8.Yen A., Sanchez R., Oblender M., Raimer S. Leukemia cutis: Darier's sign in a neonate with acute lymphoblastic leukemia. J Am Acad Dermatol. 1996;34:375–378. doi: 10.1016/s0190-9622(07)80012-0. [DOI] [PubMed] [Google Scholar]
- 9.Soslow R.A., Baergen R.N., Warnke R.A. B-lineage lymphoblastic lymphoma is a clinicopathologic entity distinct from other histologically similar aggressive lymphomas with blastic morphology. Cancer. 1999;85:2648–2654. [PubMed] [Google Scholar]
- 10.Shafer D., Wu H., Al-Saleem T. Cutaneous precursor B-cell lymphoblastic lymphoma in 2 adult patients: clinicopathologic and molecular cytogenetic studies with a review of the literature. Arch Dermatol. 2008;144:1155–1162. doi: 10.1001/archderm.144.9.1155. [DOI] [PubMed] [Google Scholar]