Abstract
Background
Toxicity of calcium channel blockers leads to high patient mortality and there is no effective antidote. The benefit of using 20% lipid emulsion and sugammadex has been reported. The present study measured the effect of sugammadex and 20% lipid emulsion on hemodynamics and survival in a rat model of verapamil toxicity.
Material/Methods
In this single-blinded randomized control study, rats were separated into 4 groups of 7 rats each: Sugammadex (S), Sugammadex plus 20% lipid emulsion (SL), 20% lipid emulsion (L), and control (C). Heart rates and mean arterial pressures were monitored and noted each minute until death.
Results
Average time to death was 21.0±9.57 minutes for group C, 35.57±10.61 minutes for group S, 37.14±16.6 minutes for group L and 49.86±27.56 minutes for group SL. Time to death was significantly longer in other groups than in the control group (p<0.05).
Conclusions
Verapamil overdose is has a comparatively high mortality rate and there is no effective antidote. Treatment generally involves gastric decontamination and symptomatic treatment to counteract the drug’s negative effects. In animal studies sugammadex and lipid emulsion had a positive effect on survival in patients with calcium channel blocker toxicity. Sugammadex and intralipid increased survival in a rat model of verapamil toxicity. The combination of both drugs may decrease cardiotoxicity. Sugammadex alone or combined with 20% lipid emulsion reduce the need for inotropic agents. The mechanism requires clarification with larger studies.
MeSH Keywords: Drug Overdose; Fat Emulsions, Intravenous; gamma-Cyclodextrins; Verapamil
Background
In the Annual Report of the American Association of Poison Control Centers, around 5000 cases of intoxication with calcium channel blockers (CCB) were reported in 2013, although this figure is over 11 000 when multi-drug ingestion is considered. More than half of these patients required hospitalization. The same publication reported 29 deaths (mortality rate 0.58%) due to CCB [1]. There are studies reporting up to 10% CCB overdose mortality in children [2]. Verapamil and diltiazem are the most frequently used medications in CCB group. Preparations of slow-prolonged release verapamil can be deadly, even in small quantities, especially in children.
CCB overdose can lead to cardiovascular symptoms, such as hypotension, bradycardia, myocardial depression, as well as neurological (e.g., syncope and confusion), endocrinological (e.g., acidosis and hyperglycemia), and respiratory (e.g., respiratory depression and noncardiogenic pulmonary edema) problems [3,4]. Standard treatment consists of gastrointestinal decontamination, high-dose insulin, calcium, vasopressors, and glucagon, although use of pacemakers, intra-aortic balloon pumps, extracorporeal membrane oxygenation, and venovenous hemodiafiltration has also been reported [5–9]. There are also animal studies and case reports that have reported successful use of lipid emulsion, beta-cyclodextrin, sugammadex (a modified gamma-cyclodextrin), levosimendan, aminopyridine, and methylene blue [10–17]. There is, however, no definitive antidote.
Overdose of calcium channel blockers has high mortality and their use for suicide has been increasing. In this study our primary aim was to evaluate the effect of sugammadex, lipid emulsion, and sugammadex plus lipid emulsion on the survival time in a rat model of verapamil toxicity. Our secondary aim was to investigate their effect on hemodynamic parameters in the same model.
Material and Methods
Study design
In this randomized, blinded, controlled trial, we compared the effect of sugammadex, lipid emulsion, and sugammadex plus lipid emulsion on hemodynamic parameters and survival time in a rat model of verapamil toxicity. The study was approved by Yeditepe University School of Medicine Animal Studies Ethics Board and was performed at Yeditepe University School of Medicine Experimental Research Center in Istanbul, Turkey.
Twenty-eight Sprague-Dawley rats weighing 180–250 grams were kept in a temperature (22–24ºC) and humidity-controlled environment with free access to food and water. During the experimental period, the care of laboratory animals was in accordance with international guidelines.
Verapamil (Verapamil Ratiopharm; 5 mg/2 mL, Ratiopharm GmbH, Germany), sugammadex (Bridion; 200 mg/2 mL, Schering- Plough, Turkey), and 20% lipid emulsion (ClinOleic 500 mL, Eczacıbaşı-Baxter, Turkey) were purchased for use in this study.
Study protocol
All rats’ weights and ages were noted and rats were randomly separated into 4 groups: control group (group C), sugammadex group (group S), lipid emulsion group (group L), and lipid emulsion plus sugammadex group (group SL). Each group consisted of 7 rats.
Rats were anesthetized using 60 mg/kg intraperitoneal ketamine. Following a period of 6–11 minutes for hemodynamic stabilization and depth of anesthesia, rats were placed on a warm surface (38ºC) and 1 L/m O2 was administered. After electrocardiographic (ECG) monitoring via alligator clips, the right femoral area was surgically dissected and P50 cannulas were placed in the femoral artery and vein. To counteract any blood loss up to this time, 1 ml of saline solution was administered. For medication and fluid infusions, a CMA 400 syringe pump (CMA Microdialysis, Kista, Sweden) was used. ECG and mean arterial pressure (MAP) measurements were performed using Lab Chart Power Lab 8/30 (AD Instruments, New South Wales, Australia).
We administered 37.5 mg/kg/hr dose of verapamil (2.5 mg/ml) via the femoral vein to all rats. T0 was the time when the infusion started, and infusion continued until death was confirmed. Rats’ heart rates on ECG and mean arterial pressure were noted at each minute. Death was accepted as asystole on ECG or no respiratory effort for over 1 minute.
In group C, 5 minutes after beginning of verapamil infusion, rats were given 12.4 mL/kg normal saline for a period of 5 minutes. This amount represents a 20% volume based on an average rat blood volume of 62 mL/kg. In group S, following a 16 mg/kg sugammadex bolus, saline was given to complete the total dose of 12.4 mL/kg. In group L, 20% intralipid emulsion was administered at 12.4 mL/kg. In group SL, following 16 mg/kg sugammadex bolus, 20% lipid emulsion was administered in an amount to complete a total of 12.4 mL/kg.
Infusion dosages and volumes for verapamil intoxication were performed as reported previously [10,14,18]. The data recorder who noted infusion times, time of death, total verapamil dosage (mg/kg), mean arterial pressures, and heart rate information was blinded to group identity.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 22 (IBM SPSS, Turkey). The Kolmogorov-Smirnov test was used to evaluate the distribution of data and parameters were found not to show normal distribution. Parameters were compared using the Kruskal-Wallis test and Mann-Whitney U test. Intergroup comparison was performed using the Friedman test and Wilcoxon signed rank test. Statistical significance was accepted as p<0.05.
Results
Age, weight, temperature, and basal respiratory rates were similar for all groups (p>0.05). Average time to death was 21.0±9.57 minutes for group C, 35.57±10.61 minutes for group S, 37.14±16.6 minutes for group L, and 49.86±27.56 minutes for group SL. There was a statistically significant difference between these groups (p<0.05). When inter-group comparison was performed, the time to death was significantly shorter in group C (p<0.05). There was no statistically significant difference between groups S, L or SL. Similarly, while the average lethal dose was significantly different in group C when compared to other groups (p<0.05), there was no difference between groups S, L, or SL (p>0.05) (Table 1). Figure 1 shows the box plot graphic for time to death, Figure 2 shows the Kaplan-Meier analysis, and Figure 3 shows the relation between administered dose and number of living rats.
Table 1.
Baseline characteristics.
| Group C | Group S | Group L | Group SL | P | |
|---|---|---|---|---|---|
| Ave ±SD (median) | Ave ±SD (median) | Ave ±SD (median) | Ave ±SD (median) | ||
| Age (day) | 137.43±6 (139) | 138.71±3.04 (138) | 138.57±4.76 (140) | 137.71±3.99 (138) | 0.774 |
| Weight (gr) | 212.86±24.3 (200) | 214.29±16.18 (210) | 217.14±17.04 (230) | 218.57±10.69 (220) | 0.942 |
| Respiratory rate (Breaths/min) | 83.14±3.48 (83) | 82.14±3.48 (82) | 83±2.31 (82) | 81±3.61 (82) | 0.649 |
| Temperature (Cº) | 36.51±0.52 (36.4) | 36.43±0.51 (36.4) | 36.4±0.56 (36.4) | 36.6±0.31 (36.5) | 0.882 |
| Time to death (Min) | 21.0±9.57 (19) | 35.57±10.61 (37) | 37.14±16.6 (30) | 49.86±27.56 (37) | 0.049* |
| Lethal dose of Verapamil (mg/kg) | 13.13±5.98 (11.88) | 22.24±6.63 (23.13) | 23.22±10.37 (18.75) | 31.16±17.22 (23.13) | 0.048* |
Kruskal-Wallis Test
p<0.05.
Figure 1.
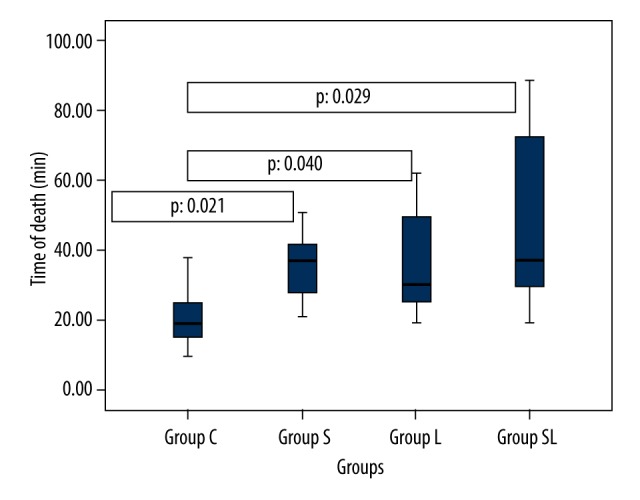
Box plot of time of death by saline, sugammadex, lipid, and sugammadex-lipid group status. Median indicated by black line, and upper and lower quartiles indicated by box edges.
Figure 2.
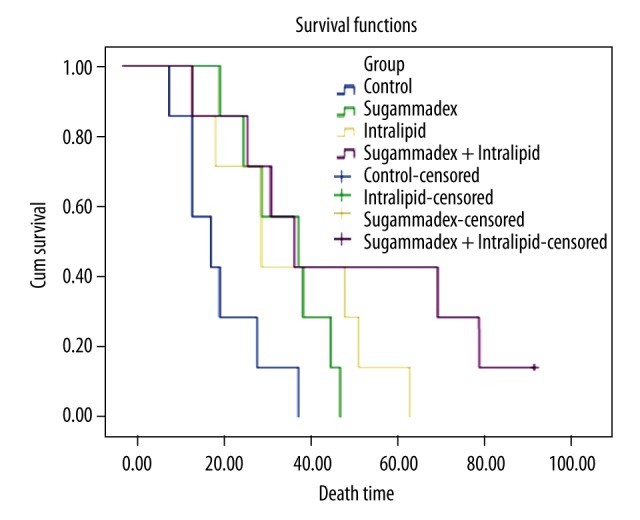
Kaplan-Meier survival analyses of groups.
Figure 3.
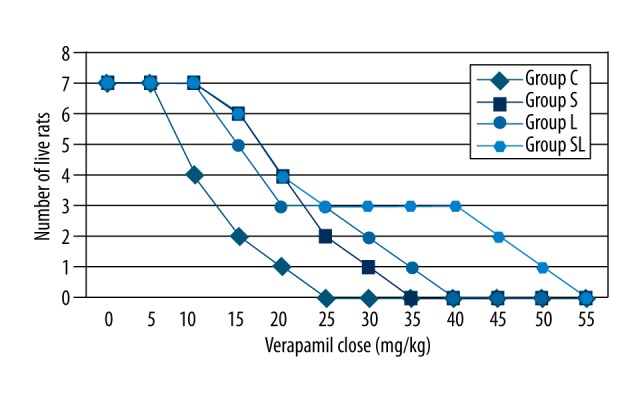
Dose-response curves for all groups.
When time to death data was analyzed, rats in group SL were found to live longer than other groups. While only 1 rat survived longer than 30 minutes in group C, only 2 rats died before 30 minutes in the remaining groups. The number of rats died before 20 minutes was 4 for group C, 1 each for group L and SL, and none in group S (Figures 1, 2).
Tables 2 and 3 show average heart rate and MAP at the time when verapamil infusion was commenced (T0), the time when drugs and/or saline infusion were started (T5), and the time when infusion was discontinued (T10). While there was no difference in heart rates at T0 or T5 between groups, at T10 heart rates of groups S and SL were significantly higher compared to groups C and L (p<0.05) (Table 2). No difference was observed between groups at T0, T5, or T10 for MAP (p>0.5) (Table 3).
Table 2.
Comparison of T0, T5, and T10 heart rates between groups.
| Group C | Group S | Group L | Group SL | 1P | |
|---|---|---|---|---|---|
| Ave ±SD (median) | Ave ±SD (median) | Ave ±SD (median) | Ave ±SD (median) | ||
| T0 | 346.57±52.4 (340) | 368.14±54.62 (382) | 328.86±60.49 (345) | 366.86±103.52 (394) | 0.622 |
| T5 | 309.71±55.61 (302) | 310.86±35.45 (317) | 290.57±69.09 (288) | 309.14±59.17 (321) | 0.911 |
| T10 | 172.86±94.51 (182) | 276.57±49.95 (288) | 185.43±40.44 (197) | 225.57±77.58 (246) | 0.038* |
| 2p | 0.001** | 0.102 | 0.001** | 0.156 |
Kruskal-Wallis Test;
Friedman Test
p<0.05
p<0.01.
Table 3.
Comparison of T0, T5, and T10 MAP between groups.
| Mean arterial pressure (mmHg) | Group C | Group S | Group L | Group SL | 1P |
|---|---|---|---|---|---|
| Ave ±SD (median) | Ave ±SD (median) | Ave ±SD (median) | Ave ±SD (median) | ||
| T0 | 110.01±17.3 (110) | 111.64±25.74 (95.68) | 118.47±15.36 (128.1) | 108.4±16.88 (108) | 0.534 |
| T5 | 95.16±13.68 (97.04) | 97.5±25.53 (88.21) | 94.3±20.07 (94) | 98.31±15.19 (95) | 0.947 |
| T10 | 43.04±25.63 (48) | 71.68±21.38 (72) | 52.6±10.89 (51) | 67.75±18.91 (65.85) | 0.079 |
| 2p | 0.002** | 0.004** | 0.002** | 0.001** |
Kruskal-Wallis Test;
Friedman Test,
p<0.05,
p<0.01.
Heart rates and average MAP from T0 until asystole is shown in Figures 4 and 5. Heart rates and average MAP from T0 until asystole showed a decreasing trend and were similar in all groups. However, a slight increase in heart rate and MAP was seen after sugammadex infusion in group S, and it was stable for longer than in other groups (Figures 3, 4). When compared with the second group, in which sugammadex was administered, heart rate and MAP were slightly higher in group SL than in groups C and L.
Figure 4.
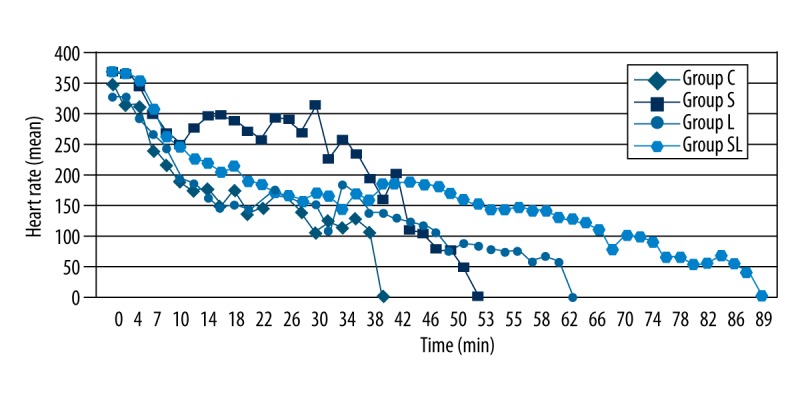
Heart rate versus time for groups.
Figure 5.
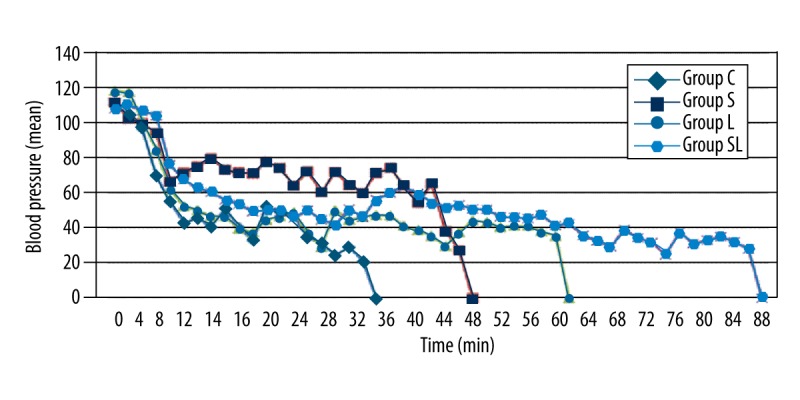
Mean arterial pressure (mmHg) versus time for groups.
ECG analysis showed sinus bradycardia to be the dominant rhythm. In most rats, asystole followed sinus bradycardia. Block or atrial fibrillation was not observed. In many rats, QRS morphology and ventricular escape beats were seen when heart rates fell below 100/m.
No animal had respiratory arrest leading to cardiac arrest. Following asystole, most rats were observed to have some respiratory effort in the form of gasping. Apnea was the last event in all animals.
Discussion
Verapamil overdose is comparatively deadly and there is no effective antidote. Treatment generally involves gastric decontamination and symptomatic treatment to counteract the drug’s negative effects [3–5]. Research continues on drugs that can be used in verapamil toxicity. In intoxications caused by lipophilic drugs, 20% intralipid is frequently used. A study performed in 2003 found that 20% intralipid administered to dogs after bupivacaine-induced cardiac arrest increased the success rate of resuscitation [19]. In 2006, Tebbutt et al. [10] found that intravenous infusion of 20% lipid emulsion increased time to death and LD50 in a rat model of verapamil toxicity. More recently, several case reports and series reported beneficial effects of intravenous lipid emulsion in serious verapamil overdose [5,20–24]. The “lipid sink” theory is the most accepted explanation for why intravenous lipid emulsion seems to be effective in verapamil and other lipophilic drugs [13,22–24].
There are some studies on the effect of cyclodextrins in verapamil toxicity [16–18]. Mottram et al. [16,17] demonstrated prolonged survival in a verapamil toxicity model when Sulfobutylether-β-cyclodextrin (SBE-CD), an agent known to bind to verapamil, was administered. Ozbilgin et al. [18] reported that sugammadex, a modified gamma cyclodextrin, at 16 mg/kg dose, prolonged survival and increased LD in rats when administered for verapamil toxicity, similar to intralipid and SBE-CD.
Use of sugammadex is increasing. It is known to encapsulate steroidal non-depolarizing muscle relaxants used for anesthesia in order to reverse the effect of these drugs [23,25]. Although it encapsulates verapamil 120–700 times less than rocuronium, its beneficial effects have been shown for verapamil toxicity [18,25].
Our study found that when compared to a control group, the administration of intravenous lipid, sugammadex, or both prolonged survival and increased verapamil LD in animals. However, there was no statistically significant difference between survival or LD among groups S, L, and SL. When other groups were compared with group SL for mean survival time, there was a 2.5-fold increase for group C, and a nearly 1.5-fold increase for groups S and L. There is a correlation between our results and those reported by Ozbilgin et al. [18] in terms of survival and verapamil LD in the sugammadex (16 mg/kg) group. Compared to our results, Tebbutt et al. [10] reported survival times that were 4 minutes longer for the control group and 8 minutes longer in the lipid group. This difference could be due to the difference in type of animal and dosage of intraperitoneal ketamine. In our study we used ClinOleic 20% lipid emulsion but Tebbutt et al. [10] used 20% intralipid. The difference in the brand and content of these 2 lipid solutions could also explain the difference in survival. Evans et al. [26] compared these 2 types of lipid emulsions in local anesthetic toxicity and found no significant difference. Research comparing different types of lipid emulsions could be beneficial in toxicology.
There are few experimental studies reporting invasive arterial pressure monitoring in verapamil overdose [15,27]. Apart from comparing survival between groups, we also monitored the changes in heart rate and arterial blood pressure during verapamil infusion to determine the requirement for positive inotropic agents and, if necessary, their timing. When compared to other groups, there was a pronounced difference for group S and a less pronounced difference for group SL for heart rate and MAP. When this data is taken into account, the administration of sugammadex in verapamil overdose could lead to lower requirement for inotropic agents. Similarly, Ozbilgin et al. [18] found that heart rate decrease was least in the sugammadex 16 mg/kg group. Because sugammadex does not seem to affect hemodynamics when used to reverse the effect of steroidal muscle relaxants in anesthesia, this effect could be due to the rapid binding of sugammadex to verapamil in the circulatory system. However, our data suggest that less positive inotropic agents are be required when only sugammadex or sugammadex plus 20% lipid emulsion is infused for verapamil overdose.
The suggested dosage for sugammadex, which this study and a previous study [18] has demonstrated to increase survival for verapamil toxicity in rats, is 1–4 mg/kg doses for reversing the effect of nondepolarizing muscle relaxants, although doses of 8–16–32 mg/kg can be used for reversal deep neuromuscular blockage. In accordance with data from Ozbilgin et al. [18] that shows efficacy for verapamil toxicity in rat model, we used 16 mg/kg dosage in this study. Although our data has demonstrated that these doses increase survival in rats and keep hemodynamic parameters more stable for a longer time, most possibly through its effect on binding verapamil. However, further studies are required to determine the optimum dosage for its clinical use in humans.
The reported dosage of 20% lipid emulsion for rat models of verapamil toxicity is 12.4 ml/kg [10]. However, its usage in human toxicity is different and it is generally used as a 24-hour or longer continuous infusion following a bolus dose [29,30]. There are no verapamil toxicity rat models with lipid interventions as a continuous infusion following a bolus. Several studies have demonstrated the effect of lipid emulsion for verapamil toxicity in humans [5,29,30].
In our study we compared the use of sugammadex alone, lipid emulsion alone, and the combination of sugammadex and lipid emulsion. Our study is the first to combine sugammadex and lipid emulsion. Although we found that survival was increased in all groups when compared to the control group, there was no statistically significant difference for the combination of sugammadex and lipid emulsion.
Limitations
It is possible that intraperitoneal ketamine used for anesthesia in rats could lead to hemodynamic changes. However, procedures such as insertion of femoral artery and venous cannula, plus the requirement for rats to stay immobile during infusion, lead to the necessity of anesthesia.
The lack of mechanical ventilation support for animals in this study is another limitation. Although this study was planned to include mechanical ventilation support with blood gasses and blood glucose monitoring throughout all procedures, technical difficulties and financial limitations did not make this possible. However, if we do not take into account any effect of bradypnea, and considering that the last event for all rats was respiratory arrest, we do not believe that mechanical ventilation would have changed the end results.
We were not able to determine the effect of early complications leading to death in this study. We could not exclude rats that had adverse effect to intravenous lipid emulsion, such as hypersensitivity reaction or pulmonary or cerebral fat emboli in groups L and SL [10,21–23,28].
Sprague-Dawley rats have no or at most a very short calcium-dependent phase II action potential plateau and may not reproduce the cardiotoxic effects of CCBs seen in humans. Treatments found to have good efficacy in other animal models (such as high-dose insulin) were not tested. Monitoring could have been improved by including pressure-volume assessment to estimate LV contractility, and blood verapamil concentrations.
Although there are reports on the use of lipid emulsion in verapamil toxicity in humans, there are no reports for the use of sugammadex. Therefore, further studies are required to demonstrate the effectiveness and optimal dosage of sugammadex for verapamil toxicity in humans, as well as evaluating the combination of sugammadex and lipid emulsion.
Conclusions
This study demonstrated that rat survival in verapamil toxicity is prolonged with the application of intravenous lipid emulsion and sugammadex separately or as combined treatment. In all cases, the cardiotoxic effect of verapamil was delayed. The administration of sugammadex at 16 mg/kg alone or combined with 20% intravenous lipid emulsion could lead to a lessened requirement for positive inotropic agents. The clarification of the mechanism of these effects requires larger studies.
Footnotes
Conflict of Interest
The authors report no conflict of interests.
Source of support: Departmental sources
Financial support
The authors have no financial interests related to this work.
References
- 1.Mowry JB, Spyker DA, Cantilena LR, et al. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol. 2014;52:1032–83. doi: 10.3109/15563650.2014.987397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charpentier C, Flandrois M, Labombarda F, et al. [Verapamil intoxication: Beware of the delayed effect]. Arch Pediatr. 2014;21:1344–47. doi: 10.1016/j.arcped.2014.09.013. [in French] [DOI] [PubMed] [Google Scholar]
- 3.Mégarbane B, Karyo S, Abidi K, et al. Predictors of mortality in verapamil overdose: usefulness of serum verapamil concentrations. Basic Clin Pharmacol Toxicol. 2011;108:385–89. doi: 10.1111/j.1742-7843.2010.00666.x. [DOI] [PubMed] [Google Scholar]
- 4.Lip GY, Ferner RE. Poisoning with anti-hypertensive drugs: calcium antagonists. J Hum Hypertens. 1995;9:155–61. [PubMed] [Google Scholar]
- 5.St-Onge M, Dubé P-A, Gosselin S, et al. Treatment for calcium channel blocker poisoning: a systematic review. Clin Toxicol. 2014;52:926–44. doi: 10.3109/15563650.2014.965827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akıncı E, Akıllı NB, Koylu R, Gonen MO. Successful resuscitation of a patient with continuous venovenous hemodiafiltration following intoxication from verapamil and trandolapril. Kaohsiung J Med Sci. 2014;30:321–22. doi: 10.1016/j.kjms.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Janion M, Stepień A, Sielski J, Gutkowski W. Is the intra-aortic balloon pump a method of brain protection during cardiogenic shock after drug intoxication? J Emerg Med. 2010;38:162–67. doi: 10.1016/j.jemermed.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 8.Daubin C, Lehoux P, Ivascau C, et al. Extracorporeal life support in severe drug intoxication: a retrospective cohort study of seventeen cases. Crit Care. 2009;13:R138. doi: 10.1186/cc8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masson R, Colas V, Parienti J-J, et al. A comparison of survival with and without extracorporeal life support treatment for severe poisoning due to drug intoxication. Resuscitation. 2012;83:1413–17. doi: 10.1016/j.resuscitation.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Tebbutt S, Harvey M, Nicholson T, Cave G. Intralipid prolongs survival in a rat model of verapamil toxicity. Acad Emerg Med. 2006;13:134–39. doi: 10.1197/j.aem.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Aggarwal N, Kupfer Y, Seneviratne C, Tessler S. Methylene blue reverses recalcitrant shock in β-blocker and calcium channel blocker overdose. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2012-007402. pii: bcr2012007402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graudins A, Wong KKL. Comparative hemodynamic effects of levosimendan alone and in conjunction with 4-aminopyridine or calcium chloride in a rodent model of severe verapamil poisoning. J Med Toxicol. 2010;6:85–93. doi: 10.1007/s13181-010-0073-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang C, Kim DH, Kim SC, et al. The effects of intravenous lipid emulsion on prolongation of survival in a rat model of calcium channel blocker toxicity. Clin Toxicol (Phila) 2015;53(6):540–44. doi: 10.3109/15563650.2015.1045979. [DOI] [PubMed] [Google Scholar]
- 14.Abraham MK, Scott SB, Meltzer A, Barrueto F., Jr Levosimendan does not improve survival time in a rat model of verapamil toxicity. J Med Toxicol. 2009;5:3–7. doi: 10.1007/BF03160973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurola J, Jouni K, Heli L, et al. Effect of levosimendan in experimental verapamil – induced myocardial depression. Scand J Trauma Resusc Emerg Med. 2010;18:12. doi: 10.1186/1757-7241-18-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mottram AR, Bryant SM, Aks SE. Dose-dependent response to cyclodextrin infusion in a rat model of verapamil toxicity. West J Emerg Med. 2012;13:63–67. doi: 10.5811/westjem.2011.3.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mottram AR, Bryant SM, Aks SE. Effect of cyclodextrin infusion in a rat model of verapamil toxicity. Am J Ther. 2011;18:371–74. doi: 10.1097/MJT.0b013e3181ea3173. [DOI] [PubMed] [Google Scholar]
- 18.Ozbilgin S, Ozbilgin M, Kucukoztas B, et al. Evaluation of the effectiveness of sugammadex for verapamil intoxication. Basic Clin Pharmacol Toxicol. 2013;113:280–85. doi: 10.1111/bcpt.12089. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg G, Ripper R, Feinstein DL, Hoffman W. Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity. Reg Anesth Pain Med. 2003;28:198–202. doi: 10.1053/rapm.2003.50041. [DOI] [PubMed] [Google Scholar]
- 20.Sebe A, Dişel NR, Açıkalın Akpınar A, Karakoç E. Role of intravenous lipid emulsions in the management of calcium channel blocker and β-blocker overdose: 3 years experience of a university hospital. Postgrad Med. 2015;127:119–24. doi: 10.1080/00325481.2015.1012480. [DOI] [PubMed] [Google Scholar]
- 21.Doepker B, Healy W, Cortez E, Adkins EJ. High-dose insulin and intravenous lipid emulsion therapy for cardiogenic shock induced by intentional calcium-channel blocker and Beta-blocker overdose: a case series. J Emerg Med. 2014;46:486–90. doi: 10.1016/j.jemermed.2013.08.135. [DOI] [PubMed] [Google Scholar]
- 22.Cao D, Heard K, Foran M, Koyfman A. Intravenous lipid emulsion in the emergency department: a systematic review of recent literature. J Emerg Med. 2015;48:387–97. doi: 10.1016/j.jemermed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Presley JD, Chyka PA. Intravenous lipid emulsion to reverse acute drug toxicity in pediatric patients. Ann Pharmacother. 2013;47:735–43. doi: 10.1345/aph.1R666. [DOI] [PubMed] [Google Scholar]
- 24.Ozcan MS, Weinberg G. Intravenous lipid emulsion for the treatment of drug toxicity. J Intensive Care Med. 2012;29:59–70. doi: 10.1177/0885066612445978. [DOI] [PubMed] [Google Scholar]
- 25.Ledowski T, Ong JS, Flett T. Neuromuscular monitoring, muscle relaxant use, and reversal at a tertiary teaching hospital 2.5 years after introduction of sugammadex: changes in opinions and clinical practice. Anesthesiol Res Pract. 2015;2015:367937. doi: 10.1155/2015/367937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans JA, Wallis SC, Dulhunty JM, Pang G. Binding of local anaesthetics to the lipid emulsion Clinoleic™ 20% Anaesth Intensive Care. 2013;41:618–22. doi: 10.1177/0310057X1304100507. [DOI] [PubMed] [Google Scholar]
- 27.Lynch MJ, Katz KD, Callaway CW, Logue ES. Survival of verapamil-poisoned rats treated with triiodothyronine. J Med Toxicol. 2010;6:94–99. doi: 10.1007/s13181-010-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine M, Skolnik AB, Ruha A-M, et al. Complications following antidotal use of intravenous lipid emulsion therapy. J Med Toxicol. 2014;10:10–14. doi: 10.1007/s13181-013-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin C, Gonzalez H, Ruiz S, et al. Acute respiratory distress syndrome following verapamil overdose treated with intravenous lipid emulsion: a rare life-threatening complication. Ann Fr Anesth Reanim. 2014;33(6):e101–2. doi: 10.1016/j.annfar.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Sampson CS, Bedy SM. Lipid emulsion therapy given intraosseously in massive verapamil overdose. Am J Emerg Med. 2015 doi: 10.1016/j.ajem.2015.04.061. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


