Introduction
Frontal fibrosing alopecia (FFA) is a form of cicatricial alopecia that predominantly affects perimenopuasal and postmenopausal women.1, 2, 3, 4 Although the precise cause is unknown, it is currently classified as a primary lymphocytic cicatricial alopecia that is closely related to lichen planopilaris (LPP). FFA not only causes scarring hair loss but also frequently causes skin atrophy within the frontal hairline.5, 6
Until recently, the treatment for FFA has mirrored the treatment algorithms used for other primary lymphocytic scarring alopecias. Topical steroids, steroid injections, hydroxychloroquine, doxycycline, tetracycline and mycophenolate mofetil have been the main treatments. However, in the last few years, an increasing number of reports have suggested a beneficial role for the 5 alpha reductase inhibitory medications, finasteride and dutasteride.4, 6, 7, 8
To date, the published studies of FFA treatment outcomes have focused on hair follicles—whether they are lost, stabilized, or promoted to regrow. The other important feature of the condition—cutaneous atrophy—has not received much attention. Here, I report a patient with FFA who experienced not only marked frontal hair regrowth with the 5α-reductase inhibitor, finasteride, but also a marked reversal of cutaneous atrophy.
Case report
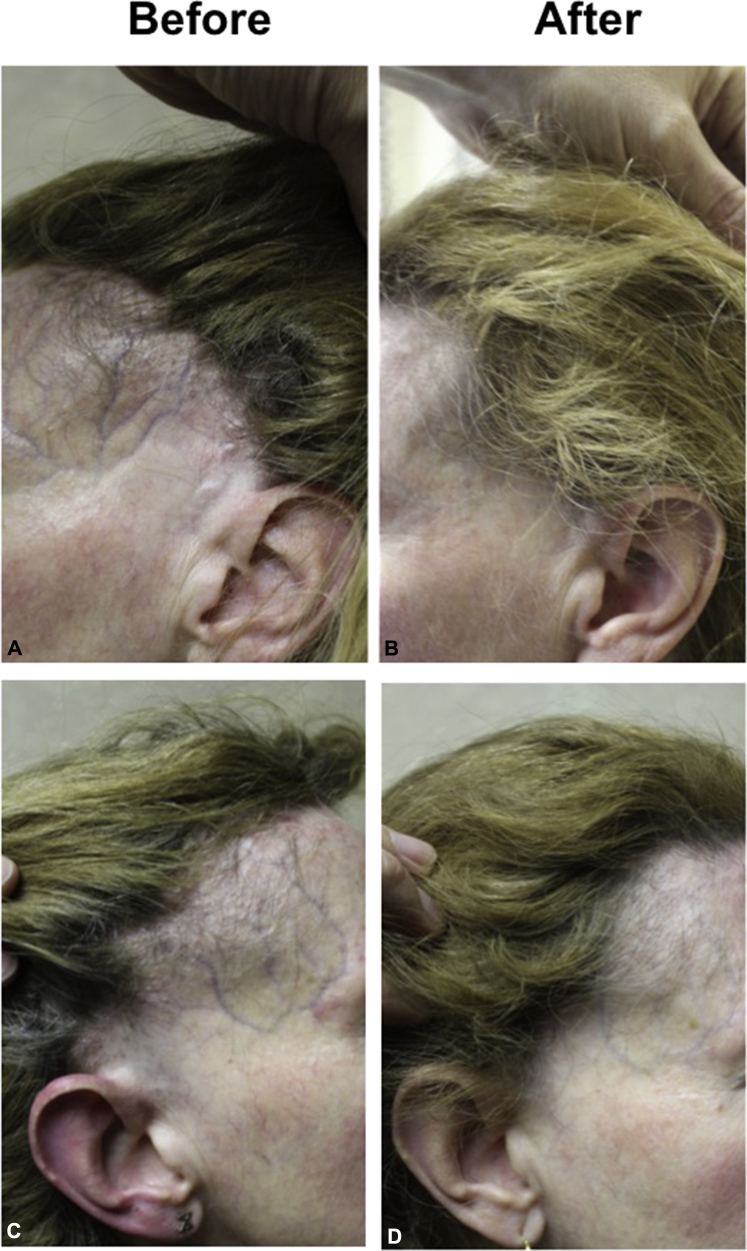
A 51-year-old woman presented with a 9-year history of asymptomatic frontal hair loss (Fig 1, A and C, before treatment). Hair loss started at age 42 in the preauricular area and extended to the entire frontal hairline. The patient was premenopausal at the time of first hair loss and entered menopause at age 49. Eyebrows were reduced in density but still present. A proportion of existing hair follicles in the receded hairline displayed perifollicular erythema and perifollicular scale. Marked atrophy was noted along the frontal hairline, and facial veins were visible (Fig 1, A and C). Biopsy findings confirmed the diagnosis of a lymphocytic cicatricial scarring alopecia consistent with the clinical diagnosis of frontal fibrosing alopecia. Results of blood work, including iron and thyroid studies, were normal. Initial treatments, including hydroxychloroquine (6 month trial); betamethasone valerate, 0.1 % cream (3 weeks); and tacrolimus, 0.1 % ointment (2 months), were not helpful and did not lead to any clinical change. The patient then started finasteride, 2.5 mg daily, and within 3 months experienced a reduction in redness and reversal of skin atrophy followed by hair regrowth in the fronto-temporal scalp. Further improvements were noted at 1-year follow-up (Fig 1, C and D).
Fig 1.
Hair regrowth and reversal of atrophy in a patient with frontal fibrosing alopecia. A and C, Before introduction of finasteride. B and D, 12 months posttreatment with finasteride, 2.5 mg. Note hair regrowth and reduction in atrophy.
In addition to author's assessment and patient's assessment of hair regrowth after finasteride treatment, clinical measurements also supported hair regrowth in the frontal hairline. In the author's practice, changes to frontal hairline in patients with frontal fibrosing alopecia are followed with use of clinical photography, dermoscopy, and a series of standardized measurements. For assessing the frontal hairline, the author draws a line (often with a crayon) from the lateral canthus to the root of the helix (LC-RH line). For most individuals, this distance is between 6 and 7 cm. Three additional hatch marks are then drawn perpendicular to this line at 2 cm, 4 cm, and 6 cm starting from the lateral canthus. Four separate perpendicular measurements are then taken from the LC-RH line to the patient's hairline: one at the lateral canthus and 3 at 2, 4, and 6 cm from the lateral canthus. For the patient in this report, these measurements (right side) were 7.6 cm, 7.0 cm, 5.5 cm, and 3 cm before treatment and 7.5 cm, 6.5 cm, 3.5 cm, and 1.5 cm after treatment with finasteride.
Discussion
Emerging evidence suggests that 5α-reductase inhibitors may be among the most effective treatments for FFA.4, 6, 7, 8 Although these drugs are not approved by the US Food and Drug Administration for use in women and must not be used in women of childbearing potential, they are increasingly used off label for treatment of postmenopausal FFA. Recent studies by Vano-Galvan et al4 support the notion that partial hair regrowth may be possible for a significant proportion of FFA patients treated with 5α-reductase inhibitors. Specifically, 52 of 111 FFA patients (47 %) experienced hair regrowth after treatment with these drugs.4 To date, hair regrowth does not appear to be a feature of any other class of drugs besides the 5α-reductase inhibitors.
It is well recognized that atrophy is a part of the clinical presentation of FFA. Atrophy can also be a side effect of topical steroids or steroid injections used to treat FFA. In our patient, atrophy was present before initiation of the short course of topical midpotency steroids; thus, atrophy cannot be attributed to use of topical steroids. Moreover, reversal of atrophy cannot be attributed to cessation of topical steroid therapies. The timing of improvement of both atrophy and hair regrowth strongly favor this as an effect of finasteride therapy.
Descriptive studies and rating scales to document atrophy have not been undertaken. Of the main published FFA studies, only a brief mention is made to the atrophy6, 9 or presence of dilated veins in women with FFA.5, 10 It is increasingly clear that disease activity scales often applied for the closely related condition, LPP, such as the Lichen Planopilaris Activity Index are inadequate for evaluating treatment responses in FFA.3 The Lichen Planopilaris Activity Index does not account for hair regrowth and places significant emphasis on disease symptoms and the positive pull test, both of which are less frequently a feature of FFA than LPP.6 New activity scales are needed that take into account variables such as patient symptoms, clinical signs (perifollciular scale and erythema), symptoms, speed of hairline advancement, hair regrowth, and possibly changes in skin atrophy. It would be helpful in the future to assess changes in skin atrophy before and after treatment with histology or ultrasonography. The assessment of skin atrophy by clinical examination is an important limitation of this study.
This case further documents the marked changes in hair regrowth that are possible with use of 5α-reductase inhibitors and raises the possibility that reversal of cutaneous atrophy may also be a bona fide associated treatment outcome to monitor.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Tan K.T., Messenger A.G. Frontal fibrosing alopecia: clinical presentations and prognosis. Br J Dermatol. 2009;160:75–79. doi: 10.1111/j.1365-2133.2008.08861.x. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald A., Clark C., Holmes S. Frontal fibrosing alopecia: a review of 60 cases. J Am Acad Dermatol. 2012;67:955–961. doi: 10.1016/j.jaad.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 3.Samrao A., Chew A.-L., Price V. Frontal fibrosing alopecia: a clinical review of 36 patients. Br J Dermatol. 2010;163:1296–1300. doi: 10.1111/j.1365-2133.2010.09965.x. [DOI] [PubMed] [Google Scholar]
- 4.Vano-Galvan S., Moina-Ruiz A.M., Serrano-Falcon C. Frontal fibrosing alopecia: A multicenter review of 355 patients. J Am Acad Dermatol. 2014;70:670–678. doi: 10.1016/j.jaad.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Banka N., Mubki T., Bunagan M.J. Frontal fibrosing alopecia: a retrospective clinical review of 62 patients with treatment outcome and long-term follow-up. Int J Dermatol. 2014;53(11):1324–1330. doi: 10.1111/ijd.12479. [DOI] [PubMed] [Google Scholar]
- 6.Tosti A., Piraccini B.M., Iorizzo M., Misciali C. Frontal fibrosing alopecia in postmenopausal women. J Am Acad Dermatol. 2005;52:55–60. doi: 10.1016/j.jaad.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Ladizinski B., Bazakas A., Selim A. Frontal fibrosing alopecia: A retrospective review of 19 patients seen at Duke University. J Am Acad Dermatol. 2013;68:749–755. doi: 10.1016/j.jaad.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Katoulis A., Georgala S., Bozi E., Papadavid E., Kalogeromitros D., Stavrianeas N. Frontal fibrosing alopecia: treatment with oral dutasteride and topical pimecrolimus. J Eur Acad Dermatol Venereol. 2009;23:580–582. doi: 10.1111/j.1468-3083.2008.02963.x. [DOI] [PubMed] [Google Scholar]
- 9.Moreno-Ramirez D., Camacho Martinez F. Frontal fibrosing alopecia: a survey in 16 patients. J Eur Acad Dermatol Venereol. 2005;19:700–705. doi: 10.1111/j.1468-3083.2005.01291.x. [DOI] [PubMed] [Google Scholar]
- 10.Vano-Galvan S., Rodrigues-Barata A.R., Urech M. Depression of the frontal veins: a new clinical sign of frontal fibrosing alopecia. J Am Acad Dermatol. 2015;72:1087–1088. doi: 10.1016/j.jaad.2015.02.1129. [DOI] [PubMed] [Google Scholar]