Summary
Background
The aim of the paper was to compare radiographs and MRI in assessment of active and chronic inflammatory changes in the sacroiliac joints in patients with chronic back pain and suspected axial spondyloarthritis.
Moreover, the aim was to determine which of the two methods is more accurate in diagnosing individual inflammatory changes in the sacroiliac joints and whether there is a correlation between radiographs and MRI in their identification.
Material/Methods
The analysis was conducted in a group of 101 patients, including 61 women and 40 men, referred to radiographs and MR examinations by rheumatologists due to chronic back pain.
AP images of the lumbar region of the spine were performed in each patient in the supine position. The images included the sacroiliac joints. Changes in the SIJs were assessed based on the New York criteria of 1966. In MR examination, the SIJs were assessed in terms of the presence of active and chronic inflammatory changes described by the ASAS. The statistical analysis of the variables tested was conducted in the Excel and Statistica systems.
Results
In relation to the final clinical diagnosis of axSpA, MRI had higher sensitivity and specificity than radiography in diagnosing sacroiliitis (sensitivity: 71% vs. 22%, specificity: 90% vs. 94% on radiographs according to New York criteria. In relation to MRI, radiographs resulted in 40% of incorrect sacroiliitis diagnoses (both false positive and false negative results). In as many as 50% of cases (7/14), MRI failed to confirm the presence of inflammatory changes in the sacroiliac joints observed in radiography according to the modNY criteria (false positive results on radiographs).
Both examinations are characterised by very low agreement, which is near to random, in assessing individual features of sacroiliitis, such as sclerosis, change in the joint space width, erosions and ankylosis.
Conclusions
1. Radiographs do not allow early inflammatory lesions indicating sacroiliitis to be diagnosed, which leads to diagnostic delay. MRI is the method of choice in diagnosing early sacroiliitis and detecting structural lesions, in particular sclerosis and erosions. 2. Radiographs and MRI are characterised by low, near to random, agreement in the detectability of the individual inflammatory changes in the sacroiliac joints.
MeSH Keywords: Magnetic Resonance Imaging, Sacroiliac Joint, Sacroiliitis, Spondylarthropathies, X-Ray Film
Background
Spondyloarthritis (SpA) is a group of inflammatory rheumatoid diseases which includes five disease entities: ankylosing spondylitis (AS), reactive arthritis, psoriatic arthritis, arthritis associated with inflammatory bowel diseases (IBD) and undifferentiated spondyloarthritis [1]. Depending on the prevailing symptoms associated with musculo-skeletal system involvement, each of the aforementioned entities may have an axial or peripheral form.
In axial SpA (axSpA), the sacroiliac joints (SIJs) become inflamed first. Radiographs, apart from clinical assessment, have been the basis for diagnosing sacroiliitis for many years. Due to their unsatisfactory diagnostic value (particularly in initial stages of the disease), the ASAS (the Assessment of SpondyloArthritis International Society) introduced new classification criteria for sacroiliitis in 2009. Apart from radiographic findings, they also included magnetic resonance imaging (MRI) of the SIJs [2].
The ASAS classification criteria for axSpA are used in patients with chronic back pain (CBP) that persists for more than 3 months and the first occurrence of which was before the age of 45 [1,3]. The further diagnostic process of axSpA may have two dimensions: clinical arm or imaging arm.
As for the imaging arm, the disease can be diagnosed based on sacroiliitis features seen on radiographs or MR images together with at least one clinical sign of spondyloarthritis present [2]. Sacroiliitis is diagnosed in radiography based on the modified New York (modNY) criteria for ankylosing spondylitis [2,4]. On MRI images, bone marrow oedema (BME) is a necessary criterion to diagnose sacroiliitis [5–7].
Currently, numerous research teams are conducting studies on the diagnostic value of the ASAS classification criteria for axSpA. Their results indicate that MRI is superior to X-ray in diagnosing and monitoring early axSpA and confirm the superiority of radiographs in assessing chronic inflammatory changes.
Aim
The aim of the paper was to compare radiographs and MRI in assessment of active and chronic inflammatory changes in the sacroiliac joints in patients with chronic back pain and suspected axial spondyloarthritis as well as to determine which of the two methods is more accurate in diagnosing individual inflammatory changes in the sacroiliac joints.
Material and Methods
The analysis was conducted in a group of 101 patients, including 61 females and 40 males, referred to radiographic and MR examinations of the sacroiliac joints by rheumatologists due to chronic back pain [2]. The age of the subjects ranged from 19 to 71 years (lower quartile Q1 29 years, upper quartile Q3 52 years).
Methods to assess radiographs of the sacroiliac joints
The radiographs were performed with the use of a DRX-Evolution Carestream system. AP images of the lumbar spine were performed in each patient in the supine position. The images included the sacroiliac joints, lumbar spine and the last vertebrae of the thoracic region of the spine.
The SIJs were assessed with the use of the New York criteria of 1966 (Table 1) which are the basis for the radiological classification criteria for axial SpA of ASAS [4].
Table 1.
New York criteria for sacroiliitis.
| Grade | Evaluation |
|---|---|
| 0 | Normal sacroiliac joints |
| I | Suspected changes – blurry margins of the joint space |
| II | Minimal changes – visible periarticular sclerosis, single erosions; only when the joint space is blurred, with no change in joint space width |
| III | Advanced changes – numerous erosions, distinct periarticular sclerosis, widened articular space, possible partial bony ankylosis |
| IV | Total bony ankylosis |
Methods to assess MRI of the sacroiliac joints
MRI images were obtained with the use of a Magnetom Avanto Syngo B17 scanner by Siemens using a Body Matrix coil (Tim Coil). The examination protocol included:
pilot sequences in coronal, transverse and sagittal planes in order to plan the examination;
T2-weighted images in the sagittal plane;
T1-weighted, T1FS, T2-weighted, T2TIRM images in the coronal oblique plane parallel to the posterior aspect of the S2 vertebral body;
PD-weighted images in the transverse oblique plane perpendicular to the posterior aspect of the S2 vertebral body;
contrast-enhanced fat-saturated T1-weighted images in the coronal oblique plane conducted only if bone marrow oedema was identified or if high signal was recorded in the joint space or/and in the syndesmosis in T2-weighted and T2TIRM images.
The sacroiliac joints were assessed in terms of the presence of active and chronic inflammatory changes described in the ASAS criteria (Table 2) [2–7].
Table 2.
Division of inflammatory changes in the sacroiliac joints in MRI according to the ASAS.
| Active inflammatory changes | Chronic inflammatory changes |
|---|---|
| Bone marrow oedema/osteitis | Subchondral sclerosis |
| Capsulitis | Erosions |
| Synovitis | Fatty transformation of the bone marrow |
| Enthesitis | Bony bridges/ankylosis |
In total, 202 joints were evaluated in search for the following changes:
bone marrow oedema;
synovitis;
enthesitis;
capsulitis;
subchondral sclerosis;
change in joint space width (dilatation/narrowing);
erosions;
fatty transformation of the bone marrow;
osseous bridges, bony ankylosis.
The following features were evaluated in the comparative analysis of radiography and MRI:
blurred margins of the joint space visible on radiographs with bone marrow oedema in MRI;
subchondral sclerosis;
change in joint space width;
erosions;
bony ankylosis.
The study was approved by the Ethics Committee of the Warsaw Medical University.
Statistical methods
The statistical analysis of the variables tested was conducted in the Excel and Statistica (version 10) systems. In order to compare the two diagnostic methods, the authors calculated the percentage agreement and the Cohen’s kappa coefficient at 95% confidence level (95% CI). The value of the Cohen’s kappa coefficient within the range 0–1 was interpreted on the basis of the scale proposed by J.R. Landis and G.G. Koch [8].
Results
Results of the analysis of SIJs radiographs and MRI in assessment of active and chronic inflammatory changes in the sacroiliac joints in patients with chronic back pain were presented in Tables 3–6.
Table 3.
Comparison of X-ray and MRI examinations in identifying inflammatory changes with the use of the Cohen’s kappa coefficient.
| MRI | Total | |||
| Radiographs | Blurred margins/oedema | Absent | Present | |
| Absent | 105 | 36 | 141 | |
| Present | 29 | 26 | 55 | |
| Total | 134 | 62 | 196 | |
| Comparison | Radiographs and MRI agreement=67% Cohen’s kappa coefficient=0.21; CI: 0.05–0.37 |
|||
| MRI | Total | |||
| Radiographs | Subchondral sclerosis | Absent | Present | |
| Absent | 76 | 59 | 135 | |
| Present | 17 | 44 | 61 | |
| Total | 93 | 103 | 196 | |
| Comparison | Radiographs and MRI agreement=61% Cohen’s kappa coefficient=0.24; CI: 0.11–0.37 |
|||
| MRI | Total | |||
| Radiographs | Change in joint space width | Absent | Present | |
| Absent | 172 | 6 | 178 | |
| Present | 12 | 6 | 18 | |
| Total | 184 | 12 | 196 | |
| Comparison | Radiographs and MRI agreement=91.0% Cohen’s kappa coefficient=0.35;CI: 0.07–0.64 |
|||
| MRI | ||||
| Radiographs | Erosions | Absent | Present | Total |
| Absent | 164 | 26 | 190 | |
| Present | 3 | 3 | 6 | |
| Total | 167 | 29 | 196 | |
| Comparison | Radiographs and MRI agreement=85% Cohen’s kappa coefficient=0.13;CI: 0.0–0.42 |
|||
| MRI | ||||
| Radiographs | Bony Ankylosis | Absent | Present | Total |
| Absent | 193 | 2 | 195 | |
| Present | 0 | 1 | 1 | |
| Total | 193 | 3 | 196 | |
| Ccomparison | Radiographs and MRI agreement=99.0% Cohen’s kappa coefficient=0.5; CI: 0.0–1.0 |
|||
Table 4.
Comparison of radiographs according to the modNY criteria with MRI.
| MRI | Total | |||
| X-ray according to modNY | No of patients | Normal image | Sacroiliitis | |
| Normal image | 53 (53%) | 33 (33%) | 86 (86%) | |
| Sacroiliitis | 7 (7%) | 7 (7%) | 14 (14%) | |
| Total | 60 (60%) | 40 (40%) | 100 (100%) | |
| Comparison | X-ray and MRI agreement=60% Cohen’s kappa coefficient=0.06; CI: 0–0.29 |
|||
CI – confidence interval.
Table 5.
Sacroiliitis diagnosis in relation to the final clinical diagnosis.
| Patients | Total | |||
| Radiographs +/− ray according to modNY | SIJ image | No axSpA | axSpA | |
| Normal | 47 (47%) | 39 (39%) | 86 (86%) | |
| Sacroiliitis | 3 (3%) | 11 (11%) | 14 (14%) | |
| Total | 50 (50%) | 50 (50%) | 100 (100%) | |
| Comparison | Sensitivity 22% (11/50), positive predictive value 79% (11/14), LR+ 3.67 Specificity 94% (47/50), negative predictive value 55% (47/86), LR – 0.83 |
|||
| Patients | Total | |||
| MRI | SIJ image | No axSpA | axSpA | |
| Normal | 45 (45%) | 15 (15%) | 60 (59%) | |
| Sacroiliitis | 5 (5%) | 36 (35%) | 41 (41%) | |
| Total | 50 (50%) | 51 (50%) | 101 (100%) | |
| Comparison | Sensitivity 71% (36/51), positive predictive value 88% (36/41), LR+ 7.1 Specificity 90% (45/50), negative predictive value 75% (45/60), LR –0.32 |
|||
Table 6.
Individual features on radiographs and in MRI in relation to the final clinical diagnosis.
| Patients | Total | Sensitivity | Specificity | Predictive value | ||||
|---|---|---|---|---|---|---|---|---|
| No axSpA | axSpA | Positive | Negative | |||||
| X-ray | Subchondral sclerosis | 16 (39%) | 25 (61%) | 41 (41%) | 50% | 68% | 61% | 58% |
| MRI | Subchondral sclerosis | 25 (42%) | 35 (35%) | 60 (59%) | 69% | 50% | 58% | 61% |
| X-ray | Erosions | 1 (2%) | 4 (8%) | 5 (5%) | 8% | 98% | 80% | 52% |
| MRI | Erosions | 3 (13%) | 20 (87%) | 23 (23%) | 39% | 94% | 87% | 60% |
| X-ray | Subchondral sclerosis | 1 (39%) | 25 (61%) | 41 (41%) | 50% | 68% | 61% | 58% |
| MRI | Change in joint space width | 1 (10%) | 9 (90%) | 10 (10%) | 18% | 98% | 90% | 54% |
| X-ray | Bone ankylosis | 0 (0%) | 1 (100%) | 1 (1%) | 2% | 100% | 100% | 51% |
| MRI | Bone ankylosis | 1 (25%) | 3 (75%) | 4 (4%) | 6% | 98% | 75% | 51% |
| MRI | Active erosions | 3 (14%) | 18 (86%) | 21 (21%) | 35% | 94% | 86% | 59% |
| All joints | 50 (50%) | 50 (52%) | 100 (100%) | |||||
Comparative analysis of individual inflammatory changes in the SIJs identified on radiographs and MR images was presented in Table 3.
Evaluation of agreement in identifying inflammatory changes in the SIJs on radiographs vs. MR images was presented in Table 4. The agreement of the two methods was obtained in 60% of patients (Cohen’s kappa coefficient was 0.06) (Table 4l Figures 1A–1D, 2A–2E).
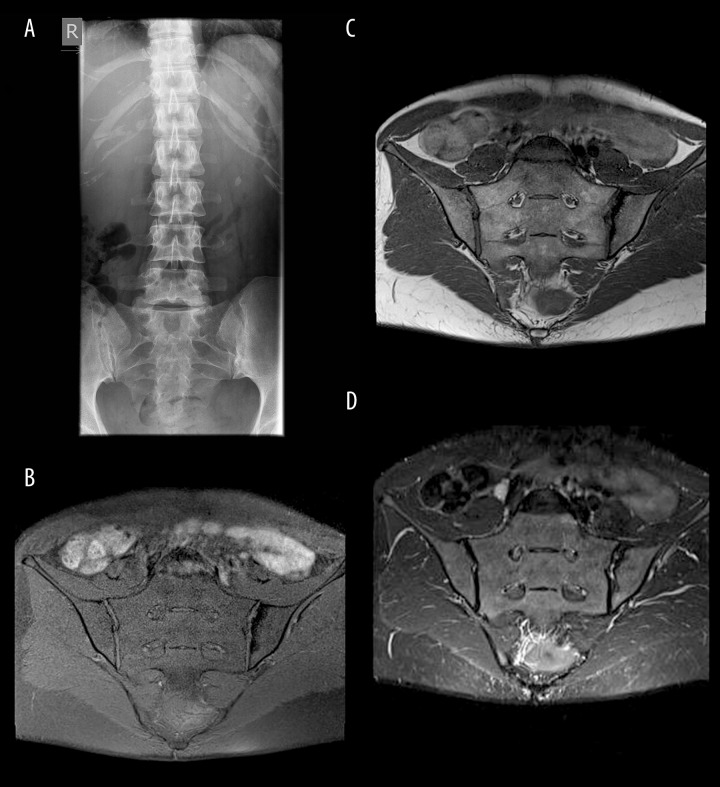
Figure 1.
(A) SIJs radiograph: the right joint - blurry margins, visible subchondral sclerosis, slightly dilated joint space in its postero-inferior part – grade II according to the NY criteria; left joint – blurry, uneven margins of the joint with erosions and subchondral sclerosis – grade III according to the NY criteria; (B–D) SIJs MRI, TSE sequences, coronal oblique planes in T1FS-weighted (B), T1-weighted (C) and T2 TIRM (D) images: right joint – normal; left joint – no active inflammatory changes, the only abnormality is subchondral sclerosis in the left ilium.
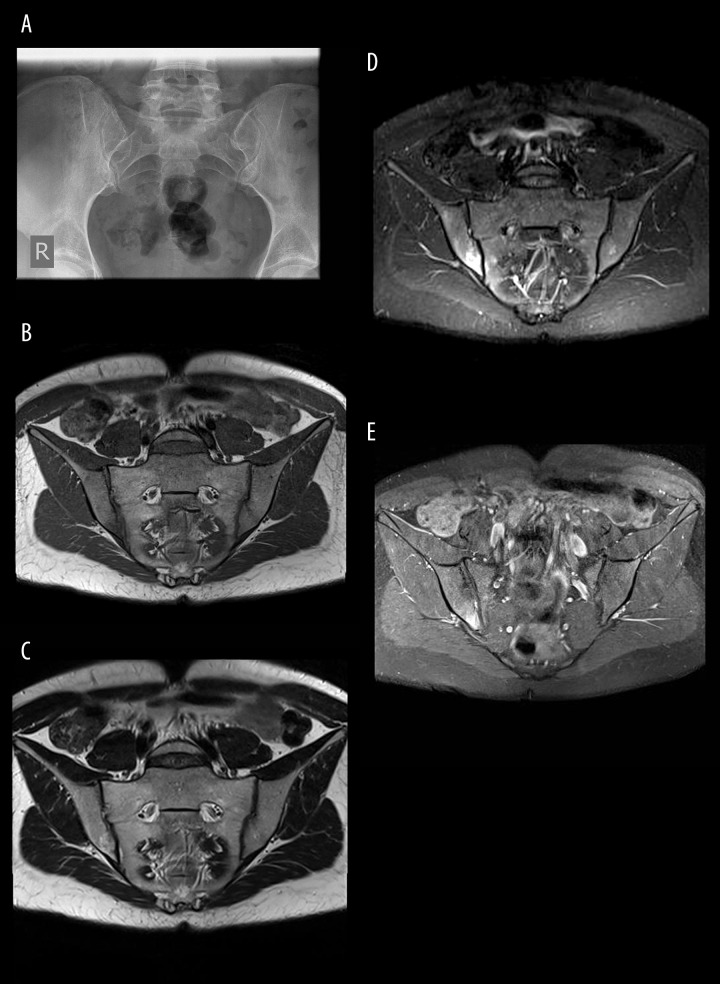
Figure 2.
(A) SIJs radiograph: normal image of the SIJs – grade 0 sacroiliitis according to the NY criteria. (B–E) SIJs MRI, TSE sequences, coronal oblique planes in T1-weighted (B), T2-weighted (C), T2 TIRM (D) and T1 FS CM (E) images: bone marrow oedema in the right SIJ that mainly encompasses the ilium and to a lesser extent the sacrum.
Diagnostic value of MRI and radiography in relation to the final clinical diagnosis of axSpA was shown in Table 5. The sensitivity and specificity of the two imaging examinations in relation to the final clinical diagnosis of axSpA were (Table 5):
22% and 94% respectively for radiographs according to the modNY criteria;
71% and 90% respectively for MRI.
Finally, Table 6 presents the sensitivity and specificity of the individual inflammatory changes in the SIJs on radiographs and in MRI in relation to the final clinical diagnosis of axSpA.
Discussion
The basic radiological sign that allows sacroiliitis to be diagnosed in MRI is bone marrow oedema (BME) [2–10]. It is visible as an area of increased signal intensity in T2-weighted images with or without fat saturation and in T2 TIRM images. The development of BME probably results from the presence of cellular infiltrates and hyperaemia which cause pathological accumulation of fluid in tissues. These regions are hypointense in T1-weighted and T1FS images and undergo enhancement following contrast agent administration, which attests to the presence of inflammatory vessels in the given area [2–7].
It might be hypothesised that bone marrow oedema observed in MRI can correlate with the presence of blurry joint space in radiography. It is believed that this earliest radiographic sign of sacroiliitis results from the loss of cortical layer margin and, probably, from resorption and demineralisation of the subcortical cancellous bone. As a result, periarticular osteoporosis develops [11]. As in the case of BME in MRI, it probably results from the presence of inflammatory infiltrates in the subchondral layer of the bone marrow. In the authors’ own studies, the correlation of BME (on MRI images) and blurry joint space margins (on radiographs) was low and very low (κ=0.21) (Table 3). Thus, it was not confirmed that blurry margins of the joint space correlate with BME in MRI.
In relation to MRI, which is considered to be superior to radiography in identifying sacroiliitis, the percentage of erroneous diagnoses of sacroiliitis in radiography according to the modNY criteria reached 40% (40/100). This included 7% (7/100) of patients with false positive results and 33% (33/100) of patients with false negative results. MRI failed to confirm the presence of inflammatory changes in the SIJs which were observed on radiographs according to the modNY criteria in as many as 50% (7/14) of cases (Table 4). These results are of considerable significance in the light of further management and indicate that basing the diagnosis on radiographs according to the current ASAS classification criteria carries the risk of establishing an erroneous diagnosis of sacroiliitis. This is also confirmed by recent analyses [12,13]. Van der Berg et al. [12] found that in patients with a recent-onset inflammatory back pain, both trained readers and local rheumatologists and radiologists agreed only moderately on the recognition of radiographic sacroiliitis. Also, a significant proportion of locally recognized ankylosing spondylitis patients were not confirmed as having AS by central reading (false positive), while a small minority of patients were false negative, indicating the necessity of re-evaluating the role of radiographic sacroiliitis as a diagnostic criterion for axial SpA [12].
Subchondral sclerosis is the most frequently diagnosed chronic change in the SIJs. In the authors’ own study, it was seen on radiographs in 61 of 196 joints (31%), and on MRI scans – in 103 of 196 joints (53%) (Table 3). The sensitivity of X-ray in relation to MRI was 43% (44/103), and the specificity was 82% (76/93) (Table 3), which indicates that MRI is superior to radiographs in terms of the detectability of sclerotic changes. Puhakka et al. [14] found sclerosis in 29 of 41 patients with the diagnosed disease (71%). In own study, the results were similar: MRI detected sclerosis in 60 of 100 patients (60%), including 35 cases with clinically confirmed disease (58%) (sensitivity – 69%, specificity – 50%) (Table 6). On radiographs, sclerosis in the SIJs was observed in 41 of 100 patients (38%), including 25 with ultimately confirmed axSpA (61%) (sensitivity and specificity: 50% and 68%) (Table 6). The agreement of both methods in the assessment of subchondral sclerosis was 61%, and the Cohen’s kappa coefficient reached κ=0.24 (Table 3). The agreement of both examinations was low, which – considering all the data – is the highest result achieved so far.
The presence of erosions in SIJs is another chronic and specific feature of axSpA [15]. MRI and CT are characterised by higher sensitivity in visualising them than radiography [5,6,7,14]. Moreover, MRI detects them earlier than radiographs [15,16]. The authors’ own results confirm these findings. MRI detected significantly more erosions than radiographs. This concerned both the entire examined group and patients with confirmed axSpA. In relation to the clinical diagnosis, the sensitivity and specificity of MRI in detecting erosions were 39% and 94% respectively whereas the respective values for radiography reached merely 8% and 98% (Table 6). According to the literature reports, erosions are found in MRI in 73.1–83% of the joints in patients with axSpA [11,14,]. In this study, MRI visualised erosions in 15% of the joints (29/196) (Table 3), i.e. in 23 patients, including 20 patients with finally diagnosed SpA (39%) (Table 6) whereas radiography visualised the pathology in merely 3% of the joints (6/196), i.e. in 5 patients, including 4 patients with the clinically confirmed disease (8%). In the study conducted by Weber et al. [15] MRI detected erosions in 90.7% of patients with confirmed axSpA, 3.8% of patients with chronic back pain but without confirmed axSpA, and in 1.7% of healthy individuals. In the authors’ own study, MRI detected erosions in 6% (3/50) of patients with and without axSpA (Table 6) whereas radiography visualised the pathology in 2% of cases (1/50) (Table 3). The agreement of both methods in detecting erosions was very low (κ=0.13) despite the percentage agreement of 85% which reflects the high number of joints without erosions in both examinations (Table 3).
According to certain reports, the addition of erosions to the ASAS criteria (which currently include only active inflammatory changes) would increase the sensitivity of the criteria from 67% to 81% with no decline in their specificity [15].
Another element analysed in this study was change in joint space width, i.e. its dilatation or narrowing, which was detected in MRI in 6% of the joints (12/196) (Table 3) in 10 of 101 patients (10%), including 9 patients with clinically confirmed axSpA (sensitivity and specificity were: 18% and 98% respectively, PPV and NPV: 90% and 54% respectively) (Table 6). Puhakka et al. [14] detected joint space width change in 26 of 41 patients in MRI (63%). In the authors’ own study, radiography revealed change in joint space width in 9% of the joints (18/196) (Table 3), i.e. in 11 patients, including 9 patients with the clinically confirmed disease. The sensitivity and specificity of radiography was comparable to MRI: 18% and 96% (PPV and NPV: 82% and 53%) (Table 6). The results indicate that the lack of change in joint space width does not rule out the disease, but its presence strongly correlates with it. The agreement of radiographs and MR images in terms of joint space width assessment was 91% and the Cohen’s kappa coefficient was low and amounted to κ=0.35 (Table 3). These relatively good results (compared to low agreement of radiographs and MRI observed in the analyses of other parameters) probably result from the fact that most patients showed no changes in the width of joint space (MRI: 94%, 184/196, radiographs: 90%, 178/196). Both methods were consistent in confirming the presence of normal joint space width in 172 of 196 joints evaluated in both examinations (88%) and its changes in merely 6 joints (3%) (Table 3).
As for the detectability of the most advanced structural lesions, i.e. partial or total bony ankylosis, this study did not confirm the opinions on the higher diagnostic value of radiography [2]. Partial bony ankylosis was found in MRI in merely 2% of the joints (3/196) (Table 3), in 4 of 100 patients (4%), including 3 patients with the clinically confirmed disease (sensitivity and specificity were: 6% and 98% respectively; PPV and NPV: 75% and 51% respectively) (Table 6). Radiographs revealed SIJ ankylosis in merely 1 joint (0.5%, 1/196) (Table 3) (sensitivity and specificity of X-ray: 2% and 100%; PPV and NPV: 100% and 51%) (Table 6). Song et al. [11] and Puhakka et al. [14] also found bony ankylosis in MRI in only single cases: in 6.9% of the joints [11] and in 1 patient (2%) [14]. In the authors’ own material, the agreement of radiography and MRI in detecting bony ankylosis was moderate (κ=0.49; agreement: 99%) (Table 3). Moreover, in the group of 15 patients without the signs of sacroiliitis on radiographs and MR examinations but with a disease confirmed on the basis of a positive HLA-B27 test and additional clinical features (fulfilling the clinical arm of the ASAS criteria), one patient showed bony ankylosis and fatty bone marrow transformation in MR examination without active inflammatory changes. This is merely one case but, at the same time, another example suggesting that it is needed to include chronic inflammatory features in the interpretation of MRI images while diagnosing axSpA.
In relation to the final clinical diagnosis of axSpA, MRI was characterised by superior sensitivity and specificity than radiography in diagnosing sacroiliitis (sensitivity and specificity were: 71% and 90% vs. 22% and 94% respectively) (Table 5).
Summary
The comparative analysis of radiographs and MR examinations revealed their very low or low agreement in identifying inflammatory changes in the SIJs. The highest agreement was noted in visualising sclerosis, the level of which was, however, still low (κ=0.24). It was followed by blurry joint space margins observed on radiographs and bone marrow oedema seen in MRI (which is hypothetically consistent with it). Their coexistence was low or very low (κ=0.21).
The comparative analysis of SIJs radiographs and MRI revealed that MRI is a good method to evaluate not only active inflammatory changes, such as bone marrow oedema, capsulitis, synovitis and enthesitis, but also structural lesions, such as sclerosis, erosions and partial or total bony ankylosis, which in this study were detected in a greater number of patients by MRI than by radiography. This is consistent with only single published studies [12,14]. Based on our own results, it can be stated that the addition of structural lesions to axSpA classification criteria could considerably improve the detectability of axSpA. Moreover, the position of radiography in axSpA diagnosis also needs re-evaluation due to the presence of false positive and false negative results in correlation with MRI images.
Conclusions
Radiographs do not allow early inflammatory lesions indicating sacroiliitis to be diagnosed, which leads to diagnostic delay. MRI is the method of choice in diagnosing early sacroiliitis and detecting structural lesions, in particular sclerosis and erosions.
Radiographs and MRI are characterised by low, near to random, agreement in the detectability of the individual inflammatory changes in the sacroiliac joints.
References
- 1.Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–83. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 2.Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68(Suppl 2):ii1–i44. doi: 10.1136/ard.2008.104018. [DOI] [PubMed] [Google Scholar]
- 3.Braun J, Van Der Heijde D. Imaging and scoring in ankylosing spondylitis. Best Pract Res Clin Rheumatol. 2002;16:573–604. [PubMed] [Google Scholar]
- 4.Van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–68. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 5.Rudwaleit M, Jurik AG, Hermann K-GA, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68(10):1520–27. doi: 10.1136/ard.2009.110767. [DOI] [PubMed] [Google Scholar]
- 6.Sudoł-Szopinska I, Urbanik A. Diagnostic imaging of sacroiliac joints and the spine in the course of spondyloarthropathies. Pol J Radiol. 2013;78(2):43–49. doi: 10.12659/PJR.889039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuller-Weiderkamm C, Mascarenas V, Sudoł-Szopińska I, et al. Imaging and interpretation of Axial Spondyloarthritis: The Radiologist’s perspective – consensus of the Arthritis Subcommittee of the ESSR. Semin Musculoskelet Radiol. 2014;18(3):265–80. doi: 10.1055/s-0034-1375569. [DOI] [PubMed] [Google Scholar]
- 8.Fleiss J. Statistical Methods for Rates and Proportions. 2nd ed. New York: John Wiley; 1981. [Google Scholar]
- 9.Song I-H, Hermann KG, Haibel H, et al. Relationship between active inflammatory lesions in the spine and sacroiliac joints and new development of chronic lesions on whole-body MRI in early axial spondyloarthritis: results of the ESTHER trial at week 48. Ann Rheum Dis. 2011;70(7):1257–63. doi: 10.1136/ard.2010.147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudoł-Szopińska I, Kontny E, Maśliński W, et al. The pathogenesis of rheumatoid arthritis in radiological studies. Part I: Formation of inflammatory infiltrates within the synovial membrane. J Ultrason. 2012;12(48):202–13. doi: 10.15557/JoU.2012.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sudoł-Szopińska I, Zaniewicz-Kaniewska K, Warczyńska A, et al. The pathogenesis of rheumatoid arthritis in radiological studies. Part II: Imaging studies in rheumatoid arthritis. J Ultrason. 2012;12(49):319–28. doi: 10.15557/JoU.2012.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Berg R, Lenczner G, Feydy A, et al. Agreement between clinical practice and trained central reading in reading of sacroiliac joints on plain pelvic radiographs. Results from the DESIR cohort. Arthritis Rheumatol. 2014;66:2403–11. doi: 10.1002/art.38738. [DOI] [PubMed] [Google Scholar]
- 13.Sudoł-Szopińska I, Kwiatkowska B, Włodkowska-Korytkowska M, et al. Diagnostics of sacroiliitis according to ASAS criteria: A comparative evaluation of conventional radiographs and MRI in patients with a clinical suspicion of spondyloarthropathy. Preliminary results. Pol J Radiol. 2015;80:266–76. doi: 10.12659/PJR.892529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puhakka KB, Jurik AG, Egund N, et al. Imaging of sacroiliitis in early seronegative spondylarthropathy. Assessment of abnormalities by MR in comparison with radiography and CT. Acta Radiol. 2003;44:218–29. doi: 10.1080/j.1600-0455.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 15.Weber U, Lambert RGW, Østergaard M, et al. The diagnostic utility of magnetic resonance imaging in spondylarthritis: an international multicenter evaluation of one hundred eighty-seven subjects. Arthritis Rheum. 2010;62(10):3048–58. doi: 10.1002/art.27571. [DOI] [PubMed] [Google Scholar]
- 16.Weber U, Pedersen SJ, Østergaard M, et al. Can erosions on MRI of the sacroiliac joints be reliably detected in patients with ankylosing spondylitis? – A cross-sectional study. Arthritis Res Ther. 2012;14(3):R124. doi: 10.1186/ar3854. [DOI] [PMC free article] [PubMed] [Google Scholar]