Abstract
Background
Puerarin has protective effects on ischemia-reperfusion injury, but the underlying mechanisms are not fully revealed. This study explored the effect of puerarin on the expression of Bcl-2 associated athanogene 3 (BAG3) in an in vitro model of anoxia/reoxygenation injury (A/RI) in neonate rat primary cardiomyocytes and the functions of BAG3 in A/RI.
Material/Methods
BAG3 expression in cardiomyocytes with or without puerarin pre-treatment was quantified using qRT-PCR and Western blot analysis. The effects of BAG3 on A/RI were studied by measuring the activity of lactate dehydrogenase (LDH) and creatine phosphate kinase (CPK), the concentration of malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px). The effects of BAG3 on autophagy and apoptosis of the cardiomyocytes after A/RI were further studied.
Results
Puerarin significantly promoted BAG3 expression in the rat primary cardiomyocytes after A/RI. Enforced BAG3 expression presented similar effects as puerarin pre-treatment in attenuating A/RI in terms of CPK, LDH, MDA, SOD, GSH-Px, ROS generation, and cell viability. BAG3 overexpression significantly stimulated autophagy in cardiomyocytes after A/RI, which presented protective effects on A/RI in terms of cell viability and apoptosis. Autophagy inhibition partly abrogated the protective effects of BAG3.
Conclusions
Puerarin can directly increase BAG3 transcription and translation in cardiomyocytes after A/RI. The elevated BAG3 expression presents protective effects on A/RI at least through enhancing autophagy and reducing apoptosis, which is a novel protective mechanism of puerarin in ARI.
MeSH Keywords: Autophagy; Myocytes, Cardiac; Reperfusion Injury
Background
Acute myocardial infarction (AMI) can result in irreversible injury and necrosis of myocardial cells [1,2]. Although early reperfusion to the ischemic area can rescue the ischemic myocardium, it also leads to ischemia-reperfusion injury, which might be a result of inflammation and re-introduction of oxidative stress [3–5]. In fact, ischemia-reperfusion injury is one of the major problems in treatment of myocardial infarction [6–8].
Puerarin is an isoflavone found in a number of plants and herbs, such as the kudzu root [9], and is used to treat cardio-cerebrovascular diseases [10]. Some recent studies suggest that puerarin has protective effects on cerebral and myocardial ischemia [11,12] as well as brain and cardiac tissues against ischemia and reperfusion injury [13–15]. The possible mechanisms include the antioxidant role of puerarin as a scavenger of active oxygen radicals [16], inhibiting the inflammatory response [13,17], and stimulating the production of nitric oxide and phosphorylation of endothelial nitric oxide synthase [9,18]. In fact, there might be some other unidentified mechanisms since a natural compound usually activates or inhibits multiple target genes directly or indirectly.
The Bcl-2 associated athanogene (BAG) family of proteins function as co-chaperones via assisting molecular chaperones to recruit target proteins [19]. BAG3 is a member of this family predominantly expressed in skeletal and cardiac muscle [20]. It can interact with Bcl-2 to inhibit apoptosis [20]. In addition, it is also involved in autophagy regulation in cardiomyocytes via interacting with the heat-shock protein family of proteins and HspB8 to facilitate the removal of misfolded and degraded proteins [19,21]. In this study, the effect of puerarin on the expression of BAG3 in an in vitro model of anoxia/reoxygenation injury (A/RI) in neonate rat primary cardiomyocytes was investigated. In addition, the protective effect of BAG3 on A/RI and possible underlying mechanisms was further studied.
Material and methods
Cell culture
Procedures involving animals were reviewed and approved by the Animal Care and Use Committee of Tengzhou Central People’s Hospital. All animal studies were performed in accordance with the ethical standards according to the Declaration of Helsinki. Primary cardiomyocytes were prepared from ventricles of 2-day-old Sprague-Dawley rats purchased from Nanchang University School of Medical according to the methods introduced in one previous study [22]. Briefly, the ventricles of the rat pups were minced into pieces, dissociated into single-cell suspension, and pre-plated onto 60-mm primaria culture dishes pre-coated in 1% gelatin (37°C, 30 min). The non-adherent cardiomyocytes were collected and plated on gelatin-coated 60-mm culture dishes at a density of 1×106 cells per dish and cultured in DMEM supplemented with 15% FCS, 100 U/ml of penicillin, and streptomycin. During the first 3 days of culture, 5-bromo-2′-deoxyuridine was added to the culture medium to inhibit the growth of residual fibroblasts.
Cell transfection
The ready-to-use lentival-BAG3 expression particles were purchased from Santa Cruz Biotech (sc-72602-V, Santa Cruz, CA, USA). To overexpress BAG3, the primary cardiomyocytes were infected with the lentiviral particles with the presence of 8 μg/ml polybrene according to manufacturer’s protocol (Sigma-Aldrich, St Louis, MO, USA).
Anoxia and reoxygenation injury (A/RI) model
The cardiomyocytes with or without BAG3 overexpression were exposed to anoxia via adding fresh anoxia medium (NaH2PO4 0.9 mmol/l, NaHCO3 6.0 mmol/l, NaCl 98.5 mmol/l, KCl 10.0 mmol/l, MgSO4 1.2 mmol/l, CaCl2 1.8 mmol/l, sodium lactate 40 mmol/l, HEPES 20 mmol/l and pH 6.8) and then incubated in an incubator containing 95% N2 and 5% CO2 for 4 hours. After that, the medium was changed to a reoxygenation medium containing NaCl 129.5 mmol/l, NaH2PO4 0.9 mmol/l, NaHCO3 20 mmol/l, KCl 5.0mmol/l, CaCl2 1.8mmol/l, MgSO4 1.2 mmol/l, glucose 5.5 mmol/l, and HEPES 20 mmol/l (pH 7.4), and then the cells were cultured in normoxia (5% CO2) for 2 hours. In the negative control group, the cells were consistently cultured in fresh growth culture medium in normoxia for 6 hours. To study the protective effects of puerarin on A/RI, the cells were pre-treated with 50, 100, or 200 μM puerarin (Fangming Pharmaceutical, Heze, Shandong, China) 24 hours before A/RI.
To study the effect of autophagy on A/RI, the primary cardiomyocytes without BAG3 overexpression were treated with 50 μM rapamycin (Rapa, Sigma-Aldrich) or 5 mM 3-methyladenine (3-MA, Sigma-Aldrich) 1 hour before A/RI. In addition, to investigate whether inhibition of autophagy abrogates the protective effect of puerarin, the cells with BAG3 overexpression or pre-treated with 200 μM puerarin were treated with 5 mM 3-MA 1 hour before A/RI. Then, the cells were subjected to analysis of autophagy, cell viability, and apoptosis.
QRT-PCR analysis of BAG3 expression
Total RNA from primary cardiomyocytes after indicating treatments were extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s instructions. Then, complementary DNA (cDNA) was synthesized using the PrimeScript RT reagent kit (TaKaRa, Dalian, China). To measure the level of BAG3 mRNA, qRT-PCR was performed by using the following primers: forward, 5′-CTCCATTCCGGTGATACACGA-3′, reverse, 5′-TGGTGGGTCTGGTACTCCC-3′ and SYBR Premix Ex Taq II (TaKaRa) in an ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). GAPDH was used as the internal control. The expression of BAG3 was calculated by the 2−ΔΔCt method.
Western blot analysis
Cell samples were firstly lysed using a lysis buffer (Beyotime, Shanghai, China). Then, the concentration of total protein in the samples were quantified using a BCA protein assay kit (Beyotime). The samples were separated on 10% SDS-PAGE gel and then transferred to PVDF membranes. The membranes were then blocked in 5% nonfat milk in TBST at room temperature for 1 hour and then incubated with anti-BAG3 (1:500, ab135892, Abcam, Cambridge, MA, USA), anti-LC3B (1:3000, ab51520, Abcam), anti-p62/SQSTM1 (1:1000, #8025, Cell Signaling, Danvers, MA, USA), or anti-β-actin (1:1000, ab8227, Abcam) overnight at 4°. After washing with TBST, the membranes were incubated with HRP-conjugated secondary antibodies for another 1 hour at room temperature. Then the protein signals were visualized using the ECL Western blotting substrate (Promega, Madison, WI, USA) and the band intensity was quantified using ImageQuant 5.2 (GE Healthcare, Piscataway, NJ, USA). The relative gray-scale value of BAG3 versus β-actin in negative control group was set as 1.
Measurement of biochemical parameters
After A/R treatment, the culture media or cell lysate supernatants were collected. Then, the activity of lactate dehydrogenase (LDH) and creatine phosphate kinase (CPK) in cell culture and the concentration of cellular malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) were determined using commercially available assay kits (USCN Life Science, Wuhan, China) according to the manufacturer’s instruction.
Measurement of intracellular reactive oxygen species (ROS)
To determine the effect of puerarin and BAG3 on ROS generation in the cardiomyocytes after ARI, the Cellular Reactive Oxygen Species Detection Assay Kit (ab113851, Abcam) was used. The fluorescence intensity of each group was determined using a FACS Calibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions.
Analysis of cell viability and cell apoptosis
Viability of the cell after A/R treatment was determined using the Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) according the manufacturer’s instructions. To measure the proportion of apoptotic cells, the Annexin V-FITC Apoptosis Detection Kit (V13241, Invitrogen) was used in combination with a FACS Calibur flow cytometer (BD Biosciences).
Statistical analysis
Data are presented as means ± standard deviation (SD) based on at least 3 repeats. One-way ANOVA was performed to compare means in multiple group experiments. Comparison between groups was performed using the unpaired t test. A two-sided p value of <0.05 was considered statistically significant.
Results
Puerarin enhances BAG3 expression in rat primary cardiomyocytes after A/RI
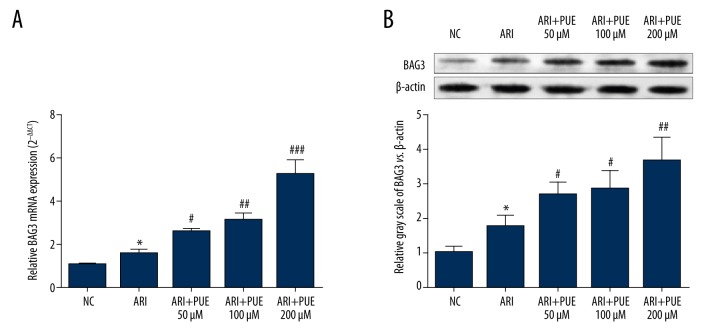
The important role of BAG3 in the homeostasis of myocytes has been gradually recognized [20]. In this study, we firstly explored the influence of puerarin on BAG3 expression after A/RI. The results showed that elevated BAG3 is a natural response of cardiomyocytes after A/RI (Figure 1A, 1B). Puerarin pre-treatment significantly enhanced the transcription and translation of BAG3 (Figure 1A, 1B). In addition, there is a dose-dependent effect of puerarin on the expression of BAG3 (Figure 1A, 1B). BAG3 expression in cardiomyocytes pre-treated with 200 μM puerarin was about 2 times higher than that in the A/RI group (Figure 1A, 1B).
Figure 1.
Puerarin enhances BAG3 expression in rat primary cardiomyocytes after A/RI. (A, B) QRT-PCR analysis (A) and Western blot analysis (B) of BAG3 expression in rat primary cardiomyocytes without A/RI and in the cells with or without pre-treatment of puerarin (50/100/200 μM) after A/RI. * Comparison with NC; # comparison with A/RI. * and # p<0.05, ** and ## p<0.01.
BAG3 overexpression decreases LDH and CPK activity and increases cell viability
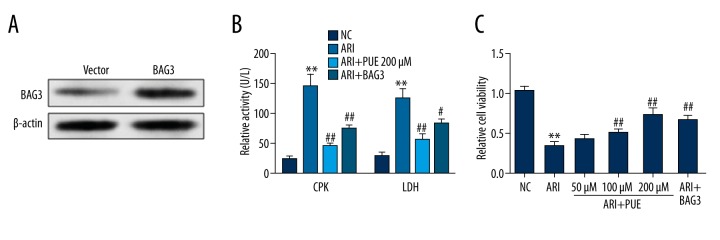
Then, we investigated the role of BAG3 on injury and viability of cardiomyocytes after A/RI. The primary cardiomyocytes were firstly transfected with BAG3 lentiviral particles for overexpression (Figure 2A). A/RI significantly promoted the activity of both LDH and CPK in the supernatants of cell culture, suggesting cardiomyocyte injury (Figure 2B). In contrast, the supernatants of cardiomyocytes pre-treated with 200 μM puerarin or with BAG3 overexpression had significantly lower LDH and CPK activity than the A/RI group (Figure 2B), which indicates attenuated cardiomyocyte injury. We also investigated the effect of BAG3 on cell viability after A/RI. The results showed that BAG3 overexpression significantly restored the loss of cell viability due to A/RI (Figure 2C).
Figure 2.
BAG3 overexpression decreases LDH and CPK activity and increases cell viability. (A) Western blot analysis (B) of BAG3 expression in rat primary cardiomyocytes infected with BAG3 expression lentiviral particles. (B) The activity of lactate dehydrogenase (LDH) and creatine phosphate kinase (CPK) activity in the culture medium of cardiomyocytes without A/RI or with 200 μM puerarin pre-treatment or BAG3 overexpression after A/RI. C. The relative cell viability of cardiomyocytes without A/RI or with 50/100/200 μM puerarin pre-treatment or BAG3 overexpression after A/RI. * Comparison with NC; # comparison with A/RI. * and # p<0.05, ** and ## p<0.01.
BAG3 overexpression reduces ROS generation and decreases MDA content but increases SOD and GSH-Px concentration in cells after A/RI
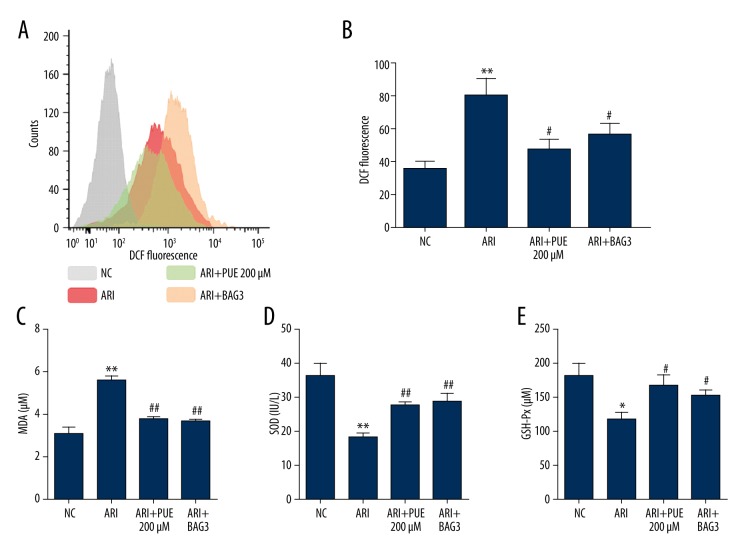
ROS generation is also a marker of cell injury. In this study, we demonstrated that A/RI resulted in significantly increased ROS generation (Figure 3A, 3B), which suggests consequent oxidative stress damage. However, BAG3 overexpression significantly reduced ROS generation after A/RI (Figure 3A, 3B). Then, we examined the change of the myocardial lipid peroxidative product MDA and concentration of SOD and GSH-Px, 2 important antioxidases in cardiomyocytes after A/RI. The results showed that A/RI significantly increased MDA, but decreased both SOD and GSH-Px (Figure 3C–3E). BAG3 overexpression reduced the level of MDA and partly restored the concentration of SOD and GSH-Px (Figure 3C–3E).
Figure 3.
BAG3 overexpression reduces ROS generation and decreases MDA content but increases SOD and GSH-Px activity in cells after A/RI. (A) Flow cytometric histograms of DCF fluorescence indicating reactive oxygen species (ROS) generation in cardiomyocytes without A/RI or with 200 μM puerarin pre-treatment or BAG3 overexpression after A/RI. (B) Column bar graph of cell fluorescence for DCF. Data were expressed as the mean ±SD for 3 independent experiments. (C–E) Alteration of MDA (C), SOD (D) and GSH-Px (E) in cardiomyocytes without A/RI or with 200 μM puerarin pre-treatment or BAG3 overexpression after A/RI. * Comparison with NC; # comparison with A/RI. * and # p<0.05, ** and ## p<0.01.
BAG3 overexpression increases autophagy and reduces apoptosis after ARI, which act as a protective mechanism of cell survival
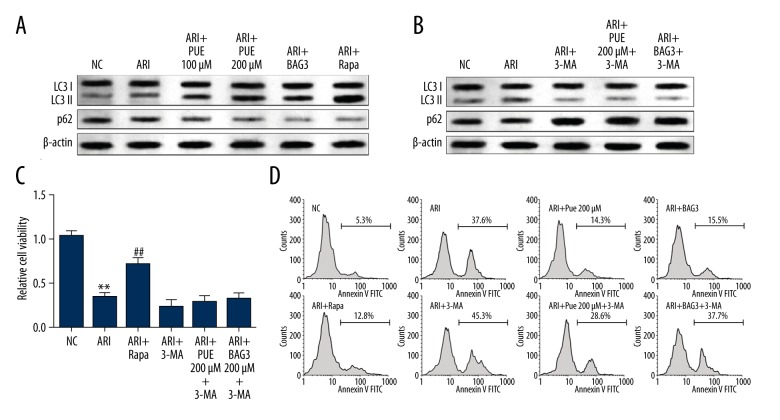
BAG3 is a protein related to both autophagy and apoptosis regulation [20,21,23]. Therefore, we decided to study the involvement of autophagy in the protective effect of puerarin on A/RI. Increased autophagy is a spontaneous response of cardiomyocytes after A/RI (Figure 4A). BAG3 overexpression significantly enhanced the level of autophagy after A/RI (Figure 4A). 3-MA, an autophagy inhibitor that blocks autophagosome formation, can significantly suppress spontaneously increased autophagy due to A/RI and puerarin or BAG3-induced autophagy (Figure 4B). Elevating the level of autophagy by using rapamycin significantly promoted cell viability (Figure 4C). However, inhibiting autophagy partly abrogated the effect of BAG3 on promoting cell viability (Figure 4C). By performing flow cytometry analysis of cell apoptosis, we also observed that enhancing autophagy attenuated cell apoptosis, while reducing autophagy promoted cell apoptosis (Figure 4D). Inhibiting autophagy partly abrogated the effect of BAG3 on reducing cell apoptosis after A/RI (Figure 4D).
Figure 4.
BAG3 overexpression increases autophagy and decreases apoptosis after ARI, which acts as a protective mechanism of cell survival. (A) Western blot analysis of LC3 and p62 expression in cardiomyocytes without A/RI or with 100/200 μM puerarin pre-treatment, BAG3 overexpression or rapamycin pre-treatment after ARI. (B) Western blot analysis of LC3 and p62 expression in cardiomyocytes without A/RI, with A/RI and 3-MA pre-treatment or with 200 μM puerarin pre-treatment or BAG3 overexpression in combination with 3-MA after A/RI. (C, D) CCK-8 assay of relative cell viability (C) and flow cytometry analysis of cell apoptosis (D) of cardiomyocytes after indicating treatments. * Comparison with NC; # comparison with A/RI. * and # p<0.05, ** and ## p<0.01.
Discussion
The protective effect of puerarin on myocardial ischemia and cardiac tissues against ischemia and reperfusion injury have been reported in previous studies [15,24,25]. The possible mechanisms include inhibition of the L-type calcium current of ventricular myocytes [25], opening of mitochondrial ATP-sensitive potassium channels, inhibition of mitochondrial permeability transition pore opening, and inhibiting the production of proinflammatory cytokines [15,26]. Another recent study found that puerarin can increase the expression of protein kinase C epsilon, a critical cardioprotective protein, thereby attenuating A/RI injury [24]. In this study, we also confirmed that protective effects of puerarin on A/RI in terms of CPK, LDH, SOD, GSH-Px, ROS generation, and cell viability.
There might be some other unidentified mechanisms, since a natural compound usually activates or inhibits multiple target genes directly or indirectly. BAG3 is an important protein contributing to homeostasis of cardiomyocytes [20]. In this study, we firstly explored the regulative role of puerarin on the expression of BAG3. The results showed that puerarin significantly promoted BAG3 expression after A/RI in the rat primary cardiomyocytes. Enforced BAG3 expression presented similar effects as puerarin treatment in attenuating A/RI, suggesting the protective effects of puerarin is at least partly mediated by BAG3.
A/RI is closely associated with free radicals and other reactive oxygen species [26], leading to peroxidation of the lipids of cell membranes. The level of MDA formation correlates with lipid peroxidation and thus indirectly reflects the degree of cell damage [27]. CPK is released from damaged myocardial cells and the elevated activity of CPK is also a cardiac-specific marker of acute myocardial infarction [14]. Following cell damage, the accumulation of damaged proteins usually triggers the signaling of necrosis and apoptosis. Some recent studies suggest that induction of autophagy leads to inhibition of apoptosis after myocardial ischemia/reperfusion injury [28]. Autophagy is a highly conserved recycling process of cytoplasmic components, such as long-lived proteins and organelles to maintain cellular homeostasis [29]. Previous studies reported that BAG3 is a protein involved in autophagy modulation. Carra et al. found that BAG3 can promote macroautophagy via association with HspB8 [21]. BAG3 overexpression leads to facilitated degradation of huntingtin protein Htt43Q, while BAG3 knockdown suppresses the degradation via HspB8 [30]. In addition, BAG3 overexpression promotes the formation of LC3-II, but BAG3 knockdown presents an inverse effect [30]. In fact, HspB8 can recognize the misfolded proteins. The HspB8-BAG3 complex helps to eliminate damaged or unstable proteins through macroautophagy machinery [30]. Besides this mechanism, BAG3 can also be coupled with the chaperone Hsp70 and the co-chaperone ubiquitin ligase carboxyl terminal of Hsp70/Hsp90 interacting protein (CHIP), thereby facilitating the sequestration of misfolded proteins into autophagosomes [31]. In addition, BAG3 has also been reported as an apoptosis inhibitor through multiple mechanism, such as synergizing with Bcl-2 and with Bcl-XL [20], modulating the NF-κB pathway [32], and downregulating miR-29b, which leads to upregulation of the anti-apoptosis protein Mcl-l [33]. Besides BAG3, a recent study reported that puerarin attenuates pressure overload-induced cardiac hypertrophy via blockade of PI3K/Akt and JNK signaling pathways [34]. In fact, blockade of PI3K/Akt can decrease the activation of mTOR, an important inhibitor of autophagy induction [35]. This might also be a mechanism by which puerarin induces higher levels of autophagy.
Therefore, we decided to further investigate the role of BAG3 in A/RI of the cardiomyocytes. Our data showed that BAG3 overexpression significantly stimulated autophagy in cardiomyocytes after A/RI, which also presented protective effects on A/RI in terms of cell viability and apoptosis. Autophagy inhibition partly abrogated the protective effects of BAG3. These data demonstrated that the protective effects of puerarin on ARI is partly mediated by enhancing BAG3 expression, which functions as a stimulator of autophagy. Currently, puerarin has been approved by the State Food and Drug Administration in China for clinical therapy of cardiovascular and other diseases [10]. This study found that autophagy simulating might be an important mechanism underlying the therapeutic effect of puerarin on anoxia/reoxygenation injury. Therefore, in the future, it is meaningful to investigate the use of autophagy stimulator in combination with puerarin to enhance the therapeutic effects.
Conclusions
Puerarin can directly increase BAG3 transcription and translation in cardiomyocytes after A/RI. The elevated BAG3 expression presents protective effects on A/RI through enhancing autophagy and reducing apoptosis, which is a novel protective mechanism of puerarin in A/RI.
Footnotes
Source of support: Departmental sources
References
- 1.Lunder M, Janic M, Ziberna L, et al. A low-dose atorvastatin and losartan combination directly improves aortic ring relaxation and diminishes ischaemic-reperfusion injury in isolated rat hearts. Med Sci Monit. 2012;18(9):BR366–74. doi: 10.12659/MSM.883347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yilmaz Y, Taken K, Atar M, et al. Protective effect of curcumin on priapism and ischemia-reperfusion injury in rats. Eur Rev Med Pharmacol Sci. 2015;19:4664–70. [PubMed] [Google Scholar]
- 3.Sirotkovic-Skerlev M, Plestina S, Bilic I, Kovac Z. Pathophysiology of ischaemia-reperfusion injury. Lijec Vjesn. 2006;128:87–95. [in Croatian] [PubMed] [Google Scholar]
- 4.He XH, Li QW, Wang YL, et al. Transduced PEP-1-heme oxygenase-1 fusion protein reduces remote organ injury induced by intestinal ischemia/reperfusion. Med Sci Monit. 2015;21:1057–65. doi: 10.12659/MSM.893924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Li RJ, Lv GY, Liu HQ. The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci. 2015;19:2036–47. [PubMed] [Google Scholar]
- 6.Yellon DM, Baxter GF. Protecting the ischaemic and reperfused myocardium in acute myocardial infarction: Distant dream or near reality? Heart. 2000;83:381–87. doi: 10.1136/heart.83.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szczesny B, Modis K, Yanagi K, et al. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide. 2014;41:120–30. doi: 10.1016/j.niox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long Y, Wang P, Sun H, et al. Effect of low-dose cyclophosphamide on endoplasmic reticulum stress and inflammatory reaction of acute renal ischemia reperfusion injury. Eur Rev Med Pharmacol Sci. 2015;19:3751–56. [PubMed] [Google Scholar]
- 9.Luo CF, Hou N, Tian J, et al. Metabolic profile of puerarin in rats after intragastric administration of puerarin solid lipid nanoparticles. Int J Nanomedicine. 2013;8:933–40. doi: 10.2147/IJN.S39349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou YX, Zhang H, Peng C. Puerarin: A review of pharmacological effects. Phytother Res. 2014;28:961–75. doi: 10.1002/ptr.5083. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Chen S, Shen Y, et al. Puerarin induces angiogenesis in myocardium of rat with myocardial infarction. Biol Pharm Bull. 2006;29:945–50. doi: 10.1248/bpb.29.945. [DOI] [PubMed] [Google Scholar]
- 12.Wang N, Zhang Y, Wu L, et al. Puerarin protected the brain from cerebral ischemia injury via astrocyte apoptosis inhibition. Neuropharmacology. 2014;79:282–89. doi: 10.1016/j.neuropharm.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Zhou F, Wang L, Liu P, et al. Puerarin protects brain tissue against cerebral ischemia/reperfusion injury by inhibiting the inflammatory response. Neural Regen Res. 2014;9:2074–80. doi: 10.4103/1673-5374.147934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenjun H, Jing W, Tao L, et al. The protective effect of puerarin on myocardial infarction reperfusion injury (MIRI): A meta-analysis of randomized studies in rat models. Med Sci Monit. 2015;21:1700–6. doi: 10.12659/MSM.894312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan HY, Gao Q, Yao H, Xia Q. The protective role and the mechanisms of puerarin on isolated rat heart during ischemia/reperfusion. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2006;22:455–59. [in Chinese] [PubMed] [Google Scholar]
- 16.Han RM, Tian YX, Becker EM, et al. Puerarin and conjugate bases as radical scavengers and antioxidants: Molecular mechanism and synergism with beta-carotene. J Agric Food Chem. 2007;55:2384–91. doi: 10.1021/jf062796c. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Mei Z, Qian J, et al. Puerarin partly counteracts the inflammatory response after cerebral ischemia/reperfusion via activating the cholinergic anti-inflammatory pathway. Neural Regen Res. 2013;8:3203–15. doi: 10.3969/j.issn.1673-5374.2013.34.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yener AU, Sehitoglu MH, Ozkan MT, et al. Effects of kefir on ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci. 2015;19:887–96. [PubMed] [Google Scholar]
- 19.Gurusamy N, Lekli I, Gherghiceanu M, et al. BAG-1 induces autophagy for cardiac cell survival. Autophagy. 2009;5:120–21. doi: 10.4161/auto.5.1.7303. [DOI] [PubMed] [Google Scholar]
- 20.Knezevic T, Myers VD, Gordon J, et al. BAG3: A new player in the heart failure paradigm. Heart Fail Rev. 2015;20:423–34. doi: 10.1007/s10741-015-9487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carra S, Seguin SJ, Landry J. HspB8 and Bag3: A new chaperone complex targeting misfolded proteins to macroautophagy. Autophagy. 2008;4:237–39. doi: 10.4161/auto.5407. [DOI] [PubMed] [Google Scholar]
- 22.Watkins SJ, Borthwick GM, Arthur HM. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim. 2011;47:125–31. doi: 10.1007/s11626-010-9368-1. [DOI] [PubMed] [Google Scholar]
- 23.Gamerdinger M, Kaya AM, Wolfrum U, et al. BAG3 mediates chaperone-based aggresome-targeting and selective autophagy of misfolded proteins. EMBO Rep. 2011;12:149–56. doi: 10.1038/embor.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang L, Liu D, Yi X, et al. The protective effects of puerarin in cardiomyocytes from anoxia/reoxygenation injury are mediated by PKCepsilon. Cell Biochem Funct. 2014;32:378–86. doi: 10.1002/cbf.3026. [DOI] [PubMed] [Google Scholar]
- 25.Guo XG, Chen JZ, Zhang X, Xia Q. Effect of puerarin on L-type calcium channel in isolated rat ventricular myocytes. Zhongguo Zhong Yao Za Zhi. 2004;29:248–51. [in Chinese] [PubMed] [Google Scholar]
- 26.Feng ZQ, Wang YY, Guo ZR, et al. The synthesis of puerarin derivatives and their protective effect on the myocardial ischemia and reperfusion injury. J Asian Nat Prod Res. 2010;12:843–50. doi: 10.1080/10286020.2010.505563. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Qiao H, Li Y, Li L. Protective roles of puerarin and Danshensu on acute ischemic myocardial injury in rats. Phytomedicine. 2007;14:652–58. doi: 10.1016/j.phymed.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 28.Hamacher-Brady A, Brady NR, Logue SE, et al. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–57. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 29.Carew JS, Kelly KR, Nawrocki ST. Autophagy as a target for cancer therapy: New developments. Cancer Manag Res. 2012;4:357–65. doi: 10.2147/CMAR.S26133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carra S, Seguin SJ, Lambert H, Landry J. HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. J Biol Chem. 2008;283:1437–44. doi: 10.1074/jbc.M706304200. [DOI] [PubMed] [Google Scholar]
- 31.Doong H, Vrailas A, Kohn EC. What’s in the ‘BAG’? – A functional domain analysis of the BAG-family proteins. Cancer Lett. 2002;188:25–32. doi: 10.1016/s0304-3835(02)00456-1. [DOI] [PubMed] [Google Scholar]
- 32.Ammirante M, Rosati A, Arra C, et al. IKK{gamma} protein is a target of BAG3 regulatory activity in human tumor growth. Proc Natl Acad Sci USA. 2010;107:7497–502. doi: 10.1073/pnas.0907696107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugio A, Iwasaki M, Habata S, et al. BAG3 upregulates Mcl-1 through downregulation of miR-29b to induce anticancer drug resistance in ovarian cancer. Gynecol Oncol. 2014;134:615–23. doi: 10.1016/j.ygyno.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 34.Yuan Y, Zong J, Zhou H, et al. Puerarin attenuates pressure overload-induced cardiac hypertrophy. J Cardiol. 2014;63:73–81. doi: 10.1016/j.jjcc.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012;8:903–14. doi: 10.4161/auto.19653. [DOI] [PMC free article] [PubMed] [Google Scholar]