Abstract
Systemic sclerosis is a rare autoimmune disorder with significant morbidity and mortality due to multi-organ system involvement. Early diagnosis and screening for organ involvement is critical as earlier treatment appears to improve function and may impact mortality. The purpose of this article is to address some of the commonly asked questions by rheumatologists on systemic sclerosis.
Introduction
Systemic sclerosis (SSc) is an autoimmune disorder that affects various organs via inflammation, vascular damage, and fibrosis [1]. It can affect the heart, lungs, kidneys, gastrointestinal tract, and blood vessels. It is not a common disorder, and due to its variable multi-organ system involvement, it is not always an easy diagnosis to make. Early diagnosis is critical as there is no cure, but patients can be managed based on organ involvement. The objective of this review is to answer some of the most commonly asked questions regarding SSc in order to provide clinicians with the tools necessary to make a diagnosis of SSc and provide basics of treatment for the various organs involved.
1. Does this patient have SSc?
Scleroderma is divided into 3 large categories. The first category is localized scleroderma and morphea, which include linear scleroderma and deep morphea. The second category is scleroderma-like skin disorders including eosinophilic fasciitis, scleredema, scleromyxedema, and nephrogenic systemic fibrosis. The final category is SSc, which includes limited cutaneous systemic sclerosis (lcSSc), diffuse cutaneous systemic sclerosis (dcSSc), sine scleroderma and overlap syndrome.
When determining if a patient has SSc, there are signs, symptoms, and laboratory findings that can help with a diagnosis of SSc. In SSc, approximately 95% have sclerodactyly, 90% have Raynaud’s phenomenon (RP), and 95% have a positive anti-nuclear antibody (ANA) by immunofluorescence (IF) [2]. However, if ANA testing by multiplex bead technology is used, it can give a false negative ANA in up to 50% of patients with SSc due to lack of measurement of anti-RNA polymerase III, anti-Th/To and U3-RNP, which are associated with ANA with nucleolar pattern [3, 4]. If a patient with skin thickening does not have sclerodactyly, RP, or a positive ANA, one must think of a scleroderma-like skin disorder [2]. Patients with morphea (localized or generalized) or disorders such as eosinophilic fasciitis do not have sclerodactyly and generally do not have RP or a positive ANA, although they do co-exist based on prevalence in the population. SSc is a clinical diagnosis, but use of serological testing and nailfold capillaroscopy can be helpful with diagnosis. Skin biopsy is not required. A skin biopsy or deep tissue biopsy should only be performed if you suspect a scleroderma-like disorder (e.g., eosinophilic fasciitis and scleromyxedema) and need to confirm the diagnosis.
2. How do I classify patients into lcSSc and dcSSc?
Patients with SSc usually have the following phases of skin disease: edematous, fibrotic induration, and atrophic. Of the patients with SSc, approximately 55% have lcSSc, 35% have dcSSc, and 10% have overlap and sine scleroderma [5]. The morbidity and mortality are different between these groups. The difference between lcSSc and dcSSc depends on the extent of skin involvement. Patients with SSc with skin thickening distal to the elbows and knees, with or without face involvement, are classified as lcSSc, and those with distal skin thickening who also have thickening of upper arms, anterior chest, abdomen and/or thighs, with or without face involvement, are classified as dcSSc [2]. CREST syndrome is not synonymous with lcSSc as long standing dcSSc can also have CREST syndrome.
The classification criteria for SSc have recently been revised (Table 1) [6]. The purpose of classification criteria is to enroll patients in clinical and translational research to have a standardized or relatively homogenous population. Since the development of the American College of Rheumatology (ACR) 1980 criteria, there have been advances in SSc-specific serologies and nailfold capillaroscopy examination. In addition, the 1980 criteria missed 20% to 66% of patients diagnosed by expert clinicians [7, 8, 9]. The 2013 ACR/European League Against Rheumatism criteria are more sensitive and specific compared to the ACR 1980 criteria (Table 1) [6]. This new criteria is a point system where a score of 9 or higher classifies a patient as having SSc.
Table 1.
The American College of Rheumatology/European League Against Rheumatism Criteria of the Classification of Systemic Sclerosis.
| Criteria | Sub-criteria | Score |
|---|---|---|
| Skin thickening of fingers of both hands extending proximal to the metacarpolphalangeal (MCP) joints | 9 | |
| Skin thickening of fingers only (only count higher score) | - Puffy fingers - Sclerodactyly of the fingers (distal to the MCP joints but proximal to the proximal interphalangeal (PIP) joints |
2 4 |
| Fingertip lesions (only count higher score) | - Digital tip ulcers - Fingertip pitting scars |
2 3 |
| Telangectasia | 2 | |
| Abnormal nailfold capillaries | 2 | |
| Lung involvement (maximum score of 2) | - PAH - ILD |
2 2 |
| RP | 3 | |
| SSc-associated autoantibodies (anti-centromere, anti-topoisomerase I (anti-Scl-70), anti-RNA polymerase III) | 3 |
The total score is calculated by adding the highest score in each category. Those with a score of at least 9 are classified as having SSc.
Modified from [6].
3. How do I diagnose patients with early SSc?
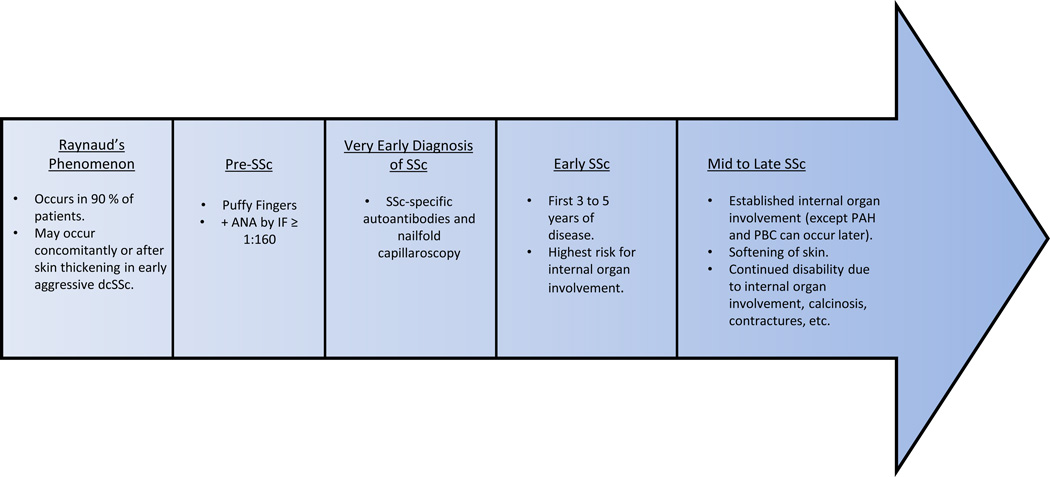
Early diagnosis of SSc is critical given its significant morbidity and mortality resulting from multi-system organ involvement. Although the classification criteria can guide providers, classification and diagnostic criteria are not synonymous. The following symptoms, signs, and laboratory testing should raise suspicion for a patient with SSc: RP, puffy fingers, and positive ANA. To make a definitive diagnosis, the patient should be further evaluated for the presence of SSc-specific autoantibodies and the presence of nailfold capillary abnormalities consistent with scleroderma pattern (Figure 1) [10]. However, in early, aggressive dcSSc, RP may occur concomitantly or after the onset of skin thickening.
Figure 1.
The natural history of SSc.
Autoantibodies are used for diagnosis and prognosis in SSc. There are many different autoantibodies seen in SSc, but there are 7 commonly assessed (Table 2). All of these autoantibodies have commercially available assays for detection, except for anti-U3RNP and anti Th/To. However, anti-Th/To and anti-U3RNP antibodies are considered to be present if there is a nucleolar pattern on ANA by IF testing. Thirty to 60% of patients with SSc have anti-centromere antibodies, anti-topoisomerase I antibodies, and anti-RNA polymerase III antibodies (Table 2) [11, 12]. Therefore, it is not uncommon to have patients with SSc who may not have these autoantibodies. Also, it is rare to have two SSc-specific autoantibodies in a single patient.
Table 2.
Autoantibodies in SSc with common disease manifestation associations, frequency in various populations and testing options.
| Common autoantibodies seen in SSc |
Disease manifestation associations |
North American Caucasian frequency of autoantibody presence in SSc |
North American African-American frequency of autoantibody presence in SSc |
Available commercial assays for autoantibodies |
|---|---|---|---|---|
| Anti-Scl-70/Anti-topoisomerase I | dcSSc and severe pulmonary fibrosis | 20% | 11% | Yes |
| Anti-centromere | lcSSc and PAH | 21% | 11% | Yes |
| Anti-RNA polymerase III* | dcSSc and scleroderma renal crisis | 24% | 11% | Yes |
| Anti-PM-Scl | SSc and inflammatory myopathy overlap | 2% | 0% | Yes |
| Anti-U1 RNP | mixed connective tissue disease/overlap syndrome (MCTD) | 14% | 26% | Yes |
| Anti-U3RNP* | ILD and PAH | 4% | 37% | No |
| Anti-Th/To* | lcSSc, severe pulmonary fibrosis and PAH | 5% | 0% | No |
4. What testing should be performed in patients suspected of having SSc?
To assist with diagnosis and prognosis, we routinely perform ANA by IF and obtain SSc autoantibodies including anti-centromere, anti-Scl-70, anti-RNA polymerase III, and anti-PM-Scl. Nailfold capillaroscopy examination should also be performed to look for SSc pattern. The SSc pattern is further divided into early, active and late patterns but mainly is used for research purposes. The positive predictive value of patients with SSc pattern on nailfold capillaroscopy along with positive SSc antibodies in one large longitudinal cohort was 79.5% [13].
Patients with early SSc, including both dcSSc and lcSSc, are at the highest risk for developing internal organ involvement during the initial 3 to 5 years after their first disease manifestation other than RP. The development of tendon friction rubs, joint contractures, myopathy, interstitial lung disease (ILD), myocardial involvement, and renal crisis occurs within the first 5 years of disease. Those with increasing skin thickness are noted to be at increased risk of internal organ involvement as well. There are also certain predictors of diffuse skin involvement and internal organ involvement, which are concerning for rapidly progressive SSc (Table 3) [14]. After 5 years of disease is typically when pulmonary arterial hypertension (PAH) occurs in those with lcSSc or dcSSc, and when those with lcSSc can develop primary biliary cirrhosis (PBC).
Table 3.
Predictors of Rapidly Progressive SSc (diffuse skin involvement and/or internal organ involvement)
| dcSSc |
|
| Renal crisis |
|
| Severe ILD |
|
| PAH |
|
| Myocardial involvement |
|
| Digital ulcers |
|
Modified from [14].
5. How do I manage progressive skin involvement with and without internal organ involvement?
We have recently published an up-to-date review of management of skin and internal organ involvement in SSc [1]. The following sections briefly discuss management.
To assess skin involvement, we routinely perform the modified Rodnan skin score (MRSS). It assesses 17 different areas of skin by subjective palpation of skin. Skin thickness is scored from 0 to 3, with 0 being no thickening, 1 mild thickening, 2 moderate thickening and 3 severe thickening. The total possible score ranges from 0 to 51 [2]. Assessing the extent of skin thickness is essential because increased thickness is related to visceral involvement and increased mortality. Methotrexate use has been supported in two randomized controlled trials (RCT) to improve MRSS compared to placebo. In patients with early dcSSc, EULAR/EUSTAR recommends the use of methotrexate [15]. Although there is increasing use of mycophenolate mofetil for dcSSc there is lack of a RCT. In our practice, we use methotrexate orally or via a subcutaneous route at doses up to 30mg/week, especially in patients with no to mild ILD and inflammatory polyarthritis (seen in approximately 30% of patients). We reserve mycophenolate mofetil for progressive skin disease with mild to moderate ILD without evidence of inflammatory polyarthritis, and pulse cyclophosphamide for progressive skin disease with moderate to severe ILD (typically anti-Scl-70 antibody positive) [1]. Duration of treatment for skin fibrosis, based on natural history, is typically 3 to 5 years.
6. Does every patient with SSc-ILD need treatment? How should I treat SSc-ILD and for how long?
Cardiopulmonary involvement, either due to ILD or PAH, is the leading cause of death of SSc patients [16]. Progressive ILD tends to occur early in disease and is a predictor of mortality. In patients with severe pulmonary fibrosis (FVC <50%), the most rapid decline occurs within the first 4 to 5 years of disease. ILD typically affects the bases of the lungs. Up to 90% of patients with SSc have features of ILD on high resolution CT (HRCT). Severe restrictive lung disease occurs in 42% of dcSSc patients and 22% of lcSSc patients [17]. However, not every patient with SSc associated ILD needs immunosuppressive therapy [1]. Those with SSc who are dyspneic and within the first 3 to 5 years of disease after they have developed their first sign or symptom attributable to SSc should be treated if they have had a decline in their FVC of 10% over the last 12 months, an FVC < 70–75% at time of diagnosis, or moderate fibrosis (>20%) or total lung involvement on HRCT. Our approach to treatment of mild ILD is with mycophenolate mofetil at 2 to 3 grams/day for 3 to 5 years. For moderate to severe ILD with normal renal function, we prescribe monthly pulse cyclophosphamide at 500 to 750mg/m2 for 6 to 12 months. After completion of cyclophosphamide infusions, we usually switch to mycophenolate mofetil at 2 to 3 grams daily or azathioprine at 2 to 3 mg/kg/day (if mycophenolate mofetil is not approved or is unavailable) with a plan to continue this for 3 to 5 years. Spirometry with DLCO should be repeated every 3 to 4 months while on treatment [1, 18]. In resistant cases, rituximab can be prescribed as a salvage therapy based on small studies, or patients can be offered autologous hematopoietic stem cell transplantation [19].
7. How do I screen for PAH?
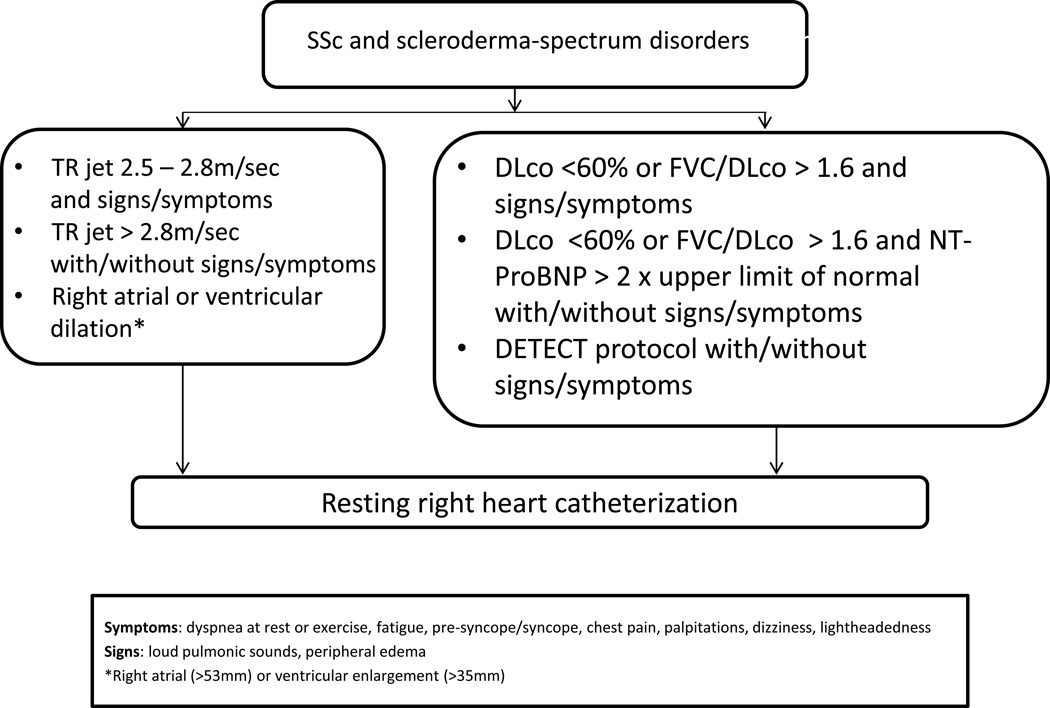
PAH is characterized by elevated pulmonary arterial pressure, which can eventually lead to right ventricular failure. Historically it had a 3 year survival rate of 50% to 60% and is a cause of death in approximately 8 to 12% of patients [1]. Survival has improved in recent times [20], which is likely related to increased awareness and earlier treatment. It is thought that poor prognosis may be due to late diagnosis of PAH [21]. We recommend that all patients with SSc or other connective tissue diseases with scleroderma features be screened for PAH [22]. Initial screening tests to order include transthoracic echocardiogram, spirometry with DLCO, and NT-Pro BNP. If patients have a DLCO <60% and have a disease duration of >3 years, the DETECT algorithm should be used (Figure 2) [22].
Figure 2.
Recommendations – Referral for RHC
Modified from [22].
The gold standard for diagnosis of PAH is RHC. Patients with SSc should have echo with Doppler and spirometry with DLCO yearly [22]. There are 3 main therapies for PAH: endothelian receptor antagonists (bosentan, macitentan, and ambrisentan), phosphodiesterase-5 (PDE5) inhibitors/soluble guanylate cyclase stimulators (sildenafil, tadalafil, and riociguat), and prostacyclins (iloprost, epoprostenol, and treprostinil) [1].
8. Who is at risk for scleroderma renal crisis? Does prophylactic ACE inhibitor use prevent scleroderma renal crisis?
Scleroderma renal crisis (SRC) is a feared complication in early SSc. It should be suspected in any patient with SSc with a new increase in blood pressure and rising creatinine. The classic presentation is early disease with progressive skin disease with the presence of anti-RNA polymerase III antibody. The treatment of choice is ACE inhibitors. However, the prophylactic use of ACE inhibitors has not shown improved outcomes and is generally not recommended [23]. Patients suspected of having SRC should be admitted to a hospital and treated with short acting ACE inhibitors, such as captopril. The goal is to normalize blood pressure (<120–140/ 70–90), so the dose should be quickly up-titrated every few hours as needed. If ACE inhibitors are not achieving goal blood pressure control, other agents such as calcium channel blockers or furosemide may be used. Beta blockers are generally contraindicated. Dialysis can be used as needed, but if someone develops the need for chronic dialysis, they should wait 18 months prior to renal transplant [1].
9. How do I manage resistant RP and digital ulcers?
RP is the most prevalent manifestation of SSc. It is present in approximately 95% of patients with SSc. The main pharmacologic treatment regimen of RP and digital ulcers are calcium channel blockers. Nifedipine has the most evidence supporting its use currently, but amlodipine is frequently used. PDE5 inhibitor use is supported by meta-analysis and topical nitroglycerin patches and fluoxetine have also been used [1, 24].
For digital ulcers and RP attacks, PDE5 inhibitors, such as sildenafil and tadalafil, and prostacyclins have been shown to be helpful. The standard regimen for prostacyclin infusions include epoprostenol infusion for 6 hours daily as an outpatient (in Europe) or inpatient (in US) for 3 to 5 days. In cases of threatened ischemic digit, we recommend to admit the patient to a hospital and begin prostacyclin for 5 days along with aspirin or heparin and a PDE5 inhibitor. If the ischemia is not improving on that regimen, then digital sympathectomy and/or botulinum toxin injection can be considered as well [1].
10. How can I manage gastrointestinal tract involvement in SSc?
SSc involvement of the gastrointestinal (GI) tract is common and affects up to 90% of patients. GI tract involvement ranges from asymptomatic disease to dysmotility causing severe malnutrition and has a varying time of onset and speed of progression [25, 26]. There are no treatments currently available to reverse the effects of SSc on the GI tract, but there are multiple therapies to assist in symptom management to improve quality of life.
The esophagus is the most commonly involved organ of the GI tract in SSc and is often involved early in disease. Gastroesophageal reflux disease (GERD) is due to esophageal dysmotility, gastric dysmotility and/or abnormal function of the lower esophageal sphincter (LES) [25, 27]. Treatment of GERD is to provide symptomatic relief, prevent the complications of erosions such as strictures and malignancy, and prevent aspiration events that may lead to or exacerbate ILD [25]. Non-pharmacologic therapy should be advocated for all patients and involves the avoidance of aggravating foods, avoidance of eating prior to sleep, and elevating the head of the bed [25, 27]. Medications typically used for GERD treatment include proton pump inhibitors (PPI) and/or H2 receptor antagonists (H2RA). PPI are often used initially and if no response at once daily dosing, the dose can be increased to twice daily, and even a different PPI can be tried. If symptoms are still not controlled with twice daily PPI, a H2RA can be added in the evening and a referral to a gastroenterologist for endoscopy and/or esophageal pH testing should be made [28]. Patients with Barrett’s esophagus should be screened regularly with endoscopy with biopsies to evaluate for malignancy.
Gastric involvement is manifested by gastric antral vascular ectasia (GAVE) and gastroparesis. GAVE presents with iron deficiency anemia and/or gross hemorrhage. Treatment is with iron supplementation, blood transfusions, and/or endoscopic interventions such as laser photocoagulation and argon plasma coagulation (APC) [29]. Gastroparesis occurs in approximately 50% of patients with SSc [30]. Non-pharmacologic therapy includes appropriate hydration, electrolyte balance, avoidance of fatty foods, low fiber diet, and frequent small meals. Before pharmacologic therapy is instituted, an objective diagnosis should be confirmed. Pharmacologic therapy includes prokinetic and anti-emetic medications. Various prokinetics include metoclopramide, domperidone, and erythromycin; however, these agents have significant potential side effects, which must be carefully reviewed for the appropriateness in each patient case. Gastroparesis refractory to pharmacologic management resulting in significant malnutrition may eventually require supplemental enteral nutrition through a jejunostomy tube or even parenteral nutrition if the small bowel is not functional [25, 31, 32].
Intestinal dysmotility affects a significant portion of patients with SSc which can result in small intestinal bacterial overgrowth (SIBO) and less commonly intestinal pseudo-obstruction, and pneumatosis cystoides intestinales. SIBO should be suspected if there is evidence of malnutrition. SIBO can be diagnosed by jejunal aspirates or hydrogen breath testing, but some providers will treat empirically with antibiotics if the diagnosis is suspected due to difficulty with testing [29]. The duration of antibiotic therapy for treatment of SIBO depends on the response and often requires rotating of antibiotics to prevent resistance [25]. Probiotics have been used as well in SIBO. If malnutrition progresses, parenteral nutrition may need to be considered. Intestinal pseudo-obstruction can be managed with bowel rest, nutritional support, electrolyte correction, antibiotics if SIBO is suspected, prokinetics, and consideration of decompression [33].
SSc patients can have colorectal involvement presenting as constipation or fecal incontinence. Initially, patients present with constipation from slow colonic motility but later can develop diarrhea due to SIBO. Fecal incontinence can affect up to 38% of patients due to connective tissue deposition and impaired functioning of the internal anal sphincter [25, 30]. Treatment of fecal incontinence includes management of diarrhea, biofeedback training, pelvic floor exercises and sacral nerve stimulation [25].
11. Can biologics be used in SSc?
Various biologics have been tried for various organ manifestations in SSc. There have been case reports and case series showing promising data. Observational studies and a small RCT revealed that rituximab is beneficial in SSc-ILD and skin fibrosis [19, 34]. In addition, a significant improvement in calcinosis has been noticed with rituximab [35, 36]. Some studies show that TNF-α antagonists, such as infliximab and etanercept, can result in improvement in inflammatory arthritis. However, there have been some reports raising concern for TNF-α antagonists possibly increasing fibrosis, malignancy risk, and causing drug-induced lupus in SSc patients [34, 37, 38]. Tocilizumab and abatacept have been effective in inflammatory arthritis in a EUSTAR study [39].
12. What is the role of autologous hematopoietic stem cell transplantation in aggressive SSc?
An upcoming treatment option for patients with early progressive SSc is autologous hematopoietic stem cell transplantation (HSCT). The rationale of HSCT is to eradicate the immune response causing disease with the goal of creating a new and non-auto reactive immune system. The ASTIS trial was a RCT of HSCT versus monthly pulse cyclophosphamide in patients with early dcSSc. The results of this study revealed that patients who received HSCT had an improved long-term event-free survival and overall survival at the median follow up of approximately 6 years and improvements in MRSS, FVC% and patient reported outcomes at 2 years. However, the HSCT group had more treatment related mortality (10.1%), all within the first 60 days of transplant. There was an association of smoking and an increase in mortality. SSc patients who are ideal candidates for HSCT may be those non-smokers whose disease did not respond to standard treatment along with either of the following characteristics: dcSSc within the first 4 to 5 years with mild to moderate organ involvement or lcSSc with progressive visceral involvement [40]. Currently, HSCT is performed as part of an IRB protocol at various scleroderma centers in the United States.
13. Is pregnancy safe in SSc?
SSc affects more woman than men and often manifests during childbearing years, making pregnancy an important topic to discuss with women of childbearing age who have SSc. It was previously thought that pregnancy in SSc was associated with deleterious outcomes for the mother and the child; however, new studies have emerged over time revealing that pregnancies in SSc can have good maternal and fetal outcomes. However, pregnant woman with SSc do have higher risk of pre-term delivery, intrauterine growth restriction and low birth weight [41]. In a retrospective case control study, there was an increase in frequency of premature delivery in patients with SSc or RA compared to healthy controls and it occurred more frequently in those SSc patients who had disease onset prior to pregnancy, and the delivery of small, full term infants occurred more frequently in patients with SSc than RA and healthy controls [42]. In a prospective SSc pregnancy study, 65% of those with early dcSSc had preterm delivery [42]. In an Italian multicenter study of 99 women with SSc who were pregnant, they were at a significantly increased risk for pre-term delivery, intrauterine growth restriction, and very low birth weight compared to controls [41].
Pregnancy does not cause progressive visceral involvement in SSc often; for example, in a prospective study by Steen, 60% had stable disease, 20% had improvement in disease, and 20% had some worsening of disease during pregnancy. Also, pregnancy does not appear to affect the 10 year survival compared to the survival of those with SSc who were not pregnant [43]. However, SRC is the most common cause of maternal death during pregnancy. Pregnant women with SSc most at risk for SRC have early dcSSc. ACE inhibitors have successfully treated SRC during pregnancy and must be used to prevent maternal death, despite the risk of congenital malformations with using an ACE inhibitor during pregnancy. RP is often improved during pregnancy, but can worsen post-partum. Pregnant patients with SSc with cardiopulmonary involvement are at high risk similar to those patients without SSc who have cardiopulmonary disease [42].
Thus, women with SSc who become pregnant are at high risk for premature and small, full-term infants. Those who have early disease, dcSSc, anti-Scl-70 antibody and/or anti-RNA polymerase III antibody are particularly at increased risk [41, 42, 43]. Those women with dcSSc, and particularly early disease, should be encouraged to delay pregnancy until later in disease when their risk for visceral complications is lower [41, 42]. Any patient with SSc who becomes pregnant should be followed closely by a maternal fetal medicine specialist.
Conclusion
SSc is a disease with significant morbidity and mortality that has varied disease presentations often leading to late diagnosis. In this review, we have answered some commonly asked questions a rheumatologist may have regarding SSc. There is increasing evidence for the importance of detection of disease earlier in onset, particularly with the evaluation for positive ANA by IF, RP, puffy fingers, SSc-specific autoantibodies and nailfold capillaroscopy suggestive of SSc pattern. Once a diagnosis of SSc has been made, aggressive and serial screening for organ involvement is essential as we now have various organ based treatment options, which can lead to improved prognosis.
Acknowledgments
CONFLICTS
Dr. Khanna has/had consultancy relationship with and/or has received research funding from Actelion, Bayer, Biogen Idec, Bristol Myers Squibb, Celgene, Cytori, EMD Serono, Genentech/ Roche, Gilead, Glaxo SmithKline, InterMune, Lycera, Medac, Sanofi-Aventis/Genzyme, and United Therapeutics.
FUNDING: D. Khanna is supported by NIH/NIAMS K24 AR063120
References
- 1.Nagaraja V, Denton CP, Khanna D. Old medications and new targeted therapies in systemic sclerosis. Rheumatology (Oxford) 2014 doi: 10.1093/rheumatology/keu285. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clements PJ. Systemic sclerosis (scleroderma) and related disorders: clinical aspects. Baillieres Best Pract Res Clin Rheumatol. 2000;14(1):1–16. doi: 10.1053/berh.1999.0074. [DOI] [PubMed] [Google Scholar]
- 3.Shanmugam VK, Swistowski DR, Saddic N, et al. Comparison of indirect immunofluorescence and multiplex antinuclear antibody screening in systemic sclerosis. Clin Rheumatol. 2011;30(10):1363–1368. doi: 10.1007/s10067-011-1766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho KT, Reveille JD. The clinical relevance of autoantibodies in scleroderma. Arthritis Res Ther. 2003;5(2):80–93. doi: 10.1186/ar628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements PJ, Furst DE. Systemic Sclerosis. Baltimore, MD: Williams and Wilkins; 2003. [Google Scholar]
- 6.Van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72(11):1747–1755. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 7.Hachulla E, Launay D. Diagnosis and classification of systemic sclerosis. Clin Rev Allergy Immunol. 2011;40(2):78–83. doi: 10.1007/s12016-010-8198-y. [DOI] [PubMed] [Google Scholar]
- 8.Lonzetti LS, Joyal F, Raynauld JP, et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum. 2001;44(3):735–736. doi: 10.1002/1529-0131(200103)44:3<735::AID-ANR125>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 9.Walker JG, Pope J, Baron M, et al. The development of systemic sclerosis classification criteria. Clin Rheumatol. 2007;26(9):1401–1409. doi: 10.1007/s10067-007-0537-x. [DOI] [PubMed] [Google Scholar]
- 10.Avouac J, Fransen J, Walker UA, et al. Preliminary criteria for the very early diagnosis of systemic sclerosis: results of a Delphi Consensus Study from EULAR Scleroderma Trials and Research Group. Ann Rheum Dis. 2011;70(3):476–481. doi: 10.1136/ard.2010.136929. [DOI] [PubMed] [Google Scholar]
- 11.Kuwana M, Okano Y, Kaburaki J, et al. Racial differences in the distribution of systemic sclerosis-related serum antinuclear antibodies. Arthritis Rheum. 1994 Jun;37(6):902–906. doi: 10.1002/art.1780370619. [DOI] [PubMed] [Google Scholar]
- 12.Meyer OC, Fertig N, Lucas M, et al. Disease subsets, antinuclear antibody profile, and clinical features in 127 French and 247 US adult patients with systemic sclerosis. J Rheumatol. 2007 Jan;34(1):104–109. [PubMed] [Google Scholar]
- 13.Koenig M, Joyal F, Fritzler MJ, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud's phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008;58(12):3902–3912. doi: 10.1002/art.24038. [DOI] [PubMed] [Google Scholar]
- 14.Khanna D, Denton CP. Evidence-based management of rapidly progressing systemic sclerosis. Best Pract Res Clin Rheumatol. 2010 Jun;24(3):387–400. doi: 10.1016/j.berh.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowal-Bielecka O, Landewé R, Avouac J, et al. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR) Ann Rheum Dis. 2009;68(5):620–628. doi: 10.1136/ard.2008.096677. [DOI] [PubMed] [Google Scholar]
- 16.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66(7):940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nihtyanova SI, Schreiber BE, Ong VH, et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol. 2014 Jun;66(6):1625–1635. doi: 10.1002/art.38390. [DOI] [PubMed] [Google Scholar]
- 18.Rao V, Khanna D. Scleroderma and Fibrosing Disorders: Advances in Management. Int J Adv Rheumatol. 2010;8(2):53–62. [Google Scholar]
- 19.Daoussis D, Liossis SN, Tsamandas AC, et al. Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology (Oxford) 2010;49(2):271–280. doi: 10.1093/rheumatology/kep093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung L, Chen H, Khanna D, et al. Dyspnea assessment and pulmonary hypertension in patients with systemic sclerosis: utility of the University of California, San Diego, Shortness of Breath Questionnaire. Arthritis Care Res (Hoboken) 2013;65(3):454–463. doi: 10.1002/acr.21827. [DOI] [PubMed] [Google Scholar]
- 21.Humbert M, Yaici A, de Groote P, et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival. Arthritis Rheum. 2011;63(11):3522–3530. doi: 10.1002/art.30541. [DOI] [PubMed] [Google Scholar]
- 22.Khanna D, Gladue H, Channick R, et al. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum. 2013;65(12):3194–3201. doi: 10.1002/art.38172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penn H, Howie AJ, Kingdon EJ, et al. Scleroderma renal crisis: patient characteristics and long-term outcomes. QJM. 2007;100(8):485–494. doi: 10.1093/qjmed/hcm052. [DOI] [PubMed] [Google Scholar]
- 24.Roustit M, Blaise S, Allanore Y, et al. Phosphodiesterase-5 inhibitors for the treatment of secondary Raynaud's phenomenon: systematic review and meta-analysis of randomised trials. Ann Rheum Dis. 2013;72(10):1696–1699. doi: 10.1136/annrheumdis-2012-202836. [DOI] [PubMed] [Google Scholar]
- 25.Butt S, Emmanuel A. Systemic sclerosis and the gut. Expert review of gastroenterology & hepatology. 2013;7(4):331–339. doi: 10.1586/egh.13.22. [DOI] [PubMed] [Google Scholar]
- 26.Sallam H, McNearney TA, Chen JD. Systematic review: pathophysiology and management of gastrointestinal dysmotility in systemic sclerosis (scleroderma) Alimentary pharmacology & therapeutics. 2006;23(6):691–712. doi: 10.1111/j.1365-2036.2006.02804.x. [DOI] [PubMed] [Google Scholar]
- 27.Ebert EC. Esophageal disease in scleroderma. Journal of clinical gastroenterology. 2006;40(9):769–775. doi: 10.1097/01.mcg.0000225549.19127.90. [DOI] [PubMed] [Google Scholar]
- 28.Tutuian R, Castell DO. Nocturnal Acid Breakthrough -- Approach to Management. Med Gen Med. 2004;6(4):11. [PMC free article] [PubMed] [Google Scholar]
- 29.Gyger G, Baron M. Gastrointestinal manifestations of scleroderma: recent progress in evaluation, pathogenesis, and management. Curr Rheumatol Rep. 2012;14(1):22–29. doi: 10.1007/s11926-011-0217-3. [DOI] [PubMed] [Google Scholar]
- 30.Domsic R, Fasanella K, Bielefeldt K. Gastrointestinal manifestations of systemic sclerosis. Dig Dis Sci. 2008;53(5):1163–1174. doi: 10.1007/s10620-007-0018-8. [DOI] [PubMed] [Google Scholar]
- 31.Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108(1):18–37. doi: 10.1038/ajg.2012.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forbes A, Marie I. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatology (Oxford) 2009;48(Suppl 3):iii36–iii39. doi: 10.1093/rheumatology/ken485. [DOI] [PubMed] [Google Scholar]
- 33.Rose S, Young MA, Reynolds JC. Gastrointestinal manifestations of scleroderma. Gastroenterol Clin North Am. 1998;27(3):563–594. doi: 10.1016/s0889-8553(05)70021-2. [DOI] [PubMed] [Google Scholar]
- 34.Cappelli S, Bellando-Randone S, Guiducci S, et al. Is immunosuppressive therapy the anchor treatment to achieve remission in systemic sclerosis? Rheumatology (Oxford) 2014;53(6):975–987. doi: 10.1093/rheumatology/ket312. [DOI] [PubMed] [Google Scholar]
- 35.de Paula DR, Klem FB, Lorencetti PG, et al. Rituximab-induced regression of CREST-related calcinosis. Clin Rheumatol. 2013;32(2):281–283. doi: 10.1007/s10067-012-2124-z. [DOI] [PubMed] [Google Scholar]
- 36.Daoussis D, Antonopoulos I, Liossis SN, et al. Treatment of systemic sclerosis-associated calcinosis: a case report of rituximab-induced regression of CREST-related calcinosis and review of the literature. Semin Arthritis Rheum. 2012;41(6):822–829. doi: 10.1016/j.semarthrit.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Omair MA, Phumethum V, Johnson SR. Long-term safety and effectiveness of tumour necrosis factor inhibitors in systemic sclerosis patients with inflammatory arthritis. Clin Exp Rheumatol. 2012;30(2 Suppl 71):S55–S59. [PubMed] [Google Scholar]
- 38.Phumethum V, Jamal S, Johnson SR. Biologic therapy for systemic sclerosis: a systematic review. J Rheumatol. 2011;38(2):289–296. doi: 10.3899/jrheum.100361. [DOI] [PubMed] [Google Scholar]
- 39.Elhai M, Meunier M, Matucci-Cerinic M, et al. Outcomes of patients with systemic sclerosis-associated polyarthritis and myopathy treated with tocilizumab or abatacept: a EUSTAR observational study. Ann Rheum Dis. 2013;72(7):1217–1220. doi: 10.1136/annrheumdis-2012-202657. [DOI] [PubMed] [Google Scholar]
- 40.Khanna D, Georges GE, Couriel DR. Autologous hematopoietic stem cell therapy in severe systemic sclerosis: ready for clinical practice? JAMA. 2014 Jun 25;311(24):2485–2487. doi: 10.1001/jama.2014.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taraborelli M, Ramoni V, Brucato A, et al. Brief report: successful pregnancies but a higher risk of preterm births in patients with systemic sclerosis: an Italian multicenter study. Arthritis Rheum. 2012;64(6):1970–1977. doi: 10.1002/art.34350. [DOI] [PubMed] [Google Scholar]
- 42.Steen VD. Pregnancy in scleroderma. Rheum Dis Clin North Am. 2007;33(2):345–358. doi: 10.1016/j.rdc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Lidar M, Langevitz P. Pregnancy issues in scleroderma. Autoimmun Rev. 2012;11(6–7):A515–A519. doi: 10.1016/j.autrev.2011.11.021. [DOI] [PubMed] [Google Scholar]