Abstract
Background
Risk of venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), may be increased in liver cirrhosis. We conducted a single-center study to explore the epidemiology, risk factors, and in-hospital mortality of VTE in Chinese patients with liver cirrhosis.
Material/Methods
All patients with liver cirrhosis who were consecutively admitted to our hospital between January 2011 and December 2013 were retrospectively included.
Results
Of 2006 patients with liver cirrhosis included, 9 patients were diagnosed with or developed VTE during hospitalization, including 5 patients with a previous history of DVT, 1 patient with either a previous history of DVT or new onset of PE, and 3 patients with new onset of VTE (PE, n=1; DVT, n=2). Risk factors for VTE included a significantly higher proportion of hypertension and significantly higher red blood cells, hemoglobin, alanine aminotransferase, aspartate aminotransferase, prothrombin time (PT), international normalized ratio (INR), D-dimer, and Child-Pugh scores. The in-hospital mortality was significantly higher in patients with VTE than those without VTE (33.3% [3/9] versus 3.4% [67/1997], P<0.001).
Conclusions
VTE was observed in 0.4% of patients with liver cirrhosis during hospitalization and it significantly increased the in-hospital mortality. Elevated PT/INR aggravated the risk of VTE.
MeSH Keywords: Liver Cirrhosis, Prevalence, Venous Thrombosis
Background
Traditionally, patients with liver cirrhosis have an auto-anticoagulation status because they often have an elevated prothrombin time (PT) or international normalized ratio (INR) [1–4]. If so, a cirrhotic patient should rarely experience thrombotic events. However, the accumulated evidence suggests that cirrhotic patients have an increased risk of venous thromboembolism (VTE), which is defined as deep vein thrombosis (DVT) and pulmonary embolism (PE) [5–25]. Case-control studies have also confirmed that cirrhotic patients are more likely to develop VTE than the general population [26]. Our recent systematic review and meta-analysis found that about 1% of patients with chronic liver diseases had and developed VTE during their hospitalizations [27]. However, the relevant data are almost all from Western countries; by comparison, few studies have been conducted in Chinese populations. The most common etiology of liver cirrhosis was hepatitis B virus in China [28]. On the other hand, there was no consensus about the risk factors of VTE in liver cirrhosis. Herein, we conducted a retrospective observational study to evaluate the epidemiology and risk factors of VTE in Chinese hospitalized patients with liver cirrhosis and to explore whether VTE might influence the in-hospital mortality of liver cirrhosis.
Material and Methods
In this retrospective observational study, all patients with a diagnosis of liver cirrhosis who were consecutively admitted to the General Hospital of Shenyang Military Area between January 2011 and December 2013 were identified by searching the international classification codes (ICD)-9, ICD-10, and discharge diagnoses in the Department of Information. Diagnosis of liver cirrhosis was primarily established according to the history of liver disease, clinical presentations, laboratory tests, and abdominal imaging. Patients with malignancy were excluded. Repeated admission was not excluded. Some patients had been included in our previous studies [29–32]. The study protocol was approved by the Ethics Committee of our hospital The number was K(2015)30. Informed written consents were waived.
VTE was defined as DVT and PE. Considering that the potential pathogenesis and prognosis might be different between VTE and portal venous system thrombosis [33,34], portal venous system thrombosis was not considered in the present study. The medical records were thoroughly searched by an investigator to identify the history and new onset of VTE (XZ). The data accuracy was checked by another investigator (XQ). Diagnosis of VTE was established according to the clinical presentations and imaging examinations.
Additional data were collected by our study group, including the age, sex, blood pressure at admission, history of liver cirrhosis, etiology of liver disease, acute gastrointestinal bleeding (AUGIB), ascites, hepatic encephalopathy, and laboratory tests (red blood cell count, hemoglobin, white blood cell count, platelets, total bilirubin, albumin, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, glutamyl transpeptidase, blood urea nitrogen, creatinine, potassium, sodium, PT/INR, activated partial thromboplastin time, and D-dimer). Arterial hypertension was classified into grade I, II, and III according to the guideline [35]. Ascites and hepatic encephalopathy were graded according to the guidelines [36,37]. Child-Pugh and model for end-stage liver diseases (MELD) scores were calculated according to the previous criteria [38,39]. In-hospital death was reviewed. Causes of death were also recorded.
Statistical analysis
All statistical analyses were performed by SPSS Statistics version 17.0.0. Continuous data are expressed as mean ± standard deviation and median with range and were compared by the independent sample t test. Categorical data were expressed as frequency (percentage) and were compared by the chi-square test or Fisher exact test. A bar chart was also drawn to compare the in-hospital mortality between patients with and without VTE. Two-sided p<0.05 was considered to be statistically significant.
Results
Patients
Overall, 2006 patients were included in our study. A majority of patients were male, had hepatitis B virus and alcohol abuse, and had Child-Pugh class A and B (Table 1). Among them, 5 patients had a previous history of lower extremity DVT, 1 patient had a previous history of lower extremity DVT and developed new onset of PE during hospitalization, 1 patient developed new onset of PE during hospitalization, and 2 patients developed new onset of DVT during hospitalization. Thus, the prevalence of VTE in liver cirrhosis during hospitalization was 0.4% (9/2006) and the incidence of VTE was 0.2% (4/2006).
Table 1.
Patients characteristics.
| Variables | All patients (n=2006) | VTE in total (n=9) | No VTE in total (n=1997) | P value | |||
|---|---|---|---|---|---|---|---|
| No. Pts available | Results | No. Pts available | Results | No. Pts available | Results | ||
| Age (years) | 2006 | 56.24±12.18; 55.78 (6.20–95.13) | 9 | 56.94±13.36; 56.50 (40.07–84.35) | 1997 | 56.23±12.18; 55.77 (6.20–95.13) | 0.862 |
| Sex (male/female), n (%) | 2006 | 1330 (66.3%)/ 676 (33.7%) | 9 | 8 (88.9%)/ 1 (11.1%) | 1997 | 1322 (66.2%)/ 675 (33.8%) | 0.151 |
| History of liver cirrhosis, n (%) | 1897 | 8 | 1889 | 0.184 | |||
| <1 year | 690 (36.4%) | 5 (62.5%) | 685 (36.3%) | ||||
| 1–5 year | 769 (40.5%) | 3 (37.5%) | 766 (40.6%) | ||||
| >5 year | 438 (23.1%) | 0 (0.0%) | 438 (23.2%) | ||||
| Causes of liver diseases, n (%) | 1999 | 8 | 1991 | 0.991 | |||
| HBV alone | 565 (28.3%) | 4 (50.0%) | 561 (28.2%) | ||||
| HCV alone | 140 (7.0%) | 0 (0.0%) | 140 (7.0%) | ||||
| HBV+HCV | 13 (0.7%) | 0 (0.0%) | 13 (0.7%) | ||||
| Alcohol alone | 492 (24.6%) | 2 (25.0%) | 490 (24.6%) | ||||
| HBV + alcohol | 138 (6.9%) | 0 (0.0%) | 138 (6.9%) | ||||
| HCV + alcohol | 20 (1.0%) | 0 (0.0%) | 20 (1.0%) | ||||
| HBV + HCV + alcohol | 5 (0.3%) | 0 (0.0%) | 5 (0.3%) | ||||
| Autoimmune | 83 (4.2%) | 0 (0.0%) | 83 (4.2%) | ||||
| Drug | 31 (1.6%) | 0 (0.0%) | 31 (1.6%) | ||||
| Cholestatic | 54 (2.7%) | 0 (0.0%) | 54 (2.7%) | ||||
| Others | 28 (1.4%) | 0 (0.0%) | 28 (1.4%) | ||||
| Unknown | 428 (21.4%) | 2 (25.0%) | 426 (21.4%) | ||||
| HBV + PBC | 2 (0.1%) | 0 (0.0%) | 2 (0.1%) | ||||
| Blood pressure at admission, n (%) | 1990 | 8 | 1982 | <0.001 | |||
| Normal range | 1552 (78.0%) | 4 (50.0%) | 1548 (78.1%) | ||||
| Hypotension | 51 (2.6%) | 0 (0.0%) | 51 (2.6%) | ||||
| Hypertension – Grade I | 274 (13.8%) | 2 (25.0%) | 272 (13.7%) | ||||
| Hypertension – Grade II | 85 (4.3%) | 2 (25.0%) | 83 (4.2%) | ||||
| Hypertension – Grade III | 28 (1.4%) | 0 (0.0%) | 28 (1.4%) | ||||
| AUGIB, n (%) | 1994 | 563 (28.2%) | 8 | 0 (0.0%) | 1986 | 563 (28.3%) | 0.075 |
| Ascites, n (%) | 1991 | 8 | 1983 | 0.077 | |||
| No | 1023 (51.4%) | 1 (12.5%) | 1022 (51.5%) | ||||
| Mild | 217 (10.9%) | 2 (25.0%) | 215 (10.8%) | ||||
| Moderate and severe | 751 (37.7%) | 5 (62.5%) | 746 (37.6%) | ||||
| Hepatic encephalopathy, n (%) | 1991 | 8 | 1983 | 0.733 | |||
| No | 1848 (92.8%) | 8 (100.0%) | 1840 (92.8%) | ||||
| Grade I–II | 117 (5.9%) | 0 (0.0%) | 117 (5.9%) | ||||
| Grade II–IV | 26 (1.3%) | 0 (0.0%) | 26 (1.3%) | ||||
| Red blood cell (1012/L) | 1960 | 3.11±0.86; 3.07 (0.93–6.78) | 9 | 3.67±0.74; 3.41 (2.89–5.19) | 1951 | 3.11±0.86; 3.06 (0.93–6.78) | 0.049 |
| Hemoglobin (g/L) | 1960 | 95.01±30.03; 93.00 (23.0–218.0) | 9 | 118.03±19.99; 121.00 (91.0–155.0) | 1951 | 94.91±30.03; 93.00 (23.0–218.0) | 0.021 |
| White blood cell (109/L) | 1961 | 5.40±4.14; 4.20 (0.30–46.10) | 9 | 7.02±4.26; 6.40 (3.20–17.00) | 1952 | 5.39±4.13; 4.20 (0.30–46.10) | 0.238 |
| Platelet (109/L) | 1959 | 100.97±86.83; 76.00 (5–1278) | 9 | 75.22±45.91; 57.00 (28–180) | 1950 | 101.09±86.96; 76.00 (5–1278) | 0.373 |
| Total bilirubin (umol/L) | 1926 | 39.47±59.63; 21.85 (1.9–707.7) | 9 | 62.20±87.84; 33.10 (3.4–285.6) | 1917 | 39.36±59.48; 21.80 (1.9–707.7) | 0.252 |
| Albumin (g/L) | 1901 | 32.14±6.90; 32.10 (0.4–52.8) | 9 | 29.36±8.06; 33.00 (17.4–39.7) | 1892 | 32.15±6.89; 32.10 (0.4–52.8) | 0.225 |
| Alanine aminotransferase (U/L) | 1925 | 39.35±65.78; 26.00 (3.0–1460) | 9 | 180.67±434.01; 29.00 (9.0–1335.0) | 1916 | 38.69±58.87; 26.00 (3.0–1460) | <0.001 |
| Aspartate aminotransferase (U/L) | 1924 | 57.48±102.45; 36.00 (8.0–2454) | 9 | 227.00±434.75; 55.00 (17.0–1366.0) | 1915 | 56.68±98.08; 36.00 (8.0–2454) | <0.001 |
| Alkaline phosphatase (U/L) | 1921 | 113.70±96.58; 86.00 (12.8–980.0) | 9 | 108.31±60.28; 89.00 (41.0–215.0) | 1912 | 113.73±96.72; 86.00 (12.8–980.0) | 0.867 |
| Glutamyl transpeptidase (U/L) | 1916 | 113.92±202.34; 48.00 (1.5–4562) | 9 | 52.67±24.54; 59.00 (8.0–84.0) | 1907 | 114.21±202.77; 48.00 (1.5–4562) | 0.363 |
| Blood urea nitrogen (mmol/L) | 1881 | 7.97±7.01; 5.89 (1.42–76.02) | 9 | 6.97±4.03; 6.14 (2.96–16.36) | 1872 | 7.97±7.02; 5.89 (1.42–76.02) | 0.669 |
| Creatinine (umol/L) | 1880 | 87.16±116.78; 60.00 (2.8–1473) | 9 | 75.56±41.15; 63.00 (48.0–182.0) | 1871 | 87.22±117.03; 60.00 (2.8–1473) | 0.765 |
| Potassium (mmol/L) | 1910 | 4.08±0.54; 4.04 (2.20–7.87) | 9 | 4.31±0.61; 4.10 (3.84–5.11) | 1901 | 4.08±0.54; 4.04 (2.20–7.87) | 0.184 |
| Sodium (mmol/L) | 1909 | 138.15±4.49; 138.80 (116.3–160.8) | 9 | 136.12±5.21; 137.30 (125.7–142.2) | 1900 | 138.16±4.48; 138.80 (116.3–160.8) | 0.175 |
| Prothrombin time (second) | 1881 | 16.25±4.64; 15.20 (10.5–94.6) | 9 | 19.97±6.57; 19.20 (12.7–31.8) | 1872 | 16.23±4.63; 15.20 (10.5–94.6) | 0.016 |
| Activated partial thromboplastin time (second) | 1879 | 43.18±11.92; 41.50 (20.0–180.0) | 9 | 45.70±18.91; 40.10 (28.3–87.3) | 1870 | 43.17±11.89; 41.50 (20.0–180.0) | 0.526 |
| International normalized ratio | 1875 | 1.34±0.57; 1.20 (0.76–13.40) | 8 | 1.75±0.82; 1.53 (0.98–3.22) | 1867 | 1.33±0.57; 1.20 (0.76–13.40) | 0.039 |
| D-dimer (ug/ml) | 609 | 0.76±1.32; 0.30 (0–15.5) | 4 | 5.75±6.86; 3.60 (0.3–15.5) | 605 | 0.73±1.17; 0.30 (0–9.0) | 0.022 |
| Child-Pugh score | 1781 | 7.53±2.04; 7.00 (5–15) | 7 | 9.14±1.68; 9.00 (6–11) | 1774 | 7.53±2.04; 7.00 (5–15) | 0.037 |
| Child-Pugh class, n (%) | 1781 | 7 | 1774 | 0.437 | |||
| A | 649 (36.4%) | 1 (14.3%) | 648 (36.5%) | ||||
| B | 827 (46.4%) | 4 (57.1%) | 823 (46.4%) | ||||
| C | 305 (17.1%) | 2 (28.6%) | 303 (17.1%) | ||||
| MELD score | 1794 | 7.40±7.48; 5.96 (−14.28–51.64) | 7 | 12.41±10.48; 10.53 (−1.16–26.40) | 1787 | 7.38±7.47; 5.95 (−14.28–51.64) | 0.076 |
HBV – hepatitis B virus; HCV – hepatitis C virus; PBC – primary biliary cirrhosis; AUGIB – acute upper gastrointestinal bleeding; MELD – model for end stage liver disease.
Comparison between patients with and without VTE
Compared with those without VTE, patients with VTE had a significantly higher proportion of hypertension and significantly higher red blood cells, hemoglobin, alanine aminotransferase, aspartate aminotransferase, PT, INR, D-dimer, and Child-Pugh scores (Table 1). MELD score was statistically similar between the 2 groups.
In-hospital mortality between patients with and without VTE
The in-hospital mortality was 3.5% (70/2006). Causes of death are shown in Table 2. The in-hospital mortality was significantly higher in patients with VTE than in those without VTE (33.3% [3/9] versus 3.4% [67/1997], P<0.001) (Figure 1). The overall mortality due to VTE was 0.09% (2/2006) and VTE accounted for 2.8% of all causes of death (2/70).
Table 2.
Causes of in-hospital death.
| Causes | All patients | VTE in total | No VTE in total |
|---|---|---|---|
| Massive gastrointestinal bleeding | 23 | 0 | 23 |
| Liver failure | 12 | 1 | 11 |
| Liver failure plus multiple organ failure | 11 | 0 | 11 |
| Multiple organ failure | 7 | 0 | 7 |
| Massive gastrointestinal bleeding plus multiple organ failure | 4 | 0 | 4 |
| Pulmonary embolism | 2 | 2 | 0 |
| Renal failure | 2 | 0 | 2 |
| Sudden cardiac arrest | 2 | 0 | 2 |
| Cerebral infarction plus multiple organ failure | 2 | 0 | 2 |
| Cerebral infarction plus massive gastrointestinal bleeding | 1 | 0 | 1 |
| Massive gastrointestinal bleeding plus liver failure | 1 | 0 | 1 |
| Respiratory failure | 1 | 0 | 1 |
| Sepsis | 1 | 0 | 1 |
| Severe pulmonary infection | 1 | 0 | 1 |
Figure 1.
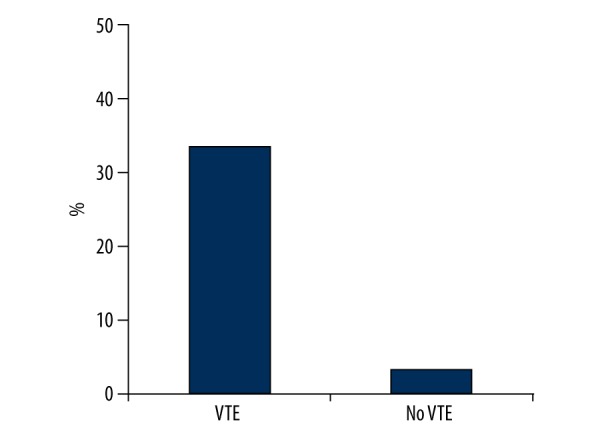
In-hospital mortality between patients with and without VTE.
Discussion
Our study demonstrated that the prevalence and incidence of VTE during hospitalization was 0.4% and 0.2% in Chinese patients with liver cirrhosis, respectively. This finding was relatively lower than our recent systematic review that the incidence of VTE in patients with chronic liver diseases was 1% (95% confidence interval: 0.7–1.3%), and the prevalence of VTE was 1% (95% confidence interval: 0.7–1.2%) [27]. This discrepancy might be explained by the heterogeneous sample size and etiology of liver cirrhosis among studies; despite this, we preferred to emphasize another major finding – that patients with VTE had an approximately 10-fold higher risk of in-hospital death than those without VTE. Certainly, a small number of patients with VTE might restrict the interpretation of comparative analysis. Considering a statistically significant difference of in-hospital mortality between the 2 groups, we had to acknowledge the importance of early diagnosis and treatment of VTE in liver cirrhosis.
We also attempted to analyze the risk factors for VTE in liver cirrhosis. D-dimer, red blood cells, hemoglobin, alanine aminotransferase, aspartate aminotransferase, PT, INR, and Child-Pugh scores were significantly associated with the presence of VTE. Except for D-dimer, which has been a routine diagnostic test for VTE [40], and arterial hypertension, which is an important determinant of VTE in the general population [41], the risk factors for VTE should be interpreted carefully. First, patients with VTE had significantly higher red blood cell and hemoglobin than those without VTE, possibly because none of patients with VTE had AUGIB, but 28.3% of patients without VTE had AUGIB. Thus, we might speculate that AUGIB was rarely complicated in patients with VTE. Second, patients with VTE had significantly higher alanine aminotransferase and aspartate aminotransferase than those without VTE. Similarly, patients with VTE also had significantly higher Child-Pugh scores than those without VTE. Additionally, higher total bilirubin and lower albumin were observed in patients with VTE, but the difference was not statistically significant. Thus, the relationship between severity of liver dysfunction and probability of VTE is suggested. Third, patients with VTE had significantly higher PT and INR than those without VTE. This finding is seemingly counterintuitive, but was largely consistent with the modern concept that PT and INR do not reflect the global coagulation status, and that elevated PT and INR does not protect cirrhotic patients from the development of VTE. This has been repeatedly reported in previous studies and was recently reviewed [42–44]. This finding can be explained by the fact that PT and INR mirror the liver synthesis of the procoagulants only, whereas other parameters associated with an overall decrease of liver function can be more accurate surrogates for impaired production of procoagulants as well as natural anticoagulants.
Except for our findings, previous studies should be deeply and systematically discussed. We described a detailed search strategy in our recent systematic review of the epidemiology of VTE in liver diseases [27]. Studies were eligible if they compared the characteristics between liver disease patients with and without VTE. Notably, we just summarized the risk factors of VTE in the patients with liver diseases, but not in the general population. Indeed, in the latter condition, the presence of liver disease might be one of the variables included. Thus, the following items about the risk factors of VTE were collected: variables included in the univariable analyses, significant factors calculated in the univariate analyses, the variables included in the multivariate analyses, and the independent predictors calculated in the multivariate analyses. Their frequencies were counted. The odds or risk ratio for each factor was also recorded to clarify whether the independent factors increased or decreased the risk of VTE in liver diseases.
Overall, 7 individual studies evaluated the risk factors of predicting the development of VTE in patients with liver diseases in univariate analyses, and 5 of them also conducted multivariate analyses [7,9,11,12,18,19,24] (Table 3). A total of 19 significant risk factors were reported in univariate analyses. They included age, race, Charlson co-morbidity index, insurance, encephalopathy, variceal bleeding, ascites, coagulopathy, hypo-osmolality, malnutrition, total parenteral nutrition, mechanical ventilation, central venous line placement, diabetes mellitus, albumin, creatinine, aspartate aminotransferase, alanine aminotransferase, and hematocrit. A total of 16 independent risk factors were reported in multivariate analyses. The race with the highest risk of VTE was black, followed by white and Hispanics. A higher Charlson co-morbidity index, malnutrition, central venous line placement, active malignancy, trauma or surgery during hospitalization, history of VTE, and diabetes mellitus were also associated with an increased risk of VTE in patients with liver diseases. The presence of encephalopathy, variceal bleeding, ascites, coagulopathy, and hypo-osmolality, Medicaid insurance, and use of VTE prophylaxis were associated with a decreased risk of VTE.
Table 3.
Summary of univariate and multivariate analyses regarding the risk factors of VTE in liver diseases.
| First author, journal (year) | Univariate analysis | No. observed/significant variables in univariate analyses | Multivariate analysis | No. observed/significant variables in multivariate analyses |
|---|---|---|---|---|
| Aldawood A, Thromb J (2011) | Yes | 29/0 | No | Not applicable |
| Ali M, Dig Dis Sci (2011) | Yes | 22/13 | Yes | 10/10 |
| Barclay SM, Pharmacotherapy (2013) | Yes | 4/0 | Yes | 8/4 |
| Dabbagh O, Chest (2010) | Yes | 3/0 | No | Not applicable |
| Lesmana CRA, Hepatol Int (2010) | Yes | 17/1 | Yes | 4/1 |
| Northup PG, Am J Gastroenterol (2006) | Yes | 13/1 | Yes | 5/1 |
| Walsh KA, Ann Pharmacother (2013) | Yes | 18/5 | Yes | 7/1 |
A lower albumin level was identified as an independent predictor of VTE in 2 studies by Northup [19] and Walsh [24]. In the study by Northup, the albumin level was significantly lower in cirrhotic patients with VTE than in those without VTE (mean: 2.85 g/dL, 95%CI: 2.70–3.01 versus mean: 3.10 g/dL, 95%CI: 2.96–3.23, P=0.01); in the study by Walsh, the albumin level was significantly lower in patients with VTE than in those without VTE (median: 2.1 g/dL, interquartile range: 1.7–2.6 versus median: 2.4 g/dL, interquartile range: 2–2.8, P=0.02). By contrast, another two studies did not identify the albumin level as a significant predictor of VTE in the multivariate analyses [11,18]. In the study by Lesmana, the proportion of an albumin level <3 mg/dL was lower in patients with DVT than in those without DVT (75% [9/12] versus 49.2% [120/244], P=0.081), but the difference was not statistically significant. No detailed data was reported in the study by Barclay.
Although a firm conclusion was not achieved, these preliminary findings from our team and others are helpful for the identification of high-risk patients. However, these data were scattered and needed to be integrated into a score system or predictive index. Ideally, a prospective study should include a training cohort to produce a predictive model to stratify the risk of the development of VTE and a validation cohort to confirm its accuracy.
The retrospective nature was a major limitation of our study. Although we selected all patients consecutively admitted to our hospital and had very few exclusion criteria, the patient selection bias should not be neglected in a retrospective study. Second, laboratory and radiological examinations for the diagnosis of VTE were not routinely performed. Prospective studies might be warranted to establish the accurate epidemiology of VTE, especially asymptomatic VTE. Third, the regional and ethnic difference in the epidemiology of VTE could not be evaluated in this single-center study.
Conclusions
VTE was observed in 0.4% of patients with liver cirrhosis during hospitalization and it significantly increased the in-hospital mortality. Degree of liver dysfunction might be significantly associated with the presence of VTE in liver cirrhosis. More importantly, elevated PT/INR did not protect from the development of VTE, but increased the risk of VTE in liver cirrhosis. Due to potential study limitations, these findings should be cautiously interpreted and further validated.
Abbreviations
- PT
prothrombin time
- INR
international normalized ratio
- VTE
venous thromboembolism
- DVT
deep vein thrombosis
- PE
pulmonary embolism
- ICD
international classification code
- AUGIB
acute upper gastrointestinal bleeding
- MELD
model for end-stage liver diseases
Footnotes
Source of support: This study was partially supported by the grant from the National Natural Science Foundation of China (no. 81500474) and Natural Science Foundation of Liaoning Province (no. 2015020409)
Conflict of interest
None.
References
- 1.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365(2):147–56. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 2.Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis. 2002;22(1):83–96. doi: 10.1055/s-2002-23205. [DOI] [PubMed] [Google Scholar]
- 3.Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116(6):878–85. doi: 10.1182/blood-2010-02-261891. [DOI] [PubMed] [Google Scholar]
- 4.Northup PG, Caldwell SH. Coagulation in liver disease: a guide for the clinician. Clin Gastroenterol Hepatol. 2013;11(9):1064–74. doi: 10.1016/j.cgh.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 5.De Stefano V, Rossi E. Venous thromboembolism in patients with liver diseases. Intern Emerg Med. 2015;10(4):489–91. doi: 10.1007/s11739-015-1215-7. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed S, Mehta V, Vendetti N, et al. Risk of venous thromboembolism (VTE) in patients with chronic hepatitis C (CHC) Pharmacoepidemiology Drug Saf. 2012;21:94–103. [Google Scholar]
- 7.Aldawood A, Arabi Y, Aljumah A, et al. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J. 2011;9(1):1. doi: 10.1186/1477-9560-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Dorzi HM, Bhat S, Tamim H, et al. Practices of venous thromboembolism prophylaxis and incidence in critically ill cirrhotic patients. American Journal of Respiratory and Critical Care Medicine. 2010;181(1):A1638. [Google Scholar]
- 9.Ali M, Ananthakrishnan AN, McGinley EL, Saeian K. Deep vein thrombosis and pulmonary embolism in hospitalized patients with cirrhosis: a nationwide analysis. Dig Dis Sci. 2011;56(7):2152–59. doi: 10.1007/s10620-011-1582-5. [DOI] [PubMed] [Google Scholar]
- 10.Anthony Lizarraga W, Dalia S, Reinert SE, Schiffman FJ. Venous thrombosis in patients with chronic liver disease. Blood Coagul Fibrinolysis. 2010;21(5):431–35. doi: 10.1097/MBC.0b013e328337b3ba. [DOI] [PubMed] [Google Scholar]
- 11.Barclay SM, Jeffres MN, Nguyen K, Nguyen T. Evaluation of pharmacologic prophylaxis for venous thromboembolism in patients with chronic liver disease. Pharmacotherapy. 2013;33(4):375–82. doi: 10.1002/phar.1218. [DOI] [PubMed] [Google Scholar]
- 12.Dabbagh O, Oza A, Prakash S, et al. Coagulopathy does not protect against venous thromboembolism in hospitalized patients with chronic liver disease. Chest. 2010;137(5):1145–49. doi: 10.1378/chest.09-2177. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Fuster MJ, Abdilla N, Fabia MJ, et al. Venous thromboembolism and liver cirrhosis. Rev Esp Enferm Dig. 2008;100(5):259–62. doi: 10.4321/s1130-01082008000500002. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 14.Girleanu I, Trifan A, Cojocariu C, et al. The risk of thrombotic events in patients with liver cirrhosis. Rev Med Chir Soc Med Nat Iasi. 2012;116(4):991–96. [PubMed] [Google Scholar]
- 15.Gulley D, Teal E, Suvannasankha A, et al. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci. 2008;53(11):3012–17. doi: 10.1007/s10620-008-0265-3. [DOI] [PubMed] [Google Scholar]
- 16.Kohsaka S, Nagai T, Yaegashi M, Fukuda K. Pulmonary embolism and deep venous thrombosis in hospitalized patients with liver cirrhosis. Hepatol Res. 2012;42(4):433–34. doi: 10.1111/j.1872-034X.2011.00941.x. [DOI] [PubMed] [Google Scholar]
- 17.Kumar G, Kumar N, Deshmukh A, et al. Is cirrhosis protective for venous thromboembolism? Analysis from a national Inpatient Sample. Chest. 2010;138(4):935A. [Google Scholar]
- 18.Lesmana CR, Inggriani S, Cahyadinata L, Lesmana LA. Deep vein thrombosis in patients with advanced liver cirrhosis: a rare condition? Hepatol Int. 2010;4(1):433–38. doi: 10.1007/s12072-010-9166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Northup PG, McMahon MM, Ruhl AP, et al. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol. 2006;101(7):1524–28. doi: 10.1111/j.1572-0241.2006.00588.x. quiz 1680. [DOI] [PubMed] [Google Scholar]
- 20.Ponziani FR, Zocco MA, Garcovich M, et al. Epidemiology of venous thrombotic events and pulmonary embolism among hospitalized cirrhotic patients: A single center experience. Digestive and Liver Disease. 2013;45:S41. [Google Scholar]
- 21.Saleh T, Matta F, Alali F, Stein PD. Venous thromboembolism with chronic liver disease. Am J Med. 2011;124(1):64–68. doi: 10.1016/j.amjmed.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Shah NL, Northup PG, Caldwell SH. A clinical survey of bleeding, thrombosis, and blood product use in decompensated cirrhosis patients. Ann Hepatol. 2012;11(5):686–90. [PubMed] [Google Scholar]
- 23.Smith CB, Hurdle AC, Kemp LO, et al. Evaluation of venous thromboembolism prophylaxis in patients with chronic liver disease. J Hosp Med. 2013;8(10):569–73. doi: 10.1002/jhm.2086. [DOI] [PubMed] [Google Scholar]
- 24.Walsh KA, Lewis DA, Clifford TM, et al. Risk factors for venous thromboembolism in patients with chronic liver disease. Ann Pharmacother. 2013;47(3):333–39. doi: 10.1345/aph.1R496. [DOI] [PubMed] [Google Scholar]
- 25.Wu H, Nguyen GC. Liver cirrhosis is associated with venous thromboembolism among hospitalized patients in a nationwide US study. Clin Gastroenterol Hepatol. 2010;8(9):800–5. doi: 10.1016/j.cgh.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 26.Sogaard KK, Horvath-Puho E, Gronbaek H, et al. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol. 2009;104(1):96–101. doi: 10.1038/ajg.2008.34. [DOI] [PubMed] [Google Scholar]
- 27.Qi X, Ren W, Guo X, Fan D. Epidemiology of venous thromboembolism in patients with liver diseases: a systematic review and meta-analysis. Intern Emerg Med. 2015;10(2):205–17. doi: 10.1007/s11739-014-1163-7. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Fan D. Hepatitis B in China. Lancet. 2007;369(9573):1582–83. doi: 10.1016/S0140-6736(07)60723-5. [DOI] [PubMed] [Google Scholar]
- 29.Qi X, Li H, Chen J, Xia C, et al. Serum liver fibrosis markers for predicting the presence of gastroesophageal varices in liver cirrhosis: A retrospective cross-sectional study. Gastroenterol Res Pract. 2015 doi: 10.1155/2015/274534. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi X, Peng Y, Li H, Dai J, Guo X. Diabetes is associated with an increased risk of in-hospital mortality in liver cirrhosis with acute upper gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2015;27(4):476–77. doi: 10.1097/MEG.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 31.Peng Y, Qi X, Dai J, et al. Child-Pugh versus MELD score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis. Int J Clin Exp Med. 2015;8(1):751–57. [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu C, Qi X, Li H, et al. Correlation of serum liver fibrosis markers with severity of liver dysfunction in liver cirrhosis: a retrospective cross-sectional study. Int J Clin Exp Med. 2015;8(4):5989–98. [PMC free article] [PubMed] [Google Scholar]
- 33.De Stefano V, Martinelli I. Splanchnic vein thrombosis: clinical presentation, risk factors and treatment. Intern Emerg Med. 2010;5(6):487–94. doi: 10.1007/s11739-010-0413-6. [DOI] [PubMed] [Google Scholar]
- 34.Qi X, Li H, Liu X, Yao H, et al. Novel insights into the development of portal vein thrombosis in cirrhosis patients. Expert Rev Gastroenterol Hepatol. 2015:1–12. doi: 10.1586/17474124.2015.1083856. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25(6):1105–87. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 36.Moore KP, Wong F, Gines P, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38(1):258–66. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 37.Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy – definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35(3):716–21. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 38.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 39.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60(8):646–49. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 40.Rodger MA, Le Gal G, Wells P, et al. Clinical decision rules and D-Dimer in venous thromboembolism: current controversies and future research priorities. Thromb Res. 2014;134(4):763–68. doi: 10.1016/j.thromres.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 41.Tsai J, Grant AM, Beckman MG, et al. Determinants of venous thromboembolism among hospitalizations of US adults: a multilevel analysis. PLoS ONE. 2015;10(4):e0123842. doi: 10.1371/journal.pone.0123842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buresi M, Hull R, Coffin CS. Venous thromboembolism in cirrhosis: a review of the literature. Can J Gastroenterol. 2012;26(12):905–8. doi: 10.1155/2012/175849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aggarwal A, Puri K, Liangpunsakul S. Deep vein thrombosis and pulmonary embolism in cirrhotic patients: systematic review. World J Gastroenterol. 2014;20(19):5737–45. doi: 10.3748/wjg.v20.i19.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang ZJ, Costa KA, Novelli EM, Smith RE. Venous thromboembolism in cirrhosis. Clin Appl Thromb Hemost. 2014;20(2):169–78. doi: 10.1177/1076029612461846. [DOI] [PMC free article] [PubMed] [Google Scholar]


