Abstract
Objectives
Interstitial lung disease (ILD) is common in connective tissue disease (CTD) and is the leading cause of mortality. Investigators have used certain outcome measures in randomized controlled trials (RCTs) in CTD-ILD, but the lack of a systematically-developed, CTD-specific index that captures all measures, relevant and meaningful to CTD-ILD patients, has left a large and conspicuous gap in CTD-ILD research.
Methods
The CTD-ILD Working Group, under the aegis of OMERACT, has completed a consensus group exercise to reach harmony on core domains and items for inclusion in RCTs in CTD-ILD. During the OMERACT 12 meeting, consensus was sought after on the domains and core items for inclusion in RCTs. In addition, consensus was pursued on efinition of response in RCTs. Consensus was defined as ≥ 75% consensus.
Results
OMERACT 12 participants endorsed the domains with minimal modifications. Clinically meaningful progression for CTD-ILD was proposed as ≥10% relative decline in forced vital capacity (FVC)% or ≥5–<10% relative decline in FVC% and ≥15% relative decline in diffusion capacity in carbon monoxide (DLCO)%.
Conclusion
There is consensus on the domains for inclusion in RCTs in CTD-ILD and definition of clinically meaningful progression. Data-driven approaches will need to validate this in different cohorts and RCTs.
Key Indexing Terms: Lung diseases, Interstitial lung disease, Outcome assessment, OMERACT
Interstitial lung disease (ILD) induces overwhelming morbidity and is the leading cause of mortality in patients with connective tissue disease (CTD) (1, 2). Certain CTDs are more likely to be associated with ILD (e.g. systemic sclerosis (SSc), idiopathic inflammatory myopathy (IIM) and rheumatoid arthritis (RA)), but all CTD patients are at risk for developing ILD, and ILD may be the first or only manifestation of a CTD(3). There are currently no approved treatments for CTD-ILD and drug development for CTD-ILD is challenged by its variable presentation, heterogeneous disease course, devastating morbidity, and considerable mortality (3). There have been very few randomized controlled trials (RCTs) in CTD-ILD and further advancements are adversely affected by the lack of well-defined consensus driven outcome measures (4, 5). In a well-designed RCT of cyclophosphamide vs. placebo in SSc-ILD (Scleroderma Lung Study-1), modest changes were evident in lung physiology (forced vital capacity and total lung capacity) and in patient-reported outcomes (5). This is reminiscent of the 1980s when RA trials were being conducted without consensus on a group of core set outcome measures to assess efficacy. The lack of uniform outcome measures impedes drug development and hampers meta-analyses to assess efficacy. This has been a major obstacle to the conduct and interpretation of RCTs in CTD-ILD. Similar challenges were present in idiopathic pulmonary fibrosis (IPF) but there have been recent success with positive trials (6, 7).
Because of the above issues, there is a keen and growing interest in the rheumatology and pulmonary communities to identify and test promising therapies that target CTD-ILD. Investigators have used certain outcome measures in RCTs in CTD-ILD, but the lack of a systematically-developed, CTD-specific index that captures all measures, relevant and meaningful to CTD-ILD patients, has left a large and conspicuous gap in CTD-ILD research. Although the CTDs where ILD develops are complex and heterogeneous, manifestations of ILD share similar symptomatic, physiologic, and radiographic features suggesting that development of a single response index may be possible. The CTD-ILD Working Group, under the aegis of OMERACT, has completed a consensus group exercise to reach harmony on core domains and items for inclusion in RCTs in CTD-ILD that involved patient partners in well-structured focus groups to develop themes that are important to patients, and have initiated analyses in large international cohorts of CTD-ILD.
Background information
Consensus methodology to develop outcome measures for RCTs in CTD-ILD
The CTD-ILD working group has completed consensus development (including a detailed Delphi process, patient focus groups and a nominal group technique (NGT) meeting among participating health care providers and patient partners) to propose and select domains and items (outcome measures) for multi-center RCTs in CTD-ILD and IPF and the original manuscript is published elsewhere (8). Briefly, this initiative included an international interdisciplinary network comprised of rheumatology, pulmonary, thoracic radiology and pathology experts in ILD; patients with CTD-ILD or IPF participated at each stage of this initiative. There was a 4-tier web-based Delphi exercise for identification of domains and items followed by the NGT to reach consensus (8). A core set including the following domains: pulmonary physiology (including function), pulmonary imaging, survival, dyspnea, cough and health-related quality of life was proposed as appropriate for consideration for use in a hypothetical 1-year multicenter RCT for CTD-ILD (Table 1). Existing items (instruments) were proposed and voted on during the NGT exercise (see dyspnea and cough domains) with careful evaluation of the proposed items as they relate to the OMERACT filter 2.0 (reviewed in (7)). In addition, there was discussion regarding the need to develop ILD-specific instruments (which are included in the research agenda).
Table 1.
Consensus domain and instrument for CTD-ILD and IPF groups (Modified from 7)
| Domains and Instrument | CTD-ILD consensus | IPF consensus |
|---|---|---|
| Dyspnea | ||
| MRC chronic dyspnea scale | 75% | 92% |
| Dyspnea 12 | 88% | 70% |
| UCSD-SBQ | NA | 80% |
| Cough | ||
| Leicester cough monitor | 79% | 82% |
| HRQoL | ||
| Short form 36 | 100% | 82% |
| SGRQ | 87% | 82% |
| VAS-PtGA | 96% | NA |
| Lung Imaging | ||
| Overall extent of ILD on HRCT | 92% | 100% |
| Lung physiology | ||
| Forced vital capacity | 100% | 100% |
| Diffusion capacity of lung | 91% | 100% |
| Survival | ||
| All cause mortality | 100% | 100% |
Footnote: CTD-ILD: Connective tissue disease associated interstitial lung disease; HRCT: High resolution CT; HRQoL: health related quality of life; IPF: idiopathic pulmonary fibrosis; MRC: Medical Research Council; PtGA: Patient global assessment; SGRQ: St George’s Respiratory Questionnaire; UCSD-SBQ: University of California San Diego Shortness of Breath Questionnaire; VAS: visual analogue scale.
Patient Perspective
Since the last OMERACT CTD-ILD Workshop, qualitative interviews have been completed in 45 patients in 6 types of CTD-ILD across the US and Canada. Cough and dyspnea were found central to the CTD-ILD experience and patients considered both as very important measures to be evaluated in RCTs. Further, the patient participant focus groups provided ILD-specific content, context and language essential for development and validation of patient-reported outcome measures (8). Life impact of CTD-ILD on activity, participation, patients’ perceptions, family/caregivers, work, and overall health-related quality of life were explored. Psychosocial themes related to life impact included self-efficacy, living with uncertainty, and struggle over self-identity. Living with uncertainty was a theme where patients described confusion regarding their diagnosis and prognosis; discussions emphasized the need for improved communication to aid patients’ perceptions and understanding of their health/health condition. This manuscript has been submitted for publication (9) (unpublished observation).
Developing definitions of response
Candidate measures of efficacy have been proposed for IPF that attempt to address the inconsistent relationship between pulmonary function trends [i.e. serial such as forced vital capacity (FVC)] and outcomes important to patients, especially survival and changes in symptoms. A “time to worsening” definition has been proposed in IPF that measures time to occurrence of clinically meaningful events including acute IPF exacerbation, IPF-related death, lung transplantation and/or hospitalization for respiratory decompensation. The excellent short-term survival in SSc-ILD (the most studied CTD in RCTs) and other CTD-ILDs (such as RA) and the rarity of performance of lung transplantation reduce the utility of this definition of outcome and response in CTD-ILD. For example there were only 7 deaths over 2 years in the Scleroderma Lung Study and none in the first year (10). An intermediate measure of poor clinical course is termed “progression-free survival”, specifically the time to first occurrence of either ≥10% relative decline in FVC% predicted or ≥5–10% relative decline in FVC% predicted and ≥15% relative decline in carbon monoxide diffusion capacity (DLCO)% predicted or death has been proposed as a possible composite outcome index for CTD-ILD (11).
OMERACT 12 Workshop presentations
Three brief presentations highlighted data on the topics discussed above: results from the consensus process and NGT meeting, the patient participant focus groups, data-driven approaches in each CTD-ILD to validate proposed domains/ items, and a proposal for a clinically meaningful definition of progression as an endpoint in 1 year CTD-ILD RCTs. These were followed by 3 breakout sessions—2 breakout groups focused on core domains/ items for a 1-year multicenter RCT and a “progression-free survival” definition and one breakout group focused on patient perspectives. The patient perspective group focused on the benefits and limitations of standardization of patient / physician communication protocols and whether coping and self-efficacy should be captured in the context of a 1-year RCT and observational studies.
Discussion on core domains/ items and “progression-free survival” definition
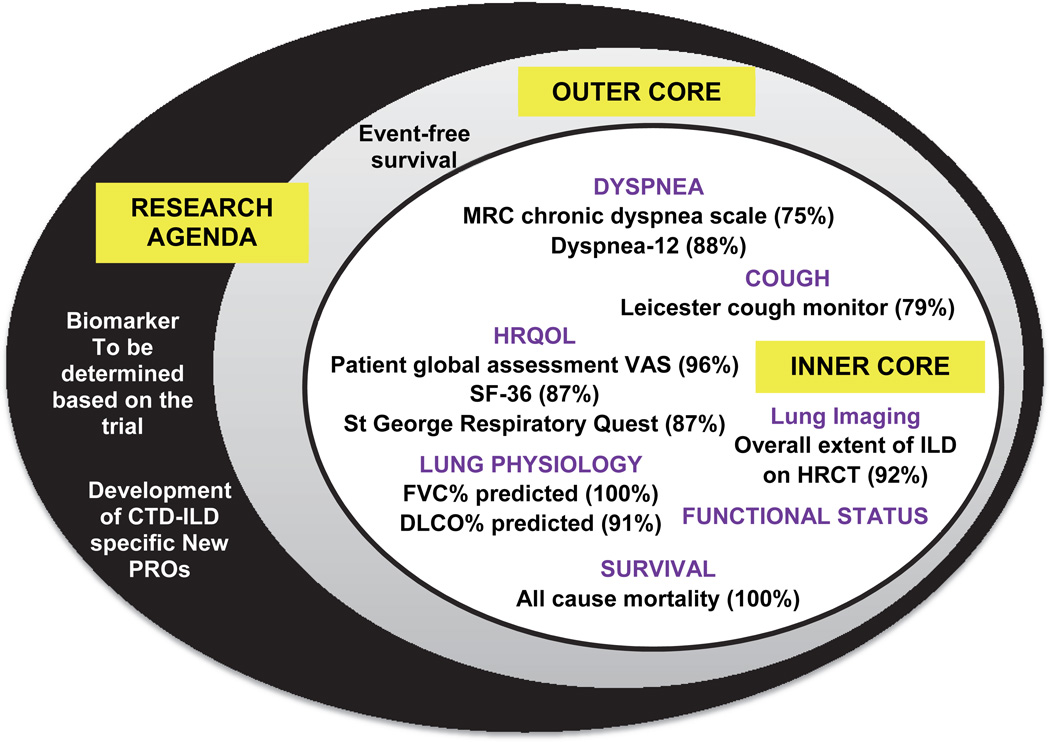
There was consensus on the preliminary core set of domains and research agenda (Figure); 45 of 46 (98%) voters concurred. It was suggested to separate functional status from lung physiology and to include this as a separate domain in the inner core. It was also acknowledged that some of the existing core items (instruments), especially for cough and dyspnea do not meet the OMERACT 2.0 filter (12) and research should be conducted to develop CTD-ILD specific patient-reported outcomes for their assessment (98% [45 of 46 attendees] concurred with one abstention). Further there was consensus [98% with one abstention] that a disease-specific measure of health-related quality of life and instrument[s] to assess life impact should be included in the research agenda.
Figure.
Proposed core set measures for Connective tissue disease associated interstitial lung disease 1 year randomized controlled trials (Modified from 7).
Regarding progression-free survival, participants recommended that survival be separated from disease progression, as it is difficult to demonstrate a relationship between the two in a clinical trial. The break out groups suggested to use the term “clinically meaningful progression” and agreed with the proposed definition of ≥10% relative decline in FVC% predicted or ≥5 to <10% relative decline in FVC% predicted and ≥15% relative decline in DLCO% predicted; 87% agreed with 6 abstaining (of 46 votes). Several points were emphasized: a clear distinction should be made between a surrogate vs. a clinical outcome measure and that progression should not be synonymous with decline as future therapies may stabilize and/or even improve pulmonary physiology. For RCTs, it was emphasized to standardize the outcome measures (e.g., the American Thoracic Society/ European Respiratory Society recommendations on performance/evaluation of PFTs)(13). The next steps are to validate this definition and assess psychometric properties of core domains and items (Figure) in large observational studies and RCTs already underway in cohorts of RA, SSc, and IIM-associated ILDs. The overall goal is to develop composite indices in different CTD-ILDs but we acknowledge the heterogeneity of CTD-ILDs may impede a single measure across different CTD-ILDs. Different CTD-ILDs may have different composite indices such as a composite for change in disease bulk (decline in FVC, decline in DLCO, change on HRCT), clinically significant events (severe decline/hospital admissions/mortality), or combination of both. This will largely depend on the underling ILD. For example, a patient with SSc-usual interstitial pneumonia may have overtly irreversible disease; b) CTD-ILD with definite organizing pneumonia that is reversible; and c) indeterminate ILD (such as IIM-non-specific interstitial pneumonia) - reversibility possible although unlikely. The differences in end-points potentially will likely need multidisciplinary review by a rheumatologist, a pulmonologist and an experienced radiologist to determine whether a patient fell into a key sub-group, which might influence the choice of the primary end-point and use of a composite index. This type of data-driven approach will inform such decisions.
Discussion in the patient-perspective breakout group
Self-efficacy and coping were discussed as separate, but related aspects of how patients manage their ILD. Coping referred to a patients’ behavioral or cognitive efforts related to managing ILD, whereas self-efficacy referred to a patient’s self-perception and judgment of how a situation can be managed. OMERACT attendees agreed that coping and self-efficacy were not unique to CTD-ILD patients and that a special interest group (SIG) to discuss these aspects across multiple chronic rheumatologic diseases should be established.
Communication between providers and patients living with CTD-ILD was discussed to identify aspects at the time of diagnosis of ILD that would provide the basis for a meaningful understanding regarding prognosis and management decisions. Patients with a CTD-ILD expressed the need for a timely discussion at diagnosis of ILD and provision of sufficient information related to ILD; particularly discussions concerning results such as pulmonary function tests as knowledge of disease activity/severity had an important impact on self-efficacy.
In conclusion, important advances have been made by the CTD-ILD group in the past 2 years. The next steps include validation of consensus driven definitions of domains/ items and clinically meaningful progression.
Acknowledgments
Source of Support
InterMune, Biogen Idec, EMD Serono, Sigma-Tau Pharmaceuticals and the Mayo Clinic. Dr. Khanna was supported by NIH/NIAMS K24 AR063120
Footnotes
Name of Attributed Department and Institution
Department of Internal Medicine, Division of Rheumatology, University of Michigan Scleroderma Program
Contributor Information
Dinesh Khanna, University of Michigan Scleroderma Program, Ann Arbor, Michigan, USA.
Shikha Mittoo, University of Toronto, Toronto, Canada.
Rohit Aggarwal, Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Susanna M Proudman, Royal Adelaide Hospital and Associate Professor Discipline of Medicine, University of Adelaide, Adelaide, Australia.
Nicola Dalbeth, University of Auckland, Auckland, New Zealand.
Eric Matteson, Mayo Clinic, Rochester, Minnesota, USA.
Kevin Brown, National Jewish Hospital, Denver, Colorado, USA.
Kevin Flaherty, Medicine, University of Michigan, Ann Arbor, Michigan.
Athol U Wells, Royal Bromptom Hospital and National Heart and Lung Institute, London, United Kingdom.
James R Seibold, Scleroderma Research Consultants, Litchfield, Connecticut, USA.
Vibeke Strand, Stanford University, Palo Alto, California, USA.
References
- 1.Winstone TA, Assayag D, Wilcox PG, Dunne JV, Hague CJ, Leipsic J, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: A systematic review. Chest. 2014;146:422–436. doi: 10.1378/chest.13-2626. [DOI] [PubMed] [Google Scholar]
- 2.Kim EJ, Collard HR, King TE., Jr Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest. 2009;136:1397–1405. doi: 10.1378/chest.09-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryson T, Sundaram B, Khanna D, Kazerooni EA. Connective tissue disease-associated interstitial pneumonia and idiopathic interstitial pneumonia: similarity and difference. Seminars in ultrasound CT, and MR. 2014;35:29–38. doi: 10.1053/j.sult.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Seibold JR, Denton CP, Furst DE, Guillevin L, Rubin LJ, Wells A, et al. Randomized, prospective, placebo-controlled trial of bosentan in interstitial lung disease secondary to systemic sclerosis. Arthritis and rheumatism. 2010;62:2101–2108. doi: 10.1002/art.27466. [DOI] [PubMed] [Google Scholar]
- 5.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–2666. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 6.King TE, Jr, Albera C, Bradford WZ, Costabel U, du Bois RM, Leff JA, et al. All-cause mortality rate in patients with idiopathic pulmonary fibrosis. Implications for the design and execution of clinical trials. American journal of respiratory and critical care medicine. 2014;189:825–831. doi: 10.1164/rccm.201311-1951OC. [DOI] [PubMed] [Google Scholar]
- 7.Vancheri C, du Bois RM. A progression-free end-point for idiopathic pulmonary fibrosis trials: lessons from cancer. The European respiratory journal. 2013;41:262–269. doi: 10.1183/09031936.00115112. [DOI] [PubMed] [Google Scholar]
- 8.Saketkoo LA, Mittoo S, Huscher D, Khanna D, Dellaripa PF, Distler O, et al. Connective tissue disease related interstitial lung diseases and idiopathic pulmonary fibrosis: provisional core sets of domains and instruments for use in clinical trials. Thorax. 2014;69:428–436. doi: 10.1136/thoraxjnl-2013-204202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mittoo S, Frankel S, LaSage D, Strand V, Shah A, Christopher-Stine L, et al. Patient Perspective: an Anchor for Future Metric Development and Improved Approaches to Healthcare Delivery in Connective Tissue Disease Related Interstitial Lung Disease (CTD-ILD) Rheumatology. 2014 doi: 10.2174/1573398X11666150619182624. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tashkin DP, Elashoff R, Clements PJ, Roth MD, Furst DE, Silver RM, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. American journal of respiratory and critical care medicine. 2007;176:1026–1034. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna D, Brown KK, Clements PJ, Elashoff R, Furst DE, Goldin J, et al. Systemic sclerosis-associated interstitial lung disease-proposed recommendations for future randomized clinical trials. Clinical and experimental rheumatology. 2010;28:S55–S62. [PubMed] [Google Scholar]
- 12.Boers M, Kirwan JR, Wells G, Beaton D, Gossec L, d'Agostino MA, et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol. 2014;67:745–753. doi: 10.1016/j.jclinepi.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique-1995 update. Am J Respir Crit Care Med. 1995;152:2185–2198. doi: 10.1164/ajrccm.152.6.8520796. [DOI] [PubMed] [Google Scholar]